Abstract
Evidence suggests that chronic low level cadmium exposure impairs the function of insulin-producing β cells and may be associated with type-2 diabetes mellitus. Herein, we describe the cadmium content in primary human islets and define the uptake kinetics and effects of environmentally relevant cadmium concentrations in cultured β cells. The average cadmium content in islets from 10 non-diabetic human subjects was 29 ± 7 nmol/g protein (range 7 to 72 nmol/g protein). Exposure of the β-cell line MIN6 to CdCl2 concentrations between 0.1 and 1.0 µmol/L resulted in a dose- and time-dependent uptake of cadmium over 72 h. This uptake resulted in an induction of metallthionein expression, likely enhancing cellular cadmium accumulation. Furthermore, cadmium accumulation resulted in an inhibition of glucose stimulated insulin secretion in MIN6 cells and primary mouse islets. Our results indicate that this impairment in β-cell function is not due to an increase in cell death or due to an increase in oxidative stress. We conclude that mouse β cells accumulate cadmium in a dose- and time-dependent manner over a prolonged time course at environmentally relevant concentrations. This uptake leads to a functional impairment of β-cell function without significant alterations in cell viability, expression of genes important for β-cell function or increase in oxidative stress.
Keywords: cadmium, insulin secretion, metallothionein, zinc transporters, β cells
Introduction
Impaired function of insulin-producing pancreatic β cells in the setting of insulin resistance is the main underlying cause of type-2 diabetes. β-cell dysfunction is thought to be multifactorial, with the interaction of genetic susceptibility, age, lifestyle, insulin resistance and environmental factors leading to progressive β-cell failure. Some evidence suggests that chronic low-level exposure to cadmium (Cd) in the environment may be associated with an increased risk for developing dysglycemia and diabetes mellitus.1,2 From a biological standpoint, Cd and to a lesser degree mercury (Hg) are of interest in the biology of insulin-secreting β cells given their similarity to zinc (Zn) and the importance of Zn in β-cell physiology. The divalent metals Cd and Hg are members of the same group as Zn in the periodic table and, therefore, exhibit similar chemical characteristics. In multiple cell types, Cd2+, and to a lesser degree Hg2+ have been shown to compete with Zn2+ for several of its binding proteins and transporters.3-10 β cells have high intracellular concentrations of Zn, particularly within secretory vesicles, where zinc facilitates the packaging of insulin into hexamers and may play a role in the processing of pro-insulin into mature insulin.11-15 Given that Zn is co-secreted with insulin, β cells have to maintain a high turnover of Zn, shuttling large quantities of Zn through the cytoplasm into the secretory vesicle to replenish Zn co-secreted with insulin.16,17 Given the abov,e we hypothesized that β cells may be more susceptible to the accumulation and potential toxicity of this group of divalent metals than other cell types. Therefore, our initial studies sought to determine the content of Cd and Hg in primary human islets from non-diabetic subjects. Additionally, we examined the accumulation kinetics of CdCl2 and HgCl2 in mouse β cells. These initial studies showed a significantly higher native content of Cd in human islets samples compared with Hg. Furthermore, mouse islets accumulated Cd more avidly than Hg. We therefore conducted more detailed studies to examine the accumulation kinetics and physiological effects of low level Cd exposure in insulin-producing β cells.
Results
Concentration of divalent metals in human islet
Low-level exposure to Hg and Cd is widely prevalent. At the same time, the divalent ions of these metals have been shown to compete with Zn transport and buffering mechanisms. Given this, our initial studies examined the content of the heavy metals Cd and Hg in human islets. Additional metals including Zn, copper (Cu) and nickel (Ni) were measured in order to validate our methods by comparing their expected concentration ratios with previously published reports. The concentration of Cd in islet samples was significantly higher than that of Hg (Table 1). Variations in divalent metal content were noted between the 10 human islet samples examined, suggesting a substantial influence of environmental exposure and other parameters such as genetic variations on islet cell metal content. Metal content in solutions used in the isolation, purification, culture and transport of islets contained no significant quantities of the metals of interest Hg and Cd (Table S1).
Table 1. Concentration of Cd, Hg, Cu, Ni and Zn in viable human islets from 10 non-diabetic subjects.
| Subject # (age, gender, BMI) | Cd | Hg | Cu | Ni | Zn |
|---|---|---|---|---|---|
| Human islets #1 (28y, m, BMI 24) |
16.1 nmol/g protein |
unavailable |
unavailable |
unavailable |
unavailable |
| Human islets #2 (27y, m, BMI 26) |
7.4 nmol/g protein |
unavailable |
unavailable |
unavailable |
unavailable |
| Human islets #3 (no data available) |
9.5 nmol/g protein |
unavailable |
unavailable |
unavailable |
unavailable |
| Human islets #4 (62 yo m, BMI 18.8) |
23.6 nmol/g protein |
5.0 nmol/g protein |
1397.0 nmol/g protein |
207.6 nmol/g protein |
4656.5 nmol/g protein |
| Human islets #5 (54y, f, BMI 28.4) |
15.8 nmol/g protein |
4.6 nmol/g protein |
470.7 nmol/g protein |
228.6 nmol/g protein |
18061.2 nmol/g protein |
| Human islets #6 (35y, m, BMI 46.1) |
23.8 nmol/g protein |
< 4.00nmol/g protein |
505.1 nmol/g protein |
153.5 nmol/g protein |
10981.7 nmol/g protein |
| Human islets #7 (52y, f, BMI 29.2) |
51.90 nmol/g protein |
< 4.0 nmol/g protein |
307.0 nmol/g protein |
169.7 nmol/g protein |
10432.7 nmol/g protein |
| Human islets #8 (44y, f, BMI 22.50) |
19.1 nmol/g protein |
< 4.0 nmol/g protein |
105.7 nmol/g protein |
32.8 nmol/g protein |
3645.7 nmol/g protein |
| Human islets #9 (39y, f, BMI 29.80) |
48.5 nmol/g protein |
< 4.0 nmol/g protein |
249.6 nmol/g protein |
110.7 nmol/g protein |
29861.5 nmol/g protein |
| Human islets #10 (56y, f, BMI 25.20) |
71.9 nmol/g protein |
< 4.0 nmol/g protein |
328.9 nmol/g protein |
66.6 nmol/g protein |
16916.4 nmol/g protein |
| Average human islet content |
28.8* ± 6.7 nmol/g protein |
< 4.0 nmol/g protein |
480.6 ± 160.9 nmol/g protein |
138.5 ± 27.3 nmol/g protein |
13508.0 ± 3420.0 nmol/g protein |
| Negative control: Cultured MIN6 cells (n = 4) | < 4.0 nmol/g protein | < 4.0 nmol/g protein | 266.8 ± 36.6 nmol/g protein | 58.1 ± 15.5 nmol/g protein | 3813.2 ± 560.2 nmol/g protein |
Values are reported as μmol/gram lysate protein. Values in cultured MIN6 cells are listed for comparison. Values for solutions used in the isolation, purification and transport of islets are reported in the supplemental material.
Accumulation kinetics of Hg and Cd
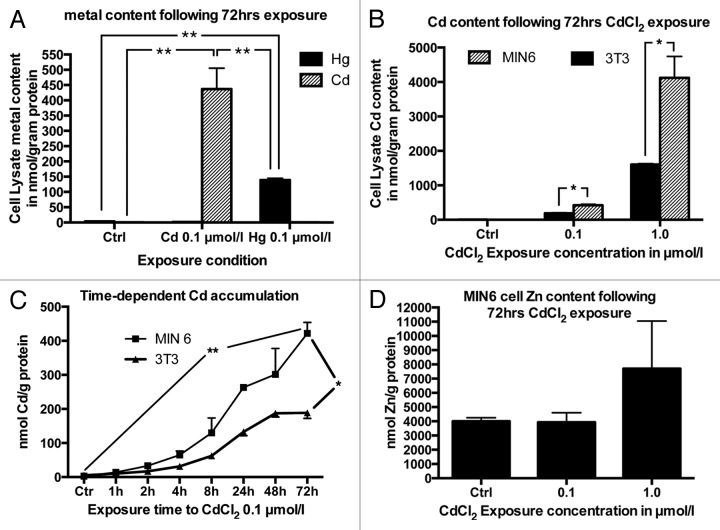
Next, we set out to confirm the higher accumulation capacity of β cells to Cd compared with Hg. To this end the mouse β-cell line MIN6 was exposed to CdCl2 or HgCl2 0.1 µmol/L for 48 h. The cells accumulated significantly more Cd compared with Hg under these circumstances to concentrations of 437 ± 69 and 139 ± 6 nmol/g protein respectively (Fig. 1A). Given this result and the higher Cd concentration in primary human islets, subsequent studies focused on examining the uptake kinetics and effects of Cd accumulation. In order to compare the uptake of Cd into β cells to that in a non-β-cell line, uptake in MIN6 cells was compared with that in the mouse fibroblast cell line 3T3. MIN6 cells and 3T3 cells were grown in MIN6 medium and exposed to final CdCl2 concentrations of 0, 0.1 or 1 µmol/L for 72 h. Both cells lines accumulated Cd in a dose dependent manner (Fig. 1B). MIN6 cells exposed to 0.1 and 1.0 µmol/L CdCl2 for 72 h accumulated Cd with lysate Cd concentrations of 422 ± 32 and 4125 ± 617 nmol/g protein respectively. For comparison, the mouse 3T3 fibroblasts accumulated Cd to a significantly lower concentration of 188 ± 16 and 1608 ± 26 nmol/g of protein under the same conditions.
Figure 1. (A) Comparison between Cd and Hg accumulation in MIN6 cells following exposure to Cd or Hg 0.1 µmol/L for 48 h (n = 4). (B) Comparison between 3T3 and MIN6 cell Cd accumulation following exposure to 0.1 or 1 µmol/L CdCl2 for 72 h compared with non-exposed control cells (n = 3). (C) Cd accumulation in MIN6 and 3T3 cells following exposure to 0.1 µmol/L CdCl2 for 1, 2, 4, 8, 24, 48 or 72 h (n = 3), (D) MIN6 cell Zn content following Cd exposure for 72 h: Changes in Zn concentration following exposure to 0.1 or 1 µmol/L CdCl2 for 72 h (n = 3). All metal concentrations are expressed as µmol/g protein *, p < 0.05, **, p < 0.01. The Student’s t-test with Bonferroni correction was used for comparison of Cd, Hg and Zn concentrations at 72 h (A, B and D). Two-way ANOVA was used to compare the time dependent rate of Cd accumulation between 3T3 and MIN6 cells (C).
We then performed studies to determine the time course of Cd accumulation. After exposure to 0.1 µmol/L CdCl2 for 1, 2, 4, 8, 24, 48 and 72 h, MIN6 and 3T3 cells accumulated Cd in a time dependent manner (Fig. 1C). The total amount of Cd accumulated as well as the accumulation rate in 3T3 cells was significantly lower than that of MIN6 cells.
No significant changes in Zn concentration were seen in MIN6 cells following exposure to CdCl2 except for a trend toward a higher Zn concentration following exposure to the higher CdCl2 concentration of 1.0 µmol/L, likely related to an upregulation of the Zn buffering protein metallothionein (see below). MIN6 cell Zn concentrations following exposure to 0, 0.1 or 1.0 µmol/L CdCl2 were 4005 ± 251, 3933 ± 676 and 7702 ± 3344 nmol/g protein, respectively (Fig. 1D).
mRNA levels of ZnT and ZIP class Zn transporters and DMT-1 in MIN6 and 3T3 cells
Cd transport into cells may be mediated, in part, by some members of the family of Zn transporters such as SLC39A8 (ZIP8), SLC39A14 (ZIP14) and SLC30A1 (ZnT1).6,8,18-20 Additionally, some reports suggest a role for the divalent metal transporter-1 (DMT-1) in cellular Cd influx in some cell types.21,22 To begin to determine possible reasons for the difference in Cd accumulation in MIN6 and 3T3 cells, expression of the genes encoding members of the ZIP and ZnT classes of Zn transporters as well as DMT-1 in MIN6, primary mouse islets and 3T3 cells was determined. As expected, 3T3 cells had lower overall levels of mRNAs encoding Zn transporters of the SLC39 (ZIP) and SLC30 (ZnT) family compared with MIN6 and primary mouse islet cells (Table 2). Moreover, ZnT8 (encoded by slc30A8), which was previously shown to be important for Zn transport into insulin secretory vesicles in β cells11,23-26 was highly expressed in MIN6 and primary mouse islet cells but not expressed in 3T3 cells. Other transporters that were noted to have a higher expression level in MIN6 and islet cells compared with 3T3 cells included SLC39A5 (ZIP5), SLC30A3 (ZnT3), SLC30A2 (ZnT2) as well as DMT-1. Levels of SLC39A8 (ZIP8), one of the transporters thought to potentially mediate Cd transport, were higher in MIN6 cells compared with both primary islets cells and 3T3 cells.
Table 2. Relative mRNA expression levels of Zn transporters of the ZnT and ZIP class as well as DMT-1 in various cells.
| |
Mouse islets |
MIN6 cells |
3T3 cells |
|---|---|---|---|
| Mean ± SEM | Mean ± SEM | Mean ± SEM | |
|
ZIP1 |
8.40 × 10-02 ± 1.07 × 10-02 |
1.47 × 10-02 ± 6.83 × 10-04 |
6.73 × 10-02 ± 3.57 × 10-03 |
|
ZIP2 |
4.94 × 10-05 ± 1.27 × 10-05 |
3.72 × 10-05 ± 1.26 × 10-05 |
1.27 × 10-05 ± 1.37 × 10-06 |
|
ZIP3 |
4.83 × 10-03 ± 2.61 × 10-04 |
2.94 × 10-03 ± 5.42 × 10-04 |
2.02 × 10-03 ± 2.14 × 10-04 |
|
ZIP4 |
7.05 × 10-04 ± 3.31 × 10-04 |
2.21 × 10-05 ± 2.21 × 10-05 |
1.50 × 10-05 ± 1.05 × 10-06 |
|
ZIP5 |
5.14 × 10-04 ± 1.52 × 10-04 |
1.29 × 10-05 ± 1.29 × 10-05 |
1.77 × 10-07 ± 5.74 × 10-08 |
|
ZIP6 |
9.84 × 10-04 ± 1.76 × 10-04 |
1.07 × 10-03 ± 2.27 × 10-04 |
2.76 × 10-04 ± 1.92 × 10-05 |
|
ZIP7 |
2.13 × 10-01 ± 4.76 × 10-02 |
1.04 × 10-01 ± 1.31 × 10-02 |
4.81 × 10-02 ± 1.80 × 10-03 |
|
ZIP8 |
1.33 × 10-03 ± 2.30 × 10-04 |
1.95 × 10-02 ± 3.06 × 10-03 |
7.15 × 10-03 ± 3.45 × 10-04 |
|
ZIP9 |
4.36 × 10-03 ± 1.40 × 10-03 |
3.78 × 10-03 ± 6.17 × 10-04 |
4.21 × 10-03 ± 2.39 × 10-04 |
|
ZIP10 |
5.64 × 10-04 ± 1.37 × 10-04 |
1.28 × 10-03 ± 1.37 × 10-04 |
4.29 × 10-03 ± 1.17 × 10-04 |
|
ZIP11 |
3.77 × 10-03 ± 1.37 × 10-03 |
2.89 × 10-04 ± 2.90 × 10-06 |
1.49 × 10-03 ± 1.15 × 10-04 |
|
ZIP12 |
3.33 × 10-08 ± 3.33 × 10-08 |
3.33 × 10-08 ± 3.33 × 10-08 |
1.14 × 10-07 ± 4.14 × 10-08 |
|
ZIP13 |
8.59 × 10-03 ± 2.68 × 10-03 |
3.56 × 10-03 ± 4.33 × 10-04 |
6.21 × 10-03 ± 6.88 × 10-04 |
|
ZIP14 |
2.35 × 10-03 ± 2.58 × 10-05 |
1.49 × 10-03 ± 2.81 × 10-04 |
3.01 × 10-03 ± 1.71 × 10-04 |
|
ZnT1 |
4.38 × 10-03 ± 1.71 × 10-03 |
2.79 × 10-03 ± 4.10 × 10-04 |
1.34 × 10-03 ± 7.04 × 10-05 |
|
ZnT2 |
4.88 × 10-04 ± 7.66 × 10-05 |
1.29 × 10-05 ± 1.08 × 10-05 |
2.34 × 10-06 ± 1.92 × 10-06 |
|
ZnT3 |
1.82 × 10-05 ± 1.41 × 10-05 |
5.68 × 10-06 ± 3.17 × 10-06 |
6.95 × 10-08 ± 8.89 × 10-09 |
|
ZnT4 |
1.75 × 10-03 ± 1.34 × 10-04 |
2.27 × 10-03 ± 1.14 × 10-04 |
1.68 × 10-02 ± 5.13 × 10-04 |
|
ZnT5 |
1.37 × 10-02 ± 2.28 × 10-03 |
2.60 × 10-02 ± 3.27 × 10-03 |
7.75 × 10-03 ± 3.61 × 10-04 |
|
ZnT6 |
5.64 × 10-03 ± 8.13 × 10-04 |
5.20 × 10-03 ± 7.52 × 10-04 |
9.87 × 10-03 ± 3.96 × 10-04 |
|
ZnT7 |
1.01 × 10-02± 5.94 × 10-04 |
5.82 × 10-03 ± 8.71 × 10-04 |
1.62 × 10-02 ± 2.79 × 10-04 |
|
ZnT8 |
5.76 × 10-02± 2.33 × 10-02 |
1.16 × 10-01 ± 1.89 × 10-02 |
0.00 ± 0.00 |
|
ZnT9 |
2.59 × 10-03 ± 6.62 × 10-04 |
2.86 × 10-03 ± 2.01 × 10-04 |
8.00 × 10-03 ± 6.33 × 10-05 |
|
ZnT10 |
0.00 ± 0.00 |
0.00 ± 0.00 |
4.52 × 10-07 ± 3.06 × 10-07 |
| DMT-1 | 2.56 × 10-02 ± 6.98 × 10-03 | 3.38 × 10-02 ± 1.90 × 10-02 | 9.56 × 10-03 ± 9.22 X 10-05 |
mRNA levels of Zn transporters of the ZnT and ZIP families as well as DMT-1 in primary mouse islets, MIN6 cells and 3T3 relative to the house keeping genes β actin and 18S (n = 3).
Changes in metallothionein (MT) expression in response to exposure to Cd
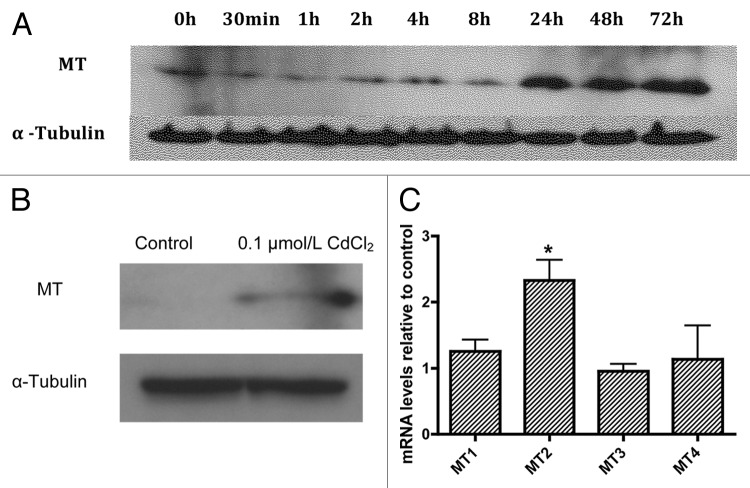
Next we examined whether expression of metallothionein (MT), the main known intracellular Cd buffering protein, is increased following exposure to 0.1µmol/L CdCl2. MT expression was determined using western blot analysis following exposure of MIN6 cells to CdCl2 0.1 µmol/L for 30 min or 1, 2, 4, 8, 24, 48 or 72 h (Fig. 2A). Increased MT expression was observed beginning at the 24 h time point and remained stable over the ensuing 48 h. The increased expression of MT following exposure to CdCl2 0.1 µmol/L was confirmed in primary mouse islets by western blot (Fig. 2B) and quantitative real time PCR (Fig. 2C) with MT protein and MT2 mRNA both showing significant increases. Contrary to prior reports showing upregulation of both MT2 and MT1 expression in various mouse tissues following Cd exposure,27 we did not observe a significant increase in MT1 mRNA levels.
Figure 2. Representative western blot analysis of metallothionein expression in MIN6 cells following exposure or not of MIN6 cells to 0.1µmol/L CdCl2 for 30 min or 1, 2, 4, 8, 24, 48 or 72 h (A, representative of n = 3). Changes in protein levels of total metallothionein (B, representative of n = 3) and mRNA levels of the metallothionein genes MT1 through MT4 (C, n = 5) in dispersed primary mouse islets following exposure to CdCl2 0.1 µmol/L for 48 h relative to control cells. *, p < 0.05 using the Student’s t-test with Bonferroni correction.
Effect of Cd exposure on glucose stimulated insulin secretion in MIN6 cells
In static incubation experiments, exposure of MIN6 cells to CdCl2 concentrations of 0.1, 0.5 and 1.0 µmol/L for 48 h only showed a trend toward a concentration dependent decrease in glucose stimulated insulin secretion (GSIS) with GSIS ratios of 5.6 ± 0.8, 2.8 ± 1.0 and 1.4 ± 0.1, respectively, compared with 5.6 ± 0.6 in the control (p < 0.05 for 1.0 µmol/L CdCl2 compared with control, Fig. 3A).
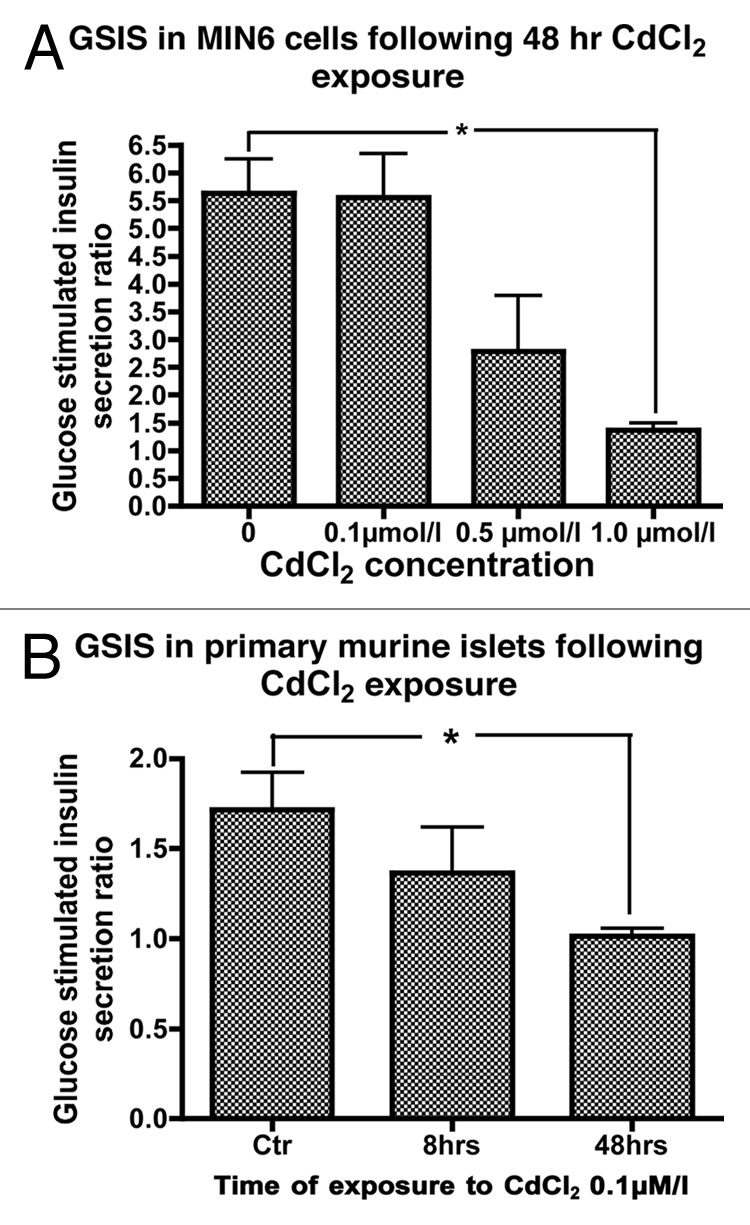
Figure 3. (A) GSIS in MIN6 cells following exposure or not to 0.1, 0.5 or 1.0 µmol/L CdCl2 for 48 h (n = 5, each in duplicate). *, p < 0.05 using Student’s t test with Bonferroni correction. (B) Glucose stimulated insulin secretion (GSIS) in primary mouse islet cells following exposure to 0.1 µmol/L CdCl2 for 8 or 48 h compared with control cells (n = 4, each in duplicate).
Effect of Cd exposure in dispersed primary mouse islet cells
Having established that MIN6 cells accumulate Cd followed by inhibition of GSIS, we sought to determine whether Cd had a similar effect on primary mouse islets. We therefore examined the effect of Cd exposure on GSIS in primary dispersed mouse islets. A non-significant trend for decreased GSIS was observed following exposure of dispersed mouse islet cells to CdCl2 0.1 µmol/L for 8 h with a decrease in GSIS from 1.7 ± 0.2 to 1.4 ± 0.3 in exposed cells. However, exposure of dispersed primary mouse islets to CdCl2 0.1 µmol/L for 48 h resulted in a significant decrease in GSIS to 1.0 ± 0.04 (Fig. 3B).
Effect of Cd exposure on the viability of dispersed primary mouse islet cells
To rule out generalized cell toxicity as the cause of the Cd-induced decrease in GSIS, the viability of dispersed mouse islet cells following exposure to 0.1µmol/L CdCl2 for 8 and 48 h was examined. Significant changes in cell viability at these time points were not observed. The cell viability index at 8 and 48 h was 809 ± 86 and 849 ± 100 compared with the control (783 ± 111, Fig. 4A). There was also no change in expression of the heavy metal toxicity marker Heat Shock Protein 70 (HSP70) at 48 h as determined by western blot analysis (Fig. 4B).
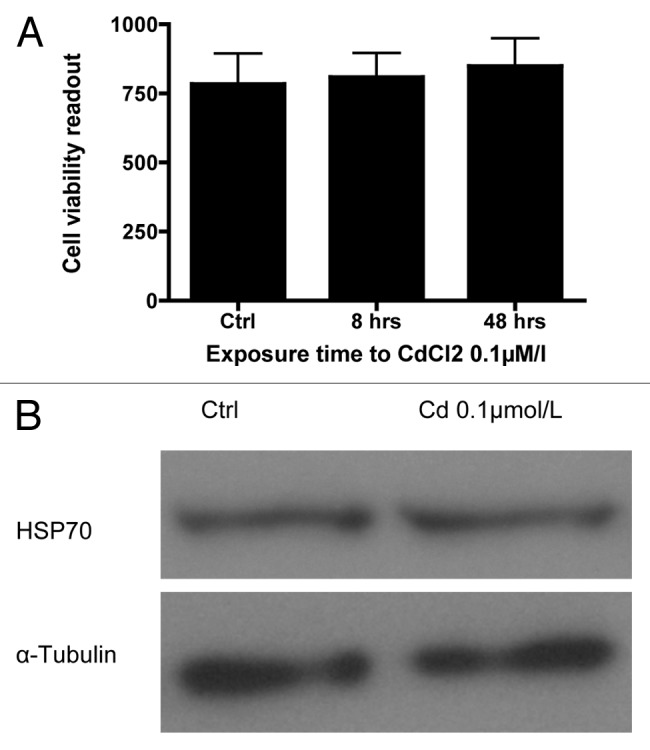
Figure 4. (A) Viability of dispersed primary mouse islet cells at 48 h: Ratio of viable to dead cells in dispersed primary mouse islets using the Multitox® assay following exposure to 0.1 µmol/L CdCl2 for 8 or 48 h compared with control (n = 3, each in triplicate). (B) Representative blot of the expression of the heavy metal toxicity marker Heat Shock Protein 70 (HSP70) in dispersed primary mouse islets following exposure to 0.1 µmol/L CdCl2 for 48 h compared with non-exposed control cells.
No effect of Cd exposure on the expression of genes most relevant to β-cell function in dispersed primary murine islets
To examine the effect of Cd exposure on the expression of genes central to normal β-cell function as well as Zn homeostasis in primary mouse islets, the expression of the genes encoding Insulin 1 and 2 (INS-1 and -2), MAFA, MAFB, was examined following exposure to 0.1µmol/L CdCl2 for 48 h. Given prior reports of altered regulation of genes relevant to β cell Zn and, possibly, Cd homeostasis, namely SLC39A10 (encoding ZIP10) and SLC30A1 (encoding ZnT1),19,28,29 we also examined the expression of these genes. Additionally, we examined the effect of exposure to CdCl2 0.1µmol/L on the mRNA level of SLC30A8 (ZnT8) and DMT-1 mRNA. All the genes examined showed no significant difference in mRNA expression levels apart from the increase in MT2 expression as noted above (Fig. S1).
Effect of Cd exposure on oxidative stress in primary dispersed mouse islets and MIN6 cells
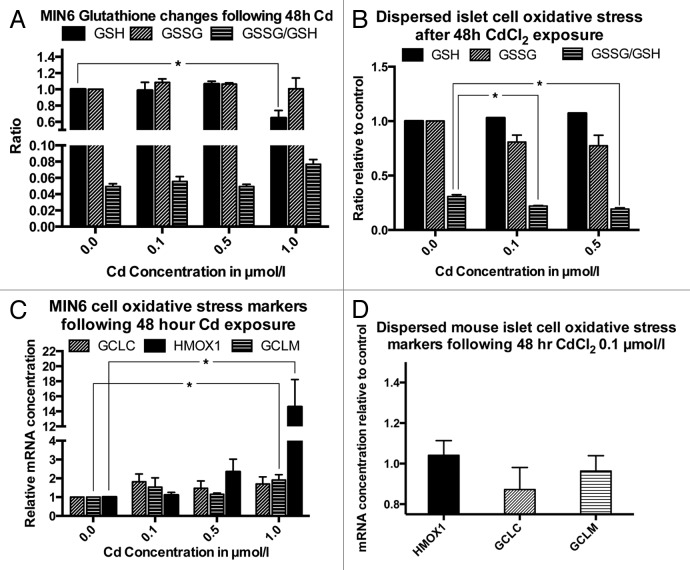
To examine the influence of Cd accumulation on cellular oxidative stress, we determined the levels of GSH, GSSG and the ratio of GSH/GSSG in MIN6 cells and primary dispersed mouse islets following exposure to various CdCl2 concentrations for 48 h. In MIN6 cells, these studies showed evidence for mild oxidative stress only following exposure to a CdCl2 concentration of 1.0 µmol/L, but not at the lower CdCl2 concentrations of 0.1 and 0.5 µmol/L. This increase in oxidative stress was evident from a significant decrease of GSH in cells exposed to 1.0 µmol/L to 64.7% ± 9% of the GSH level in untreated control cells (p < 0.05, Fig. 5A). There was also a non-significant trend toward an increase in the GSSG/GSH ratio from 0.05 ± 0.003 in the untreated control to 0.08 ± 0.006 under the same conditions. In contrast, there was no evidence for an increase in oxidative stress markers in dispersed primary mouse islet cells following exposure to CdCl2 at concentrations of 0.1 and 0.5 µmol/L. Surprisingly, the GSSG/GSH ratio in these cells decreased significantly at both CdCl2 concentrations from 0.31 ± 0.015 in the control cells to 0.22 ± 0.005 and 0.19 ± 001 in cells exposed to 0.1 and 0.5 µmol/L CdCl2 respectively, likely indicating an upregulation of cellular protective mechanisms (p < 0.05, Fig. 5B). Measurement of mRNA levels of the cellular oxidative response factors GCLC, GCLM and HMOX1 showed an increase of GCLM and HMOX1 mRNA levels only in MIN6 cells exposed to 1.0 µmol/L of CdCl2 (p < 0.05, Fig. 5C). No changes were observed in dispersed primary mouse islet cells exposed to 0.1 µmol/L CdCl2 for 48 h (Fig. 5D).
Figure 5. Oxidative stress and oxidative stress markers following exposure to CdCl2 for 48 h: GSH, GSSG (both normalized to the non-exposed control) and GSSG/GSH ratio in MIN6 cells (A, n = 4, each in triplicate) and dispersed primary mouse islet cells (B, n = 3, each in triplicate). mRNA levels of the oxidative response genes GCLC, HMOX1 and GCLM in MIN6 cells (C, n = 4) and in dispersed primary mouse islets (n = 4). *, p < 0.05 using Student’s t-test with Bonferroni correction.
Discussion
Zinc (Zn) is the second most abundant transition metal in mammals30-32 and is utilized in numerous cellular processes.33 Zn is of particular importance in the physiology of insulin-producing β cells given its unique role in this cell type.11-13,23,34 Beta cells have high intracellular concentrations of Zn, particularly within secretory vesicles, where zinc facilitates the packaging of insulin into hexamers.11-14 Given that Zn is co-secreted with insulin, β cells are required to maintain a high Zn turnover rate. In order to achieve this, β cells require the trafficking of Zn across cell and vesicular membranes. This is thought to occur mainly through members of the Zinc-Transporter (ZnT, SLC30A) and Zrt-Irt-like Protein (ZIP, SLC39A) transporter families. ZnTs and ZIPs facilitate Zn diffusion out of or into the cytoplasm, respectively.33,35 Within the cell, Zn is mostly bound to buffering proteins, of which metallothionein (MT) is thought to have the highest capacity, thereby acting as a buffer and reservoir for intracellular Zn.36-38 Zn, Cd and Hg belong to the same group of divalent metals in the periodic table and have similar chemical properties. It is therefore not surprising that Cd and—to a lesser extent—Hg have been shown to compete with Zn for transporters of the ZnT and ZIP families as well as for binding to MT.3-10,19,28,29,39-43 Specifically, the Zn transporters ZIP8, ZIP14 and ZnT1 have been shown to transport Cd at high rates.6,18-20 Kinetic and competition studies by He et al. showed that ZIP8 has a high capacity to transport Cd but a lower capacity for Hg transport.8 Although we do not have conclusive data from knockout or knockdown models, it is conceivable that Zn transporters play a significant role in β-cell heavy metal accumulation in β cells analogous to tubular renal cells and testicular cells where ZIP8 has been showing to play a significant role in Cd accumulation and toxicity.6,18 Another potential mechanism for Cd accumulation in β cells is DMT-1, a transporter previously reported to have a relatively high capacity for Cd transport but –to our knowledge- no prior reports of Hg transport capacity.21,22 Our current study shows a relatively high abundance of ZIP8 and DMT-1 mRNA in β cells. Under this assumption, our results showing a less avid accumulation of Hg compared with Cd are expected given the lower affinity of Hg for MT, DMT-1 and ZIP class transporters. To our knowledge, our report is the first description of human islet content of the metals measured in the current study.
Given this difference in the accumulation of Cd and Hg, our subsequent studies focused on the accumulation kinetics and effects of Cd. These studies showed that the mouse β-cell line MIN6 accumulates Cd in a time- and dose-dependent manner over 72 h. To the best of our knowledge, these studies are the first report exploring the accumulation kinetics of Cd in insulin-producing β cells. For comparison, we show that a mouse fibroblast cell line accumulated Cd less avidly with a lower rate of accumulation and a lower steady-state peak concentration. Cd has been shown to bind MT with an affinity that exceeds that of Zn while at the same time inducing expression of MT.5,7,39,43-48 Cd binding to MT is commonly thought of as a cellular defense mechanism by reducing the level of free intracellular Cd.40,41,43,45,49 However, the induction of MT expression previously reported by others and confirmed by our current studies is thought to prolong the intracellular half-life of Cd substantially.7,47,49 It is likely that the induction of MT expression starting at 24 h contributes to the substantial increase in the Cd accumulation observed following 24, 48 and 72 h of exposure.
In our experiments, Cd accumulation did not alter the total cell Zn concentration. Remarkably, this was also true following exposure of MIN6 cells to a high concentration of CdCl2, 1.0 µmol/L, which resulted in accumulation of Cd to concentrations approaching those of Zn. It is likely that the observed increase in MT levels compensated for any displacement of Zn from MT binding sites by Cd, thereby maintaining a stable overall Zn concentration despite displacement of Zn from some of its MT binding sites. It has been described that Zn bound to MT’s seven Zn binding sites are donated to other acceptor proteins in a fixed, predetermined order.50,51 Given the likely role of MT as a Zn reservoir,38,52,53 it is uncertain what the net effect of Zn displacement from MT in combination with increased MT expression on the intracellular distribution and availability of Zn will be. It has been reported that Zn is displaced by Cd from MT in a similar, predetermined order, with the lower affinity Zn atoms displaced first.7,48 It is therefore likely that the Zn distribution and fluxes within the cell are altered by Cd accumulation. One of the important findings in this study was the inhibition of GSIS by Cd in β cells. Further studies will have to clarify the underlying mechanism for this effect. Importantly, a general cytotoxic effect or an increase in general oxidative stress as an underlying factor is unlikely, as the expression of Heat Shock Protein-70 (HSP70), which has been reported as a marker of β-cell toxicity,54-57 was unaltered following exposure to 0.1 µmol/L Cd. Moreover, Cd did not have a negative effect on islet cell viability. Additionally, the expression of several genes relevant for β-cell function examined in our studies was unaltered. This suggests that the observed impairment in the ability of primary mouse β cells to secrete insulin in response to glucose following Cd exposure is a result of a functional disturbance of a cellular process relevant for normal β-cell function. Our studies showed no clear evidence for an increase in oxidative stress in response to Cd accumulation except at the relatively high CdCl2 concentration of 1.0 µmol/L. In contrast, we observed a decrease in GSSG/GSH ratio in dispersed primary mouse islets exposed to CdCl2, likely due to an upregulation of cellular protective mechanisms other than GCLC, GCLM and HMOX1. It is possible that the observed induction of MT expression contributed to this effect, but it is unlikely to be the only responsible mechanism. The difference in oxidative stress levels between primary mouse islet cells and MIN6 cells following Cd accumulation suggests a substantial difference in their response to Cd-induced oxidative stress. Given these results, it is possible that oxidative stress contributed to the observed decrease in MIN6 GSIS at the highest Cd exposure concentration of 1.0 µmol/L. However, it is unlikely to be a significant factor in the reduced GSIS in dispersed primary mouse islets following exposure to the much lower CdCl2 concentration of 0.1 µmol/L.
The impairment in β-cell function observed in our studies following exposure to a relatively low concentration of Cd is consistent with prior reports of a diabetogenic effect of Cd in vivo. Edwards et al. reported that exposure of adult Sprague-Dawley rats to Cd at a dose of 0.6 mg/kg/day, 5 d a week for 12 weeks resulted in an increase in fasting glucose and HbA1c and a decrease in serum insulin levels at week 12 but not earlier than week 11, suggesting a gradual accumulation of Cd and subsequent β-cell dysfunction.58 Whether Cd accumulation in β cells plays a role in promoting β-cell dysfunction or the development of frank diabetes mellitus under normal environmental Cd exposure is unclear. Nilsson et al. had reported the acute accumulation and effect of Cd following exposure to 2.5 or 5 µmol/L for 1 h.59 These studies are not comparable given the substantially higher concentration and shorter exposure duration, especially given the gradual accumulation of Cd and upregulation of MT observed in our studies. To our knowledge, no prior studies on Cd accumulation and its effects on β-cell function in native islets comparable to our current studies have been reported.
Human Cd exposure in the general population below the threshold generally considered as toxic is highly prevalent. Reports of serum or blood Cd concentrations in the population range from 0.0009 to 0.087 μmol/L,2,60-67 which is consistent with the lower concentration of Cd used in our studies. These exposure levels are mostly considered to be below the toxic exposure levels, which are defined based on the nephrotoxic effect of Cd. We consider our findings of significant β-cell accumulation of Cd and the measureable quantity of Cd in primary human islets as being of potential relevance to the pathophysiology of diabetes mellitus. Indeed, some epidemiologic reports in humans suggest that chronic low-level Cd exposure may be linked to altered glucose homeostasis, impaired β-cell function and an increased risk for type 2 diabetes. A sub analysis on participants in the NHANESIII observational cohort study reported an association between elevated urinary Cd levels and impaired fasting glucose levels as well as frank diabetes mellitus.1 Swaddiwudhipong et al. reported a 5-y observational study in a cohort of 436 persons exposed to high environmental Cd concentrations. They reported an increase incidence of diabetes in the 217 persons with continued high Cd exposure compared with the 219 persons who lowered their Cd intake through dietary interventions.68 Furthermore, Afridi et al. reported increased Cd levels in scalp hair of subjects with diabetes mellitus compared with non-diabetic controls.2 The causal relationship of these observations to diabetes mellitus remains to be explored. Our data provide a basis for further investigating the effect of Cd on insulin-producing β cells. It is unclear if the toxic effect of Cd on β cells has a threshold level below which Cd does not exert an adverse effect on β cells or whether the effect follows a continuous dose response curve. Assuming that the effect of Cd on β cells has a threshold, the “lowest observed adverse effect level” (LOAEL) for the inhibition of GSIS was between 0.1 and 1.0 µmol/L in our experiments for primary mouse islets and MIN6 cells respectively. Formulas that are commonly employed to extrapolate acceptable reference concentrations (RfC) used to define a tolerable environmental exposure levels have traditionally incorporated safety margins between 100 and 1,000.69 The concentration of Cd observed in MIN6 cells following exposure to CdCl2 0.1 and 1.0 µmol/L was 14.5 and 145 times higher respectively than the average Cd concentration observed in human islet samples from non-diabetic subjects. It was also approximately 6 and 60 times higher than the highest Cd concentration observed in subject # 10, the subject with the highest islet Cd concentration in our cohort. These concentrations fall well within the commonly employed safety margins discussed above. Given this, further studies to investigate whether the Cd concentrations found in human islets have a significant impact on normal human β-cell physiology are warranted. We acknowledge limitations of our studies. First, using short-term studies in cultured cells does not fully overcome the challenges associated with attempting to recapitulate the impact of chronic exposure in vivo. Furthermore, we were unable to compare the degree of Cd accumulation in primary mouse islets with that in MIN6 cells due to technical limitations. Also, our study was limited to islets from non-diabetic subjects and limited demographic and geographical data are available. Despite these limitations, the study provides important new information on Cd accumulation in islets under normal environmental conditions and its impact on islet function.
We conclude that primary human islets from individuals exposed to normal environmental conditions contain measureable quantities of Cd. Cultured mouse β cells accumulate Cd gradually over more than 72 h. This accumulation leads to the selective activation of only some of the known protective cellular response mechanisms, namely the upregulation of the metal buffering protein metallothionein, but not others such as ZnT-1, thereby prolonging the intracellular half-life of Cd. Cd accumulation led to an alteration of β-cell function in mouse islet cells without inducing general cell toxicity or oxidative stress at environmentally relevant Cd concentrations. Further studies will be required to elucidate whether this effect is relevant under normal environmental exposure levels and to elucidate the underlying mechanism.
Materials and Methods
Accruement of human islets
Viable human islets where provided through the NIH/NIDDK sponsored Integrated Islet Distribution Program (IIDP) via overnight shipment from IIDP centers in the United States. Islets were processed immediately upon arrival. Additional samples of freshly isolated islets were provided by the Northwestern University Division of Transplant Surgery. All studies involving human samples were approved by the institutional review board in our institution. Cell lysates and samples of the transport medium were stored in trace metal free 1.5 mL tubes at -20°C until the time of analysis.
MIN6 and 3T3 cell culture
MIN6 cells were a gift from Dr. Junichi Miyazaki (Osaka University Medical School) and were grown and maintained as described previously.24,70 MIN6 cells between passages 28 and 35 were used. 3T3 cells (ATCC) were cultured using the same culture conditions and medium. 3T3 cells between passages 50 and 59 were used. Both cell types were cultured in cell culture medium containing DMEM supplemented with 15% heat inactivated FBS (Hyclone), 100 U/mL penicillin, 100 μg/mL streptomycin and 2 mmol/l glutamine and 50 µM β mercaptoethanol (Invitrogen).
Dispersed mouse islet cell culture
Islets from 10 to 14 week old male C57Bl/6 mice (Jackson Laboratories) were isolated, dispersed and cultured as described previously.24 All studies involving the use of animals were by the Northwestern University Animal Care and Use Committee.
Studies examining the accumulation and effects of Cd or Hg
For studies examining the accumulation of Cd2+ or, Hg2+ in cultured cells, culture medium was changed 48hrs after plating the cells. Cells were harvested 72 h later. Prior to cell harvest. CdCl2 100 µmol dissolved in PBS was added to the medium at appropriate volumes to achieve the intended end concentration 1, 2, 4, 8, 24, 48 or 72 h prior to cell harvest. Control cells were treated with a corresponding volume of vehicle (PBS). Accurate exposure concentrations were verified by analyzing medium samples by ICP-MS.
Western blot analysis
Western blot analysis of cell lysates was performed as described previously,24 with modifications as described herein. For Heat Shock Protein 70 (HSP70), lysates containing 20 µg of protein were suspended in βME-containing Laemmli buffer, heated to 95°C for 10 min and cooled to room temperature. 10% SDS-PAGE gel electrophoresis was performed followed by detection per standard protocol using antibodies against mouse HSP70 (Cell Signaling) at a dilution of 1:1,000 in TBS-T with 5% bovine serum albumin (BSA). For metallothionein immunoblotting, 40 µg of protein (20 µg for dispersed mouse islets) was supplemented with 0.1 mol/l DTT and heated to 95 °C for 10 min in Kimura buffer (0.04M Tris, pH 8.8, 1.6% SDS, 10% glycerol final concentration), cooled to 50°C, supplemented with iodoacetamide (final concentration 0.1 mol/l), incubated at 50°C for 15 min and subjected to 15% SDS-PAGE followed by immunoblotting and detection as previously described using a 1:500 dilution of monoclonal mouse-anti metallothionein antibody (Dako).
Glucose stimulated insulin secretion (GSIS) studies
Glucose stimulated insulin secretion was performed following incubation without or with CdCl2 as previously described.24 Briefly, cells were grown in tissue culture plates. The appropriate volume of a 100 µmol/L CdCl2 solution in PBS was added to the medium either 8 or 48 h prior to GSIS to achieve the desired concentration and exposure duration prior to GSIS at 48 h. At the 48 h, culture medium was removed and the cells washed with PBS twice. Next, the cells were equilibrated in HEPES-buffered Krebs solution (HK: 130 mmol/l NaCl, 3.6 mmol/l KCl, 0.5 mmol/l NaH2PO4, 0.5 mmol/l MgSO4, 1.5 mmol/l CaCl2, 1% HEPES, 0.1% BSA, pH 7.4) containing 5.6 mmol/l glucose for 30 min. This was followed by baseline incubation in HK containing 2.8 mmol/l glucose for 30 min and then stimulation by incubation in HK containing 16.7 mmol/l glucose for 30 min. After each of the incubation periods in baseline or stimulated conditions, 200 µL of the supernatant were collected. Samples were stored at -80°C for later measurement of insulin content.
Analysis of Zn and Cd concentration in cell lysates
Cultured MIN6 and 3T3 cells were harvested by washing the plates with PBS twice. Cells were incubated in 0.05% trypsin + EDTA (Invitrogen) at 37°C × 4 min for cell separation and removal of divalent metals from the cell surface. After neutralization with DME medium (Invitrogen), cells were transferred to acid-washed 600 μl plastic tubes. Cells were pelleted by centrifugation at 500 RCF for 3 min and washed with PBS. The pelleting and washing in PBS was repeated twice. Hydrolysis was performed by adding trace metal grade 1 N NaOH (Fisher), incubation at 99°C for 15 min and neutralized with trace metal grade 1 N HCl (Fisher). The protein concentration of the lysates was measured using the micro-BCA assay (Pierce/Thermo Fisher Scientific). Inductively coupled plasma mass spectrometry (ICP-MS) was used to measure the content of the elements reported.
Quantification of Ni, Cu, Zn, Cd and Hg was accomplished using ICP-MS of cell lysates. Specifically, lysates were diluted in metal-free 15 mL polypropylene conical tubes (VWR Scientific) with ultra-pure trace metal grade water (Fisher Scientific), trace element concentrated nitric acid (TraceSelect 70%, Fisher Scientific), trace element hydrochloric acid (TraceSelect ≥ 37%, Fisher Scientific) and multi-element internal standard containing 208Bi, 165Ho, 113,115In, 6Li, 45Sc, 159Tb and 89Y (Inorganic Ventures, Christiansburg) to produce a final solution of 2.0% nitric acid (v/v), 2.0% hydrochloric acid (v/v) and 5.0 ng/g internal standard. Fully quantitative element standards were prepared starting from a custom mixed multi-element standard (Inorganic Ventures) containing 10 µg/mL Ca, Mg, Co, Cr, Ni, Cu, Zn, Cd, Hg and 50 µg/mL Au (to bind Hg) using eight fully quantitative concentrations ranging from 0.1 to 90 ng/g standard concentrations containing 2.0% nitric acid (v/v), 2.0% hydrochloric acid (v/v) and 5.0 ng/g internal standard. All samples and standards were run within 24 h of preparation due to the low stability of Hg in solution.
ICP-MS was performed on a computer-controlled (Plasmalab software) Thermo X series II ICP-MS (Thermo Fisher Scientific) operating in standard (Xt) mode equipped with Xt sample (nickel) and skimmer (platinum) cones and a CETAC 260 autosampler (Omaha). Instrument performance was optimized and checked daily using a multi-element tune solution, autotune sequence and performance report analysis meeting manufacturer’s specifications. Each sample was acquired using one survey run (10 sweeps) and three main (peak jumping) runs (100 sweeps). The isotopes selected for analysis were 60Ni,63,65Cu,66,68Zn, 111,114Cd and 200, 202Hg (using 45Sc, 89Y, 115In and 165Ho as internal standards for data interpolation). Fully quantitative standards were run prior to and following unknown sample runs for quality control purposes.
Cell viability measurement using the Multitox assay
Cell viability was measured using the double fluorescence Multitox® assay (Promega) as according to the manufacturers recommendations and as previously described.24 Briefly, dispersed mouse islet cells were seeded and cultured in black, opaque fluorescence 96-well tissue culture plates (BD-Falcon) in 100 µL medium containing CdCl2 at the specified concentration. Cell viability was assessed 48 h later by adding 100 µL of the assay mix to each well and incubated at 37°C for 2 h. Fluorescence was measured on a fluorescence plate reader at excitation wavelength (Ex) 400 nm/emission wavelength (Em) 505 nm and Ex 485/Em 520, which measured fluorescence from proteases in viable and dead cells, respectively. Ratios of the fluorescence readouts were calculated. Changes in the cell viability index correspond to changes in the ratio of live to dead cells.
Real time PCR
Previously-reported primers for housekeeping genes and Zn transporters of the ZnT and ZIP family were used.24 Additionally, the following mouse primer pairs were used: insulin-I F: 5′-GCAAGCAGGTCATTGTTTCAAC-3′ and R: 5′-AAGCCTGGGTGGGTTTGG-3′71; insulin-II F: 5′-CCACCCAGGCTTTTGTCAAA-3′ and R: 5′-CCCAGCTCCAGTTGTTCCAC-3′71; MafA F: 5′-CCTGTAGAGGAAGCCGAGGAA-3′ and R: 5′-CCTCCCCCAGTCGAGTATAGC-3′72; MafB F: 5′-CAACAGCTACCCACTAGCCA-3′ and R: 5′-GGCGAGTTTCTCGCACTTGA-3′71; IRS-2 F: 5′-GCGGCCTCATCTTCTTCACT-3′, and R: 5′-AACTGAAGTCCAGGTTCATATAGTCA-3′73; DMT-1 F: 5′-AGGAAGTGCGGGAAGCCAATAAGTA-3′, and R: 5′-ACACGACAAAGACATTGATGATGAA-3′22; HMOX1 F: 5′-AACAAGCAGAACCCAGTCTATGC-3′, and R: 5′-AGGTAGCGGGTATATGCGTGGGCC-3′74; GCLC F: 5′- ATGTGGACACCCGATGCAGTATT-3′, and R: 5′- TGTCTTGCTTGTAGTCAGGATGGTTT-3′74; GCLM F: 5′- GCCACCAGATTTGACTGCCTTT-3′, and R: 5′- CAGGGATGCTTTCTTGAAGAGCTT-3′.74 The delta Ct method was used to calculate relative expression levels of the target genes. The normalized values for 18S and β Actin mRNA were calculated for each target gene and the average of the two calculations was reported. All cDNA samples were diluted to achieve similar 18S and β Actin Ct values within one cycle in order to minimize the effect of differences in primer efficiencies.
Measurement of cellular oxidized and reduced glutathione
Reduced glutathione (GSH) and oxidized glutathione (GSSG) as well as the ratio of GSH to GSSG were measured using the GSH/GSSG-Glo™ Assay (Promega). Briefly, dispersed mouse islet cells or MIN6 cells were seeded and cultured in white, opaque wall, clear bottom 96-well tissue culture plates (Greiner Bio-One) in 90 µL medium containing CdCl2 at the specified concentration. GSH and GSSG was measured 48 h later by following the manufacturer’s instructions. GSH and GSSG values were normalized to the values from the non-treated control within each experiment to adjust for variations in cell density between experiments.
Statistical analysis
Results are reported as means ± SEM unless otherwise stated. The “n” represents the number of independent experiments performed. Two-sided unpaired Student’s t-test was used to compare parameters to control. The Bonferroni correction was used where appropriate. Two-way ANOVA was used to compare the time course of Cd accumulation in 3T3 and MIN6 cells. Pvalues ≤ 0.05 and 0.005 were considered statistically significant and highly significant respectively. (STATA-IC V.10.1 and Graphpad Prism V6.0b).
For simplification, the symbols Cd and Zn were used throughout the article when referring to their respective ions.
Supplementary Material
Acknowledgments
This study was funded by a Northwestern Memorial Foundation MD-Scientist Fellowship in Genetic Medicine award and the Northwestern Memorial Foundation/NUCATS Dixon Young Investigator Award and a NIEHS/NIH grant 1K08ES020880-01 for M.E. The study was also funded by two NIH grants: R37GM038784 and U01CA151461 for T.V.O., P50HD044405 (M.U. and S.B.) and RO1HD057450 (M.U.). Human islets were provided through the NIH sponsored Integrated Islet Distribution Program (IIDP). ICP-MS Metal analysis was performed at the Northwestern University Quantitative Bioelemental Imaging Center generously supported by NASA Ames Research Center NNA04CC36G. We thank the following individuals and institutions for providing samples of the solutions used in the process of isolating human islets for meta-analysis: City of Hope: Ismail H.Al-Abdullah, PhD, Fouad Kandeel, MD, PhD, Noe Gonzales; the University of Wisconsin: Laura Zitur, Luis A. Fernandez, MD; The University of Miami: Omaima Malik MD, Aisha Khan, PhD, Camillo Ricordi, MD.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/islets/article/23101
References
- 1.Schwartz GG, Il’yasova D, Ivanova A. Urinary cadmium, impaired fasting glucose, and diabetes in the NHANES III. Diabetes Care. 2003;26:468–70. doi: 10.2337/diacare.26.2.468. [DOI] [PubMed] [Google Scholar]
- 2.Afridi HI, Kazi TG, Kazi N, Jamali MK, Arain MB, Jalbani N, et al. Evaluation of status of toxic metals in biological samples of diabetes mellitus patients. Diabetes Res Clin Pract. 2008;80:280–8. doi: 10.1016/j.diabres.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 3.Eddins D, Petro A, Pollard N, Freedman JH, Levin ED. Mercury-induced cognitive impairment in metallothionein-1/2 null mice. Neurotoxicol Teratol. 2008;30:88–95. doi: 10.1016/j.ntt.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerson RJ, Shaikh ZA. Uptake and binding of cadmium and mercury to metallothionein in rat hepatocyte primary cultures. Biochem J. 1982;208:465–72. doi: 10.1042/bj2080465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schultze P, Wörgötter E, Braun W, Wagner G, Vasák M, Kägi JH, et al. Conformation of [Cd7]-metallothionein-2 from rat liver in aqueous solution determined by nuclear magnetic resonance spectroscopy. J Mol Biol. 1988;203:251–68. doi: 10.1016/0022-2836(88)90106-4. [DOI] [PubMed] [Google Scholar]
- 6.Dalton TP, He L, Wang B, Miller ML, Jin L, Stringer KF, et al. Identification of mouse SLC39A8 as the transporter responsible for cadmium-induced toxicity in the testis. Proc Natl Acad Sci U S A. 2005;102:3401–6. doi: 10.1073/pnas.0406085102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elinder CG, Nordberg M, Palm B, Björk L, Jönsson L. Cadmium, zinc, and copper in rabbit kidney metallothionein--relation to kidney toxicity. Environ Res. 1987;42:553–62. doi: 10.1016/S0013-9351(87)80222-0. [DOI] [PubMed] [Google Scholar]
- 8.He L, Girijashanker K, Dalton TP, Reed J, Li H, Soleimani M, et al. ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: characterization of transporter properties. Mol Pharmacol. 2006;70:171–80. doi: 10.1124/mol.106.024521. [DOI] [PubMed] [Google Scholar]
- 9.Wiśniewska JM, Trojanowska B, Piotrowski J, Jakubowski M. Binding of mercury in the rat kidney by metallothionein. Toxicol Appl Pharmacol. 1970;16:754–63. doi: 10.1016/0041-008X(70)90081-5. [DOI] [PubMed] [Google Scholar]
- 10.Antala S, Dempski RE. The human ZIP4 transporter has two distinct binding affinities and mediates transport of multiple transition metals. Biochemistry. 2012;51:963–73. doi: 10.1021/bi201553p. [DOI] [PubMed] [Google Scholar]
- 11.Lemaire K, Ravier MA, Schraenen A, Creemers JW, Van de Plas R, Granvik M, et al. Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc Natl Acad Sci U S A. 2009;106:14872–7. doi: 10.1073/pnas.0906587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dodson G, Steiner D. The role of assembly in insulin’s biosynthesis. Curr Opin Struct Biol. 1998;8:189–94. doi: 10.1016/S0959-440X(98)80037-7. [DOI] [PubMed] [Google Scholar]
- 13.Chimienti F, Devergnas S, Pattou F, Schuit F, Garcia-Cuenca R, Vandewalle B, et al. In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. J Cell Sci. 2006;119:4199–206. doi: 10.1242/jcs.03164. [DOI] [PubMed] [Google Scholar]
- 14.Figlewicz DP, Forhan SE, Hodgson AT, Grodsky GM. 65Zinc and endogenous zinc content and distribution in islets in relationship to insulin content. Endocrinology. 1984;115:877–81. doi: 10.1210/endo-115-3-877. [DOI] [PubMed] [Google Scholar]
- 15.Huang XF, Arvan P. Intracellular transport of proinsulin in pancreatic beta-cells. Structural maturation probed by disulfide accessibility. J Biol Chem. 1995;270:20417–23. doi: 10.1074/jbc.270.35.20417. [DOI] [PubMed] [Google Scholar]
- 16.Liuzzi JP, Cousins RJ. Mammalian zinc transporters. Annu Rev Nutr. 2004;24:151–72. doi: 10.1146/annurev.nutr.24.012003.132402. [DOI] [PubMed] [Google Scholar]
- 17.Liuzzi JP, Bobo JA, Lichten LA, Samuelson DA, Cousins RJ. Responsive transporter genes within the murine intestinal-pancreatic axis form a basis of zinc homeostasis. Proc Natl Acad Sci U S A. 2004;101:14355–60. doi: 10.1073/pnas.0406216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang B, Schneider SN, Dragin N, Girijashanker K, Dalton TP, He L, et al. Enhanced cadmium-induced testicular necrosis and renal proximal tubule damage caused by gene-dose increase in a Slc39a8-transgenic mouse line. Am J Physiol Cell Physiol. 2007;292:C1523–35. doi: 10.1152/ajpcell.00409.2006. [DOI] [PubMed] [Google Scholar]
- 19.Ohana E, Sekler I, Kaisman T, Kahn N, Cove J, Silverman WF, et al. Silencing of ZnT-1 expression enhances heavy metal influx and toxicity. J Mol Med (Berl) 2006;84:753–63. doi: 10.1007/s00109-006-0062-4. [DOI] [PubMed] [Google Scholar]
- 20.Girijashanker K, He L, Soleimani M, Reed JM, Li H, Liu Z, et al. Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: similarities to the ZIP8 transporter. Mol Pharmacol. 2008;73:1413–23. doi: 10.1124/mol.107.043588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garrick MD, Dolan KG, Horbinski C, Ghio AJ, Higgins D, Porubcin M, et al. DMT1: a mammalian transporter for multiple metals. Biometals. 2003;16:41–54. doi: 10.1023/A:1020702213099. [DOI] [PubMed] [Google Scholar]
- 22.Kim DW, Kim KY, Choi BS, Youn P, Ryu DY, Klaassen CD, et al. Regulation of metal transporters by dietary iron, and the relationship between body iron levels and cadmium uptake. Arch Toxicol. 2007;81:327–34. doi: 10.1007/s00204-006-0160-7. [DOI] [PubMed] [Google Scholar]
- 23.Chimienti F, Favier A, Seve M. ZnT-8, a pancreatic beta-cell-specific zinc transporter. Biometals. 2005;18:313–7. doi: 10.1007/s10534-005-3687-9. [DOI] [PubMed] [Google Scholar]
- 24.El Muayed M, Billings LK, Raja MR, Zhang X, Park PJ, Newman MV, et al. Acute cytokine-mediated downregulation of the zinc transporter ZnT8 alters pancreatic beta-cell function. J Endocrinol. 2010;206:159–69. doi: 10.1677/JOE-09-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicolson TJ, Bellomo EA, Wijesekara N, Loder MK, Baldwin JM, Gyulkhandanyan AV, et al. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes. 2009;58:2070–83. doi: 10.2337/db09-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wijesekara N, Dai FF, Hardy AB, Giglou PR, Bhattacharjee A, Koshkin V, et al. Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia. 2010;53:1656–68. doi: 10.1007/s00125-010-1733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrews GK. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem Pharmacol. 2000;59:95–104. doi: 10.1016/S0006-2952(99)00301-9. [DOI] [PubMed] [Google Scholar]
- 28.Hasumi M, Suzuki K, Matsui H, Koike H, Ito K, Yamanaka H. Regulation of metallothionein and zinc transporter expression in human prostate cancer cells and tissues. Cancer Lett. 2003;200:187–95. doi: 10.1016/S0304-3835(03)00441-5. [DOI] [PubMed] [Google Scholar]
- 29.Günther V, Lindert U, Schaffner W. The taste of heavy metals: gene regulation by MTF-1. Biochim Biophys Acta. 2012;1823:1416–25. doi: 10.1016/j.bbamcr.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Milne DB, Ralston NV, Wallwork JC. Zinc content of blood cellular components and lymph node and spleen lymphocytes in severely zinc-deficient rats. J Nutr. 1985;115:1073–8. doi: 10.1093/jn/115.8.1073. [DOI] [PubMed] [Google Scholar]
- 31.Wallwork JC, Johnson LK, Milne DB, Sandstead HH. The effect of interactions between dietary egg white protein and zinc on body weight, bone growth and tissue trace metals in the 30-day-old rat. J Nutr. 1983;113:1307–20. doi: 10.1093/jn/113.7.1307. [DOI] [PubMed] [Google Scholar]
- 32.Ward NI, Mason JA. Neuron activation analysis techniques for identifying Elemental Status in Alzheimer's Disease. J Radioanal Nucl Chem. 1987;113:515–26. doi: 10.1007/BF02050527. [DOI] [Google Scholar]
- 33.Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking, and signals. J Biol Chem. 2006;281:24085–9. doi: 10.1074/jbc.R600011200. [DOI] [PubMed] [Google Scholar]
- 34.Chausmer AB. Zinc, insulin and diabetes. J Am Coll Nutr. 1998;17:109–15. doi: 10.1080/07315724.1998.10718735. [DOI] [PubMed] [Google Scholar]
- 35.Outten CE, O’Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–92. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 36.Colvin RA, Fontaine CP, Laskowski M, Thomas D. Zn2+ transporters and Zn2+ homeostasis in neurons. Eur J Pharmacol. 2003;479:171–85. doi: 10.1016/j.ejphar.2003.08.067. [DOI] [PubMed] [Google Scholar]
- 37.Finney LA, O’Halloran TV. Transition metal speciation in the cell: insights from the chemistry of metal ion receptors. Science. 2003;300:931–6. doi: 10.1126/science.1085049. [DOI] [PubMed] [Google Scholar]
- 38.Suhy DA, Simon KD, Linzer DI, O’Halloran TV. Metallothionein is part of a zinc-scavenging mechanism for cell survival under conditions of extreme zinc deprivation. J Biol Chem. 1999;274:9183–92. doi: 10.1074/jbc.274.14.9183. [DOI] [PubMed] [Google Scholar]
- 39.Choi CY, An KW, Nelson ER, Habibi HR. Cadmium affects the expression of metallothionein (MT) and glutathione peroxidase (GPX) mRNA in goldfish, Carassius auratus. Comp Biochem Physiol C Toxicol Pharmacol. 2007;145:595–600. doi: 10.1016/j.cbpc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Habeebu SS, Liu J, Liu Y, Klaassen CD. Metallothionein-null mice are more sensitive than wild-type mice to liver injury induced by repeated exposure to cadmium. Toxicol Sci. 2000;55:223–32. doi: 10.1093/toxsci/55.1.223. [DOI] [PubMed] [Google Scholar]
- 41.Habeebu SS, Liu J, Liu Y, Klaassen CD. Metallothionein-null mice are more susceptible than wild-type mice to chronic CdCl(2)-induced bone injury. Toxicol Sci. 2000;56:211–9. doi: 10.1093/toxsci/56.1.211. [DOI] [PubMed] [Google Scholar]
- 42.Heuchel R, Radtke F, Georgiev O, Stark G, Aguet M, Schaffner W. The transcription factor MTF-1 is essential for basal and heavy metal-induced metallothionein gene expression. EMBO J. 1994;13:2870–5. doi: 10.1002/j.1460-2075.1994.tb06581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y, Liu J, Habeebu SM, Waalkes MP, Klaassen CD. Metallothionein-I/II null mice are sensitive to chronic oral cadmium-induced nephrotoxicity. Toxicol Sci. 2000;57:167–76. doi: 10.1093/toxsci/57.1.167. [DOI] [PubMed] [Google Scholar]
- 44.Nordberg GF. Historical perspectives on cadmium toxicology. Toxicol Appl Pharmacol. 2009;238:192–200. doi: 10.1016/j.taap.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 45.Masters BA, Kelly EJ, Quaife CJ, Brinster RL, Palmiter RD. Targeted disruption of metallothionein I and II genes increases sensitivity to cadmium. Proc Natl Acad Sci U S A. 1994;91:584–8. doi: 10.1073/pnas.91.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waalkes MP, Harvey MJ, Klaassen CD. Relative in vitro affinity of hepatic metallothionein for metals. Toxicol Lett. 1984;20:33–9. doi: 10.1016/0378-4274(84)90179-6. [DOI] [PubMed] [Google Scholar]
- 47.Waisberg M, Joseph P, Hale B, Beyersmann D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology. 2003;192:95–117. doi: 10.1016/S0300-483X(03)00305-6. [DOI] [PubMed] [Google Scholar]
- 48.Zangger K, Oz G, Otvos JD, Armitage IM. Three-dimensional solution structure of mouse [Cd7]-metallothionein-1 by homonuclear and heteronuclear NMR spectroscopy. Protein Sci. 1999;8:2630–8. doi: 10.1110/ps.8.12.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 50.Jiang LJ, Maret W, Vallee BL. The glutathione redox couple modulates zinc transfer from metallothionein to zinc-depleted sorbitol dehydrogenase. Proc Natl Acad Sci U S A. 1998;95:3483–8. doi: 10.1073/pnas.95.7.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang LJ, Vasák M, Vallee BL, Maret W. Zinc transfer potentials of the alpha - and beta-clusters of metallothionein are affected by domain interactions in the whole molecule. Proc Natl Acad Sci U S A. 2000;97:2503–8. doi: 10.1073/pnas.97.6.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feng W, Cai J, Pierce WM, Franklin RB, Maret W, Benz FW, et al. Metallothionein transfers zinc to mitochondrial aconitase through a direct interaction in mouse hearts. Biochem Biophys Res Commun. 2005;332:853–8. doi: 10.1016/j.bbrc.2005.04.170. [DOI] [PubMed] [Google Scholar]
- 53.Mason AZ, Perico N, Moeller R, Thrippleton K, Potter T, Lloyd D. Metal donation and apo-metalloenzyme activation by stable isotopically labeled metallothionein. Mar Environ Res. 2004;58:371–5. doi: 10.1016/j.marenvres.2004.03.082. [DOI] [PubMed] [Google Scholar]
- 54.Cannino G, Ferruggia E, Luparello C, Rinaldi AM. Mitochondrial compartment: a possible target of cadmium effects on breast epithelial cells. Mol Cell Biochem. 2009;328:75–84. doi: 10.1007/s11010-009-0076-7. [DOI] [PubMed] [Google Scholar]
- 55.Hsiao CJ, Stapleton SR. Early sensing and gene expression profiling under a low dose of cadmium exposure. Biochimie. 2009;91:329–43. doi: 10.1016/j.biochi.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 56.Luparello C, Sirchia R, Longo A. Cadmium as a transcriptional modulator in human cells. Crit Rev Toxicol. 2011;41:75–82. doi: 10.3109/10408444.2010.529104. [DOI] [PubMed] [Google Scholar]
- 57.Yamada H, Koizumi S. DNA microarray analysis of human gene expression induced by a non-lethal dose of cadmium. Ind Health. 2002;40:159–66. doi: 10.2486/indhealth.40.159. [DOI] [PubMed] [Google Scholar]
- 58.Edwards JR, Prozialeck WC. Cadmium, diabetes and chronic kidney disease. Toxicol Appl Pharmacol. 2009;238:289–93. doi: 10.1016/j.taap.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nilsson T, Rorsman F, Berggren PO, Hellman B. Accumulation of cadmium in pancreatic beta cells is similar to that of calcium in being stimulated by both glucose and high potassium. Biochim Biophys Acta. 1986;888:270–7. doi: 10.1016/0167-4889(86)90225-9. [DOI] [PubMed] [Google Scholar]
- 60.Olsson IM, Bensryd I, Lundh T, Ottosson H, Skerfving S, Oskarsson A. Cadmium in blood and urine--impact of sex, age, dietary intake, iron status, and former smoking--association of renal effects. Environ Health Perspect. 2002;110:1185–90. doi: 10.1289/ehp.021101185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benoff S, Hauser R, Marmar JL, Hurley IR, Napolitano B, Centola GM. Cadmium concentrations in blood and seminal plasma: correlations with sperm number and motility in three male populations (infertility patients, artificial insemination donors, and unselected volunteers) Mol Med. 2009;15:248–62. doi: 10.2119/molmed.2008.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bulat ZP, Dukic-Cosic D, Dokic M, Bulat P, Matovic V. Blood and urine cadmium and bioelements profile in nickel-cadmium battery workers in Serbia. Toxicol Ind Health. 2009;25:129–35. doi: 10.1177/0748233709104488. [DOI] [PubMed] [Google Scholar]
- 63.Link B, Gabrio T, Piechotowski I, Zöllner I, Schwenk M. Baden-Wuerttemberg Environmental Health Survey (BW-EHS) from 1996 to 2003: toxic metals in blood and urine of children. Int J Hyg Environ Health. 2007;210:357–71. doi: 10.1016/j.ijheh.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 64.Järup L, Rogenfelt A, Elinder CG, Nogawa K, Kjellström T. Biological half-time of cadmium in the blood of workers after cessation of exposure. Scand J Work Environ Health. 1983;9:327–31. doi: 10.5271/sjweh.2404. [DOI] [PubMed] [Google Scholar]
- 65.Dakeshita S, Kawai T, Uemura H, Hiyoshi M, Oguma E, Horiguchi H, et al. Gene expression signatures in peripheral blood cells from Japanese women exposed to environmental cadmium. Toxicology. 2009;257:25–32. doi: 10.1016/j.tox.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 66.Ebert-McNeill A, Clark SP, Miller JJ, Birdsall P, Chandar M, Wu L, et al. Cadmium intake and systemic exposure in postmenopausal women and age-matched men who smoke cigarettes. Toxicol Sci. 2012;130:191–204. doi: 10.1093/toxsci/kfs226. [DOI] [PubMed] [Google Scholar]
- 67.Ruiz P, Mumtaz M, Osterloh J, Fisher J, Fowler BA. Interpreting NHANES biomonitoring data, cadmium. Toxicol Lett. 2010;198:44–8. doi: 10.1016/j.toxlet.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 68.Swaddiwudhipong W, Limpatanachote P, Mahasakpan P, Krintratun S, Punta B, Funkhiew T. Progress in cadmium-related health effects in persons with high environmental exposure in northwestern Thailand: a five-year follow-up. Environ Res. 2012;112:194–8. doi: 10.1016/j.envres.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 69.Casarett LJ, Doull J, Klaassen CD. Casarett and Doull's toxicology: the basic science of poisons. New York: McGraw-Hill, 2008:116-28. [Google Scholar]
- 70.Miyazaki J, Araki K, Yamato E, Ikegami H, Asano T, Shibasaki Y, et al. Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology. 1990;127:126–32. doi: 10.1210/endo-127-1-126. [DOI] [PubMed] [Google Scholar]
- 71.Artner I, Le Lay J, Hang Y, Elghazi L, Schisler JC, Henderson E, et al. MafB: an activator of the glucagon gene expressed in developing islet alpha- and beta-cells. Diabetes. 2006;55:297–304. doi: 10.2337/diabetes.55.02.06.db05-0946. [DOI] [PubMed] [Google Scholar]
- 72.Raum JC, Hunter CS, Artner I, Henderson E, Guo M, Elghazi L, et al. Islet beta-cell-specific MafA transcription requires the 5′-flanking conserved region 3 control domain. Mol Cell Biol. 2010;30:4234–44. doi: 10.1128/MCB.01396-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hussain MA, Porras DL, Rowe MH, West JR, Song WJ, Schreiber WE, et al. Increased pancreatic beta-cell proliferation mediated by CREB binding protein gene activation. Mol Cell Biol. 2006;26:7747–59. doi: 10.1128/MCB.02353-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang H, Liu H, Davies KJ, Sioutas C, Finch CE, Morgan TE, et al. Nrf2-regulated phase II enzymes are induced by chronic ambient nanoparticle exposure in young mice with age-related impairments. Free Radic Biol Med. 2012;52:2038–46. doi: 10.1016/j.freeradbiomed.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.