Abstract
Brain iron overload has a detrimental role in brain injury after intracerebral hemorrhage (ICH). Lipocalin 2 (LCN2), a siderophore-binding protein, is involved in cellular iron transport. The present study investigated changes in LCN2 expression after ICH and the role of iron in those changes. Male Sprague-Dawley rats had an intracaudate injection of autologous blood (ICH) or iron. Control rats received a needle insertion or saline injection. Some ICH animals were treated with either vehicle or deferoxamine, an iron chelator. Brain LCN2 expression was determined by Western blot analysis and immunohistochemistry. Real time PCR was also used to confirm brain LCN2 mRNA expression. The number of LCN2 positive cells was markedly increased in the ipsilateral basal ganglia and cortex after ICH and most LCN2 positive cells were astrocytes. Western blots showed that brain LCN2 levels were higher at days 1, 3 and 7 in the ipsilateral hemisphere after ICH (70 to 80 fold higher than contralateral hemisphere or sham-operated rats at 3 days), and declined to lower levels at day 14. Iron, but not saline injection also caused brain LCN2 upregulation (a more than 100-fold increase). In addition, systemic treatment of deferoxamine reduced ICH-induced LCN2 upregulation (p<0.05). These results suggest iron has a role in brain LCN2 upregulation following ICH. LCN2 upregulation after ICH may be part of the response to clear iron released from the hematoma during clot resolution.
Keywords: cerebral hemorrhage, lipocalin-2, iron, deferoxamine, immunohistochemistry
1. Introduction
Intracerebral hemorrhage (ICH) is a devastating form of stroke with high morbidity and mortality (Qureshi et al., 2009; Rincon and Mayer, 2012). Evidence suggests that iron-overload is involved in ICH-induced brain damage resulting in perihematoma edema, neuronal death and brain atrophy (Keep et al., 2012; Xi et al., 2006). We have demonstrated that deferoxamine, an iron chelator, reduces ICH-induced brain injury in young and aged rats (Okauchi et al., 2010; Xi et al., 2006), as well as in pigs (Gu et al., 2009). Clinical studies also found that high levels of serum ferritin, an iron storage protein, are independently associated with severe brain edema and poor prognosis in ICH patients (Mehdiratta et al., 2008; Perez de la Ossa et al., 2010), suggesting a role of iron in ICH-induced brain injury(Selim et al., 2011).
Lipocalin 2 (LCN2), also known as neutrophil gelatinase associated lipocalin (NGAL) or 24p3, is a siderophore-binding protein. LCN2 mediates iron uptake in cells expressing the LCN2 cell surface receptor, LCN2R (24p3R) (Devireddy et al., 2005). Recently, the function of LCN2 within the CNS has started to be elucidated. In vivo studies have demonstrated LCN2 expression in the choroid plexus in response to peripheral lipopolysaccharide administration (Marques et al., 2008) and excitotoxic brain injury (Chia et al., 2011). Recent studies showed that LCN2 plays a detrimental role in the CNS after spinal cord injury (Rathore et al., 2011) but it appears to play a protective role in experimental autoimmune encephalomyelitis (Berard et al., 2012).
In the present study, we examined the time course of brain LCN2 expression in a rat model of ICH. The role of iron in ICH-induced LCN2 expression was also examined.
2. Results
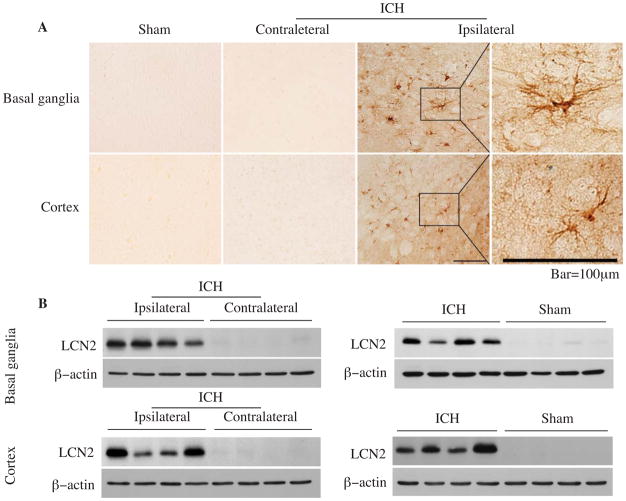
ICH-induced changes in LCN2 were measured by immunohistochemistry and Western blotting. LCN2 positive cells were found in the ipsilateral basal ganglia but not the contralateral basal ganglia at days 1, 3, 7 and 14 after ICH (Fig. 1A). The LCN2 protein levels determined by Western blotting in the ipsilateral basal ganglia were significantly increased at day 1, remained at high levels at days 3 and 7, and the decreased markedly at day 14 after ICH (Fig. 1B). Similar results were found in the ipsilateral cortex following ICH (Fig. 1C).
Figure 1.
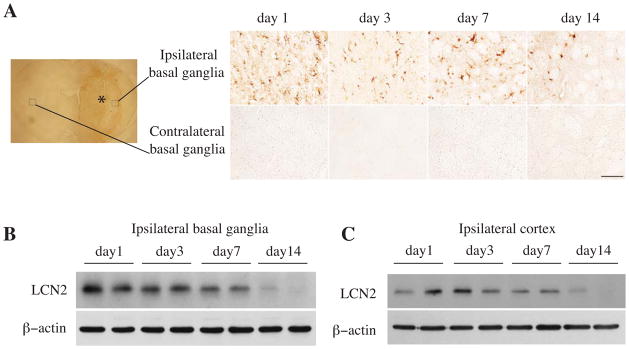
Time course showing LCN2 immunoreactivity in the ipsi- and contralateral basal ganglia (A), and the protein levels of LCN2 and β-actin in the ipsilateral basal ganglia (B) and cortex (C) after an injection of 100μl autoglous blood into the right caudate. The asterisk indicates the hematoma, the boxes indicate the locations where higher powered immunohistochemistry images were taken. Scale bar=100 μm.
LCN2 immunoreactivity was very weak in sham-operated brain but increased significantly after blood injection. LCN2 positive cells were astrocyte-like (Fig. 2A). Western blots, with LCN2 normalized to β-actin, indicated that LCN2 protein levels in the ipsilateral basal ganglia were 71-fold higher than those in the contralateral basal ganglia (LCN2/β-actin ratio: 2.70 ± 0.46 vs. 0.04 ± 0.01, p<0.001) and 84-fold higher than in sham-operated animals (0.92 ± 0.14 vs. 0.01 ± 0.01, p<0.001) at 3 days after ICH (Fig. 2B). Similar results were also found in the cortex (Fig. 2B).
Figure 2.
LCN2 immunoreactivity in the ipsilateral basal ganglia and cortex after a sham operation, and in the contra- and ipsilateral basal ganglia and cortex after ICH at day 3(A), scale bar=100μm. The protein levels of LCN2 and β-actin determined by Western blotting in the basal ganglia and cortex (B) 3 days after sham operation or blood injection.
Double-labeling was used to determine which types of cells express LCN2 after ICH. We found that LCN2 positive cells predominantly colocalized with glial fibrillary acid protein (GFAP) positive cells after ICH. There were only a few LCN2 positive cells that colocalized with neuronal nuclear antigen (NeuN) in cortex (Fig. 3). These results suggest that LCN2 is mainly expressed in astrocytes following ICH.
Figure 3.
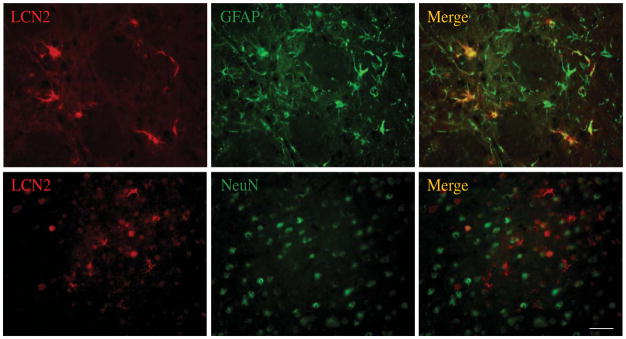
LCN2, glial fibrillary acidic protein (GFAP) and NeuN immunoreactivity and the colocalization of LCN2 with GFAP or NeuN by double labeling in the ipsilateral basal ganglia at day 3 after 100μl blood infused into the right basal ganglia. Scale bar=50μm.
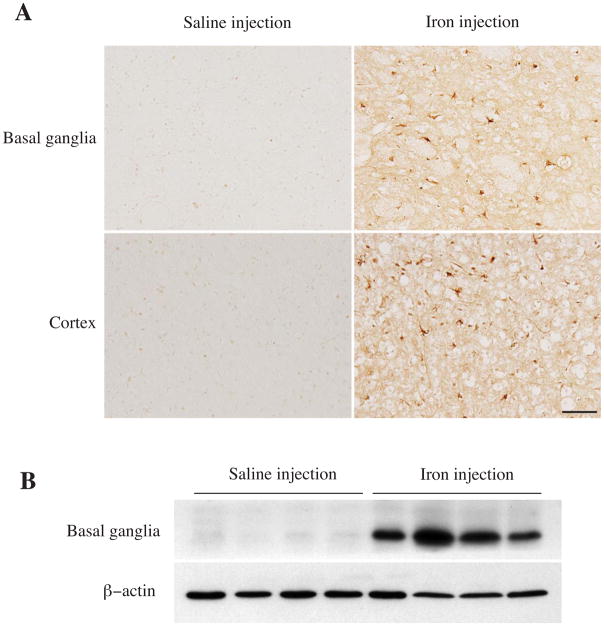
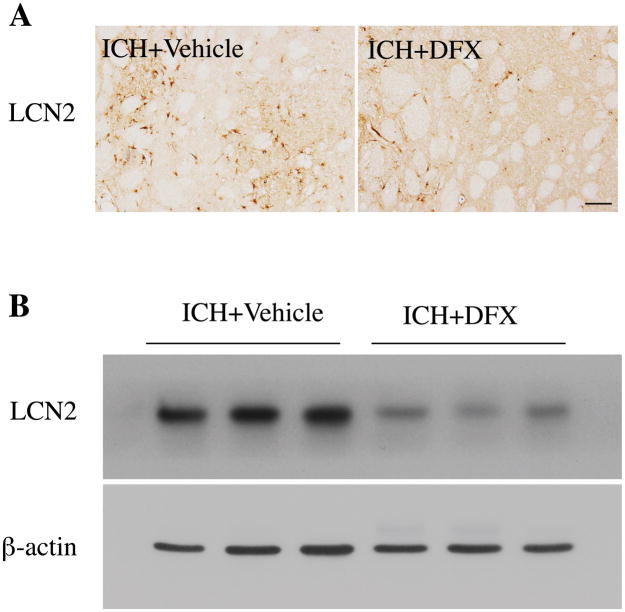
To test whether iron can induce LCN2 expression, 30 μL of ferrous chloride (1 mmol/L) or saline was injected into the rats’ right basal ganglia. There were many more LCN2 positive cells in the ipsilateral basal ganglia after iron injection compared to saline injection (Fig. 4A). Iron injection caused a 136-fold increase of LCN2 protein levels at day 3 (ratio of LCN2/β-actin: 1.77 ± 0.43 vs. 0.013 ± 0.004 in saline injection, p<0.01, Fig. 4B). To examine whether ICH-induced LCN2 expression can be attenuated by iron chelation, rats were treated with deferoxamine or vehicle (2 hours and then every 12 hours for 7 days) after ICH. Deferoxamine reduced ICH-induced LCN2 upregulation at day 7 (ratio of LCN2/β-actin: 0.44 ± 0.23 vs. 1.73 ± 0.5 in vehicle, p<0.05, Fig. 5).
Figure 4.
LCN2 immunoreactivity in the ipsilateral basal ganglia and cortex, and the protein levels of LCN2 and β-actin in the ipsilateral basal ganglia at day 3 after 30 μl of ferrous iron (1.0 mmol/L) or saline injection into the rat basal ganglia. Scale bar=100μm.
Figure 5.
(A) LCN2 immunohistochemistry of the ipsilateral basal ganglia and (B) Western blot of that tissue from rats treated with deferoxamine (DFX) or vehicle for 7 days after 100μl blood was injected into the right caudate. Scale bar=100μm.
To further elucidate whether the brain produces LCN2 after ICH, quantitative real-time PCR of LCN2 gene expression was examined in triplicate using Eppendorf Mastercycler Realplex Detection System. Real time PCR showed that LCN2 mRNA expression in the ipsilateral basal ganglia was increased 6.5-fold compared to the contralateral basal ganglia at 24 hours after ICH (p< 0.05).
3. Discussion
The major findings of the present study are: 1) brain LCN2 levels are markedly increased after ICH; 2) most LCN2 positive cells after ICH are astrocytes; 3) iron can upregulate brain LCN2; and 4) deferoxamine reduces ICH-induced LCN2 upregulation. These findings suggest an important role of iron in brain LCN2 upregulation after ICH and potentially a role of LCN2 in handling iron that is released from the hematoma after ICH.
LCN2 expression in the brain is prominently increased at an early stage of ICH, which may be associated with acute brain injury. There was a very marked increase in LCN2 in the perihematomal zone of basal ganglion and cortex occurs 1 day after ICH. Brain LCN2 levels were high for 7 days and decreased around 14 days after ICH. Although levels of LCN2 mRNA and protein expression are low in most regions of the normal brain (Chia et al., 2011), LCN2 is rapidly inducible (Liu and Nilsen-Hamilton, 1995). Recent work showed that LCN2 expression can be induced in the center never system following injury and neuroinflammation (Berard et al., 2012; Chia et al., 2011; Ip et al., 2011). Our study also confirmed that the brain can produce LCN2.
Many factors may cause brain LCN2 upregulation after ICH. Our results showed that intracerebral injection of iron increases brain LCN2 levels and deferoxamine, an iron chelator, reduces ICH-induced upregulation of brain LCN2. These results indicate a role of iron in regulating LCN2 expression following ICH. However, LCN2 is an acute phase protein and other factors, including tumor necrosis factor-alpha (TNF-α) and thrombin, two major factors which cause brain injury after ICH (Bodmer et al., 2012), may also upregulate brain LCN2 in the acute phase. For example, TNF-α is increased in the brain two hours after ICH (Hua et al., 2006) and TNF-α can upregulate LCN2 (Naude et al., 2012). Future studies should determine whether thrombin has a role in ICH-induced upregulation of brain LCN2.
The role of LCN2 in ICH is unclear, but LCN2 upregulation in the brain after ICH may affect iron homeostasis. It is well known that iron overload following ICH causes brain damage. LCN2 transfers iron from intracellular to the extracellular medium via binding with intracellular siderophore, thereby reducing intracellular iron concentration. A report has suggested that LCN2 could be a mediator of an alternative, transferrin-independent pathway for cellular iron delivery (Yang et al., 2002). The iron chelator, deferoxamine, protects endotoxin-induced toxicity and mortality in LCN2 deficient mice (Srinivasan et al., 2012). Future studies should determine whether LCN2 can enhance brain iron clearance following ICH.
We observed that LCN2 was mainly expressed in astrocytes after ICH. Our results are supported by other studies. In the case of kainite-induced brain injury, LCN2 is highly expressed in the reactive astrocytes in the injured hippocampus (Chia et al., 2011). Similarly, after spinal cord contusion injury, Rathore et al. found a strong induction of LCN2 in astrocytes but not microglia (Rathore et al., 2011). After ICH, the breakdown of hemoglobin in the hematoma results in the release of iron. Thus, there is an increase in perihematomal iron, that can be detected by Perls’ staining, which persists for weeks and is mostly present in glial cells (Wu et al., 2003). LCN2 upregulation in perihematomal astrocytes suggests that it may be involved in, or a response to, iron accumulation.
We also found some expression of LCN2 in neurons after ICH. LCN2 is also found to be expressed in neurons and up-regulated in the hippocampus by stress (Mucha et al., 2011). Neuronal expression of LCN2 has been observed following exposure to trauma (Rathore et al., 2011) or neuroinflammation (Berard et al., 2012; Chia et al., 2011; Ip et al., 2011). After ICH, Perls’ staining indicates that there is a build-up of iron in some neurons(Wu et al., 2003). Whether LCN2 participates in that uptake and in the neuronal injury that follows ICH deserves further investigation.
In conclusion, LCN2 is markedly upregulated in the brain after ICH. As an intracerebral iron injection induces brain LCN2 and iron chelation reduces ICH-induced LCN2 upregulation, iron appears to play a central role in the LCN2 response to hemorrhage. LCN2 may be involved in iron homeostasis after cerebral hemorrhage and modifying brain LCN2 levels may affect ICH-induced brain iron overload and brain injury.
4. Experimental Procedures
Animal preparation and intracerebral injection
Animal use protocols were approved by the University of Michigan Committee on the Use and Care of Animals. A total of 96 male Sprague-Dawley rats (weighed 275–350g, Charles River Laboratories, Portage, MI USA) were used in these studies. Animals were housed under standard 12:12-h light-dark conditions and allowed free access to food and water. Animals were anesthetized with pentobarbital (45 mg/kg, i.p.). Septic precautions were used in all surgical procedures and rectal temperature was maintained at 37.5°C using a feedback-controlled heating pad. A polyethylene catheter (PE-50) was inserted into the right femoral artery to monitor arterial blood pressure and blood gases and to obtain blood for intracerebral infusion. The animals were positioned in a stereotactic frame (Kopf Instruments) and a cranial burr hole (1mm) was drilled. Rats received an autologous blood injection into the right basal ganglia (coordinates: 0.2 mm anterior, 3.5 mm lateral, and 5.5 mm ventral to the bregma) through a 26-gauge needle at a rate of 10 μL/min using a microinfusion pump.
Experimental Groups
This study was divided into four parts. In the first part, rats received either an intracerebral injection of 100 μL autologous whole blood or a needle insertion (sham). Rats were euthanized 1, 3, 7, or 14 days later for Western blot analysis (n=4 for each time point) and immunohistochemistry (n=4 for each time point). In the second part, rats had an infusion of 30 μL ferrous chloride (1 mmol/L) or saline. Rat brains were sampled at 3 days after intracerebral injection and used for Western blot analysis (n=4) and immunohistochemistry (n=4). In the third part, rats had an intracerebral infusion of 100μL autologous whole blood and were treated with either deferoxamine (100 mg/kg, i.p. at 2 hours after ICH and then every 12 hours for 7 days) or vehicle. Rats were euthanized at day 7 for Western blot analysis (n=4) and immunohistochemistry (n=4). In the fourth part, rats had a 100 μl blood injection in the right basal ganglia and were killed at 24 hours for RNA isolation and real time PCR analysis.
Western blot analysis
Western blot analysis was performed as previously described (Xi et al., 1999). Briefly, brain tissue was immersed in Western sample buffer and sonicated. Protein concentration was determined by Bio-Rad protein assay kit and 50-μg protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a Hybond-C pure nitrocellulose membrane (Amersham). Membranes were probed with primary antibody: goat anti-lipocalin 2 antibody (1:200 dilution, R&D Systems). The secondary antibody was rabbit anti-goat IgG (Bio-Rad; 1:2,500 dilution). The antigen-antibody complexes were visualized with the ECL chemiluminescence system (Amersham) and exposed to Kodak X-OMAT film. The relative densities of bands were analyzed with NIH ImageJ.
Immunohistochemistry
Immunohistochemistry was performed as described previously (Xi et al., 1999). Briefly, rats were anesthetized and subjected to intracardiac perfusion with 4% paraformaldehyde in 0.1mol/L phosphate-buffered saline (pH 7.4). Brains were removed and kept in 4% paraformaldehyde for 6 h, then immersed in 30% sucrose for 3–4 days at 4 °C. After embedding in a mixture of 30% sucrose and OCT (SAKURA Finetek, USA), 18-μm sections were taken on a cryostat. Immunohistochemistry staining was then performed using the avidin-biotin complex technique. The primary antibody was goat anti-lipocalin 2 antibody (1:200 dilution, B&D Systems). The secondary antibody was rabbit anti-goat IgG (Bio-Rad; 1:2,500 dilution).
Immunofluorescent double-labeling
For immunofluorescent double-labeling, the primary antibodies were goat anti-lipocalin 2 (1:200 dilution, R&D Systems), mouse anti-NeuN (1:200 dilution, Millipore), and mouse anti-GFAP (1:200 dilution, Millipore). Rhodamine-conjugated donkey anti-goat (Invitrogen; 1:500 dilution), fluorescein isothiocyanate (FITC)-labeled horse anti-mouse (Vector; 1:500 dilution) and donkey anti-rabbit antibodies were used as secondary antibodies. The double-labeling was analyzed using a fluorescence microscope (Xi et al., 1999).
Quantitative real-time polymerase chain reaction (qPCR)
Total RNA was prepared from 20–30mg the ipsilateral basal ganglia tissue using an RNeasy Mini kit (Qiagen), and stored at −80°C. 2μg mRNA was used for cDNA synthesis using High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). Quantitative real-time PCR of gene expression were run in triplicate using TaqMan PCR Master Mix (Roche). No template controls were prepared by omitting to add cDNA template to the reaction. Real-time quantitative primers were designed and purchased from Applied Biosystems (Life Technologies Corporation). The rat LCN2 primers were 5′ GAT TCG TCA GCT TTG CCA AGT 3′ (forward primer) and 5′ CAT TGG TCG GTG GGA ACA G 3′ (reverse primer). A positive standard curve for each primer was obtained using serially-diluted cDNA sample mixture. The quantity of gene expression was calculated using standard samples and normalized with GAPDH.
Statistical Analysis
All data in this study are presented as mean ± SD. Data were analyzed with Student’s t-test and analysis of variance. The P value of less than 0.05 was considered as significant level.
Highlights.
Effects of intracerebral hemorrhage (ICH) on lipocalin 2 examined in a rat model
ICH caused a marked (70–80 fold) increase in brain lipocalin 2 levels
Intracerebral iron injection also markedly increased brain lipocalin 2 (136 fold)
Deferoxamine (iron chelator) reduced ICH-induction of lipocalin 2
Lipocalin 2 may play an important role in iron homeostasis after ICH
Acknowledgments
This study was supported by grants NS-039866, NS-057539, NS-073595 and NS-079157 from the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- ICH
intracerebral hemorrhage
- LCN2
lipocalin2
- GFAP
glial acidic fibrillary protein
Footnotes
Disclosures:
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berard JL, Zarruk JG, Arbour N, Prat A, Yong VW, Jacques FH, Akira S, David S. Lipocalin 2 is a novel immune mediator of experimental autoimmune encephalomyelitis pathogenesis and is modulated in multiple sclerosis. Glia. 2012;60:1145–59. doi: 10.1002/glia.22342. [DOI] [PubMed] [Google Scholar]
- Bodmer D, Vaughan KA, Zacharia BE, Hickman ZL, Connolly ES., Jr The Molecular Mechanisms that Promote Edema After Intracerebral Hemorrhage. Translational Stroke Research. 2012;3:S52–S61. doi: 10.1007/s12975-012-0162-0. [DOI] [PubMed] [Google Scholar]
- Chia WJ, Dawe GS, Ong WY. Expression and localization of the iron-siderophore binding protein lipocalin 2 in the normal rat brain and after kainate-induced excitotoxicity. Neurochemistry international. 2011;59:591–9. doi: 10.1016/j.neuint.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Devireddy LR, Gazin C, Zhu X, Green MR. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005;123:1293–305. doi: 10.1016/j.cell.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Gu Y, Hua Y, Keep RF, Morgenstern LB, Xi G. Deferoxamine reduces intracerebral hematoma-induced iron accumulation and neuronal death in piglets. Stroke. 2009;40:2241–3. doi: 10.1161/STROKEAHA.108.539536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Y, Wu J, Keep RF, Nakamura T, Hoff JT, Xi G. Tumor necrosis factor-alpha increases in the brain after intracerebral hemorrhage and thrombin stimulation. Neurosurgery. 2006;58:542–50. doi: 10.1227/01.NEU.0000197333.55473.AD. discussion 542–50. [DOI] [PubMed] [Google Scholar]
- Ip JP, Nocon AL, Hofer MJ, Lim SL, Muller M, Campbell IL. Lipocalin 2 in the central nervous system host response to systemic lipopolysaccharide administration. Journal of neuroinflammation. 2011;8:124. doi: 10.1186/1742-2094-8-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurology. 2012;11:720–31. doi: 10.1016/S1474-4422(12)70104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Nilsen-Hamilton M. Identification of a new acute phase protein. The Journal of biological chemistry. 1995;270:22565–70. doi: 10.1074/jbc.270.38.22565. [DOI] [PubMed] [Google Scholar]
- Marques F, Rodrigues AJ, Sousa JC, Coppola G, Geschwind DH, Sousa N, Correia-Neves M, Palha JA. Lipocalin 2 is a choroid plexus acute-phase protein. Journal of cerebral blood flow and metabolism. 2008;28:450–5. doi: 10.1038/sj.jcbfm.9600557. [DOI] [PubMed] [Google Scholar]
- Mehdiratta M, Kumar S, Hackney D, Schlaug G, Selim M. Association between serum ferritin level and perihematoma edema volume in patients with spontaneous intracerebral hemorrhage. Stroke. 2008;39:1165–70. doi: 10.1161/STROKEAHA.107.501213. [DOI] [PubMed] [Google Scholar]
- Mucha M, Skrzypiec AE, Schiavon E, Attwood BK, Kucerova E, Pawlak R. Lipocalin-2 controls neuronal excitability and anxiety by regulating dendritic spine formation and maturation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:18436–41. doi: 10.1073/pnas.1107936108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naude PJ, Nyakas C, Eiden LE, Ait-Ali D, van der Heide R, Engelborghs S, Luiten PG, De Deyn PP, den Boer JA, Eisel UL. Lipocalin 2: novel component of proinflammatory signaling in Alzheimer’s disease. FASEB Journal. 2012;26:2811–23. doi: 10.1096/fj.11-202457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okauchi M, Hua Y, Keep RF, Morgenstern LB, Schallert T, Xi G. Deferoxamine treatment for intracerebral hemorrhage in aged rats: therapeutic time window and optimal duration. Stroke. 2010;41:375–82. doi: 10.1161/STROKEAHA.109.569830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez de la Ossa N, Sobrino T, Silva Y, Blanco M, Millan M, Gomis M, Agulla J, Araya P, Reverte S, Serena J, Davalos A. Iron-related brain damage in patients with intracerebral hemorrhage. Stroke. 2010;41:810–3. doi: 10.1161/STROKEAHA.109.570168. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632–44. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore KI, Berard JL, Redensek A, Chierzi S, Lopez-Vales R, Santos M, Akira S, David S. Lipocalin 2 plays an immunomodulatory role and has detrimental effects after spinal cord injury. The Journal of neuroscience. 2011;31:13412–9. doi: 10.1523/JNEUROSCI.0116-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincon F, Mayer SA. Intracerebral Hemorrhage: Clinical Overview and Pathophysiologic Concepts. Translational Stroke Research. 2012;3:S10–S24. doi: 10.1007/s12975-012-0175-8. [DOI] [PubMed] [Google Scholar]
- Selim M, Yeatts S, Goldstein JN, Gomes J, Greenberg S, Morgenstern LB, Schlaug G, Torbey M, Waldman B, Xi G, Palesch Y. Safety and tolerability of deferoxamine mesylate in patients with acute intracerebral hemorrhage. Stroke. 2011;42:3067–74. doi: 10.1161/STROKEAHA.111.617589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan G, Aitken JD, Zhang B, Carvalho FA, Chassaing B, Shashidharamurthy R, Borregaard N, Jones DP, Gewirtz AT, Vijay-Kumar M. Lipocalin 2 deficiency dysregulates iron homeostasis and exacerbates endotoxin-induced sepsis. Journal of Immunology. 2012;189:1911–9. doi: 10.4049/jimmunol.1200892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Hua Y, Keep RF, Nakamura T, Hoff JT, Xi G. Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke. 2003;34:2964–9. doi: 10.1161/01.STR.0000103140.52838.45. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hua Y, Xiang J, Hoff JT. Attenuation of thrombin-induced brain edema by cerebral thrombin preconditioning. Stroke. 1999;30:1247–55. doi: 10.1161/01.str.30.6.1247. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet neurology. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- Yang J, Goetz D, Li JY, Wang W, Mori K, Setlik D, Du T, Erdjument-Bromage H, Tempst P, Strong R, Barasch J. An iron delivery pathway mediated by a lipocalin. Molecular cell. 2002;10:1045–56. doi: 10.1016/s1097-2765(02)00710-4. [DOI] [PubMed] [Google Scholar]