Abstract
Although the causes of inflammatory arthritis elude us, aberrant cytokine expression has been linked to joint pathology. Consequently, several approaches in the clinic and/or in clinical trials are targeting cytokines, e.g. tumor necrosis factor (TNF), interleukin 23 (IL-23) and interleukin 17 (IL-17), with the goal of antagonizing their respective biologic activity through therapeutic neutralizing antibodies. Such, cytokine signaling-dependent molecular networks orchestrate synovial inflammation on multiple levels including differentiation of myeloid cells to osteoclasts, the central cellular players in arthritis-associated pathologic bone resorption. Hence, understanding of the cellular and molecular mechanisms elicited by synovial cytokine networks that dictate recruitment, differentiation and activation of osteoclast precursors and osteoclasts, respectively, is central to shaping novel therapeutic options for inflammatory arthritis patients. In this article we are discussing the complex signaling interactions involved in the regulation of inflammatory arthritis and it’s associated bone loss with a focus on interleukin 27 (IL-27). The present review will discuss the primary bone-degrading cell, the osteoclast, and on how IL-27, directly or indirectly, modulates osteoclast activity in autoimmune-driven inflammatory joint diseases.
Introduction
Bone remodeling is the process whereby a healthy skeleton is constantly renewed throughout adult life. This life-long process under physiological conditions is maintained by two different cell types, which exhibit opposing functions. The osteoclast, which supports removal of old bone, by bone resorption and the osteoblast, which supports bone formation by bone apposition.
Differentiation of osteoclasts from its hematopoietic precursors is regulated by receptor activator for nuclear factor κ B ligand (RANKL) and macrophage colony stimulating factor (MCSF); both of which are secreted by the osteoblasts under physiological conditions [1, 2]. M-CSF stimulates RANK expression in osteoclast precursor cells and supports osteoclast survival by preventing apoptosis thereby allowing RANKL to promote osteoclast formation [3, 4]. RANKL is a trans-membrane protein expressed by activated osteoblasts, synovial fibroblasts and T cells. RANKL-induced osteoclastogenesis is inhibited by osteoprotegerin (OPG), a soluble decoy receptor for RANKL, which is also produced by a variety of cells, including osteoblasts, synovial fibroblasts, B-cells and T-cells [5]. OPG-deficient mice are severely osteoporotic [6], while OPG-transgenic mice are osteopetrotic suggesting that the RANKL/RANK/OPG axis tightly regulates osteoclast formation and bone resorption [7].
Inflammatory arthritis is generally characterized by, bone erosions, osteopenia, soft-tissue swelling, lymphocyte infiltration into the joint area, and uniform joint space loss. Bone erosion is prominent in Rheumatoid arthritis (RA), Juvenile Idiopathic Arthritis (JIA), Psoriatic Arthritis (PsA) and in these inflammatory joint conditions a significant macrophage and T-cell infiltrate commonly occurs. The extent of synovial macrophage infiltration correlates strongly with the degree of joint erosion in arthritis [8]. This correlation may in part reflect the fact that synovial macrophages constitute a subset of osteoclast precursor population. There is a plethora of evidence suggesting that synovial macrophages differentiate in vitro to fully functional osteoclasts after RANKL stimulation, suggesting that the macrophage infiltration into the joint increases the number of osteoclast precursors locally [9]. Moreover macrophages are a source of TNF and IL-1; importantly, TNF induces osteoclastogenesis in RANK deficient mice and induces multi-nucleated cell formation from osteoclast precursors in the BM macrophage population suggesting that TNF can promote osteoclast formation independently of RANKL [10, 11]. Apart from the cytokines produced by activated macrophages, synovial T cells also have a prominent role in arthritis pathogenesis and are involved in osteoclast-mediated bone resorption [12].
Although the precise contribution to bone destruction of infiltrating inflammatory T cell subsets is not fully defined, it is evidently largely dependent on the cytokines produced that promote osteoclast differentiation. Different T cell subsets express a repertoire of cytokines with opposing functions in osteoclast biology [12]. Th1 cells express TNF and IFNγ, which can synergize or inhibit RANKL-induced osteoclastogenesis. Th17 cells are considered osteoclastogenic due to their ability to secrete pro-osteoclastogenic factors including soluble RANKL. T cell differentiation and the resulting cytokine milieu of pro-osteoclastogenic and anti-osteoclastogenic factors is therefore of immense importance in the synovial tissue and inflammatory arthritis.
IL-27 plays a major role in the regulation of T cell differentiation through which it affects both RANKL-dependent and RANKL-independent osteoclastogenesis pathways. Discovering the cellular and molecular mechanisms that dictate recruitment and activation of osteoclasts in inflammatory arthritis is central to preventing this disabling condition. The IL-27 regulatory action in T cells and its direct actions on osteoclast precursors may hold the key to identify novel pathways in bone destruction in inflammatory arthritis.
IL-27
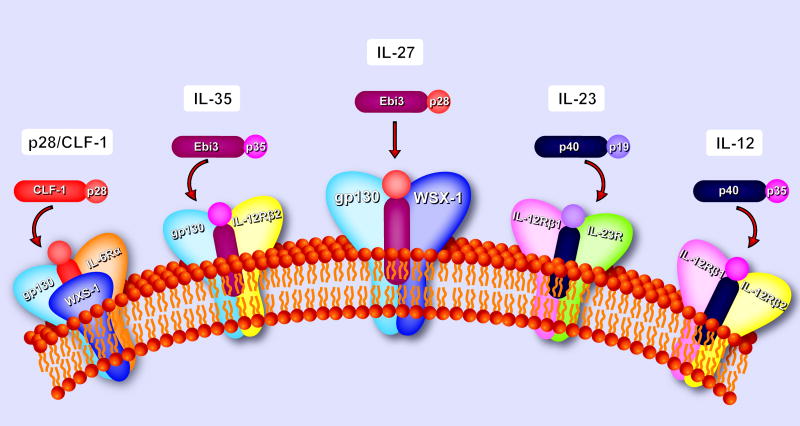
Interleukin-27 (IL-27) was first described about 9 years ago as a novel cytokine, structurally and architecturally related to IL-12 and IL-23 [11, 13]. Two different molecular entities are required for formation of functional IL-27. One is the Epstein Barr-Virus-induced gene 3 (Ebi3), which contains two cytokine binding domains but lacks membrane anchoring motifs and a cytoplasmic tail and has no described activity on its own [14]. Ebi3 associates with a predicted four-alpha helix bundle cytokine-like protein, termed p28, to form functional IL-27.
Human Ebi3 and p28 are encoded in separate genomic loci, on chromosomes 19p13.3 and 16p11.2, respectively, and mouse Ebi3 and p28 on chromosomes 17qD and 7qF3, respectively, hence Ebi3 and p28 are independent genes. The predominant co-producers of Ebi3 and p28 proteins appear to be activated dendritic cells (DC). Particularly following activation of TLR2, TLR4 and TLR9, expression of Ebi3 in DCs is induced in a MyD88-dependent fashion [15]. While p28 gene expression seems to be induced by TLR4 signaling, particularly in macrophages p28 is also activated downstream of the TLR3/TRIF-dependent pathway [16, 17]. In addition, p28 expression can be activated by type I Interferon-dependent signaling networks involving IRF1 and IRF3 [18]. In human DCs, p28 expression is activated by commensal gram-negative but not gram-positive bacteria [19]. Therefore, regulation of gene expression of the two IL-27 components shows some overlap but also some differences. P28-independent expression of Ebi3 can occur in murine CD4+CD25+FoxP3+ Treg cells when Ebi3 partners with co-expressed IL-12p35 to form IL-35 [20–22]. Whether or not IL-35 can be produced and secreted from human cells is debated at present [23–25]. Conversely, p28 also seems to be able to bind an alternative DC-derived partner, CLF-1, to form another composite factor with cytokine-like activity on NK cells [26]. In addition, a recent study has suggested that p28 by itself possesses bioactivity as a natural antagonist of cytokine signaling through gp130 [27].
Recently it was shown that IL-35 signaled through a unique heterodimer of receptor chains IL-12Rβ2 and gp130 or homodimers of each chain [28]. The p35 subunit of IL-35 is shared with IL-12, a heterodimeric cytokine composed of the p35 and p40 subunits. P35 is expressed ubiquitously and constitutively at low levels, whereas the p40 subunit is expressed by phagocytic cells [29]. Although biologically active IL-12 must express both p40 and p35 subunits, p40 can be secreted independently of p35 and produced as a monomer or as a homodimer (p80) [29]. Monomeric p40 associates with p19 to form IL-23 which signals through IL-23R and IL-12Rβ1 [30, 31]. The IL-23R is expressed on the surface of activated lymphoid cells such as T cells and NK cells, along with cells of myeloid origin, including dendritic cells, macrophages and monocytes [30].
Several recent studies point to sharing of not only cytokine subunits but, in addition, sharing of promiscuous signaling receptors among several different composite cytokines [26, 32]. Thus, alternative functions of cytokine subunits, as individual proteins or as part of composite factors acting through variable receptors, can be viewed as a fascinating example of how evolution has generated multiple differential activities and specificities with utilizing a limited number of gene products. However, the promiscuity of Ebi3 and p28 does undoubtedly complicate studies attempting to selectively define functions of the single genes p28 or Ebi3, or of IL-27. Studies of considerable complexity may be required to further dissect contributions of the various factors that involve Ebi3 and/or p28.
IL-27 receptors
IL-27 engages two type-I trans-membrane proteins of the hematopoietic cytokine receptor family [33]. First, glycoprotein 130 (gp130), a ubiquitously expressed receptor chain that is shared with IL-6-family cytokines [34], and second, a receptor termed WSX-1 or TCCR, which seems to be broadly but not ubiquitously expressed, with preferential mRNA expression observed in lymphoid tissues [35]. Interestingly, the interactions between IL-27 and WSX-1 appear readily detectable by biochemical methods, however, gp130 possesses undetectable affinity for IL-27 [36], and involvement of gp130 in IL-27-dependent signaling thus far could only be demonstrated by functional assays [33].
It should be noted that while the majority of IL-27-related literature investigates its effects on various subsets of CD4+ T cells, WSX-1 is also expressed by other cell types. Accordingly, IL-27-mediated effects have been described on monocytes and mast cells [33], CD8+ T cells [37, 38], B cells [39, 40], NK cells [41], DCs [13], and neutrophils [42]. Together with the fascinating interplay between these plethora of heterodimeric ligands and receptors IL-27 biology becomes all the more complex Figure 1.
Figure 1. The IL-27 and IL-27 receptor.
Schematic representation of IL-27 and the IL-27 receptor complex, demonstrating a significant homology with other IL-12 receptor family members. IL-27 shares Ebi3 with IL-35 whilst p28 may signal on its own or form complexes with CLF-1. IL-27 receptor complex shares gp130 with IL-35 and IL-6 receptors. IL-12Rβ2 is shared among IL-12, IL-23, and IL-35 receptors further increasing the possibility of signaling complexes.
IL-27 directly modulates bone loss via the Osteoclast
Osteoclasts, the only specialized bone degrading cells, are large 20 to 100 μm multinucleated cells containing three to 100 nuclei with many mitochondria, lysosomes, dense granules, vesicles, and an extensive Golgi network required for the synthesis and secretion of factors required to degrade the bone matrix and subsequent phagocytosis of the resorbed products [43]. Tartrate-resistant acid phosphatase (TRAP) [44], cathepsin K [45], calcitonin receptor [46], and the ανβ3 integrin [47] are characteristic gene products of the mature osteoclast and facilitate the process of bone resorption [48]. The induction of these genes is directly regulated by nuclear factor of activated T cells (NFATc1). NFATc1 forms an osteoclast-specific transcriptional complex containing AP-1 (Fos/Jun), PU.1 and MITF for the efficient induction of osteoclast-specific genes reviewed by Takayanagi [49].
The initial event in bone resorption is the attachment of the mature osteoclast to the bone matrix by cell surface ανβ3 integrins which bind to a variety of extracellular matrix proteins including vitronectin, osteopontin, and bone sialoprotein [50]. Once attached to bone, the osteoclast generates an isolated extracellular microenvironment between itself and the bone surface by creating a “sealing” zone structure unique to the osteoclast. Bone resorption depends upon acidification of this extracellular compartment within the sealed zone which leads to demineralization of the inorganic bone component and subsequent organic matrix degradation by cysteine proteases [51]. The expanded membrane adjacent to the bone (ruffled border) creates additional surface area for massive H+ transport performed by the vacuolar (V-Type) electrogenic H+-ATPase [52]. The proton source is carbonic acid produced by carbonic anhydrase type II intracellular pH is balanced by a passive chloride-bicarbonate exchange in the basolateral membrane [52]. The resorbed material is transcytosed through the osteoclast [53, 54].
Mouse hematopoietic stem cells (HSCs) express both IL-27R subunits, gp130 and WSX-1[55]. Expression of mRNA IL-27 receptor subunits WSX-1 and gp130 was induced by MCSF in mouse bone marrow macrophages (osteoclast precursors) and direct engagement of IL-27R by IL-27 negatively regulated osteoclastogenesis [56]. In the same study Kamiya and colleagues showed that expression of IL-27 and IL-23 receptor subunits were also expressed in primary osteoblasts. However neither IL-27 nor IL-23 showed any significant effects on alkaline phosphatase, or RANKL, Runx2 or Osteoclacin expression and osteoblast proliferation. In the Kamiya et al study the authors found that IL-27 (and IL-23) had inhibitory effects in osteoclastogenesis in murine bone marrow cultures that were mediated by T lymphocytes. Although IFNγ was elevated in these cultures the authors showed similar inhibitory effects on osteoclast formation with IL-23 where there was no observed elevation of IFNγ, suggesting that more precise studies are required to clarify the molecular mechansism of this inhibition. IFNγ signaling mediates the degradation of the RANK adapter protein TRAF6, resulting in strong inhibition of RANKL-induced activation of NFκB and Jun N-terminal kinase [14]. The involvement of IFNγ as a potential inhibitory mechanism, in IL-27-mediated inhibition of osteoclastogenesis has received considerable attention. Another study confirmed that IL-27 inhibited RANKL-induced osteoclast formation in vitro and IL-27-Fc ameliorated bone destruction in a collagen-induced arthritis (CIA) in vivo model. Interestingly IL-27-Fc did not ameliorate collagen-induced arthritis (CIA) arthritis in IFNγ−/− mice, suggesting that the IL-27 inhibitory effect in osteoclastogenesis and bone destruction is IFNγ dependent [57]. Previous similar data have also been observed using human osteoclast assays where IL-27 induced STAT1 protein expression and phosphorylation. STAT1 activation correlated with inhibition of RANKL-induced c-Fos and NFATc1; transcription factors, indispensable for osteoclastogenesis [58]. In the same study, IL-27 could not inhibit RANKL-induced osteoclastogenesis in the presence of a STAT1 inhibitor and small interfering RNA partially rescued the inhibition of osteoclastogenesis by IL-27. Again it was confirmed that IL-27/IFNγ/STAT1 signaling does not interfere with the degradation of TRAF6 through the ubiquitin-proteasome system, but through the downregulation of c-fos [58]. Taken together these data suggest that IL-27 inhibits osteoclast formation via IFNγ but this IFNγ mediated inhibition of osteoclastogenesis is independent of TRAF6 and dependent on STAT1. Whilst additional experiments are required to elucidate the role of IFNγ in IL-27 osteoclast inhibition, other groups have suggested alternative plausible mechanisms.
In a study performed by Kalliolias et al, the presence of WSX-1 mRNA in freshly isolated mouse bone marrow macrophages and mouse splenocytes was confirmed and WSX-1 was also detected in both CD14+ and CD14- fractions of human peripheral blood mononuclear cells (PBMC). Again IL-27 stimulation of the osteoclast precursors negatively regulated osteoclastogenesis [59]. In this elegant study Kalliolias et al, described that direct stimulation of human osteoclast precursors with IL-27 inhibits RANKL-induced osteoclastogenesis in a dose dependent manner. IL-27 prevented RANKL-induced IκBα phosphorylation and degradation. Interestingly this study revealed that IL-27 inhibited NFATc1 and RANK receptor and this correlated well with downregulation of the triggering receptor expressed on myeloid cells 2 (TREM-2). TREM-2 delivers intracellular signals through the adaptor DAP12 to regulate myeloid cell function both within and outside the immune system.
TREM-2 associates with the adaptor DNAX activating protein of 12 kDa (DAP12), a transmembrane adapter, recognized for its role in transducing activation signals for an extended array of receptors in NK cells, granulocytes, monocytes/macrophages, and dendritic cells [60]. DAP12 is required for surface expression and signaling by TREM-2 and via DAP12 it mediates downstream signaling through a cytoplasmic ITAM domain, which can recruit Syk and activate PI3K, phospholipase C, and Vav signaling cascades [61–63]. DAP12-signaling pathways are indispensable for osteoclast formation and inflammatory arthritis [64, 65]. Moreover, we have recently shown that the Myeloid DAP12-associating lectin (MDL)-1 associates with DAP12 to transduce signals that regulate synovial inflammation and bone erosion associated with autoimmune arthritis [66]. Collectively this data suggests the possibility that IL-27 may regulate ITAM-mediated costimulatory signals to control both synovial inflammation and osteoclastogenesis.
In a recent study it was shown that IL-27 suppresses macrophage responses to TNF and IL-1 [59]. We and others have shown that TNF and IL-1 can induce osteoclast formation from synovial macrophages independently of RANKL [67]. Moreover IL-1 signals through a TRAF-6-Src protein complex which regulates the cytoskeletal reorganization essential for osteoclast activation and bone resorption [68]. Therefore the anti-inflammatory function of IL-27 in inflammatory arthritis is partly explained with a negative regulation of RANKL-independent osteoclastogenesis pathway, and provides an additional mechanism by which IL-27 may negatively regulate osteoclast formation. Although the direct action of IL-27 in both human and mouse osteoclast precursors is still elusive and multiple mechanisms have been proposed the literature largely agrees that IL-27 is a negative regulator of osteoclastogenesis in all in vitro systems described to date.
IL-27 indirectly modulates bone loss via T cells
IL-27 and Th1 cells
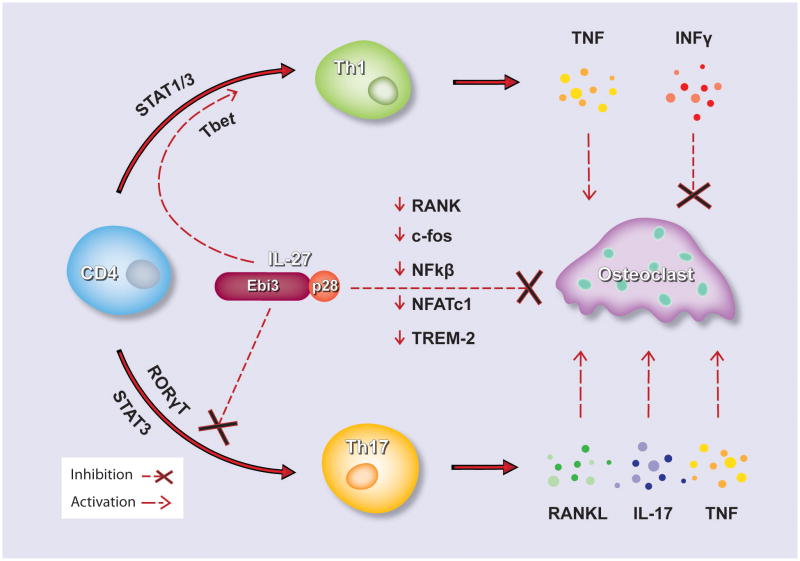
Early studies suggested a role of IL-27 in proliferation and Th1 commitment of naïve CD4+ T cells via activation of STAT1/STAT3 and T-bet (Tbx21), the master transcription factor for Th1 commitment [31, 33, 69–71]. Prior to identification of a ligand for the then orphan receptor WSX-1, two groups independently had reported compromised initiation of Th1 responses in WSX-1-deficient mice after challenge with the intracellular pathogens L. major and L. monocytogenes, both of which require an efficient Th1 response for host protection [72, 73]. However, subsequent studies provided evidence to suggest that the dominant in vivo role of IL-27 does not primarily relate to early phase support, but to late phase control of the immune response. WSX-1-deficient animals infected with T. gondii controlled the pathogen initially but later succumbed to excessive CD4+ T cell-mediated systemic inflammation [74]. Similarly, unlike WT mice, WSX-1-deficient mice showed over-expression of IL-6 and TNF and developed cytokine-mediated liver damage in a T. cruzi infection model [71]. Severe inflammation associated with elevated production of various cytokines in WSX-1-deficient mice was reported in a number of Th1-dependent and independent in vivo animal models [75–80]. The IL-27 prominent role in the proliferation and Th1 commitment of naïve CD4+ cells induces the production of Th1 signature cytokine IFNγ, along with IL-2, IL-10 and TNF. The role of these cytokines in osteoclastogenesis, as previously discussed, is diverse; however the net result of Th1 activation on osteoclast differentiation is indeed inhibitory [12, 81].
IL-27 and Th17 cells
Th17 cells are considered to be an osteoclastogenic T helper subtype due to their ability to synthesize and secrete RANKL, which directly induces osteoclast formation [81]. Moreover Th17 signature cytokine IL-17 can upregulate RANKL expression in osteoblasts and induce osteoclast formation in cocultures of mouse hematopoietic cells and primary osteoblasts [82]. We and others have shown that IL-17 is able to induce the expression of RANK receptor on human and mouse macrophages therefore increasing the potential of macrophages present in the inflammatory infiltrate to differentiate into osteoclasts [83, 84]. Other reports have also suggested that additional pathways where IL-17 induces TNF may also be implicated in osteoclastogenesis elicited by IL-17 [85]. In addition the direct production of TNF by double producers RANKL-TNF Th17 cells make the Th17 subset osteoclastogenic and its inhibition by IL-27 would have dramatic suppressive effects on bone destruction.
IL-27 counteracts the Th17 differentiation pathway by inhibition RORγT transcription factor essential for Th17 differentiation [86–88]. Several studies suggest that IL-27 promotes the differentiation of a Foxp3- T regulatory type 1 (Tr1) cell, which express IFNγ and IL-10 [89–93]. Other groups have shown that IL-27 inhibits cell surface expression of RANKL on naive CD4+ T cells activated by T cell receptor ligation and the secretion of soluble RANKL [94]. In this report the inhibitory effect was mediated in part by STAT3 but not by STAT1 or IL-10. Moreover RANKL expression is not reduced in Th17 cells differentiated in the presence of IL-27 and IL-27 only minimally inhibits RANKL expression in differentiated Th17 cells [94]. Taken together, these results indicate that IL-27 inhibits RANKL expression in CD4+ T cells, which could contribute to the suppressive effects of IL-27 on the inflammatory bone destruction (Figure 2).
Figure 2. Direct and indirect modulation of osteoclast differentiation by IL-27.
Schematic representation depicting the molecular events of IL-27 direct inhibition of osteoclast differentiation by downregulation of key osteoclast specific genes RANK, c-fos, NFκB, NFATc1 and TREM-2. Indirect inhibition of osteoclast formation is also achieved, by promoting Th1 cell differentiation and inhibiting the differentiation of osteoclastogenic subset Th17 and the secretion of pro-osteoclastogenic factors RANKL, IL-17 and TNF.
Concluding Remarks
Although IL-27 negatively regulates osteoclast formation and bone resorption its role in inflammatory arthritis remains puzzling. These aforementioned studies, at least in part, explain the immune-regulatory properties of IL-27 in different settings and collectively support the concept that IL-27 acts as an immune-modulatory cytokine in most circumstances of chronic tissue inflammation. However, a recent study suggests pro-inflammatory activities of IL-27 in a model of intestinal inflammation after T cell transfer into WSX-1-deficient or WT mice [95]. Consistent with this concept, plasma concentration of p28 was found to be significantly higher in RA patients than in control subjects [96]. In another study p28 was also found to be elevated in synovial fluid macrophages and synovial tissues of RA patients [96–98]. Other groups have reported that circulating IL-27-producing CD14+ cells infiltrate inflamed joints of rheumatoid arthritis and negatively regulate inflammation, suggesting an anti-inflammatory role for IL-27 in joint pathology [99]. In contrast, recently it was reported that IL-17 which exacerbates CIA also induces the expression of IL-27 questioning the significance of IL-27 in the process of bone destruction in autoimmune diseases such as rheumatoid arthritis [100]. In contrast to the negative regulation of osteoclasts other groups have shown contradictory data whereas IL-27 induces a Th1 immune response and susceptibility to experimental arthritisand psoriasis [101, 102].
As IL-27 has demonstrated biologic effects both at the early and the late phase of an immune response and also was shown to regulate several different CD4+ T cell subsets, absence of IL-27-mediated biology seems to have variable impacts on inflammation in differential experimental settings that in some instances perhaps appear puzzling. Considering the heterogeneity of the cellular targets and the complexity of the ligand-receptor systems, defining the biology of IL-27 still poses a challenge and dictates the need of further research in order to reconcile that paradox. Detailed understanding of these cellular and molecular interactions will yield insights into regulation of arthritis that can be exploited for therapeutic interventions.
Acknowledgments
Funding Statement: The authors would like to thank Thanh Nguyen for graphic design and Erika Suzuki for help with reference management. IEA is the recipient of the Sontag Fellowship of Arthritis National Research Foundation. Research reported in this publication was partly supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01AR62173 to IEA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We apologize to colleagues for omissions imposed by space limitations.
Biographies

Iannis Adamopoulos received his DPhil at Wolfson College, University of Oxford, United Kingdom, for his work on defining the cellular and molecular mechanisms of bone destruction, where he was also the recipient of a prestigious research into aging scholarship. He continued his post-doctoral training at Washington University of St Louis and at the DNAX Research Institute, Palo Alto, California on immune cytokines and their effects on osteoclast differentiation. Dr Adamopoulos is currently an Assistant Professor at University of California at Davis and a principal investigator at the Institute of Pediatric and Regenerative Medicine at Shriners Hospitals for Children-Northern California. In 2011 he was named the Sontag Fellow of the Arthritis National Research Foundation for his work on IL-23 and IL-17 in osteoclast regulation. His research effort is focused on elucidating the molecular mechanisms that regulate bone loss in inflammatory arthritis.

Stefan Pflanz received his PhD in Molecular Biology and Biochemistry from University of Aachen, Germany, where he studied structural and functional features required for cytokine receptor activation in the IL-6 cytokine family. He subsequently joined DNAX Research Institute, Palo Alto, California, for his postdoctoral fellowship where he worked on discovery of novel cytokines and was the first to report on IL-27. Since, he has served as research scientist in the pharmaceutical industry. Dr Pflanz’s assignments included group leader of target research at Microment Inc, and head of cytokine biology at Schering-Plough Biopharma. He is presently heading the immune regulation group at Gilead Sciences. His current research interests are geared towards understanding the cellular and molecular mechanisms through which HBV and HIV affect host innate and adaptive immunity to establish viral persistence.
Footnotes
Disclaimer
SP is an employee of Gilead Sciences, Inc. IEA has received salary and/or consulting fees from MERCK. Inc and TRL USA, and declares no other conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 2.Quinn JM, Elliott J, Gillespie MT, Martin TJ. A combination of osteoclast differentiation factor and macrophage-colony stimulating factor is sufficient for both human and mouse osteoclast formation in vitro. Endocrinology. 1998;139:4424–7. doi: 10.1210/endo.139.10.6331. [DOI] [PubMed] [Google Scholar]
- 3.Arai F, Miyamoto T, Ohneda O, Inada T, Sudo T, Brasel K, et al. Commitment and Differentiation of Osteoclast Precursor Cells by the Sequential Expression of c-Fms and Receptor Activator of Nuclear Factor {kappa}B (RANK) Receptors. J Exp Med. 1999;190:1741–54. doi: 10.1084/jem.190.12.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuller K, Owens JM, Jagger CJ, Wilson A, Moss R, Chambers TJ. Macrophage colony-stimulating factor stimulates survival and chemotactic behavior in isolated osteoclasts. J Exp Med. 1993;178:1733–44. doi: 10.1084/jem.178.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillespie M. Impact of cytokines and T lymphocytes upon osteoclast differentiation and function. Arthritis Res Ther. 2007;9:103. doi: 10.1186/ar2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, et al. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–8. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–19. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 8.Yanni G, Whelan A, Feighery C, Bresnihan B. Synovial tissue macrophages and joint erosion in rheumatoid arthritis. Annals of the rheumatic diseases. 1994;53:39–44. doi: 10.1136/ard.53.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adamopoulos IE, Sabokbar A, Wordsworth BP, Carr A, Ferguson DJ, Athanasou NA. Synovial fluid macrophages are capable of osteoclast formation and resorption. The Journal of pathology. 2006;208:35–43. doi: 10.1002/path.1891. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Sarosi I, Yan X-Q, Morony S, Capparelli C, Tan H-L, et al. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. PNAS. 2000;97:1566–71. doi: 10.1073/pnas.97.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azuma Y, Kaji K, Katogi R, Takeshita S, Kudo A. Tumor Necrosis Factor-alpha Induces Differentiation of and Bone Resorption by Osteoclasts. J Biol Chem. 2000;275:4858–64. doi: 10.1074/jbc.275.7.4858. [DOI] [PubMed] [Google Scholar]
- 12.Adamopoulos IE, Bowman EP. Immune regulation of bone loss by Th17 cells. Arthritis Res Ther. 2008;10:225. doi: 10.1186/ar2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pflanz STJ, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, et al. IL-27, a Heterodimeric Cytokine Composed of EBI3 and p28 Protein, Induces Proliferation of Naive CD4+ T cells. Immunity. 2002;16:779–90. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 14.Devergne O, Hummel M, Koeppen H, Le Beau MM, Nathanson EC, Kieff E, et al. A novel interleukin-12 p40-related protein induced by latent Epstein-Barr virus infection in B lymphocytes. Journal of virology. 1996;70:1143–53. doi: 10.1128/jvi.70.2.1143-1153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wirtz SBC, Fantini MC, Nieuwenhuis EE, Tubbe I, Galle PR, Schild H-J, et al. EBV-Induced Gene 3 Transcription Is Induced by TLR Signaling in Primary Dendritic Cells via NF-κB Activation. J Immunol. 2005;174:2814–24. doi: 10.4049/jimmunol.174.5.2814. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Guan X, Ma X. Regulation of IL-27 p28 gene expression in macrophages through MyD88- and interferon-gamma-mediated pathways. J Exp Med. 2007;204:141–52. doi: 10.1084/jem.20061440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molle CNM, Flamand V, Renneson J, Trottein F, De Wit D, et al. IL-27 synthesis induced by TLR ligation critically depends on IFN regulatory factor 3. J Immunol. 2007;178:7607–15. doi: 10.4049/jimmunol.178.12.7607. [DOI] [PubMed] [Google Scholar]
- 18.Pirhonen JSJ, Julkunen I, Matikainen S. IFN-alpha regulates Toll-like receptor-mediated IL-27 gene expression in human macrophages. J Leukoc Biol. 2007;82:1185–92. doi: 10.1189/jlb.0307157. [DOI] [PubMed] [Google Scholar]
- 19.Smits HHvBA, Hessle C, Westland R, de Jong E, Soeteman E, et al. Commensal Gram-negative bacteria prime human dendritic cells for enhanced IL-23 and IL-27 expression and enhanced Th1 development. Eur J Immunol. 2004;34:1371–80. doi: 10.1002/eji.200324815. [DOI] [PubMed] [Google Scholar]
- 20.Devergne OBM, Kieff E. Epstein-Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proc Natl Acad Sci USA. 1997;94:12041–046. doi: 10.1073/pnas.94.22.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collison LWWC, Kuo TT, Boyd K, Wang Y, Vignali KM, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–69. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 22.Collison LWCV, Henderson AL, Giacomin PR, Guy C, Bankoti J, et al. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11:1093–101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu FTF, He Y, Liu H. Detectable expression of IL-35 in CD4+ T cells from peripheral blood of chronic hepatitis B patients. Clin Immunol. 2011;139:1–5. doi: 10.1016/j.clim.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Seyerl MKS, Majdic O, Seipelt J, Jindra C, Schrauf C, Stöckl J. Human rhinoviruses induce IL-35-producing Treg via induction of B7-H1 (CD274) and sialoadhesin (CD169) on DC. Eur J Immunol. 2010;40:321–9. doi: 10.1002/eji.200939527. [DOI] [PubMed] [Google Scholar]
- 25.Bardel ELF, Charlot-Rabiega P, Coulomb-L’Herminé A, Devergne O. Human CD4+ CD25+ Foxp3+ regulatory T cells do not constitutively express IL-35. J Immunol. 2008;181:6898–905. doi: 10.4049/jimmunol.181.10.6898. [DOI] [PubMed] [Google Scholar]
- 26.Crabé SG-GA, Tormo AJ, Duluc D, Lissilaa R, Guilhot F. The IL-27 p28 subunit binds cytokine-like factor 1 to form a cytokine regulating NK and T cell activities requiring IL-6R for signaling. J Immunol. 2009;183:7692–702. doi: 10.4049/jimmunol.0901464. [DOI] [PubMed] [Google Scholar]
- 27.Stumhofer JSTE, Quinn WJ, 3rd, Hosken N, Spudy B, Goenka R. A role for IL-27p28 as an antagonist of gp130-mediated signaling. Nat Immunol. 2010;11:1119–26. doi: 10.1038/ni.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collison LW, Delgoffe GM, Guy CS, Vignali KM, Chaturvedi V, Fairweather D, et al. The composition and signaling of the IL-35 receptor are unconventional. Nat Immunol. 2012;13:290–9. doi: 10.1038/ni.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watford WTHB, Bream JH, Kanno Y, Muul L, O’Shea JJ. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol Rev. 2004;202:139–56. doi: 10.1111/j.0105-2896.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- 30.Oppmann BLR, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–25. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 31.Parham CCM, Timans J, Vaisberg E, Travis M, Cheung J, Pflanz S, Zhang R, Singh KP, Vega F, To W, Wagner J, O’Farrell AM, McClanahan T, Zurawski S, Hannum C, Gorman D, Rennick DM, Kastelein RA, de Waal Malefyt R, Moore KW. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699–708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 32.Jones LL, Chaturvedi V, Uyttenhove C, Van Snick J, Vignali DAA. Distinct subunit pairing criteria within the heterodimeric IL-12 cytokine family. Molecular Immunology. 2012;51:234–44. doi: 10.1016/j.molimm.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pflanz SHL, Mattson J, Rosales R, Vaisberg E, Bazan JF. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–31. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 34.Silver JSHC. gp130 at the nexus of inflammation, autoimmunity, and cancer. J Leukoc Biol. 2010;88:1145–56. doi: 10.1189/jlb.0410217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sprecher CAGF, Baumgartner JW, Presnell SR, Schrader SK, Yamagiwa T. Cloning and characterization of a novel class I cytokine receptor. Biochem Biophys Res Commun. 1998;246:82–90. doi: 10.1006/bbrc.1998.8576. [DOI] [PubMed] [Google Scholar]
- 36.Scheller JSB, Hölscher C, Yoshimoto T, Rose-John S. No inhibition of IL-27 signaling by soluble gp130. Biochem Biophys Res Commun. 2005;326:724–8. doi: 10.1016/j.bbrc.2004.11.098. [DOI] [PubMed] [Google Scholar]
- 37.Morishima NOT, Asakawa M, Kamiya S, Mizuguchi J, Yoshimoto T. Augmentation of effector CD8+ T cell generation with enhanced granzyme B expression by IL-27. J Immunol. 2005;175:1686–93. doi: 10.4049/jimmunol.175.3.1686. [DOI] [PubMed] [Google Scholar]
- 38.Mayer KDMK, Reiley W, Wittmer S, Kohlmeier JE, Pearl JE, et al. Cutting edge: T-bet and IL-27R are critical for in vivo IFN-gamma production by CD8 T cells during infection. J Immunol. 2008;180:693–7. doi: 10.4049/jimmunol.180.2.693. [DOI] [PubMed] [Google Scholar]
- 39.Yoshimoto TOK, Morishima N, Kamiya S, Owaki T, Asakawa M. Induction of IgG2a class switching in B cells by IL-27. J Immunol. 2004;173:2479–85. doi: 10.4049/jimmunol.173.4.2479. [DOI] [PubMed] [Google Scholar]
- 40.Larousserie FCP, Bardel E, Froger J, Kastelein RA, Devergne O. Differential effects of IL-27 on human B cell subsets. J Immunol. 2006;176:5890–7. doi: 10.4049/jimmunol.176.10.5890. [DOI] [PubMed] [Google Scholar]
- 41.Liu LWS, Shan B, Shao L, Sato A, Kawamura K, Li Q, Ma G, Tagawa M. IL-27-mediated activation of natural killer cells and inflammation produced antitumour effects for human oesophageal carcinoma cells. Scand J Immunol. 2008;68:22–9. doi: 10.1111/j.1365-3083.2008.02111.x. [DOI] [PubMed] [Google Scholar]
- 42.Wirtz STI, Galle PR, Schild HJ, Birkenbach M, Blumberg RS, Neurath MF. Protection from lethal septic peritonitis by neutralizing the biological function of interleukin 27. J Exp Med. 2006;203:1875–81. doi: 10.1084/jem.20060471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holtrop ME, King GJ. The ultrastructure of the osteoclast and its functional implications. Clin Orthop. 1977:177–96. [PubMed] [Google Scholar]
- 44.Minkin C. Bone acid phosphatase: tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcif Tissue Int. 1982;34:285–90. doi: 10.1007/BF02411252. [DOI] [PubMed] [Google Scholar]
- 45.Drake FH, Dodds RA, James IE, Connor JR, Debouck C, Richardson S, et al. Cathepsin K, but not cathepsins B, L, or S, is abundantly expressed in human osteoclasts. The Journal of biological chemistry. 1996;271:12511–6. doi: 10.1074/jbc.271.21.12511. [DOI] [PubMed] [Google Scholar]
- 46.Hattersley G, Chambers TJ. Calcitonin receptors as markers for osteoclastic differentiation: correlation between generation of bone-resorptive cells and cells that express calcitonin receptors in mouse bone marrow cultures. Endocrinology. 1989;125:1606–12. doi: 10.1210/endo-125-3-1606. [DOI] [PubMed] [Google Scholar]
- 47.Davies J, Warwick J, Totty N, Philp R, Helfrich M, Horton M. The osteoclast functional antigen, implicated in the regulation of bone resorption, is biochemically related to the vitronectin receptor. J Cell Biol. 1989;109:1817–26. doi: 10.1083/jcb.109.4.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teitelbaum SL. Bone Resorption by Osteoclasts. Science. 2000;289:1504–8. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 49.Takayanagi H. The Role of NFAT in Osteoclast Formation. Annals of the New York Academy of Sciences. 2007;1116:227–37. doi: 10.1196/annals.1402.071. [DOI] [PubMed] [Google Scholar]
- 50.Horton MA, Taylor ML, Arnett TR, Helfrich MH. Arg-Gly-Asp (RGD) peptides and the anti-vitronectin receptor antibody 23C6 inhibit dentine resorption and cell spreading by osteoclasts. Exp Cell Res. 1991;195:368–75. doi: 10.1016/0014-4827(91)90386-9. [DOI] [PubMed] [Google Scholar]
- 51.Zaidi M, Troen B, Moonga BS, Abe E. Cathepsin K, osteoclastic resorption, and osteoporosis therapy. J Bone Miner Res. 2001;16:1747–9. doi: 10.1359/jbmr.2001.16.10.1747. [DOI] [PubMed] [Google Scholar]
- 52.Blair HC, Teitelbaum SL, Ghiselli R, Gluck S. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science. 1989;245:855–7. doi: 10.1126/science.2528207. [DOI] [PubMed] [Google Scholar]
- 53.Salo J, Lehenkari P, Mulari M, Metsikko K, Vaananen HK. Removal of osteoclast bone resorption products by transcytosis. Science. 1997;276:270–3. doi: 10.1126/science.276.5310.270. [DOI] [PubMed] [Google Scholar]
- 54.Nesbitt SA, Horton MA. Trafficking of matrix collagens through bone-resorbing osteoclasts. Science. 1997;276:266–9. doi: 10.1126/science.276.5310.266. [DOI] [PubMed] [Google Scholar]
- 55.Seita J, Asakawa M, Ooehara J, Takayanagi S-i, Morita Y, Watanabe N, et al. Interleukin-27 directly induces differentiation in hematopoietic stem cells. Blood. 2008;111:1903–12. doi: 10.1182/blood-2007-06-093328. [DOI] [PubMed] [Google Scholar]
- 56.Kamiya S, Nakamura C, Fukawa T, Ono K, Ohwaki T, Yoshimoto T, et al. Effects of IL-23 and IL-27 on osteoblasts and osteoclasts: inhibitory effects on osteoclast differentiation. Journal of Bone and Mineral Metabolism. 2007;25:277–85. doi: 10.1007/s00774-007-0766-8. [DOI] [PubMed] [Google Scholar]
- 57.Park JS, Jung YO, Oh HJ, Park SJ, Heo YJ, Kang CM, et al. Interleukin-27 suppresses osteoclastogenesis via induction of interferon-γ. Immunology. 2012 doi: 10.1111/j.1365-2567.2012.03622.x. n/an/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Furukawa MTH, Takito J, Yoda M, Sakai S, Hikata T, et al. IL-27 abrogates receptor activator of NF-kappa B ligand-mediated osteoclastogenesis of human granulocyte-macrophage colony-forming unit cells through STAT1-dependent inhibition of c-Fos. J Immunol. 2009;183:2397–406. doi: 10.4049/jimmunol.0802091. [DOI] [PubMed] [Google Scholar]
- 59.Kalliolias GDZB, Triantafyllopoulou A, Park-Min KH, Ivashkiv LB. Interleukin-27 inhibits human osteoclastogenesis by abrogating RANKL-mediated induction of nuclear factor of activated T cells c1 and suppressing proximal RANK signaling. Arthritis Rheum. 2010;62:402–13. doi: 10.1002/art.27200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lanier LLCB, Wu J, Leong C, Phillips JH. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature. 1998;391:703–7. doi: 10.1038/35642. [DOI] [PubMed] [Google Scholar]
- 61.Daws MR, Lanier LL, Seaman WE, Ryan JC. Cloning and characterization of a novel mouse myeloid DAP12-associated receptor family. Eur J Immunol. 2001;31:783–91. doi: 10.1002/1521-4141(200103)31:3<783::aid-immu783>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 62.Vivier E, Nunes JA, Vely F. Natural killer cell signaling pathways. Science. 2004;306:1517–9. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- 63.Zou W, Reeve JL, Liu Y, Teitelbaum SL, Ross FP. DAP12 couples c-Fms activation to the osteoclast cytoskeleton by recruitment of Syk. Molecular cell. 2008;31:422–31. doi: 10.1016/j.molcel.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zou W, Zhu T, Craft CS, Broekelmann TJ, Mecham RP, Teitelbaum SL. Cytoskeletal dysfunction dominates in DAP12-deficient osteoclasts. Journal of cell science. 2010;123:2955–63. doi: 10.1242/jcs.069872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaifu T, Nakahara J, Inui M, Mishima K, Momiyama T, Kaji M, et al. Osteopetrosis and thalamic hypomyelinosis with synaptic degeneration in DAP12-deficient mice. The Journal of clinical investigation. 2003;111:323–32. doi: 10.1172/JCI16923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Joyce-Shaikh B, Bigler ME, Chao CC, Murphy EE, Blumenschein WM, Adamopoulos IE, et al. Myeloid DAP12-associating lectin (MDL)-1 regulates synovial inflammation and bone erosion associated with autoimmune arthritis. J Exp Med. 2010;207:579–89. doi: 10.1084/jem.20090516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adamopoulos I, Sabokbar A, Wordsworth B, Carr A, Ferguson D, Athanasou N. Synovial fluid macrophages are capable of osteoclast formation and resorption. The Journal of pathology. 2006;208:35–43. doi: 10.1002/path.1891. [DOI] [PubMed] [Google Scholar]
- 68.Nakamura I, Kadono Y, Takayanagi H, Jimi E, Miyazaki T, Oda H, et al. IL-1 regulates cytoskeletal organization in osteoclasts via TNF receptor-associated factor 6/c-Src complex. J Immunol. 2002;168:5103–9. doi: 10.4049/jimmunol.168.10.5103. [DOI] [PubMed] [Google Scholar]
- 69.Hibbert LPS, De Waal Malefyt R, Kastelein RA. IL-27 and IFN-alpha signal via Stat1 and Stat3 and induce T-Bet and IL-12Rbeta2 in naive T cells. J Interferon Cytokine Res. 2003;23:513–22. doi: 10.1089/10799900360708632. [DOI] [PubMed] [Google Scholar]
- 70.Lucas SGN, Li J, de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci USA. 2003;100:15047–52. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hamano SHK, Miyazaki Y, Ishii K, Yamanaka A, Takeda A, et al. WSX-1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity. 2003;19:657–67. doi: 10.1016/s1074-7613(03)00298-x. [DOI] [PubMed] [Google Scholar]
- 72.Chen SHD, Shi X, Shen N, Gu Y, Bao C. The relationship between Th1/Th2-type cells and disease activity in patients with systemic lupus erythematosus. Chin Med J (Engl) 2000;113:877–80. [PubMed] [Google Scholar]
- 73.Yoshida HHS, Senaldi G, Covey T, Faggioni R, Mu S, et al. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. 2001;15:569–78. doi: 10.1016/s1074-7613(01)00206-0. [DOI] [PubMed] [Google Scholar]
- 74.Villarino AHL, Lieberman L, Wilson E, Mak T, Yoshida H, Kastelein RA, Saris C, Hunter CA. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645–55. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 75.Artis DJL, Joyce K, Saris C, Villarino A, Hunter CA, Scott P. Cutting edge: early IL-4 production governs the requirement for IL-27-WSX-1 signaling in the development of protective Th1 cytokine responses following Leishmania major infection. J Immunol. 2004;172:4672–5. doi: 10.4049/jimmunol.172.8.4672. [DOI] [PubMed] [Google Scholar]
- 76.Artis DVA, Silverman M, He W, Thornton EM, Mu S, et al. The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. J Immunol. 2004;173:5626–34. doi: 10.4049/jimmunol.173.9.5626. [DOI] [PubMed] [Google Scholar]
- 77.Yamanaka AHS, Miyazaki Y, Ishii K, Takeda A, Mak TW, et al. Hyperproduction of proinflammatory cytokines by WSX-1-deficient NKT cells in concanavalin A-induced hepatitis. J Immunol. 2004;172:3590–6. doi: 10.4049/jimmunol.172.6.3590. [DOI] [PubMed] [Google Scholar]
- 78.Miyazaki YIH, Matsumura M, Matsumoto K, Nakano T, Tsuda M, et al. Exacerbation of experimental allergic asthma by augmented Th2 responses in WSX-1-deficient mice. J Immunol. 2005;175:2401–7. doi: 10.4049/jimmunol.175.4.2401. [DOI] [PubMed] [Google Scholar]
- 79.Hölscher CHA, Rückerl D, Yoshimoto T, Yoshida H, Mak T, Saris C, Ehlers S. The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J Immunol. 2005;174:3534–44. doi: 10.4049/jimmunol.174.6.3534. [DOI] [PubMed] [Google Scholar]
- 80.Robinson KKR, Pidgeon EL, Shakib S, Patel S, Polson RJ. Helicobacter pylori-induced peptic ulcer disease is associated with inadequate regulatory T cell responses. Gut. 2008;57:1375–85. doi: 10.1136/gut.2007.137539. [DOI] [PubMed] [Google Scholar]
- 81.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–82. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. The Journal of clinical investigation. 1999;103:1345–52. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Adamopoulos IE, Chao CC, Geissler R, Laface D, Blumenschein W, Iwakura Y, et al. Interleukin-17A upregulates receptor activator of NF-kappaB on osteoclast precursors. Arthritis Res Ther. 2010;12:R29. doi: 10.1186/ar2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tyagi AM, Srivastava K, Mansoori MN, Trivedi R, Chattopadhyay N, Singh D. Estrogen Deficiency Induces the Differentiation of IL-17 Secreting Th17 Cells: A New Candidate in the Pathogenesis of Osteoporosis. PloS one. 2012;7:e44552. doi: 10.1371/journal.pone.0044552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yago T, Nanke Y, Ichikawa N, Kobashigawa T, Mogi M, Kamatani N, et al. IL-17 induces osteoclastogenesis from human monocytes alone in the absence of osteoblasts, which is potently inhibited by anti-TNF-α antibody: A novel mechanism of osteoclastogenesis by IL-17. Journal of Cellular Biochemistry. 2009;108:947–55. doi: 10.1002/jcb.22326. [DOI] [PubMed] [Google Scholar]
- 86.Batten MLJ, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–36. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 87.Stumhofer JSLA, Wilson EH, Huang E, Tato CM, Johnson LM, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–45. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 88.Diveu CMM, Boniface K, Stumhofer JS, Sathe M, Joyce-Shaikh B, et al. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol. 2009;182:5748–56. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- 89.Stumhofer JSSJ, Laurence A, Porrett PM, Harris TH, Turka LA, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–71. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 90.Fitzgerald DCCB, Touil T, Harle H, Grammatikopolou J, Das Sarma J, et al. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:3268–75. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 91.Awasthi ACY, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–9. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 92.Neufert CBC, Wirtz S, Fantini MC, Weigmann B, Galle PR, Neurath MF. IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. Eur J Immunol. 2007;37:1809–16. doi: 10.1002/eji.200636896. [DOI] [PubMed] [Google Scholar]
- 93.Batten MKN, Li J, Walter MJ, de Sauvage FJ, Ghilardi N. Cutting edge: IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. J Immunol. 2008;180:2752–6. doi: 10.4049/jimmunol.180.5.2752. [DOI] [PubMed] [Google Scholar]
- 94.Kamiya S, Okumura M, Chiba Y, Fukawa T, Nakamura C, Nimura N, et al. IL-27 suppresses RANKL expression in CD4+ T cells in part through STAT3. Immunology letters. 2011;138:47–53. doi: 10.1016/j.imlet.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 95.Cox JHKN, Ramamoorthi N, Diehl L, Batten M, Ghilardi N. IL-27 promotes T cell-dependent colitis through multiple mechanisms. J Exp Med. 2011;208:115–23. doi: 10.1084/jem.20100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wong CK, Chen da P, Tam LS, Li EK, Yin YB, Lam CW. Effects of inflammatory cytokine IL-27 on the activation of fibroblast-like synoviocytes in rheumatoid arthritis. Arthritis Res Ther. 2010;12:R129. doi: 10.1186/ar3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Niedbala W, Cai B, Wei X, Patakas A, Leung BP, McInnes IB, et al. Interleukin 27 attenuates collagen-induced arthritis. Annals of the rheumatic diseases. 2008;67:1474–9. doi: 10.1136/ard.2007.083360. [DOI] [PubMed] [Google Scholar]
- 98.Shahrara S, Huang Q, Mandelin AM, 2nd, Pope RM. TH-17 cells in rheumatoid arthritis. Arthritis Res Ther. 2008;10:R93. doi: 10.1186/ar2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tanida S, Yoshitomi H, Ishikawa M, Kasahara T, Murata K, Shibuya H, et al. IL-27-producing CD14(+) cells infiltrate inflamed joints of rheumatoid arthritis and regulate inflammation and chemotactic migration. Cytokine. 2011;55:237–44. doi: 10.1016/j.cyto.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 100.Baek SH, Lee SG, Park YE, Kim GT, Kim CD, Park SY. Increased synovial expression of IL-27 by IL-17 in rheumatoid arthritis. Inflammation research: official journal of the European Histamine Research Society [et al] 2012 doi: 10.1007/s00011-012-0534-7. [DOI] [PubMed] [Google Scholar]
- 101.Cao Y, Doodes PD, Glant TT, Finnegan A. IL-27 induces a Th1 immune response and susceptibility to experimental arthritis. J Immunol. 2008;180:922–30. doi: 10.4049/jimmunol.180.2.922. [DOI] [PubMed] [Google Scholar]
- 102.Shibata S, Tada Y, Asano Y, Yanaba K, Sugaya M, Kadono T, et al. IL-27 Activates Th1-Mediated Responses in Imiquimod-Induced Psoriasis-Like Skin Lesions. J Invest Dermatol. 2012 doi: 10.1038/jid.2012.313. [DOI] [PubMed] [Google Scholar]