Abstract
Introduction
Currently, the only treatment for celiac disease is a gluten free diet, and there is an increased desire for alternative therapies. In vitro and in vivo models of celiac disease have been generated in order to better understand the pathogenesis of celiac disease, and this review will discuss these models as well as the testing of alternative therapies using these models.
Areas Covered
The research discussed describes the different in vitro and in vivo models of celiac disease that currently exist and how they have contributed to our understanding of how gluten can stimulate both innate and adaptive immune responses in celiac patients. We also provide a summary on the alternative therapies that have been tested with these models and discuss whether subsequent clinical trials were done based on these tests done with these models of celiac disease.
Expert Opinion
Only a few of the alternative therapies that have been tested with animal models have gone on to clinical trials; however, those that did go on to clinical trial have provided promising results from a safety standpoint. Further trials are required to determine if some of these therapies may serve as an effective adjunct to a gluten free diet to alleviate the adverse affects associated with accidental gluten exposure. A “magic-bullet” approach may not be the answer to celiac disease, but possibly a future cocktail of these different therapeutics may allow celiac patients to consume an unrestricted diet.
Keywords: celiac, gliadin, gluten, in vitro, in vivo, model, monkey, mouse, nondietary, rat, T cell, therapy, treatment
1. Introduction
Celiac disease is prevalent in most of the industrialized world and is increasing with time1. Ingestion of wheat-derived gluten by celiac patients results in immune mediated injury of the intestines that is characterized by intestinal permeability, villous atrophy, and an inflammatory infiltration of the lamina propria that consists primarily of lymphocytes and plasma cells2, 3. Intestinal permeability in celiac disease can occur as a result of a number of reasons, one of which is the release of zonulin that disrupts the tight junctions in the epithelial layer3. The increased intestinal permeability in celiac disease allows for paracellular transfer of gluten derived peptides to the lamina propria, resulting in the presentation of gluten derived peptides to, and subsequent activation of, T cells. Previous publications have focused on the gluten-derived epitopes that are presented by the most predominant variant of DQ2 in celiac disease, DQ2.5,and have shown that there is a specific set of peptides derived from α-gliadin that are immunogenic4. These peptides are rendered more immunogenic by complexing with intestinally derived tissue transglutaminase (tTG), resulting in the deamidation of select epitopes5. Intestinal plasma cells produce autoantibodies directed against the self-protein, (tTG); these antibodies are used as a diagnostic marker for disease.
Gluten derived peptides can also stimulate non-T cells directly, including epithelial cells, monocytes, and dendritic cells 6–8. These cells can produce a variety of inflammatory cytokines in response to stimulation with gluten7, 8 including the expression of IL-15 by epithelial cells, which has been shown to play a crucial role in the activation of NK T cells and subsequent development of enteropathy in celiac disease 6, 9. Thus, the stimulation of both non-T cells (innate immune response) and T cells (adaptive immune response) by gluten contribute to the development of intestinal inflammation in celiac disease.
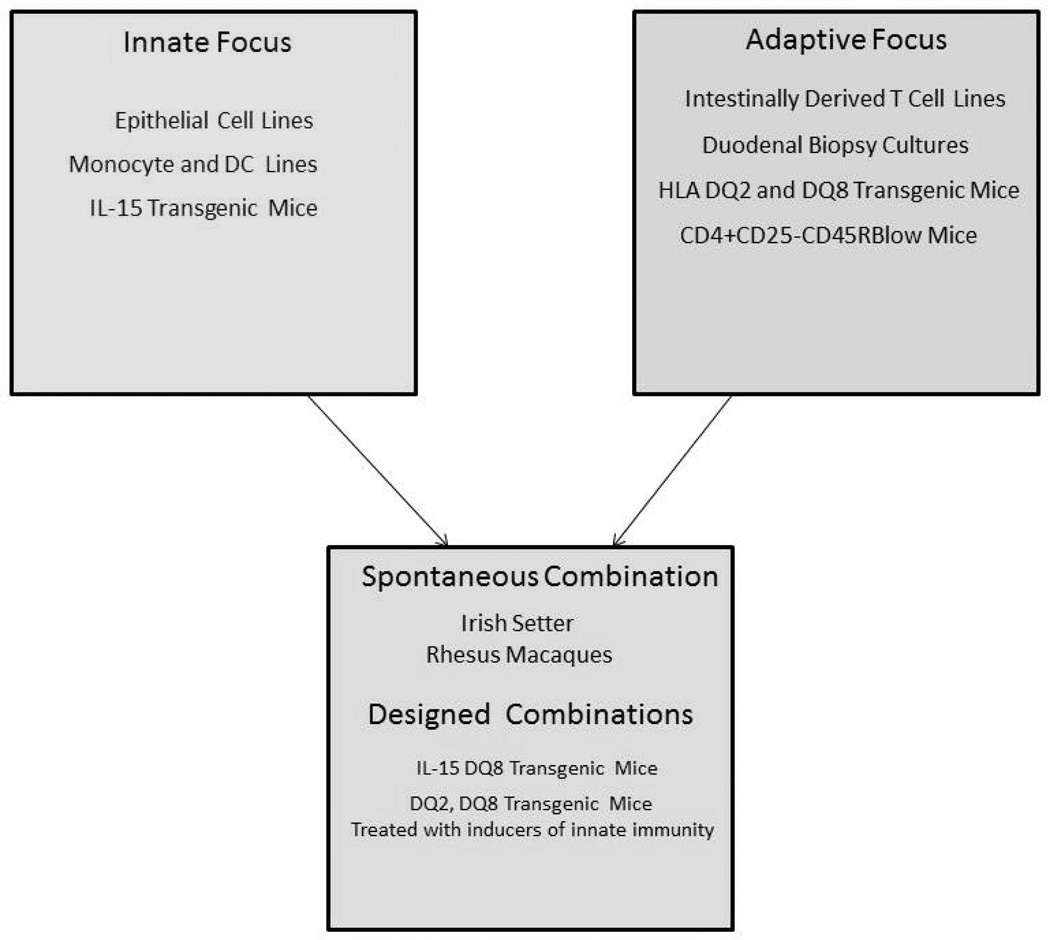
In order to understand how both the innate immune response as well as the adaptive immune response to gluten can combine to manifest into celiac disease, models of the cellular responses as well as in vivo models of the disease have been generated (Figure 1). An increasing number of animal models are now being used to test novel therapies that target the different pathways induced by gluten stimulation. This review builds upon our previous review in 2009 10 and summarizes the recent advances in in vitro and in vivo models of celiac disease and their application in clinical trials of potential therapies.
Figure 1.
Diagram of which models best address innate immunity associated with gluten, which adaptive, and which models have been used to address both simultaneously.
2. In Vitro Models of Celiac Disease
2.1 Cell Lines
Many studies demonstrate that gluten can induce inflammatory responses in the first line of defense in the intestine, the epithelial layer. This includes the release of zonulin, leading to disruption of tight junction proteins in the epithelial layer, and subsequent intestinal permeability 3. In vitro models of an epithelial layer consistent with celiac disease include Caco-2 and IEC-6 epithelial cell lines. Exposure of these cells to gluten results in very diverse responses. These include physical changes, such as cytoskeletal rearrangement and disruption of the tight junction integrity, but also altered expression of inflammatory cytokines11. Recent studies have shown that a peptide derived from the α-gliadin molecule, called p31–p43 (a stimulator of innate responses in celiac disease), induces expression of cell surface IL-15 by Caco-2 cells, and thereby stimulates the proliferation of T cells 9. This is in contrast to the p57–68 peptide, which does not induce cell surface expression of IL15 by Caco-2 cells, but does activate T cells when presented in the context of DQ2, which would be an adaptive immune response. This difference is due to the ability of p31–43 (also called the innate peptide) to disrupt endocytic vesicle trafficking in epithelial cells, whereas p57–68 does not 12–14. Even more intriguing is that p31–43 (but not p57–68) also induces the expression of tissue transglutaminase 2 by Caco-2 cells, finally providing a potential cellular source for TG2 15.
Caco-2 cells have also allowed for increased understanding of how the intestinal microbiome plays a role in the pathogenesis of celiac disease. Celiac patients have increased levels of Bacteroides in their microbiome in contrast to normals 16, 17. When Caco-2 cells are exposed to Bacteroides fragilis and gliadin, there is a subsequent increase in intestinal permeability as well as increased production of TNFα and IL-1β18. These findings in Caco-2 cells demonstrate how intestinal microbiota in conjunction with gliadin may potentiate the inflammatory cascade associated with celiac disease.
Monocytes and dendritic cells (DCs) have also been used as models of celiac disease; they are unique in that they can respond to gluten directly as well as support inflammatory T helper cells that are gluten responsive. With respect to monocytes responding to gluten in an innate fashion, monocytes cultured with proteolytic fragments of gliadin (PT-gliadin) will produce the inflammatory cytokines TNFα and IL-8 7. Similarly, when DCs are cultured with PT-gliadin, they secrete the inflammatory cytokines IL-6, IL-8, and TNFα8. Culture with PT-gliadin also promotes migration of the DCs in response to CCL19 and CCL21 8. Both are chemokines important in trafficking to lymph nodes 19. Similar to the studies done with bacteria and Caco-2 cells, a very recent publication demonstrated that co-culture of DCs with gliadin and different enterobacteria led to different cytokine profiles 20. Culturing gliadin treated DCs with Shigella or E. Coli led to increased production of IL-12 and TNFα as compared to gliadin treated DCs incubated with B. Longum. Yet when cultured with IL-15 and PT-gliadin, monocytes can also shape the adaptive immune response to wheat by producing IL-1β, IL-6, IL-15, IL-23, TNFα and CCL20, and activating Th17 and Th1 responses to gliadin 21. This last result introduces the concept of using a mix of antigen presenting cells (APC) and T cells to create an in vitro model of adaptive immunity to gluten and gliadin.
2.2 APC/T Cell Mixes
Mixes of APCs with intestinally derived T cells from celiac patients have been used extensively to model the presentation of gluten derived epitopes in celiac disease. This includes identifying the specific alleles of DQ2 and DQ8 that are capable of presenting gliadin derived immunogenic epitopes, as well as identifying the specific gliadin derived immunogenic epitopes that are presented by these alleles 22, 23. They were also used to demonstrate that transglutaminase rendered certain epitopes more immunogenic through the deamidation process 5. Gene dosage analyses and evaluation of the contributions of specific amino acids inside the peptide groove of the MHC II allele to the binding of different gliadin epitopes were also done with the APC/T cell in vitro model 24, 25. Most recently, this model was used to determine that T cells from DQ2.2+ celiac patients recognize a glutenin derived epitope that is entirely different from the α-gliadin derived 33mer peptide found to be very immunogenic in DQ2.5 celiac patients 4. Similarly, DQ9, which deviates from DQ8 by only one amino acid at position β57, will affect presentation of gliadin derived epitopes. Specifically, DQ9 restricted T cell responses will not respond to the DQ8 α-gliadin or γ-gliadin epitopes, only the DQ8 glutenin derived epitope, DQ8-glut-14. Thus, the APC/T cellmodel is providing crucial data on the adaptive immune responses to gluten that can then be used to treat patients at an individual level.
2.3 Mucosal Biopsy Cultures
The previous models utilized cells that were isolated separately from one another; in contrast, intestinal biopsy cultures extracted from celiac patients incorporate all of the cell-cell interactions present in the small intestine. This model is most likely the oldest of all the models described in this review26. Studies done with this model range from analyzing the T cell stimulatory potential of different fractions of gluten and gliadin27, 28 to understanding how gluten derived peptides are transported across the surface epithelium 29. One recent study that utilized this model determined that regulatory T cells exist in the mucosa of untreated celiac patients, but are rendered dysfunctional by overexpression of IL-15 30. Another recent study that used duodenal biopsy culture demonstrated that Th17 cells are generated in celiac disease31.
Mucosal biopsy cultures have also been used to determine the immunogenicity of wheat species other than the common Triticum Aestivum as well as other species used in cereals 32–34. In one study, Triticum monococcum was found to stimulate the expression of IFNγ, and with one cultivar (Monlis), the expression of IL15 35. In contrast, oat species (Avena Potenza and Avena genziana) did not increase expression of IL15 by enterocytes, nor proliferation of crypt epithelial cells, but did increase IFNγ expression in intestinally derived T cells 34. Quinoa, of the genus Chenopodium, considered to be safe for celiac patients, also was capable of stimulating mucosal biopsy cultures from celiac patients to express IFNγ; albeit one cultivar, Pasankalla, did not as compared to untreated cultures 33. Since IL-15 is derived from epithelial cells and IFNγ from T cells, these studies demonstrate that the mucosal biopsy culture model proves to be quite useful in determining both innate and adaptive responses to gluten derived from alternative cereals, and as such their potential toxicity for celiac disease patients. The potential toxicity for celiac patients can then be tested using a short term challenge, as was done in the study on Triticum monococcum35.
3. In Vivo Models of Celiac Disease
Although in vitro models have provided great understanding and insight into the pathogenesis of celiac disease at a cellular level, they cannot fully model the systemic development of celiac disease. While the best in vitro model is probably the intestinal biopsy culture, which incorporates the effect of cell-cell interactions and signaling, this model may lack hormone and neurologic signals delivered by other organs or cell systems. To ensure such signals are not overlooked, animal models of celiac disease have been developed. This section describes the animal models of celiac disease that have been recently used.
3.1 Spontaneous Models
The spontaneous animal models are models in which no sensitization is required for development of disease. Most research on spontaneous animal models has been done on the dog and monkey models, although there has been one publication on a potential spontaneous horse model of celiac disease36. In the dog model, Irish setters develop partial villous atrophy and intraepithelial lymphocyte (IEL) infiltration in response to consumption of gluten 37–39. Rhesus macaques will also develop similar pathology in response to gluten consumption; however, the MHC II is not associated with the gluten dependent pathology in either model 40, 41. Of great interest is that one of the rhesus macaques developed dermatitis similar to dermatitis herpetiformis, the skin manifestation of celiac disease 42. This monkey spontaneously developed antibodies specific for epidermal transglutaminase and tissue transglutaminase, similar to DH patients 43, and the dermatitis resolved after administration of a gluten free diet. However, in contrast to the mouse model of DH, IgA deposition at the dermal papillae was not associated with subepidermal splitting 44. Also, the gluten dependent enteropathy that developed in other rhesus macaques did not present in the one monkey that developed the gluten dependent dermatitis41, 45.
3.2 Induced Models
The rat model continues to be used today in a number of studies. In this model, germ-free Wistar AVN rats are administered gliadin immediately after birth46. This leads to shortening of villi, crypt hyperplasia, and increased numbers of intestinal CD8αβ+ IELs 46. Recent papers that have utilized this model have evaluated the role of intestinal bacteria in the development of celiac disease47, 48. In Laparra et al., administration of Bifidobacterium longum was shown to protect against the effects of gliadin sensitization of the rats47. Specifically, TNFα was significantly decreased, but IL10 was significantly increased in jejunal tissue sections, thereby significantly decreasing the inflammation induced by gliadin alone in the jejunum. Olivares et al. used MALDITOF-TOF peptide fingerprinting analysis to confirm that feeding the rats B. longum results in the up-regulation of anti-inflammatory processes and that this is enough to partially ameliorate gliadin induced stress when both B. longum and gliadin are administered to the rats 48. In a separate study, it was determined that co-administration of Shigella or E. coli with gliadin resulted in an increase in the impairment of tight junctions and resulted in translocation of gliadin peptides into the lamina propria49. These results support the theory that the intestinal microbiome plays a crucial role in the development of celiac disease.
There are also mouse models where enteropathy is induced. In one mouse model by Freitag et al., this induction was achieved by transferring CD4+CD25-CD45RBlow cells from gliadin sensitized mice to recipient Rag 1−/− mice 50. Another new model involved Balb/c mice that were bred for three generations on a gluten free diet; subsequent offspring were then weaned and raised on a gluten free chow for up to 10 weeks of age. The resultant mice were placed onto a standard chow for 30 days. These mice had increased numbers of infiltrating CD3+ IELs with a decreased (villous height)/(crypt depth) ratio 51.
3.3 Transgenic Models
In order to analyze specific pathways involved in the development of celiac disease, transgenic and knock out mice have been generated. The transgenes and knock out constructs utilized are many, but the most widely used are mice that express the HLA genes DQ8 or DQ2 that are tightly associated with celiac disease 44, 52–72. These mice have resulted in the greatest number of publications on animal models of celiac disease and continue to be used in a number of studies addressing mechanism as well as novel therapies for celiac disease. Briefly, these mice have demonstrated that the HLA molecules DQ8 and DQ2 can contribute to the development of a potent inflammatory T cell response against gliadin, but that this alone is not sufficient for the development of gluten dependent enteropathy characterized by shortened villi. Other mitigating factors are necessary for this to occur, and the studies with transgenic mice have demonstrated (for the most part) that perturbations of the innate immune response in the intestine are necessary for features of gluten dependent enteropathy to develop. These include the administration of cholera toxin, which acts as an adjuvant, and indomethacin, which causes small intestinal pathology. Poly I:C stimulates the innate immune response through TLR3 73, and was used in a mouse line in which TG2 was knocked out 74. This latter manuscript by Dafik et el demonstrated that TG2 is not required for the development of poly I:C generated villous atrophy; instead, activation of TG2 is probably a consequence of villous atrophy and not a cause of villous atrophy. Of increasing interest are the transgenic mice that overexpress IL-15. One line of IL-15 mice has human IL15 inserted behind an enterocyte specific promoter (T3b), and this resulted in increased numbers of CD8+ cells infiltrating the small intestine 75. Interestingly, these mice developed anti tTG IgA in the absence of a gluten-specific CD4+ T cell response76. A different line of IL-15 mice used a minimal MHC class I Dd promoter for the expression of IL-15; in a later study, these mice were then crossed with DQ8 transgenic mice 55. The resultant IL-15 DQ8 transgenic mice developed increased numbers of CD3+ IELs in response to feeding with gliadin55. This latter manuscript demonstrates how combining an adaptive response to gluten with a chronic perturbation of the innate immune system (IL-15 overexpression) results in a model that is more similar to celiac disease than either alone.
4. Testing Novel Therapies
Ultimately the goal to any disease specific research is to find a cure or reliable treatment to suppress the disease process. A gluten free diet is currently the only therapy available for patients with celiac disease. While effective, it is difficult to maintain due to cost, availability of products, social acceptance, and patient knowledge of what defines gluten-free. In addition to these patient factors, there is the issue of "hidden gluten" which results from contamination during manufacturing 77. The definition of gluten free also varies from country to country and is due to the absence of an internationally safe threshold of gluten for patients with CD 78.
Given these obstacles, as many as 50% of patients with celiac disease who adhere to a gluten free diet do not achieve histologic remission79. Lack of histologic remission may be due to daily consumption of 50 mg of gluten, the equivalence of 1/100TH of a slice of bread77, 79. This amount is not insignificant, as it is estimated that patients adherent to a gluten free diet will inadvertently consume an average of 5 to 50 mg of gluten each day as a result of gluten contamination 77.
These issues have led to increased interest in therapeutics which may either replace or supplement a gluten free diet to diminish the adverse effects associated with accidental gluten exposure. Just as in vitro and in vivo models have helped advance understanding of the complex pathophysiology which leads to the development of celiac disease; such models are also paramount to testing new therapies. Of note, not all in vivo and in vitro models discussed in earlier portions of this paper have been utilized in testing new therapeutics. The following section will focus on recent developments in therapeutics and the models that have been utilized to ensure their safety and efficacy.
4.1 Wheat Alternatives and Alterations – Sorghum, C173, Hydrolyzed wheat, Transamidated wheat
Recognizing that immunogenic gluten is predominantly derived from wheat, other avenues of therapeutic research have investigated different alternatives to wheat as well as ways to alter wheat, rendering it less immunogenic to celiac patients.
Sorghum is a cereal grain related to maize that has been integral to the diet of people in Africa and Asia for thousands of years. Sorghum has recently been used to make multiple wheat-free products, including breads, tortillas, cookies, and flatbreads. In vitro organ culture studies of sorghum failed to show an increase in inflammatory markers. A five day challenge in two celiac patients did not result in gastrointestinal symptoms or serologic changes. 80. Further studies in larger populations and which include assessment of histology are needed before further recommendations can be made on the safety of sorghum for celiac patients.
Quinoa has often been touted as a safe grain for celiac patients and while most cultivars of quinoa are safe consumption, this is not true for all. Incubation of duodenal organ cultures from celiac patients on a gluten free diet with prolamins from different quinoa cultivars demonstrated that four out of fifteen cultivars had celiac-toxic epitopes with values within the range considered acceptable for gluten free designation. However, two cultivars had epitopes capable of stimulating the adaptive and innate immune responses33. Quinoa is not necessarily off the list of acceptable items for celiac patients; however it is important to note that not all quinoa is equally gluten-free.
The creation of gluten-free wheat would seem to be a logical therapeutic avenue; however, this is a challenging endeavor as gluten plays a key role in determining the visco-elasticity and polymerization of bread 81. Another approach to creating celiac-safe wheat is to breed and hybridize different wheat species to create a new species devoid of the immunogenic gluten derived proteins. Different wheat species and cultivars vary significantly in the levels of T-cell-stimulatory gluten epitopes 82.
C173 is an experimental wheat line bred from crossing two mutant plants with spontaneous deletions of several gliadins and glutenins; specifically Gli-A2, Gli-D1, and Gli-D3. Incubation of duodenal mucosal biopsies from treated and healed celiac patients with C173 did not decrease the villous to crypt ratio but was associated with increased levels of IFN-gamma, IL-2, IL-10, and anti-tTg antibodies in the collected supernatant 83. Lack of histologic changes would suggest that this wheat is less toxic; however, the continued production of inflammatory cytokines is of great concern. With increased and continued exposure to such wheat, histologic changes could potentially develop over time; therefore C173 may not be appropriate for celiac patients.
Another mechanism to render wheat less toxic is to ferment the wheat with specific combinations of lactobacilli and fungal proteases with the goal of completely hydrolyzing gluten 84. Hydrolyzed wheat flour still maintains its mechanical properties and therefore it can be used to make bread, pasta, and sweets 85, 86. Incubation of duodenal mucosa from celiac patients on a gluten free diet and healthy controls with this fermented wheat flour did not increase IFN-gamma mRNA levels 87. When consumed by celiac patients, this fermented bread does not change intestinal permeability 84. In a randomized trial of 6 patients, those who ingested 200 grams per day of fully hydrolyzed baked goods (containing 8ppm residual gluten) for 60 days did not report symptoms, nor did they demonstrate changes in morphology or serology86. A similar study involving ingestion of 200 grams per day of fully hydrolyzed sweet baked goods did not show a change in clinical complaints, serology, or intestinal permeability 87.
Another method to create less immunogenic wheat involves transamidation of the α-gliadin derived peptides by incubating commercial wheat flour with microbial transglutaminase and lysine methyl ester. Tests to evaluate the safety and efficacy of this altered wheat include T cell lines derived from duodenal biopsy cultures and clinical trials. T cell lines derived from 12 celiac patients were incubated with this altered wheat for 48 hours. Some of these patients had been treated with a gluten free diet, some had not. As a result of this incubation, INF-gamma expression was inhibited and binding of immunogenic peptides to DQ2 was decreased, though not completely eliminated 32. More recently, a randomized, single blinded, controlled 90 day trial examined the effect of such altered wheat (3.7 grams of transamidated gluten per day) in celiac patients previously maintained on a gluten free diet. Clinical relapse in the form of symptoms, changes in intestinal permeability, serology, histology, and intestinal IFN-gamma mRNA was diminished in the group receiving the deamidated wheat in comparison to the celiac patients receiving regular flour 88. While results are promising, they are not sufficiently adequate to suggest that transamidated wheat is ready to replace a gluten free diet.
These studies demonstrate that celiac safe wheat that retains necessary mechanical properties may be feasible; though further studies are required in larger populations to ensure that continued and prolonged consumption of such wheat does not result in delayed serologic and histology changes. It is unlikely that these specialized wheat varieties would be a financially acceptable option to patients with celiac disease.
4.2 Changing the face of gluten – Glutenases and Gluten Binders
It is well recognized that gluten triggers the inflammatory cascade associated with celiac disease. One therapeutic approach is to either change the gluten so that it is no longer immunogenic or to change the body’s ability to “see” and react to the gluten. This section will discuss the new developments in glutenases and gluten binders as they pertain to these therapeutic mechanisms since our last paper.
Glutenases are enzymes that splice glutamine and proline residues on gluten and thereby decrease the immunogencity of gluten. They are intended as supplements to a gluten free diet, with the aim of diminishing harmful effects associated with accidental gluten contamination. We will be discussing recent advances in the more promising glutenases; ALV003, AN-PEP, and Stan-1.
ALV003 contains two glutenases – a glutamine-specific cysteine endoprotease derived from germinating barley seeds (EP-B2) and a proline-specific prolyl endoprotease from Sphingomonas capsulate (SC-PEP)79. When studied in rats, ALV003 retains enzymatic activity in acidic environments such as the stomach and duodenum 89. Phase 1 clinical trials in healthy and celiac patients, have shown that 300 mg of ALV003 is safe and can degrade 88 ± 5% of 1 gram of gluten 90. A phase 2A clinical trial showed that celiac patients who consumed 2 grams of gluten daily with ALV003 for 6 weeks experienced no serologic changes as well as fewer symptoms and morphological changes, in comparison to celiac patients receiving placebo 91. Encouraging results from this study have prompted plans for a phase 2b study.
AN-PEP consists of a prolyl endopeptidase derived from Aspergillus Niger. In vitro studies have demonstrated that it retains activity at pHs comparable with the gastrointestinal lumen, is resistant to pepsin degradation, and is an effective glutenase 92. Use of a multi-compartmental in-vitro system simulating the gastrointestinal tract (stomach, duodenum, jejunum, ileum) has shown that “ingestion” of bread with AN-PEP leads to effective degradation of gluten in an acidic environment during the time span typically required for mechanical and chemical digestion in the stomach. In vitro studies have also shown that ANPEP eliminates gluten’s ability to stimulate T cells 93.
Based on these in vitro findings, a couple of clinical trials are underway. A randomized double-blind control trial studying the efficacy of AN-PEP to detoxify 8 grams of gluten in a commercial food product via assessment of histology and serology has been completed and results have been accepted for publication 94. Plans are underway for a randomized, double-blind crossover study to evaluate the effect of caloric density on AN-PEP’s enzymatic properties. This study is not yet recruiting participants95.
Another glutenase cocktail combines aspergillopepsin (ASP) from Aspergillus Niger in conjunction with dipeptidyl peptidase IV (DPPIV), an exopeptidase. ASP is not specific for immunotoxic gluten epitopes, but may aid in degradation of larger proteins into smaller peptides, thereby exposing target residues faster to more specific enzymes. DPPIV enhances ASP’s ability to degrade gluten; however it is inactive at a pH < 4 and therefore it requires concomitant use of antacids96. ASP may have a role as an adjunct to endopeptidases such as ALV003 and EP-B2.
STAN 1 is another enzyme cocktail commonly used in food supplements and appears to be an effective glutenase. A randomized, double-blind placebo controlled study found that ingestion of STAN-1 and 1 gram of gluten per day for 12 weeks did not change serology in celiac patients who previously had persistent positive serology despite adherence to a gluten free diet 97.
Glutenases physically change gluten so it loses its immunogenicity. Another mechanism to prevent gluten related immune responses is to use a polymeric binder designed to bind gluten. In the process of binding gluten, degradation and absorption are prevented, thereby allowing gluten to pass through the intestinal tract seemingly unnoticed.
Poly (hydroxythylmethacrylate-co-styrene sodium sulfonate) [P(HEMA-co-SS)] is a polymeric binder which effectively binds α-gliadin in an acidic environment. Testing of this binder in HLA-DQ8 mice receiving a gluten rich diet has shown decreased number of CD3+ IELs and decreased barrier dysfunction 59, 60. Incubation of duodenal biopsy cultures with partially digested gliadin and P(HEMA-co-SS) led to decreased secretion of TNF-α. 60. Clinical trials have not yet been started for this novel therapy.
4.3 Modifying the Microbiome – Probiotics and helminths
Knowing that microbiota and some parasites can affect intestinal permeability and/or exert an immunomodulatory effect, there is increased interest in identifying organisms which may reverse or block such key components of celiac disease pathogenesis. The use of probiotics and helminths are now being investigated as possible therapeutics for celiac disease.
Just as Caco-2 cell lines have shown that Bacteroides fragilis can potentiate the inflammatory response 18, this cell line has also been used to investigate bacteria which may ameliorate this response. Bifidobacterium longum is frequently diminished in the microbiome of celiac patients in comparison to normals. The addition of Bifidobacterium longum to Caco-2 cells exposed to gliadin is associated with decreased TNFα production and therefore may be associated with a decreased inflammatory potential 98.
Probiotics have also been studied extensively in HLA transgenic mice and rats47, 48, 64, 65. All of these studies indicate that probiotic therapy can ameliorate the inflammation induced by gluten and be useful as a supportive therapy for the gluten free diet; however, probiotics will probably not rise to the level of a monotherapy and will need to be administered indefinitely. For use as a monotherapy, further expansion on bioengineered bacteria would be necessary53.
Building upon these in vitro and in vivo models, a randomized, double-blind placebo controlled study investigated the use of Bifidobacterium infantis as a probiotic in celiac patients. Results of this study have been accepted for publication and will be available in the near future. In this study, celiac patients who were recently diagnosed by serology received two capsules (4× 109 colony forming units) of Bifidobacterium infantis with each meal while consuming 12 grams of gluten per day over a three week period. Discussion with the authors reveals that there was no change in intestinal permeability and post-trial duodenal biopsies showed villous atrophy. There was, however, a trend towards lower serologic values following treatment with this probiotic, as well as significant improvements in symptoms suggesting that this probiotic may be advantageous in celiac patients with more gastrointestinal complaints 99.
In addition to bacteria, helminths have also been considered as possible intestinal immunomodulators. Previous in vivo mouse models of peanut allergy have demonstrated that helminths are safe and are capable of suppressing the development of peanut specific IgE 100. Pig whipworm (Trichuris suis) and human hookworm have also been studied in IBD patients.
Expanding this possible therapy to the celiac population, a Phase 1a/2b trial investigated the ability of human hookworm (Necator Americanus) to inhibit immune responsiveness of healthy celiac patients when exposed to gluten. Patients were inoculated with the helminth and 20 weeks later were given a five day gluten challenge. Infection with this helminth was well tolerated from a safety and side effect profile. This intervention did not appear to prevent histologic or systemic inflammatory responses (as indicated by the presence of gluten-specific INF-gamma producing peripheral blood mononuclear cells) when comparing cases to controls, though there appeared to be a trend favoring reduced histologic damage and inflammatory response 101.
Approximately one year following this initial trial, the control patients were invited to participate in a follow-up trial which examined the cytokine profile that follows helminth infection and gluten challenge. Results of this study demonstrated decreased production of pro-inflammatory cytokines (IFN-gamma and IL-17A), suggesting that hookworm infection may shift the immune response towards a TH2 phenotype as opposed to a TH1/TH7 inflammatory response 102.
4.4 Protecting tight junctions and preventing changes in intestinal permeability – Larazotide acetate
Gluten is capable of inducing changes in tight junctions and intestinal permeability, a key step in the pathogenesis of celiac disease.TG2 inhibitors and Larazotide are therapeutics which aim to maintain the integrity of tight junctions as a way of preventing downstream inflammatory cascades 103, 104. TG2 inhibitors inhibit changes in tight junction permeability in Caco-2 cells 103 and are also effective in reducing the production of anti-TG2 antibodies and corresponding crypt cell proliferation when studied in duodenal biopsy cultures from untreated (gluten containing diet) celiac patients103.
Larazotide acetate is a synthetic peptide designed to prevent opening of tight junctions and has been shown to prevent gliadin associated changes in tight junctions and intestinal permeability in Caco-2 cells 104. Similar results were obtained in HLA-DQ8 transgenic mice, with which administration of larazotide acetate inhibited gliadin induced infiltration of inflammatory cells and maintained tight junction integrity 104.
Larazotide Acetate (aka AT1001) has undergone multiple Phase 2 clinical studies. Twelve milligrams of AT1001 was safe and well tolerated by 21 celiac patients while consuming a gluten containing diet. Preliminary results from this trial suggested AT1001 can prevent changes in intestinal permeability, decrease stimulation of IFN-gamma, and decrease gastrointestinal symptoms105. Following this study, a dose-ranging, placebo-controlled study at 10 clinical sites investigated AT1001 in 86 celiac patients. Patients were randomized to receive either AT1001 (0.25 mg, 1 mg, 4 mg, or 8mg) or placebo three times a day. Intestinal permeability was tested, but was highly variable with no difference observed between those on a gluten free diet and those on a gluten containing diet. Like the previous study, AT1001 was well tolerated and appeared to be effective at reducing gastrointestinal symptoms. The latter effect appeared to be most potent at the 0.25 and 4 mg doses 106. A Phase 2B study is currently recruiting patients to evaluate the efficacy and safety of different doses of AT1001 (0.5, 1 and 2 mg three times a day) in patients with celiac disease already on a gluten free diet, but with persistent symptoms107.
4.5 Blocking the inflammatory cascade – Ascorbate and anti-IL15 therapy
Another method of treating celiac disease is to target different key molecules in the inflammatory cascade. The addition of ascorbate (vitamin C) to gliadin exposed duodenal biopsies from treated (GFD) celiac patients was associated with diminished production of IFNγ, TNFα, IL-6, IFNα, IL-15 and IL-17. 108 Antibodies targeting the IL-15 receptor or IL-15 itself were successful in inhibiting villous atrophy in one of the IL-15 overexpressing mouse lines109, 110.
4.6 Vaccines - Nexvax2®
Stemming from experience with food and environmental allergies, another possible therapy is to create a vaccine that might induce gluten tolerance. Nexvax2® is a vaccine consisting of three deamidated gluten peptide sequences which are recognized by the majority of gluten-specific T cells. Nexvax2® has been studied in HLA DQ2-dependent mouse models of gluten immunity.111 A recent Phase 1 study investigated the safety of weekly injections of Nexvax2® in celiac patients on a gluten free diet. The vaccine was l well tolerated, although some gluten related side effects appeared to be more common with the higher doses. These gluten related side effects are likely due to the presence of deamidated gluten peptide sequences in the vaccine. Patients who received the vaccine also developed IFNγ producing anti-gluten T cells112.
4.7 Immunomodulators (CCX282b, aka Traficet-EN®)
A hallmark feature of celiac disease is the lymphocytic infiltration of the intestinal epithelium. Lymphocytes are directed to the intestine via stimulation of chemokine receptor 9 (CCR9) by its only ligand, CCL25. CCR9 has been implicated in the development of inflammatory bowel disease, celiac disease, and primary sclerosing cholangitis. CCX282-B is an antagonist of CCR9 and has been studied as a therapeutic agent for inflammatory bowel disease in the settings of Molt-4 cells and TNFαΔARE mice (mouse model for Crohns disease)113. A recent phase 2 clinical trial investigated the efficacy of CCX282-B in celiac patients. Patients received either CCX282-B (250 mg by mouth twice daily) or placebo for 13 weeks while consuming a gluten rich diet. Endpoints included histologic response, degree of lymphocytic infiltration, serologic changes, and patient reported symptoms. This study has been completed, but results are not yet available 107.
5. Expert Opinion
While celiac disease is one of the better understood autoimmune diseases, there are still a number of questions regarding the role of genetics, the intestinal microbiome, inflammatory cascades, and how environmental factors contribute to the development of this disease and its variable spectrum of phenotypic expression. Currently, the only therapy available is a gluten free diet, which, while effective, can be quite difficult for patients to follow, resulting in significant expenses, frustration, and feelings of social isolation. Use of in vitro and in vivo models including cell lines (Caco-2 and IEC-6), intestinal biopsy cultures, spontaneous in vivo models (dog and monkey), induced models (germ-free Wister AVN rats and mice), and transgenic models (mice) have allowed for significant advances in the understanding of the complex pathophysiology of this disease and testing of new and alternative therapies for celiac disease.
The use of helminths and probiotics are intriguing new alternative therapies for celiac disease. Their use is based on the “hygiene hypothesis” as well as increased recognition that the intestinal microbiota plays an interactive role with the gut immune system. Increased recognition of the mutualistic relationship humans share with intestinal microbiota has also led to increased understanding of how they can exert an immunomodulatory effect on the gut and how in the absence of such immunomodulation, there is an increased predisposition towards autoimmunity114. Clinical trials evaluating the use of such organisms have been published; further studies will be needed to define the appropriate dose and to determine if these interventions can deliver histologic, serologic, and symptomatic protection for celiac patients in the face of a gluten challenge. Certainly this concept is exciting as it makes the leap to restore balance to the gut’s immune system.
The search for celiac-safe wheat has been making progress, though creating wheat which retains important mechanical properties and is palatable are significant food engineering tasks. Current experimental wheat lines may be safer than regular wheat, but recent studies do not support that this wheat is safe enough for celiac patients. Truly celiac-safe wheat may be developed over time, though we anticipate such wheat may be quite expensive and may be cost prohibitive.
Glutenases and gluten binders continue to have great promise as an adjunctive therapy to a gluten free diet with the goal of mitigating adverse effects associated with accidental gluten exposures. A major limitation to the use of such products is that they would need to withstand the acidic environment of the stomach and also effectively degrade or bind all gluten-derived immunogenic peptides before the chyme enters the duodenum. These requirements are substantial and probably would prohibit the use of glutenases and gluten binders as a monotherapy in a gluten rich diet. Until a therapeutic agent is developed that would permit celiac patients to consume an unrestricted diet, glutenases and binders could play an important role as adjunctive therapy.
Therapies which target specific pathophysiologic processes (ie: changes in intestinal permeability (TG2 inhibitors, Larazotide acetetate), lymphocytic tracking to the intestinal epithelium (CCX282B), and inhibitors of inflammatory cascade markers (ascorbate, IL-15 antibodies), will likely hold the ultimate promise of treatment. It is also important to note that a “magic-bullet” may not be the solution to a complex disease such as celiac disease. Therapy may one day include a cocktail of medicines which addresses different aspects of the inflammatory cascade.
As we increase our knowledge of the complex pathophysiology underlying celiac disease, we will be able to create new disease targeted therapies. The in vitro and in vivo models discussed in this article will certainly remain as the first line of testing for all of these novel therapies. Ultimately, the goal would be to find a cure which reverses the immunologic cascade and thereby abolish the need for gluten avoidance.
Article Highlights.
Many more studies are using in vitro models as opposed to in vivo models to characterize the ability of gluten to stimulate and innate immune response.
A couple of new animal models of celiac disease have been generated, including one that uses horses.
HLA transgenic mice have been used in the majority of articles that utilize animal models of celiac disease.
A number of clinical trials have come to completion using the alternative therapies tested in the animal models. These have been (or soon will be) published.
Acknowledgments
Declaration of Interest
Authors declare support from the NIH and the Mayo Foundation. One author is also a consultant for Alvine Pharmaceuticals.
Footnotes
Declaration of Interest: The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Bibliography
* of interest
** of high interest
- 1. Rubio-Tapia A, Kyle RA, Kaplan EL, Johnson DR, Page W, Erdtmann F, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology. 2009 Jul;137(1):88–93. doi: 10.1053/j.gastro.2009.03.059. **Basis for why it is important to come up with effective alternative therapies for celiac disease.
- 2.Jabri B, Sollid LM. Mechanisms of disease: immunopathogenesis of celiac disease. Nat Clin Pract Gastroenterol Hepatol. 2006 Sep;3(9):516–525. doi: 10.1038/ncpgasthep0582. [DOI] [PubMed] [Google Scholar]
- 3.Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. 2011 Jan;91(1):151–175. doi: 10.1152/physrev.00003.2008. [DOI] [PubMed] [Google Scholar]
- 4.Bodd M, Kim CY, Lundin KE, Sollid LM. T-cell response to gluten in patients with HLA-DQ2.2 reveals requirement of peptide-MHC stability in celiac disease. Gastroenterology. 2012 Mar;142(3):552–561. doi: 10.1053/j.gastro.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Molberg O, McAdam SN, Korner R, Quarsten H, Kristiansen C, Madsen L, et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat Med. 1998 Jun;4(6):713–717. doi: 10.1038/nm0698-713. [DOI] [PubMed] [Google Scholar]
- 6.Abadie V, Sollid LM, Barreiro LB, Jabri B. Integration of genetic and immunological insights into a model of celiac disease pathogenesis. Annu Rev Immunol. 2011;29:493–525. doi: 10.1146/annurev-immunol-040210-092915. [DOI] [PubMed] [Google Scholar]
- 7.Jelinkova L, Tuckova L, Cinova J, Flegelova Z, Tlaskalova-Hogenova H. Gliadin stimulates human monocytes to production of IL-8 and TNF-alpha through a mechanism involving NF-kappaB. FEBS Lett. 2004 Jul 30;571(1–3):81–85. doi: 10.1016/j.febslet.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 8.Palova-Jelinkova L, Rozkova D, Pecharova B, Bartova J, Sediva A, Tlaskalova-Hogenova H, et al. Gliadin fragments induce phenotypic and functional maturation of human dendritic cells. Journal of Immunology. 2005 Nov 15;175(10):7038–7045. doi: 10.4049/jimmunol.175.10.7038. [DOI] [PubMed] [Google Scholar]
- 9.Barone MV, Zanzi D, Maglio M, Nanayakkara M, Santagata S, Lania G, et al. Gliadin-mediated proliferation and innate immune activation in celiac disease are due to alterations in vesicular trafficking. PLoS One. 2011;6(2):e17039. doi: 10.1371/journal.pone.0017039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marietta EV, Schuppan D, Murray JA. In vitro and in vivo models of celiac disease. Expert Opin Drug Dis. 2009 Nov;4(11):1113–1123. doi: 10.1517/17460440903307417. [DOI] [PubMed] [Google Scholar]
- 11.Clemente MG, De Virgiliis S, Kang JS, Macatagney R, Musu MP, Di Pierro MR, et al. Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut. 2003 Feb;52(2):218–223. doi: 10.1136/gut.52.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reinke Y, Behrendt M, Schmidt S, Zimmer KP, Naim HY. Impairment of protein trafficking by direct interaction of gliadin peptides with actin. Exp Cell Res. 2011 Sep 10;317(15):2124–2135. doi: 10.1016/j.yexcr.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 13.Zimmer KP, Fischer I, Mothes T, Weissen-Plenz G, Schmitz M, Wieser H, et al. Endocytotic segregation of gliadin peptide 31–49 in enterocytes. Gut. 2010 Mar;59(3):300–310. doi: 10.1136/gut.2008.169656. [DOI] [PubMed] [Google Scholar]
- 14.Barone MV, Nanayakkara M, Paolella G, Maglio M, Vitale V, Troiano R, et al. Gliadin peptide P31–43 localises to endocytic vesicles and interferes with their maturation. PLoS One. 2010;5(8):e12246. doi: 10.1371/journal.pone.0012246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caputo I, Secondo A, Lepretti M, Paolella G, Auricchio S, Barone MV, et al. Gliadin Peptides Induce Tissue Transglutaminase Activation and ER-Stress through Ca(2+) Mobilization in Caco-2 Cells. PLoS One. 2012;7(9):e45209. doi: 10.1371/journal.pone.0045209. *Use of Caco-2 Cells to demonstrate a possible link between the innate and adaptive responses to gluten.
- 16.Collado MC, Donat E, Ribes-Koninckx C, Calabuig M, Sanz Y. Specific duodenal and faecal bacterial groups associated with paediatric coeliac disease. J Clin Pathol. 2009 Mar;62(3):264–269. doi: 10.1136/jcp.2008.061366. [DOI] [PubMed] [Google Scholar]
- 17.Nadal I, Donat E, Ribes-Koninckx C, Calabuig M, Sanz Y. Imbalance in the composition of the duodenal microbiota of children with coeliac disease. J Med Microbiol. 2007 Dec;56(Pt 12):1669–1674. doi: 10.1099/jmm.0.47410-0. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez E, Laparra JM, Sanz Y. Discerning the role of Bacteroides fragilis in celiac disease pathogenesis. Applied and environmental microbiology. 2012 Sep;78(18):6507–6515. doi: 10.1128/AEM.00563-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol. 2005 Sep;6(9):895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 20.De Palma G, Kamanova J, Cinova J, Olivares M, Drasarova H, Tuckova L, et al. Modulation of phenotypic and functional maturation of dendritic cells by intestinal bacteria and gliadin: relevance for celiac disease. J Leukoc Biol. 2012 Aug 13; doi: 10.1189/jlb.1111581. [DOI] [PubMed] [Google Scholar]
- 21.Harris KM, Fasano A, Mann DL. Monocytes differentiated with IL-15 support Th17 and Th1 responses to wheat gliadin: implications for celiac disease. Clinical immunology. 2010 Jun;135(3):430–439. doi: 10.1016/j.clim.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molberg O, Kett K, Scott H, Thorsby E, Sollid LM, Lundin KE. Gliadin specific, HLA DQ2-restricted T cells are commonly found in small intestinal biopsies from coeliac disease patients, but not from controls. Scand J Immunol. 1997 Sep;46(3):103–109. doi: 10.1046/j.1365-3083.1997.d01-93.x. [DOI] [PubMed] [Google Scholar]
- 23. Lundin KE, Scott H, Hansen T, Paulsen G, Halstensen TS, Fausa O, et al. Gliadin-specific, HLA-DQ(alpha 1*0501,beta 1*0201) restricted T cells isolated from the small intestinal mucosa of celiac disease patients. J Exp Med. 1993 Jul 1;178(1):187–196. doi: 10.1084/jem.178.1.187. *Use of duodenal biopsies reveals a potential link between innate and adaptive responses in celiac disease.
- 24.Vader W, Stepniak D, Kooy Y, Mearin L, Thompson A, van Rood JJ, et al. The HLA-DQ2 gene dose effect in celiac disease is directly related to the magnitude and breadth of gluten-specific T cell responses. Proc Natl Acad Sci U S A. 2003 Oct 14;100(21):12390–12395. doi: 10.1073/pnas.2135229100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia J, Siegel M, Bergseng E, Sollid LM, Khosla C. Inhibition of HLA-DQ2-mediated antigen presentation by analogues of a high affinity 33-residue peptide from alpha2-gliadin. J Am Chem Soc. 2006 Feb 15;128(6):1859–1867. doi: 10.1021/ja056423o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindfors K, Rauhavirta T, Stenman S, Maki M, Kaukinen K. In vitro models for gluten toxicity: relevance for celiac disease pathogenesis and development of novel treatment options. Exp Biol Med (Maywood) 2012 Feb 1;237(2):119–125. doi: 10.1258/ebm.2011.011294. [DOI] [PubMed] [Google Scholar]
- 27.de Ritis G, Auricchio S, Jones HW, Lew EJ, Bernardin JE, Kasarda DD. In vitro (organ culture) studies of the toxicity of specific A-gliadin peptides in celiac disease. Gastroenterology. 1988 Jan;94(1):41–49. doi: 10.1016/0016-5085(88)90607-5. [DOI] [PubMed] [Google Scholar]
- 28.Howdle PD, Ciclitira PJ, Simpson FG, Losowsky MS. Are all gliadins toxic in coeliac disease? An in vitro study of alpha, beta, gamma, and w gliadins. Scand J Gastroenterol. 1984 Jan;19(1):41–47. [PubMed] [Google Scholar]
- 29.Lebreton C, Menard S, Abed J, Moura IC, Coppo R, Dugave C, et al. Interactions among secretory immunoglobulin A, CD71, and transglutaminase-2 affect permeability of intestinal epithelial cells to gliadin peptides. Gastroenterology. 2012 Sep;143(3):698–707. doi: 10.1053/j.gastro.2012.05.051. e1–4. [DOI] [PubMed] [Google Scholar]
- 30. Zanzi D, Stefanile R, Santagata S, Iaffaldano L, Iaquinto G, Giardullo N, et al. IL-15 interferes with suppressive activity of intestinal regulatory T cells expanded in Celiac disease. Am J Gastroenterol. 2011 Jul;106(7):1308–1317. doi: 10.1038/ajg.2011.80. *Use of duodenal biopsies reveals a potential link between innate and adaptive responses in celiac disease.
- 31.Castellanos-Rubio A, Santin I, Martin-Pagola A, Irastorza I, Castano L, Vitoria JC, et al. Long-term and acute effects of gliadin on small intestine of patients on potentially pathogenic networks in celiac disease. Autoimmunity. 2010 Mar;43(2):131–139. doi: 10.3109/08916930903225229. [DOI] [PubMed] [Google Scholar]
- 32.Gianfrani C, Siciliano RA, Facchiano AM, Camarca A, Mazzeo MF, Costantini S, et al. Transamidation of wheat flour inhibits the response to gliadin of intestinal T cells in celiac disease. Gastroenterology. 2007 Sep;133(3):780–789. doi: 10.1053/j.gastro.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 33.Zevallos VF, Ellis HJ, Suligoj T, Herencia LI, Ciclitira PJ. Variable activation of immune response by quinoa (Chenopodium quinoa Willd.) prolamins in celiac disease. The American journal of clinical nutrition. 2012 Aug;96(2):337–344. doi: 10.3945/ajcn.111.030684. [DOI] [PubMed] [Google Scholar]
- 34.Maglio M, Mazzarella G, Barone MV, Gianfrani C, Pogna N, Gazza L, et al. Immunogenicity of two oat varieties, in relation to their safety for celiac patients. Scand J Gastroenterol. 2011 Oct;46(10):1194–1205. doi: 10.3109/00365521.2011.603159. [DOI] [PubMed] [Google Scholar]
- 35.Gianfrani C, Maglio M, Rotondi Aufiero V, Camarca A, Vocca I, Iaquinto G, et al. Immunogenicity of monococcum wheat in celiac patients. The American journal of clinical nutrition. 2012 Dec;96(6):1339–1345. doi: 10.3945/ajcn.112.040485. [DOI] [PubMed] [Google Scholar]
- 36. van der Kolk JH, van Putten LA, Mulder CJ, Grinwis GC, Reijm M, Butler CM, et al. Gluten-dependent antibodies in horses with inflammatory small bowel disease (ISBD) Vet Q. 2012 Apr 10; doi: 10.1080/01652176.2012.675636. **Potential new animal model of celiac disease.
- 37.Batt RM, Carter MW, McLean L. Wheat-sensitive enteropathy in Irish setter dogs: possible age-related brush border abnormalities. Res Vet Sci. 1985 Jul;39(1):80–83. [PubMed] [Google Scholar]
- 38.Batt RM, McLean L, Carter MW. Sequential morphologic and biochemical studies of naturally occurring wheat-sensitive enteropathy in Irish setter dogs. Dig Dis Sci. 1987 Feb;32(2):184–194. doi: 10.1007/BF01297107. [DOI] [PubMed] [Google Scholar]
- 39.Hall EJ, Batt RM. Dietary modulation of gluten sensitivity in a naturally occurring enteropathy of Irish setter dogs. Gut. 1992 Feb;33(2):198–205. doi: 10.1136/gut.33.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polvi A, Garden OA, Houlston RS, Maki M, Batt RM, Partanen J. Genetic susceptibility to gluten sensitive enteropathy in Irish setter dogs is not linked to the major histocompatibility complex. Tissue Antigens. 1998 Dec;52(6):543–549. doi: 10.1111/j.1399-0039.1998.tb03085.x. [DOI] [PubMed] [Google Scholar]
- 41.Bethune MT, Borda JT, Ribka E, Liu MX, Phillippi-Falkenstein K, Jandacek RJ, et al. A non-human primate model for gluten sensitivity. PLoS One. 2008;3(2):e1614. doi: 10.1371/journal.pone.0001614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sestak K, Mazumdar K, Midkiff CC, Dufour J, Borda JT, Alvarez X. Recognition of Epidermal Transglutaminase by IgA and Tissue Transglutaminase 2 Antibodies in a Rare Case of <em>Rhesus</em> Dermatitis. J Vis Exp. 2011;(58) doi: 10.3791/3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sardy M, Karpati S, Merkl B, Paulsson M, Smyth N. Epidermal transglutaminase (TGase 3) is the autoantigen of dermatitis herpetiformis. J Exp Med. 2002 Mar 18;195(6):747–757. doi: 10.1084/jem.20011299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marietta E, Black K, Camilleri M, Krause P, Rogers RS, 3rd, David C, et al. A new model for dermatitis herpetiformis that uses HLA-DQ8 transgenic NOD mice. J Clin Invest. 2004 Oct;114(8):1090–1097. doi: 10.1172/JCI21055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bethune MT, Ribka E, Khosla C, Sestak K. Transepithelial transport and enzymatic detoxification of gluten in gluten-sensitive rhesus macaques. PLoS One. 2008;3(3):e1857. doi: 10.1371/journal.pone.0001857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stepankova R, Tlaskalova-Hogenova H, Sinkora J, Jodl J, Fric P. Changes in jejunal mucosa after long-term feeding of germfree rats with gluten. Scand J Gastroenterol. 1996 Jun;31(6):551–557. doi: 10.3109/00365529609009127. [DOI] [PubMed] [Google Scholar]
- 47. Laparra JM, Olivares M, Gallina O, Sanz Y. Bifidobacterium longum CECT 7347 modulates immune responses in a gliadin-induced enteropathy animal model. PLoS One. 2012;7(2):e30744. doi: 10.1371/journal.pone.0030744. **Demonstrates, using a rat model, the ability of a probiotic in suppressing some of gluten’s deleterious effects.
- 48.Olivares M, Laparra M, Sanz Y. Oral administration of Bifidobacterium longum CECT 7347 modulates jejunal proteome in an in vivo gliadin-induced enteropathy animal model. J Proteomics. 2012 Sep 27; doi: 10.1016/j.jprot.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 49.Cinova J, De Palma G, Stepankova R, Kofronova O, Kverka M, Sanz Y, et al. Role of intestinal bacteria in gliadin-induced changes in intestinal mucosa: study in germ-free rats. PloS one. 2011;6(1):e16169. doi: 10.1371/journal.pone.0016169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Freitag TL, Rietdijk S, Junker Y, Popov Y, Bhan AK, Kelly CP, et al. Gliadin-primed CD4+CD45RBlowCD25- T cells drive gluten-dependent small intestinal damage after adoptive transfer into lymphopenic mice. Gut. 2009 Dec;58(12):1597–1605. doi: 10.1136/gut.2009.186361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Papista C, Gerakopoulos V, Kourelis A, Sounidaki M, Kontana A, Berthelot L, et al. Gluten induces coeliac-like disease in sensitised mice involving IgA, CD71 and transglutaminase 2 interactions that are prevented by probiotics. Lab Invest. 2012 Apr;92(4):625–635. doi: 10.1038/labinvest.2012.13. [DOI] [PubMed] [Google Scholar]
- 52.Black KE, Murray JA, David CS. HLA-DQ determines the response to exogenous wheat proteins: a model of gluten sensitivity in transgenic knockout mice. Journal of Immunology. 2002 Nov 15;169(10):5595–5600. doi: 10.4049/jimmunol.169.10.5595. [DOI] [PubMed] [Google Scholar]
- 53.Huibregtse IL, Marietta EV, Rashtak S, Koning F, Rottiers P, David CS, et al. Induction of antigen-specific tolerance by oral administration of Lactococcus lactis delivered immunodominant DQ8-restricted gliadin peptide in sensitized nonobese diabetic Abo Dq8 transgenic mice. J Immunol. 2009 Aug 15;183(4):2390–2396. doi: 10.4049/jimmunol.0802891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hovhannisyan Z, Weiss A, Martin A, Wiesner M, Tollefsen S, Yoshida K, et al. The role of HLA-DQ8 beta57 polymorphism in the anti-gluten T-cell response in coeliac disease. Nature. 2008 Nov 27;456(7221):534–538. doi: 10.1038/nature07524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. DePaolo RW, Abadie V, Tang F, Fehlner-Peach H, Hall JA, Wang W, et al. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature. 2011 Mar 10;471(7337):220–224. doi: 10.1038/nature09849. **Unique combination of transgenes to address both inate and adaptive responses to gluten.
- 56.Verdu EF, Huang X, Natividad J, Lu J, Blennerhassett PA, David CS, et al. Gliadin-dependent neuromuscular and epithelial secretory responses in gluten-sensitive HLA-DQ8 transgenic mice. Am J Physiol Gastrointest Liver Physiol. 2008 Jan;294(1):G217–G225. doi: 10.1152/ajpgi.00225.2007. [DOI] [PubMed] [Google Scholar]
- 57.Natividad JM, Huang X, Slack E, Jury J, Sanz Y, David C, et al. Host responses to intestinal microbial antigens in gluten-sensitive mice. PloS one. 2009;4(7):e6472. doi: 10.1371/journal.pone.0006472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Galipeau HJ, Rulli NE, Jury J, Huang X, Araya R, Murray JA, et al. Sensitization to Gliadin Induces Moderate Enteropathy and Insulitis in Nonobese Diabetic-DQ8 Mice. J Immunol. 2011 Oct 15;187(8):4338–4346. doi: 10.4049/jimmunol.1100854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pinier M, Verdu EF, Nasser-Eddine M, David CS, Vezina A, Rivard N, et al. Polymeric binders suppress gliadin-induced toxicity in the intestinal epithelium. Gastroenterology. 2009 Jan;136(1):288–298. doi: 10.1053/j.gastro.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 60.Pinier M, Fuhrmann G, Galipeau HJ, Rivard N, Murray JA, David CS, et al. The copolymer P(HEMA-co-SS) binds gluten and reduces immune response in gluten-sensitized mice and human tissues. Gastroenterology. 2012 Feb;142(2):316–325. doi: 10.1053/j.gastro.2011.10.038. e1–12. [DOI] [PubMed] [Google Scholar]
- 61.Silva MA, Jury J, Sanz Y, Wiepjes M, Huang X, Murray JA, et al. Increased bacterial translocation in gluten-sensitive mice is independent of small intestinal paracellular permeability defect. Dig Dis Sci. 2012 Jan;57(1):38–47. doi: 10.1007/s10620-011-1847-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.D'Arienzo R, Maurano F, Luongo D, Mazzarella G, Stefanile R, Troncone R, et al. Adjuvant effect of Lactobacillus casei in a mouse model of gluten sensitivity. Immunol Lett. 2008 Aug 15;119(1–2):78–83. doi: 10.1016/j.imlet.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 63.D'Arienzo R, Stefanile R, Maurano F, Luongo D, Bergamo P, Mazzarella G, et al. A deregulated immune response to gliadin causes a decreased villus height in DQ8 transgenic mice. Eur J Immunol. 2009 Dec;39(12):3552–3561. doi: 10.1002/eji.200839161. [DOI] [PubMed] [Google Scholar]
- 64.D'Arienzo R, Stefanile R, Maurano F, Mazzarella G, Ricca E, Troncone R, et al. Immunomodulatory effects of Lactobacillus casei administration in a mouse model of gliadin-sensitive enteropathy. Scand J Immunol. 2011 Oct;74(4):335–341. doi: 10.1111/j.1365-3083.2011.02582.x. [DOI] [PubMed] [Google Scholar]
- 65.D'Arienzo R, Maurano F, Lavermicocca P, Ricca E, Rossi M. Modulation of the immune response by probiotic strains in a mouse model of gluten sensitivity. Cytokine. 2009 Dec;48(3):254–259. doi: 10.1016/j.cyto.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 66.Senger S, Maurano F, Mazzeo MF, Gaita M, Fierro O, David CS, et al. Identification of immunodominant epitopes of alpha-gliadin in HLA-DQ8 transgenic mice following oral immunization. J Immunol. 2005 Dec 15;175(12):8087–8095. doi: 10.4049/jimmunol.175.12.8087. [DOI] [PubMed] [Google Scholar]
- 67.Senger S, Luongo D, Maurano F, Mazzeo MF, Siciliano RA, Gianfrani C, et al. Intranasal administration of a recombinant alpha-gliadin down-regulates the immune response to wheat gliadin in DQ8 transgenic mice. Immunol Lett. 2003 Aug 5;88(2):127–134. doi: 10.1016/s0165-2478(03)00069-5. [DOI] [PubMed] [Google Scholar]
- 68.Bergamo P, Gogliettino M, Palmieri G, Cocca E, Maurano F, Stefanile R, et al. Conjugated linoleic acid protects against gliadin-induced depletion of intestinal defenses. Mol Nutr Food Res. 2011 Sep;55(Suppl 2):S248–S256. doi: 10.1002/mnfr.201100295. [DOI] [PubMed] [Google Scholar]
- 69.de Kauwe AL, Chen Z, Anderson RP, Keech CL, Price JD, Wijburg O, et al. Resistance to celiac disease in humanized HLA-DR3-DQ2-transgenic mice expressing specific anti-gliadin CD4+ T cells. Journal of Immunology. 2009 Jun 15;182(12):7440–7450. doi: 10.4049/jimmunol.0900233. [DOI] [PubMed] [Google Scholar]
- 70.Ciccocioppo R, Rossi M, Pesce I, Ricci G, Millimaggi D, Maurano F, et al. Effects of gliadin stimulation on bone marrow-derived dendritic cells from HLA-DQ8 transgenic MICE. Dig Liver Dis. 2008 Dec;40(12):927–935. doi: 10.1016/j.dld.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 71.Chen D, Ueda R, Harding F, Patil N, Mao Y, Kurahara C, et al. Characterization of HLA DR3/DQ2 transgenic mice: a potential humanized animal model for autoimmune disease studies. Eur J Immunol. 2003 Jan;33(1):172–182. doi: 10.1002/immu.200390020. [DOI] [PubMed] [Google Scholar]
- 72. Du Pre MF, Kozijn AE, van Berkel LA, ter Borg MN, Lindenbergh-Kortleve D, Jensen LT, et al. Tolerance to ingested deamidated gliadin in mice is maintained by splenic, type 1 regulatory T cells. Gastroenterology. 2011 Aug;141(2):610–620. doi: 10.1053/j.gastro.2011.04.048. 20 e1–2. *Use of a new line of HLA-DQ2 transgenic mice reveals important information on regulatory T cells in gluten sensitivity.
- 73.Zhou R, Wei H, Sun R, Tian Z. Recognition of double-stranded RNA by TLR3 induces severe small intestinal injury in mice. Journal of Immunology. 2007 Apr 1;178(7):4548–4556. doi: 10.4049/jimmunol.178.7.4548. [DOI] [PubMed] [Google Scholar]
- 74.Dafik L, Albertelli M, Stamnaes J, Sollid LM, Khosla C. Activation and inhibition of transglutaminase 2 in mice. PLoS One. 2012;7(2):e30642. doi: 10.1371/journal.pone.0030642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ohta N, Hiroi T, Kweon MN, Kinoshita N, Jang MH, Mashimo T, et al. IL-15-dependent activation-induced cell death-resistant Th1 type CD8 alpha beta+NK1.1+ T cells for the development of small intestinal inflammation. Journal of Immunology. 2002 Jul 1;169(1):460–468. doi: 10.4049/jimmunol.169.1.460. [DOI] [PubMed] [Google Scholar]
- 76.Yokoyama S, Takada K, Hirasawa M, Perera LP, Hiroi T. Transgenic mice that overexpress human IL-15 in enterocytes recapitulate both B and T cell-mediated pathologic manifestations of celiac disease. J Clin Immunol. 2011 Dec;31(6):1038–1044. doi: 10.1007/s10875-011-9586-7. [DOI] [PubMed] [Google Scholar]
- 77.Catassi C, Fabiani E, Iacono G, D'Agate C, Francavilla R, Biagi F, et al. A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. The American journal of clinical nutrition. 2007 Jan;85(1):160–166. doi: 10.1093/ajcn/85.1.160. [DOI] [PubMed] [Google Scholar]
- 78.Akobeng AK, Thomas AG. Systematic review: tolerable amount of gluten for people with coeliac disease. Aliment Pharmacol Ther. 2008 Jun 1;27(11):1044–1052. doi: 10.1111/j.1365-2036.2008.03669.x. [DOI] [PubMed] [Google Scholar]
- 79.Tye-Din JA, Anderson RP, Ffrench RA, Brown GJ, Hodsman P, Siegel M, et al. The effects of ALV003 pre-digestion of gluten on immune response and symptoms in celiac disease in vivo. Clinical immunology. 2010 Mar;134(3):289–295. doi: 10.1016/j.clim.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 80.Ciacci C, Maiuri L, Caporaso N, Bucci C, Del Giudice L, Rita Massardo D, et al. Celiac disease: in vitro and in vivo safety and palatability of wheat-free sorghum food products. Clin Nutr. 2007 Dec;26(6):799–805. doi: 10.1016/j.clnu.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 81.van den Broeck HC, van Herpen TW, Schuit C, Salentijn EM, Dekking L, Bosch D, et al. Removing celiac disease-related gluten proteins from bread wheat while retaining technological properties: a study with Chinese Spring deletion lines. BMC Plant Biol. 2009;9:41. doi: 10.1186/1471-2229-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spaenij-Dekking L, Kooy-Winkelaar Y, van Veelen P, Drijfhout JW, Jonker H, van Soest L, et al. Natural variation in toxicity of wheat: potential for selection of nontoxic varieties for celiac disease patients. Gastroenterology. 2005 Sep;129(3):797–806. doi: 10.1053/j.gastro.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 83.Carroccio A, Di Prima L, Noto D, Fayer F, Ambrosiano G, Villanacci V, et al. Searching for wheat plants with low toxicity in celiac disease: Between direct toxicity and immunologic activation. Dig Liver Dis. 2011 Jan;43(1):34–39. doi: 10.1016/j.dld.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 84.Di Cagno R, De Angelis M, Auricchio S, Greco L, Clarke C, De Vincenzi M, et al. Sourdough bread made from wheat and nontoxic flours and started with selected lactobacilli is tolerated in celiac sprue patients. Applied and environmental microbiology. 2004 Feb;70(2):1088–1096. doi: 10.1128/AEM.70.2.1088-1096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.di Cagno R, de Angelis M, Alfonsi G, de Vincenzi M, Silano M, Vincentini O, et al. Pasta made from durum wheat semolina fermented with selected lactobacilli as a tool for a potential decrease of the gluten intolerance. J Agric Food Chem. 2005 Jun 1;53(11):4393–4402. doi: 10.1021/jf048341+. [DOI] [PubMed] [Google Scholar]
- 86.Greco L, Gobbetti M, Auricchio R, Di Mase R, Landolfo F, Paparo F, et al. Safety for patients with celiac disease of baked goods made of wheat flour hydrolyzed during food processing. Clin Gastroenterol Hepatol. 2011 Jan;9(1):24–29. doi: 10.1016/j.cgh.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 87.Di Cagno R, Barbato M, Di Camillo C, Rizzello CG, De Angelis M, Giuliani G, et al. Gluten-free sourdough wheat baked goods appear safe for young celiac patients: a pilot study. J Pediatr Gastroenterol Nutr. 2010 Dec;51(6):777–783. doi: 10.1097/MPG.0b013e3181f22ba4. [DOI] [PubMed] [Google Scholar]
- 88.Mazzarella G, Salvati VM, Iaquinto G, Stefanile R, Capobianco F, Luongo D, et al. Reintroduction of gluten following flour transamidation in adult celiac patients: a randomized, controlled clinical study. Clin Dev Immunol. 2012;2012 doi: 10.1155/2012/329150. 329150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gass J, Bethune MT, Siegel M, Spencer A, Khosla C. Combination enzyme therapy for gastric digestion of dietary gluten in patients with celiac sprue. Gastroenterology. 2007 Aug;133(2):472–480. doi: 10.1053/j.gastro.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 90. Siegel M, Garber ME, Spencer AG, Botwick W, Kumar P, Williams RN, et al. Safety, tolerability, and activity of ALV003: results from two phase 1 single, escalating-dose clinical trials. Dig Dis Sci. 2012 Feb;57(2):440–450. doi: 10.1007/s10620-011-1906-5. *Highlights the potential use of glutenases in treating celiac disease.
- 91.Lahdeaho M, Maki M, Kaukinen K, Laurila K, A M, Adelman D. ALV003, a novel gluteanase, attneuates gluten-induced small intestinal mucosal injury in Celiac Disease patients: a randomized phase 2A clinical trial. In: Federation UEG, editor. United European Gastroenterology Week; 2011. Stockholm: 2011. p. 57. [Google Scholar]
- 92.Stepniak D, Spaenij-Dekking L, Mitea C, Moester M, de Ru A, Baak-Pablo R, et al. Highly efficient gluten degradation with a newly identified prolyl endoprotease: implications for celiac disease. Am J Physiol Gastrointest Liver Physiol. 2006 Oct;291(4):G621–G629. doi: 10.1152/ajpgi.00034.2006. [DOI] [PubMed] [Google Scholar]
- 93.Mitea C, Havenaar R, Drijfhout JW, Edens L, Dekking L, Koning F. Efficient degradation of gluten by a prolyl endoprotease in a gastrointestinal model: implications for coeliac disease. Gut. 2008 Jan;57(1):25–32. doi: 10.1136/gut.2006.111609. [DOI] [PubMed] [Google Scholar]
- 94.VU University Medical Center. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); [2000- [cited 2012 Apr 16]]. Effect of Aspergillus Niger Prolyl Endoprotease (AN-PEP) Enzyme on the Effects of Gluten Ingestion in Patients with Coeliac Disease. Available from: http://clinicaltrials.gov/show/NCT00810654 NLM Identifier: NCT00810654. [Google Scholar]
- 95.DSM Food Specialties. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); [2000- [cited 2012 Apr 16]]. Effect of AN-PEP Enzyme on Gluten Digestion. Available from: http://clinicaltrials.gov/show/NCT01335503 NLM Identifier: NCT01335503. [Google Scholar]
- 96.Ehren J, Moron B, Martin E, Bethune MT, Gray GM, Khosla C. A food-grade enzyme preparation with modest gluten detoxification properties. PLoS One. 2009;4(7):e6313. doi: 10.1371/journal.pone.0006313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Korponay-Szabo IR, Tumpek J, Gyimesi J, Laurila K, Papp M, Maki M, et al. FOOD-GRADE GLUTEN DEGRADING ENZYMES TO TREAT DIETARY TRANSGRESSIONS IN COELIAC ADOLESCENTS. J Pediatr Gastroenterol Nutr. 2010 Jun;50:E68-E. [Google Scholar]
- 98.Olivares M, Laparra M, Sanz Y. Influence of Bifidobacterium longum CECT 7347 and gliadin peptides on intestinal epithelial cell proteome. J Agric Food Chem. 2011 Jul 27;59(14):7666–7671. doi: 10.1021/jf201212m. [DOI] [PubMed] [Google Scholar]
- 99.Smecuol E, Sugai E, Niveloni S, Vazquez H, Pedreira S, Mazure R, et al. Permeability, zonulin production, and enteropathy in dermatitis herpetiformis. Clin Gastroenterol Hepatol. 2005 Apr;3(4):335–341. doi: 10.1016/s1542-3565(04)00778-5. [DOI] [PubMed] [Google Scholar]
- 100.Bashir ME, Andersen P, Fuss IJ, Shi HN, Nagler-Anderson C. An enteric helminth infection protects against an allergic response to dietary antigen. Journal of Immunology. 2002 Sep 15;169(6):3284–3292. doi: 10.4049/jimmunol.169.6.3284. [DOI] [PubMed] [Google Scholar]
- 101. Daveson AJ, Jones DM, Gaze S, McSorley H, Clouston A, Pascoe A, et al. Effect of hookworm infection on wheat challenge in celiac disease--a randomised double-blinded placebo controlled trial. PLoS One. 2011;6(3):e17366. doi: 10.1371/journal.pone.0017366. **Intriguing new therapy for celiac disease based on animal models of food allergies.
- 102.McSorley HJ, Gaze S, Daveson J, Jones D, Anderson RP, Clouston A, et al. Suppression of inflammatory immune responses in celiac disease by experimental hookworm infection. PLoS One. 2011;6(9):e24092. doi: 10.1371/journal.pone.0024092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rauhavirta T, Oittinen M, Kivisto R, Mannisto PT, Garcia-Horsman JA, Wang Z, et al. Are Transglutaminase 2 Inhibitors Able to Reduce Gliadin-Induced Toxicity Related to Celiac Disease? A Proof-of-Concept Study. J Clin Immunol. 2012 Aug 10; doi: 10.1007/s10875-012-9745-5. [DOI] [PubMed] [Google Scholar]
- 104. Gopalakrishnan S, Durai M, Kitchens K, Tamiz AP, Somerville R, Ginski M, et al. Larazotide acetate regulates epithelial tight junctions in vitro and in vivo. Peptides. 2012 Feb 27; doi: 10.1016/j.peptides.2012.02.015. *Use of both an in vitro and in vivo model to test a novel therapy based on gluten’s stimulation of an innate immune response.
- 105.Paterson BM, Lammers KM, Arrieta MC, Fasano A, Meddings JB. The safety, tolerance, pharmacokinetic and pharmacodynamic effects of single doses of AT-1001 in coeliac disease subjects: a proof of concept study. Aliment Pharmacol Ther. 2007 Sep 1;26(5):757–766. doi: 10.1111/j.1365-2036.2007.03413.x. [DOI] [PubMed] [Google Scholar]
- 106.Leffler DA, Kelly CP, Abdallah HZ, Colatrella AM, Harris LA, Leon F, et al. A randomized, double-blind study of larazotide acetate to prevent the activation of celiac disease during gluten challenge. Am J Gastroenterol. 2012 Oct;107(10):1554–1562. doi: 10.1038/ajg.2012.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Alba Therapeutics. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); [2000- [cited 2012 Oct 31]]. A Double-blind Placebo-controlled Study to Evaluate Larazotide Acetate for the Treatment of Celiac Disease. Available from http://clinicaltrials.gov/ct2/show/NCT01396213?term=01396213&rank=1 NLM Identifier: NCT01396213. [Google Scholar]
- 108.Bernardo D, Martinez-Abad B, Vallejo-Diez S, Montalvillo E, Benito V, Anta B, et al. Ascorbate-dependent decrease of the mucosal immune inflammatory response to gliadin in coeliac disease patients. Allergol Immunopathol (Madr) 2012 Jan-Feb;40(1):3–8. doi: 10.1016/j.aller.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 109.Yokoyama S, Watanabe N, Sato N, Perera PY, Filkoski L, Tanaka T, et al. Antibody-mediated blockade of IL-15 reverses the autoimmune intestinal damage in transgenic mice that overexpress IL-15 in enterocytes. Proc Natl Acad Sci U S A. 2009 Sep 15;106(37):15849–15854. doi: 10.1073/pnas.0908834106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Malamut G, El Machhour R, Montcuquet N, Martin-Lanneree S, Dusanter-Fourt I, Verkarre V, et al. IL-15 triggers an antiapoptotic pathway in human intraepithelial lymphocytes that is a potential new target in celiac disease-associated inflammation and lymphomagenesis. J Clin Invest. 2010 Jun 1;120(6):2131–2143. doi: 10.1172/JCI41344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Keech CL, Dromey J, Chen ZJ, Anderson RP, McCluskey J. Immune Tolerance Induced By Peptide Immunotherapy in An HLA Dq2-Dependent Mouse Model of Gluten Immunity. Gastroenterology. 2009 May;136(5):A57-A. [Google Scholar]
- 112.Brown GJ, Daveson J, Marjason JK, Ffrench RA, Smith D, Sullivan M, et al . A Phase 1 study to determine safety, tolerability, and bioactivityof Nexvax2® in HLA DQ-2+ volunteers with celiac diseas following a long-term, strict gluten-free diet. Gastroenterology. 2011;140:S437–S438. [Google Scholar]
- 113.Walters MJ, Wang Y, Lai N, Baumgart T, Zhao BN, Dairaghi DJ, et al. Characterization of CCX282-B, an orally bioavailable antagonist of the CCR9 chemokine receptor, for treatment of inflammatory bowel disease. J Pharmacol Exp Ther. 2010 Oct;335(1):61–69. doi: 10.1124/jpet.110.169714. [DOI] [PubMed] [Google Scholar]
- 114.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012 Jun 8;336(6086):1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]