Abstract
MDA-9 (melanoma differentiation associated gene-9)/Syntenin is a PDZ domain-containing adaptor protein involved in multiple diverse cellular processes including organization of protein complexes in the plasma membrane, intracellular trafficking and cell surface targeting, synaptic transmission, and cancer metastasis. In the present study, we analyzed the expression pattern of MDA-9/syntenin during mouse development. MDA-9/syntenin was robustly expressed with tight regulation of its temporal and spatial expression during fetal development in the developing skin, spinal cord, heart, lung and liver, which are regulated by multiple signaling pathways in the process of organogenesis. Recent studies also indicate that MDA-9/syntenin is involved in the signaling pathways crucial during development such as Wnt, Notch and FGF. Taken together, these results suggest that MDA-9/syntenin may play a prominent role during normal mouse development in the context of cell proliferation as well as differentiation through modulating multiple signaling pathways as a crucial adaptor protein. Additionally, temporal regulation of MDA-9/syntenin expression may be required during specific stages and in specific tissues during development.
Keywords: MDA-9/syntenin, development, mouse embryo, adaptor protein
Introduction
Melanoma differentiation associated gene-9 (MDA-9) was first discovered by our group as a gene regulated during terminal differentiation of human melanoma cells by combinatorial treatment of IFN-β and the protein kinase C activator mezerein (Lin et al. 1998, 1996). Another group subsequently cloned MDA-9 using yeast two-hybrid screening as an interacting partner of syndecans, and named the protein syntenin (Grootjans et al. 1997). MDA-9/syntenin is a PDZ domain containing scaffolding protein involved in organization of protein complexes in the plasma membranes, thereby regulating a plethora of biological functions (Sarkar et al. 2008). MDA-9/syntenin is also involved in the early secretory pathway and associated with the endoplasmic reticulum, intermediate compartment and cis-Golgi to facilitate intracellular trafficking and targeting of receptors and secreted proteins such as proTGF-α (Beekman and Coffer 2008; Fernandez-Larrea et al. 1999). MDA-9/syntenin mediates IL-5-induced Sox4 activation through its direct interaction with IL-5Rα and Sox4, supporting a potential role in B-cell development and differentiation (Geijsen et al. 2001). MDA-9/syntenin also mediates Notch ligand Δ1-induced cohesiveness of epidermal stem cells through direct interaction of Δ1 with MDA-9/syntenin that maintains Δ1 on the cell surface (Estrach et al. 2007). Moreover, MDA-9/syntenin is involved in synaptic transmission via interaction with glutamate receptors, and axonal outgrowth by interacting with Unc51.1 and Rab5 (Hirbec et al. 2002; Enz and Croci 2003; Tomoda et al. 2004).
Recent reports have emphasized crucial roles of MDA-9/syntenin in tumor progression and metastasis (Sarkar et al. 2008). MDA-9/syntenin has been shown to contribute to metastatic progression of uveal melanoma, melanoma, and cancer of the breast and stomach by activating various oncogenic signaling pathways such as FAK, MAPKs, Akt, Src, and NF-κB (Helmke et al. 2004; Boukerche et al. 2007; Boukerche et al. 2008; Boukerche et al. 2010; Koo et al. 2002; Gangemi et al. 2012). In addition to activation of oncogenic signals, MDA-9/syntenin interacts with and probably inhibits tumor suppressor proteins such as sch-1 (a isoform of the NF2 protein) and r-PTPη (a receptor-type tyrosine phosphatase) even though its functional significance was not investigated (Jannatipour et al. 2001; Iuliano et al. 2001). In total, these findings indicate that MDA-9/syntenin serving as a scaffolding protein interacts with various proteins that may play a fundamental and global role in normal cell physiology, suggesting that its aberrant regulation could be crucial in various diseases as cancer metastasis.
In the present study, in order to provide initial insights into the potential roles of MDA-9/syntenin in normal development we examined its expression pattern in the embryonic skin, spinal cord, heart, lung and liver of mice in which progenitor cells exist for differentiation and cell proliferation for each tissue and organ formation.
Materials and methods
Animals and tissue preparation
C57BL/6NCR mice were time-mated and pregnant females were sacrificed to collect embryos at different embryonic stages (E8.5-E18.5). Our animal facility artificially provides a 12 h light and 12 h dark cycle. Timing of mating most likely takes place in the dark. The vaginal plugs were found early in the morning. This was considered as the first day of pregnancy and designated as E0.5. The fetuses were isolated from the uterus and dissected under a microscope. They were fixed in 4% (W/V) paraformaldehyde. The VCU Institutional Animal Care and Use Committee approved this study.
Extraction of total RNA and Real-Time PCR
Total RNA was extracted using TRIzol reagent (Invitrogen) and cDNA was synthesized by SuperScript III Reverse transcriptase (Invitrogen). Real-time PCR was performed using ABI 7900 fast real-time PCR system and TaqMan Gene Expression Assays for mouse MDA-9/syntenin (Mm00489742_m1, Applied Biosystems) and β-actin (4352933E, Applied Biosystems) according to the manufacturer’s instructions. PCR mixtures were incubated at 50 °C for 2 min and 95 °C for 10 min, and then each cDNA was synthesized by two steps at 95 °C for 15 min and 60 °C for 1 min during 40 cycles.
Immunohistochemistry
The Embryos from E12.5 to E18.5 were embedded in paraffin and 4-μm sections were prepared. Paraffin sections were deparaffinized in xylene and rehydrated through a graded series of ethanol from absolute ethanol to 80% ethanol and finally washed in PBS. After rinsing in PBS, endogenous hydrogen peroxidase was quenched with 3% H2O2 in PBS for 1h. The sections were incubated with 3% skim milk for 1h and developed by avidin-biotin-peroxidase complexes with DAB substrate solution (Vector Laboratories). The sections were incubated with anti-MDA-9/syntenin (1:30) antibody (Santa Cruz Biotechnology, Inc.) overnight at 4°C and treated with anti-rabbit IgG biotinylated secondary antibody for 1h at room temperature after rinsing thoroughly in PBS-T. Following incubation with peroxidase complex reagent for 1h, the immunoreactive signals in sections were detected by DAB substrate (DAKO) and counterstained briefly with hematoxylin (Zymed Laboratories Inc.). The sections were then dehydrated, mounted and photographed.
Results and discussion
Expression of mda-9/syntenin in mouse embryos at E8.5, E9.5 and E10.5
For the initial assessment of mda-9/syntenin expression pattern during early development stages, mRNAs were extracted from the whole wild-type mouse embryos at E8.5, E9.5 and E10.5 and subjected to quantitative real-time PCR. mda-9/syntenin mRNA was detected in varying degrees at these three stages, with much higher expression in E9.5 and E10.5 than in E8.5 (Fig. 1). MDA-9/syntenin is an interacting molecule of cell-surface heparan sulfate syndecans, which is involved in cell-cell and cell-matrix adhesion, signal transduction from the cell surface to the interior, and trafficking of lipoproteins and lipases, thus suggesting its prominent roles in cell growth, development, and differentiation (Grootjans et al. 1997). Based on these results and the robust expression of mda-9/syntenin in the early developmental stages we hypothesize that this gene plays a pertinent role in progenitor cell differentiation and/or proliferation of early embryos.
Fig. 1.
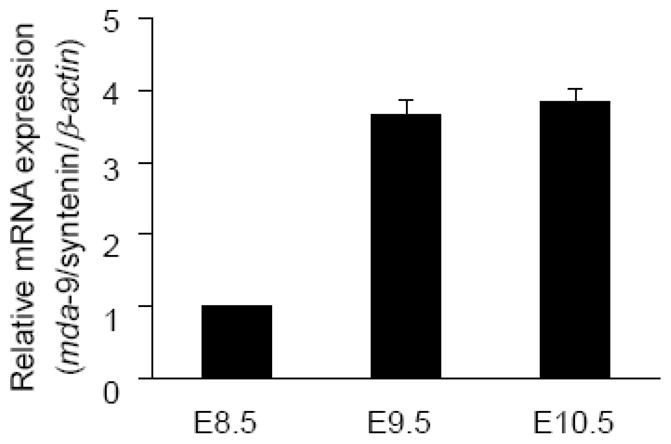
Expression of mda-9/syntenin in the mouse embryo. Real-time PCRs at embryonic stage E8.5 to E10.5 were performed at least three times in duplicate or triplicate, and the minimal number of embryos (n) used at each stage was 9. The expression of mda-9/syntenin was normalized by β-actin, and the error bars indicate SEM.
Expression of MDA-9/syntenin in the developing skin and hair follicle
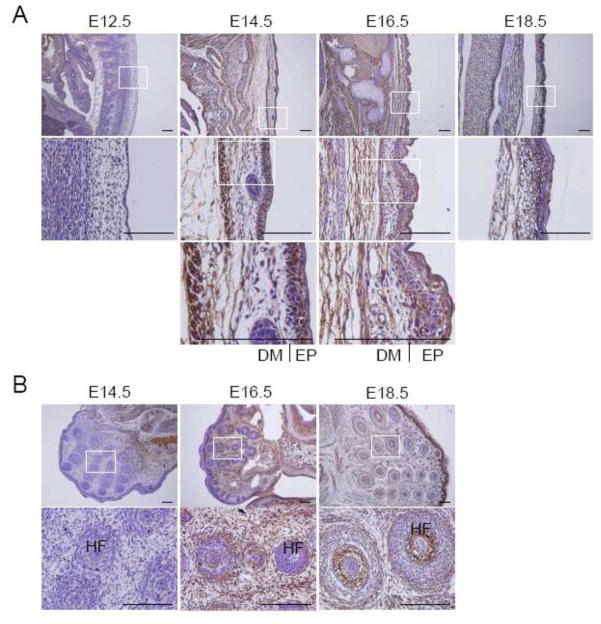
The skin is composed of dermis and epidermis, and is responsible for the development of the hair follicles, sebaceous glands, mammary glands, teeth and external genitalia (Fuchs and Raghavan 2002). Morphology and thickness in the skin during the developmental period undergo well-defined changes. The skin at E12.5 is a less developed thin layer of early epidermis, while embryos between E14.5 and E18.5 have a more stratified and thick layers in both the epidermis and dermis (Fig. 2a). Expression of MDA-9/syntenin in the epidermis was strong in most regions at E14.5 and only in the basal layer at E16.5, but barely detectable at E18.5 (Fig. 2a). Compared to the epidermis, the dermis displayed a negligible signal at E14.5, but the expression of MDA-9/syntenin was apparently increased at E16.5 and E18.5 (Fig. 2a). MDA-9/syntenin is known to mediate Delta1-induced cohesiveness of epidermal stem cells and to promote metastasis of melanoma by activating c-Src (Boukerche et al. 2008; Estrach et al. 2007). Furthermore, Notch signals trigger terminal differentiation of the developing epidermis (Blanpain et al. 2006; Nguyen et al. 2006). These results suggest that MDA-9/syntenin may be involved in the process of differentiation during skin development.
Fig. 2.
Expression of MDA-9/syntenin in the skin and hair follicle. (a) The dorsal skin at E12.5 to E18.5 was dissected, and expression of MDA-9/syntenin was examined. The skin was thin layered at E12.5, and stratified dermis and epidermis were appeared from E14.5. (b) The hair follicle was first detected at E14.5, and expression of MDA-9/syntenin was observed at late stage in most developing hair follicles. Every enlarged image in the lower panel corresponds to the boxed area in the upper panel. Scale bars indicate 100 μm. DM: dermis, EP: epidermis and HF: hair follicle.
Hair follicles as skin appendages begin to develop at E14.5, and appear to be in the form of a hair germ cell at E16.5, and are characterized by an inner root sheath and engulfment of dermal papilla within the hair bulb on E18.5 (Fuchs and Raghavan 2002; Owens et al. 2008). We examined the region near the mouth, where many developing hair follicles take shape, the number increasing so that by E18.5 the majority of the face is covered by hair follicles (Fig. 2b). Expression of MDA-9/syntenin at the hair follicles was negligible at E14.5, but significant expression was detected in both inner and outer root sheaths at E16.5 and E18.5 (Fig. 2b). Interestingly, MDA-9/syntenin was also noticeable in connective tissues surrounding hair follicles at E16.5 and E18.5 (Fig. 2b). Early in development, the epithelium grows down into the dermis and the hair follicles also require intimate interactions and cellular signaling with the epidermis (Jahoda 1992; Risek et al. 1992). The instructive signals for hair follicle formation are derived from but not limited to the pathways of Wnt/β-catenin, Hedgehog, FGF and BMP (Fuchs and Raghavan 2002; Owens et al. 2008). Recent studies indicate that MDA-9/syntenin impacts on the trafficking and restriction of FGF signals (Baietti et al. 2012; Zimmermann et al. 2005). Together these results suggest that MDA-9/syntenin may play a key role in the development of hair follicle by regulating FGF signals.
Expression of MDA-9/syntenin in the spinal cord
We next examined MDA-9/syntenin expression in the developing spinal cord. Segmenting cartilage primordium of vertebra began to be form at E12.5, but there was no distinct expression of MDA-9/syntenin at this stage in this region (Fig. 3). Intriguingly, a strong signal was detected from cartilage primordium of the spinal column and connective tissues surrounding the spinal column at E14.5 and E16.5, and the degree of expression apparently diminished at E18.5 (Fig. 3). In addition, MDA-9/syntenin stained in the nucleus pulposus in the middle of spinal disc at E18.5, but not in the annulus fibrosus surrounding the nucleus pulposus (Fig. 3). There are two distinctive cell types, chondrocyte-like cells and notochordal cells that are mainly found in the nucleus pulposus located in the intervertebral disk. Nucleus pulposus is required for generating and maintaining the disk structure, and embryonic nucleus pulposus is formed from the embryonic notochord (Setton and Chen 2006). Notochords begin to segregate along the anteroposterior axis at E12.5, cells of the notochord aggregate in areas where the nucleus pulposus is forming at E15.5, and the cells by E16.5 form the disk-shaped condensation between the vertebrae (Choi et al. 2008). Therefore, further studies are required to determine exactly which of the cells in the nucleus pulposus express MDA-9/syntenin. Taken together, these findings suggest that MDA-9/syntenin could be important in the development of the spinal cord.
Fig. 3.
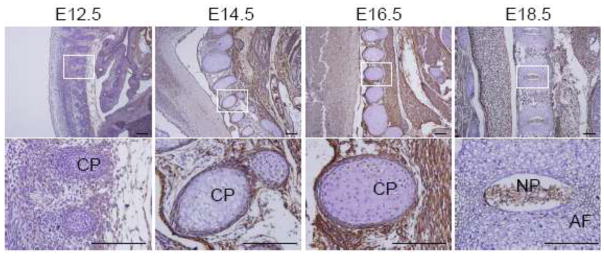
Expression of MDA-9/syntenin in the spinal cord. The spinal cord was stained with anti-MDA-9/syntenin in E12.5 to E18.5 embryos. Strong expression of MDA-9/syntenin was detected near root ganglia surrounding spinal column at E14.5 and E16.5, and in the nucleus pulposus at E18.5. Every enlarged image in the lower panel corresponds to the boxed area in the upper panel. Scale bars indicate 100 μm. CP: cartilage primordium, AF: annulus fibrosus and NP: nucleus pulposus.
Expression of MDA-9/syntenin in the developing heart
We also examined the developing heart between E12.5 and E18.5. The heart is the first functional organ during embryogenesis and its formation is initiated in the cardiac crescent at about E7.5 in mice, and the cardiac crescent from cardiac precursor cells converges along the ventral midline of the embryos to form the linear heart tube at around E8.5, which undergoes fusions to form the primary linear heart tube (Olson and Schneider 2003; Webb et al. 1998). Then the tube loops to form primary chamber at E9.5, the chamber separation occurs on E10.5 – E15.5 to form a four-chamber heart, and the heart has complete four chamber separated by interatria septum, interventricular septum and the atrioventricular valves during later stages of development (from E16.5 to birth) (Olson and Schneider 2003; Webb et al. 1998). As shown in Fig. 4, in particular, MDA-9/syntenin was robustly expressed at stages from E12.5 to E16.5 with a peak expression at E16.5 in both compact and trabeculated myocardia of all atrial and ventricular regions of the developing heart. However, expression of MDA-9/syntenin was apparently diminished thereafter and a reduced signal was evident in most regions at E18.5 (Fig. 4). Wnt, activin/Nodal, BMPs, FGFs, and Notch signaling pathways have been investigated extensively for their roles during cardiogenesis (Foley and Mercola 2004). Especially, Wnt and Notch pathways play pivotal roles in development of multiple tissues including the heart through regulation of cell proliferation, differentiation, migration, and gene expression (Logan and Nusse 2004; Lin et al. 2007; High and Epstein 2008). MDA-9/syntenin is also involved in regulation of Wnt, Notch and FGF pathways (Baietti et al. 2012; Zimmermann et al. 2005; Estrach et al. 2007; Luyten et al. 2008). These findings suggest that MDA-9/syntenin may be a key player for cardiogenesis by modulating cross talk between and activities of Wnt, Notch and FGF signaling pathways.
Fig. 4.
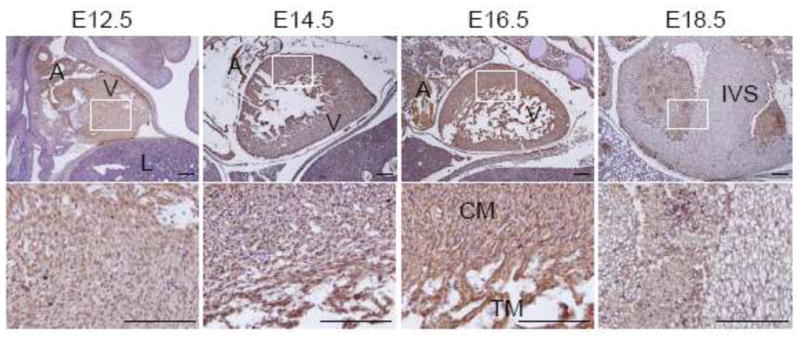
Expression of MDA-9/syntenin in the developing heart. Immunohistochemistry was performed at each section at E12.5 to 18.5 of the developing heart. Expression of MDA-9/syntenin at each stage is shown. Every enlarged image in the lower panel corresponds to the boxed area in the upper panel. Scale bars indicate 100 μm. A: atrium, V: ventricle, L: lung, CM: compact myocardium, TM: trabeculated myocardium and IVS: interventricular septum.
Expression of MDA-9/syntenin in the developing lung
Lung development of mice has been histologically divided into four chronological stages, pseudoglandular stage (E9.5 – E16.6), canalicular stage (E16.6 – E17.4), terminal sac stage (E17.4 – postnatal day (P) 5), and alveolar stage (P5 – P30) (Warburton et al. 2000). We examined the expression of MDA-9/syntenin in the lung between E14.5 – E18.5 (Fig. 5). Interestingly, a strong signal was detected in the lung mesenchymal and endothelial cells at all the developmental stages we examined, but not in the lung epithelial cells (Fig. 5). Mesenchymal-epithelial inductive signaling to induce epithelial proliferation, chemotaxis and organ-specific gene expression is crucial in determining respiratory organogenesis. Soluble factors secreted by lung mesenchyme comprise a complete inducer of lung morphogenesis, and signaling by IGF, EGF and TGF-β/BMP, extracellular matrix components, and integrin pathways also controls lung morphogenesis and cell lineage differentiation (Warburton et al. 2000). MDA-9/syntenin as a PDZ domain-containing adaptor protein is involved in regulating cell-cell and cell-matrix interaction, signal transduction from the cell surface to the interior, intracellular trafficking and cell surface targeting (Das et al. 2012). In summary, these results suggest that MDA-9/syntenin may play a key role as an adaptor protein in lung development through modulating complex signaling pathways.
Fig. 5.
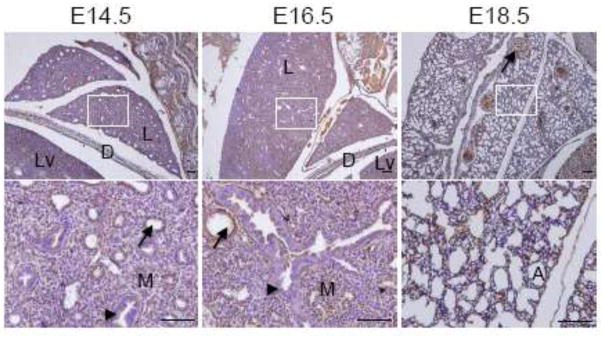
Expression of MDA-9/syntenin in the embryonic lung. The developing lung at E14.5 to E18.5 was dissected, and expression of MDA-9/syntenin was examined. MDA-9/syntenin is also partially localized in the lung. Every enlarged image in the lower panel corresponds to the boxed area in the upper panel. Scale bars indicate 100 μm. L: lung, Lv: liver, D: diaphragm, M: mesenchymal cells, A: alveoli, arrows: blood vessels and arrowheads: epithelial cells.
Expression of MDA-9/syntenin in the developing liver
We also examined the developing liver between E12.5 and E18.5. As shown in Fig. 6, the liver has a well-differentiated and denser architecture compared to other organs at E12.5. In addition, a fissure divides the liver into an upper and a lower lobe. Hepatic and hematopoietic cells are easily distinguishable in the fetal liver, and hepatic cells increase in relative abundance between E12.5 and E18.5 (Fig. 6). Unlike other organs, except the heart, MDA-9/syntenin was evenly detected in the hepatic cells of the liver at the developmental stages we examined, and there was relatively weak expression in the liver at E12.5 compared to later stages of mouse development, while its expression was greatly increased by E18.5 (Fig. 6). These results suggest that MDA-9/syntenin may normally be expressed in actively proliferating cells and tissues like liver with a strong regenerative ability. In addition, MDA-9/syntenin was robustly expressed in endothelial lining cells of the hepatic veins as shown in the lung blood vessels (Fig. 6). A recent study showed that MDA-9/syntenin and syndecan-1 play a role in the angiogenic process (Yuan et al. 2004). These findings indicate that MDA-9/syntenin may be important in the vascular development of mouse embryos.
Fig. 6.
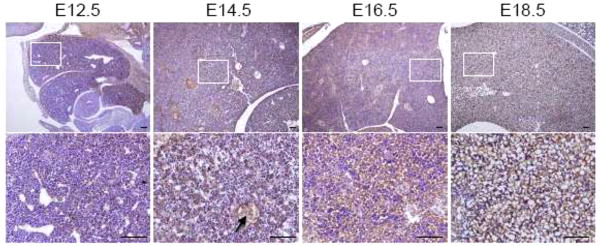
Expression of MDA-9/syntenin in the developing liver. Immunohistochemistry was performed at each section at E12.5 to 18.5 of the developing liver. Expression of MDA-9/syntenin at each stage is shown. Every enlarged image in the lower panel corresponds to the boxed area in the upper panel. Scale bars indicate 100 μm. Arrows: hepatic vein.
In the present study, we examined the expression pattern of MDA-9/syntenin during mouse development from embryonic stage E8.5 through E18.5. MDA-9/syntenin expression was significantly detected and abundant in the developing skin, spinal cord, heart, lung and liver. The expression pattern indicates that MDA-9/syntenin may contribute to cell proliferation and differentiation during the early developmental period, and that its temporal and spatial expression is strictly regulated during fetal development. MDA-9/syntenin is recognized as an important scaffolding-PDZ domain-containing adaptor protein which mediates cell-cell and cell-matrix interaction as well as multiple signaling pathways involved in cell shape, migration and invasion of cells, B-cell development, intracellular trafficking, synaptic transmission, axonal outgrowth, and cancer metastasis (Das et al. 2012; Sarkar et al. 2008). Taken together, these results suggest that MDA-9/syntenin may contribute to normal mouse development through modulating multiple signaling pathways involved in cell proliferation and differentiation as a crucial adaptor protein, and further detailed studies of MDA-9/syntenin function during development as well as in normal and pathologic physiology are warranted.
Acknowledgments
The present study was supported in part by National Institutes of Health, National Cancer Institute Grant R05 CA097318 and the Thelma Newmeyer Corman Endowment to P.B.F., National Institutes of Health, National Cancer Institute Grant R01 CA138540 to D.S., Elsa U Pardee Foundation (548424) to S.D., and by a National Research Foundation of Korea grant (No. 2011-0006220) to S-G.L. P.B.F. holds the Thelma Newmeyer Corman Endowed Chair in Cancer Research at the VCU Massey Cancer Center. D.S. is a Harrison Scholar in the VCU Massey Cancer Center.
References
- Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E, Zimmermann P, David G. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14(7):677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- Beekman JM, Coffer PJ. The ins and outs of syntenin, a multifunctional intracellular adaptor protein. J Cell Sci. 2008;121(Pt 9):1349–1355. doi: 10.1242/jcs.026401. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Pasolli HA, Fuchs E. Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev. 2006;20(21):3022–3035. doi: 10.1101/gad.1477606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukerche H, Aissaoui H, Prevost C, Hirbec H, Das SK, Su ZZ, Sarkar D, Fisher PB. Src kinase activation is mandatory for MDA-9/syntenin-mediated activation of nuclear factor-kappaB. Oncogene. 2010;29(21):3054–3066. doi: 10.1038/onc.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukerche H, Su ZZ, Emdad L, Sarkar D, Fisher PB. mda-9/Syntenin regulates the metastatic phenotype in human melanoma cells by activating nuclear factor-kappaB. Cancer Res. 2007;67(4):1812–1822. doi: 10.1158/0008-5472.CAN-06-3875. [DOI] [PubMed] [Google Scholar]
- Boukerche H, Su ZZ, Prevot C, Sarkar D, Fisher PB. mda-9/Syntenin promotes metastasis in human melanoma cells by activating c-Src. Proc Natl Acad Sci U S A. 2008;105(41):15914–15919. doi: 10.1073/pnas.0808171105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KS, Cohn MJ, Harfe BD. Identification of nucleus pulposus precursor cells and notochordal remnants in the mouse: implications for disk degeneration and chordoma formation. Dev Dyn. 2008;237(12):3953–3958. doi: 10.1002/dvdy.21805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK, Bhutia SK, Kegelman TP, Peachy L, Oyesanya RA, Dasgupta S, Sokhi UK, Azab B, Dash R, Quinn BA, Kim K, Barral PM, Su ZZ, Boukerche H, Sarkar D, Fisher PB. MDA-9/syntenin: a positive gatekeeper of melanoma metastasis. Front Biosci. 2012;17:1–15. doi: 10.2741/3911. [DOI] [PubMed] [Google Scholar]
- Enz R, Croci C. Different binding motifs in metabotropic glutamate receptor type 7b for filamin A, protein phosphatase 1C, protein interacting with protein kinase C (PICK) 1 and syntenin allow the formation of multimeric protein complexes. Biochem J. 2003;372(Pt 1):183–191. doi: 10.1042/BJ20021750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrach S, Legg J, Watt FM. Syntenin mediates Delta1-induced cohesiveness of epidermal stem cells in culture. J Cell Sci. 2007;120(Pt 16):2944–2952. doi: 10.1242/jcs.016253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Larrea J, Merlos-Suarez A, Urena JM, Baselga J, Arribas J. A role for a PDZ protein in the early secretory pathway for the targeting of proTGF-alpha to the cell surface. Mol Cell. 1999;3 (4):423–433. doi: 10.1016/s1097-2765(00)80470-0. [DOI] [PubMed] [Google Scholar]
- Foley A, Mercola M. Heart induction: embryology to cardiomyocyte regeneration. Trends Cardiovasc Med. 2004;14(3):121–125. doi: 10.1016/j.tcm.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Raghavan S. Getting under the skin of epidermal morphogenesis. Nat Rev Genet. 2002;3(3):199–209. doi: 10.1038/nrg758. [DOI] [PubMed] [Google Scholar]
- Gangemi R, Mirisola V, Barisione G, Fabbi M, Brizzolara A, Lanza F, Mosci C, Salvi S, Gualco M, Truini M, Angelini G, Boccardo S, Cilli M, Airoldi I, Queirolo P, Jager MJ, Daga A, Pfeffer U, Ferrini S. Mda-9/syntenin is expressed in uveal melanoma and correlates with metastatic progression. PLoS One. 2012;7(1):e29989. doi: 10.1371/journal.pone.0029989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijsen N, Uings IJ, Pals C, Armstrong J, McKinnon M, Raaijmakers JA, Lammers JW, Koenderman L, Coffer PJ. Cytokine-specific transcriptional regulation through an IL-5Ralpha interacting protein. Science. 2001;293(5532):1136–1138. doi: 10.1126/science.1059157. [DOI] [PubMed] [Google Scholar]
- Grootjans JJ, Zimmermann P, Reekmans G, Smets A, Degeest G, Durr J, David G. Syntenin, a PDZ protein that binds syndecan cytoplasmic domains. Proc Natl Acad Sci U S A. 1997;94 (25):13683–13688. doi: 10.1073/pnas.94.25.13683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmke BM, Polychronidis M, Benner A, Thome M, Arribas J, Deichmann M. Melanoma metastasis is associated with enhanced expression of the syntenin gene. Oncol Rep. 2004;12 (2):221–228. [PubMed] [Google Scholar]
- High FA, Epstein JA. The multifaceted role of Notch in cardiac development and disease. Nat Rev Genet. 2008;9(1):49–61. doi: 10.1038/nrg2279. [DOI] [PubMed] [Google Scholar]
- Hirbec H, Perestenko O, Nishimune A, Meyer G, Nakanishi S, Henley JM, Dev KK. The PDZ proteins PICK1, GRIP, and syntenin bind multiple glutamate receptor subtypes. Analysis of PDZ binding motifs. J Biol Chem. 2002;277(18):15221–15224. doi: 10.1074/jbc.C200112200. [DOI] [PubMed] [Google Scholar]
- Iuliano R, Trapasso F, Sama I, Le Pera I, Martelli ML, Lembo F, Santoro M, Viglietto G, Chiariotti L, Fusco A. Rat protein tyrosine phosphatase eta physically interacts with the PDZ domains of syntenin. FEBS Lett. 2001;500 (1–2):41–44. doi: 10.1016/s0014-5793(01)02580-7. [DOI] [PubMed] [Google Scholar]
- Jahoda CA. Induction of follicle formation and hair growth by vibrissa dermal papillae implanted into rat ear wounds: vibrissa-type fibres are specified. Development. 1992;115 (4):1103–1109. doi: 10.1242/dev.115.4.1103. [DOI] [PubMed] [Google Scholar]
- Jannatipour M, Dion P, Khan S, Jindal H, Fan X, Laganiere J, Chishti AH, Rouleau GA. Schwannomin isoform-1 interacts with syntenin via PDZ domains. J Biol Chem. 2001;276(35):33093–33100. doi: 10.1074/jbc.M105792200. [DOI] [PubMed] [Google Scholar]
- Koo TH, Lee JJ, Kim EM, Kim KW, Kim HD, Lee JH. Syntenin is overexpressed and promotes cell migration in metastatic human breast and gastric cancer cell lines. Oncogene. 2002;21(26):4080–4088. doi: 10.1038/sj.onc.1205514. [DOI] [PubMed] [Google Scholar]
- Lin JJ, Jiang H, Fisher PB. Characterization of a novel melanoma differentiation-associated gene, mda-9, that is down-regulated during terminal cell differentiation. Mol Cell Different. 1996;4:17. [Google Scholar]
- Lin JJ, Jiang H, Fisher PB. Melanoma differentiation associated gene-9, mda-9, is a human gamma interferon responsive gene. Gene. 1998;207 (2):105–110. doi: 10.1016/s0378-1119(97)00562-3. [DOI] [PubMed] [Google Scholar]
- Lin L, Cui L, Zhou W, Dufort D, Zhang X, Cai CL, Bu L, Yang L, Martin J, Kemler R, Rosenfeld MG, Chen J, Evans SM. Beta-catenin directly regulates Islet1 expression in cardiovascular progenitors and is required for multiple aspects of cardiogenesis. Proc Natl Acad Sci USA. 2007;104(22):9313–9318. doi: 10.1073/pnas.0700923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Luyten A, Mortier E, Van Campenhout C, Taelman V, Degeest G, Wuytens G, Lambaerts K, David G, Bellefroid EJ, Zimmermann P. The postsynaptic density 95/disc-large/zona occludens protein syntenin directly interacts with frizzled 7 and supports noncanonical Wnt signaling. Mol Biol Cell. 2008;19(4):1594–1604. doi: 10.1091/mbc.E07-08-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen BC, Lefort K, Mandinova A, Antonini D, Devgan V, Della Gatta G, Koster MI, Zhang Z, Wang J, Tommasi di Vignano A, Kitajewski J, Chiorino G, Roop DR, Missero C, Dotto GP. Cross-regulation between Notch and p63 in keratinocyte commitment to differentiation. Genes Dev. 2006;20(8):1028–1042. doi: 10.1101/gad.1406006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EN, Schneider MD. Sizing up the heart: development redux in disease. Genes Dev. 2003;17(16):1937–1956. doi: 10.1101/gad.1110103. [DOI] [PubMed] [Google Scholar]
- Owens P, Han G, Li AG, Wang XJ. The role of Smads in skin development. J Invest Dermatol. 2008;128(4):783–790. doi: 10.1038/sj.jid.5700969. [DOI] [PubMed] [Google Scholar]
- Risek B, Klier FG, Gilula NB. Multiple gap junction genes are utilized during rat skin and hair development. Development. 1992;116 (3):639–651. doi: 10.1242/dev.116.3.639. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Boukerche H, Su ZZ, Fisher PB. mda-9/Syntenin: more than just a simple adapter protein when it comes to cancer metastasis. Cancer Res. 2008;68(9):3087–3093. doi: 10.1158/0008-5472.CAN-07-6210. [DOI] [PubMed] [Google Scholar]
- Setton LA, Chen J. Mechanobiology of the intervertebral disc and relevance to disc degeneration. J Bone Joint Surg Am. 2006;88(Suppl 2):52–57. doi: 10.2106/JBJS.F.00001. [DOI] [PubMed] [Google Scholar]
- Tomoda T, Kim JH, Zhan C, Hatten ME. Role of Unc51.1 and its binding partners in CNS axon outgrowth. Genes Dev. 2004;18(5):541–558. doi: 10.1101/gad.1151204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton D, Schwarz M, Tefft D, Flores-Delgado G, Anderson KD, Cardoso WV. The molecular basis of lung morphogenesis. Mech Dev. 2000;92 (1):55–81. doi: 10.1016/s0925-4773(99)00325-1. [DOI] [PubMed] [Google Scholar]
- Webb S, Brown NA, Anderson RH. Formation of the atrioventricular septal structures in the normal mouse. Circ Res. 1998;82 (6):645–656. doi: 10.1161/01.res.82.6.645. [DOI] [PubMed] [Google Scholar]
- Yuan K, Hong TM, Chen JJ, Tsai WH, Lin MT. Syndecan-1 up-regulated by ephrinB2/EphB4 plays dual roles in inflammatory angiogenesis. Blood. 2004;104(4):1025–1033. doi: 10.1182/blood-2003-09-3334. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Zhang Z, Degeest G, Mortier E, Leenaerts I, Coomans C, Schulz J, N’Kuli F, Courtoy PJ, David G. Syndecan recycling [corrected] is controlled by syntenin-PIP2 interaction and Arf6. Dev Cell. 2005;9(3):377–388. doi: 10.1016/j.devcel.2005.07.011. [DOI] [PubMed] [Google Scholar]