Abstract
Background
Identification of angiotensin-(1-12) [Ang-(1-12)] in forming Ang II by a non-renin dependent mechanism has increased knowledge on the paracrine/autocrine mechanisms regulating cardiac expression of Ang peptides. This study now describes in humans the identity of the enzyme accounting for Ang-(1-12) metabolism in the left ventricular (LV) tissue of normal subjects.
Methods and Results
Reverse phase HPLC characterized the products of 125I-Ang-(1-12) metabolism in plasma membranes (PMs) from human LV in the absence and presence of inhibitors for chymase (chymostatin), angiotensin converting enzyme (ACE) 1 (lisinopril) and 2 (MLN-4760) and neprilysin (SHC39370). In the presence of the inhibitor cocktail ≥ 98 ± 2% of cardiac 125I-Ang-(1-12) remained intact, whereas exclusion of chymostatin from the inhibitor cocktail led to significant conversion of Ang-(1-12) into angiotensin II. In addition, chymase-mediated hydrolysis of 125I-Ang I was higher compared to Ang-(1-12). Negligible Ang-(1-12) hydrolysis occurred by ACE, ACE2, and neprilysin. A high chymase activity was detected for both 125I-Ang-(1-12) and 125I-Ang I substrates.
Conclusions
Chymase accounts for the conversion of Ang-(1-12) and Ang I to Ang II in normal human LV. These novel findings expand knowledge of the alternate mechanism by which Ang-(1-12) contributes to the production of cardiac angiotensin peptides.
Keywords: ACE2, cardiac myocytes, angiotensin converting enzyme inhibitors, angiotensin II, heart disease, proangiotensin 12, angiotensin-(1-7)
Introduction
The concept that angiotensin II (Ang II) synthesis occurs in multiple organs is now established and construed as primary evidence to explain the role of the renin angiotensin system (RAS) in adverse cardiovascular remodeling, stroke, and renal failure (1). Previous seminal studies demonstrating the existence of divergent biochemical mechanisms, that within the circulating and tissue RAS, account for the formation of the vasodilator and anti-trophic heptapeptide angiotensin-(1-7) [Ang-(1-7)] provided newer insights as to the mechanisms by which the system in the tissues regulates cardiac and vascular homeostasis (2). The further identification of an angiotensin converting enzyme (ACE) homologue [ACE2], insensitive to blockade with ACE inhibitors, and functioning to primarily metabolize Ang II into Ang-(1-7) (3–6) revealed that this servo control system is more complex than originally suspected, as both Ang-(1-7) and ACE2 acts to negatively influence the vasoconstrictor, trophic, and pro-inflammatory actions of Ang II (7).
While past studies showed that Ang II formation from angiotensin I (Ang I) in humans occurs through a non-ACE pathway (8), the importance of this alternate Ang II-forming mechanism in terms of therapeutic approaches has remained relatively ignored. The recent description of an alternate processing pathway for Ang II production upstream from Ang I now expands current concepts of the biochemical mechanisms engaged in the tissue production of Ang II. Nagata et al. (9) first identified in rats an extended form of Ang I, [Angiotensin-(1-12), Ang-(1-12)], able to generate Ang II through ACE. In realizing the potential impact of their observations to the understanding of the tissue actions of Ang II, a series of studies from our laboratory extended Nagata et al. (9) observations by showing: a)- increased expression of Ang-(1-12) in cardiac myocytes of spontaneously hypertensive rats (SHR) compared with the WKY normotensive strain (10); b)- that renin did not contribute to cardiac Ang II formation from Ang-(1-12) (11); and c)- that increased cardiac chymase expression in the hypertrophy heart of adult SHR (10) was associated with increased Ang-(1-12) metabolism by chymase in neonatal cardiac myocytes from the same hypertensive rat strain (12). The potential relevance of these findings to human cardiac pathology was further strengthened by our recent demonstration of Ang-(1-12) expression in left atrial tissue of patients undergoing open-heart surgery for the correction of resistant atrial fibrillation (13). The objective of the current study was to ascertain the enzymatic pathway accounting for the biotransformation of Ang-(1-12) into angiotensin peptides in normal cardiac tissue. This study was warranted by the necessity to uncover whether increased chymase expression occurred only in conditions of cardiac pathology or whether it is the fundamental pathway for Ang II formation in both normal and disease human hearts.
Methods
Ethic Statement
Samples of left ventricular (LV) tissue were obtained from Imgenex Laboratories (San Diego, CA) from six normal humans involved in motor vehicle accidents (MVA). All MVA patients had normal histological examination of the tissue, no history of cardiovascular disease, and no reported evidence of medications at the time of death (Table 1). The use of these tissues was approved by the University of Alabama at Birmingham Institutional Review Board.
Table 1.
Characteristics of Normal Human Heart Tissue
| Age
|
Gender
|
Cause of Death
|
Medications
|
|
|---|---|---|---|---|
| Normal Tissue: | ||||
| Sample 1 | 49 | male | MVA | None documented |
| Sample 2 | 38 | female | MVA | None documented |
| Sample 3 | 61 | male | MVA | None documented |
| Sample 4 | 42 | male | MVA | None documented |
| Sample 5 | 42 | male | MVA | None documented |
| Sample 6 | 25 | male | MVA | None documented |
Abbreviations: MVA, motor vehicle accident.
Reagents
Human Ang-(1-12) (>99% purity) was purchased from GenScript USA Inc. (Piscataway, NJ). Chymostatin (chymase inhibitor), lisinopril (ACE inhibitor), amastatin, bestatin, benzyl succinate and p-chloromercuribenzoate (PCMB) were purchased from Sigma-Aldrich Co. (St. Louis, MO). SCH39370 (neprilysin inhibitor) was obtained from Merck Inc. (West Point, PA). MLN-4760 (ACE2 inhibitor) was obtained from Millennium Pharmaceuticals (Cambridge, MA). 125Iodine was purchased from PerkinElmer Life and Analytical Sciences, Inc. (Waltham, MA). All other chemicals used in this study were of analytical grade and were obtained from Sigma (St. Louis, MO) and Fisher Scientific (Atlanta, GA).
Iodination (125I) of Ang peptides
The iodination of Ang peptides [Ang-(1-12), Ang I, Ang II and Ang-(1-7)] using the oxidant chloramine-T were done in small plastic tubes at room temperature as described elsewhere (12;13). Briefly, 10 μL of 1 mM angiotensin peptides were added to 20 μL of PBS and 10 μL of 1 mCi of Na[125I] (Perkin-Elmer, Waltham, MA ). The iodination reaction was started by adding 10 μL of chloramines-T solution (10–15 mg/10 mL) to the mixture for ~20–30 sec. The reaction was stopped by adding 50 μL of sodium bisulfate solution (30 mg/10 mL MilliQ water). The iodinated Ang peptide was separated from free Na[125I] by passing the mixture through an activated Sep-Pak column. The Sep-Pak eluted iodinated Ang peptide was further purified with reverse-phase high performance liquid chromatography (HPLC) and only monoiodinated Ang peptides [125I-Ang-(1-12), 125I-Ang I, 125I-Ang II and 125I-Ang-(1-7)] were used in these studies. During each experiment, the purity of radiolabelled 125I-Ang substrates was checked on the HPLC to verify that peptides are intact and not degraded before they were added to the PMs. Highly purified form of 125I-Ang substrates (≥98% purity) were only used in our metabolism studies.
Plasma membrane preparation
Native and soluble plasma membranes (PMs) were prepared at 4° C as described previously (12;13). Briefly, frozen LV tissues (50–100 mg) were homogenized at 4° C in 1 mL reaction buffer (25 mM HEPES, 125 mM NaCl and 10 μM ZnCl2, pH 7.4) using a Qiagen Tissue Lyzer (Valencia, CA) for 90 second at 25 Hz. The homogenate was centrifuged at low spin (200 g) for 1 min at 4° C to remove the connective tissues and cell debris. The supernatant was transferred into a new tube and centrifuged at 28,000 g for 20 min at 4° C. The pellet (native membranes) was washed twice by resuspending it in the reaction buffer, centrifuged, and stored at −80° C till its use for metabolism studies.
ACE and ACE2 activity were assayed in solubilized membrane. Native PMs were solubilized in a reaction buffer containing 0.5% triton X-100 on ice overnight. The soluble portion of the native membrane was separated from insoluble portion by centrifugation (28,000 g for 20 min at 4° C). The protein concentrations in each cardiac PMs (native and soluble) were measured by Bradford Reagent using bovine serum albumin as the standard, and the results were normalized in terms of per mg protein.
Metabolism Studies
The metabolism of human 125I-Ang-(1-12) and other Ang peptides by LV PMs (native PMs for chymase and neprilysin and soluble PMs for ACE & ACE2) were studied under different combinations of RAS inhibitors as described previously (13). Briefly, the soluble or native PMs (25–50 μg per reaction mixture) were preincubated for 10 min in a reaction buffer under various combinations of enzyme and peptidases inhibitors (see Table 2). After preincubation of PMs with different combinations of RAS inhibitors, highly purified human 125I-Ang-(1-12), 125I-Ang I or 125I-Ang II [1 nmol/L each; ≥98% purity] was added to the reaction medium and incubated for specific time points (30 min for chymase, 60 min for ACE/NEP, and 120 min for ACE2) at 37° C. These time points were chosen on the basis of pilot studies showing full conversion of Ang-(1-12) into the products at each of the respective incubation periods. At the end of the incubation time, the reaction was stopped by adding equal volume of 1% phosphoric acid, mixed well, centrifuged (28,000 g for 20 min to remove the native PMs or 16, 000 g for 1 min in case soluble PMs) and stored at 4° C. On the day of Ang peptide analysis, the samples were filtered before separation by HPLC. We used a linear gradient from 10% to 50% mobile phase B at a flow rate of 0.35 mL/min at 32° C. The solvent system consisted of 0.1% phosphoric acid (mobile phase A) and 80% acetonitriles/0.1% phosphoric acid (mobile phase B). The eluted 125I products were monitored by an in-line flow-through gamma detector (BioScan Inc., Washington, DC). Products were identified by comparison of retention time of synthetic [125I] standard peptides and the data were analyzed with the Shimadzu LCSolution (Kyoto, Japan) acquisition software. Experiments were performed three or more times. The metabolic products are shown as percent of Ang peptides fraction generated from the parent substrate by specific RAS enzymes. The RAS enzyme activity was calculated based on the amount of parent 125I-Ang substrate hydrolyzed into specific 125I-Ang products by the LV PMs isolated from normal tissues at 37° C with or without the presence of specific RAS inhibitors. RAS enzyme activities are reported as fmoles of Ang product generated from the parent 125I-Ang substrate (fmol × min−1 × mg−1).
Table 2.
Outline of Enzyme Inhibitors Used in the Experiments
| Group
|
Inhibitors added (50 μM each)
|
|---|---|
| All RAS inhibitors: | RAS inhibitors [chymostatin for chymase; lisinopril for angiotensin converting enzyme; SCH39370 for neprilysin; and MLN-4760 for angiotensin converting enzyme 2]; amastatin, bestatin, benzyl succinate, and P-chloromercuribenzoate (PCMB). |
| Minus RAS inhibitors: | All above inhibitors except one of the RAS inhibitors [chymostatin/lisinopril/SCH39370/MLN-4760] omitted at a time from the reaction mixture. |
Western Blot Analysis and Immunohistochemistry
The expression of chymase protein in human normal LV PM samples (n=4) were assessed by immunoblot technique as previously described by us (13). Briefly, the LV PMs were separated by gel electrophoresis (10% gel) and transferred to polyvinylidene defluoridated membranes (PVDF). The PVDF membranes were probed with a primary monoclonal anti-human chymase antibody (CMA1 antibody from R&D System, Minneapolis, MN, Cat # MAB4099; 2 μg/mL). After incubation with the primary antibody, the membranes were probed with the horseradish peroxidase-conjugated secondary antibody (anti-mouse, 1:3000; Pierce Inc., Rockford, IL, USA). Immune complexes were visualized using ECL plus detection reagents (Thermo Scientific Pierce Protein Biology Products, Rockford, Illinois). The blots were probed for equal loading using RedAlert (14).
Statistical Analysis
Experiments were repeated three or more times. All values are reported as means ± SEM. The Student’s t-test and repeated-measures ANOVA followed by a Turkey’s post hoc test for multiple comparisons were used to determine significant differences at P < 0.05 using GraphPad Prism 5.0 software (San Diego, CA).
Results
125I-Ang peptide hydrolysis
Under the conditions described in Methods, there was excellent separation of the radiolabeled products derived from the addition of either 125I-Ang-(1-12) or 125I-Ang I in both the presence and absence of the inhibitor cocktail.
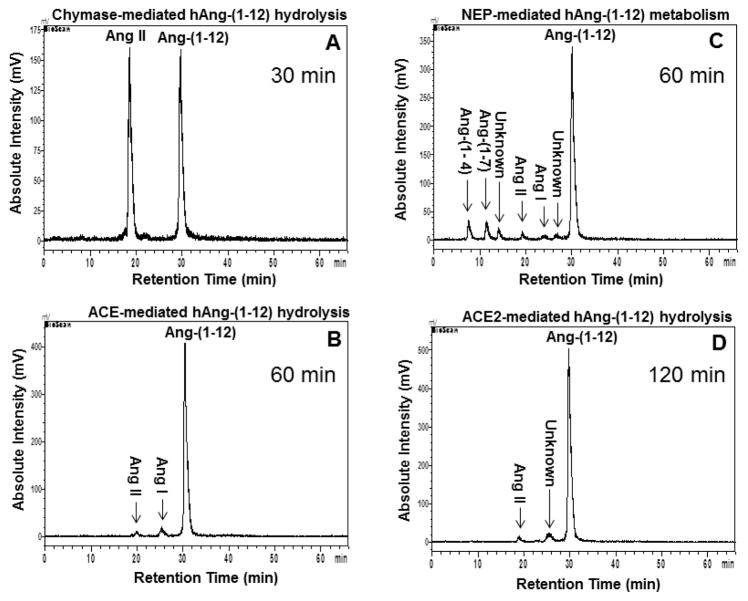
In the presence of all RAS inhibitors a single peak corresponding to the Ang-(1-12) retention time was found on the chromatogram [≥ 98 ± 2% of 125I-Ang-(1-12) remained intact]. Figure 1 (panel A) shows Ang II as the only peptide peak detected from the hydrolysis of 125I-Ang-(1-12) when chymostatin was excluded from the cocktail of inhibitors. Removal of either lisinopril (ACE inhibitor), SCH39370 (neprilysin inhibitor) or MLN-4760 (ACE2 inhibitor), results in the appearance of very minor peaks corresponding to the retention times for Ang I, Ang II, Ang-(1-7) (Figure 1, panels B, C and D, respectively). These data show that chymase is the preferred enzyme accounting for the formation of Ang II from Ang-(1-12) in normal human hearts.
Figure 1.
125I-Ang-(1-12) hydrolysis by Normal Left Ventricular Plasma Membranes. Chromatograms show the hydrolysis of 125I-Ang-(1-12) in normal human left ventricular. Panel A: Without chymostatin (chymase-mediated hydrolysis, 30 min), Panel B: Without lisinopril (ACE-mediated hydrolysis, 60 min), Panel C: Without SCH39370 (NEP-mediated hydrolysis, 60 min), and Panel D: Without MLN-4760 (ACE2-mediated hydrolysis, 120 min). Results are representative of three or more separate metabolism experiments for each human sample.
Table 3 shows the average results of 125I-Ang-(1-12) hydrolysis by LV tissue of normal hearts. Chymase constitutes the primary 125I-Ang-(1-12) metabolizing enzyme in normal LV tissue while ACE, neprilysin and ACE2 showed essentially no hydrolytic activity on Ang-(1-12) as a substrate (Table 3).
Table 3.
Metabolism of 125I-Ang-(1-12)* by RAS enzymes in Plasma Membranes Isolated from Left Ventricular Tissues
| Enzyme/Peptide
|
Ang-(1-12)*
|
Ang I
|
Ang II
|
Ang-(1-7)
|
Ang-(1-4)
|
Unknown
|
|---|---|---|---|---|---|---|
| Chymase Contribution: (chymostatin absent) | ||||||
| 53 ± 7 | ND | 47 ± 7 | ND | ND | ND | |
| Angiotensin Converting Enzyme Contribution (lisinopril absent) | ||||||
| 93 ± 1 | 5 ± 1 | 2 ± 1 | ND | ND | ND | |
| Neprilysin Contribution: (SCH39370 absent) | ||||||
| 87 ± 2 | 1.0 ± 0.2 | 2.0 ± 0.3 | 3 ± 1 | 3.0 ± 0.7 | 4 ± 1 | |
| Angiotensin Converting Enzyme 2 Contribution: (MLN-4760 absent) | ||||||
| 95 ± 1 | ND | 1.0 ± 0.2 | ND | ND | 4 ± 1 | |
HPLC of human 125I-Ang-(1-12) metabolic products generated by PMs prepared from human LV tissue incubated with or without the presence of RAS inhibitors. The 125I-Ang-(1-12) substrate was ≥98% intact before adding to the PM for metabolism reaction. Values are expressed as % (Mean ± SEM) of Ang peptides generated from parent 125I-Ang-(1-12) substrate.
Represents amount of parent 125I-Ang-(1-12) substrate that remained unmetabolized at the end of reaction.
ND = not detected.
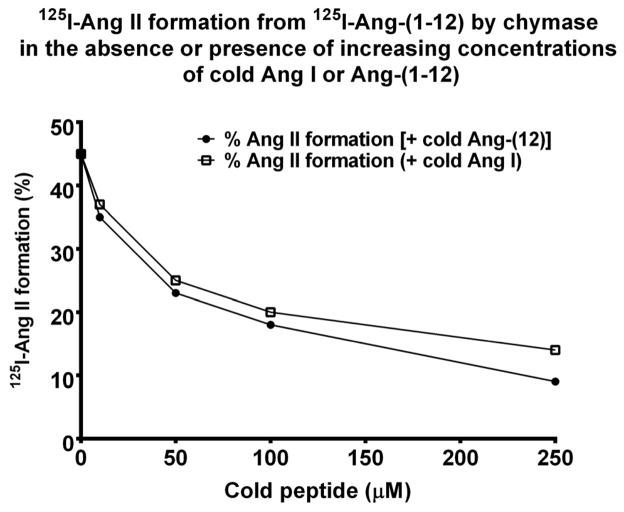
Table 4 documents the products generated by PMs when 125I-Ang I rather than 125I-Ang-(1-12) is used as a substrate. Chymase-mediated Ang II formation from the 125I-Ang I substrate (80 ± 5%, Table 4) was the predominant metabolite formed by the human LV samples. The fraction of Ang II produced from 125I-Ang I metabolism is higher than that resulting from the incubation with 125I-Ang-(1-12) (Table 3), a finding that corroborates prior evidence of chymase as a human Ang II forming enzyme from Ang I (8;15;16). In agreement with prior findings, Figure 2 shows the formation of 125I-Ang II from 125I-Ang-(1-12) by chymase in the presence of increasing concentration of unlabeled Ang I or human Ang-(1-12) peptide [0–250 μM]. In the presence of unlabeled Ang I or Ang-(1-12) 125I-Ang II formation from 125I-Ang-(1-12) by chymase was equally reduced. ACE-mediated Ang II formation from Ang I by normal PMs was higher (15 ± 3%) when compared to Ang-(1-12) (2 ± 1%). Similar to Ang-(1-12) (Table 3), Ang I was also poorly metabolized by neprilysin (Table 4).
Table 4.
Metabolism of 125I-Ang I by Plasma Membranes Isolated from Left Ventricular Tissue
| Enzyme/Peptide
|
Ang I
|
Ang II
|
Ang-(1-7)
|
Ang-(1-4)
|
Unknown
|
|---|---|---|---|---|---|
| Chymase Contribution: (chymostatin absent) | |||||
| 20 ± 5 | 80 ± 5 | ND | ND | ND | |
| Angiotensin Converting Enzyme Contribution: (lisinopril absent) | |||||
| 85 ± 3 | 15 ± 3 | ND | ND | ND | |
| Neprilysin Contribution: (SCH39370 absent) | |||||
| 82 ± 2 | 6 ± 1 | 7 ± 1 | 4 ± 1 | 1 ± 0.2 | |
Values are expressed as % (Mean ± SEM) of Ang peptides generated from parent 125I-Ang substrate (purity ≥98%).
Percent of corresponding 125I-Ang I substrate that remained un-metabolized at the end of reaction. Each experiment was done at least three times and the results are the average of six normal human subjects.
ND = not detected.
Figure 2.
HPLC analysis of 125I-Ang II formation (%) from 125I-Ang-(1-12) substrate by chymase in the absence or presence of cold Ang-(1-12) or Ang I. As described in Methods, the reaction mixture contained 50 μg of plasma membrane plus ALL RAS inhibitors (except chymostatin) plus substrate incubated in the absence or presence of increasing concentration of cold Ang-(1-12) or Ang I (range: 0–250 μM for 30 min at 37° C.
The effect of using 125I-Ang II as a substrate was evaluated by removal of the potent ACE2 inhibitor (MLN-4760) from the inhibitor cocktail. ACE2-mediated hydrolysis of 125I-Ang II substrate into Ang-(1-7) product formation averaged 9.5 ± 1.8%. ACE2 mediated conversion of labeled Ang I was not investigated in these studies since the enzyme has a very low affinity for the decapeptide (5).
Relative Contributions of RAS enzymes in LV PMs
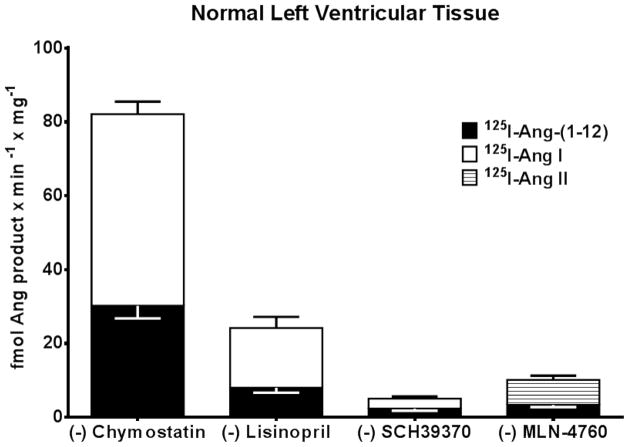
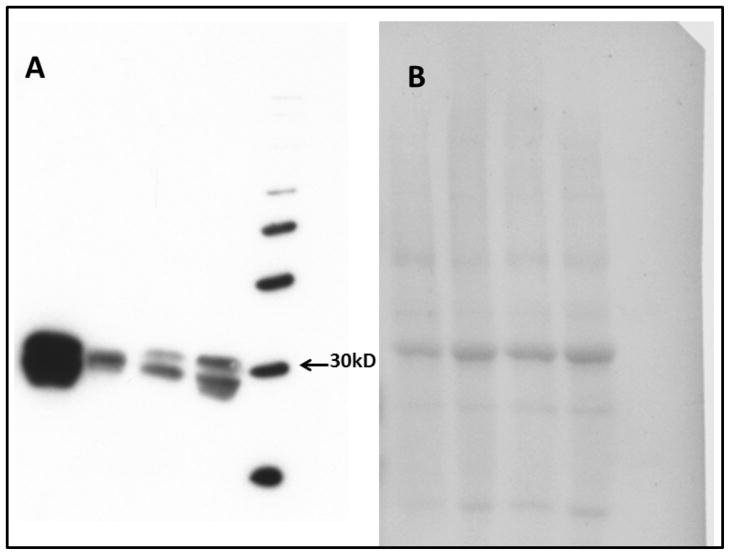
Figure 3 shows the relative expression of RAS enzymes activities in the LV PMs isolated from human samples. Enzymes activities were calculated from the specific Ang products generated from either Ang-(1-12) or Ang I (for chymase, ACE and neprilysin) or from Ang-(1-12) or Ang II (for ACE2) hydrolysis under different combinations of RAS inhibitors. In keeping with the metabolism studies, cardiac chymase activity was several folds higher as compared to ACE, neprilysin, and even ACE2. Chymase activity accounting for Ang II formation from both radiolabeled Ang-(1-12) and Ang I substrate hydrolysis averaged 30.2 ± 3.4 fmol × min−1 × mg−1 and 51.9 ± 3.4 fmol × min−1 × mg−1, respectively (Figure 3). The generation of Ang II from Ang-(1-12) by ACE activity is much lower when compared to chymase in normal LV (8.0 ± 1.3 fmol × min−1 × mg−1). On the other hand, higher ACE activity was detected in normal LV (16.2 ± 3.0 fmol × min−1 × mg−1) when 125I-Ang I was employed as a substrate instead of 125I-Ang-(1-12). Low neprilysin activity was detected either using Ang-(1-12) or Ang I as substrate in normal subjects. Neprilysin activity detected in normal PMs averaged 2.4 ± 0.6 fmol × min−1 × mg−1 using 125I-Ang-(1-12) substrate, and 2.7 ± 0.6 fmol × min−1 × mg−1 for 125I-Ang I substrate. ACE2 activity was also low using 125I-Ang-(1-12) as substrate in normal PMs (3.4 ± 0.6 fmol × min−1 × mg−1) but significantly higher ACE2 activity was detected for 125I-Ang II substrate [6.8 ± 1.1 fmol × min−1 × mg−1as compared to 125I-Ang-(1-12) substrate]. Chymase protein expression by Western blot technique is shown in Figure 4.
Figure 3.
RAS enzyme activities calculated based on the amount of parent 125I-Ang substrate hydrolyzed into products by PMs prepared from human left ventricular tissue with or without the presence of specific RAS inhibitors. Values are Mean ± SEM from parent 125I-Ang substrate. Each experiment was done at least three times and the results are the average of six normal human subjects.
Figure 4.
Panel A, Chymase protein expression in human left ventricular tissue (n=4).
Discussion
This study provides the first characterization of the enzymatic pathways for Ang II and Ang-(1-7) production from Ang-(1-12) in LV tissue obtained from normal subjects. The results obtained in these experiments underscore the characteristics of the human cardiac membrane-bound enzymes accounting for the generation of angiotensin peptides from Ang-(1-12). Chymase, functioning as an ectoenzyme within the plasma membrane of LV cells, has a predominant activity in degrading either Ang-(1-12) or Ang I in normal heart tissue. ACE, neprilysin, or ACE2 do not contribute significantly to the formation of Ang II from Ang-(1-12) in normal hearts. In addition, we now show that chymase-mediated Ang II formation from Ang-(1-12) does not require an intermediate processing to Ang I. The existence of an alternate processing pathway for angiotensin peptide formation from Ang-(1-12) in human hearts is an important step in establishing whether direct production of Ang II from Ang-(1-12) contributes to adverse cardiac remodeling and failure (7;17) bypassing an intermedia processing into Ang I.
Our earlier demonstration of chymase expression and function in Ang-(1-12) metabolism in cardiac myocytes obtained from the atrial tissue of subjects with the diagnosis of atrial fibrillation (13) had posit the question of whether this metabolic pathway was active only in the diseased human heart. The current study now clearly demonstrates that normal human LV tissue, via a chymase pathway, has the capacity for direct generation of Ang II from Ang-(1-12). The past demonstration (7;13) that inhibition of Ang-(1-12) conversion into Ang II in the human atria by chymostatin was comparable to the results obtained with a highly selective orally active chymase inhibitor (TEI-F00806) (18;19) affirms that chymase is the cardiac enzyme accounting for the conversion of Ang-(1-12) into Ang II. In agreement with other studies we also showed that both human Ang I and human Ang-(1-12) competed equally for chymase to generate Ang II. Similarly, the chymase-mediated hydrolytic 125I-Ang II formation from 125I-Ang I, under same experimental conditions, was also equally reduced in the presence of increasing concentration of cold Ang-(1-12).
The identification of the dodecapeptide Ang-(1-12) as a source for angiotensin peptides generation was first reported by Nagata et al. (9) in the circulation and cardiovascular tissues of Wistar rats. These investigators further showed that administration of this extended form of Ang I elicited pressor and vasoconstrictor response that were blocked by either an ACE inhibitor or the Ang II receptor blocker candesartan. The catalytic activity of ACE in Ang-(1-12) metabolism was confirmed in additional studies in the circulation of WKY and SHR (20), isolated rat arteries (21), the rat brain (22), and in the serum and kidney of a congenic rat model of hypertension due to increased tissue renin expression (23). On the other hand, two other studies implicated chymase as a source for Ang II from Ang-(1-12) (12;24). In an isolated heart model of ischemia-reperfusion in the rat, inhibition of chymase with chymostatin significantly reduced Ang II production and attenuated Ang-(1-12)-induced vasoconstriction and myocardial damage following ischemia while ACE inhibition was without effect (24). In the other study we showed that while ACE and neprilysin accounted for Ang-(1-12) metabolism in the medium of neonatal myocytes from WKY rats, an additional role for chymase was found in SHR (12). This latter study suggested a chymase participation in conditions of acute or chronic cardiac stress.
It may be argued that studies of Ang-(1-12) metabolism in plasma membranes may differ from the conversion pathways existing in intact tissue. A comparative study in intact cardiac tissue from normal hearts is not practical or even possible as human fresh tissue will be impossible to recover. On the other hand, previous studies of Ang-(1-12) metabolism in the isolated perfused rat heart (11), rat neonatal cardiac myocytes (12), and those reported by Prosser et al. (21;24) in Sprague Dawley heart and the vasculature do not suggest differences in Ang-(1-12) processing between intact cardiac tissue and isolated membranes. This interpretation agrees with the previous demonstration that both chymase and Ang-(1-12) were expressed endogenously in rat hypertensive neonatal cardiac myocytes (12) and diseased human atrial tissues (13).
Western blot findings in the human tissue confirmed the presence of the enzyme in cardiac tissue. It is known that chymase is stored in a macromolecular complex with heparin proteoglycan within mast cell granules and is more resistant to inhibit by macromolecular natural serine protease inhibitors such as a-antitrypsin (25;26). The chymase protein remains complexed even after degranulation from mast cells. The discrete cardiac chymase expression in the heart tissue suggest that chymase-dependent Ang-(1-12) processing may occur in the extracellular cardiac milieu although we also showed that Ang-(1-12) uptake by cardiac neonatal myocytes (12). Our studies also showed that levels of chymase expression differed from sample to sample, a finding that has been observed previously by others (27;28). Furthermore, the amount of Ang II formation from Ang-(1-12) by chymase activity was consistent with the chymase protein expression in these human samples. While this warrants further study, the variability of enzyme activity might be related to factors such age and gender. There was also some variability in the molecular weight of chymase protein expression in the studied tissues. More than one band for human cardiac chymase (major bands with molecular weight ~27–32 Kd and minor protein bands ~17–19Kd) have been reported by others (29;30) while Frith et al. (31) showed two chymase bands and a degraded product (32 Kd and 27 Kd) in human wounded cells crude lysates.
From the preceding it is now clear that there is a fundamental difference in the mechanisms of Ang II production and enzymatic pathways for cardiac Ang-(1-12) metabolism between rodents and humans, as both our current and past studies (13;15) and those reported by others (32–36) showed that chymase rather than ACE is the primary pathway for the production of Ang II in humans. In support for this interpretation, the alpha chymase gene is expressed in humans and baboons while both the alpha and beta forms of the chymase gene are expressed in rats and mouse (15). While the alpha chymase has highly specific and efficient Ang II-forming activity, the beta chymase shows broad substrate specificity and do not form Ang II (35). Differences in the processing enzymes accounting for Ang-(1-12) metabolism may be influenced also by the route of processing: i.e., intravascular versus the tissue interstitial compartments. Our data and those obtained elsewhere demonstrate that while Ang-(1-12) metabolism in the circulation is mediated by ACE (9;12;20;23), chymase is the primary enzyme accounting for the cleavage of this substrate in human heart tissue. We further showed that ACE2 does not contribute to Ang II formation from Ang-(1-12), although less efficiently, it is reported to convert Ang I to Ang-(1-9) (4;37).
While a robust clinical and experimental literature established the importance of the ACE-mediated Ang II production in the regulation of blood pressure and cardiorenal function, the role of this enzyme as a primary loci for Ang II formation from Ang I remains an open question as conversion of Ang I to Ang II in tissues can proceed despite complete ACE inhibition (35) and the effectiveness of these drugs in the prevention of cardiac arrhythmias, and the progression of cardiac dysfunction is not completely certain (16;17;38–40). Since the original observation by Urata et al. (8) of chymase as an Ang II-forming enzyme, evidence for its direct role in the pathogenesis of human cardiovascular disease continues to increase (15;19;27–29;34;36;41–47). The current studies and those reported by us previously (13) expand knowledge of the cardiac processing pathways accounting for Ang II formation via the actions of chymase rather than ACE or even neprilysin. The current findings further illustrate the complexity of alternate pathways for Ang II production and species differences. These data warrants reconsideration of current therapies for suppressing the pathological actions of Ang II in humans through the further evaluation of the potential benefits of chymase inhibition in human cardiovascular disease. The discovery of a chymase-dependent primary pathway for the direct formation of Ang II from Ang-(1-12) by chymase in the human heart implies a distinct pathophysiological role of this Ang II-forming enzyme particularly in conditions in which the catalytic activities of renin or ACE are inhibited. In summary, in LV tissue obtained from normal hearts, the enzymatic pathway accounting for the production of Ang II entails the direct cleavage of Ang-(1-12) via chymase.
Acknowledgments
The studies reported here were supported by a grant (HL-051952) from the Heart, Lung, Blood Institute of the National Institutes of Health. We also acknowledge partial support provided by the Farley-Hudson Foundation, Jacksonville, NC.
Footnotes
There are no financial disclosures or conflict of interests declared by any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferrario CM, Ahmad S, Joyner J, Varagic J. Advances in the renin angiotensin system focus on angiotensin-converting enzyme 2 and angiotensin-(1-7) Adv Pharmacol. 2010;59:197–233. doi: 10.1016/S1054-3589(10)59007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrario CM. New physiological concepts of the renin-angiotensin system from the investigation of precursors and products of angiotensin I metabolism. Hypertension. 2010 Feb;55(2):445–52. doi: 10.1161/HYPERTENSIONAHA.109.145839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002 Jun 20;417(6891):822–8. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 4.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000 Sep 1;87(5):E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 5.Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J. 2004 Oct 1;383(Pt 1):45–51. doi: 10.1042/BJ20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wysocki J, Ye M, Rodriguez E, Gonzalez-Pacheco FR, Barrios C, Evora K, et al. Targeting the degradation of angiotensin II with recombinant angiotensin-converting enzyme 2: prevention of angiotensin II-dependent hypertension. Hypertension. 2010 Jan;55(1):90–8. doi: 10.1161/HYPERTENSIONAHA.109.138420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrario CM. ACE2: More of Ang-(1-7) or less Ang II? Curr Opin Nephrol Hypertens. 2011 Jan;20(1):1–6. doi: 10.1097/MNH.0b013e3283406f57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urata H, Kinoshita A, Misono KS, Bumpus FM, Husain A. Identification of a highly specific chymase as the major angiotensin II-forming enzyme in the human heart. J Biol Chem. 1990 Dec 25;265(36):22348–57. [PubMed] [Google Scholar]
- 9.Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K. Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochem Biophys Res Commun. 2006 Dec 1;350(4):1026–31. doi: 10.1016/j.bbrc.2006.09.146. [DOI] [PubMed] [Google Scholar]
- 10.Jessup JA, Trask AJ, Chappell MC, Nagata S, Kato J, Kitamura K, et al. Localization of the novel angiotensin peptide, angiotensin-(1-12), in heart and kidney of hypertensive and normotensive rats. Am J Physiol Heart Circ Physiol. 2008 Jan;294(6):H2614–H2618. doi: 10.1152/ajpheart.91521.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trask AJ, Jessup JA, Chappell MC, Ferrario CM. Angiotensin-(1-12) is an alternate substrate for angiotensin peptide production in the heart. Am J Physiol Heart Circ Physiol. 2008 May;294(5):H2242–H2247. doi: 10.1152/ajpheart.00175.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmad S, Varagic J, Westwood BM, Chappell MC, Ferrario CM. Uptake and metabolism of the novel peptide angiotensin-(1-12) by neonatal cardiac myocytes. PLoS One. 2011;6(1):e15759. doi: 10.1371/journal.pone.0015759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad S, Simmons T, Varagic J, Moniwa N, Chappell MC, Ferrario CM. Chymase-dependent generation of angiotensin II from angiotensin-(1-12) in human atrial tissue. PLoS One. 2011;6(12):e28501. doi: 10.1371/journal.pone.0028501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eslami A, Lujan J. J Vis Exp. 44. 2010. Western blotting: sample preparation to detection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dell’Italia LJ, Husain A. Dissecting the role of chymase in angiotensin II formation and heart and blood vessel diseases. Curr Opin Cardiol. 2002 Jul;17(4):374–9. doi: 10.1097/00001573-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Husain A. The chymase-angiotensin system in humans. J Hypertens. 1993 Nov;11(11):1155–9. [PubMed] [Google Scholar]
- 17.Disertori M, Barlera S, Staszewsky L, Latini R, Quintarelli S, Franzosi MG. Systematic Review and Meta-Analysis: Renin-Angiotensin System Inhibitors in the Prevention of Atrial Fibrillation Recurrences. An Unfulfilled Hope. Cardiovasc Drugs Ther. 2011 Oct 19; doi: 10.1007/s10557-011-6346-0. [DOI] [PubMed] [Google Scholar]
- 18.Maeda Y, Inoguchi T, Takei R, Sawada F, Sasaki S, Fujii M, et al. Inhibition of chymase protects against diabetes-induced oxidative stress and renal dysfunction in hamsters. Am J Physiol Renal Physiol. 2010 Dec;299(6):F1328–F1338. doi: 10.1152/ajprenal.00337.2010. [DOI] [PubMed] [Google Scholar]
- 19.Pat B, Chen Y, Killingsworth C, Gladden JD, Shi K, Zheng J, et al. Chymase inhibition prevents fibronectin and myofibrillar loss and improves cardiomyocyte function and LV torsion angle in dogs with isolated mitral regurgitation. Circulation. 2010 Oct 12;122(15):1488–95. doi: 10.1161/CIRCULATIONAHA.109.921619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagata S, Kato J, Kuwasako K, Asami M, Kitamura K. Plasma and tissue concentrations of proangiotensin-12 in rats treated with inhibitors of the renin-angiotensin system. Hypertens Res. 2012 Feb;35(2):234–8. doi: 10.1038/hr.2011.165. [DOI] [PubMed] [Google Scholar]
- 21.Prosser HC, Richards AM, Forster ME, Pemberton CJ. Regional vascular response to ProAngiotensin-12 (PA12) through the rat arterial system. Peptides. 2010 Aug;31(8):1540–5. doi: 10.1016/j.peptides.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Arnold AC, Isa K, Shaltout HA, Nautiyal M, Ferrario CM, Chappell MC, et al. Angiotensin-(1-12) requires angiotensin converting enzyme and AT1 receptors for cardiovascular actions within the solitary tract nucleus. Am J Physiol Heart Circ Physiol. 2010 Sep;299(3):H763–H771. doi: 10.1152/ajpheart.00345.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westwood BM, Chappell MC. Divergent pathways for the angiotensin-(1-12) metabolism in the rat circulation and kidney. Peptides. 2012 Apr 3; doi: 10.1016/j.peptides.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prosser HC, Forster ME, Richards AM, Pemberton CJ. Cardiac chymase converts rat proAngiotensin-12 (PA12) to angiotensin II: effects of PA12 upon cardiac haemodynamics. Cardiovasc Res. 2009 Apr 1;82(1):40–50. doi: 10.1093/cvr/cvp003. [DOI] [PubMed] [Google Scholar]
- 25.Takai S, Shiota N, Sakaguchi M, Muraguchi H, Matsumura E, Miyazaki M. Characterization of chymase from human vascular tissues. Clin Chim Acta. 1997 Sep 8;265(1):13–20. doi: 10.1016/s0009-8981(97)00114-9. [DOI] [PubMed] [Google Scholar]
- 26.Takai S, Jin D, Sakaguchi M, Miyazaki M. Chymase-dependent angiotensin II formation in human vascular tissue. Circulation. 1999 Aug 10;100(6):654–8. doi: 10.1161/01.cir.100.6.654. [DOI] [PubMed] [Google Scholar]
- 27.Dell’Italia LJ, Meng QC, Balcells E, Straeter-Knowlen IM, Hankes GH, Dillon R, et al. Increased ACE and chymase-like activity in cardiac tissue of dogs with chronic mitral regurgitation. Am J Physiol. 1995 Dec;269(6 Pt 2):H2065–H2073. doi: 10.1152/ajpheart.1995.269.6.H2065. [DOI] [PubMed] [Google Scholar]
- 28.Dell’Italia LJ, Meng QC, Balcells E, Wei CC, Palmer R, Hageman GR, et al. Compartmentalization of angiotensin II generation in the dog heart. Evidence for independent mechanisms in intravascular and interstitial spaces. J Clin Invest. 1997 Jul 15;100(2):253–8. doi: 10.1172/JCI119529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urata H, Healy B, Stewart RW, Bumpus FM, Husain A. Angiotensin II-forming pathways in normal and failing human hearts. Circ Res. 1990 Apr;66(4):883–90. doi: 10.1161/01.res.66.4.883. [DOI] [PubMed] [Google Scholar]
- 30.Urata H, Boehm KD, Philip A, Kinoshita A, Gabrovsek J, Bumpus FM, et al. Cellular localization and regional distribution of an angiotensin II-forming chymase in the heart. J Clin Invest. 1993 Apr;91(4):1269–81. doi: 10.1172/JCI116325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Firth JD, Uitto VJ, Putnins EE. Mechanical induction of an epithelial cell chymase associated with wound edge migration. J Biol Chem. 2008 Dec 12;283(50):34983–93. doi: 10.1074/jbc.M801975200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin D, Takai S, Yamada M, Sakaguchi M, Miyazaki M. The functional ratio of chymase and angiotensin converting enzyme in angiotensin I-induced vascular contraction in monkeys, dogs and rats. Jpn J Pharmacol. 2000 Dec;84(4):449–454. doi: 10.1254/jjp.84.449. [DOI] [PubMed] [Google Scholar]
- 33.Kinoshita A, Urata H, Bumpus FM, Husain A. Multiple determinants for the high substrate specificity of an angiotensin II-forming chymase from the human heart. J Biol Chem. 1991 Oct 15;266(29):19192–7. [PubMed] [Google Scholar]
- 34.Kokkonen JO, Saarinen J, Kovanen PT. Angiotensin II formation in the human heart: An ACE or non-ACE-mediated pathway? Ann Med. 1998 Aug;30(Suppl 1):9–13. [PubMed] [Google Scholar]
- 35.Liao Y, Husain A. The chymase-angiotensin system in humans: Biochemistry, molecular biology and potential role in cardiovascular diseases. Can J Cardiol. 1995 Aug;11(Suppl F):13F–19F. [PubMed] [Google Scholar]
- 36.Urata H, Hoffmann S, Ganten D. Tissue angiotensin II system in the human heart. Eur Heart J. 1994 Dec;15(Suppl D):68–78. doi: 10.1093/eurheartj/15.suppl_d.68. [DOI] [PubMed] [Google Scholar]
- 37.Campbell DJ, Zeitz CJ, Esler MD, Horowitz JD. Evidence against a major role for angiotensin converting enzyme-related carboxypeptidase (ACE2) in angiotensin peptide metabolism in the human coronary circulation. J Hypertens. 2004 Oct;22(10):1971–6. doi: 10.1097/00004872-200410000-00020. [DOI] [PubMed] [Google Scholar]
- 38.Ferrario CM. Commentary on Tikellis et al: There is more to discover about angiotensin-converting enzyme. Hypertension. 2003 Mar;41(3):390–1. doi: 10.1161/01.HYP.0000060688.57053.7E. [DOI] [PubMed] [Google Scholar]
- 39.Schneider MP, Hua TA, Bohm M, Wachtell K, Kjeldsen SE, Schmieder RE. Prevention of atrial fibrillation by renin-angiotensin system inhibition a meta-analysis. J Am Coll Cardiol. 2010 May 25;55(21):2299–307. doi: 10.1016/j.jacc.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 40.van den Meiracker AH. No advantage of the combination of ACE-inhibition and angiotensin receptor blockade in patients with high cardiovascular risk. Ned Tijdschr Geneeskd. 2008 Jun 14;152(24):1358–60. [PubMed] [Google Scholar]
- 41.Dell’Italia LJ, Balcells E, Meng QC, Su X, Schultz D, Bishop SP, et al. Volume-overload cardiac hypertrophy is unaffected by ACE inhibitor treatment in dogs. Am J Physiol. 1997 Aug;273(2 Pt 2):H961–H970. doi: 10.1152/ajpheart.1997.273.2.H961. [DOI] [PubMed] [Google Scholar]
- 42.Jin D, Takai S, Yamada M, Sakaguchi M, Kamoshita K, Ishida K, et al. Impact of chymase inhibitor on cardiac function and survival after myocardial infarction. Cardiovasc Res. 2003 Nov 1;60(2):413–20. doi: 10.1016/s0008-6363(03)00535-2. [DOI] [PubMed] [Google Scholar]
- 43.Jin D, Takai S, Okamoto Y, Muramatsu M, Miyazaki M. Chymase-derived angiotensin II and arrhythmias after myocardial infarction. Nihon Yakurigaku Zasshi. 2004 Aug;124(2):77–82. doi: 10.1254/fpj.124.77. [DOI] [PubMed] [Google Scholar]
- 44.Kokkonen JO, Lindstedt KA, Kovanen PT. Role for chymase in heart failure: Angiotensin II-dependent or independent mechanisms? Circulation. 2003 May 27;107(20):2522–4. doi: 10.1161/01.CIR.0000074786.92067.AA. [DOI] [PubMed] [Google Scholar]
- 45.Li M, Liu K, Michalicek J, Angus JA, Hunt JE, Dell’Italia LJ, et al. Involvement of chymase-mediated angiotensin II generation in blood pressure regulation. J Clin Invest. 2004 Jul;114(1):112–20. doi: 10.1172/JCI20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsumoto T, Wada A, Tsutamoto T, Ohnishi M, Isono T, Kinoshita M. Chymase inhibition prevents cardiac fibrosis and improves diastolic dysfunction in the progression of heart failure. Circulation. 2003 May 27;107(20):2555–8. doi: 10.1161/01.CIR.0000074041.81728.79. [DOI] [PubMed] [Google Scholar]
- 47.Urata H, Nishimura H, Ganten D. Mechanisms of angiotensin II formation in humans. Eur Heart J. 1995 Dec;16(Suppl N):79–85. doi: 10.1093/eurheartj/16.suppl_n.79. [DOI] [PubMed] [Google Scholar]