Abstract
Background
Perfluoroalkyl substances (PFASs) are widespread pollutants that have been associated with adverse health effects although not on a consistent basis. Diet has been considered the main source of exposure. The aim of the present study was to identify determinants of four plasma PFASs in pregnant Norwegian women.
Methods
This study is based in the Norwegian Mother and Child Cohort Study (MoBa) conducted by the Norwegian Institute of Public Health. Our sample included 487 women who enrolled in MoBa from 2003–2004. A questionnaire regarding sociodemographic, medical, and reproductive history was completed at 17 weeks gestation and a dietary questionnaire was completed at 22 weeks gestation. Maternal plasma samples were obtained around 17 weeks of gestation. Plasma concentrations of four PFASs (perfluorooctane sulfonate (PFOS), perfluorooctanoate (PFOA), perfluorohexane sulfonate (PFHxS), and perfluorononanoate (PFNA)) were examined in relation to demographic, lifestyle, dietary, and pregnancy-related covariates. Predictors were identified by optimizing multiple linear regression models using Akaike’s information criterion (AIC).
Results
Parity was the determinant with the largest influence on plasma PFAS concentrations, with r2 between 0.09 and 0.32 in simple regression models. In optimal multivariate models, when compared to nulliparous women, parous women had 46%, 70%, 19%, and 62% lower concentrations of PFOS, PFOA, PFHxS, and PFNA respectively (p<0.001 except for PFHxS, p<0.01). In all these models, duration of breastfeeding was associated with reduced PFAS levels. PFOA showed the largest reduction from breastfeeding, with a 2–3% reduction per month of breastfeeding in typical cases. Levels of PFOS, PFOA, and PFNA increased with time since most recent pregnancy. While pregnancy-related factors were the most important predictors, diet was a significant factor explaining up to 4% of the variance. One quartile increase in estimated dietary PFAS intake was associated with plasma PFOS, PFOA, PFHxS, and PFNA concentration increases of 7.2%, 3.3%, 5.8% and 9.8%, respectively, resulting in small, although non-trivial absolute changes in PFAS concentrations.
Conclusion
The history of previous pregnancies and breastfeeding were the most important determinants of PFASs in this sample of pregnant women.
Keywords: Perfluoroalkyl substances, reproductive history, pregnancy, dietary exposure
1. Introduction
Perfluoroalkyl substances (PFASs) have been manufactured for over 50 years for use in a variety of consumer products such as stain repellents, leather and fabric coatings, paper coatings, and in fire-fighting foams (Kovarova and Svobodova, 2008). These substances are characterized by a fully fluorinated carbon chain of varying length attached to a functional end group. Most PFASs are highly persistent and have long half lives in the environment and humans. For example, two of the most common PFASs, perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA), have median half-lives in human blood of approximately 2–5 years (Bartell et al., 2010; Olsen et al., 2007). These substances have a high binding affinity for albumin and are thus found primarily in blood, liver, and kidney (Jones et al., 2003; Zhang et al., 2009). Since the year 2000, data from Europe and the US indicate a clear decline in blood levels of PFOS, but a less dramatic decline in PFOA levels (Glynn et al., 2012; Haug et al., 2009b; Kato et al., 2009; Schröter-Kermani et al., 2012). These data are consistent with a phase-out of PFOS by the major manufacturer between 2000 and 2002 and a more gradual phase-out plan for PFOA (United States Environmental Protection Agency, 2010).
Although plasma levels of PFASs are decreasing in human populations, whether they are related to health outcomes in humans is under active investigation (Halldorsson et al., 2012; Melzer et al., 2010; Shankar et al., 2012). Of particular relevance here are the many epidemiologic studies among reproductive-aged women (Fei et al., 2010; Maisonet et al., 2012; Vestergaard et al., 2012; Whitworth et al., 2012a; Whitworth et al., 2012b), in which a complete understanding of factors affecting plasma levels could help reduce bias.
PFOS and PFOA have been detected in numerous food items (Haug et al., 2010a; Schecter et al., 2010) and diet is considered an important determinant of PFASs body burden (Domingo, 2012; Trudel et al., 2008). Recent studies of estimated PFOS and PFOA exposure demonstrated that consumption of food is likely to be an important source among background exposed adult populations (Haug et al., 2011a; Trudel et al., 2008). Previous studies of determinants of PFAS concentrations have focused primarily on the contribution from diet, and the results have been inconsistent. Halldorsson et al (2008) found that red meat, animal fats, and snack foods were the most important determinants of plasma PFAS levels in a sample of Danish pregnant women, while Rylander et al. (2009) and Haug et al. (2010b) both identified seafood as the most important dietary predictor in separate samples of the Norwegian population.
Few previous studies have included a thorough investigation of the contribution of factors other than diet to PFAS plasma levels. Because PFOS and PFOA cross the placenta (Fromme et al., 2010; Gützkow et al., 2012) and are found in breast milk (Haug et al., 2011a; Thomsen et al., 2010), the impact of pregnancy-related variables on women’s PFASs body burden are of particular interest. Several studies report on the importance of breast milk consumption in determining infants’ PFASs exposure (Haug et al., 2011a; Liu et al., 2011; Thomsen et al., 2010; Trudel et al., 2008). Therefore, one must consider the impact of breastfeeding on reducing the maternal body burden. As the epidemiologic literature surrounding potential adverse health effects of PFASs expands, it becomes increasingly important to carry out more comprehensive studies of determinants of PFAS levels. Such knowledge will provide a better understanding of important routes of PFASs exposure, but will also provide insight into the most important covariates for inclusion in epidemiologic investigations of associations between exposure and disease.
The aim of the present study was to identify determinants of plasma PFAS concentrations in a sample of pregnant Norwegian women.
2. Materials and methods
2.1 Study population and design
The present study is based on the Norwegian Mother and Child Cohort study (MoBa), initiated by and maintained at the Norwegian Institute of Public Health (Magnus et al., 2006). In brief, MoBa is a nation-wide pregnancy cohort that included 108,000 pregnancies enrolled from 1999 to 2008. Pregnant women were recruited through a postal invitation in connection with a routine ultrasound examination offered to all women around 17 weeks of gestation. Overall, 38.5% of invited women consented to participate. The women were asked to provide biological samples and to answer questionnaires during pregnancy. Pregnancy and birth records from the Medical Birth Registry of Norway (MBRN) were linked with the MoBa database (Magnus et al., 2006). The study was approved by the Regional Committee for Ethics in Medical Research and the Norwegian Data Inspectorate, and informed consent was obtained from each participant before the study.
The present study was based on MoBa data file version 4.301 and used data from MoBa subjects included in a prior case-base study of PFASs and subfecundity (Whitworth et al., 2012b). In the previous study, two groups were selected from all MoBa subjects who: enrolled in 2003–2004, delivered a live born child, provided a plasma sample at enrolment, and reported time to pregnancy. The first group (the base group) consisted of a random sample (n=550), while the second group (the case group) was a random sample of subfecund (time-to-pregnancy >12 months) women (n=400). Only the subjects selected in the base group are included in the primary analyses of the present study. Further, we also excluded women who did not complete the MoBa food frequency questionnaire (FFQ) (n=5) and women who reported energy intakes outside the valid range (n=4) of 4.5 to 20 MJ/day, previously established for MoBa (Meltzer et al., 2008). Of the remaining 541 women, 487 had complete data on all covariates and were included in the analysis. Among the 63 excluded women, the proportion of women with low educational attainment was larger than among the included women (50% versus 33% in the two lower education categories), but there were no differences between the groups with regard to other sociodemographic, life style or pregnancy related variables, or PFAS concentrations (data not shown).
2.2 Blood sampling and PFAS analyses
Maternal plasma samples were collected at the time of enrolment and shipped from the collection site to Oslo by mail at ambient temperature. Because PFASs are chemically stable, changes in PFAS plasma concentrations while in transit are believed to be negligible (Frömel and Knepper, 2010). At the Biorepository, plasma was separated, aliquoted and stored at −80 degrees Celsius. Although most of the existing epidemiologic literature regarding PFASs focuses on PFOS and PFOA, our analytical method is sensitive and can detect as many as 19 PFASs. Concentrations of PFOS, PFOA, perfluorohexane sulfonate (PFHxS), and perfluorononanoate (PFNA) and nine other PFASs were detected in 150 μl of plasma using high performance liquid chromatography/tandem mass spectrometry at the Norwegian Institute of Public Health. Details of the analytical method have been published elsewhere (Haug et al., 2009a). A total of 50 quality assurance/quality control (QA/QC) plasma samples from a single pool were analysed in 17 sample batches alongside the specimens. The laboratory technicians were blinded to their identity, and the QA/QC samples were indistinguishable from the plasma samples from MoBa subjects. The median concentrations of PFOS, PFOA, PFHxS and PFNA in the (QA/QC) plasma samples in the 50 pooled specimens were 19.3 ng/mL and 3.7 ng/mL, 2.4 and 0.62, respectively. For PFOS, the within-batch correlation of variation was 4.5% and the between-batch correlation of variation was 11.3%. For PFOA, the within-batch correlation of variation was 3.5% and the between-batch correlation of variation was 6.7%. For PFNA, the within-batch correlation of variation was 6.0% and the between-batch correlation of variation was 15.6%. For PFHxS, the within-batch correlation of variation was 3.9% and the between-batch correlation of variation was 13.2%. Values of PFOS, PFOA and PFNA were found in all plasma samples, while two values of PFHxS were below the limit of quantification (0.05 ng/ml). We chose to include in our analyses the four PFASs most frequently detected.
2.3 Questionnaires
Data on individual characteristics and dietary intakes were obtained from detailed self-administered questionnaires (available online at http://www.fhi.no/eway/?pid=238). The first questionnaire (Q1), completed at enrolment around gestational week 17, covered information about sociodemographic, lifestyle and health factors, including data regarding previous pregnancies. The second questionnaire (Q2), completed around gestational week 22, was a FFQ designed to capture dietary habits and intake of dietary supplements during the first four to five months of pregnancy, and has been described in detail elsewhere (Meltzer et al., 2008).
2.4 Dietary variables
The FFQ included questions about intake of 255 food items or dishes, with a special emphasis on seafood items. For each food item, the frequency of consumption was reported by selecting one out of 8–10 frequencies, ranging from never to several times monthly, weekly or daily. Consumption frequencies were converted into food amounts (g/day) by the use of standard Norwegian portion sizes. Food and nutrient calculations were performed with the use of FoodCalc software (Lauritsen, 2005) and the Norwegian food composition table (Norwegian Food Safety Authority et al., 2006). The FFQ has been previously validated (Brantsæter et al., 2008a; Brantsæter et al., 2010). The food items in the FFQ were first combined into 100 non-overlapping food groups based on structure, nutrient profile or culinary usage as in previous MoBa studies (Brantsæter et al., 2008b; Brantsæter et al., 2012). Food groups were further combined into 13 non-overlapping groups based on relevance and previous knowledge about potential dietary sources of PFASs (Halldorsson et al., 2008; Haug et al., 2010b). Estimated maternal dietary intakes of PFOS (ng/day) and PFOA (ng/day) were calculated using values of PFOS and PFOA concentrations previously measured in samples of Norwegian food items and drinking water (Haug et al., 2010a). Although values for PFHxS and PFNA have been measured in some Norwegian food items and drinking water, these substances were present in much lower concentrations than FPOS and PFOA and dietary intakes were not calculated.
2.5 Other variables
We examined the influence of numerous non-dietary variables on PFAS concentrations. Sociodemographic and lifestyle variables included: age at delivery (<25, 25–29, 30–34, 35+ years), marital status (married/cohabitating vs. single), length of education (<12, 12, 13–16, 17+ years), urban vs. rural residence as defined according to definitions obtained from Statistics Norway, smoking prior to pregnancy (non-smokers, occasional smokers and daily smokers), alcohol use during pregnancy (yes vs. no), type of housing (flat, farm, or detached), and native language (Norwegian vs. other). Additionally, self-reported height and pre-pregnancy weight were used to calculate body mass index (BMI; kg/m2). BMI was then divided into four categories defined by the World Health Organization (<18.5, 18.5–24.9, 25–29.9, 30+ kg/m2). Maternal and paternal combined annual income was also included (both <300,000, one ≥300,000, and both ≥300,000 NOK). In addition, we examined several pregnancy-related variables including: parity (nulliparous, 1, 2, 3+), pregnancy weight gain at 17 weeks (decreased weight, no change, 1–3, 4+ kg gain), gestational week of blood draw (<16, 17–18, 19–20, 21+ weeks), and marine fatty acids (n-3) supplement use (yes vs. no). For parous women, the duration of breastfeeding was reported in Q1 for each child born prior to the present pregnancy, and the total number of months spent breastfeeding all previous children (referred to as total breastfeeding duration) was calculated. Birth dates for older children were obtained from the MBRN and the time interval since the most recent pregnancy, defined as the number of months from the birth of the previous child to conception of the index child, was calculated for each woman.
2.6 Statistical methods
All results reported are based on a complete case analysis. Because the distributions of PFAS concentrations were skewed, all analyses were based on the natural log transformations (ln) of the PFAS variables. In univariate analyses, the examination of linear trend for ordered categorical variables was based on the Wald test for the categorical variable included as an ordinal term in the regression model, while for nominal categorical variables, we examined differences between categories using the Kruskal-Wallis test.
Dietary data were explored using both continuous variables as well as ranked categories. All dietary variables were energy adjusted by the residual method (Willett et al., 1997).
Determinants of PFAS concentrations were identified separately for PFOS, PFOA, PFHxS, and PFNA. We applied a multiple regression approach starting with all covariates (16–20, including candidates for interaction terms) in the model. We then applied stepwise backward variable exclusion using Akaike’s information criterion (AIC) for (Akaike-) optimal model fit. AIC is defined by the following formula AIC=2k-ln(L), where k is the number of parameters in the model, and L is the value of the likelihood function for the estimated model. Minimizing the AIC value provides a means for model selection that is optimal, where the reward for goodness of fit is balanced with a penalty for increasing the number of parameters (Claeskens and Hjort, 2008). Typically, with several hundreds of cases, the AIC-optimal model will include terms that are not significantly different from zero in the ordinary p<0.05 sense. However, with our data, the AIC approach was similar in effect to selecting optimal models with candidate variables having p values < 0.10, and occasionally the standard penalty for including variables was increased to exclude factors having little influence. Model fit was additionally assessed using several diagnostic plots.
We also performed two sensitivity analyses. In the first, we repeated the main analyses using the multiple imputations option in SPSS software to deal with missing data on covariates (n=54). Also, to examine whether subjects’ selection for the previous study of subfecundity (case vs. base) affected our results, we repeated the analyses including the 400 subjects selected in the case group and controlled for subject selection (case vs. base).
For the multiple regression models with ln(PFAS) as the dependent variable, beta values correspond to the expected geometric mean of the non-transformed outcome variable associated with the independent variable, relative to the mean in the referent category of the independent variable, where [(exp(beta)]−1)*100 is the % change in PFAS for a one unit change in the independent variable.
We used predictive analytics software (PASW) for Windows, version 17.0 (IBM® SPSS®, USA) for descriptive statistical analyses and the statistical software R, version 2.11.0 to fit optimized multiple regression models. A significance level of p=0.05 was used if not otherwise stated.
3. Results
3.1 Descriptive statistics
PFOS, PFOA and PFNA were detectable in all women, while two women had PFHxS values below the limit of quantification (0.05 ng/ml) and were excluded. The median plasma concentrations were as follows: PFOS: 12.8 ng/ml (mean, 13.7 ng/ml; interquartile range (IQR), 10.1–16.6 ng/ml), PFOA: 2.11 ng/ml (mean, 2.35 ng/ml; IQR, 1.54–2.93 ng/ml), PFHxS: 0.60 ng/ml (mean, 0.76 ng/ml; IQR, 0.43–0.86 ng/ml), and PFNA: 0.39 ng/ml (mean, 0.42 ng/ml; IQR, 0.28–0.51 ng/ml).
Plasma concentrations of all four PFAS increased with maternal educational attainment and household income, while the direction of the correlation with other demographic and lifestyle variables differed by substance (Table 1). Concentrations of PFOS and PFOA increased with age. Plasma PFOA concentration was higher in smokers than in non-smokers. The median concentration of each PFAS was highest in the obese group, but the increase with BMI was statistically significant only for PFHxS. The concentrations of PFOA, PFHxS, and PFNA were lower in women living in rural vs. urban areas, and in women living in farmhouses vs. other housing. All plasma PFAS concentrations were strongly correlated with the pregnancy related variables: parity, time interval since the most recent pregnancy, and total breastfeeding duration (Table 1).
Table 1.
Unadjusted plasma PFAS concentrations (ng/ml) by maternal demographic, lifestyle, and pregnancy related characteristics.
| PFOS | PFOA | PFHxS3 | PFNA | |||
|---|---|---|---|---|---|---|
|
| ||||||
| N | % | Median (ng/mL) | ||||
| Demographic variables | ||||||
| Maternal age at delivery | ||||||
| < 25 years | 32 | 6.6 | 12.9 | 2.70 | 0.60 | 0.39 |
| 25 – 29 years | 161 | 33.1 | 13.9 | 2.40 | 0.62 | 0.40 |
| 30 – 34 years | 232 | 47.6 | 11.9 | 1.91 | 0.56 | 0.38 |
| 35+ years | 62 | 12.7 | 12.8 | 1.93 | 0.60 | 0.44 |
| P-value 1 | 0.015 | <0.01 | 0.27 | 0.77 | ||
| Marital status | ||||||
| Married/cohabitant | 480 | 98.6 | 12.8 | 2.11 | 0.60 | 0.40 |
| Single | 7 | 1.4 | 10.3 | 2.12 | 0.38 | 0.24 |
| P-value 2 | 0.15 | 0.88 | 0.40 | 0.12 | ||
| Maternal education | ||||||
| <12 years | 31 | 6.4 | 9.9 | 1.87 | 0.43 | 0.28 |
| 12 years | 127 | 26.1 | 12.1 | 2.06 | 0.55 | 0.35 |
| 13 – 16 years | 228 | 46.8 | 13.2 | 2.14 | 0.62 | 0.42 |
| 17 + years | 101 | 20.7 | 13.8 | 2.32 | 0.65 | 0.42 |
| P-value 2 | <0.01 | 0.02 | <0.01 | <0.01 | ||
| Residence | ||||||
| Rural | 254 | 52.2 | 12.4 | 1.95 | 0.54 | 0.37 |
| Urban | 233 | 47.8 | 13.3 | 2.28 | 0.65 | 0.41 |
| P-value 2 | 0.16 | 0.01 | <0.001 | 0.01 | ||
| Native language | ||||||
| Norwegian | 445 | 91.4 | 12.8 | 2.14 | 0.60 | 0.40 |
| Other | 42 | 8.6 | 12.2 | 1.91 | 0.62 | 0.39 |
| P-value 2 | 0.84 | 0.78 | 0.47 | 0.68 | ||
| Lifestyle variables | ||||||
| Smoking prior to pregnancy | ||||||
| Non smoker | 351 | 72.1 | 12.8 | 1.99 | 0.60 | 0.40 |
| Occasional smoker | 49 | 10.1 | 13.8 | 2.12 | 0.60 | 0.43 |
| Daily smoker | 87 | 17.9 | 12.0 | 2.30 | 0.64 | 0.37 |
| P-value 2 | 0.23 | 0.02 | 0.42 | 0.27 | ||
| Alcohol during pregnancy | ||||||
| No | 389 | 79.9 | 12.8 | 2.11 | 0.59 | 0.39 |
| Yes | 98 | 20.1 | 13.0 | 2.04 | 0.66 | 0.40 |
| P-value 2 | 0.74 | 0.73 | 0.08 | 0.09 | ||
| Pre-pregnancy BMI, | ||||||
| <18.5 kg/m2 | 10 | 2.1 | 10.7 | 1.88 | 0.45 | 0.29 |
| 18.5 – 24.9 kg/m2 | 311 | 63.9 | 12.3 | 2.06 | 0.60 | 0.39 |
| 25 – 29.9 kg/m2 | 113 | 23.2 | 13.1 | 2.08 | 0.60 | 0.38 |
| 30+ kg/m2 | 53 | 10.9 | 14.2 | 2.40 | 0.67 | 0.43 |
| P-value 1 | 0.13 | 0.30 | 0.01 | 0.22 | ||
| Income | ||||||
| Both <300 000 NOK | 129 | 26.5 | 12.5 | 2.16 | 0.59 | 0.36 |
| One ≥300 000 NOK | 233 | 47.8 | 12.8 | 1.99 | 0.57 | 0.38 |
| Both ≥300 000 NOK | 125 | 25.7 | 13.3 | 2.41 | 0.67 | 0.44 |
| P-value 1 | 0.02 | 0.02 | 0.04 | <0.01 | ||
| House | ||||||
| Flat, semidetached | 191 | 39.2 | 13.7 | 2.38 | 0.67 | 0.41 |
| Farm | 28 | 5.7 | 12.4 | 1.68 | 0.48 | 0.34 |
| Detached | 268 | 55.0 | 12.6 | 1.94 | 0.56 | 0.39 |
| P-value 2 | 0.38 | <0.001 | <0.001 | 0.06 | ||
| Pregnancy related variables | ||||||
| Parity | ||||||
| Nulliparous | 207 | 42.5 | 14.8 | 2.90 | 0.71 | 0.45 |
| 1 | 194 | 39.8 | 11.6 | 1.67 | 0.52 | 0.34 |
| 2 | 69 | 14.2 | 11.3 | 1.82 | 0.47 | 0.34 |
| 3+ | 17 | 3.5 | 12.4 | 1.66 | 0.49 | 0.30 |
| P-value 1 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Time interval since most recent pregnancy | ||||||
| <12 months | 15 | 3.1 | 9.3 | 1.02 | 0.46 | 0.20 |
| 12 – 24 months | 73 | 15.0 | 10.7 | 1.44 | 0.48 | 0.30 |
| 2 – 4 years | 72 | 14.8 | 11.4 | 1.61 | 0.49 | 0.34 |
| 4+ years | 120 | 24.6 | 12.4 | 2.97 | 0.54 | 0.40 |
| Nulliparous | 207 | 42.5 | 14.8 | 2.90 | 0.71 | 0.45 |
| P-value 1 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Total breastfeeding duration | ||||||
| Nulliparous | 207 | 42.5 | 14.8 | 2.90 | 0.71 | 0.45 |
| No | 16 | 3.3 | 13.4 | 2.07 | 0.62 | 0.40 |
| 1 – 12 months | 140 | 28.7 | 12.1 | 1.80 | 0.54 | 0.36 |
| 13 – 24 months | 102 | 20.9 | 10.7 | 1.53 | 0.49 | 0.30 |
| >24 mo | 22 | 4.5 | 10.9 | 1.53 | 0.42 | 0.32 |
| P-value 1 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Weight change week at 17 | ||||||
| Decreased weight | 48 | 9.9 | 12.8 | 2.20 | 0.53 | 0.42 |
| No change | 34 | 7.0 | 12.8 | 2.46 | 0.70 | 0.37 |
| 1–3 kg gain | 213 | 43.7 | 13.4 | 2.00 | 0.61 | 0.39 |
| 4+ kg gain | 192 | 39.4 | 12.2 | 2.08 | 0.60 | 0.40 |
| P-value 1 | 0.35 | 0.47 | 0.98 | 0.87 | ||
| Gestational age blood draw | ||||||
| <16 w | 98 | 20.1 | 13.8 | 2.06 | 0.65 | 0.40 |
| 17–18 w | 188 | 38.6 | 12.3 | 2.07 | 0.60 | 0.39 |
| 19–20 w | 162 | 33.3 | 13.0 | 2.17 | 0.59 | 0.40 |
| 21+ | 39 | 8.0 | 12.9 | 2.06 | 0.60 | 0.39 |
| P-value 1, 2 | 0.63 | 0.99 | 0.58 | 0.71 | ||
| Nausea with vomiting (week 22) | ||||||
| No | 357 | 73.3 | 12.8 | 2.11 | 0.61 | 0.39 |
| Yes | 130 | 26.7 | 12.7 | 2.11 | 0.60 | 0.40 |
| P-value 2 | 0.81 | 0.81 | 0.40 | 0.99 | ||
| Marine fatty acids supplement use (fish oil/fish liver oil) | ||||||
| No | 229 | 47.0 | 12.5 | 1.99 | 0.59 | 0.37 |
| Yes | 258 | 53.0 | 13.1 | 2.28 | 0.62 | 0.41 |
| P-value 2 | 0.18 | 0.02 | 0.12 | <0.01 | ||
P-value represents the linear trend from an ordinary linear model (Wald test) and ln(PFAS)
P-value represents the difference between groups based on the Kruskall-Wallis test
For PFHxS n=485
The median estimated dietary intakes of PFOS and PFOA were 44.6 ng/day (0.66 ng/kg bw/day) and 34.2 ng/day (0.52 ng/kg bw/day), respectively. Estimated intakes of both PFOS and PFOA were statistically significantly correlated with plasma PFOS and PFOA as well as with PFHxS and PFNA concentrations, indicating that the four PFASs share similar dietary sources (Table 2).
Table 2.
Spearman correlations between estimated dietary intake of PFOS and PFOA and PFAS plasma concentrations, n=487
| Plasma concentrations
|
|||||
|---|---|---|---|---|---|
| PFOS | PFOA | PFHxS2 | PFNA | ||
| Estimated intakes1 | Median | Spearman correlation coefficients r (95% CI) | |||
| PFOS | 44.6 ng/day | 0.25 (0.16, 0.33) | 0.13 (0.04, 0.22) | 0.16 (0.07, 0.25) | 0.26 (0.17, 0.34) |
| PFOA | 34.2 ng/day | 0.23 (0.14, 0.31) | 0.17 (0.09, 0.26) | 0.22 (0.13, 0.30) | 0.28 (0.20, 0.36) |
PFOS and PFOA estimated from food intakes by FFQ and a database of PFAS concentrations in Norwegian food items
For PFHxS n=485
In unadjusted analyses, all plasma PFAS concentrations were associated with intakes of shellfish and oily fish, but the associations were relatively weak, and differed according to parity. Associations between plasma PFASs and the other food groups varied even more (Table 3). Plasma PFOS levels were not correlated with any food group other than seafood. Plasma PFOA levels correlated positively with the intake of oils in nulliparous subjects. Plasma PFHxS correlated positively with oils in nulliparous subjects, and plasma PFNA correlated positively with eggs, vegetables (nulliparous subjects), and oils. In most cases, with positive associations, the relative differences between medians of PFAS levels in the highest and lowest intake quartiles in a food group were 20–30% or less.
Table 3.
Unadjusted plasma PFAS concentrations (median, ng/ml) in nulliparous and parous women, by quartiles of food consumption. N= 487
| PFOS | PFOA | PFHxS | PFNA | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Food group1 | pari=0 | pari>0 | pari=0 | pari>0 | pari=0 | pari>0 | pari=0 | pari>0 |
| Shellfish, g/day | ||||||||
| Quartile 1 (0, 0.1) | 12.9 | 10.8 | 2.84 | 1.56 | 0.67 | 0.49 | 0.39 | 0.33 |
| Quartile 2 (0.1, 2.7) | 13.4 | 10.8 | 2.99 | 1.71 | 0.64 | 0.54 | 0.44 | 0.31 |
| Quartile 3 (2.7, 5.4) | 15.6 | 12.0 | 2.90 | 1.75 | 0.78 | 0.50 | 0.48 | 0.34 |
| Quartile 4 (5.4+) | 16.6 | 12.1 | 3.02 | 1.83 | 0.92 | 0.51 | 0.50 | 0.37 |
| P-value 3 | 0.001 | 0.07 | 0.08 | 0.05 | <0.001 | 0.39 | <0.001 | 0.02 |
| Oily fish, g/day | ||||||||
| Quartile 1 (0, 4.0) | 14.1 | 10.0 | 2.98 | 1.69 | 0.69 | 0.48 | 0.41 | 0.29 |
| Quartile 2 (4.0, 8.8) | 13.4 | 10.9 | 2.98 | 1.62 | 0.66 | 0.50 | 0.45 | 0.34 |
| Quartile 3 (8.8, 16) | 15.9 | 12.0 | 2.90 | 1.73 | 0.87 | 0.51 | 0.53 | 0.33 |
| Quartile 4 (16+) | 15.2 | 12.6 | 2.87 | 1.87 | 0.74 | 0.57 | 0.45 | 0.39 |
| P-value 3 | 0.08 | 0.001 | 0.69 | 0.13 | 0.14 | 0.02 | 0.04 | <0.001 |
| Lean fish, g/day | ||||||||
| Quartile 1 (0, 10) | 13.4 | 11.5 | 2.88 | 1.84 | 0.70 | 0.48 | 0.45 | 0.33 |
| Quartile 2 (10, 18) | 15.2 | 12.5 | 3.11 | 1.63 | 0.71 | 0.50 | 0.45 | 0.36 |
| Quartile 3 (19, 29) | 16.1 | 11.8 | 2.90 | 1.70 | 0.70 | 0.55 | 0.47 | 0.33 |
| Quartile 4 (29+) | 15.8 | 10.8 | 2.62 | 1.62 | 0.83 | 0.49 | 0.43 | 0.33 |
| P-value 3 | 0.09 | 0.20 | 0.42 | 0.14 | 0.98 | 0.96 | 0.50 | 0.62 |
| Eggs, g/day | ||||||||
| Quartile 1 (0, 4.5) | 13.9 | 10.1 | 2.91 | 1.63 | 0.66 | 0.51 | 0.42 | 0.32 |
| Quartile 2 (4.5, 7.4) | 16.1 | 11.3 | 3.09 | 1.74 | 0.85 | 0.53 | 0.44 | 0.31 |
| Quartile 3 (7.5, 10) | 14.0 | 11.9 | 2.69 | 1.64 | 0.68 | 0.48 | 0.46 | 0.33 |
| Quartile 4 (10+) | 15.9 | 11.4 | 3.02 | 1.82 | 0.83 | 0.52 | 0.50 | 0.37 |
| P-value 3 | 0.74 | 0.10 | 0.75 | 0.38 | 0.19 | 0.18 | 0.10 | 0.01 |
| Poultry, g/day | ||||||||
| Quartile 1 (0, 12) | 14.5 | 12.2 | 2.92 | 1.71 | 0.74 | 0.48 | 0.48 | 0.30 |
| Quartile 2 (12, 18) | 13.9 | 11.3 | 2.81 | 1.71 | 0.66 | 0.47 | 0.42 | 0.33 |
| Quartile 3 (18, 30) | 16.1 | 11.9 | 2.98 | 1.80 | 0.85 | 0.55 | 0.47 | 0.35 |
| Quartile 4 (30+) | 14.8 | 10.9 | 2.97 | 1.63 | 0.71 | 0.55 | 0.44 | 0.34 |
| P-value 3 | 0.91 | 0.91 | 0.90 | 0.59 | 0.52 | 0.04 | 0.82 | 0.15 |
| Beef, g/day | ||||||||
| Quartile 1 (0, 14) | 14.5 | 10.9 | 2.95 | 1.69 | 0.85 | 0.60 | 0.44 | 0.37 |
| Quartile 2 (14, 21) | 15.7 | 11.6 | 2.90 | 1.64 | 0.73 | 0.51 | 0.46 | 0.33 |
| Quartile 3 (22, 29) | 14.7 | 12.1 | 2.95 | 1.70 | 0.81 | 0.49 | 0.45 | 0.34 |
| Quartile 4 (30+) | 13.7 | 11.2 | 2.87 | 1.78 | 0.67 | 0.47 | 0.47 | 0.31 |
| P-value 3 | 0.31 | 0.71 | 0.70 | 0.23 | 0.23 | 0.01 | 0.82 | 0.66 |
| Pork, g/day | ||||||||
| Quartile 1 (0, 7.5) | 14.4 | 11.3 | 2.85 | 1.60 | 0.66 | 0.46 | 0.43 | 0.32 |
| Quartile 2 (7.6, 13) | 14.8 | 11.9 | 3.06 | 1.69 | 0.68 | 0.53 | 0.44 | 0.34 |
| Quartile 3 (13, 20) | 15.5 | 11.8 | 2.86 | 1.76 | 0.77 | 0.52 | 0.46 | 0.34 |
| Quartile 4 (21+) | 14.4 | 11.2 | 3.05 | 1.77 | 0.80 | 0.53 | 0.47 | 0.35 |
| P-value 3 | 0.64 | 0.91 | 0.91 | 0.22 | 0.10 | 0.13 | 0.82 | 0.16 |
| Milk/dairy, g/day | ||||||||
| Quartile 1 (0, 145) | 13.5 | 11.7 | 3.04 | 1.84 | 0.67 | 0.59 | 0.44 | 0.36 |
| Quartile 2 (146, 310) | 15.7 | 10.7 | 2.92 | 1.81 | 0.84 | 0.47 | 0.50 | 0.31 |
| Quartile 3 (312, 488) | 14.8 | 11.7 | 2.97 | 1.69 | 0.71 | 0.50 | 0.48 | 0.34 |
| Quartile 4 (489+) | 15.3 | 12.1 | 2.81 | 1.54 | 0.68 | 0.49 | 0.41 | 0.33 |
| P-value 3 | 0.63 | 0.48 | 0.52 | 0.05 | 0.16 | 0.58 | 0.72 | 0.99 |
| Bread, g/day | ||||||||
| Quartile 1 (0, 178) | 13.8 | 10.7 | 3.05 | 1.58 | 0.64 | 0.50 | 0.41 | 0.31 |
| Quartile 2 (180, 220) | 15.2 | 11.9 | 3.06 | 1.82 | 0.82 | 0.51 | 0.48 | 0.36 |
| Quartile 3 (220, 265) | 16.9 | 11.9 | 2.87 | 1.66 | 0.77 | 0.55 | 0.48 | 0.33 |
| Quartile 4 (266+) | 13.6 | 11.8 | 2.68 | 1.84 | 0.74 | 0.48 | 0.42 | 0.35 |
| P-value 3 | 0.80 | 0.37 | 0.17 | 0.50 | 0.38 | 0.28 | 0.78 | 0.10 |
| Fruit/berries, g/day | ||||||||
| Quartile 1 (0, 158) | 14.6 | 11.2 | 2.82 | 1.56 | 0.68 | 0.49 | 0.39 | 0.33 |
| Quartile 2 (158, 244) | 15.2 | 11.5 | 3.10 | 1.63 | 0.78 | 0.50 | 0.46 | 0.33 |
| Quartile 3 (245, 342) | 14.5 | 12.0 | 2.87 | 1.80 | 0.82 | 0.55 | 0.48 | 0.34 |
| Quartile 4 (343+) | 14.3 | 11.9 | 2.92 | 1.88 | 0.71 | 0.49 | 0.47 | 0.34 |
| P-value 3 | 0.79 | 0.66 | 0.49 | 0.08 | 0.10 | 0.31 | 0.10 | 0.52 |
| Vegetables, g/day | ||||||||
| Quartile 1 (0, 97) | 13.7 | 11.3 | 2.87 | 1.82 | 0.68 | 0.49 | 0.39 | 0.32 |
| Quartile 2 (98, 143) | 14.7 | 11.9 | 2.85 | 1.64 | 0.67 | 0.50 | 0.43 | 0.34 |
| Quartile 3 (143, 203) | 15.2 | 11.4 | 3.29 | 1.56 | 0.74 | 0.44 | 0.54 | 0.31 |
| Quartile 4 (204+) | 15.6 | 12.2 | 3.05 | 1.81 | 0.88 | 0.55 | 0.46 | 0.36 |
| P-value 3 | 0.41 | 0.18 | 0.14 | 0.37 | 0.02 | 0.45 | 0.04 | 0.34 |
| Oils, g/day | ||||||||
| Quartile 1 (0, 1.0) | 14.7 | 10.5 | 2.78 | 1.59 | 0.62 | 0.43 | 0.42 | 0.27 |
| Quartile 2 (1.0, 1.5) | 15.4 | 11.7 | 2.80 | 1.66 | 0.74 | 0.49 | 0.42 | 0.33 |
| Quartile 3 (1.5, 3.2) | 14.5 | 11.9 | 3.14 | 1.74 | 0.70 | 0.53 | 0.45 | 0.39 |
| Quartile 4 (3.2+) | 14.7 | 11.8 | 3.08 | 1.81 | 0.90 | 0.55 | 0.51 | 0.36 |
| P-value 3 | 0.46 | 0.11 | 0.07 | 0.48 | 0.001 | 0.05 | 0.02 | 0.04 |
| Butter/margarine, g/day | ||||||||
| Quartile 1 (0, 5.1) | 15.1 | 10.8 | 2.86 | 1.63 | 0.77 | 0.54 | 0.44 | 0.34 |
| Quartile 2 (5.2, 13) | 15.7 | 12.8 | 3.06 | 1.89 | 0.73 | 0.51 | 0.48 | 0.39 |
| Quartile 3 (13, 22) | 13.6 | 11.2 | 2.63 | 1.70 | 0.65 | 0.48 | 0.45 | 0.31 |
| Quartile 4 (22+) | 14.6 | 11.3 | 3.09 | 1.63 | 0.78 | 0.49 | 0.45 | 0.33 |
| P-value 3 | 0.91 | 0.93 | 0.82 | 0.20 | 0.12 | 0.66 | 0.36 | 0.89 |
Pari=0; Nulliparous, Pari>0: Parous
All food intakes are energy adjusted
For PFHxS n=485
P-value represents the linear trend from an ordinary linear model (Wald test) and ln(PFAS)
3.2 Regression analyses
Table 4 summarizes AIC-optimised linear model fits for natural log-transformed plasma levels of PFOS, PFOA, PFHxS, and PFNA. Models include dietary exposure in the form of the combined estimated dietary intake of PFOS and PFOA. The total variance explained by the models for PFOS, PFOA, PFHxS, and PFNA was: 21%, 47.9%, 17.5%, and 24.6%, respectively. Parity (nulliparous vs. parous) was the most important covariate for all PFASs. In comparison to parous women, on average, nulliparous women had 70% higher PFOA concentration. Similarly, nulliparous women had 47% higher PFOS, 19% higher PFHxS, and 62% higher PFNA concentration. We considered the parameterization of parity using three categories (nulliparous, primiparous, and multiparous), but the coefficient representing the decrease in PFAS concentrations among multiparous (compared with primiparous) women was small (data not shown). Therefore, for parsimony, we used only two categories (parous vs nulliparous).
Table 4.
Determinants of plasma concentrations (ng/ml) of PFOS, PFOA, PFHxS and PFNA in 487 pregnant women the Norwegian Mother and Child Cohort Study (MoBa), 2003–2004.
| Dependent variable | Determinants | Beta (95% CI) | Percent change in outcome (ng/mL) per unit exposure (95% CI)1 | Cumulative adjusted R squared (%) |
|---|---|---|---|---|
| Ln(PFOS) | Constant | 2.65 (2.41, 2.89) | ||
| Nulliparity | ||||
| Yes | Reference | Reference | ||
| No | −0.63 (−0.91, −0.34)*** | −46.7 (−59.9, −29.0) | 9.8 | |
| Ln(time since most recent pregnancy), years 2 | 0.14 (0.06, 0.21)*** | 9.3 (4.3, 14.4) | 12.8 | |
| Total breastfeeding duration, months 3 | −0.024 (−0.04, −0.006)** | −1.2 (−2.0, −0.28) | 13.7 | |
| Maternal education | ||||
| <12 years | Reference | Reference | ||
| 12 years | 0.22 (0.08, 0.36)** | 24.6 (8.1, 43.6) | ||
| 13 – 16 years | 0.21 (0.07, 0.35)** | 23.5 (7.6, 41.7) | ||
| 17 + years | 0.29 (0.14, 0.43)*** | 33.0 (14.9, 54.0) | 16.3 | |
| Smoking prior to pregnancy | ||||
| Non smoker | Reference | Reference | ||
| Occasional smoker | −0.04 (−0.15, 0.07) | −3.7 (−13.5, 7.3) | ||
| Daily smoker | −0.10 (−0.19, −0.01)* | −9.4 (−17.0, −1.0) | 16.9 | |
| Marital status | ||||
| Married/cohabitant | Reference | Reference | ||
| Single | −0.25 (−0.52, 0.02)† | −22.1 (−40.6, 2.1) | 17.3 | |
| Estimated summed PFOS+PFOA intake, Increase per quartile | 0.07 (0.04, 0.10)*** | 7.2 (4.2, 10.4) | 20.8 | |
| Ln(PFOA) | Constant | 1.53 (1.33, 1.72) | ||
| Nulliparity | ||||
| Yes | Reference | Reference | ||
| No | −1.21 (−1.47, −0.94)*** | −70.1 (−77.0, −61.1) | 31.8 | |
| Ln(time since most recent pregnancy), years 2 | 0.25 (0.19, 0.32)*** | 17.9 (13.0, 23.0) | 42.5 | |
| Total breastfeeding duration, months 3 | −0.05 (−0.07, −0.03)*** | −2.4 (−3.2, −1.6) | 46.2 | |
| Urban residence | ||||
| No | Reference | Reference | ||
| Yes | 0.06 (0.003, 0.12)* | 6.6 (0.35, 13.2) | 46.6 | |
| Household income | ||||
| Both < 300 000 NOK | Reference | Reference | ||
| One ≥ 300 000 NOK | 0.05 (−0.03, 0.12) | 4.7 (−2.6, 12.6) | ||
| Both ≥ 300 000 NOK | 0.12 (0.04, 0.20)** | 12.6 (3.6, 22.3) | 47.2 | |
| Gestational weight gain, up to week 17, kg | −0.01 (−0.02, 0.001)† | −0.9 (−1.9, 0.09) | 47.4 | |
| Estimated summed PFOS+PFOA intake, Increase per quartile | 0.032 (0.006, 0.06)* | 3.3 (0.6, 6.1) | 47.9 | |
| Ln(PFHxS) 4 | Intercept | −1.15 (−1.50, −0.79) | ||
| Nulliparity | ||||
| Yes | Reference | Reference | ||
| No | −0.20 (−0.34, −0.07)** | −18.5 (−29.0, −6.4) | 10.6 | |
| Total breastfeeding duration, months 3 | −0.04 (−0.06, −0.013)** | −1.9 (−3.1, −0.7) | 12.2 | |
| Maternal education | ||||
| <12 years | Reference | Reference | ||
| 12 years | 0.19 (−0.02, 0.40) | 20.19 (−1.9, 49.0) | ||
| 13 – 16 years | 0.24 (0.04, 0.44)* | 27.0 (3.8, 55.3) | ||
| 17 + years | 0.34 (0.12, 0.55)** | 40.2 (13.0, 74.0) | 13.7 | |
| Body mass index | 0.02 (0.007, 0.029)** | 1.8 (0.7, 2.9) | 15.4 | |
| Urban residence | ||||
| No | Reference | Reference | ||
| Yes | 0.13 (0.04, 0.23)** | 14.2 (3.7, 25.7) | 16.5 | |
| Estimated summed PFOS+PFOA intake, Increase per quartile | 0.06 (0.014, 0.10)* | 5.8 (1.4, 10.5) | 17.5 | |
| Ln(PFNA) | Intercept | −1.22 (−1.69, −0.75) | ||
| Nulliparity | ||||
| Yes | Reference | Reference | ||
| No | −0.97 (−1.32, −0.62)*** | −62.1 (−73.3, −46.1) | 9.4 | |
| Ln(Time since most recent pregnancy), years 2 | 0.21 (0.12, 0.30)*** | 14.7 (8.2, 21.5) | 15.4 | |
| Total breastfeeding duration, months 3 | −0.02 (−0.05, −0.001)* | −1.1 (−2.2, −0.02) | 15.8 | |
| Maternal education | ||||
| <12 years | Reference | Reference | ||
| 12 years | 0.31 (0.14, 0.49)*** | 36.6 (14.8, 62.7) | ||
| 13 – 16 years | 0.28 (0.12, 0.45)*** | 32.9 (12.4, 57.1) | ||
| 17 + years | 0.37 (0.19, 0.55)*** | 45.1 (21.4, 73.5) | 18.7 | |
| Marital status | ||||
| Married/cohabitant | Reference | |||
| Single | −0.36 (−0.70, −0.03) * | −30.5 (−50.1, −3.3) | 19.1 | |
| Maternal age at delivery | 0.013 (0.002, 0.024)* | 1.3 (0.2, 2.4) | 20.2 | |
| Marine fatty acids supplement use (fish oil/fish liver oil) | ||||
| No | Reference | Reference | ||
| Yes | 0.07 (−0.01, 0.15)† | 7.1 (−1.2, 16.1) | 20.6 | |
| Estimated summed PFOS+PFOA intake, Increase per quartile | 0.09 (0.06, 0.13)*** | 9.8 (5.9, 13.8) | 24.6 |
0.05> P-value <0.10
P-value <0.05
P-value <0.01
P-value <0.001
Percent change in the (untransformed) dependent variable for a one unit change in the independent variable [(exp(beta)]−1)*100]
For Ln (Time since most recent pregnancy, years) percent change per unit is computed for an interval increase from 1 to 2 years.
Total breastfeeding duration, months; adjusted for time since most recent pregnancy, percent change is computed for one extra month at the average pregnancy interval.
For PFHxS n=485
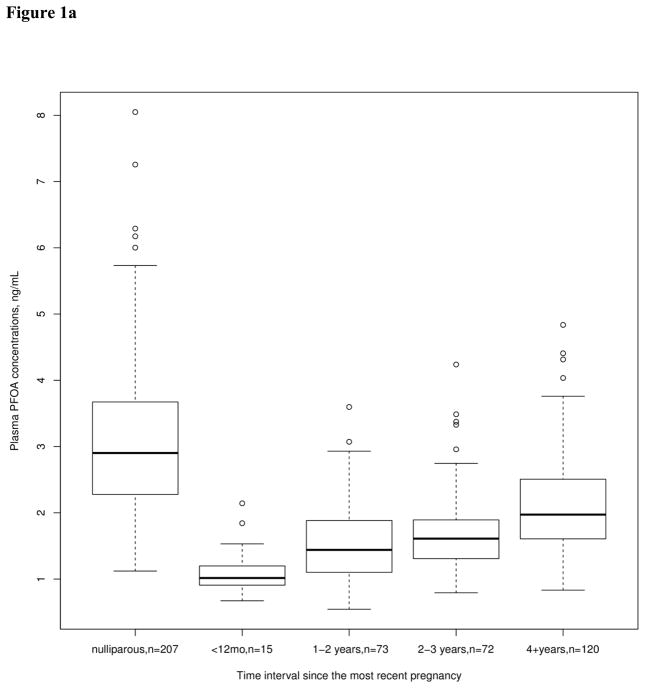
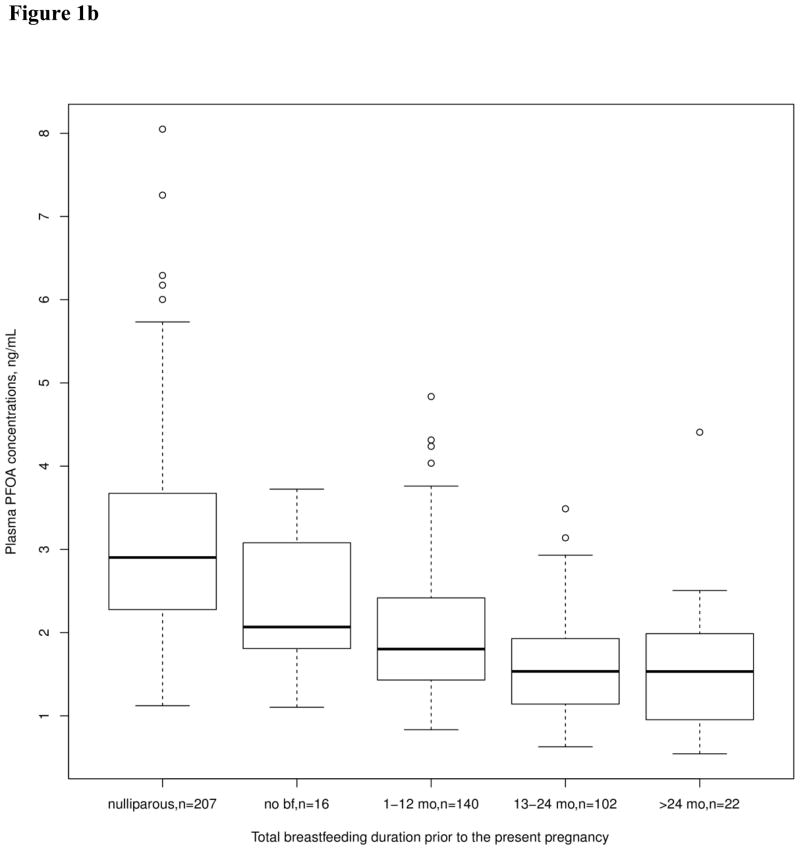
Total breastfeeding duration was correlated with reduced levels of all PFASs examined in this study. The largest reduction from breastfeeding was seen for PFOA, with a 2.4% reduction in plasma PFOA levels per month of breastfeeding. Time since the most recent pregnancy was associated with increased concentrations of PFOS, PFOA, and PFNA. Total breastfeeding duration and time since the most recent pregnancy were more strongly associated with PFOA than with the other PFAS concentrations. Figure 1 shows the unadjusted changes in plasma PFOA concentration associated with these variables. Plasma PFOA concentration is higher in nulliparous than in parous women, the concentration in parous women increases with time since the most recent pregnancy (Figure 1a), and decreases with longer duration of total breastfeeding (Figure 1b).
Figure 1.
Unadjusted plasma PFOA concentration (ng/mL) by categories representing time intervals since the most recent pregnancy (Figure 1a) and by categories representing total breastfeeding duration (Figure 1b). Box plot details: the horizontal lines indicate the median PFOA concentration; the box indicates the interquartile range (IQR) (25th percentile to 75th percentile); the whiskers represent observations within 1.5 times the IQR; and the circles indicate observations more than 1.5 times the IQR away from the box, considered outliers.
Maternal education and household income both reflect socioeconomic status, and were not selected in the same models. Maternal education was positively associated with models for plasma PFOS, PFHxS and PFNA. Women in the highest education category (17 years or more) had 33% higher PFOS, 40% higher PFHxS and 45% higher PFNA than women in the lowest category (<12 years). Household income was positively associated with the model for PFOA. In comparison with rural residence, urban residence was associated with 7% higher levels of PFOA and 14% higher levels of PFHxS. Smoking was associated with reduced levels of PFOS. Women who reported daily smoking prior to pregnancy had 9% lower PFOS concentration than non-smokers.
Because the estimated dietary intakes of PFOS and PFOA were strongly correlated (r=0.70), they were summed, and the summed dietary intake was associated with increasing concentration of all PFASs in all the multivariate models. One quartile increase in estimated dietary PFAS intake was associated with plasma PFOS, PFOA, PFHxS, and PFNA concentration increases of 7.2%, 3.3%, 5.8% and 9.8%, respectively, resulting in small, although non-trivial absolute changes in PFAS concentrations. Even though diet was a significant predictor, it contributed relatively little to the explained variance of PFAS plasma levels regardless of how it was parameterized.
When we evaluated the effect on the model of including intake from specific food groups in addition to or instead of the estimated summed PFOS and PFOA intake, only the model for PFHxS was clearly improved (R2=0.189 vs 0.175); in this case, including beef (negatively correlated), pork, oils and butter/margarine (positively correlated) improved the fit. Furthermore, the summed PFOS and PFOA intake estimate was always selected instead of the food groups upon optimization when present in the initial model, indicating that the food groups proved little additional information relative to total exposure. Shellfish and oily fish were the most influential food groups in the food groups-only PFAS models. Compared with the other PFASs, a greater number of food groups were associated with PFHxS, but the influence of each food group was small.
In each of the sensitivity analyses, with imputation of missing values on covariates and with inclusion of participants in the subfecundity group, we obtained results that were comparable to our primary analyses (Supplementary Tables 1–4).
4. Discussion
In this study of pregnant women, variables representing reproductive history (i.e. parity, the time interval since the most recent pregnancy, and total breastfeeding duration) were the major determinants of PFASs. In comparison to these variables, demographic and lifestyle variables including diet, contributed much less to the explained variance in plasma PFAS concentrations.
Parity was the determinant with the largest influence in all PFAS models. Three factors may account for this finding. First, PFASs have the ability to cross the placental barrier (Apelberg et al., 2007; Fromme et al., 2010), making direct foetal transfer a likely mechanism through which parity affects PFAS plasma concentrations. A recent study from MoBa reported PFAS concentrations in cord blood ranging from 30 to 79% of the maternal concentrations (Gützkow et al., 2012). However, the reduction in PFAS concentrations by parity apparent in our models is quantitatively larger than the reduction likely due to foetal transfer alone. A possible additional explanation could be accumulation of PFASs in the placenta, resulting in lower plasma concentrations when the placenta is removed after delivery.
Breastfeeding is another mechanism through which parous women may achieve lower PFAS concentrations compared with nulliparous women. Both PFOS and PFOA have been measured in breast milk (Haug et al., 2011a; Kärrman et al., 2007; Liu et al., 2011; Thomsen et al., 2010). A study of matched maternal serum, cord serum, and breast milk samples indicated that the postnatal exposure through human milk was higher than the prenatal exposure, particularly for PFOA (Liu et al., 2011). The authors of a recent study examined elimination rates of several compounds in breast milk during twelve months of lactation in 10 Norwegian mothers. The reduction of PFOA concentration in breast milk during the lactation period was more than twice that of PFOS (94% versus 37% in one year) (Thomsen et al., 2010).
Lastly, because a major elimination route of PFASs may be urine (Andersen et al., 2006; Harada et al., 2004; Loccisano et al., 2013), pregnant women may experience greater reduction in PFASs body burden due to an increase in the glomerular filtration rate. In healthy pregnancies, the glomerular filtration rate may increase by 40% (Dunlop, 1981; Gibson, 1973; Krutzen et al., 1992; Sims and Kranz, 1958).
It was also of note that the time interval since the most recent pregnancy was associated with increased concentrations of PFOS, PFOA and PFNA, probably reflecting re-accumulation of the chemicals with increasing time between pregnancies. Apelberg et al (2007) previously reported that parity was a determinant of PFAS concentrations in cord blood and epidemiologic studies have reported lower maternal plasma PFAS levels among parous women and women with a longer breastfeeding history (Fei et al., 2007; Fei et al., 2008). These analyses, however, were univariate, and the present analyses provide a clearer indication of the relative importance of the factors determining levels. Because of the relatively large effect of reproduction-related events on PFAS concentrations, the findings in epidemiologic studies of PFAS levels and health outcomes can easily be biased if the effects are not adequately considered in the analysis. For example, associations between PFASs and duration of breastfeeding and associations between PFASs and fecundability disappeared when the analyses were restricted to nulliparous women (Fei et al., 2010; Whitworth et al., 2012b).
The relative contribution of different exposure pathways to human exposure to PFASs has been difficult to define. Diet has been considered the most important non-occupational source of exposure to PFASs, particularly for PFOS and PFOA (Domingo, 2012; Trudel et al., 2008). Seafood has been identified as a major route of dietary exposure to PFASs in other studies from Norway (Haug et al., 2010b; Rylander et al., 2010). Haug et al., evaluated concentrations of ten PFASs in serum and food consumption in 175 men and women in the Norwegian Fish and Game Study. The study distinguished between lean fish, fish liver and shrimp, and consumption of one or more of the seafood items were associated with all PFASs (Haug et al., 2010b). Rylander et al., examined associations between the four PFASs examined in the present study and dietary habits and lifestyle in a representative group of middle-aged Norwegian women (n=315). Increased plasma concentrations of PFOS, PFHxS, and PFNA were observed in “fish eaters”. Lower concentrations of the same substances were seen in women with a “western” diet consisting of rice, pasta, water, white and red meat, chocolate, snacks, and pastries; no specific food pattern was associated with increased PFOA concentrations. In Sweden, eleven PFASs were measured in a set of archived food market basket samples from 1999, 2005, and 2010. The highest concentration was observed for PFOS in fish, meat, and egg homogenates. Although present at lower concentrations, similar contamination patterns were seen for PFHxS and PFNA, while PFOA was quantified at relatively low concentrations in food homogenates of both animal and non-animal origin (Vestergren et al., 2012). A recent report from the European Food Safety Authority summarised occurrence data for PFASs collected in 13 European countries during the period 2006 to 2012. Across food groups, PFASs were found more frequently in fish and other seafood and in meat and meat products (EFSA, 2012). Seafood items were also the main food groups associated with PFAS levels in the current study. However, the dietary variables contributed little to the overall explained variance in our models. A possible explanation could be the relatively low dietary exposure in our study population. A much higher influence of estimated intakes were seen in the previously mentioned study among men and women with much higher consumption of fish and seafood (Haug et al., 2010b). Although diet has been consistently correlated with PFASs body burden, studies across countries reveal large variation in the foods associated with their body burden. It is possible that some of this variation may be explained by drinking water, food packaging, cooking procedures (Domingo, 2012), as well as exposure to household dust (D’Hollander et al., 2010; Haug et al., 2011a; Haug et al., 2011b; Kato et al., 2009; Knobeloch et al., 2012).
Haug et al (2011a) showed that although food was the major exposure source in a group of Norwegian women, the indoor environment (air and dust) accounted for up to 50% of the total PFASs intake for several women, highlighting the importance of these exposures in determining PFASs body burden. Unfortunately, we did not have measures of indoor air or dust exposure in the present study. We found that variables related to socioeconomic status (i.e. maternal education and household income), were among the important determinants of various PFASs. We are not aware of other studies that have reported similar associations. No association between maternal socio-occupational status and PFOS or PFOA levels were observed in Danish pregnant (Fei et al., 2009). A possible explanation of this finding can be that households with women who are better educated or have higher income purchase more textiles and sports equipment containing PFASs (Herzke et al., 2012). However, the PFASs content in consumer products and possible emissions from such products remains unclear.
Women who were daily smokers had lower plasma PFOS concentration than non-smokers. This association has been inconsistently reported in previous studies. Although smoking status was not associated with plasma PFAS concentrations in either the Norwegian Fish and Game study (Haug et al., 2010b) or a study among Japanese men (Harada et al., 2004), lower levels of both PFOS and PFOA in active smokers were reported among two Danish populations (Eriksen et al., 2011; Halldorsson et al., 2008). Although Eriksen et al., (2011) reported on an inverse association between ever smoking and PFOA and PFOS levels, the study did not find an association between smoking intensity and PFAS levels, suggesting that the association may be explained by different lifestyle patterns in smokers and non-smokers.
The present study benefited from a large sample size and detailed information about a broad range of demographic, lifestyle, and pregnancy related factors, which were likely to capture a large proportion of variability in socioeconomic background and behaviour. Information about the time interval since the most recent previous pregnancy was obtained from the medical birth registry, while the other information was self-reported and may have led to misclassification with regard to, for instance, education, household income and smoking. Although the food frequency questionnaire has been thoroughly validated, the estimated intake of PFOS and PFOA were based on a relatively small database of concentrations measured in Norwegian food and drinking water, which may not be representative of all Norwegian foods. Imprecision in estimates of diet may have caused this determinant to appear less important than it is. The food group registration, on the other hand, is relatively precise and complete. Therefore, the lack of explanatory power of food groups in the multivariate models might indicate that either the dietary exposure route is not very important, or the PFOS/PFOA load varies widely with sample or food item. Another potential concern is selection bias. MoBa participants include a higher proportion of non-smokers and are older and better educated than non-participants. However, the relationships between variables were not necessarily distorted by exclusion of nonparticipants. For example, for eight exposure-disease relationships examined in the MoBa data, the associations were essentially the same as those found in an analysis of data for the entire pregnant population in Norway (Nilsen et al., 2009).
In conclusion, the history of previous pregnancies and breastfeeding were important determinants of PFAS plasma concentrations in these reproductive-aged women. The results of the present study indicate that increased elimination of PFASs occur during and after pregnancy and provide insight into the most important covariates for inclusion in epidemiologic investigations of associations between exposure and disease.
Supplementary Material
Highlights.
The history of previous pregnancies and breastfeeding were the most important determinants of PFASs in pregnant Norwegian women.
Women having given birth one or more times had 46%, 70%, 19%, and 62% lower concentrations of PFOS, PFOA, PFHxS, and PFNA respectively.
The time interval since the most recent pregnancy was associated with increased concentrations of PFOS, PFOA and PFNA, probably reflecting re-accumulation of the chemicals with increasing time between pregnancies
The duration of breastfeeding was associated with reduced levels of all PFASs, with the largest reduction in PFOA.
Diet was a significant factor explaining up to 4% of the variance in plasma PFAS concentrations.
Acknowledgments
The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health and the Ministry of Education and Research, NIH/NIEHS (contract no NO1-ES-75558), NIH/NINDS (grant no.1 UO1 NS 047537-01 and grant no. 2 UO1 NS047537-06A1), and the Norwegian Research Council/FUGE (grant no. 151918/S10). This study was supported in part by the Intramural Research Program, The National Institutes of Environmental Health Sciences (NIEHS), the National Institutes of Health (NIH). We are grateful to all the participating families in Norway who take part in this ongoing cohort study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
AL Brantsæter, Email: Anne.Lise.Brantsaeter@fhi.no.
KW Whitworth, Email: Kristina.W.Whitworth@uth.tmc.edu.
TA Ydersbond, Email: trond.arild.ydersbond@ssb.no.
LS Haug, Email: Line.Smastuen.Haug@fhi.no.
M Haugen, Email: Margaretha.Haugen@fhi.no.
HK Knutsen, Email: Helle.Knutsen@fhi.no.
C Thomsen, Email: Cathrine.Thomsen@fhi.no.
HM Meltzer, Email: Helle.Margrete.Meltzer@fhi.no.
G Becher, Email: Georg.Becher@fhi.no.
A Sabaredzovic, Email: Azemira.Sabaredzovic@fhi.no.
JA Hoppin, Email: hoppin1@niehs.nih.gov.
M Eggesbø, Email: Merete.Eggesbo@fhi.no.
MP Longnecker, Email: longnec1@niehs.nih.gov.
References
- Andersen ME, Clewell HJ, III, Tan YM, Butenhoff JL, Olsen GW. Pharmacokinetic modeling of saturable, renal resorption of perfluoroalkylacids in monkeys--probing the determinants of long plasma half-lives. Toxicology. 2006;227:156–164. doi: 10.1016/j.tox.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Apelberg BJ, Witter FR, Herbstman JB, Calafat AM, Halden RU, Needham LL, et al. Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ Health Perspect. 2007;115:1670–1676. doi: 10.1289/ehp.10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell SM, Calafat AM, Lyu C, Kato K, Ryan PB, Steenland K. Rate of decline in serum PFOA concentrations after granular activated carbon filtration at two public water systems in Ohio and West Virginia. Environ Health Perspect. 2010;118:222–228. doi: 10.1289/ehp.0901252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantsæter AL, Birgisdottir BE, Meltzer HM, Kvalem HE, Alexander J, Magnus P, et al. Maternal seafood consumption and infant birth weight, length and head circumference in the Norwegian Mother and Child Cohort Study. Br J Nutr. 2012;107:436–444. doi: 10.1017/S0007114511003047. [DOI] [PubMed] [Google Scholar]
- Brantsæter AL, Haugen M, Alexander J, Meltzer HM. Validity of a new food frequency questionnaire for pregnant women in the Norwegian Mother and Child Cohort Study (MoBa) Matern Child Nutr. 2008a;4:28–43. doi: 10.1111/j.1740-8709.2007.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantsæter AL, Haugen M, de Mul A, Bjellaas T, Becher G, van Klaveren J, et al. Exploration of different methods to assess dietary acrylamide exposure in pregnant women participating in the Norwegian Mother and Child Cohort Study (MoBa) Food Chem Toxicol. 2008b;46:2808–2814. doi: 10.1016/j.fct.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Brantsæter AL, Haugen M, Thomassen Y, Ellingsen DG, Ydersbond TA, Hagve TA, et al. Exploration of biomarkers for total fish intake in pregnant Norwegian women. Public Health Nutr. 2010;13:54–62. doi: 10.1017/S1368980009005904. [DOI] [PubMed] [Google Scholar]
- Claeskens G, Hjort NL. Model selection and model averaging. Cambridge University Press; Cambridge: 2008. [Google Scholar]
- D’Hollander W, de VP, De CW, Bervoets L. Perfluorinated substances in human food and other sources of human exposure. Rev Environ Contam Toxicol. 2010;208:179–215. doi: 10.1007/978-1-4419-6880-7_4. [DOI] [PubMed] [Google Scholar]
- Domingo JL. Health risks of dietary exposure to perfluorinated compounds. Environ Int. 2012;40:187–195. doi: 10.1016/j.envint.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Dunlop W. Serial changes in renal haemodynamics during normal human pregnancy. Br J Obstet Gynaecol. 1981;88:1–9. doi: 10.1111/j.1471-0528.1981.tb00929.x. [DOI] [PubMed] [Google Scholar]
- EFSA. Perfluoroalkylated substances in food: occurrence and dietary exposure. EFSA Journal. 2012;10:2743–2798. ( http://www.efsa.europa.eu/en/efsajournal/pub/2743.htm) [Google Scholar]
- Eriksen KT, Sorensen M, McLaughlin JK, Tjonneland A, Overvad K, Raaschou-Nielsen O. Determinants of plasma PFOA and PFOS levels among 652 Danish men. Environ Sci Technol. 2011;45:8137–8143. doi: 10.1021/es100626h. [DOI] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Lipworth L, Olsen J. Prenatal exposure to perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS) and maternally reported developmental milestones in infancy. Environ Health Perspect. 2008;116:1391–1395. doi: 10.1289/ehp.11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Lipworth L, Olsen J. Maternal levels of perfluorinated chemicals and subfecundity. Hum Reprod. 2009;24:1200–1205. doi: 10.1093/humrep/den490. [DOI] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Lipworth L, Olsen J. Maternal concentrations of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) and duration of breastfeeding. Scand J Work Environ Health. 2010;36:413–421. doi: 10.5271/sjweh.2908. [DOI] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Tarone RE, Olsen J. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ Health Perspect. 2007;115:1677–1682. doi: 10.1289/ehp.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frömel T, Knepper TP. Biodegradation of fluorinated alkyl substances. Rev Environ Contam Toxicol. 2010;208:161–177. doi: 10.1007/978-1-4419-6880-7_3. [DOI] [PubMed] [Google Scholar]
- Fromme H, Mosch C, Morovitz M, Alba-Alejandre I, Boehmer S, Kiranoglu M, et al. Pre- and postnatal exposure to perfluorinated compounds (PFCs) Environ Sci Technol. 2010;44:7123–7129. doi: 10.1021/es101184f. [DOI] [PubMed] [Google Scholar]
- Gibson HM. Plasma volume and glomerular filtration rate in pregnancy and their relation to differences in fetal growth. J Obstet Gynaecol Br Commonw. 1973;80:1067–1074. doi: 10.1111/j.1471-0528.1973.tb02981.x. [DOI] [PubMed] [Google Scholar]
- Glynn A, Berger U, Bignert A, Ullah S, Aune M, Lignell S, et al. Perfluorinated alkyl acids in blood serum from primiparous women in Sweden: serial sampling during pregnancy and nursing, and temporal trends 1996–2010. Environ Sci Technol. 2012;46:9071–9079. doi: 10.1021/es301168c. [DOI] [PubMed] [Google Scholar]
- Gützkow KB, Haug LS, Thomsen C, Sabaredzovic A, Becher G, Brunborg G. Placental transfer of perfluorinated compounds is selective--a Norwegian Mother and Child sub-cohort study. Int J Hyg Environ Health. 2012;215:216–219. doi: 10.1016/j.ijheh.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Halldorsson TI, Fei C, Olsen J, Lipworth L, McLaughlin JK, Olsen SF. Dietary predictors of perfluorinated chemicals: a study from the Danish National Birth Cohort. Environ Sci Technol. 2008;42:8971–8977. doi: 10.1021/es801907r. [DOI] [PubMed] [Google Scholar]
- Halldorsson TI, Rytter D, Haug LS, Bech BH, Danielsen I, Becher G, et al. Prenatal exposure to perfluorooctanoate and risk of overweight at 20 years of age: a prospective cohort study. Environ Health Perspect. 2012;120:668–673. doi: 10.1289/ehp.1104034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada K, Saito N, Inoue K, Yoshinaga T, Watanabe T, Sasaki S, et al. The influence of time, sex and geographic factors on levels of perfluorooctane sulfonate and perfluorooctanoate in human serum over the last 25 years. J Occup Health. 2004;46:141–147. doi: 10.1539/joh.46.141. [DOI] [PubMed] [Google Scholar]
- Haug LS, Huber S, Becher G, Thomsen C. Characterisation of human exposure pathways to perfluorinated compounds-comparing exposure estimates with biomarkers of exposure. Environ Int. 2011a;37:687–693. doi: 10.1016/j.envint.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Haug LS, Huber S, Schlabach M, Becher G, Thomsen C. Investigation on per- and polyfluorinated compounds in paired samples of house dust and indoor air from Norwegian homes. Environ Sci Technol. 2011b;45:7991–7998. doi: 10.1021/es103456h. [DOI] [PubMed] [Google Scholar]
- Haug LS, Salihovic S, Jogsten IE, Thomsen C, Van BB, Lindstrom G, et al. Levels in food and beverages and daily intake of perfluorinated compounds in Norway. Chemosphere. 2010a;80:1137–1143. doi: 10.1016/j.chemosphere.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Haug LS, Thomsen C, Becher G. A sensitive method for determination of a broad range of perfluorinated compounds in serum suitable for large-scale human biomonitoring. J Chromatogr A. 2009a;1216:385–393. doi: 10.1016/j.chroma.2008.10.113. [DOI] [PubMed] [Google Scholar]
- Haug LS, Thomsen C, Becher G. Time trends and the influence of age and gender on serum concentrations of perfluorinated compounds in archived human samples. Environ Sci Technol. 2009b;43:2131–2136. doi: 10.1021/es802827u. [DOI] [PubMed] [Google Scholar]
- Haug LS, Thomsen C, Brantsæter AL, Kvalem HE, Haugen M, Becher G, et al. Diet and particularly seafood are major sources of perfluorinated compounds in humans. Environ Int. 2010b;36:772–778. doi: 10.1016/j.envint.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Herzke D, Olsson E, Posner S. Perfluoroalkyl and polyfluoroalkyl substances (PFASs) in consumer products in Norway - A pilot study. Chemosphere. 2012;88:980–987. doi: 10.1016/j.chemosphere.2012.03.035. [DOI] [PubMed] [Google Scholar]
- Jones PD, Hu W, De CW, Newsted JL, Giesy JP. Binding of perfluorinated fatty acids to serum proteins. Environ Toxicol Chem. 2003;22:2639–2649. doi: 10.1897/02-553. [DOI] [PubMed] [Google Scholar]
- Kärrman A, Ericson I, Van BB, Darnerud PO, Aune M, Glynn A, et al. Exposure of perfluorinated chemicals through lactation: levels of matched human milk and serum and a temporal trend, 1996–2004, in Sweden. Environ Health Perspect. 2007;115:226–230. doi: 10.1289/ehp.9491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Calafat AM, Wong LY, Wanigatunga AA, Caudill SP, Needham LL. Polyfluoroalkyl compounds in pooled sera from children participating in the National Health and Nutrition Examination Survey 2001–2002. Environ Sci Technol. 2009;43:2641–2647. doi: 10.1021/es803156p. [DOI] [PubMed] [Google Scholar]
- Knobeloch L, Imm P, Anderson H. Perfluoroalkyl chemicals in vacuum cleaner dust from 39 Wisconsin homes. Chemosphere. 2012;88:779–783. doi: 10.1016/j.chemosphere.2012.03.082. [DOI] [PubMed] [Google Scholar]
- Kovarova J, Svobodova Z. Perfluorinated compounds: occurrence and risk profile. Neuro Endocrinol Lett. 2008;29:599–608. [PubMed] [Google Scholar]
- Krutzen E, Olofsson P, Back SE, Nilsson-Ehle P. Glomerular filtration rate in pregnancy: a study in normal subjects and in patients with hypertension, preeclampsia and diabetes. Scand J Clin Lab Invest. 1992;52:387–392. doi: 10.3109/00365519209088374. [DOI] [PubMed] [Google Scholar]
- Lauritsen J. [Accessed February 2006];FoodCalc [online] 2005 Available at: http://www.ibt.ku.dk/jesper/foodcalc.
- Liu J, Li J, Liu Y, Chan HM, Zhao Y, Cai Z, et al. Comparison on gestation and lactation exposure of perfluorinated compounds for newborns. Environ Int. 2011;37:1206–1212. doi: 10.1016/j.envint.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Loccisano AE, Longnecker MP, Campbell JL, Jr, Andersen ME, Clewell HJ., III Development of pbpk models for pfoa and pfos for human pregnancy and lactation life stages. J Toxicol Environ Health A. 2013;76:25–57. doi: 10.1080/15287394.2012.722523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C. Cohort profile: The Norwegian Mother and Child Cohort Study (MoBa) Int J Epidemiol. 2006;35:1146–1150. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- Maisonet M, Terrell ML, McGeehin MA, Christensen KY, Holmes A, Calafat AM, et al. Maternal concentrations of polyfluoroalkyl compounds during pregnancy and fetal and postnatal growth in British girls. Environ Health Perspect. 2012;120:1432–1437. doi: 10.1289/ehp.1003096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HM, Brantsæter AL, Ydersbond TA, Alexander J, Haugen M. Methodological challenges when monitoring the diet of pregnant women in a large study: experiences from the Norwegian Mother and Child Cohort Study (MoBa) Matern Child Nutr. 2008;4:14–27. doi: 10.1111/j.1740-8709.2007.00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer D, Rice N, Depledge MH, Henley WE, Galloway TS. Association between serum perfluorooctanoic acid (PFOA) and thyroid disease in the U.S. National Health and Nutrition Examination Survey. Environ Health Perspect. 2010;118:686–692. doi: 10.1289/ehp.0901584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen RM, Vollset SE, Gjessing HK, Skjaerven R, Melve KK, Schreuder P, et al. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol. 2009;23:597–608. doi: 10.1111/j.1365-3016.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- Norwegian Food Safety Authority, Norwegian Directorate of Health, Department of Nutrition - University of Oslo. Matvaretabellen [The Norwegian Food Composition Table] 2006 Available online at www.matportalen.no/Matvaretabellen.
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007;115:1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylander C, Sandanger TM, Frøyland L, Lund E. Dietary patterns and plasma concentrations of perfluorinated compounds in 315 Norwegian women: the NOWAC Postgenome Study. Environ Sci Technol. 2010;44:5225–5232. doi: 10.1021/es100224q. [DOI] [PubMed] [Google Scholar]
- Schecter A, Colacino J, Haffner D, Patel K, Opel M, Papke O, et al. Perfluorinated compounds, polychlorinated biphenyls, and organochlorine pesticide contamination in composite food samples from Dallas, Texas, USA. Environ Health Perspect. 2010;118:796–802. doi: 10.1289/ehp.0901347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröter-Kermani C, Muller J, Jurling H, Conrad A, Schulte C. Retrospective monitoring of perfluorocarboxylates and perfluorosulfonates in human plasma archived by the German Environmental Specimen Bank. Int J Hyg Environ Health. 2012 doi: 10.1016/j.ijheh.2012.08.004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Shankar A, Xiao J, Ducatman A. Perfluorooctanoic Acid and Cardiovascular Disease in US Adults. Arch Intern Med. 2012 doi: 10.1001/archinternmed.2012.3393. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Sims EA, Krantze KE. Serial studies of renal function during pregnancy and the puerperium in normal women. J Clin Invest. 1958;37:1764–1774. doi: 10.1172/JCI103769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen C, Haug LS, Stigum H, Frøshaug M, Broadwell SL, Becher G. Changes in concentrations of perfluorinated compounds, polybrominated diphenyl ethers, and polychlorinated biphenyls in Norwegian breast-milk during twelve months of lactation. Environ Sci Technol. 2010;44:9550–9556. doi: 10.1021/es1021922. [DOI] [PubMed] [Google Scholar]
- Trudel D, Horowitz L, Wormuth M, Scheringer M, Cousins IT, Hungerbuhler K. Estimating consumer exposure to PFOS and PFOA. Risk Anal. 2008;28:251–269. doi: 10.1111/j.1539-6924.2008.01017.x. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency. [accessed July 2012];2010 http://www.epa.gov/oppt/pfoa/pubs/stewardship/index.html.
- Vestergaard S, Nielsen F, Andersson AM, Hjollund NH, Grandjean P, Andersen HR, et al. Association between perfluorinated compounds and time to pregnancy in a prospective cohort of Danish couples attempting to conceive. Hum Reprod. 2012;27:873–880. doi: 10.1093/humrep/der450. [DOI] [PubMed] [Google Scholar]
- Vestergren R, Berger U, Glynn A, Cousins IT. Dietary exposure to perfluoroalkyl acids for the Swedish population in 1999, 2005 and 2010. Environ Int. 2012;49:120–127. doi: 10.1016/j.envint.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Whitworth KW, Haug LS, Baird DD, Becher G, Hoppin JA, Skjaerven R, et al. Perfluorinated compounds in relation to birth weight in the norwegian mother and child cohort study. Am J Epidemiol. 2012a;175:1209–1216. doi: 10.1093/aje/kwr459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth KW, Haug LS, Baird DD, Becher G, Hoppin JA, Skjaerven R, et al. Perfluorinated compounds and subfecundity in pregnant women. Epidemiology. 2012b;23:257–263. doi: 10.1097/EDE.0b013e31823b5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. [DOI] [PubMed] [Google Scholar]
- Zhang X, Chen L, Fei XC, Ma YS, Gao HW. Binding of PFOS to serum albumin and DNA: insight into the molecular toxicity of perfluorochemicals. BMC Mol Biol. 2009;10:16. doi: 10.1186/1471-2199-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.