Abstract
There is a great interest for developing poly(lactide-co-glycolide) (PLGA) based particles for targeted delivery and controlled release of encapsulated biological molecules. The PLGA particles can be used to deliver proteins, small molecule drugs and nucleotides. Furthermore, it has been shown that the co-encapsulation of multiple factors in PLGA particles generates synergistic responses, and can simultaneously provide theranostic benefits. However, the number of possible unique particle formulations that may be generated by the combination of different components in a particle increases dramatically with each new component, and currently, there is no method to generate such a vast library of unique PLGA particles. In order to address this gap, we have developed a high-throughput methodology to produce hundreds of small batches of particles. The particles are generated in the multi-well plate wells by a modified oil-in-water emulsion technique. In order to demonstrate the versatility of this technique, combinatorial formulations of six different loading concentrations of three fluorescent dyes were fabricated giving rise to 216 unique PLGA particle formulations. We demonstrate systematic and well-controlled combinatorial loading of hydrophobic molecules into the particles. This PPP methodology potentiates the generation of hundreds of different combinatorial particle formulations with multiple co-encapsulates in less than 24 h in standard polystyrene multi-well plates, thus providing rapid, low cost, high-throughput production. We envision that such a PPP library of particles encapsulating combinations of drugs and imaging modalities can subsequently be tested on small populations of cells in a high-throughput fashion, and provide personalized medicine.
Introduction
Use of the polymer, poly lactide-co-glycolide (PLGA), has had a large impact on contemporary biomedicine. PLGA has been approved by the U.S. Food and Drug Administration (FDA) for biodegradable surgical sutures and drug delivery products and, is utilized extensively for drug delivery vehicles, tissue engineering supports and combination products in pre-clinical and clinical research [1–6]. PLGA can be fabricated into scaffolds or particles for delivery of a wide range of biologically active molecules [7–9]. Release rates of encapsulates can be controlled by manipulation of molecular weight and/or the lactide to glycolide ratio of the polymer. Sizes of PLGA particles have been varied from 50 nm to 100 µm for use in drug delivery via various techniques, including solvent extraction/evaporation, phase separation (coacervation) and spray-drying [10–12]. Among these, phase separation and spray drying techniques are harsh on the encapsulates, while solvent extraction utilizes large amounts of reagents and is labor intensive. Although solvent evaporation is the most extensively usedtechnique to generate PLGA micro/nano particles, it can only produce particles in batches, one formulation at a time. Our approach utilizes a miniaturized, highly parallel solvent evaporation technique in a multiplexed configuration for parallel generation of large numbers of different formulations with combinations of multiple co-encapsulated agents. Furthermore, this method is readily scalable in terms of increasing the number of uniquely formulated batches, and leverages the use of relatively inexpensive standard contact pin printing miniarraying equipment for programmable dispensing of hydrophobic molecules into wells of a polystyrene multi-well plate. We hypothesize this PPP method will generate PLGA particles in small “micro-batches“ in a semi-automated fashion, requiring limited reagents and enabling the generation of a large multi-component particle library, appropriate for testing on small cell populations in a high-throughput fashion.
Materials and Methods
1. Materials
A 50:50 polymer composition of poly(d,l lactide-co-glycolide) (PLGA) with inherent viscosity 0.55–0.75 dL/g in hexafluoroisopropanol, HFIP (Lactel, AL, USA) was used to generate particles. Poly-vinyl alcohol (PVA) (MW ~ 100,000 g/mol, Fisher Science, Rochester, NY, USA) was utilized as an emulsion stabilizer. Phosphate buffered saline (PBS) solution (Hyclone, UT, USA) was used as the aqueous phase to form the emulsions while propylene carbonate (PC) (Fisher Scientific, NJ, USA) was used as an organic solvent to dissolve PLGA polymer. Microparticles were generated using solid-oil-water emulsion technique. Fluorescent dyes, 7-diethylamino-4-methylcoumarin (coumarin: λex= 375 nm and λem= 445 nm), 1,1',3,3,3',3'-hexamethylindodicarbocyanine iodide (cyanine:λex= 648 nm and λem= 670 nm) and rhodamine 6G (rhodamine: λex= 528 nm and λem= 550 nm) were encapsulated in the particles as representative hydrophobic molecules. Dyes were chosen so as to have minimum overlap of absorption of one dye with the emission spectrums of another, in order to reduce the quenching effect. Polystyrene 384-well plates were used as generation chambers for the particles and a Calligrapher Miniarrayer (BioRad) contact printer was used to transfer solutions into these particle generation chambers.
2. Parallel Particle Production (PPP)
A 384-well plate containing six different dilutions of the three fluorescent dyes dissolved in propylene carbonate (PC) was used as a source plate. Coumarin was dissolved in PC in concentrations of 20 mg/mL, 10 mg/mL, 5 mg/mL, 2.5 mg/mL, 1.25 mg/mL; rhodamine was dissolved in PC in concentrations 50 mg/mL, 25 mg/mL, 12.5 mg/mL, 6.25 mg/mL, 3.125 mg/mL and cyanine was dissolved in PC in concentrations of 10 mg/mL, 5 mg/mL, 2.5 mg/mL, 1.25 mg/mL, 0.625 mg/mL. A 40 µL volume of each dilution was added to the wells of 384 well plates. Additionally, a single well was filled with 40 µL volume of PC without any dye to serve as the “zero dye” control. These six dilutions of the dyes were then printed in a 384 well plate using the contact printer to form a 6 × 6× 6 matrix, with all possible combinations of the three available dyes which resulted in 216 different formulations at room temperature. The dye-printed plate was analyzed using a plate reader with appropriate filters (λex/λem= 375/445; 648/670 and 528/550 nm) to measure fluorescence of the printed dyes to ensure accurate printing. After printing the dyes, 10 µL of 3% PLGA dissolved in PC was added to the wells using a multichannel pipette. The plate was then sonicated in a sonicating water bath for 3 minutes. Next 50 µL of 5% PVA solution in PBS was added to the wells and the plate was sonicated for 5 more minutes. The plate was then incubated for 16 h under a vacuum pressure of 0.2 mTorr at 37°C to evaporate PC and water. A volume of 80 µL of de-ionized water was added to each well using a multichannel pipette and the plate was sonicated for 60 s. The plate was then centrifuged for 15 min at 1,500×G for 20 min. The supernatant was then removed and the particles re-suspended by sonicating in 50 µL/well of de-ionized water. This washing process of centrifuging, aspirating and re-suspending was repeated 5 times to remove PVA from the particles in the well. The plate was then frozen by placing over dry ice and dried under vacuum overnight. All the particle generation steps were performed in the dark to reduce photo-bleaching of the dyes.
3. Particle Analysis
For analysis, the particles were re-suspended in de-ionized water solution using a sonicating bath and printed via Miniarrayer on a plasma cleaned glass microscope slides (Fisher Scientific, NJ, USA). Particle morphology was characterized by scanning electron microscope (FEG-SEM JEOL JSM – 6335F, Major Analytical Instrumentation Center, University of Florida). Particles from different micro-batches were combined, suspended in DI water and printed using a micro array printer. The array of particles was then dried for 16 h at room temperature. Dried particles were coated with 5–10 nm thickness of gold and imaged at magnifications ranging from 30x to 60,000x. Furthermore, particle formulations were combined from separate runs, to confirm the range of particle sizes obtained from SEM and particle size was analyzed by dynamic light scattering (volume estimation) using a Nanotrac (Microtrac Inc.) particle size analyzer. Flow cytometry (BD Biosciences) was utilized to analyze the range of fluorescence intensities of >10,000 particles within each microbatch. Flow data was analyzed using FCS Express software. Appropriate gating and compensation was performed to ensure minimum overlap of the dye spectra and quadrants were chosen to quantify the percent of particles manifesting the presence of different fluorescent dyes.
4. Dendritic Cell Culture
Animal work was performed according to an approved IACUC, University of Florida (protocol number E751), protocol. Dendritic cells were generated from bone marrow of 7 week old C57Bl6/j mice using a modified 10 day protocol [25,26]. Briefly, mouse bone marrow was isolated from the femur and tibia and kept in wash media composed of DMEM/F-12 (1:1) with L-glutamine (Cellgro, Herndon, VA) and 10% fetal bovine serum (Bio-Whittaker). The ends of the bones were cut and bone marrow was flushed out with 10 mL wash media using a 25 G needle and mixed to make a homogeneous suspension. The suspension was then strained using 70 µm cell strainers (Becton Dickinson) and cells were collected by centrifugation at 330×g for 6 min. Red blood cells were lysed by incubating the cell suspension in ACK lysing buffer (Whittaker) for 5 min. The precursor cells were isolated by centrifuging at 330×g for 5 min. The precursor cells were then incubated with DC-media consisting of 20 ng/mL of GM-CSF (R&D Systems), DMEM/F12 (1:1) with L-glutamine (Cellgro, Herndon, VA) and 10% fetal bovine serum (FBS) (Hyclone), 1% sodium pyruvate (Lonza, Walkersville, MD) and 1% non-essential amino acid (Lonza, Walkersville, MD) for 2 days in a flask. The floating cells were then collected from the flask at the end of 2 days and re-seeded with fresh DC-media in a 6-well low-attachment plate (Corning Inc., NY) for 6 days. Half-media change was performed every 2 days. After 6 days of culture the cells were re-suspended in fresh media and seeded onto tissue-culture treated 6 well plates (Corning, Inc, NY) for 2 days. After 10 days, cultures of DCs were generated and characterized by flow cytometry for purity (CD11c+ > 90%), immaturity (major histocompatibility complex (MHCII+) < 6% and CD86+ < 6%), and viability by trypan blue staining (~99% viable). Dendritic cells were lifted using 5 mM solution of Na2EDTA (Fisher Scientific) in 1X PBS (Hyclone). Dendritic cells were then seeded onto the printed particle screening chip and micrographs were obtained using a Carl Zeiss 400M fluorescence microscope.
5. Particle Array Screening Chip
The particle screening chips were constructed as described by Acharya et al. [6]. Briefly, oxygen plasma etched glass coverslips were printed onto with (3-aminopropyl)-triethoxysilane (NH2 silane – Fisher Scientific) solution mixed in PBS at 1:1 volume ratio utilizing the miniarrayer, with a 400 µm diameter solid metal pin. The NH2 silane reacts with Si-O groups on the glass coverslip forming covalently grafted NH2-terminated spots. Also, when dry, the NH2 silane solution forms a film 5 – 10 microns thick. Coverslips were then coated with 200 Å of titanium followed by 250 Å of gold (Williams Advanced Materials, IL, USA). Gold coated coverslips were then sonicated in DI water for 5 min at 50°C to lift off the gold coated areas over the printed islands, exposing NH2-terminated silane arrayed spots, while leaving the gold coating intact around the islands for further processing. The coverslips were then incubated with 0.01 M, methyl-terminated alkanethiol (CH3(CH2)11SH, Sigma-Aldrich) for 1 h followed by washing with 200-proof ethyl alcohol (Fisher Scientific). Substrates were then incubated in 10% pluronic F-127 (BASF Corporation, USA) in DI water, for 4 h to render the surface around the islands cell-resistant. The coverslip was washed with DI water and particles generated via PPP technique were over-printed on the exposed islands. A dried microparticle-arrayed coverslip was rinsed with PBS and re-hydrated for 30 min with PBS-mix containing 0.05 g/L of magnesium chloride and 0.05 g/L of calcium chloride made by mixing equal parts of PBS with magnesium and calcium and PBS without magnesium and calcium (PBS50–50), before seeding immature DCs. Dendritic cells were seeded in a serum-free media in PBS50–50 for 15 min at 37°C. Dendritic cells were then rinsed with PBS having calcium and magnesium to wash-off non-adherent cells. Fresh DC-media was added onto the coverslip and adherent DCs were cultured for 24 h. Dendritic cells were fixed with 3.7% paraformaldehyde (USB Corporation, USA) for 10 min and then washed with PBS to remove excess fixing agent. Fluorescence and phase-contrast micrographs of adherent cells on the islands co-localized with particles were obtained using a 10x objective and compiled using MosaiX module of Axiovision software used for stitching the acquired images.
6. Statistical Analysis
Statistical analyses were performed using one-way ANOVA using Systat (Version 12, Systat Software, Inc., San Jose, CA). Pair-wise comparisons were made using Tukey’s Honestly-Significant-Difference, with p-values of less than or equal to 0.05 considered to be significant. Further, graphs were plotted and regression analysis performed using Sigmaplot (Version 10.0, Systat Software Inc. San Jose, CA).
Results
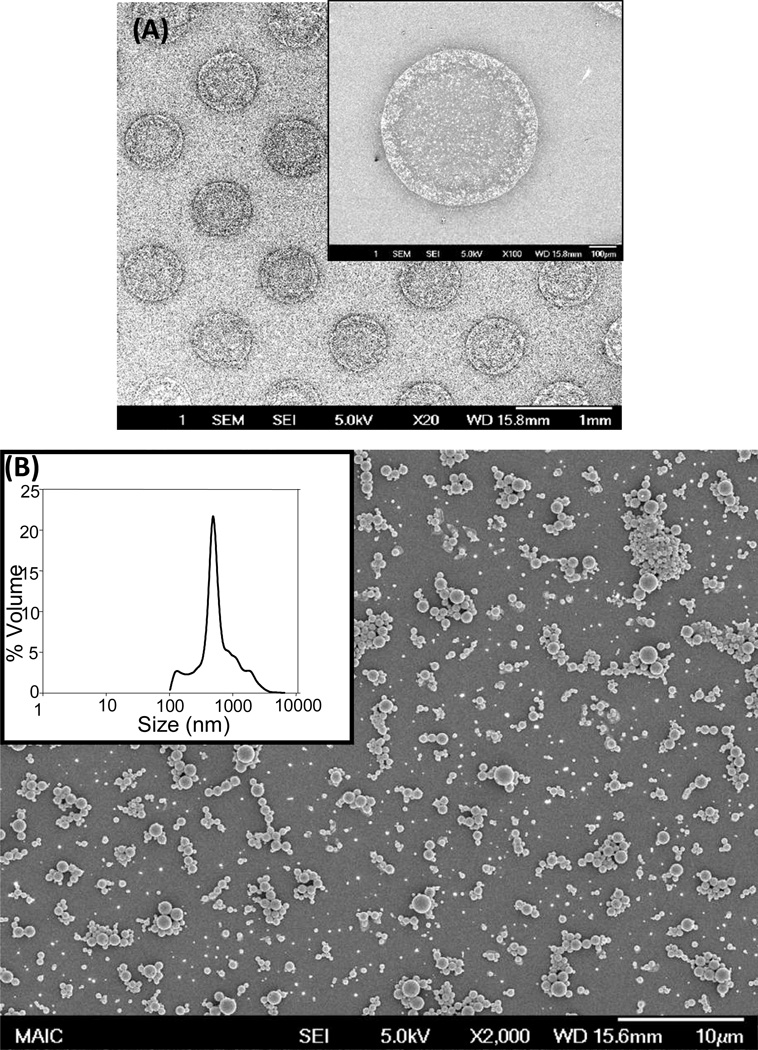
The PPP method incorporates solvent extraction using propylene carbonate as the organic phase for dissolving PLGA and small hydrophobic molecule encapsulates (e.g., fluorescent dyes), and water as the hydrophilic phase (Fig 1). Micro-batches of PPP-generated particles were pooled and characterized using scanning electron microscopy and dynamic light scattering to have a smooth, spherical morphology with an average size of ~1.0 µm. Particles generated in multi-well plates are amenable to spotting onto solid substrates via miniarrayer, demonstrated by printing 400 µm particle spots onto glass (Fig 2A,B).
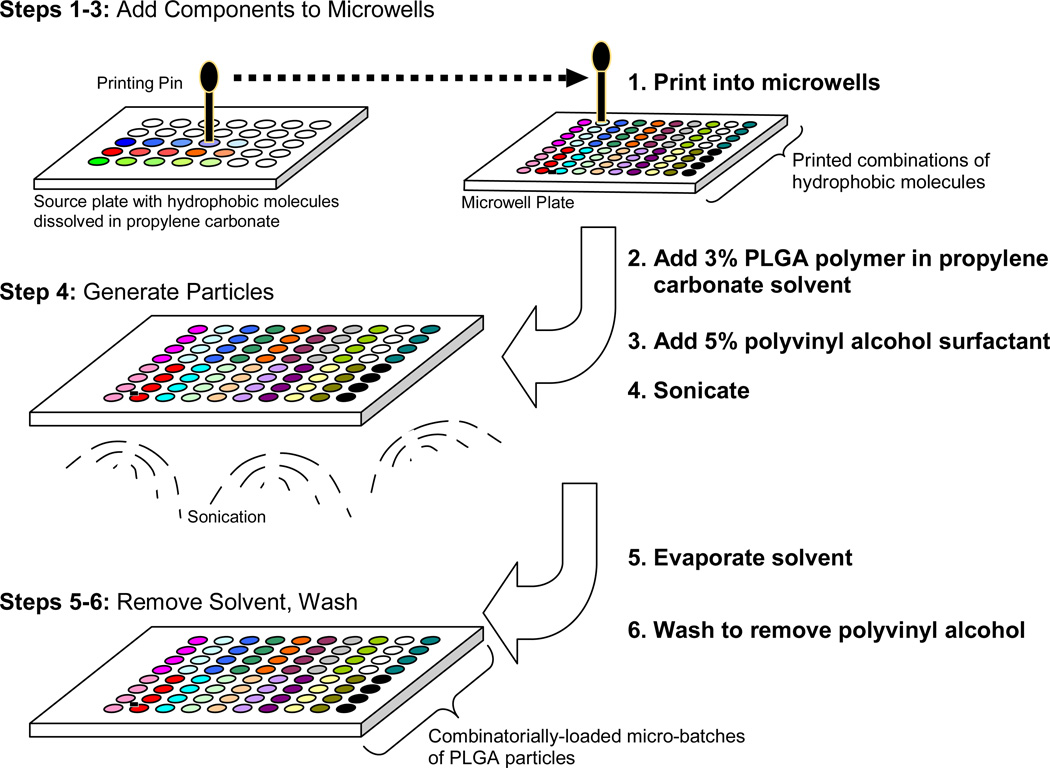
Figure 1. Parallel Particle Production.
Microparticles of poly(lactide-co-glycolide) (PLGA) co-encapsulating multiple hydrophobic components are generated using oil-in-water emulsification in a multi-well polystyrene plate serving as a bank of chambers in which hundreds of particle “micro-batches” are simultaneously generated. Fluorescent dyes are encapsulated as proof of principle, serving as a proxy combination drug formulation. The parallel particle production (PPP) method consists of: Step 1–3.) Dip-pen miniarrayer is programmed to combinatorially add multiple components to microwells: 1.) Dilutions of fluorescent dyes in solvent propylene carbonate (PC) are prepared in the source plate and these solutions are then printed into wells of a microwell plate. 2.) PLGA dissolved in PC is added to the microwells and the mixture is sonicated to homogenize. 3.) Surfactant, polyvinyl alcohol (PVA) in water is added to microwells. Step 4.) Generate Particles: Microwells, containing PVA, PLGA, PC and hydrophobic molecules (dyes) and are sonicated to form an emulsion. Step 5–6.) Remove the solvent and wash: 5.) The microwell plate is placed in a vacuum chamber and the PC solvent is evaporated. 6.) Microparticles are washed by centrifugation and buffer exchange with water to remove PVA and residual PC.
Figure 2.
Particles generated via parallel particle production have smooth morphology and an average size of ~1.0 μm. The particles were analyzed using scanning electron micrograph and dynamic light scattering to observe surface morphology and quantify particle size. A.) PLGA particles are printed and dried onto a glass coverslip using contact pin miniarrayer. B.) Particles are spherical and have a smooth morphology. A representative size distribution curve of particles quantified via dynamic light scattering analysis is shown, inset.
Programmable contact printer miniaraying technology plays a central role in the PPP method. The miniarrayer was utilized to transfer the small hydrophobic molecules from source plate wells, containing fluorescent dyes (coumarin, rhodamine and cyanine) dissolved in solvent, propylene carbonate (PC). Propylene carbonate was chosen as the organic solvent because it readily solublizes PLGA and the fluorescent dyes, but does not dissolve the polystyrene multi-well plates. Dyes were chosen such that overlap in the excitation/emission spectra was minimized. Six concentrations of the three fluorescent dyes in PC were prepared in the source plate (five 1:2 dilutions and a PC-only zero point). All possible combinations of the six dilutions (6 × 6× 6 = 216 unique formulations) were printed into the wells of a 384 multi-well plate for quantification by fluorescence. The dye solutions were deposited as adjacent spots 1 mm away from each other, and the printing pin was washed between the arraying steps. The printed dyes were then re-dissolved in PC and fluorescence intensity was quantified via plate reader. Fluorescence values of the printed dyes were plotted against the source plate concentration for each dye and flourescence intensities followed a linear relationship with respect to the source plate concentration (coefficients of determination: R2Coumarin= 0.99, R2Rhodamine= 0.99 and R2Cyanine= 0.99). This indicates dye printing is well-controlled and linear within the range tested. Furthermore, the delivered amount of each loading condition was statistically significant from every other loading condition.
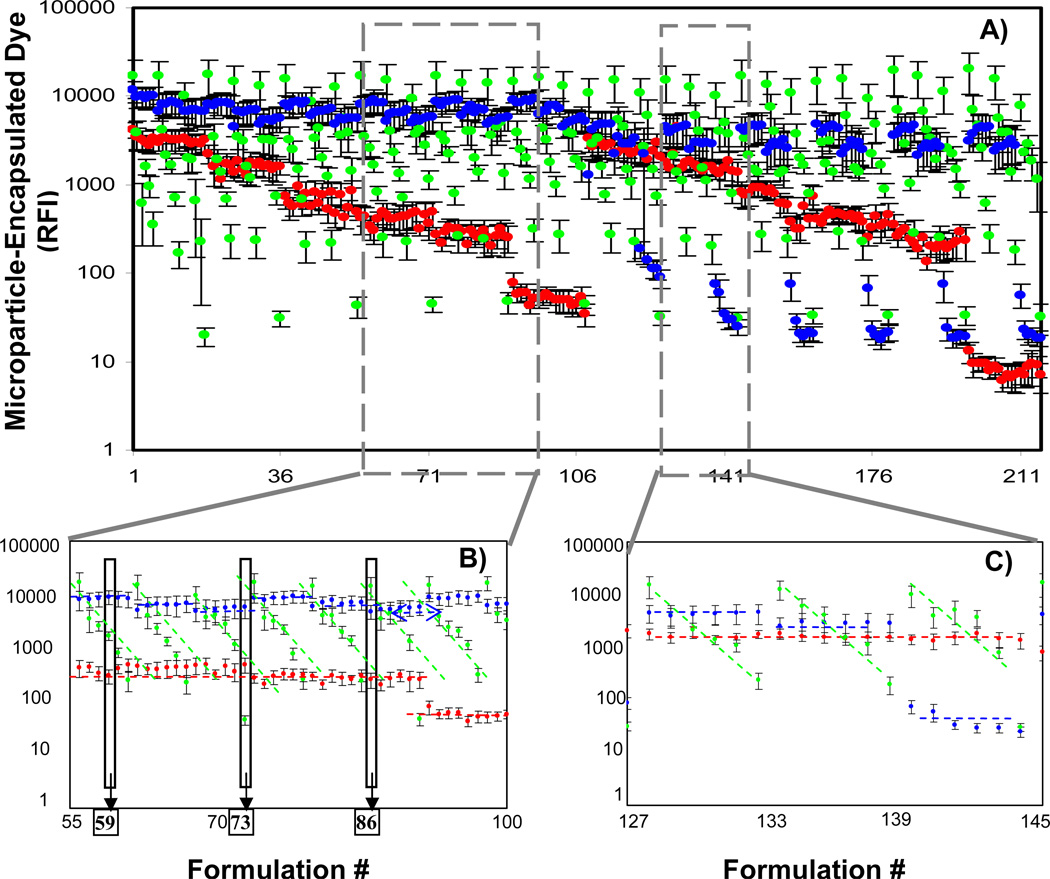
Next, fabrication of PLGA microparticles with three co-encapsulates was carried out. The three fluorescent dyes were printed into microwells in 216 combinations, and microparticles were generated by PPP. Dye loading concentrations for each of the formulations are shown (Table 1). After fabrication, microparticles were washed to remove the surfactant, polyvinyl alcohol, PVA. The particles were re-suspended in water and the fluorescence intensities of each micro-batch were quantified via fluorescence plate reader. The average relative fluorescence intensity and standard deviation is plotted for each of the 216 microparticle formulations (Fig 3A). Two representative formulation regions are shown in greater detail (Fig 3B and 3C), emphasizing the linear trends in fluorescence loading. This is indicative that the encapsulate levels were well-controlled by source plate loading concentrations and suggests that the loading of individual dye components was independent of the other dyes.
Table 1.
The 216 multi-component particle formulations generated using fluorescent dye loading concentrations is shown; A = Coumarin, B = Rhodamine and C = Cyanine.
| Formulation # | A (mg/ml) | B (mg/mL) | C (mg/mL) | Formulation # | A (mg/ml) | B (mg/mL) | C (mg/mL) | Formulation # | A (mg/ml) | B (mg/mL) | C (mg/mL) | Formulation # | A (mg/ml) | B (mg/mL) | C (mg/mL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 20 | 50 | 10 | 55 | 20 | 50 | 1.25 | 109 | 2.5 | 50 | 10 | 163 | 2.5 | 50 | 1.25 |
| 2 | 20 | 25 | 10 | 56 | 20 | 25 | 1.25 | 110 | 2.5 | 25 | 10 | 164 | 2.5 | 25 | 1.25 |
| 3 | 20 | 12.5 | 10 | 57 | 20 | 12.5 | 1.25 | 111 | 2.5 | 12.5 | 10 | 165 | 2.5 | 12.5 | 1.25 |
| 4 | 20 | 6.25 | 10 | 58 | 20 | 6.25 | 1.25 | 112 | 2.5 | 6.25 | 10 | 166 | 2.5 | 6.25 | 1.25 |
| 5 | 20 | 3.125 | 10 | 59 | 20 | 3.125 | 1.25 | 113 | 2.5 | 3.125 | 10 | 167 | 2.5 | 3.125 | 1.25 |
| 6 | 20 | 0 | 10 | 60 | 20 | 0 | 1.25 | 114 | 2.5 | 0 | 10 | 168 | 2.5 | 0 | 1.25 |
| 7 | 10 | 50 | 10 | 61 | 10 | 50 | 1.25 | 115 | 1.25 | 50 | 10 | 169 | 1.25 | 50 | 1.25 |
| 8 | 10 | 25 | 10 | 62 | 10 | 25 | 1.25 | 116 | 1.25 | 25 | 10 | 170 | 1.25 | 25 | 1.25 |
| 9 | 10 | 12.5 | 10 | 63 | 10 | 12.5 | 1.25 | 117 | 1.25 | 12.5 | 10 | 171 | 1.25 | 12.5 | 1.25 |
| 10 | 10 | 6.25 | 10 | 64 | 10 | 6.25 | 1.25 | 118 | 1.25 | 6.25 | 10 | 172 | 1.25 | 6.25 | 1.25 |
| 11 | 10 | 3.125 | 10 | 65 | 10 | 3.125 | 1.25 | 119 | 1.25 | 3.125 | 10 | 173 | 1.25 | 3.125 | 1.25 |
| 12 | 10 | 0 | 10 | 66 | 10 | 0 | 1.25 | 120 | 1.25 | 0 | 10 | 174 | 1.25 | 0 | 1.25 |
| 13 | 5 | 50 | 10 | 67 | 5 | 50 | 1.25 | 121 | 0 | 50 | 10 | 175 | 0 | 50 | 1.25 |
| 14 | 5 | 25 | 10 | 68 | 5 | 25 | 1.25 | 122 | 0 | 25 | 10 | 176 | 0 | 25 | 1.25 |
| 15 | 5 | 12.5 | 10 | 69 | 5 | 12.5 | 1.25 | 123 | 0 | 12.5 | 10 | 177 | 0 | 12.5 | 1.25 |
| 16 | 5 | 6.25 | 10 | 70 | 5 | 6.25 | 1.25 | 124 | 0 | 6.25 | 10 | 178 | 0 | 6.25 | 1.25 |
| 17 | 5 | 3.125 | 10 | 71 | 5 | 3.125 | 1.25 | 125 | 0 | 3.125 | 10 | 179 | 0 | 3.125 | 1.25 |
| 18 | 5 | 0 | 10 | 72 | 5 | 0 | 1.25 | 126 | 0 | 0 | 10 | 180 | 0 | 0 | 1.25 |
| 19 | 20 | 50 | 5 | 73 | 20 | 50 | 0.625 | 127 | 2.5 | 50 | 5 | 181 | 2.5 | 50 | 0.625 |
| 20 | 20 | 25 | 5 | 74 | 20 | 25 | 0.625 | 128 | 2.5 | 25 | 5 | 182 | 2.5 | 25 | 0.625 |
| 21 | 20 | 12.5 | 5 | 75 | 20 | 12.5 | 0.625 | 129 | 2.5 | 12.5 | 5 | 183 | 2.5 | 12.5 | 0.625 |
| 22 | 20 | 6.25 | 5 | 76 | 20 | 6.25 | 0.625 | 130 | 2.5 | 6.25 | 5 | 184 | 2.5 | 6.25 | 0.625 |
| 23 | 20 | 3.125 | 5 | 77 | 20 | 3.125 | 0.625 | 131 | 2.5 | 3.125 | 5 | 185 | 2.5 | 3.125 | 0.625 |
| 24 | 20 | 0 | 5 | 78 | 20 | 0 | 0.625 | 132 | 2.5 | 0 | 5 | 186 | 2.5 | 0 | 0.625 |
| 25 | 10 | 50 | 5 | 79 | 10 | 50 | 0.625 | 133 | 1.25 | 50 | 5 | 187 | 1.25 | 50 | 0.625 |
| 26 | 10 | 25 | 5 | 80 | 10 | 25 | 0.625 | 134 | 1.25 | 25 | 5 | 188 | 1.25 | 25 | 0.625 |
| 27 | 10 | 12.5 | 5 | 81 | 10 | 12.5 | 0.625 | 135 | 1.25 | 12.5 | 5 | 189 | 1.25 | 12.5 | 0.625 |
| 28 | 10 | 6.25 | 5 | 82 | 10 | 6.25 | 0.625 | 136 | 1.25 | 6.25 | 5 | 190 | 1.25 | 6.25 | 0.625 |
| 29 | 10 | 3.125 | 5 | 83 | 10 | 3.125 | 0.625 | 137 | 1.25 | 3.125 | 5 | 191 | 1.25 | 3.125 | 0.625 |
| 30 | 10 | 0 | 5 | 84 | 10 | 0 | 0.625 | 138 | 1.25 | 0 | 5 | 192 | 1.25 | 0 | 0.625 |
| 31 | 5 | 50 | 5 | 85 | 5 | 50 | 0.625 | 139 | 0 | 50 | 5 | 193 | 0 | 50 | 0.625 |
| 32 | 5 | 25 | 5 | 86 | 5 | 25 | 0.625 | 140 | 0 | 25 | 5 | 194 | 0 | 25 | 0.625 |
| 33 | 5 | 12.5 | 5 | 87 | 5 | 12.5 | 0.625 | 141 | 0 | 12.5 | 5 | 195 | 0 | 12.5 | 0.625 |
| 34 | 5 | 6.25 | 5 | 88 | 5 | 6.25 | 0.625 | 142 | 0 | 6.25 | 5 | 196 | 0 | 6.25 | 0.625 |
| 35 | 5 | 3.125 | 5 | 89 | 5 | 3.125 | 0.625 | 143 | 0 | 3.125 | 5 | 197 | 0 | 3.125 | 0.625 |
| 36 | 5 | 0 | 5 | 90 | 5 | 0 | 0.625 | 144 | 0 | 0 | 5 | 198 | 0 | 0 | 0.625 |
| 37 | 20 | 50 | 2.5 | 91 | 20 | 50 | 0 | 145 | 2.5 | 50 | 2.5 | 199 | 2.5 | 50 | 0 |
| 38 | 20 | 25 | 2.5 | 92 | 20 | 25 | 0 | 146 | 2.5 | 25 | 2.5 | 200 | 2.5 | 25 | 0 |
| 39 | 20 | 12.5 | 2.5 | 93 | 20 | 12.5 | 0 | 147 | 2.5 | 12.5 | 2.5 | 201 | 2.5 | 12.5 | 0 |
| 40 | 20 | 6.25 | 2.5 | 94 | 20 | 6.25 | 0 | 148 | 2.5 | 6.25 | 2.5 | 202 | 2.5 | 6.25 | 0 |
| 41 | 20 | 3.125 | 2.5 | 95 | 20 | 3.125 | 0 | 149 | 2.5 | 3.125 | 2.5 | 203 | 2.5 | 3.125 | 0 |
| 42 | 20 | 0 | 2.5 | 96 | 20 | 0 | 0 | 150 | 2.5 | 0 | 2.5 | 204 | 2.5 | 0 | 0 |
| 43 | 10 | 50 | 2.5 | 97 | 10 | 50 | 0 | 151 | 1.25 | 50 | 2.5 | 205 | 1.25 | 50 | 0 |
| 44 | 10 | 25 | 2.5 | 98 | 10 | 25 | 0 | 152 | 1.25 | 25 | 2.5 | 206 | 1.25 | 25 | 0 |
| 45 | 10 | 12.5 | 2.5 | 99 | 10 | 12.5 | 0 | 153 | 1.25 | 12.5 | 2.5 | 207 | 1.25 | 12.5 | 0 |
| 46 | 10 | 6.25 | 2.5 | 100 | 10 | 6.25 | 0 | 154 | 1.25 | 6.25 | 2.5 | 208 | 1.25 | 6.25 | 0 |
| 47 | 10 | 3.125 | 2.5 | 101 | 10 | 3.125 | 0 | 155 | 1.25 | 3.125 | 2.5 | 209 | 1.25 | 3.125 | 0 |
| 48 | 10 | 0 | 2.5 | 102 | 10 | 0 | 0 | 156 | 1.25 | 0 | 2.5 | 210 | 1.25 | 0 | 0 |
| 49 | 5 | 50 | 2.5 | 103 | 5 | 50 | 0 | 157 | 0 | 50 | 2.5 | 211 | 0 | 50 | 0 |
| 50 | 5 | 25 | 2.5 | 104 | 5 | 25 | 0 | 158 | 0 | 25 | 2.5 | 212 | 0 | 25 | 0 |
| 51 | 5 | 12.5 | 2.5 | 105 | 5 | 12.5 | 0 | 159 | 0 | 12.5 | 2.5 | 213 | 0 | 12.5 | 0 |
| 52 | 5 | 6.25 | 2.5 | 106 | 5 | 6.25 | 0 | 160 | 0 | 6.25 | 2.5 | 214 | 0 | 6.25 | 0 |
| 53 | 5 | 3.125 | 2.5 | 107 | 5 | 3.125 | 0 | 161 | 0 | 3.125 | 2.5 | 215 | 0 | 3.125 | 0 |
| 54 | 5 | 0 | 2.5 | 108 | 5 | 0 | 0 | 162 | 0 | 0 | 2.5 | 216 | 0 | 0 | 0 |
Figure 3.
Particles co-encapsulating three different fluorescent dyes are generated via parallel particle production, and their relative fluorescence values are quantified. Six concentrations of each of the dyes (coumarin, rhodamine and cyanine) were co-encapsulated in PLGA particles providing 216 unique formulations. A.) Average relative fluorescence intensity of encapsulated dyes and standard deviation for each formulation was quantified by fluorescence plate reader and plotted by formulation number. Magnified regions illustrate control over loading of co-encapsulates: B.) Formulations #55 through #100, and C.) Formulations #127 through #145. Overlay lines emphasize the trends of different concentration of the dyes: blue line - coumarin (----); green line - rhodamine (----); red line - cyanine (----). Individual formulations #59, #73 and #86 (boxes in B.), were further analyzed by flow cytometry analysis (see Figure 4).
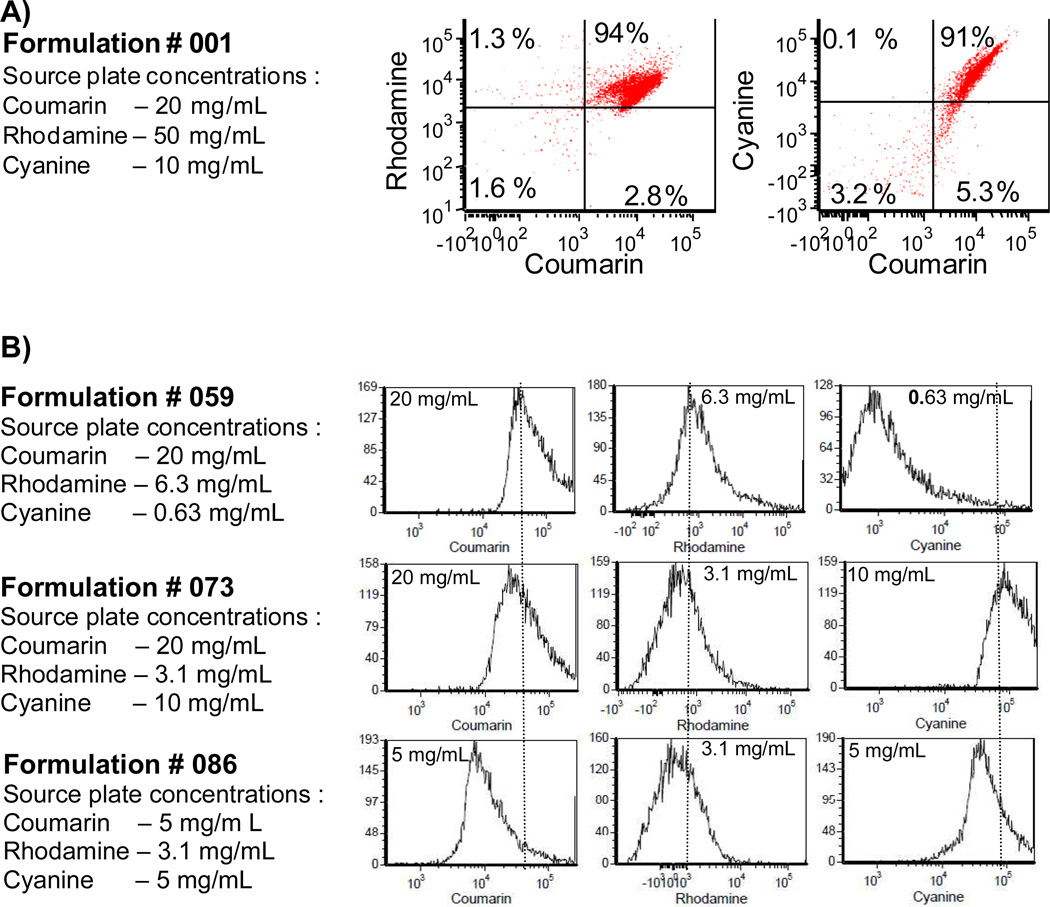
Particles were further analyzed by flow cytometry in order to characterize dye co-localization within particles on a particle-by-particle basis, and to determine variability within a micro-batch. Sum fluorescence intensity dot-plots of rhodamine versus coumarin and cyanine versus coumarin were plotted. Shown is a representative dot-plot of formulation # 001, the highest loading concentrations of the three co-encapsulates, illustrating distribution of the co-encapsulates (Fig 4A). Greater than 90% of the particles were double-positive for rhodamine and coumarin, and greater than 90% of the particles were double-positive for rhodamine and cyanine demonstrating that most of the microparticles co-encapsulated all three dyes. Furthermore, single-color histograms demonstrate that encapsulate levels in microparticle populations are generally normally distributed, and relative shifts in fluorescence intensity peaks correspond well with source plate loading concentrations (Fig 4B).
Figure 4.
Particles generated by parallel particle production co-encapsulate fluorescent dyes in a dose-dependent manner. A.) Dot-plot obtained using flow cytometry analysis demonstrates particles are >90% double-positive for each dye, indicating co-encapsulation. B.) Flow cytometry histograms of select particle formulations (see boxes in Fig 3B) demonstrate that dye encapsulation is well-controlled by the source plate concentration. Dotted lines show relative shifts in encapsulated amount.
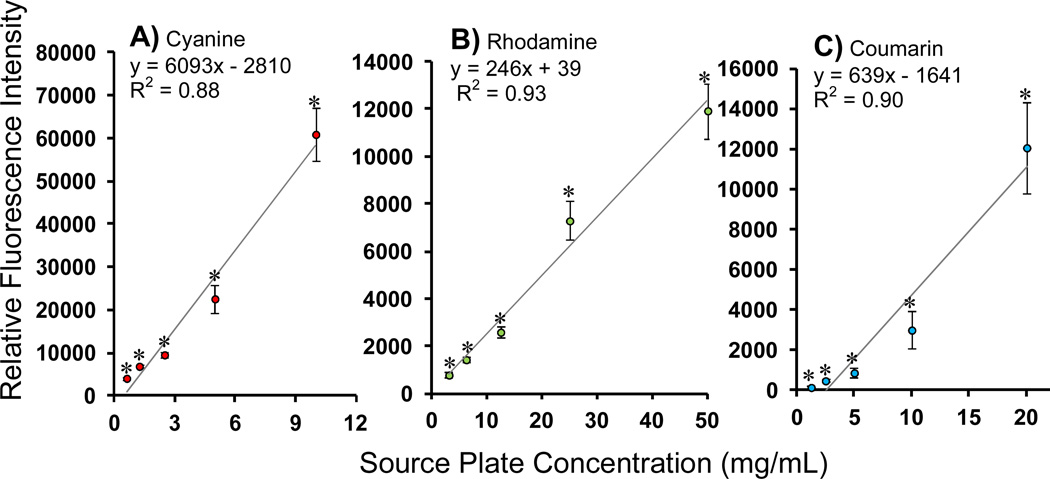
In order to investigate the possibility that co-encapsulation of multiple components may affect loading of individual components, data were pooled across the different formulations so that loaded fluorescence values from conditions of equal source plate concentrations were grouped together (including cases where differing amounts of co-encapsulates were co-loaded). Data from these pooled samples were averaged and plotted with standard deviations, and linear regression performed (Fig 5). From this analysis it is evident that co-encapsulation of each component is well-controlled, i.e., each loaded amount is significantly different from each other and loading follows a linear relationship with respect to the source plate concentration (coefficient of determination – coumarin: of R2= 0.90; rhodamine: R2= 0.93; cyanine: R2= 0.88). In order to further validate independent control over the co-encapsulation of mutliple components, comparisons were made between single-component and multi-component loading conditions via paired t-test analysis (Table 2). The paired t-test analysis resulted in a p-value > 0.5 in every case, indicating that loading in the multi-component particles is indistinguishable from loading in the single-component particles. In other words, co-encapsulation of multiple components does not affect loading of individual factors – each can be controlled independently.
Figure 5.
Loading of each encapsulate is independently controlled while simultaneously co-encapsulating multiple components. Fluorescent dyes: B.) cyanine B.) rhodamine and C.) coumarin, at 6 different loading concentrations were printed in all possible combinations in a polystyrene 384-well and encapsulated in PLGA particles thus providing 216 different formulations. For each dye loaded, loading values obtained by pooling micro-batches of equivalent loading concentrations were significantly different from the other loading concentrations (p-value < 0.05), showing a linear trend with respect to source plate concentrations.
Table 2.
P-values for paired t-test comparing single-dye formulations to multi-component conditions. P-values of > 0.05 indicate that the single-dye formulation and multi-dye formulation loading amounts are equivalent and belong in the same data-set. This indicates simultaneous loading of multiple co-encapsulates can be carried without compromising independent control over of individual component loading.
| Formulation | p-value |
|---|---|
| Coumarin – 20 mg/mL | 0.14 |
| Coumarin - 10 mg/mL | 0.57 |
| Coumarin – 5 mg/mL | 0.72 |
| Coumarin – 2.5 mg/mL | 0.38 |
| Coumarin – 1.25 mg/mL | 0.59 |
| Coumarin – 0 mg/mL | 0.67 |
| Rhodamine – 50 mg/mL | 0.14 |
| Rhodamine – 25 mg/mL | 0.96 |
| Rhodamine – 12.5 mg/mL | 0.46 |
| Rhodamine – 6.25 mg/mL | 0.25 |
| Rhodamine – 3.125 mg/mL | 0.11 |
| Rhodamine – 0 mg/mL | 0.13 |
| Cyanine – 10 mg/mL | 0.93 |
| Cyanine – 5 mg/mL | 0.75 |
| Cyanine – 2.5 mg/mL | 0.85 |
| Cyanine – 1.25 mg/mL | 0.89 |
| Cyanine – 0.625 mg/mL | 0.35 |
| Cyanine – 0 mg/mL | 0.57 |
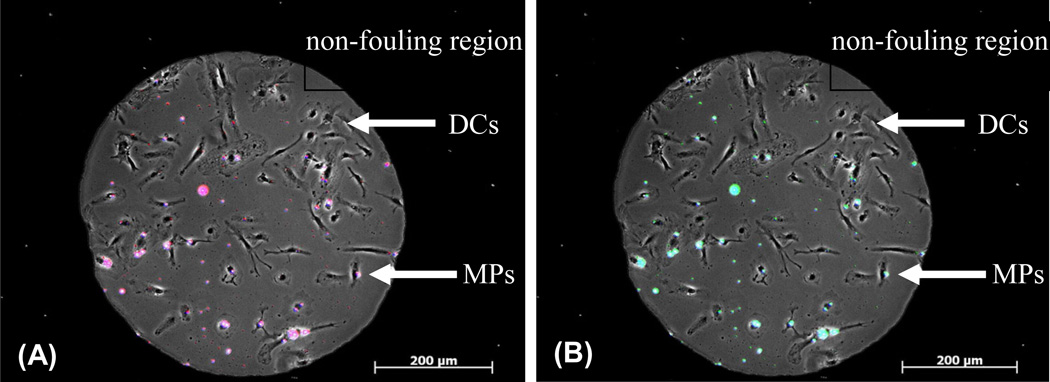
Finally, we demonstrated the utility of integrating the PPP method with a cellular microarray high-throughput screening method. Cellular microarrays consisting of surface-adsorbed microparticles co-localized with cells on isolated cell-adhesive islands surrounded by a non-fouling region were fabricated, as recently described [7]. Particles fabricated by PPP were spotted and dried onto cell-adhesive islands and dendritic cells were subsequently seeded, preferentially adhering to the adhesive islands. This combination of PPP-generated particles with the dendritic cell/microparticle array system enables the screening of large numbers of different immunomodulatory particle formulations in a high-throughput fashion, requiring very little particle material to be fabricated and small cell numbers for screening. Fluorescence and phase contrast overlay micrographs of representative cell-adhesive islands illustrate integration of PPP-generated microparticles encapsulating three different fluorescent dyes with the microparticle/cellular microarray screening method (Fig 6). Although dendritic cells were utilized for proof of principle, expansion to other cell types can be envisioned. Together, these technologies may aid in discovering synergistic combinations of otherwise non-intuitive drug combinations.
Figure 6.
Microparticles (MPs) generated using PPP method can be co-localized with dendritic cells (DCs) on a cellular array to quantify the effects of particles on DC functions in a high-throughput fashion. Particles encapsulating fluorescent dyes coumarin, rhodamine and cyanine were over-printed on an array using standard mini-arraying contact pin equipment and DCs were seeded on for 2 h. Individual spots of co-localized DCs and particles showing overlays of A.) coumarin dye (in blue) + cyanine dye (in red) = magenta; B.) coumarin dye (in blue) + rhodamine dye (in green) = cyan; demonstrating co-localization of particle formulations with cells.
Discussion
While high-throughput screening has led to identification of small molecules in solution which modulate cellular behavior, high-throughput characterization of cellular responses to controlled release systems has lagged partly due to a lack of facile particle fabrication methods. Notably, high-throughput systems have been developed to screen interactions of cells with peptide density gradients [13–15], microparticle microarrays [10], and polymer blend films [16,17]. A microfluidics approach has also recently been described to generate a particulate library of polyanhydride polymer blends [18,19]. However, currently there is no method to generate a large library of particles with multiple co-encapsulated components. Co-encapsulation of multiple biomolecules into particles has potential advantages in imaging diagnostics, theranostics, and for potentiation of immune responses [2]. In this report we demonstrate the feasibility of the PPP method of fabricating a library of PLGA particles encapsulating multiple components. These particle libraries can subsequently be screened for synergism between encapsulates.
Encapsulation of molecules using PLGA particles has long been utilized for controlled delivery in both clinical and research settings [20–23]. Several methods have been implemented to generate PLGA particles encapsulating molecules of choice, such as spray drying process, phase separation and solvent extraction methods [10–12]. Among these techniques, solvent extraction/evaporation has been most commonly utilized. However, this technique only allows for generation of particles in single bulk batches, hence, requiring large amounts of reagents, time and labor to generate even a small library of co-encapsulated combination drugs. One approach to overcome this limitation is to miniaturize the process. In order to develop such a technique, we utilized programmable contact pin miniarraying technology, capable of transferring small liquid volumes in the picoliter range, in conjuction with an appropriately-sized multi-well plate (384 multi-well, polystyrene plate) serving as a bank of chambers in which to generate particles. A sonicating water bath was used for simultaneous mixing of all micro-well chambers in the plate, and centrifugation of the plate was used to accomplish separation of particles from liquids for washing. In order to take advantage of commercially available polystyrene multi-well plates, an appropriate solvent was required which would readily dissolve PLGA but not polystyrene. A further requirement was the solvent must be capable of forming an emulsion in the presence of water and surfactant (PVA). Two solvents, triacetin and propylene carbonate (PC), were identified as suitable for generating particles in this fashion. From these two, propylene carbonate was selected due to its lower vapor pressure, which allows it to be more easily removed from the emulsion by evaporation. Furthermore, PC is moderately soluble in water (17%) which facilitates removal via the water phase during washing [24], mitigating potentially undesirable exposure to residual PC. With these key elements of this technology in place, a large number of distinct micro-batches of particles with combinatorially-loaded encapsulates is readily generated. We demonstrate that the PLGA particles generated by PPP possess similar morphology and size distribution to particles generated by a traditional large batch process. Furthermore, we show that multiple hydrophobic molecules can be encapsulated in the particles in a controlled manner, where loading of each component is independent of the others. This method enables generation of particles for screening with significant reductions in reagents, labor and time, and is generalizable to material systems other than PLGA.
Conclusions
PLGA particles encapsulating multiple hydrophobic molecules were generated in a high-throughput manner using the PPP method. The loading of hydrophobic molecules in the particles was well-controlled and 216 unique particle formulations with three co-encapsulates were generated, thus demonstrating the high-throughput nature and versatility of this technique. Furthermore, the particles generated using PPP method were incorporated into a cell-based microarray, demonstrating ease of integration with this high-throughput screening system. Such co-encapsulate particle libraries are suitable for high-throughput screening in order to identify interactions among components in the modulation of cellular functions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hamdy S, Haddadi A, Hung RW, Lavasanifar A. Targeting dendritic cells with nanoparticulate PLGA cancer vaccine formulations. Drug Deliv Rev. 2011;63:943–955. doi: 10.1016/j.addr.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 2.Singh A, Nie H, Ghosn B, Qin H, Kwak LW, Roy K. Efficient modulation of T-cell response by dual-mode, single-carrier delivery of cytokine-targeted siRNA and DNA vaccine to antigen-presenting cells. Mol Ther. 2008;16:2011–2021. doi: 10.1038/mt.2008.206. [DOI] [PubMed] [Google Scholar]
- 3.Diwan M, Tafaghodi M, Samuel J. Enhancement of immune responses by co-delivery of a CpG oligodeoxynucleotide and tetanus toxoid in biodegradable nanospheres. J Control Release. 2002;85:247–262. doi: 10.1016/s0168-3659(02)00275-4. [DOI] [PubMed] [Google Scholar]
- 4.Figueiredo M, Esenaliev R. PLGA Nanoparticles for ultrasound-mediated gene delivery to solid tumors. J Drug Deliv. 2012;2012 doi: 10.1155/2012/767839. 767839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keselowsky BG, Xia CQ, Clare-Salzler M. Multifunctional dendritic cell-targeting polymeric microparticles: engineering new vaccines for type 1 diabetes. Hum Vaccin. 2011;7:37–44. doi: 10.4161/hv.7.1.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain RA. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials. 2000;21:2475–2490. doi: 10.1016/s0142-9612(00)00115-0. [DOI] [PubMed] [Google Scholar]
- 7.Norton LW, Park J, Babensee JE. Biomaterial adjuvant effect is attenuated by antiinflammatory drug delivery or material selection. J Control Release. 2010;146:341–348. doi: 10.1016/j.jconrel.2010.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis JS, Zaveri TD, Crooks CP, Keselowsky BG. Microparticle surface modifications targeting dendritic cells for non-activating applications. Biomaterials. 2012;33:7221–7232. doi: 10.1016/j.biomaterials.2012.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choe SW, Acharya AP, Keselowsky BG, Sorg BS. Intravital microscopy imaging of macrophage localization to immunogenic particles and co-localized tissue oxygen saturation. Acta Biomater. 2010;6:3491–3498. doi: 10.1016/j.actbio.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Acharya AP, Clare-Salzler MJ, Keselowsky BG. A high-throughput microparticle microarray platform for dendritic cell-targeting vaccines. Biomaterials. 2009;30:4168–4177. doi: 10.1016/j.biomaterials.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 11.Husmann M, Schenderlein S, Lück M, Lindner H, Kleinebudde P. Polymer erosion in PLGA microparticles produced by phase separation method. Int J Pharm. 2002;242:277–280. doi: 10.1016/s0378-5173(02)00187-4. [DOI] [PubMed] [Google Scholar]
- 12.Aubert-Pouëssel A, Venier-Julienne MC, Saulnier P, Sergent M, Benoît JP. Preparation of PLGA microparticles by an emulsion-extraction process using glycofurol as polymer solvent. Pharm Res. 2004;21:2384–2391. doi: 10.1007/s11095-004-7693-3. [DOI] [PubMed] [Google Scholar]
- 13.Acharya AP, Dolgova NV, Moore NM, Xia CQ, Clare-Salzler MJ, Becker ML, et al. The modulation of dendritic cell integrin binding and activation by RGD-peptide density gradient substrates. Biomaterials. 2010;31:7444–7454. doi: 10.1016/j.biomaterials.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 14.Moore NM, Lin NJ, Gallant ND, Becker ML. The use of immobilized osteogenic growth peptide on gradient substrates synthesized via click chemistry to enhance MC3T3-E1 osteoblast proliferation. Biomaterials. 2010;31:1604–1611. doi: 10.1016/j.biomaterials.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Gallant ND, Lavery KA, Amis EJ, Becker ML. Universal Gradient Substrates for “Click” Biofunctionalization. Adv Mat. 2007;19:965–969. [Google Scholar]
- 16.Anderson DG, Levenberg S, Langer R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nat Biotechnol. 2004;22:863–866. doi: 10.1038/nbt981. [DOI] [PubMed] [Google Scholar]
- 17.Kou PM, Babensee JE. Validation of a high-throughput methodology to assess the effects of biomaterials on dendritic cell phenotype. Acta Biomater. 2010;6:2621–2630. doi: 10.1016/j.actbio.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petersen LK, Sackett CK, Narasimhan B. Novel, high throughput method to study in vitro protein release from polymer nanospheres. J Comb Chem. 2010;12:51–56. doi: 10.1021/cc900116c. [DOI] [PubMed] [Google Scholar]
- 19.Petersen LK, Chavez-Santoscoy AV, Narasimhan B. Combinatorial synthesis of and high-throughput protein release from polymer film and nanoparticle libraries. J Vis Exp. 2012;6:e3882. doi: 10.3791/3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali OA, Huebsch N, Cao L, Dranoff G, Mooney DJ. Infection-mimicking materials to program dendritic cells in situ. Nat Mater. 2009;8:151–158. doi: 10.1038/nmat2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lü JM, Wang X, Marin-Muller C, Wang H, Lin PH, Yao Q, et al. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev Mol Diagn. 2009;9:325–341. doi: 10.1586/erm.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbas AO, Donovan MD, Salem AK. Formulating poly(lactide-co-glycolide) particles for plasmid DNA delivery. J Pharm Sci. 2008;97:2448–2461. doi: 10.1002/jps.21215. [DOI] [PubMed] [Google Scholar]
- 23.Gupta RK, Chang AC, Siber GR. Biodegradable polymer microspheres as vaccine adjuvants and delivery systems. Dev Biol Stand. 1998;92:63–78. [PubMed] [Google Scholar]
- 24.Quintanar-Guerrero D, Ganem-Quintanar A, Allémann E, Fessi H, Doelker E. Influence of the stabilizer coating layer on the purification and freeze-drying of poly(D,L-lactic acid) nanoparticles prepared by an emulsion-diffusion technique. J Microencapsul. 1998;15:107–119. doi: 10.3109/02652049809006840. [DOI] [PubMed] [Google Scholar]
- 25.Acharya AP, Dolgova NV, Clare-Salzler MJ, Keselowsky BG. Adhesive substrate-modulation of adaptive immune responses. Biomaterials. 2008;29:4736–4750. doi: 10.1016/j.biomaterials.2008.08.040. [DOI] [PubMed] [Google Scholar]
- 26.Acharya AP, Dolgova NV, Xia CQ, Clare-Salzler MJ, Keselowsky BG. Adhesive substrates modulate the activation and stimulatory capacity of non-obese diabetic mouse-derived dendritic cells. Acta Biomater. 2011;7:180–192. doi: 10.1016/j.actbio.2010.08.026. [DOI] [PubMed] [Google Scholar]