Abstract
Psychosocial stress exposure is linked to a disruption of emotional regulation that can manifest as anxiety and depression. Women are more likely to suffer from such psychopathologies than men, indicating that gender-based differences in gonadal steroids may be a key factor in the etiology of stress-induced adverse health outcomes. Estradiol (E2) positively influences mood and cognition in females, an effect likely related to E2’s ability to modulate the serotonin and dopamine neurotransmitter systems. Furthermore, genetic variation due to the polymorphism in the promoter region of the gene (SLC6A4) encoding the serotonin transporter (5HTTLPR) also can influence E2’s ability to modulate behavior and physiology. However, it remains uncertain whether exposure to social stress interacts with the 5HTTLPR to influence E2-induced changes in behavior and physiology. The present study used ovariectomized adult female rhesus monkeys to investigate acute and chronic effects of E2 on central monoamine metabolite concentrations using CSF sampling. We further assessed how E2-induced changes in monoamine metabolite levels are modified by the unpredictable stress of social subordination and the 5HTTLPR polymorphism. Levels of the serotonin metabolite 5-hydroxyindoleacetic acid (5HIAA) decreased significantly during chronic E2 treatment only in dominant females with the long promoter length of SLC6A4. Chronic administration of E2 decreased levels of the dopamine metabolite dihydrophenylacetic acid (DOPAC) in a manner independent of the social status, 5HTTLPR genotype, or their interactions. Overall levels of dopamine and serotonin metabolites were increased in subordinate females but this effect of social stress was not influenced by 5HTTLPR genotype. Together, these data emphasize how E2 can modulate central neurotransmitter systems and indicate that social subordination in female monkeys is a valid model for examining how chronic psychosocial stress alters sensitivity to E2. Future studies are necessary to elaborate how changes in central neurotransmitter metabolism due to E2 and prolonged exposure to stress affect behavior and physiology.
Keywords: Psychosocial stress, estradiol, serotonin reuptake polymorphism, monkeys
Introduction
In addition to its role in reproduction and activation of sexual behavior, estradiol (E2) has anxiolytic properties (1, 2), enhances spatial learning and memory (3), and improves the ability of females to respond appropriately to danger signals in their environment (4). Estradiol modulates various pathways in the central nervous system including the limbic hypothalamic-pituitary-adrenal (LHPA) axis (5–8) and has effects on mood and cognition that are at least partly due to E2’s modulation of the monoamine neurotransmitters (9, 10). Serotonin (5HT) is implicated in the control of a number of behavioral and physiological functions. Decreased serotonergic neurotransmission has been proposed to play a key role in the etiology of depression (11). Importantly, 5HT-synthesizing neurons in the raphe and 5HT receptors throughout cortical, hypothalamic and limbic regions are modulated by E2 action (12). Estradiol can induce changes in binding of the 5HT transporter (5HTT), a protein that terminates serotonin’s synaptic effects (13). Genetic variation due to the polymorphism in the promoter region of the gene (SLC6A4) encoding the serotonin transporter (5HTTLPR) alters serotonergic activity (14). The short promoter length (s-variant) of the 5HTTLPR shows reduced transcriptional activity compared to the long promoter length (l/l) (15) and is associated with increased anxiety, aggression, and impulsivity in humans (16).
In addition to 5HT, estradiol also affects dopamine (DA) neurotransmission. In rats, amphetamine (AMPH)-stimulated DA release is greater in estrus than diestrus (17) and is decreased after ovariectomy (18). Estradiol also induces an increase in striatal DA turnover (19) and down regulates striatal D2 class DA receptor (D2R) binding in rats (20, 21). Similar findings have been reported in nonhuman primates, as gonadally-intact female monkeys have higher DA neuronal densities in the substantia nigra compared to males or ovariectomized females (22). Menstrual cycle phase also influences striatal DA receptors in female cynomolgus monkeys, as D2R availability is significantly higher in the luteal phase compared to the follicular phase in the caudate nucleus and putamen when estradiol concentrations are low (23).
What is less clear is how stress may affect estradiol-induced changes in central monoamine concentrations. Serotonin is integral to the activation (24, 25) and termination of the stress response (26–28). However, during chronic stress, corticotropin-releasing hormone (CRH) projections from the CeA to the raphe attenuate 5HT inhibition of neuronal activity in limbic targets (29, 30). Elevated glucocorticoids may also further reduce 5HT activity as cortisol increases 5HT uptake through an increase in 5HTT synthesis (31). With respect to DA, chronic psychosocial stress exposure reduces DA function, characterized by reduced D2R availability that is associated with anhedonia and increased susceptibility to addiction (32–36). The social stress of subordination in female macaques produces reduced cerebrospinal fluid (CSF) concentrations of the DA metabolite, homovanillic acid (HVA), and a reduced response of serum prolactin to haloperidol as a surrogate measure of DA (37, 38). This hypodopaminergic tone in subordinates is also associated with reduced D2R binding densities in the striatum (39, 40).
The present study used ovariectomized adult female rhesus monkeys (Macaca mulatta) to investigate effects of E2 on monoamine metabolite concentrations and how this relationship is modified by social subordination. The study was designed to determine whether social stress modifies acute or chronic E2-induced changes in endogenous monoamine levels in CSF. We hypothesize that E2’s ability to modulate 5HT and DA levels as well as their metabolites will be altered by social subordination. Furthermore, because 5HT is a target of E2 in the regulation of physiology and behavior, genetic variability in 5HT action, as occurs with the 5HTTLPR, will modify the effects of subordination on E2’s ability to alter both central 5HT and DA systems.
Materials and Methods
Animals
Previously ovariectomized adult female rhesus monkeys (n = 48) living in indoor-outdoor enclosures, measuring 3.8 by 3.8 by 3.8 m, at the Yerkes National Primate Research Center (YNPRC) Field Station were subjects. Females were housed in groups of 4 and 5 females, each containing a single male. Animals were fed Purina monkey chow (diet 5038, PMI, St Louis, MO) ad libitum twice daily and had continuous access to water. In addition, seasonal fruits and vegetables were provided daily as a supplement. The Emory University Institutional Animal Care and Use Committee approved all procedures in accordance with the Animal Welfare Act and the U.S. Department of Health and Human Services “Guide for Care and Use of Laboratory Animals.”
The ten small groups were established as previously described based upon 5HTTLPR genotype (41). Briefly, multiparous females, ranging in age from 11 to 17 yr (mean ± SEM: 13.5 ± 0.38 yr), were removed from multi-male, multi-female breeding groups at the Yerkes NPRC Field Station. All females were genotyped for 5HTTLPR (42) as previously described (43). Females homozygous for the long allele were considered l/l while females homozygous or heterozygous for the short allele were considered as short variant (s-variant) (42). Unfamiliar, unrelated females of the same 5HTTLPR genotype status (l/l or s-variant) were added to a new group over a one-week period to form five groups comprised of only l/l females and five groups comprised of only s-variant females (41). Dominance ranks were quickly established with minimal contact aggression. Males were added after the female hierarchy had been established. In the months prior to new group formation, all females were ovariectomized as a part of NIH-funded studies to determine the effects of psychosocial stress, imposed by social subordination on a number of behavioral, metabolic and reproductive outcomes (5, 41, 44–48) that required brief replacement therapy with estradiol and/or progesterone. In total, 9 dominant l/l, 9 dominant s-variant, 15 subordinate l/l, and 15 subordinate s-variant females participated in the current study.
Social stability in macaque groups, regardless of size, is maintained by a dominance hierarchy (49). Lower-ranking individuals in a social group receive a greater frequency of aggression from higher-ranking group mates and emit higher levels of submissive behaviors towards these more dominant individuals. A direct consequence of low social status in female rhesus monkeys is reduced control over both social and physical environments (50) that result in disruption of LHPA function including diminished glucocorticoid negative feedback (38, 41, 51). Therefore, social subordination in female rhesus monkeys is a well-characterized model with which to study the negative effects of chronic psychosocial stress exposure on behavior and physiology (52), including reproductive dysfunction (44, 53), immune compromise (54–56), addictive behavior (57), and cardiovascular disease (58). In the current study, the outcome of dyadic agonistic interactions between females was used to establish group dominance ranks (49). Observational data were obtained from three 30-minute observations during the first week using an established ethogram (41) to assess agonistic behavior including amount of submission and aggression received. As previously described (52), females ranked one and two were classified as dominant (n=18) and females ranked 3–5 were considered as subordinate (n= 30). Social groups had been formed and social ranks stable due to a stable social dominance structure (49) for 120 months prior to the initiation of this study.
Prior to the start of the study, females had not received hormone replacement for at least four weeks. For the present study, females were studied under two hormone replacement conditions. In one condition, females received E2 replacement therapy by surgically implanting a Silastic capsule filled with E2 subcutaneously between the scapula while anesthetized with Telazol (45). Capsule length was customized based on a female’s body weight to achieve serum concentrations of 94 ± 9 pg/ml, comparable to the mid follicular phase (59). Capsules were implanted 8 to 9 days (8.7 ± 0.5 days) prior to the collection of the initial CSF sample and were removed at the time of the second collection, approximately 4 to 8 weeks later (mean of 51.1 ± 2.5 days). The second condition was a control, no E2 replacement phase. The initial CSF collection for the no hormone replacement phase occurred, on average, 52 days after the last estradiol treatment while the second CSF sample obtained some 30 days following the first sample. The order of control versus no E2 replacement was randomized among the females.
The intent of the study was to determine the acute and chronic effects of E2 replacement on monoamine concentrations in CSF and how this may be affected by the social stress of subordination. At the time of each CSF collection, a serum sample was obtained to quantify E2 concentrations. CSF (1mL) was obtained by passive collection following sterile puncture of the cisterna magna using a 22-gauge needle while the females were anesthetized with Telazol. To accomplish this, animals were removed from their group and placed in a holding cage to obtain the serum sample followed by induction of anesthesia. All subjects were habituated to being removed from their group for conscious venipuncture using procedures in place in this lab for over 35 years (60–62). Briefly, animals were trained to move from the housing unit into a transfer box upon a cue from the research staff. Once in the transfer box, an animal is placed in a specialized cage designed for venipuncture. The cage allows a monkey to voluntarily place her leg through one of two small openings in the front of the cage. The research staff holds the leg so that a blood sample can be obtained from the saphenous vein and an injection of anesthetic administered. The order in which females in a group entered the cage was unrelated to rank and only 4–5 animals were anesthetized at one time to ensure standardization of blood and CSF collection after transfer from housing unit. Blood collection took approximately 1 minute and CSF collection was completed less than 15 minutes after Telazol administration, once sterile preparation of the cervical region was achieved. Blood contaminated CSF samples were discarded and another sample was collected from that individual animal within the following week. All animals were administered meloxicam as an analgesic following the CSF collection. Collected CSF was stored at −80°C for up to two months prior to analysis. Assays were performed in the YNPRC Biomarkers Core Lab. Serum samples were assayed for E2 to verify Silastic capsule efficacy using a modification of a previously validated commercial assay (Siemens/DPC; Los Angeles CA) (63). Using 200 μl of serum, the assay kit has a sensitivity of 5 pg/ml and an intra- and inter-assay coefficient of variation (CV) of 5.2% and 11.1%, respectively. The assay of E2 in the current study had an intra- and inter-assay CV of 7.9% and 9.6%, respectively. Monoamine concentrations in CSF, including dopamine (DA), homovanillic acid (HVA), dihydrophenylacetic acid (DOPAC), serotonin (5HT), and 5-hydroxyindoleacetic acid (5HIAA), were analyzed by HPLC as previously described (37). Intra-and inter-assay CV for DOPAC were 3.84% and 4.35% respectively, for HVA were 3.84% and 4.35% respectively, and for 5HIAA CV were 3.84% and 4.35% respectively.
Data were summarized as mean ± standard error of the mean (SEM). Data were log transformed to normalize variance. Repeated measures analysis of variance (RM-ANOVA) was used to determine how social status and E2 affected CSF monoamine concentrations over time (control vs. 1 and 5 weeks on E2) on 5HIAA, HVA, and DOPAC levels. The control number used in the analyses was determined by averaging the two samples collected during the control condition of no E2 replacement after paired t-tests were conducted to ensure that the two control samples were not significantly different (5HIAA: p=0.30; HVA: p=0.14; DOPAC: p=0.26). RM-ANOVA analyses were not performed on DA and 5HT, as levels were undetectable in the CSF samples collected by the HPLC methods used. If interaction terms were significant (e.g., status by E2 by time) post hoc comparisons were performed. All statistical tests were performed using IBM-SPSS v19. Statistical values with a p ≤ 0.05 were considered significant.
Results
Social status categorization
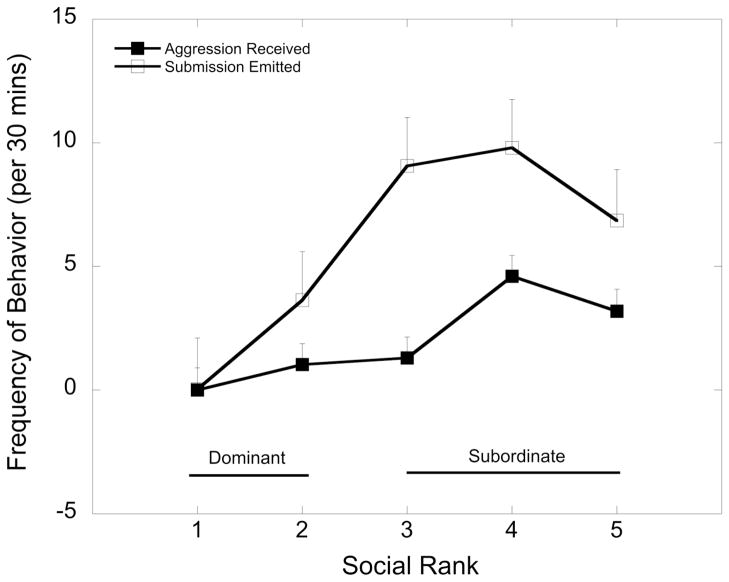
Rates of aggression received and submission emitted for monkeys at each social dominance rank position are shown in Figure 1. These data reflect agonistic behavior from three 30-minute observations during the control period of the current study. As expected, lower ranking subordinate females received significantly more aggression (F 4, 43 = 4.55, p=0.004) and emitted more submissive behaviors (F 4, 43 = 4.03, p=0.007) than higher ranking females. Categorization of females ranked 1 and 2 as dominant and those females ranked 3 through 5 as subordinate resulted in a significant main effect of social status for aggression received (F 1, 44 = 8.57, p=0.005) and submissive behaviors emitted (F 1, 44 = 13.0, p=0.001). There was no affect of 5HTTLPR genotype on rates of aggression received and submission emitted (p > 0.05).
Figure 1.
Mean ± SEM rates (per 30 min) of aggressive behavior received and submission behavior emitted by females at each social dominance rank. Rates of aggression received (p = 0.005) and submission emitted (p = 0.001) were higher in animals categorized as subordinate females (ranks 3 – 5) compared with those categorized as dominant (ranks 1 – 2).
Central levels of monoamines
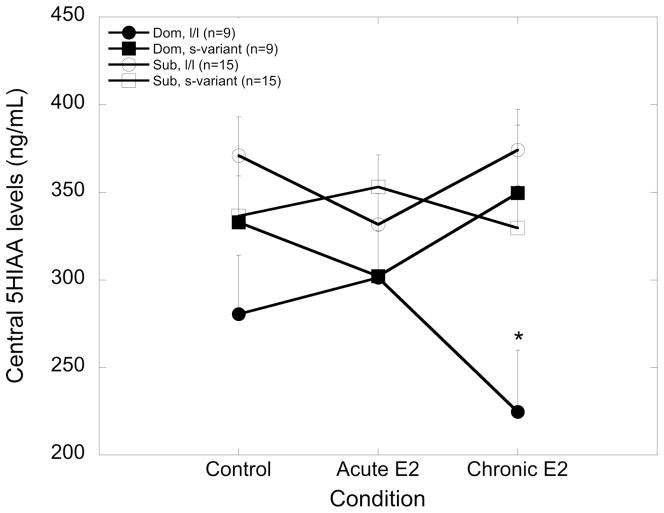
CSF levels of 5HIAA were significantly affected by social status (F1, 34 = 5.90, p = 0.021), as subordinate females had greater concentrations of 5HIAA than dominant females (349.4 ± 11.2 vs. 298.5 ± 17.7). In contrast, levels of 5HIAA were not affected by 5HTTLPR genotype or a social status by 5HTTLPR genotype interaction (p > 0.05). While there was no main effect of E2 treatment on 5HIAA concentrations (p > 0.05), there was a significant interaction between weeks on E2 replacement, social status and 5HTTLPR genotype (F1, 34 = 5.90, p = 0.021), as 5HIAA levels decreased significantly only in dominant l/l females with continued exposure to E2 treatment (Figure 2; p = 0.039).
Figure 2.
Mean ± SEM central concentrations of 5HIAA (ng/mL) at control, and following acute (7 days) and chronic (52 days) estradiol (E2) treatment for dominant l/l (closed circle), dominant s-variant (closed square), subordinate l/l (open circle) and subordinate s-variant (open square) females. The asterisk indicates that 5H1AA levels in dominant l/l females were significantly decreased after chronic E2 treatment compared to control levels (p < 0.05).
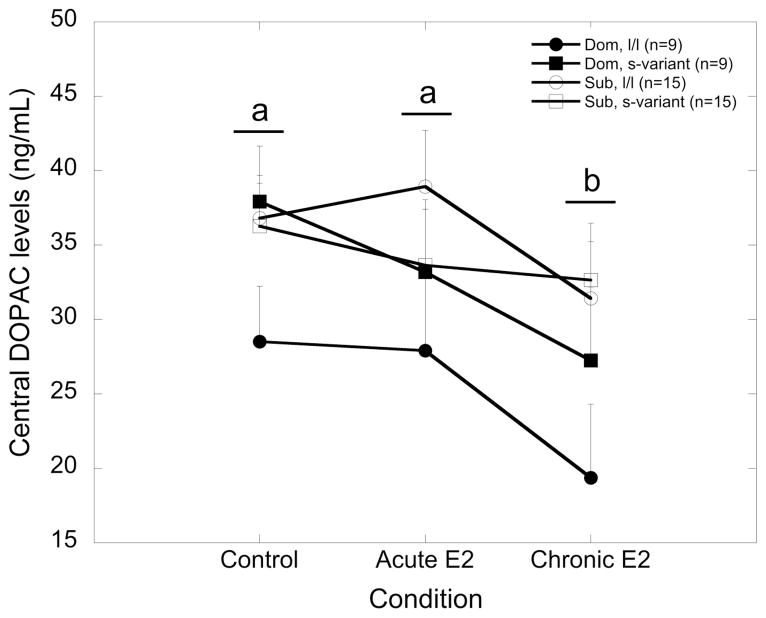
DOPAC concentrations were also significantly higher in subordinate animals compared to dominant females (34.9 ± 1.60 vs. 29.0 ± 2.06 respectively; F 1, 44 = 5.57, p = 0.023). Although this effect of status appeared to be modified by genotype, the interaction that resulted in lower DOPAC concentrations in dominant l/l females was not significant (F 1, 44 = 3.28, p = 0.077; Figure 3). Furthermore, E2 replacement significantly increased levels of DOPAC (F 2, 88 = 10.6, p < 0.001) from control by the end of E2 replacement (Figure 3). However, this main effect of E2 treatment was independent of social status, 5HTTLPR genotype, or their interaction (p > 0.05). Additionally, levels of HVA were not affected by social status, 5HTTLPR genotype or a social status by 5HTTLPR genotype interaction (p > 0.05) nor were these influenced by E2 replacement (Table 1; p > 0.05).
Figure 3.
Mean ± SEM central concentrations of DOPAC (ng/mL) at control, and following acute (7 days) and chronic (52 days) estradiol (E2) treatment for dominant l/l (closed circle), dominant s-variant (closed square), subordinate l/l (open circle) and subordinate s-variant (open square) females. The letters indicate differences in DOPAC levels across the study, as DOPAC decreased after chronic E2 treatment compared to control levels (b; p < 0.05) in all females.
Table 1.
Mean ± SEM central concentrations of HVA (ng/mL) at control, 1 wk and 5 wks on estradiol (E2) treatment for dominant l/l, dominant s-variant, subordinate l/l and subordinate s-variant females. There were no effects of social status, 5HTTLPR genotype, E2 treatment or their interactions on HVA levels (p > 0.05).
| Condition | Dom, l/l | Dom, s-variant | Sub, l/l | Sub, s-variant |
|---|---|---|---|---|
| Control | 1110.3 ± 179.1 | 1477.5 ± 196.2 | 1571.4 ± 117.3 | 1453.4 ± 121.7 |
| 1 wk of E2 | 1332.3 ± 165.8 | 1567.8 ± 181.6 | 1391.2 ± 108.5 | 1627.7 ± 112.6 |
| 5 wk of E2 | 1233.7 ± 210.7 | 1464.6 ± 230.8 | 1448.5 ± 137.9 | 1711.5 ± 143.1 |
Discussion
The results indicate that social subordination in ovariectomized female rhesus monkeys modifies the ability of E2 replacement to modulate central monoamine metabolite levels in a manner dependent on 5HTTLPR genotype. Chronic administration of the E2 attenuated levels of 5HIAA only in dominant l/l females and decreased DOPAC levels in all females. Although levels of DA and 5HT were undetectable, overall CSF concentrations of DOPAC and 5HIAA were significantly higher in subordinate females compared to dominant animals. Together, these data indicate that subordinate status, characterized by greater harassment from group mates and higher rates of submission to terminate these interactions, alters sensitivity to E2 and central levels of metabolites that are critical in the control of emotional behavior and physiology. These data underscore the importance of how ovarian status can influence behavioral and physiology by modulating central neurotransmitter systems.
Social subordination in macaques is associated with reduced control of the environment (50) and leads to a dysregulation of the LHPA axis (38, 41, 51, 64), as well as changes in behavior and physiology including reproductive dysfunction (44, 53), immune compromise (54–56), addictive behavior (57), and cardiovascular disease (58), analogous to stress-induced adverse health outcomes in women (52). Social subordination in the current study modified the effect of E2 on the 5HT and DA systems in a manner dependent on 5HTTLPR genotype. Five weeks of E2 replacement attenuated central levels of 5HIAA only in dominant l/l females and decreased levels of DOPAC in all females. Decreases in 5HIAA and DOPAC levels in response to E2 may reflect increased 5HT and DA synthesis (12) or may indicate decreased 5HT and DA breakdown due to decreased monoamine oxidase activity (MAO) (65). Data indicate that MAO gene expression increases upon ovariectomy and decreases with E2 replacement (65). Additionally, E2 increases central tryptophan hydroxylase (TPH) expression (66, 67). Together, these data support the notion that attenuation of monoamine metabolite levels described in the current study are likely due to increased synthesis and decreased degradation of 5HT and DA. However, further studies are necessary to clearly determine whether levels of 5HT and DA are increased by chronic E2 administration in relation to their metabolite levels in macaque females.
Estradiol only attenuated levels of 5HIAA in dominant l/l females. This effect of E2 on central 5HIAA levels is similar to that reported in cynomolgus macaques that express only the long variant of the 5HTTLPR (43), as 5HIAA levels in CSF are decreased during the follicular phase of the menstrual cycle compared to the luteal phase (37). The importance of the presence of E2 in uncovering the effects of the 5HTTLPR is underscored not only in the current study, but also in other studies where the hormonal status of the subjects is considered. We have previously shown that l/l 5HTTLPR females are more responsive to the serotonin reuptake inhibitor citalopram compared to s-variant females when E2 and progesterone levels are high (68). In contrast, a previous study on infant and juvenile rhesus macaques of both sexes that did not account for hormonal state found no difference in serotonergic responsivity to the 5HT-releasing agent fenfluramine between l/l and s-variant animals (69). Thus, the presence of gonadal hormones, like E2, is critical for evaluating the effects of 5HTTLPR on serotonergic function. Furthermore, the lack of an E2 effect on 5HIAA levels in s-variant females and the reduced transcriptional activity linked to the s-variant allele of the 5HTTLPR (70) indicate that the ability of E2 to attenuate 5HIAA levels in dominant l/l is probably modulated via E2’s ability to attenuate 5HTT protein (71). The lack of an E2 effect on 5HIAA in subordinate l/l females suggests that exposure to social subordination in these females alters the efficacy of E2 to regulate the serotonergic system. Consistent with this hypothesis are data showing that chronic stress attenuates 5HT inhibition of neuronal activity in limbic targets (29, 30) and alters expression of 5HT receptors critical for the regulation of behavior and physiology in monkeys (72) and in humans (73).
Chronic administration of E2 in the current study attenuated DOPAC levels in CSF independent of social status and 5HTTLPR genotype. These results corroborate previous findings in monkeys indicating that E2 influences the activity of the dopaminergic system (22, 23). Other studies in ewes show that during the nonbreeding season, E2 attenuates dopaminergic activity and results in attenuated levels of DOPAC and HVA centrally (74). Ovariectomy in rodents increases levels of DOPAC and HVA, and chronic replacement with E2 reverses these gonadectomy-related increases in the monoamine metabolite levels (75, 76). A similar manipulation in male rodents indicates that replacement of E2 following castration decreases central levels of DA metabolites (77). Lastly, the ability of E2 to attenuate DOPAC levels was not affected by social status or by 5HTTLPR genotype. While the serotonin transporter has been shown to affect the dopaminergic system as it relates to psychostimulant abuse and addiction (78) and the actions of E2 on behavior and physiology (5, 44, 68), there is no evidence that suggests that the 5HTTLPR polymorphism modulates E2’s ability to influence dopaminergic activity directly (78). The current study suggests that exposure to social subordination does not affect E2’s ability to modulate DA metabolism (37) unlike what we observed for 5HT breakdown (5HIAA).
The present study indicates that social subordination in female rhesus monkeys results in higher central levels of the neurotransmitter metabolites, 5HIAA and DOPAC. These data corroborate previous findings showing that exposure to prolonged stressors and activation of the LHPA axis increase 5HT and DA release site-specifically in the brain (34, 79, 80). We cannot completely rule out the possibility that differences in neurotransmitter systems might underlie social status ranks that emerge following the group formation process. An earlier study in rhesus macaques reported that monoamine metabolite levels remained stable before, during, and after group formation and that baseline levels of central 5HIAA were positively correlated with future high social ranking after group formation (81). However, ovarian function was not controlled for this study, making it difficult to compare with our current study where we show that ovarian hormones influence 5HIAA levels. In line with our current findings, increased serotonergic activity is also observed in subordinate cynomolgus monkeys (82) and increased 5HIAA levels are present in lower ranking talapoin monkeys (83). Indeed, one notable consequence of prolonged exposure to stress is a CRH-induced attenuation of 5HT inhibition of neuronal activity in limbic brain regions critical for the control of behavior and physiology (29, 30). It has also been shown that exposure to social subordination in monkeys (72) alters 5HT receptor expression throughout the brain, similar to that seen in people with depression (73). Furthermore, elevated levels of glucocorticoids characteristic of chronic stress exposure can act to reduce 5HT activity by increasing 5HT uptake via an increase in 5HTT synthesis (31).
The increase in central 5HIAA levels in subordinate females was concomitant with increased central levels of the DA metabolite, DOPAC. Exposure to stressors increases DA and DA metabolites levels in the brain (79) as well as alters the levels of DA receptors in prefrontal and striatal regions in rodents (32–36, 84–86). These alterations in the dopaminergic system can lead to a hypodopaminergic phenotype associated with anhedonia and increased susceptibility to addiction (32–36, 84–86). The increase in DA metabolite levels reported in this study is consistent with a stress-induced hypodopaminergic state in subordinate female monkeys that also exhibit a reduced response in serum prolactin to haloperidol as a surrogate measure of DA activity (38) and reduced D2R binding densities in the striatum (39, 40, 57). However, the current finding of increased central DOPAC levels in subordinate rhesus females is not consistent with a previous report of an attenuation of HVA levels in subordinate female cynomolgus macaques (37). The discrepancy between these two studies could be due to overall duration of exposure to social subordination, species differences, or to the fact that in the study with cynomolgus macaques, animals were being fed a high fat diet (37), which has been shown to affect the dopaminergic system in both rodents and humans (87, 88).
In conclusion, the current data indicate that exposure to social subordination in female rhesus monkeys causes higher central levels of serotonergic and dopaminergic metabolites, similar to alterations in neurotransmission implicated in the etiology of clinical depression (11). And while E2 replacement to these ovariectomized females was not able to ameliorate the effects of subordination on 5HT and DA neurotransmitter systems, the polymorphism in the 5HTT gene influenced E2’s ability to attenuate 5HIAA levels. This suggests that this genetic locus confers variability in sensitivity to E2 upon exposure to psychosocial stress in females (5, 44). It is important to note that these data should be considered preliminary, as larger numbers of subjects are necessary to establish a link between this gene variant and the stress-induced alterations in sensitivity to E2. This notwithstanding, our study supports the notion that assessing the interaction between ovarian hormone replacement and exposure to psychosocial stress in ovariectomized rhesus females serves as a effective paradigm with which to study questions relevant for hormone replacement therapy in postmenopausal women (5, 44, 68).
Acknowledgments
We thank the expert technical assistance of Jennifer Whitley, Shannon Bounar, Natalie Brutto, and Jodi Godfrey. The project was supported by NIH grants MH 081816 and, in part, RR00165. The Yerkes NPRC is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International.
References
- 1.Okada M, Hayashi N, Kometani M, Nakao K, Inukai T. Influences of ovariectomy and continuous replacement of 17beta-estradiol on the tail skin temperature and behavior in the forced swimming test in rats. Japanese journal of pharmacology. 1997;73(1):93–6. doi: 10.1254/jjp.73.93. [DOI] [PubMed] [Google Scholar]
- 2.Rocha BA, Fleischer R, Schaeffer JM, Rohrer SP, Hickey GJ. 17beta-Estradiol-induced antidepressant-like effect in the Forced Swim Test is absent in estrogen receptor-beta knockout (BERKO) mice. Psychopharmacology. 2005 doi: 10.1007/s00213-004-2078-1. [DOI] [PubMed] [Google Scholar]
- 3.Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Hormones and behavior. 1998;34(2):149–62. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- 4.Morgan MA, Schulkin J, Pfaff DW. Estrogens and non-reproductive behaviors related to activity and fear. Neuroscience and biobehavioral reviews. 2004;28(1):55–63. doi: 10.1016/j.neubiorev.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 5.Michopoulos V, Checchi M, Sharpe D, Wilson ME. Estradiol effects on behavior and serum oxytocin are modified by social status and polymorphisms in the serotonin transporter gene in female rhesus monkeys. Hormones and behavior. 2011;59(4):528–35. doi: 10.1016/j.yhbeh.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patchev VK, Almeida OF. Gonadal steroids exert facilitating and “buffering” effects on glucocorticoid-mediated transcriptional regulation of corticotropin-releasing hormone and corticosteroid receptor genes in rat brain. J Neurosci. 1996;16(21):7077–84. doi: 10.1523/JNEUROSCI.16-21-07077.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saltzman W, Hogan BK, Allen AJ, Horman BM, Abbott DH. Hypoestrogenism does not mediate social suppression of cortisol in subordinate female marmosets. Psychoneuroendocrinology. 2006;31(6):692–702. doi: 10.1016/j.psyneuen.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Wilson ME, Pazol K, Legendre A, Fisher J, Chikazawa K. Gonadal steroid modulation of the limbic - hypothalamic - pituitary - adrenal (LHPA) axis is influenced by social status in female rhesus monkeys. Endocrine. 2005;26(2) doi: 10.1385/ENDO:26:2:089. [DOI] [PubMed] [Google Scholar]
- 9.Pandaranandaka J, Poonyachoti S, Kalandakanond-Thongsong S. Anxiolytic property of estrogen related to the changes of the monoamine levels in various brain regions of ovariectomized rats. Physiology & behavior. 2006;87(4):828–35. doi: 10.1016/j.physbeh.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Renner K, Luine V. Analysis of temporal and dose-dependent effects of estrogen on monoamines in brain nuclei. Brain research. 1986;366(1–2):64–71. doi: 10.1016/0006-8993(86)91281-3. [DOI] [PubMed] [Google Scholar]
- 11.Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12(Suppl):12–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Frontiers in neuroendocrinology. 2002;23(1):41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- 13.Lu NZ, Eshleman AJ, Janowsky A, Bethea CL. Ovarian steroid regulation of serotonin reuptake transporter (SERT) binding, distribution, and function in female macaques. Molecular psychiatry. 2003;8(3):353–60. doi: 10.1038/sj.mp.4001243. [DOI] [PubMed] [Google Scholar]
- 14.Smith GS, Lotrich FE, Malhotra AK, Lee AT, Ma Y, Kramer E, Gregersen PK, Eidelberg D, Pollock BG. Effects of serotonin transporter promoter polymorphisms on serotonin function. Neuropsychopharmacology. 2004;29(12):2226–34. doi: 10.1038/sj.npp.1300552. [DOI] [PubMed] [Google Scholar]
- 15.Lesch KP, Meyer J, Glatz K, Flugge G, Hinney A, Hebebrand J, Klauck SM, Poustka A, Poustka F, Bengel D, Mossner R, Riederer P, Heils A. The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective: alternative biallelic variation in rhesus monkeys. Rapid communication. J Neural Transm. 1997;104(11–12):1259–66. doi: 10.1007/BF01294726. [DOI] [PubMed] [Google Scholar]
- 16.Murphy DL, Fox MA, Timpano KR, Moya PR, Ren-Patterson R, Andrews AM, Holmes A, Lesch KP, Wendland JR. How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. Neuropharmacology. 2008;55(6):932–60. doi: 10.1016/j.neuropharm.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becker JB, Cha JH. Estrous cycle-dependent variation in amphetamine-induced behaviors and striatal dopamine release assessed with microdialysis. Behavioural brain research. 1989;35(2):117–25. doi: 10.1016/s0166-4328(89)80112-3. [DOI] [PubMed] [Google Scholar]
- 18.Becker JB, Ramirez VD. Sex differences in the amphetamine stimulated release of catecholamines from rat striatal tissue in vitro. Brain research. 1981;204(2):361–72. doi: 10.1016/0006-8993(81)90595-3. [DOI] [PubMed] [Google Scholar]
- 19.Di Paolo T, Rouillard C, Bedard P. 17 beta-Estradiol at a physiological dose acutely increases dopamine turnover in rat brain. European journal of pharmacology. 1985;117(2):197–203. doi: 10.1016/0014-2999(85)90604-1. [DOI] [PubMed] [Google Scholar]
- 20.Bazzett TJ, Becker JB. Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain research. 1994;637(1–2):163–72. doi: 10.1016/0006-8993(94)91229-7. [DOI] [PubMed] [Google Scholar]
- 21.Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacology, biochemistry, and behavior. 1999;64(4):803–12. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- 22.Leranth C, Roth RH, Elsworth JD, Naftolin F, Horvath TL, Redmond DE., Jr Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: implications for Parkinson’s disease and memory. J Neurosci. 2000;20(23):8604–9. doi: 10.1523/JNEUROSCI.20-23-08604.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Czoty PW, Riddick NV, Gage HD, Sandridge M, Nader SH, Garg S, Bounds M, Garg PK, Nader MA. Effect of menstrual cycle phase on dopamine D2 receptor availability in female cynomolgus monkeys. Neuropsychopharmacology. 2009;34(3):548–54. doi: 10.1038/npp.2008.3. [DOI] [PubMed] [Google Scholar]
- 24.Feldman S, Weidenfeld J. The excitatory effects of the amygdala on hypothalamo-pituitary-adrenocortical responses are mediated by hypothalamic norepinephrine, serotonin, and CRF-41. Brain research bulletin. 1998;45(4):389–93. doi: 10.1016/s0361-9230(97)00384-5. [DOI] [PubMed] [Google Scholar]
- 25.Maier SF, Grahn RE, Watkins LR. 8-OH-DPAT microinjected in the region of the dorsal raphe nucleus blocks and reverses the enhancement of fear conditioning and interference with escape produced by exposure to inescapable shock. Behavioral neuroscience. 1995;109(3):404–12. doi: 10.1037//0735-7044.109.3.404. [DOI] [PubMed] [Google Scholar]
- 26.Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacology, biochemistry, and behavior. 1996;54(1):129–41. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell JB, Iny LJ, Meaney MJ. The role of serotonin in the development and environmental regulation of type II corticosteroid receptor binding in rat hippocampus. Brain research. 1990;55(2):231–5. doi: 10.1016/0165-3806(90)90204-c. [DOI] [PubMed] [Google Scholar]
- 28.Herman JP, Tasker JG, Ziegler DR, Cullinan WE. Local circuit regulation of paraventricular nucleus stress integration: glutamate-GABA connections. Pharmacology, biochemistry, and behavior. 2002;71(3):457–68. doi: 10.1016/s0091-3057(01)00681-5. [DOI] [PubMed] [Google Scholar]
- 29.Price ML, Lucki I. Regulation of serotonin release in the lateral septum and striatum by corticotropin-releasing factor. J Neurosci. 2001;21(8):2833–41. doi: 10.1523/JNEUROSCI.21-08-02833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas E, Pernar L, Lucki I, Valentino RJ. Corticotropin-releasing factor in the dorsal raphe nucleus regulates activity of lateral septal neurons. Brain research. 2003;960(1–2):201–8. doi: 10.1016/s0006-8993(02)03882-9. [DOI] [PubMed] [Google Scholar]
- 31.Tafet GE, Toister-Achituv M, Shinitzky M. Enhancement of serotonin uptake by cortisol: a possible link between stress and depression. Cogn Affect Behav Neurosci. 2001;1(1):96–104. doi: 10.3758/cabn.1.1.96. [DOI] [PubMed] [Google Scholar]
- 32.Izzo E, Sanna PP, Koob GF. Impairment of dopaminergic system function after chronic treatment with corticotropin-releasing factor. Pharmacology, biochemistry, and behavior. 2005;81 (4):701–8. doi: 10.1016/j.pbb.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 33.Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. The American journal of psychiatry. 2007;164(8):1149–59. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anisman H, Matheson K. Stress, depression, and anhedonia: caveats concerning animal models. Neuroscience and biobehavioral reviews. 2005;29(4–5):525–46. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Lucas LR, Celen Z, Tamashiro KL, Blanchard RJ, Blanchard DC, Markham C, Sakai RR, McEwen BS. Repeated exposure to social stress has long-term effects on indirect markers of dopaminergic activity in brain regions associated with motivated behavior. Neuroscience. 2004;124(2):449–57. doi: 10.1016/j.neuroscience.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 36.Sauvage M, Steckler T. Detection of corticotropin-releasing hormone receptor 1 immunoreactivity in cholinergic, dopaminergic and noradrenergic neurons of the murine basal forebrain and brainstem nuclei--potential implication for arousal and attention. Neuroscience. 2001;104(3):643–52. doi: 10.1016/s0306-4522(01)00137-3. [DOI] [PubMed] [Google Scholar]
- 37.Kaplan JR, Manuck SB, Fontenot MB, Mann JJ. Central nervous system monoamine correlates of social dominance in cynomolgus monkeys (Macaca fascicularis) Neuropsychopharmacology. 2002;26(4):431–43. doi: 10.1016/S0893-133X(01)00344-X. [DOI] [PubMed] [Google Scholar]
- 38.Shively CA. Social subordination stress, behavior, and central monoaminergic function in female cynomolgus monkeys. Biological psychiatry. 1998;44(9):882–91. doi: 10.1016/s0006-3223(97)00437-x. [DOI] [PubMed] [Google Scholar]
- 39.Grant KA, Shively CA, Nader MA, Ehrenkaufer RL, Line SW, Morton TE, Gage HD, Mach RH. Effect of social status on striatal dopamine D2 receptor binding characteristics in cynomolgus monkeys assessed with positron emission tomography. Synapse (New York, NY. 1998;29(1):80–3. doi: 10.1002/(SICI)1098-2396(199805)29:1<80::AID-SYN7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 40.Shively CA, Grant KA, Ehrenkaufer RL, Mach RH, Nader MA. Social stress, depression, and brain dopamine in female cynomolgus monkeys. Annals of the New York Academy of Sciences. 1997:807574–7. doi: 10.1111/j.1749-6632.1997.tb51972.x. [DOI] [PubMed] [Google Scholar]
- 41.Jarrell H, Hoffman JB, Kaplan JR, Berga S, Kinkead B, Wilson ME. Polymorphisms in the serotonin reuptake transporter gene modify the consequences of social status on metabolic health in female rhesus monkeys. Physiology & behavior. 2008;93(4–5):807–19. doi: 10.1016/j.physbeh.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoffman JB, Kaplan JR, Kinkead B, Berga SL, Wilson ME. Metabolic and reproductive consequences of the serotonin transporter promoter polymorphism (5-HTTLPR) in the adult female rhesus macaque (Macaca mulatta) Endocrine. 2007:31202–11. doi: 10.1007/s12020-007-0017-8. [DOI] [PubMed] [Google Scholar]
- 43.Bethea CL, Streicher JM, Mirkes SJ, Sanchez RL, Reddy AP, Cameron JL. Serotonin-related gene expression in female monkeys with individual sensitivity to stress. Neuroscience. 2005;132(1):151–66. doi: 10.1016/j.neuroscience.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 44.Michopoulos V, Berga SL, Kaplan JR, Wilson ME. Social subordination and polymorphisms in the gene encoding the serotonin transporter enhance estradiol inhibition of luteinizing hormone secretion in female rhesus monkeys. Biology of reproduction. 2009;81(6):1154–63. doi: 10.1095/biolreprod.109.079038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michopoulos V, Wilson ME. Body weight decreases induced by estradiol in female rhesus monkeys are dependent upon social status. Physiology & behavior. 2011;102(3–4):382–8. doi: 10.1016/j.physbeh.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Michopoulos V, Toufexis D, Wilson ME. Social stress interacts with diet history to promote emotional feeding in females. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collura LA, Hoffman JB, Wilson ME. Administration of human leptin differentially affects parameters of cortisol secretion in socially housed female rhesus monkeys. Endocrine. 2009 doi: 10.1007/s12020-009-9250-7. [DOI] [PubMed] [Google Scholar]
- 48.Reding K, Michopoulos V, Wallen K, Sanchez M, Wilson ME, Toufexis D. Social status modifies estradiol activation of sociosexual behavior in female rhesus monkeys. Hormones and behavior. 2012;62(5):612–20. doi: 10.1016/j.yhbeh.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bernstein S, Mason WA. Effects of age and stimulus conditions on the emotional responses of rhesus monkeys: differential responses to frustration and to fear stimuli. Developmental psychobiology. 1970;3(1):5–12. doi: 10.1002/dev.420030104. [DOI] [PubMed] [Google Scholar]
- 50.Sapolsky RM. The influence of social hierarchy on primate health. Science (New York, NY. 2005;308(5722):648–52. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- 51.Wilson ME, Fisher J, Fischer A, Lee V, Harris RB, Bartness TJ. Quantifying food intake in socially housed monkeys: social status effects on caloric consumption. Physiology & behavior. 2008;94(4):586–94. doi: 10.1016/j.physbeh.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michopoulos V, Higgins M, Toufexis D, Wilson ME. Social subordination produces distinct stress-related phenotypes in female rhesus monkeys. Psychoneuroendocrinology. 2012;37(7):1071–85. doi: 10.1016/j.psyneuen.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adams MR, Kaplan JR, Koritnik DR. Psychosocial influences on ovarian endocrine and ovulatory function in Macaca fascicularis. Physiology & behavior. 1985;35(6):935–40. doi: 10.1016/0031-9384(85)90262-8. [DOI] [PubMed] [Google Scholar]
- 54.Gust DA, Gordon TP, Wilson ME, Ahmed-Ansari A, Brodie AR, McClure HM. Formation of a new social group of unfamiliar female rhesus monkeys affects the immune and pituitary adrenocortical systems. Brain Behav Immun. 1991;5(3):296–307. doi: 10.1016/0889-1591(91)90024-5. [DOI] [PubMed] [Google Scholar]
- 55.Paiardini M, Hoffman J, Cervasi B, Ortiz AM, Stroud F, Silvestri G, Wilson ME. T-cell phenotypic and functional changes associated with social subordination and gene polymorphisms in the serotonin reuptake transporter in female rhesus monkeys. Brain Behav Immun. 2009;23(2):286–93. doi: 10.1016/j.bbi.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tung J, Barreiro LB, Johnson ZP, Hansen KD, Michopoulos V, Toufexis D, Michelini K, Wilson ME, Gilad Y. Social environment is associated with gene regulatory variation in the rhesus macaque immune system. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(17):6490–5. doi: 10.1073/pnas.1202734109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nature neuroscience. 2002;5(2):169–74. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- 58.Kaplan JR, Adams MR, Clarkson TB, Manuck SB, Shively CA, Williams JK. Psychosocial factors, sex differences, and atherosclerosis: lessons from animal models. Psychosomatic Medicine. 1996;58(6):598–611. doi: 10.1097/00006842-199611000-00008. [DOI] [PubMed] [Google Scholar]
- 59.Walker ML, Wilson ME, Gordon TP. Endocrine control of the seasonal occurrence of ovulation in rhesus monkeys housed outdoors. Endocrinology. 1984;114(4):1074–81. doi: 10.1210/endo-114-4-1074. [DOI] [PubMed] [Google Scholar]
- 60.Blank MS, Gordon TP, Wilson ME. Effects of capture and venipuncture on serum levels of prolactin, growth hormone and cortisol in outdoor compound-housed female rhesus monkeys (Macaca mulatta) Acta endocrinologica. 1983;102(2):190–5. doi: 10.1530/acta.0.1020190. [DOI] [PubMed] [Google Scholar]
- 61.Walker ML, Gordon TP, Wilson ME. Reproductive performance in capture-acclimated female rhesus monkeys (Macaca mulatta) J Med Primatol. 1982;11(5):291–302. [PubMed] [Google Scholar]
- 62.Bernstein IS, Gordon TP. Behavioral research in breeding colonies of Old World monkeys. Lab Anim Sci. 1977;27(4):532–40. [PubMed] [Google Scholar]
- 63.Pazol K, Kaplan JR, Abbott DH, Wilson ME. Practical Measurement of total and bioavailable estradiol in female macaques. Clinica Chimica Acta. 2004:340117–26. doi: 10.1016/j.cccn.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 64.Michopoulos V, Reding KM, Wilson ME, Toufexis D. Social subordination impairs hypothalamic-pituitary-adrenal function in female rhesus monkeys. Hormones and behavior. 2012 doi: 10.1016/j.yhbeh.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gundlah C, Lu NZ, Bethea CL. Ovarian steroid regulation of monoamine oxidase-A and -B mRNAs in the macaque dorsal raphe and hypothalamic nuclei. Psychopharmacology. 2002;160(3):271–82. doi: 10.1007/s00213-001-0959-0. [DOI] [PubMed] [Google Scholar]
- 66.Bethea CL, Mirkes SJ, Shively CA, Adams MR. Steroid regulation of tryptophan hydroxylase protein in the dorsal raphe of macaques. Biological psychiatry. 2000;47(6):562–76. doi: 10.1016/s0006-3223(99)00156-0. [DOI] [PubMed] [Google Scholar]
- 67.Pecins-Thompson M, Brown NA, Kohama SG, Bethea CL. Ovarian steroid regulation of tryptophan hydroxylase mRNA expression in rhesus macaques. J Neurosci. 1996;16(21):7021–9. doi: 10.1523/JNEUROSCI.16-21-07021.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Michopoulos V, Berga SL, Wilson ME. Estradiol and progesterone modify the effects of the serotonin reuptake transporter polymorphism on serotonergic responsivity to citalopram. Exp Clin Psychopharmacol. 2011;19(6):401–8. doi: 10.1037/a0025008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bethea CL, Streicher JM, Coleman K, Pau FK, Moessner R, Cameron JL. Anxious behavior and fenfluramine-induced prolactin secretion in young rhesus macaques with different alleles of the serotonin reuptake transporter polymorphism (5HTTLPR) Behav Genet. 2004;34(3):295–307. doi: 10.1023/B:BEGE.0000017873.61607.be. [DOI] [PubMed] [Google Scholar]
- 70.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science (New York, NY. 1996;274(5292):1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 71.Pecins-Thompson M, Brown NA, Bethea CL. Regulation of serotonin re-uptake transporter mRNA expression by ovarian steroids in rhesus macaques. Brain Res Mol Brain Res. 1998;53(1–2):120–9. doi: 10.1016/s0169-328x(97)00286-6. [DOI] [PubMed] [Google Scholar]
- 72.Shively CA, Friedman DP, Gage HD, Bounds MC, Brown-Proctor C, Blair JB, Henderson JA, Smith MA, Buchheimer N. Behavioral depression and positron emission tomography-determined serotonin 1A receptor binding potential in cynomolgus monkeys. Archives of general psychiatry. 2006;63(4):396–403. doi: 10.1001/archpsyc.63.4.396. [DOI] [PubMed] [Google Scholar]
- 73.Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, Huang Y, Gautier C, Mathis C. PET imaging of serotonin 1A receptor binding in depression. Biological psychiatry. 1999;46(10):1375–87. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- 74.Gayrard V, Malpaux B, Tillet Y, Thiery JC. Estradiol increases tyrosine hydroxylase activity of the A15 nucleus dopaminergic neurons during long days in the ewe. Biology of reproduction. 1994;50(5):1168–77. doi: 10.1095/biolreprod50.5.1168. [DOI] [PubMed] [Google Scholar]
- 75.Dupont A, Di Paolo T, Gagne B, Barden N. Effects of chronic estrogen treatment on dopamine concentrations and turnover in discrete brain nuclei of ovariectomized rats. Neuroscience letters. 1981;22(1):69–74. doi: 10.1016/0304-3940(81)90287-1. [DOI] [PubMed] [Google Scholar]
- 76.Bitar MS, Ota M, Linnoila M, Shapiro BH. Modification of gonadectomy-induced increases in brain monoamine metabolism by steroid hormones in male and female rats. Psychoneuroendocrinology. 1991;16(6):547–57. doi: 10.1016/0306-4530(91)90038-u. [DOI] [PubMed] [Google Scholar]
- 77.Alderson LM, Baum MJ. Differential effects of gonadal steroids on dopamine metabolism in mesolimbic and nigro-striatal pathways of male rat brain. Brain research. 1981;218(1–2):189–206. doi: 10.1016/0006-8993(81)91300-7. [DOI] [PubMed] [Google Scholar]
- 78.Filip M, Frankowska M, Zaniewska M, Golda A, Przegalinski E. The serotonergic system and its role in cocaine addiction. Pharmacol Rep. 2005;57(6):685–700. [PubMed] [Google Scholar]
- 79.Kalivas PW, Duffy P. Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain research. 1995;675(1–2):325–8. doi: 10.1016/0006-8993(95)00013-g. [DOI] [PubMed] [Google Scholar]
- 80.Doherty MD, Gratton A. Medial prefrontal cortical D1 receptor modulation of the meso-accumbens dopamine response to stress: an electrochemical study in freely-behaving rats. Brain research. 1996;715(1–2):86–97. doi: 10.1016/0006-8993(95)01557-4. [DOI] [PubMed] [Google Scholar]
- 81.Higley JD, King ST, Jr, Hasert MF, Champoux M, Suomi SJ, Linnoila M. Stability of interindividual differences in serotonin function and its relationship to severe aggression and competent social behavior in rhesus macaque females. Neuropsychopharmacology. 1996;14(1):67–76. doi: 10.1016/S0893-133X(96)80060-1. [DOI] [PubMed] [Google Scholar]
- 82.Shively CA, Fontenot MB, Kaplan JR. Social-Status, Behavior, and Central Serotonergic Responsivity in Female Cynomolgus Monkeys. American journal of primatology. 1995;37(4):333–9. doi: 10.1002/ajp.1350370408. [DOI] [PubMed] [Google Scholar]
- 83.Yodyingyuad U, de la Riva C, Abbott DH, Herbert J, Keverne EB. Relationship between dominance hierarchy, cerebrospinal fluid levels of amine transmitter metabolites (5-hydroxyindole acetic acid and homovanillic acid) and plasma cortisol in monkeys. Neuroscience. 1985;16(4):851–8. doi: 10.1016/0306-4522(85)90099-5. [DOI] [PubMed] [Google Scholar]
- 84.Macey DJ, Koob GF, Markou A. CRF and urocortin decreased brain stimulation reward in the rat: reversal by a CRF receptor antagonist. Brain research. 2000;866(1–2):82–91. doi: 10.1016/s0006-8993(00)02229-0. [DOI] [PubMed] [Google Scholar]
- 85.Harfstrand A, Fuxe K, Cintra A, Agnati LF, Zini I, Wikstrom AC, Okret S, Yu ZY, Goldstein M, Steinbusch H, et al. Glucocorticoid receptor immunoreactivity in monoaminergic neurons of rat brain. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(24):9779–83. doi: 10.1073/pnas.83.24.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36(3):165–86. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- 87.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nature neuroscience. 2010;13(5):635–41. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Brain dopamine and obesity. Lancet. 2001;357(9253):354–7. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]