Abstract
Background
Ketamine is a commonly used anesthetic but the mechanistic basis for its clinically relevant actions remains to be determined. We previously showed that HCN1 channels are inhibited by ketamine and demonstrated that global HCN1 knockout mice are two-fold less sensitive to hypnotic actions of ketamine. Although that work identified HCN1 channels as a viable molecular target for ketamine, it did not determine the relevant neural substrate.
Methods
To localize the brain region responsible for HCN1-mediated hypnotic actions of ketamine, we employed a conditional knockout strategy to delete HCN1 channels selectively in excitatory cells of the mouse forebrain. A combination of molecular, immunohistochemical and cellular electrophysiological approaches was used to verify conditional HCN1 deletion; a loss-of-righting reflex assay served to ascertain effects of forebrain HCN1 channel ablation on hypnotic actions of ketamine.
Results
In conditional knockout mice, HCN1 channels were selectively deleted in cortex and hippocampus, with expression retained in cerebellum. In cortical pyramidal neurons from forebrain-selective HCN1 knockout mice, effects of ketamine on HCN1-dependent membrane properties were absent; notably, ketamine was unable to evoke membrane hyperpolarization or enhance synaptic inputs. Finally, the half maximal effective concentration (EC50) for ketamine-induced loss-of-righting reflex was shifted to significantly higher concentrations (by ~31%).
Conclusions
These data indicate that forebrain principal cells represent a relevant neural substrate for HCN1-mediated hypnotic actions of ketamine. We suggest that ketamine inhibition of HCN1 shifts cortical neuron electroresponsive properties to contribute to ketamine-induced hypnosis.
INTRODUCTION
Anesthetic compounds represent some of the most useful drugs in the clinical arsenal. Despite extensive clinical experience and intensive experimental scrutiny, the molecular and neural mechanisms by which most of these drugs mediate their important actions remain elusive 1,2. Prevailing ideas hold that contributions of individual molecular or neuronal targets depend on the particular anesthetic compound and the specific clinical endpoint examined 1–3; this implies that understanding actions of any anesthetic drug demands information regarding how modulation of a given target within a defined neural system can yield each clinically relevant outcome 2,3. In this work, we use conditional knockout mice to test if inhibition of HCN1 channels specifically in forebrain principal cells contributes to ketamine-induced hypnosis.
In our earlier work, we made the unexpected observation that ketamine inhibited recombinant HCN1 channels at clinically relevant concentrations via a decrease in maximal current amplitude and a hyperpolarizing shift in the V1/2 of channel activation4. These inhibitory effects of ketamine were: stereoselective, with greater potency of channel inhibition obtained with the S-(+)-ketamine isomer that is also the more potent anesthetic; and subunit selective, modulating HCN1-containing homomeric or HCN1-HCN2 heteromeric channels, but not HCN2 homomeric channels. We validated this action of ketamine on an HCN1-mediated native neuronal hyperpolarization-activated cationic current (Ih) by showing that ketamine-mediated inhibition of neuronal Ih was absent in HCN1−/− mice. We also demonstrated that mice with global deletion of HCN1 were ~2-fold less sensitive to ketamine-induced hypnosis, providing strong evidence that HCN1 channels represent a relevant target for this anesthetic endpoint 4.
A role for HCN1 channels in constraining oscillatory activity in forebrain pyramidal neurons is predicted based on their depolarizing influence on membrane potential and by virtue of their ability to shunt excitatory synaptic inputs 5–7. Indeed, we found that inhibition of HCN1 channels by ketamine led to – membrane hyperpolarization and improved dendritosomatic synaptic transfer in cortical pyramidal neurons 4. Thus, insofar as these two effects membrane hyperpolarization and increased synaptic efficacy – are associated with an enhanced propensity for coherent cortical rhythms 8,9 such as those accompanying ketamine-induced hypnosis 10, we proposed that deletion of this ketamine target in cortical neurons could contribute to the diminished hypnotic actions of ketamine we observed in HCN1−/− mice 4.
Since HCN1 subunits were deleted globally in the ketamine-resistant HCN1 knockout mice used for our previous work, we sought here to use conditional knockout strategies in order to better localize the neural substrate for HCN1-dependent actions of ketamine. To this end, we took advantage of a line of mice in which HCN1 channels were deleted only from forebrain principal cells, and we focused on the hypnotic end point since that was strongly affected in global knockouts 4. Insofar as we find that forebrain-selective HCN1 knockout mice largely recapitulated the decreased sensitivity to ketamine-induced hypnosis observed previously in global HCN1 knockout animals, these new results reinforce the idea that inhibition of forebrain HCN1 channels can contribute to hypnotic actions of anesthetics.
MATERIALS AND METHODS
Animals
We used a Cre-loxP strategy to generate forebrain-selective deletion of HCN1 channels, essentially as previously described 6,11. In this system, genetic engineering is performed in mice to incorporate two so-called loxP sites flanking a region of the targeted gene (i.e., to ‘flox’ the gene); when mice bearing the floxed gene are crossed to a separate group of mice that express the bacteriophage Cre-recombinase, the enzyme will excise the region between the loxP sites, inactivating the gene 12. For this work, we obtained mice with loxP sites flanking the 4th exon of the HCN1 gene, the region that encodes the channel pore domain and S6 transmembrane segment, from Dr. Eric Kandel (Columbia University, New York, NY) 6,11. We also obtained the R1ag#5 line of transgenic mice from Dr. Scott Zeitlin (University of Virginia, Charlottesville, VA) 13,14. In these mice, Cre-recombinase is expressed selectively in forebrain principal cells under the control of the CaMKIIα-promoter 13,14, providing the opportunity to restrict Cre-mediated gene inactivation only to those cells. The CaMK-Cre mice were crossed with HCN1f/+ mice and the progeny intercrossed to obtain breeding lines in which CaMKCre:HCN1f/f mice were mated with HCN1f/f mice, yielding littermates homozygous for floxed HCN1 alleles that were either Cre-positive (forebrain-selective knockouts) or Cre-negative (control). All procedures involving animals were approved by the University of Virginia Animal Care and Use Committee (Charlottesville, VA).
Immunohistochemistry
In order to confirm HCN1 channel deletion from HCN1−/− mice and from the forebrain of HCN1f/f CamK Cre+ mice, HCN1 immunohistochemistry was performed concurrently on tissue obtained from HCN1+/+, HCN1−/−, HCN1f/f and CaMKCre:HCN1f/f mice (n=3). Mice were anesthetized with ketamine (ketamine/xylazine: 200/14 mg/kg, intramuscular injection) and perfused transcardially with Phosphate buffered saline followed by 4% paraformaldehyde/0.1M phosphate buffer. Brains were removed and post-fixed in the same solution overnight. Coronal brain sections (30 μm) including the cerebellum, hippocampus and cortex were cut on a vibrating microtome (VT1000S, Leica Microsystems) and stored in cryoprotectant (50% Phosphate buffered saline/30% ethylene glycol/20% glycerol) until they were used for immunohistochemistry. Free floating sections were washed in 0.1 M Tris-buffered saline before incubating for 30 minutes in 1% H2O2 and again washing in buffered saline. After incubating sections in blocking reagent (Basic M.O.M Immunodetection Kit, Vector Laboratories, Burlingame, CA) for 1 h, tissue was incubated overnight at 4°C in mouse anti-HCN1 (1:1000; clone N70/28; UC Davis/NIH NeuroMab Facility, Davis, CA). Thereafter, sections were incubated for 1 h in M.O.M. biotinylated anti-mouse IgG reagent followed by 1 h with avidin-biotin-peroxidase complex (Vectastain ABC Elite Kit, Vector Laboratories), before visualization with 3–3′ diaminobenzidine (DAB peroxidase substrate kit, Vector Laboratories). The 3, 3′-diaminobenzidine reaction was quenched by washing in 0.1M phosphate buffer. Sections were then mounted onto gelatinized slides, dried, dehydrated in ethanol, cleared in xylene, and cover-slipped using DPX mounting media (VWR International, Radnor, PA). Images were obtained on a Zeiss FS microscope equipped with a PixelFly digital camera (Cooke Corp., Romulus, MI) using IPLab software (Scanalytics, Spectra Services Inc., Ontario, NY).
Quantitative RT-PCR
HCN1 expression levels were determined in Cre-negative floxed control mice (HCN1f/f) and CaMKCre-expressing HCN1f/f mice (CaMKCre:HCN1f/f) by using quantitative real-time polymerase chain reaction (qRT-PCR), as described previously 15. The cortex, hippocampus and cerebellum was microdissected for RNA isolation from adult mice (n ≥ 5 animals per genotype). Total RNA was extracted from all tissues using TRIzol reagent (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. An equal quantity of RNA from each sample was reverse transcribed using Superscript II (Invitrogen) enzyme. The resulting Complementary DNA was assayed to quantify the relative abundance of various messenger RNA species using the SYBR Green Real-Time polymerase chain reaction kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. The qRT-PCR was performed from each sample in quadruplicate, using an ICycler (Bio-Rad, Hercules, CA), a previously validated HCN1 primer set (upper: AGG TTA ATC AGA TAC ATA CACC, lower: GAG TGC GTA GGA ATA TTG TTTT, 231-bp amplicon) and PCR conditions (3 min 95°C; 40 cycles: 10 s, 95°C; 40 s, 60°C; 40 s, 72°C) that yielded ≥97% efficiency. We used cyclophilin as an internal standard (upper: GGC TCT TGA AAT GGA CCC TTC, lower: CAG CCA ATG CTT GAT CAT ATT CTT, 91-bp amplicon), and control samples with no added template were included with every experiment. Each animal contributed a single data point and qRT-PCR data were analyzed using a modification of the so-called ΔΔCt normalization procedure to obtain HCN1 messenger RNA levels for each genotype, relative to cyclophilin 16.
Electrophysiological recordings from mouse cortical pyramidal neurons
Mice of either sex (14–22 days old) were anesthetized (ketamine/xylazine: 200/14 mg/kg, intramuscular injection) and transverse brain slices prepared as described previously 4,15,17. Slices were submerged in a recording chamber on a Zeiss Axioskop FS microscope (Carl Zeiss Microscopy, LLC, Thornwood, NY) and visualized with Nomarski optics; pyramidal cells were targeted for recording based on location in the slice and characteristic size and shape. For voltage clamp recording, pipettes (2–4 MΩ) were filled with (mM): KCH3O3S, 120; NaCl, 4; MgCl2, 1; CaCl2, 0.5; HEPES, 10; EGTA, 10; MgATP, 3; GTP-Tris, 0.3 (pH 7.2); for current clamp, pipette solution contained (mM): KCl, 17.5; potassium gluconate, 122.5; HEPES, 10; EGTA, 0.2; NaCl, 9; MgCl2, 1; MgATP, 3; GTP-Tris, 0.3 (pH 7.2). Recordings were obtained with an Axopatch 200B amplifier at room temperature (~24°C) in standard bath solution containing (mM): NaCl, 140; KCl, 3; HEPES, 10; CaCl2, 2; MgCl2, 2; glucose, 10; we routinely added tetrodotoxin (0.5 μM; Alomone Labs, Jerusalem, Israel), BaCl2 (200 μM) and bicuculline/strychnine (both at 30 μM; Sigma, St. Louis) to the bath, except where noted. In some experiments, ZD-7288 was used to block Ih (50 μM; Tocris Cookson, Bristol, United Kingdom). Ketamine (Ketaset, Fort Dodge Animal Health, Fort Dodge, IA) was diluted into the bath solution at the indicated concentrations.
The properties of HCN channel currents and neuronal Ih were determined from whole cell voltage clamp experiments, essentially as described 18. Under current clamp, we measured input resistance and the magnitude of depolarizing ‘sag’ from voltage response to hyperpolarizing current injection. We evoked excitatory postsynaptic potentials (EPSPs) in cortical pyramidal neurons using a pipette that was placed in the superficial layers of the cortex and connected to a stimulator (Grass S48, Grass Technologies, West Warwick, RI) via a stimulus isolation unit (Grass SIU5, Grass Technologies); the pipette was filled with standard bath solution and synaptic responses were evoked by applying 40 Hz, 5–10 V pulses (corresponding to 2–10 μA). TheEPSP summation was quantified as the ratio of the amplitudes of the last and first evoked EPSP in the train (EPSP5:EPSP1) 4,15. All data are corrected (−8 mV) for a measured liquid junction potential.
Analysis of anesthetic action in mice
Bolus injections of ketamine (5 to 20 mg/kg) were administered via the tail vein of each mouse (2 to 4 months old). Each animal was injected with only a single concentration of the drug on any given day; some animals received multiple doses (on different days, always separated by at least 1 week), but no individual animal contributed more than one data point for any given dose. Hypnosis was established by loss-of-righting reflex (LORR) that occurred within 10 s after completion of the injection and which persisted for at least 10 s thereafter 19; we also measured the latency to regain the righting reflex19.
In order to assay ketamine effects on gross sensorimotor function we used rotarod and tail flick assays, essentially as described 20. For motor function, mice were placed on a rod rotating at constant acceleration (from 5 to 40 rpm) and the length of time the animal was able to stay on the rod was recorded (Med Associates Inc, Georgia, VT), under control conditions and then immediately after receiving a sub-anesthetic dose of ketamine (2.5 mg/kg, i.v.). To measure sensitivity to painful stimuli, mice were lightly restrained in a commercially available apparatus (Tail-Flick Unit 7360, Ugo Basile, Comerio, Italy) while a radiant heat source (intensity and cutoff time were set at 90 and 12 seconds) was directed onto the tail; the time required for the mice to remove their tails from the heat source was recorded, before and after treatment with ketamine (20 mg/kg, i.v., at 30 s intervals for 5 minutes)
Data acquisition and analysis
Data were analyzed statistically with SigmaPlot 11.0 (Systat Software Inc, San Jose, CA). Results are presented as mean ± SEM. Comparisons of mean values were performed using two-way analysis of variance (ANOVA), with post-hoc pairwise comparisons used Tukey’s correction of the t-test. For evaluation of neuronal electroresponsive properties, genotype and drug treatment (ketamine, ZD7288) represented principal factors, with a repeated measure design allowing comparisons for each cell under different conditions. For behavioral effects of ketamine analyzed by two-way ANOVA with genotype and dose as principal factors (Fig. 5), each observation was treated as an independent data point since not all individual animals were assessed at each dose. Quantal dose-response data of hypnotic effects of ketamine were fitted using logistic equations in order to obtain half maximal effective concentration (EC50) values for hypnosis in Cre-positive and Cre-negative HCN1f/f mice, and those values were analyzed statistically in Prism 5.0 by ANOVA. Differences in mean values were considered significant if P<0.05.
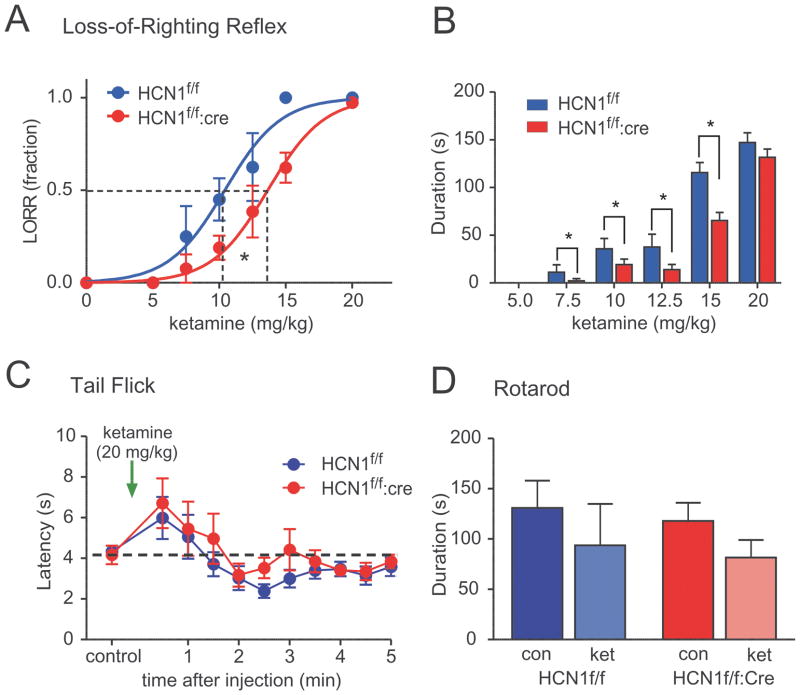
Figure 5. Deletion of HCN1 channels from forebrain reduced sensitivity of mice to hypnotic actions of ketamine.
A & B. Mice were injected with incrementing concentrations of ketamine (5–20 mg/kg, i.v.) and the fraction of HCN1f/f and CaMKCre:HCN1f/f mice that failed to right themselves (loss-of-righting reflex; LORR) was determined as a measure of hypnosis. CaMKCre:HCN1f/f mice were less sensitive to hypnotic effects of ketamine, as indicated by increased EC50 for ketamine-induced loss-of-righting reflex (A; F1,328=33.2, P<0.0001 by ANOVA) and reduced duration of the loss-of-righting reflex (B; genotype main effect, F1,211=10.4, P<0.002 by two-way ANOVA). C. The latency for mice to remove tail from a radiant heat source was essentially identical in HCN1f/f and CaMKCre:HCN1f/f mice under control conditions, and ketamine (20 mg/kg, i.v.) evoked a similar transient increase in latency in both sets of mice. D. The duration that HCN1f/f and CaMKCre:HCN1f/f mice were able to stay on an accelerating rotarod was not different before or immediately after treatment with a sub-anesthetic dose of ketamine (2.5 mg/kg, i.v.). n=31 &52, P<0.05, * HCN1f/f:cre vs. HCN1f/f.
RESULTS
In order to test the role of forebrain HCN1 expression in anesthetic actions of ketamine, we crossed HCN1f/f mice with a line of CaMKCre mice transgenic mice that express Cre-recombinase under the control of the CaMKIIα-promoter (see Methods for details of this conditional knockout strategy) 13,14. Of relevance here, CaMKIIα is expressed only in excitatory (i.e., principal) cells of the forebrain, with expression progressively increasing from barely detectable levels at birth to ~10-fold higher levels by postnatal day 21 (P21) and a further ~2.5-fold increase by P90 21. Thus, this construct drives Cre expression selectively to pyramidal neurons of the hippocampus and neocortex in the postnatal period 13,14, where it is expected to inactivate the HCN1 channel gene. It is worth noting that forebrain-selective HCN1 knockout mice were described previously to have enhanced spatial memory but otherwise normal sensorimotor function 6,11.
We first verified forebrain-specific deletion of HCN1 and loss of HCN1-dependent membrane properties in cortical pyramidal cells from CaMKCre:HCN1f/f mice, including ketamine modulation. After confirming HCN1 deletion and absence of ketamine actions on HCN1 channels in those cells, we tested the sensitivity of CaMKCre:HCN1f/f mice to ketamine-induced hypnosis and effects on sensorimotor function.
HCN1 expression is selectively deleted from forebrain of CaMKCre:HCN1f/f mice
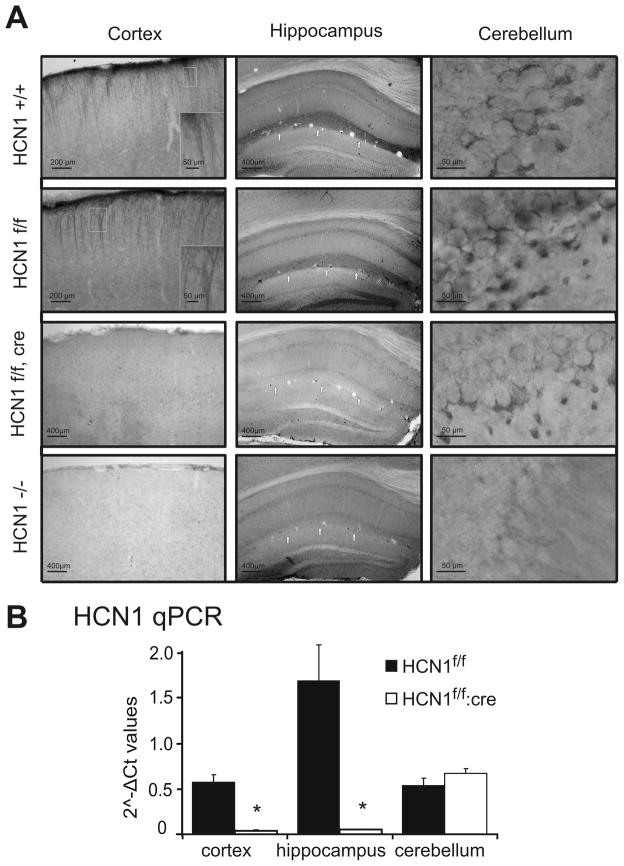
To demonstrate that the HCN1 channel gene was deleted selectively from forebrain neurons in CaMKCre:HCN1f/f mice, we performed immunohistochemistry and qRT-PCR to detect HCN1 expression. As shown in Fig. 1A, immunostaining for HCN1 was observed in the cortex, hippocampus and cerebellum from mice that were homozygous for either wild type (HCN1+/+) or floxed (HCN1f/f) alleles at the HCN1 locus; note the prominent immunostaining in apical dendrites from cortical pyramidal neurons and in the molecular layer of the hippocampal CA1 region (see arrowheads). In tissue from global HCN1 knockout mice (HCN1−/−), HCN1 expression was undetectable in all brain areas. However, consistent with forebrain selective knockout expected for CaMKCre:HCN1f/f mice 6, HCN1 staining was largely absent from cortex and hippocampus but retained in cerebellum. Corroborating these immunohistochemical results, our qRT-PCR analysis revealed that HCN1 messenger RNA was reduced by >90% in cortex and hippocampus of CaMKCre:HCN1f/f mice (decreased by 93.6% and 96.8%, respectively), with no difference in HCN1 transcript levels in cerebellum (Fig. 1B). Together, these data confirm forebrain-specific deletion of HCN1 in CaMKCre:HCN1f/f mice.
Figure 1. HCN1 is selectively deleted from forebrain neurons in CaMKCre:HCN1f/f mice.
A. HCN1 channel expression was assessed by immunohistochemistry in the cortex, hippocampus and cerebellum from wild type (HCN1+/+) or floxed (HCN1f/f) mice, from global HCN1 knockout mice (HCN1−/−) and from floxed mice expressing Cre recombinase under the CaMKIIα promoter (CaMKCre:HCN1f/f). The characteristic HCN1 expression in apical dendrites of the cortex and in stratum lacunosum-moleculare of CA1 in the hippocampus (see arrows) that is typical of wild type mice was also present in HCN1f/f mice, but was absent in both HCN1−/− and CaMKCre:HCN1f/f mice. The insets highlight dendritic staining, at 4x magnification). Note that HCN1 expression was preserved in the cerebellum of all mice except the global knockout. B. qRT-PCR shows that HCN1 transcript levels were reduced by over 90% in both the cortex and hippocampus from CaMKCre:HCN1f/f mice by comparison to HCN1f/f mice, but HCN1 expression in the cerebellum was not different. n=5, P<0.05 by two-way ANOVA, * HCN1f/f:cre vs. HCN1f/f. qRT-PCR: quantitative real-time polymerase chain reaction.
Ih is reduced and ketamine effects are diminished in cortical pyramidal neurons from CaMKCre:HCN1f/f mice
To verify deletion of HCN1 channel subunits functionally, we determined if HCN1-dependent membrane properties and HCN1-dependent ketamine actions were diminished in cortical pyramidal neurons of CaMKCre:HCN1f/f mice.
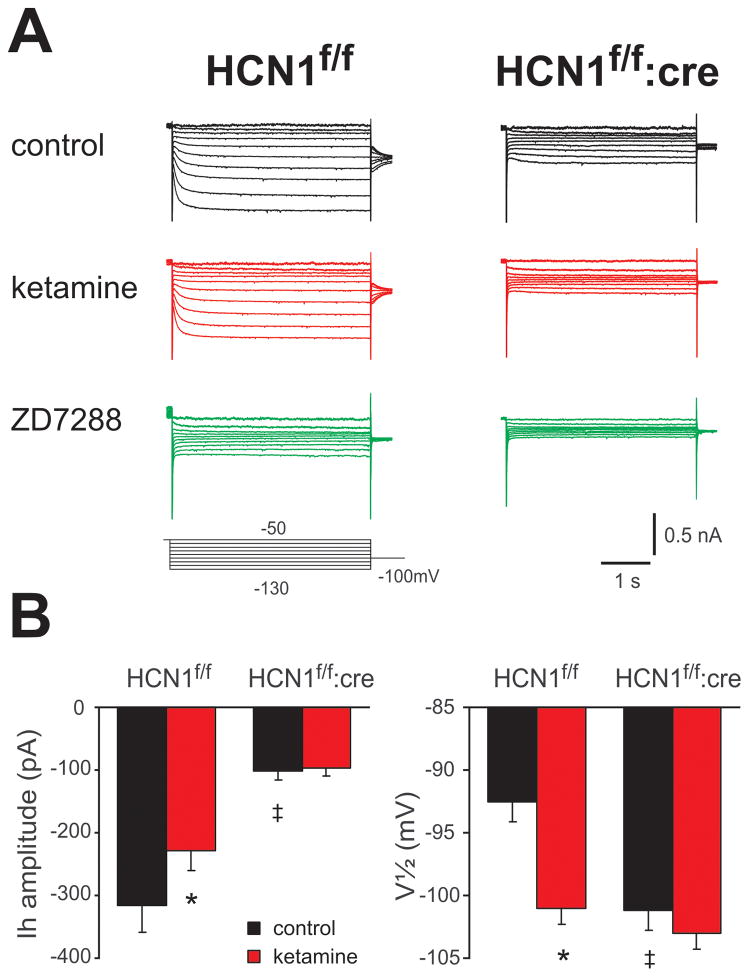
In cortical pyramidal neurons, Ih is primarily due to HCN1 and HCN2 channels, with HCN1 subunits conferring relatively faster activation kinetics and more depolarized voltage-dependence in homomeric HCN1 channels or heteromeric HCN1-HCN2 channels 17,22; homomeric HCN2 channels activate with slower kinetics and at more hyperpolarized potentials 17,22. Accordingly, as shown in the voltage clamp records of Fig. 2, Ih was smaller (Ih amplitude: 316.5 ± 42.6 pA for Cre− vs. 102.0 ± 13.4 pA for Cre+) and V1/2 of current activation was more negative (V 1/2: −92.6 ± 1.6 mV for Cre− vs. −101.2 ± 1.6 mV for Cre+) in pyramidal neurons from CaMKCre:HCN1f/f mice than in those same cells from control HCN1f/f mice (n=5 & 7 for Cre− & Cre+, P<0.05 for each variable). In addition, the residual Ih in pyramidal neurons from CaMKCre:HCN1f/f mice showed slower activation kinetics (τfast at −118 mV: 106.4 ± 24.0 ms for Cre− vs. 358.3 ± 50.0 ms for Cre+; data not shown). These results are consistent with deletion of HCN1 from pyramidal neurons of CaMKCre:HCN1f/f mice, leaving a residual Ih provided primarily by homomeric HCN2 channels.
Figure 2. Ih is diminished and effects of ketamine on Ih are reduced in cortical pyramidal neurons from CaMKCre:HCN1f/f mice.
A. Sample voltage clamp recordings of Ih in cortical pyramidal neurons from HCN1f/f and CaMKCre:HCN1f/f mice under control conditions (upper), during exposure to ketamine (20 μM, middle) and following treatment with the Ih blocker, ZD-7288 (50 μM, lower). B. Averaged data (± SE) depicting Ih amplitude (at −118 mV) and V of activation of Ih in cortical pyramidal neurons from HCN1f/f mice and CaMKCre:HCN1f/f mice. These data show that Ih was smaller with a more hyperpolarized V in cortical pyramidal neurons from CaMKCre:HCN1f/f mice; they also reveal that the ketamine-induced decrease in current amplitude and hyperpolarizing shift in V observed in cells from HCN1f/f mice were absent in CaMKCre:HCN1f/f mice. Note that ZD7288 completely blocked Ih in cells from both genotypes, so amplitude and V data after ZD7288 is not presented in B. n=5 & 7, P<0.05, * ketamine vs. control; ‡, HCN1f/f:cre vs. HCN1f/f.
We previously found that ketamine inhibits recombinant HCN channels that contain an HCN1 subunit in either HCN1 homomeric or HCN1-HCN2 heteromeric conformations; by contrast, ketamine had no appreciable effect on HCN2 homomeric channels 4. This subunit-selective action of ketamine was also apparent in its effects on native Ih in cortical pyramidal neurons, which express both HCN1 and HCN2: ketamine inhibited Ih in pyramidal neurons from wild type mice but not from global HCN1 knockout mice 4. Likewise, as shown in Fig. 2, we found that ketamine inhibited Ih in cortical pyramidal neurons from control HCN1f/f mice by evoking a hyperpolarizing shift in voltage dependence of activation (ΔV1/2: −8.4 ± 1.7 mV, from −92.6 ± 1.6 mV to −101.0 ± 1.3 mV) together with a decrease in maximal current amplitude (% inhibition: 27.6 ± 4.5 %, from 316.5 ± 42.6 pA to 228.0 ± 32.8 pA; n=5, P<0.05 for both variables) whereas ketamine had essentially no effect on the smaller and slower residual Ih in cortical pyramidal neurons from CaMKCre:HCN1f/f mice (ΔV1/2: −1.8 ± 1.0 mV, from −101.2 ± 1.6 mV to −103.0 ± 1.3 mV; % inhibition: 2.9 ± 4.2 %, from 102.0 ± 13.4 pA to 97.5 ± 11.8 pA, n=7, P<0.05, Fig. 2). After ketamine treatment, ZD7288 completely blocked all remaining Ih in cells from both genotypes. Together, these data indicate that the ketamine-sensitive HCN1 subunit was effectively deleted in cortical pyramidal neurons from CaMKCre:HCN1f/f mice, leaving a residual Ih that is unaffected by ketamine.
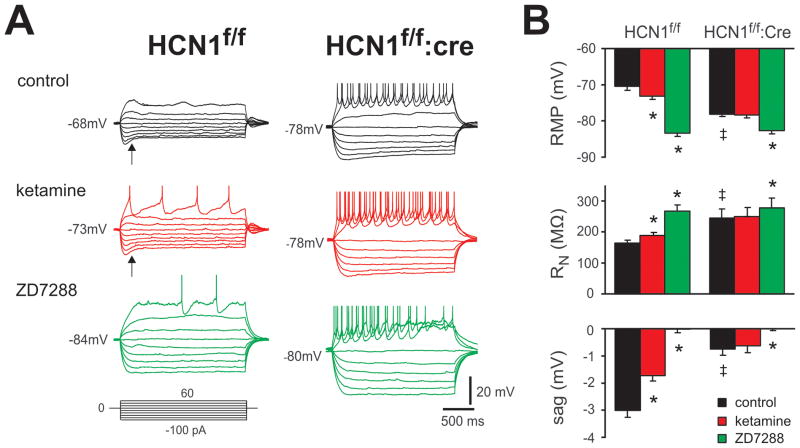
It is known that Ih contributes a resting inward current that provides a depolarizing membrane potential bias in cortical pyramidal neurons; in addition, during a step hyperpolarization, voltage-dependent activation of Ih produces a depolarizing “sag” in the membrane potential trajectory 5,22. Thus, as shown in Fig. 3, and as expected for deletion of HCN1 channels, cortical pyramidal neurons from CaMKCre:HCN1f/f mice presented with a more hyperpolarized resting membrane potential (resting membrane potential: −70.4 ± 1.1 mV for Cre− vs. −78.1 ± 0.7 mV for Cre+), greater input resistance (RN: 164.1± 8.9 MΩ for Cre− vs. 244.3 ± 29.1 MΩ for Cre+), and diminished sag (sag at −90 mV: −3.0 ± 0.3 mV for Cre− vs. −0.8 ± 0.2 mV for Cre+), by comparison to pyramidal neurons from control HCN1f/f mice (n=5 for Cre− & Cre+, P<0.05 for each variable). Moreover, whereas ketamine caused membrane hyperpolarization (resting membrane potential: from −70.4 ± 1.1 mV to −73.1 ± 0.9 mV, n=5, P<0.05,), increased input resistance (RN: from 164.1± 8.9 MΩ to 180.5 ± 11.1 MΩ, n=5, P<0.05) and reduced depolarizing sag (from −3.0 ± 0.3 mV to 1.7 ± 0.2 mV at −90 mV; n=5, P<0.05) in pyramidal neurons from control HCN1f/f mice, these actions of ketamine were occluded in pyramidal neurons from CaMKCre:HCN1f/f mice (resting membrane potential: from −78.1 ± 0.7 mV to −78.3 ± 0.8 mV; RN: from 244.3 ± 29.1 to MΩ to 248.9 ± 27.4 MΩ; sag: from −0.8 ± 0.2 mV to −0.6 ± 0.2 mV at −90 mV; n=5, P>0.05 for all variables). Again, blocking the remaining Ih with ZD7288 eliminated all genotype-dependent differences in resting membrane potential, RN or sag. Overall, these data are consistent with removal of the ketamine-sensitive HCN1 subunit in cortical pyramidal neurons from CaMKCre:HCN1f/f mice.
Figure 3. HCN1 deletion from cortical pyramidal neurons of CaMKCre:HCN1 f/f mice alters basal membrane properties and occludes ketamine modulation.
A. Sample current clamp recordings from cortical pyramidal neurons from HCN1f/f and CaMKCre:HCN1f/f mice under control conditions (upper) and during exposure to ketamine (20 μM, middle) and ZD-7288 (50 μM, lower). The depolarizing membrane sag observed during membrane hyperpolarization, a phenomenon attributed to Ih, is indicated by the arrow. Tetrodotoxin was not applied in these experiments. B. Averaged data (± SE) show that membrane potential was more hyperpolarized, RN was higher and sag was reduced in cortical pyramidal neurons from CaMKCre:HCN1f/f mice. Ketamine hyperpolarized membrane potential, increased RN and decreased sag only in cells from HCN1f/f mice. n=5 & 5, P<0.05, * ketamine vs. control; ‡, HCN1f/f:cre vs. HCN1f/f.
Ketamine modulation of synaptic properties is abolished in cortical pyramidal neurons of CaMKCre:HCN1f/f mice
In cortical pyramidal neurons, HCN1 is most prominently expressed in distal dendritic domains (e.g., see arrowheads in Fig. 1A) and the corresponding dendritic Ih provides a major current shunt that serves to dampen synaptic inputs onto those cells 7. We therefore examined if selective HCN1 deletion in cortical pyramidal neurons was associated with improved dendritosomatic synaptic transfer.
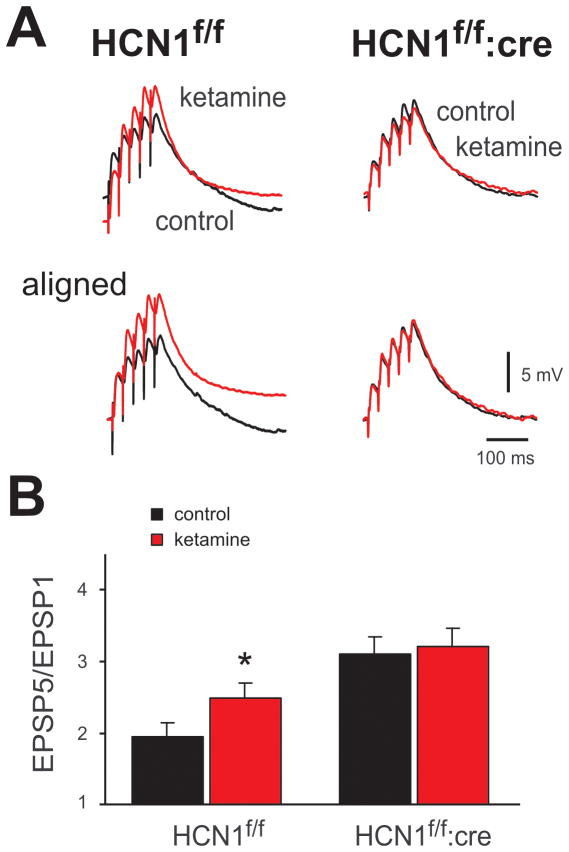
In order to evaluate effects of HCN1 deletion on basal and ketamine-modulated synaptic properties, we employed a measure of synaptic summation that is known to be influenced by dendritic Ih 7. For this, EPSPs were evoked in pyramidal neurons by 40 Hz extracellular stimulation of the superficial cortex (Fig. 4A), and EPSP temporal summation was calculated as the ratio of the final evoked EPSP (EPSP5) relative to the first EPSP (EPSP5/EPSP1; Fig. 4B); the degree of summation is most clearly evident in the aligned and normalized traces (see lower panels of Fig. 4A). Note that temporal summation of evoked EPSPs was enhanced after HCN1 deletion (EPSP5/EPSP1 ratio: 1.9 ± 0.2 for Cre− vs. 3.1 ± 0.2 for Cre+; n=5, P<0.05). Importantly, whereas ketamine increased EPSP temporal summation by ~28% (from 1.9 ± 0.2 to 2.5 ± 0.2, n = 5, p<0.05) in HCN1f/f mice, this effect of ketamine was totally eliminated in pyramidal neurons of CaMKCre:HCN1f/f mice (from 3.1 ± 0.2 to 3.2 ± 0.2, n = 5, P>0.05).
Figure 4. Ketamine fails to enhance EPSP temporal summation in cortical pyramidal neurons from CaMKCre:HCN1 f/f mice.
A. Sample voltage traces show EPSP recordings in cortical pyramidal neuron from HCN1f/f (left) and CaMKCre:HCN1f/f mice (right) in response to 40 Hz stimulation under control conditions, and during exposure to ketamine (20 μM). Lower panels: EPSPs were aligned to initial membrane potential and normalized to the amplitude of the first EPSP in the train in order to highlight ketamine effects on temporal summation. B. Averaged EPSP summation ratio (EPSP5/EPSP1) for HCN1f/f and CaMKCre:HCN1f/f mice under the indicated conditions. Ketamine enhanced EPSP summation in cortical neurons from HCN1f/f animals, but not from CaMKCre:HCN1f/f mice. *, n=5 & 5, P<0.05 by two-way RM-ANOVA. Tetrodotoxin was not applied in these experiments. EPSP: excitatory postsynaptic potential.
CaMKCre:HCN1f/f mice are significantly less sensitive to hypnotic actions of ketamine
We previously suggested that HCN1-containing channels may be important molecular substrates for hypnotic anesthetic actions of ketamine 4 because those channels are inhibited by ketamine and because the associated synaptic enhancement could promote cortical synchrony 8,9,23 like that observed during anesthetic-induced hypnosis 10. Indeed, we showed that HCN1−/− mice showed a marked decrease in sensitivity to ketamine-induced LORR, an established behavioral surrogate in mice for hypnotic anesthetic actions 1,2. However, those earlier experiments using global knockout mice could not localize that effect to deletion of HCN1 channels within forebrain neurons 4. Here, we examined if altered ketamine sensitivity was also observed in CaMKCre:HCN1f/f mice, in which HCN1 subunits were deleted only from forebrain neurons. As shown in Fig. 5A, the half-maximal dose of ketamine (EC50) that produced LORR was significantly higher in CaMKCre:HCN1f/f mice (by ~31%; 10.4 ± 0.4 mg/kg for Cre− vs. 13.7 ± 0.3 mg/kg for Cre+, n=31 & 52, p<0.0001); there was no overlap of the 95% confidence intervals for the estimated EC50 values between genotypes (9.6–11.3 mg/kg for Cre− vs. 13.0–14.3 mg/kg for Cre+). Also consistent with decreased sensitivity to ketamine, the duration of LORR evoked by ketamine was reduced in CaMKCre:HCN1f/f mice, by comparison to control HCN1f/f mice (Fig. 5B; F1,211=10.4, p<0.002). In contrast to these differences in ketamine-induced LORR, we found no genotype-dependent differences in ketamine actions on gross sensorimotor function. In a tail-flick assay to assess responses to noxious sensory stimulation (Fig. 5C), HCN1f/f and CaMKCre:HCN1f/f mice displayed essentially identical latencies to remove their tails from a painful heat source under control conditions, and both showed a similar, transient increase in latency following high dose ketamine administration (20 mg/kg). In a rotarod assay to assess motor function (Fig. 5D), both control and conditional HCN1 knockout mice were able to remain on an accelerating rod for equal durations initially, and both genotypes showed comparable decreases in duration following a sub-anesthetic dose of ketamine (2.5 mg/kg). Note that at higher ketamine doses, even those where all animals retained their righting reflex (i.e., 5 mg/kg), the mice were too incapacitated to remain on the rotarod for more than a few seconds. In sum, these data indicate that actions of ketamine on behaviors that involve spinal nociceptive reflexes or cerebellar function were unaffected in mice with forebrain-selective deletion of HCN1, as expected, whereas those mice were significantly less sensitive to ketamine-induced hypnosis.
DISCUSSION
In this paper, we have shown that selective deletion of HCN1 subunits from forebrain principal cells in mice leads to decreased sensitivity to hypnotic actions of ketamine, addressing both the molecular and neural basis for this anesthetic action. That is, this work provides additional support for our earlier proposition that HCN1-containing channels represent a behaviorally-relevant molecular target of ketamine 4. It also further restricts the potential neural substrate for HCN1-mediated hypnotic actions of ketamine to forebrain principal neurons. In this latter respect, we found that ketamine-mediated inhibition of HCN1-containing channels in cortical pyramidal neurons enhances dendritosomatic synaptic integration, an effect that would support the synaptically-mediated slow cortical rhythms that accompany the hypnotic state induced by ketamine 8–10,23. Thus, these data remain consistent with the hypothesis that cortical pyramidal neurons represent a plausible neural substrate for HCN1 contributions to ketamine-induced hypnosis.
As described by others, a number of criteria should be met for a molecular target to qualify as relevant for an anesthetic action 1,24. These include: [1] the target is modulated by the anesthetic at clinically-relevant concentrations and with a stereoselectivity that matches the in vivo actions of the drug; [2] the target is expressed in a specific anatomical location where its modulation could conceivably mediate the specific behavioral effects of the anesthetic (i.e., plausibility); and [3] elimination of the target (or disrupting its modulation by the anesthetic) diminishes the ability of the drug to achieve a specific anesthetic end point in vivo. With respect to pharmacology, we previously found a stereoselective, subunit-specific inhibition of HCN1 channels by clinically appropriate concentrations of ketamine 4; we showed that racemic ketamine shifts the V1/2 of HCN1 activation with an EC50 of ~8 μM while the S-(+)-enantiomer does so with an EC50 of ~4 μM 4, consistent with ~2-fold greater anesthetic potency of S-(+)-ketamine 25,26. Importantly, we also demonstrated by global elimination of HCN1 channels, that HCN1 knockout mice were strikingly less sensitive to the hypnotic effects of ketamine 4; this did not appear to represent a non-specific effect of HCN1 deletion since sensitivity of the mice to anesthetic effects of etomidate was unchanged 4. The current study confirms and extends that previous work in terms of plausibility by showing that this relative resistance to ketamine-induced hypnosis was largely recapitulated in mice with deletion of HCN1 channels restricted to forebrain principal cells, a brain region relevant for anesthetic-induced hypnosis. Thus, the accumulated evidence available to this point is consistent with the idea that forebrain HCN1 channels indeed contribute to ketamine-induced hypnosis.
Mechanistically, we favor the idea that the relevant forebrain cells are cortical pyramidal neurons since inhibition of dendritic HCN1 channels in those cells causes membrane hyperpolarization with enhanced synaptic efficacy, and thus yields conditions conducive to coherent cortical activity 4,8,9. It should be noted that Cre expression is not limited only to cortical pyramidal neurons in this line of mice. However, those cells represent the predominant site of HCN1 expression in the forebrain 22 and this convergence of both Cre and HCN1 expression thus favors the idea that pyramidal neurons are the likely site of action in this mouse model. Nevertheless, further refinement of the relevant neural substrate, especially between pyramidal neurons of the hippocampus and neocortex, will require mouse lines with even more restricted Cre expression.
As was seen with global HCN1−/− mice, ketamine remained capable of inducing hypnosis in CaMKCre-HCN1f/f mice, albeit at higher concentrations. Thus, other molecular targets must also be able to contribute to ketamine-induced hypnosis. In this respect, it is important to emphasize that ketamine is well known to be a potent modulator of N-Methyl-D-aspartic acid (NMDA) receptor channels 1,26. However, at present there remains no evidence from NMDA receptor knockout mice to support a role for NMDA receptor modulation in anesthetic actions of ketamine. Moreover, MK801 is a more potent and selective NMDA receptor blocker than ketamine, but is actually a poor anesthetic 27,28. Thus, despite widespread recognition of NMDA receptors as a ketamine target, the contributions of NMDA receptor antagonism to specific anesthetic actions of ketamine remain to be delineated. It is possible that inhibitory effects on NMDA receptors underlie other non-anesthetic central actions of ketamine (e.g., antidepressant or psychotomimetic) 26,29–31; it is also possible that modulation by ketamine of other molecular targets (e.g., activation of tonic gamma-aminobutyric acid (GABA) type A receptors containing α6 or δ subunits) may contribute to its other anesthetic actions 32–34.
In conclusion, these results indicate that forebrain principal cells represent the relevant neural substrate for HCN1-mediated hypnotic actions of ketamine. As knowledge of the molecular determinants for ketamine inhibition of HCN1 channels becomes available, it may be possible to develop knock-in mice that express ketamine-insensitive mutant HCN1 channels, preferably in more restricted neuronal populations, to even more rigorously define the molecular and neuronal targets for this specific clinical endpoint of ketamine.
Summary Statement.
Selective deletion of HCN1 channels in principal neurons of the mouse forebrain results in diminished sensitivity to loss-of-righting reflex produced by ketamine, establishing forebrain HCN1 channels as a behaviorally relevant neural/molecular substrate for ketamine-induced hypnosis.
MS #201205005 Final Box Summary.
What we already know about this topic
HCN1 channels are inhibited by ketamine but the neural substrate for this blockade and a full understanding of the mechanistic basis for ketamine’s clinically relevant actions remains to be determined.
What this article tells us that is new
Using a conditional knockout strategy to selectively delete HCN1, a relevant neural substrate for ketamine’s clinical action, HCN1-mediated hypnotic actions of ketamine was found in forebrain principal (excitatory) cells, consistent with the view that ketamine inhibition of HCN1 alters cortical neuron electroresponsive properties.
Acknowledgments
Support Statement: Supported by GM66181 (to Dr. Bayliss) from the National Institutes of Health (Bethesda, Maryland) and Grant 81171274 (to Dr. Chen) from the National Natural Science Foundation of China (Beijing, China), the 2009 Young Investigator Award Program (to Dr. Chen) from National Alliance for Research on Schizophrenia and Depression (Great Neck, New York), the Fundamental Research Funds for the Central Universities (to Dr. Chen) from Ministry of Education of China (Beijing, China).
References
- 1.Franks NP. Molecular targets underlying general anaesthesia. Br J Pharmacol. 2006;147:S72–S81. doi: 10.1038/sj.bjp.0706441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franks NP. General anaesthesia: From molecular targets to neuronal pathways of sleep and arousal. Nature Rev Neurosci. 2008;9:370–86. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 3.Grasshoff C, Rudolph U, Antkowiak B. Molecular and systemic mechanisms of general anaesthesia: the ‘multi-site and multiple mechanisms’ concept. Curr Opin Anaesthesiol. 2005;18:386–91. doi: 10.1097/01.aco.0000174961.90135.dc. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Shu S, Bayliss DA. HCN1 channel subunits are a molecular substrate for hypnotic actions of ketamine. J Neurosci. 2009;29:560–9. doi: 10.1523/JNEUROSCI.3481-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pape HC. Queer current and pacemaker: The hyperpolarization-activated cation current in neurons. Annu Rev Physiol. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- 6.Nolan MF, Malleret G, Dudman JT, Buhl DL, Santoro B, Gibbs E, Vronskaya S, Buzsaki G, Siegelbaum SA, Kandel ER, Morozov A. A behavioral role for dendritic integration: HCN1 channels constrain spatial inputs to distal dendrites memory and plasticity at of CA1 pyramidal neurons. Cell. 2004;119:719–32. doi: 10.1016/j.cell.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 7.Magee JC. Dendritic integration of excitatory synaptic input. Nature Rev Neurosci. 2000;1:181–90. doi: 10.1038/35044552. [DOI] [PubMed] [Google Scholar]
- 8.Hill S, Tononi G. Modeling sleep and wakefulness in the thalamocortical system. J Neurophysiol. 2005;93:1671–98. doi: 10.1152/jn.00915.2004. [DOI] [PubMed] [Google Scholar]
- 9.Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ. Computational models of thalamocortical augmenting responses. J Neurosci. 1998;18:6444–65. doi: 10.1523/JNEUROSCI.18-16-06444.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amzica F, Steriade M. Electrophysiological correlates of sleep delta waves. Electroencephalogr Clin Neurophysiol. 1998;107:69–83. doi: 10.1016/s0013-4694(98)00051-0. [DOI] [PubMed] [Google Scholar]
- 11.Nolan MF, Malleret G, Lee KH, Gibbs E, Dudman JT, Santoro B, Yin DQ, Thompson RF, Siegelbaum SA, Kandel ER, Morozov A. The hyperpolarization-activated HCN1 channel is important for motor learning and neuronal integration by cerebellar Purkinje cells. Cell. 2003;115:551–64. doi: 10.1016/s0092-8674(03)00884-5. [DOI] [PubMed] [Google Scholar]
- 12.Rossant J, Nagy A. Genome engineering: the new mouse genetics. Nat Med. 1995;1:592–4. doi: 10.1038/nm0695-592. [DOI] [PubMed] [Google Scholar]
- 13.Dragatsis I, Zeitlin S. CaMKIIalpha-Cre transgene expression and recombination patterns in the mouse brain. Genesis. 2000;26:133–5. doi: 10.1002/(sici)1526-968x(200002)26:2<133::aid-gene10>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 14.Dragatsis I, Levine MS, Zeitlin S. Inactivation of Hdh in the brain and testis results in progressive neurodegeneration and sterility in mice. Nat Genet. 2000;26:300–6. doi: 10.1038/81593. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Shu S, Kennedy DP, Willcox SC, Bayliss DA. Subunit-specific effects of isoflurane on neuronal Ih in HCN1 knockout mice. J Neurophysiol. 2009;101:129–40. doi: 10.1152/jn.01352.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X, Shu SF, Bayliss DA. Suppression of Ih contributes to propofol-induced inhibition of mouse cortical pyramidal neurons. J Neurophysiol. 2005;94:3872–83. doi: 10.1152/jn.00389.2005. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Sirois JE, Lei QB, Talley EM, Lynch C, Bayliss DA. HCN subunit-specific and cAMP-modulated effects of anesthetics on neuronal pacemaker currents. J Neurosci. 2005;25:5803–14. doi: 10.1523/JNEUROSCI.1153-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garfield JM, Bukusoglu C. Propofol and ethanol produce additive hypnotic and anesthetic effects in the mouse. Anesth Analg. 1996;83:156–61. doi: 10.1097/00000539-199607000-00027. [DOI] [PubMed] [Google Scholar]
- 20.Crawley JN. Behavioral phenotyping strategies for mutant mice. Neuron. 2008;57:809–18. doi: 10.1016/j.neuron.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Colbran RJ. Regulation and role of brain calcium/calmodulin-dependent protein kinase II. Neurochem Int. 1992;21:469–97. doi: 10.1016/0197-0186(92)90080-b. [DOI] [PubMed] [Google Scholar]
- 22.Santoro B, Chen S, Luthi A, Pavlidis P, Shumyatsky GP, Tibbs GR, Siegelbaum SA. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J Neurosci. 2000;20:5264–75. doi: 10.1523/JNEUROSCI.20-14-05264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antkowiak B. In vitro networks: Cortical mechanisms of anaesthetic action. Br J Anaesth. 2002;89:102–11. doi: 10.1093/bja/aef154. [DOI] [PubMed] [Google Scholar]
- 24.Hemmings HC, Jr, Akabas MH, Goldstein PA, Trudell JR, Orser BA, Harrison NL. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005;26:503–10. doi: 10.1016/j.tips.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Franks NP, Lieb WR. Where do general anaesthetics act? Nature. 1978;274:339–42. doi: 10.1038/274339a0. [DOI] [PubMed] [Google Scholar]
- 26.Kohrs R, Durieux ME. Ketamine: Teaching an old drug new tricks. Anesth Analg. 1998;87:1186–93. doi: 10.1097/00000539-199811000-00039. [DOI] [PubMed] [Google Scholar]
- 27.Irifune M, Katayama S, Takarada T, Shimizu Y, Endo C, Takata T, Morita K, Dohi T, Sato T, Kawahara M. MK-801 enhances gabaculine-induced loss of the righting reflex in mice, but not immobility. Can J Anaesth. 2007;54:998–1005. doi: 10.1007/BF03016634. [DOI] [PubMed] [Google Scholar]
- 28.Stabernack C, Sonner JM, Laster M, Zhang Y, Xing YL, Sharma M, Eger EI. Spinal N-Methyl-D-aspartate receptors may contribute to the immobilizing action of isoflurane. Anesth Analg. 2003;96:102–7. doi: 10.1097/00000539-200301000-00022. [DOI] [PubMed] [Google Scholar]
- 29.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psych. 2000;47:351–4. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 30.Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psych. 2006;63:856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 31.Wolff K, Winstock AR. Ketamine - From medicine to misuse. CNS Drugs. 2006;20:199–218. doi: 10.2165/00023210-200620030-00003. [DOI] [PubMed] [Google Scholar]
- 32.Hevers W, Hadley SH, Luddens H, Amin J. Ketamine, but not phencyclidine, selectively modulates cerebellar GABAA receptors containing alpha 6 and delta subunits. J Neurosci. 2008;28:5383–93. doi: 10.1523/JNEUROSCI.5443-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nature Rev Neurosci. 2004;5:709–20. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- 34.Schnoebel R, Wolff M, Peters SC, Brau ME, Scholz A, Hempelmann G, Olschewski H, Olschewski A. Ketamine impairs excitability in superficial dorsal horn neurones by blocking sodium and voltage-gated potassium currents. Br J Pharmacol. 2005;146:826–33. doi: 10.1038/sj.bjp.0706385. [DOI] [PMC free article] [PubMed] [Google Scholar]