Abstract
Background
The finding that exposure to general anesthetics (GAs) in childhood may increase rates of learning disabilities has raised a concern that anesthetics may interfere with brain development. The generation of neuronal circuits, a complex process in which axons follow guidance cues to dendritic targets, is an unexplored potential target for this type of toxicity.
Methods
GA exposures were conducted in developing neocortical neurons in culture and in early postnatal neocortical slices overlaid with fluorescently labeled neurons. Axon targeting, growth cone collapse, and axon branching were measured using quantitative fluorescence microscopy.
Results
Isoflurane exposure causes errors in Semaphorin 3A dependent axon targeting (n = 77 axons) and a disruption of the response of axonal growth cones to Semaphorin 3A (n = 2,358 growth cones). This effect occurs at clinically relevant anesthetic doses of numerous GAs with allosteric activity at γ-aminobutyric acid type A receptors, and it was reproduced with a selective agonist. Isoflurane also inhibits growth cone collapse induced by Netrin-1, but does not interfere branch induction by Netrin-1. Insensitivity to guidance cues caused by isoflurane is seen acutely in growth cones in dissociated culture, and errors in axon targeting in brain slice culture occur at the earliest point at which correct targeting is observed in controls.
Conclusion
Our results demonstrate a generalized inhibitory effect of GAs on repulsive growth cone guidance in the developing neocortex that may occur via a γ-aminobutyric acid type A receptor mechanism. The finding that GAs interfere with axon guidance, and thus potentially with circuit formation, represents a novel form of anesthesia neurotoxicity in brain development.
INTRODUCTION
Recent epidemiologic studies have demonstrated a striking correlation between childhood exposure to general anesthetics (GAs) and subsequent learning and behavioral disorders 1–5, raising concerns that GAs may adversely affect brain development 6–10. It is difficult to dissociate the effects of surgery and anesthesia in clinical research. However, in developing rodent models a combination of GAs administered at clinically relevant doses in the absence of surgery has been shown to cause persistent deficits in learning and memory 11. The functional deficits observed in behavioral testing were accompanied by two distinct phenomena: first, GAs enhanced neuronal apoptosis throughout the forebrain, and second, they produced alterations in brain function, in the form of decreased long-term potentiation in the hippocampus 11. Increases in apoptosis or apoptotic markers resulting from the administration of GAs have been verified in model systems ranging from the primate brain to organotypic rat brain slices to dissociated cell culture 12–14. However, apoptosis is widespread and adaptive in normal brain development 15,16, and so the question remains as to whether changes in the function of surviving neurons caused by GAs might be as important as the increased levels of cell death.
Normal brain development requires axons to navigate over long distances to form synapses with appropriate dendritic targets, and a range of severe cognitive defects are features of human developmental disorders that result from disrupted axon guidance 17. The axonal growth cone (AGC) is a highly motile specialized structure at the distal tip of the axon, which determines the direction of growth based on sensing of guidance cues that can be either attractive or repulsive 18. The Semaphorins are a family of diffusible guidance cues initially determined to be chemorepulsive based on their ability to collapse growth cones 19,20. Using Semaphorin 3A (Sema3A) and the rodent neocortex as a model, we tested the hypothesis that commonly used GAs might disrupt development of the nervous system by interfering with axon guidance, a process that is vital to circuit formation and normal brain function.
MATERIALS AND METHODS
Cultures
Care of animals adhered strictly to the guidelines of the National Institutes of Health and Columbia University, and institutional animal care committee approval was obtained for all experiments (Institutional Animal Care and Use Committee, Columbia University, New York, New York and Institutional Animal Care and Use Committee, The Mount Sinai School of Medicine, New York, New York). Dissociated neurons were prepared from embryonic day 18 to 19 C57BL/6 mouse neocortex 21,22. Neurons were plated at a density of 100/mm2 on cover slips coated with poly-D-lysine and laminin where noted (Sigma-Aldrich, St. Louis, MO). They were maintained in B-27/L-glutamine supplemented Neurobasal media (Invitrogen, Carlsbad, CA) and cocultured with a feeder layer of an immortalized astrocytic cell line (gift from James W. Jacobberger, PhD, Department of Molecular Biology and Microbiology, Professor, Case Western Reserve University, Cleveland, Ohio) 23. Experiments on dissociated neurons were performed at 2 to 4 days in vitro and represent at least three separate cultures, each with concurrent controls. For the slice overlay assay, 400 µm coronal slices of postnatal day 3 rat neocortex were allowed to settle on 0.4 µm Millicell inserts (Millipore, Bellerica, MA) for 4 h. Dissociated embryonic day 18 rat cortical neurons were then transfected with pmax-green fluorescent protein by electroporation using a Nucleofector (Lonza, Allendale, NJ) and applied at 7.5×104 neurons per slice for 4 h prior to anesthetic treatment 24,25. Experiments with slice overlay assays represent slices from at least four separate animals. There is some overlap between isoflurane and muscimol controls at 5 h, but in all cases controls were run concurrently to experimental conditions.
Anesthetic exposure
For isoflurane, sevoflurane, and desflurane treatment, dissociated neurons on cover slips or overlaid cortical slices were placed in airtight, humidified modular chambers (Billups-Rothenberg, Del Mar, CA) connected to an agent-specific calibrated vaporizer (Datex-Ohmeda, Madison, WI) that delivered the agent mixed with 5% carbon dioxide / 95% air carrier gas at 12 L/min 26. Nitrous oxide was delivered directly to the chambers from a tank containing 5% carbon dioxide, 25% oxygen, and 70% nitrous oxide. After a 15-min equilibration the chambers were sealed and kept at 37°C. Gas composition was measured periodically using a 5250 RGM gas analyzer (Datex-Ohmeda). Overlaid slices were treated with isoflurane, muscimol, or control carrier gas for 5 or 8 h. Dissociated cultures were incubated with anesthetics or vehicle controls for 1 h, followed by 20 min with recombinant mouse Sema3A-Fc chimera or Netrin-1 (R & D Systems, Bend, OR) to induce collapse. Pure propofol was diluted in dimethyl sulfoxide for delivery, and controls included equivalent amounts of this diluent. For branching experiments, cultures were incubated with isoflurane 1.2% and Netrin-1 for 8 h.
Cell labeling, immunocytochemistry, and microscopic analysis
Slice cultures were fixed with 4% paraformaldehyde and immunolabeled with anti-green fluorescent protein antibody (Millipore). Neurons with clearly defined axons at least 10 microns long that fell on the cortical plate between 50 and 200 µm from the dorsal edge were visualized using an Axiophot fluorescence microscope (Zeiss, Thornwood, NY) and traced using Neurolucida (MicroBrightField, Colchester, VT) for measurement of axon length and angle of trajectory. Axons were scored as either ventrally or dorsally targeted, and odds ratio (OR) indicating the likelihood of a loss of appropriate ventral targeting is reported.
Following treatment, dissociated neurons were fixed with 4% paraformaldehyde and labeled with Texas Red-conjugated phalloidin (Invitrogen) for growth cone analysis. For these studies and for later studies on axonal arborization, the axons were identified as the longest neurite, a consistent morphological determinant that is readily apparent at 2 to 4 days in vitro. 27 Axonal growth cones were classified morphologically by fluorescence miscroscopy as “extended” or “collapsed” based on standard criteria (collapsed growth cones are defined as lacking a full lamellipodia and/or exhibiting two or fewer filopodia in the direction of growth). The classification and counting of growth cones was done by an investigator who was blind to the experimental condition. Data are reported as the mean percentage of collapsed axonal growth cones per field, and the error bars denote standard deviation. For branching analysis dissociated neurons were fixed with 4% paraformaldehyde and immunolabled with anti-βIII Tubulin (Millipore) and a fluorescently tagged secondary antibody. Axonal arbors were traced and analyzed using NeuroLucida software (MicroBrightField) by an investigator blind to condition. Data are reported as mean branch number per 100 µm with error bars indicating standard deviation.
Statistical Methods
The criterion for significance in all statistical tests was p < 0.05, and all statistical analysis was performed using Prism 5 software (GraphPad, La Jolla, CA). All analyses represent complete data sets, with the exception of one overlaid slice in the muscimol group that was inadvertently destroyed during microscopy. For all slice overlay assays a two-by-two table was constructed to test the hypothesis that a loss of appropriate ventral targeting is associated with the specified pharmacologic treatment. We employed the Fisher exact test to assess for statistical significance. A student’s t test was used to test for a statistically significant difference in mean axon outgrowth length between the control and isoflurane exposed groups after an 8-h exposure. For all collapse assays we tested the hypothesis that mean percentage of growth cones which collapsed in response to Sema3A or Netrin-1 was reduced by the specified pharmacologic treatment. We performed an ANOVA with Dunnett's multiple-comparison post hoc tests to assess differences in mean collapse between groups. Similarly, in the analysis of axonal branching, we used an ANOVA with Dunnett's multiple-comparison post hoc tests to test the hypothesis that axonal branching induced by Netrin-1 was reduced by isoflurane treatment.
RESULTS
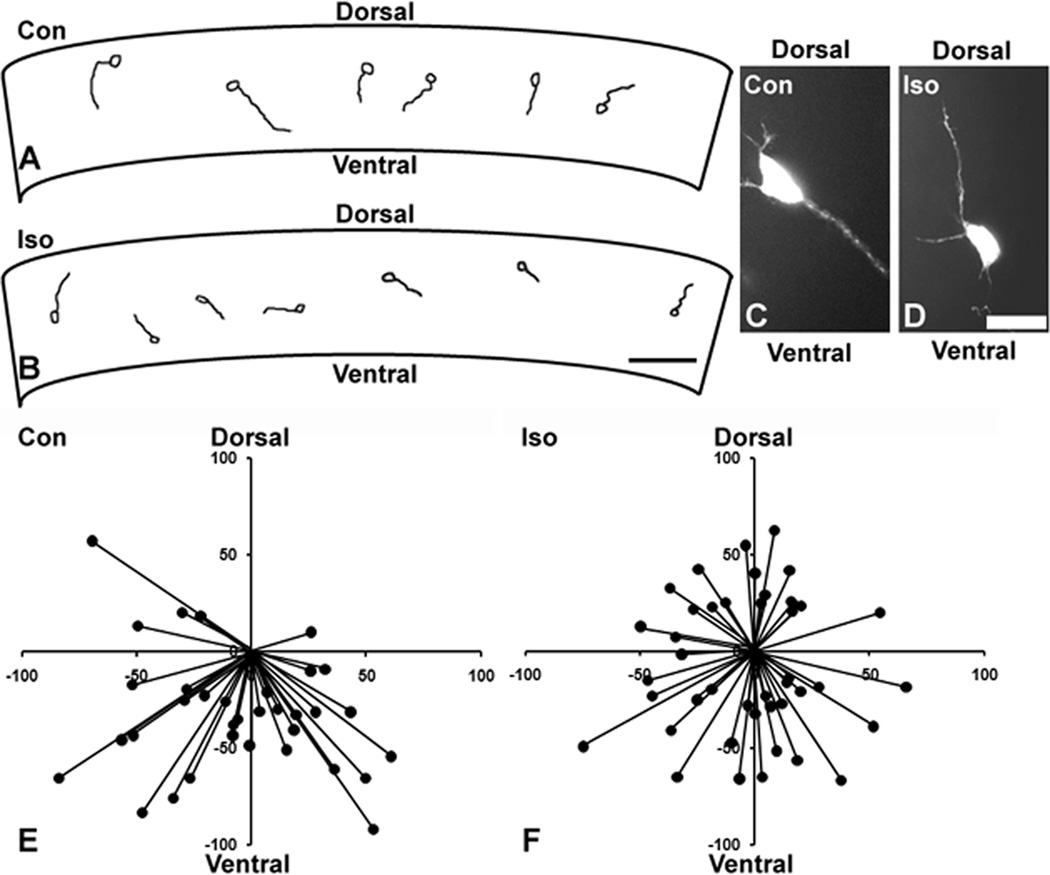
To determine whether GAs interfere with axon targeting, we chose a model system in which axons follow a clear, easily assessed trajectory. In the “slice overlay” assay, the axons of dissociated neocortical neurons applied to the cortical plate of an early postnatal coronal neocortex slice are directed ventrally towards the sub-cortical white matter by a dorsoventral gradient of Sema3A 25. We found that 85% of control axons take an appropriate ventral trajectory. In striking contrast, after 8 h of treatment with 1.2% isoflurane, only 58% of axons take a ventral trajectory, a statistically significant reduction. Figure 1 shows examples of Neurolucida tracings from a single slice for each condition (fig. 1A and B) along with representative examples of individual neurons (fig. 1C and D). A summary diagram depicting the axon paths of all neurons analyzed demonstrates that the trajectories are randomly oriented in the isoflurane-treated slice (compare fig. 1E and F). Interestingly, there was also a modest but statistically significant reduction in mean axon outgrowth length (53.0 µm for controls vs. 43.5 µm for the isoflurane group), which is consistent with previous findings in dissociated culture 28.
Figure 1. Isoflurane causes aberrant axonal guidance.
An example of a Neurolucida tracing from a single coronal slice demonstrates appropriate ventrally directed growth of most axons in a control (Con) slice (A) and no clear directional preference in a slice treated with 1.2% isoflurane (Iso) for 8h (B). Examples of individual neurons are shown in which the control axon exhibits a ventral path (C) and the axon from the isoflurane-treated slice has grown aberrantly in a dorsal direction (D). A summary diagram from all slice overlay experiments shows that nearly all control axons take a ventral trajectory (E), whereas those of the isoflurane group appear to be randomly oriented (F). n=77 axons in 11 slices for (E) and (F). Scale bar is 50 µm in (B) and 25 µm in (D); axes in (E) and (F) in µm.
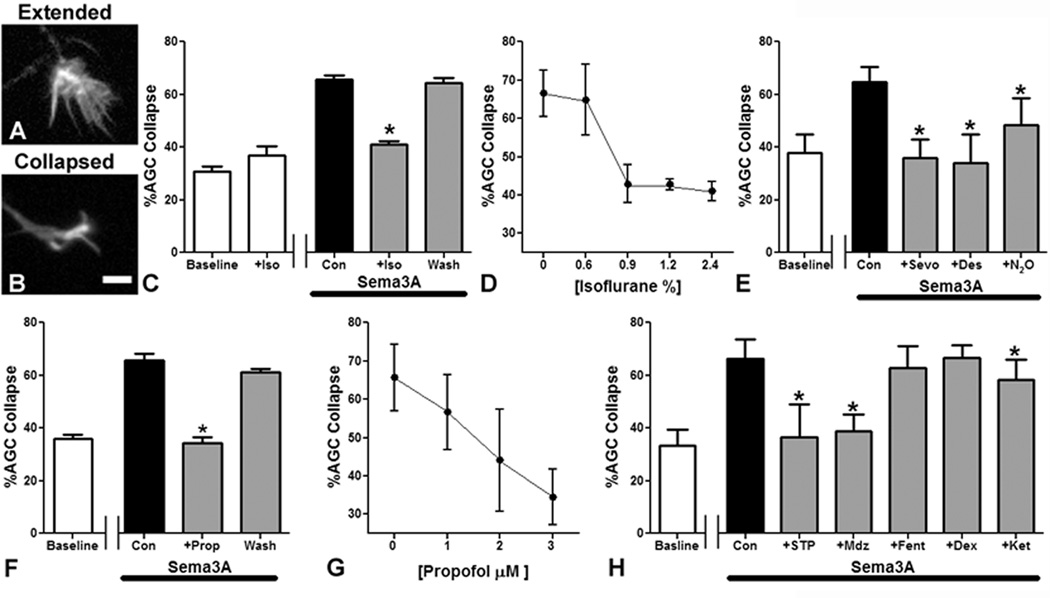
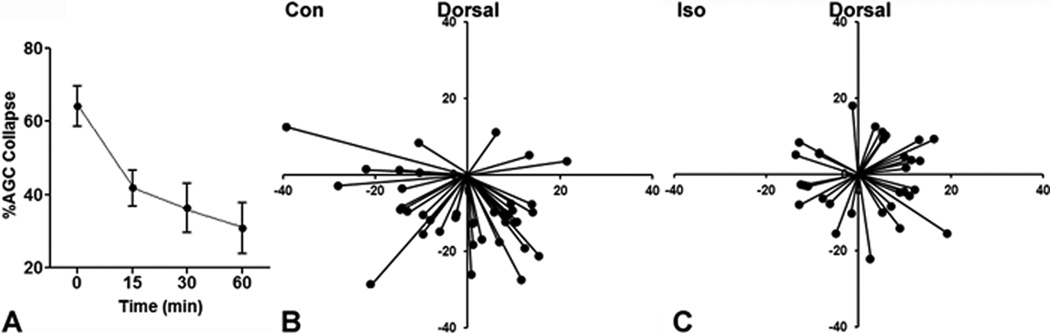
To further explore the relationship between GA treatment and errors in Sema3A axon guidance, we examined growth cone collapse in dissociated primary neocortical neurons. In this assay, a switch in growth cone morphology from predominantly extended (fig. 2A) to predominantly collapsed (fig. 2B) correlates strongly with repulsive targeting 29. Consistent with previous findings, cultures treated with Sema3A showed 60–70% AGC collapse (e.g., fig. 2C, “Con”) as compared to 30–40% at baseline for all untreated controls (e.g., fig. 2C, “Baseline”) 24. Isoflurane treatment alone does not alter growth cone morphology, but isoflurane treatment for one hour caused a statistically significant inhibition of growth cone collapse induced by Sema3A (fig. 2C). The collapse response returned to pre-treatment levels when the cells were allowed to recover for 3 h from isoflurane exposure prior to Sema3A application, indicating that the effect is reversible (fig. 2C). This inhibition of the AGC response to Sema3A is concentration-dependent across a spectrum of clinically relevant isoflurane concentrations, ranging from 0.6% to 2.4%, (fig. 2D). An identical loss of the AGC collapse response to Sema3A is also obtained with 4% sevoflurane or 12% desflurane (fig. 2E). There is a smaller response to 70% N2O (fig. 2E).
Figure 2. Commonly used general anesthetics inhibit Semaphorin3A mediated axonal growth cone collapse.
Examples of extended (A) and collapsed (B) axonal growth cones (AGCs) are shown. One hour of 2.4% isoflurane treatment caused a statistically significant reduction in Semaphorin3A (Sema3A)-induced AGC collapse and the effect is reversed 3h after washout of isoflurane prior to Sema3A application (C). Isoflurane (2.4%) alone does not induce collapse (C). Inhibition of the Sema3A induced collapse response caused by isoflurane is concentration-dependent over a clinically relevant range (D). Treatment with 4% sevoflurane (Sevo) and 12% desflurane (Des) reduces AGC collapse to levels seen in controls not treated with Sema3A, whereas nitrous oxide (N2O) has a lesser effect (E). A 1h treatment with the intravenous agent propofol at 3 µM also inhibits Sema3A induced AGC collapse, and as with isoflurane, the effect is reversed 3h after washout (F). Propofol causes a concentration-dependent decrease in AGC collapse over the range from 1 to 3 µM (G). Sodium thiopental (STP) at 50 µM and midazolam (Mdz) at 1 µM also inhibit the AGC response to Sema3A (H). In contrast, neither fentanyl (Fent) at 100 nM, nor dexmedetomidine (Dex) at 40 nM, alter the AGC response to Sema3A, and ketamine (Ket) at 20 µM causes a modest reduction in collapse (H). n=2,358 AGCs in 56 fields for (C) and (D). n=1,804 AGCs in 48 fields for (E). n=1,519 AGCs in 50 fields for (F) and (G). n=3,575 AGCs, 108 fields for (H). Scale bar in (B) is 10 µm. *Indicates p<0.05 compared to Sema3A treated controls.
To determine whether this effect is unique to volatile GAs we assayed AGC collapse in the presence of intravenous anesthetics of different classes. Propofol, the most commonly used GA alternative to the inhaled agents, exhibits a similar disruption of the AGC Sema3A collapse response, which is fully reversible and concentration-dependent between 1 and 3 µM (fig. 2F and G). A barbiturate, sodium thiopental (50 µM), and a benzodiazepine, midazolam (1 µM), both cause a complete inhibition of Sema3A induced AGC collapse as well (fig. 2H). In contrast, high doses of fentanyl (100 nM) and dexmedetomidine (40 nM) do not prevent AGC collapse, and ketamine (20 µM) has only a modest effect on the Sema3A response (fig. 2H). The known pharmacology of these GAs suggests a mechanism to explain our results. The principal molecular targets of propofol, barbiturates, and benzodiazepines are the γ-aminobutyric acid type A receptors (GABAARs) 30,31, and the potent volatile GAs also act on GABAARs 30,32–34. On the other hand, it is well established that fentanyl and dexmedetomidine have no effects at GABAARs, whereas ketamine and nitrous oxide may have some weak GABAAR activity 35,36.
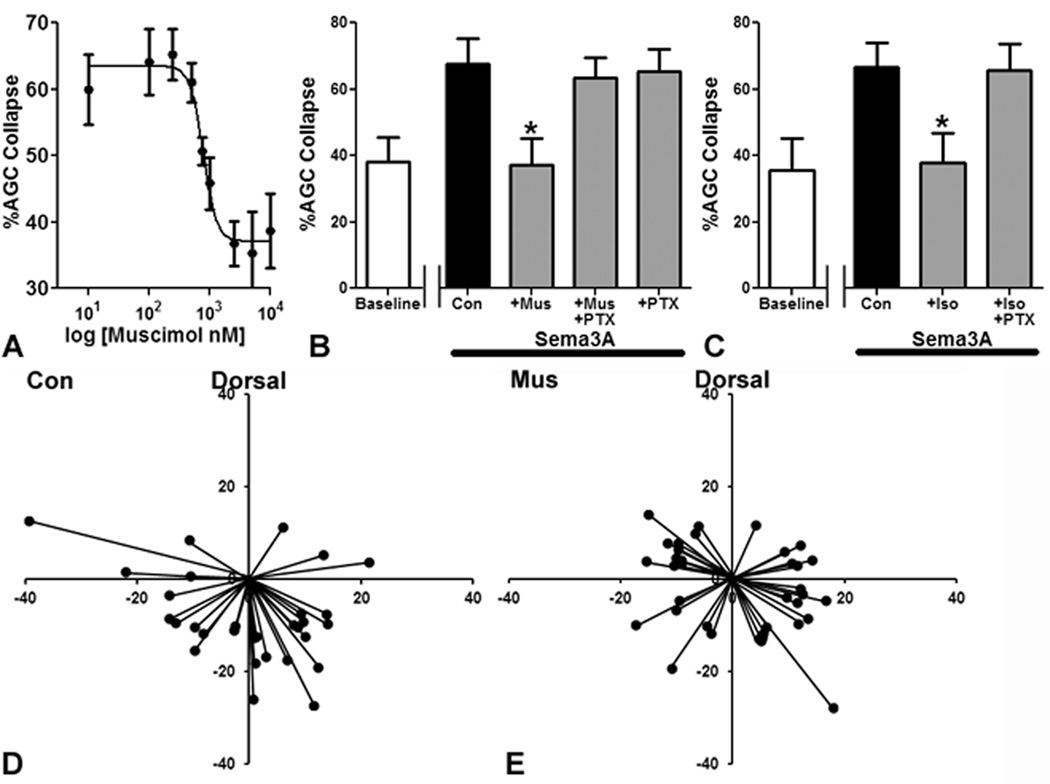
To test the hypothesis that the Sema3A response in AGCs is inhibited by GA actions at GABAARs, we assayed collapse to Sema3A in the presence of a highly specific GABAAR agonist, muscimol, at concentrations from the nanomolar to micromolar range. An inhibition curve was constructed demonstrating a clear concentration-response relationship for muscimol, with an IC50 at 0.8 µM (fig. 3A). This action of muscimol is completely inhibited by the GABAAR channel blocker picrotoxin (50 µM) (fig. 3B). The AGC collapse response to Sema3A is also fully preserved when picrotoxin is coadministered with 2.4% isoflurane (fig. 3C), consistent with the idea that the effect of isoflurane could be mediated by GABAAR activation. Next we sought to determine whether the inhibition of the growth cone responses to Sema3A by GABAAR activation can translate into a disruption of axon targeting. We found that slice overlay preparations treated with muscimol (10 µM) had a statistically significant loss of appropriate ventral targeting relative to controls (fig. 3D and E; 77% ventral for controls, 53% ventral for muscimol group, OR 2.94).
Figure 3. Semaphorin3A induced axonal growth cone collapse and appropriate axon targeting in the slice overlay model are blocked by γ-aminobutyric acid type A receptor activity.
The γ-aminobutyric acid type A receptor agonist muscimol causes a concentration-dependent inhibition of axonal growth cone (AGC) collapse (A). Treatment with the γ-aminobutyric acid type A receptor channel blocker picrotoxin (PTX) allows Semaphorin3A induced AGC collapse even with a high dose of muscimol (Mus) (10 µM) (B) or isoflurane (2.4%) (C). Normal ventral targeting seen in a control slice overlays (D) is disrupted by 5h of treatment with muscimol at 10 µM (E). n=2,883 AGCsin 84 fields for (A). n=2,329 AGCs in 60 fields for (B). n=1,910 AGCs in 52 fields for (C). n=66 axons in 11 slices for (D) and (E). Axes in (B) and (C) in µm. *Indicates p<0.05 compared to Semaphorin3A treated controls.
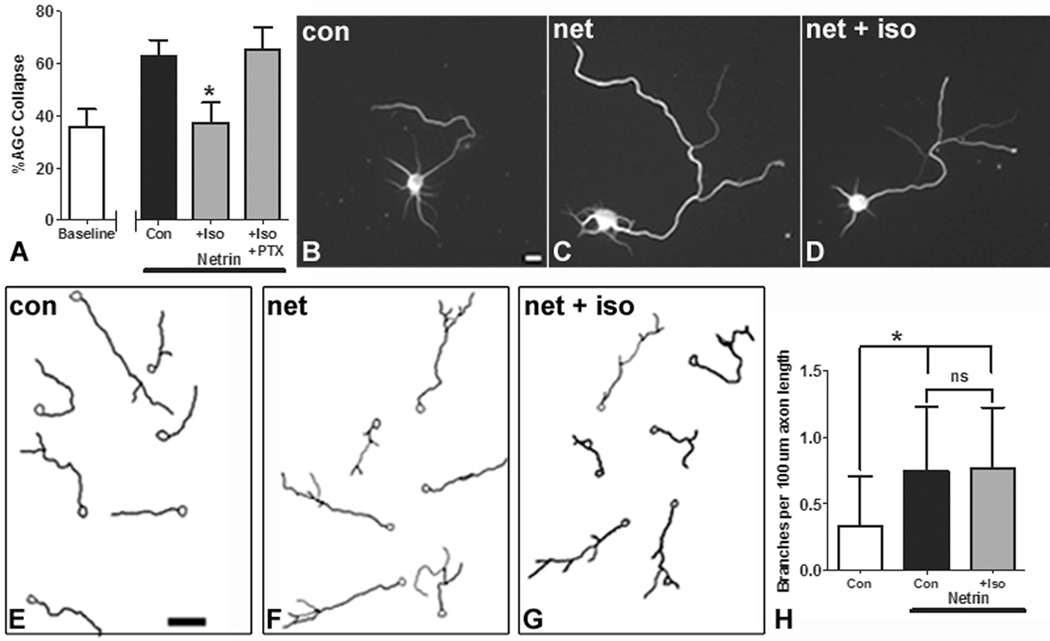
To determine whether the effect of anesthetics on the AGC is specific to Sema3A or generalized to repulsive guidance, we tested the effects of isoflurane on the response to another guidance cue with distinct receptors and signaling pathways from Sema3A. Netrin-1 is a chemoattractant in many contexts, but it functions as a chemorepellent to axons growing on a laminin substrate 37. We found that isoflurane blocked collapse induced by Netrin-1 in conjunction with a laminin substrate, and that the collapse response was restored by coadministration of picrotoxin (fig. 4A). In addition to serving as a growth cone guidance cue, Netrin-1 can alter axonal morphology by inducing branching in cortical neurons 38. To test whether anesthetics interfere with multiple functions of guidance cues, as opposed to having activity specifically at the growth cone, we assayed the effects of isoflurane on axonal branching in neurons exposed to Netrin-1. We found that Netrin-1 induces axonal branch formation (compare fig. 4B with fig. 4C, which are representative examples of single neurons; mean of 0.8 branches per 100 µm increased to 2.2 branches per 100 µm with Netrin-1) but that treatment with isoflurane neither inhibits nor enhances this effect (fig. 4D, mean 2.0 branches per 100 µm). Representative examples of fields of traced neurons are shown in figure 4E–G, and results are summarized in figure 4H. Taken together these data indicate that isoflurane generally inhibits repulsive guidance independent of the cue, but that the effects of isoflurane on guidance cue activity are specific to the growth cone.
Figure 4. Isoflurane inhibits growth cone collapse induced by Netrin-1, but does not alter branching induced by Netrin-1.
Isoflurane (2.4%) inhibits Netrin-1/Laminin induced axonal growth cone collapse, and the effect is blocked by picrotoxincotreatment (A). Isoflurane (1.2%) does not alter axon branching induced by Netrin-1. Representative examples of neurons and neurolucida tracings of their axon arbors are shown. (B) and (E) are control neurons incubated for 8h in carrier gas alone. (C) and (F) are neurons with Netrin-1 and carrier gas, showing the increase in branching induced by Netrin-1. (D) and (G) show axonal arbors of neurons cotreated with Netrin-1 and 1.2% isoflurane. An analysis of branch number shows that isoflurane treatment does not alter branching (H). n=1,418 axonal growth cones in 46 fields for (A). n=81 neurons for (H). *In (A) indicates p<0.05 compared to Netrin-1/Laminin treated controls. *In (A) Indicates p<0.05 compared to Netrin-1 treated control. *In (H) indicates p<0.05 compared to control without Netrin-1, and “ns” indicates no significant difference. Scale bar is 10 µm in (A) and 50 µm in (B).
In order to better understand the temporal relationship between isoflurane exposure and alterations in axon guidance that might be relevant to circuit formation, we examined the duration of exposure that was required to cause measurable effects. We found that an isoflurane exposure as short as 15 min, the minimum time to achieve equilibration in the closed chamber, was sufficient to render AGCs insensitive to Sema3A in the collapse assay (fig. 5A). In pilot experiments of the overlay assay we found that control preparations require a minimum of five hours to achieve consistent appropriate ventral targeting. While the effects is less dramatic than at the 8-h time point, a 5-h treatment with isoflurane was sufficient to cause a statistically significant loss of ventral targeting (fig. 5B and C, OR 3.41).
Figure 5. Isoflurane causes immediate effects on axonal growth cone sensitivity to Semaphorin3A but requires a longer exposure for effects on axon guidance.
A time-response curve is shown depicting the percent axonal growth cone collapse to Semaphorin3A at increasing time of exposure to isoflurane, which shows a loss of collapse in as little as 15 min (A). Trajectory diagrams from a slice overlay assay conducted at 5h, the minimum time for appropriate targeting of controls (B) shows that a disruption of axon guidance is seen with 1.8% isoflurane at this time point. N=598 axonal growth cones in 32 fields for (A). n=71 axons in 13 slices for (B) and (C). Axes in (B) and (C) in µm.
DISCUSSION
Our data demonstrate that both isoflurane and a selective GABAAR agonist can interfere with axon targeting. Furthermore, isoflurane and other agents which act on GABAARs, block the response of AGCs to Sema3A, and sensitivity to Sema3A can be restored by coadministration of a GABAAR antagonist. Many GAs used in clinical practice have activity at GABAARs, a ligand-gated ion channel with chloride selectivity that is comprised of a variable combination of subunits. Interestingly, ethanol, which also acts on GABAARs, is known to inhibit Sema3A growth cone collapse in a similar fashion 39, a finding which agrees with our results and suggests that they might generalize to a broad array of compounds with activity at GABAARs.
The putative link between GABAAR, Sema3A, and the complex signaling and machinery system that effects rearrangement of the growth cone cytoskeleton is not yet clear. γ-aminobutyric acid has been shown to play an important role in neuronal development 40. Also, tonic γ-aminobutyric acid activity can influence circuit formation in newly born dentate granule cells undergoing integration into existing circuits in the adult hippocampus. In this model, a depolarizing chloride current mediated by GABAARs is necessary for dendritic development to occur normally 41. Immature neurons are typically depolarized by GABAAR activation due to an outwardly directed chloride gradient maintained by the developmentally prevalent sodium potassium chloride cotransporter 1 42,43. Both Sema3A and Netrin-1 cause shifts in membrane potential, and repulsion can be blocked by electrically clamping the membrane potential 44. Thus, the most parsimonious explanation for the ability of GAs to inhibit the AGC collapse response to both Sema3A and Netrin-1/laminin is that they do so by effectively clamping the membrane potential of the growth cone at the chloride equilibrium potential. It is also possible that GABAAR mediated depolarization causes calcium entry, which in turn disrupts the localized, asymmetric calcium transients on which axon guidance is dependent 45–47.
Alternatively, the effects of GAs on axon guidance and growth cone function may be mediated by effects on the small GTPase RhoA. Isoflurane is known to activate RhoA 48, which can result in changes in the actin cytoskeleton 13. RhoA regulates cytoskeletal dynamics downstream from both Sema3A and Netrin-1 signaling 49, however our findings were replicated using GAs other than isoflurane, including agents which are chemically very dissimilar to the potent volatiles anesthetics. It is possible that other GAs might have similar effects on RhoA, but such evidence is lacking at present. Furthermore, RhoA is known to promotes axonal branching in developing cortical neurons 50. The absence of any effects of isoflurane on branching induced by Netrin-1 supports a model in which isoflurane and other GAs that act on GABAARs fundamentally alter the motile properties of the growth cone, rather than interfering generally with signaling systems such as small GTPases that are shared between different functions of the cues.
Establishing the minimum duration of anesthetic exposure that is required to cause a lasting and clinically significant alteration in brain function is of key importance in research on putative developmental anesthetic neurotoxicity. We find that the effects of anesthetics on AGC responses to Sema3A occur acutely over the course of minutes, but a model more relevant to circuit formation such as the overlay assay has a different timescale. In this setting the AGCs experience a Sema3A cue gradient which exerts an impact over a long period of growth, and an exposure on the order of hours is necessary for the effects of isoflurane to be manifested as errors in targeting. Our study is, of course, limited by the rodent model system in which brain development proceeds on the order of months, rather than years as in a human patient. Additionally, our data indicate that the effects of anesthetics on growth cones responsiveness to cues does not outlast the exposure (fig. 2C), but it is unclear what the long-term fate of aberrantly directed projections laid down during the exposure would be.
The disruption of axon guidance by GAs is a novel and potentially important mechanism of anesthesia neurotoxicity, which must be added to already existing concerns that GAs can enhance neuronal apoptosis during development. Increased apoptosis is not likely to be a feature of the model presented here since the isoflurane exposure we employed in primary cortical neuronal culture did not increase cell death measured by cell density per field or cellular metabolic rate, as measured by tetrazolium reduction (data not shown). Additionally, recent data indicates that developmental exposure to both propofol and potent volatile anesthetics such as isoflurane alters dendritic spine density, again suggesting a perturbation of normal development distinct from direct toxicity to neurons via proapoptotic properties of the GAs 51,52. However, these phenomena may all be related in the intact nervous system, as neurons which fail to make appropriate connections either through errors in axon guidance or problems with synaptogenesis are thought to be eliminated by apoptosis, presumably to protect the integrity of key circuits 16. Our data underscore the importance of examining the dose, type, and duration of GA exposure in clinical investigations of pediatric anesthetic neurotoxicity.
Summary.
What we already know about this topic
Exposure to general anesthetics during early brain development can result in persistent deficits in leaning and memory in animal models
The possible role of anesthetic effects on neuronal network formation in this phenomenon is unknown
What this article tells us that is new
Using rodent cortex brain slice and isolated neuron assays, general anesthetics known to act via GABAergic mechanisms were shown to disrupt axon guidance mechanisms
Anesthetic disruption of axon guidance could affect normal brain development, but its behavioral impact or clinical relevance require further study
Acknowledgements
The authors thank Roxana Mesias, B.A. (Research Associate, Department of Neuroscience, The Mount Sinai School of Medicine, New York, New York) for technical assistance), Sobiah I. Khan for assistance with manuscript preparation (Research Associate, Barnard College, New York, New York) H.T. Lee M.D., Ph.D. (Professor, Department of Anesthesiology, Columbia University, New York, New York) for use of an anesthesia exposure system.
Funding was provided by the Department of Anesthesiology at Columbia University, New York, New York (to CDM and SCS) and the National Institutes of Health, Bethesda, Maryland, grants GM008464 (to CDM) and NS050634 (to DLB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Block RI, Thomas JJ, Bayman EO, Choi JY, Kimble KK, Todd MM. Are anesthesia and surgery during infancy associated with altered academic performance during childhood? Anesthesiology. 2012;117:494–503. doi: 10.1097/ALN.0b013e3182644684. [DOI] [PubMed] [Google Scholar]
- 2.Dimaggio C, Sun L, Li G. Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth Analg. 2011;113:1143–1151. doi: 10.1213/ANE.0b013e3182147f42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DiMaggio C, Sun LS, Kakavouli A, Byrne MW, Li G. A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. J Neurosurg Anesthesiol. 2009;21:286–291. doi: 10.1097/ANA.0b013e3181a71f11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ing C, Dimaggio C, Whitehouse A, Hegarty MK, Brady J, von Ungern-Sternberg BS, Davidson A, Wood AJ, Li G, Sun LS. Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics. 2012;130:e476–e485. doi: 10.1542/peds.2011-3822. [DOI] [PubMed] [Google Scholar]
- 5.Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL, Warner DO. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidson AJ. Anesthesia and neurotoxicity to the developing brain: The clinical relevance. Paediatr Anaesth. 2011;21:716–721. doi: 10.1111/j.1460-9592.2010.03506.x. [DOI] [PubMed] [Google Scholar]
- 7.Istaphanous GK, Loepke AW. General anesthetics and the developing brain. Curr Opin Anaesthesiol. 2009;22:368–373. doi: 10.1097/aco.0b013e3283294c9e. [DOI] [PubMed] [Google Scholar]
- 8.Kuehn BM. FDA considers data on potential risks of anesthesia use in infants, children. JAMA. 2011;305:1749–1750. 1753. doi: 10.1001/jama.2011.546. [DOI] [PubMed] [Google Scholar]
- 9.Rappaport B, Mellon RD, Simone A, Woodcock J. Defining safe use of anesthesia in children. N Engl J Med. 2011;364:1387–1390. doi: 10.1056/NEJMp1102155. [DOI] [PubMed] [Google Scholar]
- 10.Wilder RT. Is there any relationship between long-term behavior disturbance and early exposure to anesthesia? Curr Opin Anaesthesiol. 2010;23:332–336. doi: 10.1097/ACO.0b013e3283391f94. [DOI] [PubMed] [Google Scholar]
- 11.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Zhang X, Dissen GA, Creeley CE, Olney JW. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834–841. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lemkuil BP, Head BP, Pearn ML, Patel HH, Drummond JC, Patel PM. Isoflurane neurotoxicity is mediated by p75NTR-RhoA activation and actin depolymerization. Anesthesiology. 2011;114:49–57. doi: 10.1097/ALN.0b013e318201dcb3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wise-Faberowski L, Zhang H, Ing R, Pearlstein RD, Warner DS. Isoflurane-induced neuronal degeneration: An evaluation in organotypic hippocampal slice cultures. Anesth Analg. 2005;101:651–657. doi: 10.1213/01.ane.0000167382.79889.7c. [DOI] [PubMed] [Google Scholar]
- 15.Cowan WM, Fawcett JW, O'Leary DD, Stanfield BB. Regressive events in neurogenesis. Science. 1984;225:1258–1265. doi: 10.1126/science.6474175. [DOI] [PubMed] [Google Scholar]
- 16.Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- 17.Engle EC. Human genetic disorders of axon guidance. Cold Spring Harb Perspect Biol. 2010;2:a001784. doi: 10.1101/cshperspect.a001784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolodkin AL, Tessier-Lavigne M. Mechanisms and molecules of neuronal wiring: A primer. Cold Spring Harb Perspect Biol. 2011;3:a00727. doi: 10.1101/cshperspect.a001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolodkin AL, Matthes DJ, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75:1389–1399. doi: 10.1016/0092-8674(93)90625-z. [DOI] [PubMed] [Google Scholar]
- 20.Luo Y, Raible D, Raper JA. Collapsin: A protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75:217–227. doi: 10.1016/0092-8674(93)80064-l. [DOI] [PubMed] [Google Scholar]
- 21.Banker G, Goslin K. Culturing nerve cells. 2nd Edition. Cambridge, Mass.: MIT Press; 1998. [Google Scholar]
- 22.Ma L, Song L, Radoi GE, Harrison NL. Transcriptional regulation of the mouse gene encoding the α-4 subunit of the GABAA receptor. J Biol Chem. 2004;279:40451–40461. doi: 10.1074/jbc.M406827200. [DOI] [PubMed] [Google Scholar]
- 23.Frisa PS, Goodman MN, Smith GM, Silver J, Jacobberger JW. Immortalization of immature and mature mouse astrocytes with SV40 T antigen. J Neurosci Res. 1994;39:47–56. doi: 10.1002/jnr.490390107. [DOI] [PubMed] [Google Scholar]
- 24.Mintz CD, Carcea I, McNickle DG, Dickson TC, Ge Y, Salton SR, Benson DL. ERM proteins regulate growth cone responses to Sema3A. J Comp Neurol. 2008;510:351–366. doi: 10.1002/cne.21799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polleux F, Giger RJ, Ginty DD, Kolodkin AL, Ghosh A. Patterning of cortical efferent projections by semaphorin-neuropilin interactions. Science. 1998;282:1904–1906. doi: 10.1126/science.282.5395.1904. [DOI] [PubMed] [Google Scholar]
- 26.Lee HT, Kim M, Jan M, Emala CW. Anti-inflammatory and antinecrotic effects of the volatile anesthetic sevoflurane in kidney proximal tubule cells. Am J Physiol Renal Physiol. 2006;291:F67–F78. doi: 10.1152/ajprenal.00412.2005. [DOI] [PubMed] [Google Scholar]
- 27.Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mintz CD, Smith SC, Barrett KM, Benson DL. Anesthetics interfere with the polarization of developing cortical neurons. J Neurosurg Anesthesiol. 2012;24:368–375. doi: 10.1097/ANA.0b013e31826a03a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapfhammer JP, Xu H, Raper JA. The detection and quantification of growth cone collapsing activities. Nat Protoc. 2007;2:2005–2011. doi: 10.1038/nprot.2007.295. [DOI] [PubMed] [Google Scholar]
- 30.Franks NP, Lieb WR. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994;367:607–614. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- 31.Hales TG, Lambert JJ. The actions of propofol on inhibitory amino acid receptors of bovine adrenomedullary chromaffin cells and rodent central neurones. Br J Pharmacol. 1991;104:619–628. doi: 10.1111/j.1476-5381.1991.tb12479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones MV, Brooks PA, Harrison NL. Enhancement of γ-aminobutyric acid-activated Cl- currents in cultured rat hippocampal neurones by three volatile anaesthetics. J Physiol. 1992;449:279–293. doi: 10.1113/jphysiol.1992.sp019086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakahiro M, Yeh JZ, Brunner E, Narahashi T. General anesthetics modulate GABA receptor channel complex in rat dorsal root ganglion neurons. FASEB J. 1989;3:1850–184. doi: 10.1096/fasebj.3.7.2541038. [DOI] [PubMed] [Google Scholar]
- 34.Krasowski MD, Harrison NL. The actions of ether, alcohol and alkane general anaesthetics on GABAA and glycine receptors and the effects of TM2 and TM3 mutations. Br J Pharmacol. 2000;129:731–743. doi: 10.1038/sj.bjp.0703087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dzoljic M, Van Duijn B. Nitrous oxide-induced enhancement of γ-aminobutyric acid A-mediated chloride currents in acutely dissociated hippocampal neurons. Anesthesiology. 1998;88:473–480. doi: 10.1097/00000542-199802000-00026. [DOI] [PubMed] [Google Scholar]
- 36.Lin LH, Chen LL, Zirrolli JA, Harris RA. General anesthetics potentiate γ-aminobutyric acid actions on γ-aminobutyric acidA receptors expressed by Xenopus oocytes: Lack of involvement of intracellular calcium. J Pharmacol Exp Ther. 1992;263:569–578. [PubMed] [Google Scholar]
- 37.Hopker VH, Shewan D, Tessier-Lavigne M, Poo M, Holt C. Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature. 1999;401:69–73. doi: 10.1038/43441. [DOI] [PubMed] [Google Scholar]
- 38.Dent EW, Barnes AM, Tang F, Kalil K. Netrin-1 and semaphorin 3A promote or inhibit cortical axon branching, respectively, by reorganization of the cytoskeleton. J Neurosci. 2004;24:3002–3012. doi: 10.1523/JNEUROSCI.4963-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sepulveda B, Carcea I, Zhao B, Salton SR, Benson DL. L1 cell adhesion molecule promotes resistance to alcohol-induced silencing of growth cone responses to guidance cues. Neuroscience. 2011;180:30–40. doi: 10.1016/j.neuroscience.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sernagor E, Chabrol F, Bony G, Cancedda L. GABAergic control of neurite outgrowth and remodeling during development and adult neurogenesis: General rules and differences in diverse systems. Front Cell Neurosci. 2010;4:11. doi: 10.3389/fncel.2010.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hubner CA, Lorke DE, Hermans-Borgmeyer I. Expression of the Na-K-2Cl-cotransporter NKCC1 during mouse development. Mech Dev. 2001;102:267–269. doi: 10.1016/s0925-4773(01)00309-4. [DOI] [PubMed] [Google Scholar]
- 43.LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 44.Nishiyama M, von Schimmelmann MJ, Togashi K, Findley WM, Hong K. Membrane potential shifts caused by diffusible guidance signals direct growth-cone turning. Nat Neurosci. 2008;11:762–771. doi: 10.1038/nn.2130. [DOI] [PubMed] [Google Scholar]
- 45.Gomez TM, Robles E, Poo M, Spitzer NC. Filopodial calcium transients promote substrate-dependent growth cone turning. Science. 2001;291:1983–1987. doi: 10.1126/science.1056490. [DOI] [PubMed] [Google Scholar]
- 46.Hong K, Nishiyama M, Henley J, Tessier-Lavigne M, Poo M. Calcium signalling in the guidance of nerve growth by netrin-1. Nature. 2000;403:93–98. doi: 10.1038/47507. [DOI] [PubMed] [Google Scholar]
- 47.Zheng JQ, Felder M, Connor JA, Poo MM. Turning of nerve growth cones induced by neurotransmitters. Nature. 1994;368:140–144. doi: 10.1038/368140a0. [DOI] [PubMed] [Google Scholar]
- 48.Tas PW, Gambaryan S, Roewer N. Volatile anesthetics affect the morphology of rat glioma C6 cells via RhoA, ERK, Akt activation. J Cell Biochem. 2007;102:368–376. doi: 10.1002/jcb.21294. [DOI] [PubMed] [Google Scholar]
- 49.Hu H, Marton TF, Goodman CS. Plexin B mediates axon guidance in Drosophila by simultaneously inhibiting active Rac and enhancing RhoA signaling. Neuron. 2001;32:39–51. doi: 10.1016/s0896-6273(01)00453-6. [DOI] [PubMed] [Google Scholar]
- 50.Ohnami S, Endo M, Hirai S, Uesaka N, Hatanaka Y, Yamashita T, Yamamoto N. Role of RhoA in activity-dependent cortical axon branching. J Neurosci. 2008;28:9117–9121. doi: 10.1523/JNEUROSCI.1731-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Briner A, De Roo M, Dayer A, Muller D, Habre W, Vutskits L. Volatile anesthetics rapidly increase dendritic spine density in the rat medial prefrontal cortex during synaptogenesis. Anesthesiology. 2010;112:546–556. doi: 10.1097/ALN.0b013e3181cd7942. [DOI] [PubMed] [Google Scholar]
- 52.Briner A, Nikonenko I, De Roo M, Dayer A, Muller D, Vutskits L. Developmental stage-dependent persistent impact of propofol anesthesia on dendritic spines in the rat medial prefrontal cortex. Anesthesiology. 2011;115:282–293. doi: 10.1097/ALN.0b013e318221fbbd. [DOI] [PubMed] [Google Scholar]