Abstract
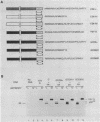
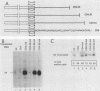
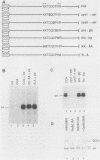
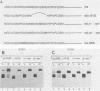
We report that the cytoplasmic domains of the T-lymphocyte glycoproteins CD4 and CD8 alpha contain short related amino acid sequences that are involved in binding the amino-terminal domain of the intracellular tyrosine protein kinase, p56lck. Transfer of as few as six amino acid residues from the cytoplasmic domain of the CD8 alpha protein to the cytoplasmic domain of an unrelated protein conferred p56lck binding to the hybrid protein in HeLa cells. The common sequence motif shared by CD4 and CD8 alpha contains two cysteines, and mutation of either cysteine in the CD4 sequence eliminated binding of p56lck.p56lck also contains two cysteine residues within its CD4-CD8 alpha-binding domain, and both are critical to the interaction with CD4 or CD8 alpha. Because the interaction does not involve disulfide bond formation, a metal ion could stabilize the complex.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amrein K. E., Sefton B. M. Mutation of a site of tyrosine phosphorylation in the lymphocyte-specific tyrosine protein kinase, p56lck, reveals its oncogenic potential in fibroblasts. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4247–4251. doi: 10.1073/pnas.85.12.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank I., Chess L. Perturbation of the T4 molecule transmits a negative signal to T cells. J Exp Med. 1985 Oct 1;162(4):1294–1303. doi: 10.1084/jem.162.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber E. K., Dasgupta J. D., Schlossman S. F., Trevillyan J. M., Rudd C. E. The CD4 and CD8 antigens are coupled to a protein-tyrosine kinase (p56lck) that phosphorylates the CD3 complex. Proc Natl Acad Sci U S A. 1989 May;86(9):3277–3281. doi: 10.1073/pnas.86.9.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekoff M., Kubo R., Grey H. M. Activation requirements for normal T cells: accessory cell-dependent and -independent stimulation by anti-receptor antibodies. J Immunol. 1986 Sep 1;137(5):1411–1419. [PubMed] [Google Scholar]
- Blue M. L., Craig K. A., Anderson P., Branton K. R., Jr, Schlossman S. F. Evidence for specific association between class I major histocompatibility antigens and the CD8 molecules of human suppressor/cytotoxic cells. Cell. 1988 Jul 29;54(3):413–421. doi: 10.1016/0092-8674(88)90204-8. [DOI] [PubMed] [Google Scholar]
- Bushkin Y., Demaria S., Le J. M., Schwab R. Physical association between the CD8 and HLA class I molecules on the surface of activated human T lymphocytes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3985–3989. doi: 10.1073/pnas.85.11.3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright C. A., Kaplan P. L., Cooper J. A., Hunter T., Eckhart W. Altered sites of tyrosine phosphorylation in pp60c-src associated with polyomavirus middle tumor antigen. Mol Cell Biol. 1986 May;6(5):1562–1570. doi: 10.1128/mcb.6.5.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiSanto J. P., Knowles R. W., Flomenberg N. The human Lyt-3 molecule requires CD8 for cell surface expression. EMBO J. 1988 Nov;7(11):3465–3470. doi: 10.1002/j.1460-2075.1988.tb03221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle C., Strominger J. L. Interaction between CD4 and class II MHC molecules mediates cell adhesion. Nature. 1987 Nov 19;330(6145):256–259. doi: 10.1038/330256a0. [DOI] [PubMed] [Google Scholar]
- Emmrich F., Strittmatter U., Eichmann K. Synergism in the activation of human CD8 T cells by cross-linking the T-cell receptor complex with the CD8 differentiation antigen. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8298–8302. doi: 10.1073/pnas.83.21.8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A. D., Bredt D. S., Pabo C. O. Tat protein from human immunodeficiency virus forms a metal-linked dimer. Science. 1988 Apr 1;240(4848):70–73. doi: 10.1126/science.2832944. [DOI] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley T. R., Sefton B. M. Analysis of the activity and phosphorylation of the lck protein in lymphoid cells. Oncogene. 1989 Mar;4(3):265–272. [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. O-linked oligosaccharides are acquired by herpes simplex virus glycoproteins in the Golgi apparatus. Cell. 1983 Mar;32(3):987–997. doi: 10.1016/0092-8674(83)90083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P., Buss J. E., Sefton B. M. Mutation of NH2-terminal glycine of p60src prevents both myristoylation and morphological transformation. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4625–4628. doi: 10.1073/pnas.82.14.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavathas P., Sukhatme V. P., Herzenberg L. A., Parnes J. R. Isolation of the gene encoding the human T-lymphocyte differentiation antigen Leu-2 (T8) by gene transfer and cDNA subtraction. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7688–7692. doi: 10.1073/pnas.81.24.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmiecik T. E., Shalloway D. Activation and suppression of pp60c-src transforming ability by mutation of its primary sites of tyrosine phosphorylation. Cell. 1987 Apr 10;49(1):65–73. doi: 10.1016/0092-8674(87)90756-2. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon B. S., Weissman S. M. cDNA sequences of two inducible T-cell genes. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1963–1967. doi: 10.1073/pnas.86.6.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Rose L. M., Spooner C. E., Beatty P. G., Martin P. J., Clark E. A. Antibodies to common leukocyte antigen p220 influence human T cell proliferation by modifying IL 2 receptor expression. J Immunol. 1985 Sep;135(3):1819–1825. [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Littman D. R., Maddon P. J., Axel R. Corrected CD4 sequence. Cell. 1988 Nov 18;55(4):541–541. doi: 10.1016/0092-8674(88)90211-5. [DOI] [PubMed] [Google Scholar]
- Littman D. R., Thomas Y., Maddon P. J., Chess L., Axel R. The isolation and sequence of the gene encoding T8: a molecule defining functional classes of T lymphocytes. Cell. 1985 Feb;40(2):237–246. doi: 10.1016/0092-8674(85)90138-2. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Littman D. R., Godfrey M., Maddon D. E., Chess L., Axel R. The isolation and nucleotide sequence of a cDNA encoding the T cell surface protein T4: a new member of the immunoglobulin gene family. Cell. 1985 Aug;42(1):93–104. doi: 10.1016/s0092-8674(85)80105-7. [DOI] [PubMed] [Google Scholar]
- Marchildon G. A., Casnellie J. E., Walsh K. A., Krebs E. G. Covalently bound myristate in a lymphoma tyrosine protein kinase. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7679–7682. doi: 10.1073/pnas.81.24.7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolskee R. F., Kavathas P., Berg P. Epstein-Barr virus shuttle vector for stable episomal replication of cDNA expression libraries in human cells. Mol Cell Biol. 1988 Jul;8(7):2837–2847. doi: 10.1128/mcb.8.7.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth J. D., Peet R., Krebs E. G., Perlmutter R. M. A lymphocyte-specific protein-tyrosine kinase gene is rearranged and overexpressed in the murine T cell lymphoma LSTRA. Cell. 1985 Dec;43(2 Pt 1):393–404. doi: 10.1016/0092-8674(85)90169-2. [DOI] [PubMed] [Google Scholar]
- Niemann H., Boschek B., Evans D., Rosing M., Tamura T., Klenk H. D. Post-translational glycosylation of coronavirus glycoprotein E1: inhibition by monensin. EMBO J. 1982;1(12):1499–1504. doi: 10.1002/j.1460-2075.1982.tb01346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norment A. M., Littman D. R. A second subunit of CD8 is expressed in human T cells. EMBO J. 1988 Nov;7(11):3433–3439. doi: 10.1002/j.1460-2075.1988.tb03217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norment A. M., Salter R. D., Parham P., Engelhard V. H., Littman D. R. Cell-cell adhesion mediated by CD8 and MHC class I molecules. Nature. 1988 Nov 3;336(6194):79–81. doi: 10.1038/336079a0. [DOI] [PubMed] [Google Scholar]
- Piwnica-Worms H., Saunders K. B., Roberts T. M., Smith A. E., Cheng S. H. Tyrosine phosphorylation regulates the biochemical and biological properties of pp60c-src. Cell. 1987 Apr 10;49(1):75–82. doi: 10.1016/0092-8674(87)90757-4. [DOI] [PubMed] [Google Scholar]
- Reynolds A. B., Vila J., Lansing T. J., Potts W. M., Weber M. J., Parsons J. T. Activation of the oncogenic potential of the avian cellular src protein by specific structural alteration of the carboxy terminus. EMBO J. 1987 Aug;6(8):2359–2364. doi: 10.1002/j.1460-2075.1987.tb02512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd C. E., Trevillyan J. M., Dasgupta J. D., Wong L. L., Schlossman S. F. The CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (pp58) from human T lymphocytes. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5190–5194. doi: 10.1073/pnas.85.14.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Samstag Y., Emmrich F., Staehelin T. Activation of human T lymphocytes: differential effects of CD3- and CD8-mediated signals. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9689–9693. doi: 10.1073/pnas.85.24.9689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson S. C., Bolen J. B., Veillette A. CD4 and p56lck can stably associate when co-expressed in NIH3T3 cells. Oncogene. 1989 Sep;4(9):1141–1143. [PubMed] [Google Scholar]
- Snow P. M., Terhorst C. The T8 antigen is a multimeric complex of two distinct subunits on human thymocytes but consists of homomultimeric forms on peripheral blood T lymphocytes. J Biol Chem. 1983 Dec 10;258(23):14675–14681. [PubMed] [Google Scholar]
- Snow P. M., Van de Rijn M., Terhorst C. Association between the human thymic differentiation antigens T6 and T8. Eur J Immunol. 1985 May;15(5):529–532. doi: 10.1002/eji.1830150520. [DOI] [PubMed] [Google Scholar]
- Tite J. P., Sloan A., Janeway C. A., Jr The role of L3T4 in T cell activation: L3T4 may be both an Ia-binding protein and a receptor that transduces a negative signal. J Mol Cell Immunol. 1986;2(4):179–190. [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Bolen J. B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988 Oct 21;55(2):301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Samelson L. E., Bolen J. B. Signal transduction through the CD4 receptor involves the activation of the internal membrane tyrosine-protein kinase p56lck. Nature. 1989 Mar 16;338(6212):257–259. doi: 10.1038/338257a0. [DOI] [PubMed] [Google Scholar]
- Voronova A. F., Buss J. E., Patschinsky T., Hunter T., Sefton B. M. Characterization of the protein apparently responsible for the elevated tyrosine protein kinase activity in LSTRA cells. Mol Cell Biol. 1984 Dec;4(12):2705–2713. doi: 10.1128/mcb.4.12.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronova A. F., Sefton B. M. Expression of a new tyrosine protein kinase is stimulated by retrovirus promoter insertion. Nature. 1986 Feb 20;319(6055):682–685. doi: 10.1038/319682a0. [DOI] [PubMed] [Google Scholar]
- Wassmer P., Chan C., Lögdberg L., Shevach E. M. Role of the L3T4-antigen in T cell activation. II. Inhibition of T cell activation by monoclonal anti-L3T4 antibodies in the absence of accessory cells. J Immunol. 1985 Oct;135(4):2237–2242. [PubMed] [Google Scholar]
- Zamoyska R., Derham P., Gorman S. D., von Hoegen P., Bolen J. B., Veillette A., Parnes J. R. Inability of CD8 alpha' polypeptides to associate with p56lck correlates with impaired function in vitro and lack of expression in vivo. Nature. 1989 Nov 16;342(6247):278–281. doi: 10.1038/342278a0. [DOI] [PubMed] [Google Scholar]
- Ziegler S. F., Levin S. D., Perlmutter R. M. Transformation of NIH 3T3 fibroblasts by an activated form of p59hck. Mol Cell Biol. 1989 Jun;9(6):2724–2727. doi: 10.1128/mcb.9.6.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]