Abstract
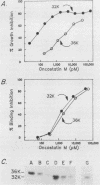
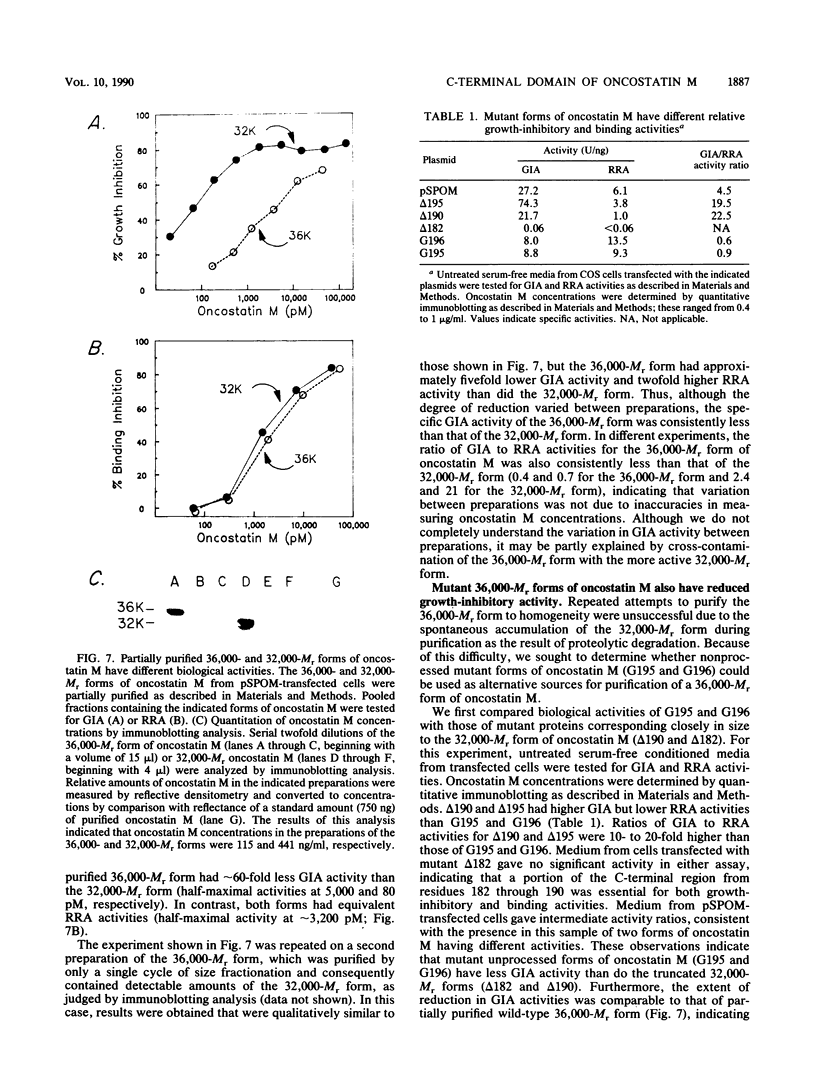
Oncostatin M is a polypeptide cytokine, produced by normal and malignant hematopoietic cells, that has several in vitro activities, including the ability to inhibit growth of cultured carcinoma cells. Here we present a structural and functional comparison of two oncostatin M-related proteins (Mr 36,000 and 32,000) secreted by COS cells transfected with oncostatin M cDNA. The smaller of these forms lacked a hydrophilic C-terminal domain comprising predominantly basic amino acids. This domain was also absent from native oncostatin M produced by U937 cells. The 32,000-Mr form of oncostatin M was not produced by cells transfected with plasmids (G195 and G196) in which a potential trypsinlike cleavage site within the hydrophilic C-terminal domain was altered by site-directed mutagenesis. A 32,000-Mr fragment was produced by trypsin treatment of the 36,000-Mr form of oncostatin M. These observations suggest that the 32,000-Mr form of oncostatin M was derived from the 227-amino-acid propeptide by proteolytic cleavage at or near the paired basic residues at positions 195 and 196. Pro-oncostatin M was equally active in radioreceptor assays as the processed form but was 5- to 60-fold less active in growth inhibition assays. Likewise, nonprocessed mutant protein encoded by plasmid G196 was equally active in the radioreceptor assays as the processed form but was five- to ninefold less active in growth inhibition assays. Thus, the highly charged C-terminal domain of pro-oncostatin M is not required for receptor binding or growth-inhibitory activity but may alter the functional properties of the molecule. Propeptide processing of oncostatin M may be important for regulating in vivo activities of this cytokine.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black R. A., Kronheim S. R., Cantrell M., Deeley M. C., March C. J., Prickett K. S., Wignall J., Conlon P. J., Cosman D., Hopp T. P. Generation of biologically active interleukin-1 beta by proteolytic cleavage of the inactive precursor. J Biol Chem. 1988 Jul 5;263(19):9437–9442. [PubMed] [Google Scholar]
- Bond J. S., Butler P. E. Intracellular proteases. Annu Rev Biochem. 1987;56:333–364. doi: 10.1146/annurev.bi.56.070187.002001. [DOI] [PubMed] [Google Scholar]
- Brown T. J., Lioubin M. N., Marquardt H. Purification and characterization of cytostatic lymphokines produced by activated human T lymphocytes. Synergistic antiproliferative activity of transforming growth factor beta 1, interferon-gamma, and oncostatin M for human melanoma cells. J Immunol. 1987 Nov 1;139(9):2977–2983. [PubMed] [Google Scholar]
- Chen L., Mory Y., Zilberstein A., Revel M. Growth inhibition of human breast carcinoma and leukemia/lymphoma cell lines by recombinant interferon-beta 2. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8037–8041. doi: 10.1073/pnas.85.21.8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Cseh K., Beutler B. Alternative cleavage of the cachectin/tumor necrosis factor propeptide results in a larger, inactive form of secreted protein. J Biol Chem. 1989 Sep 25;264(27):16256–16260. [PubMed] [Google Scholar]
- Dedhar S., Gaboury L., Galloway P., Eaves C. Human granulocyte-macrophage colony-stimulating factor is a growth factor active on a variety of cell types of nonhemopoietic origin. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9253–9257. doi: 10.1073/pnas.85.23.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Mier J. W. Lymphokines. N Engl J Med. 1987 Oct 8;317(15):940–945. doi: 10.1056/NEJM198710083171506. [DOI] [PubMed] [Google Scholar]
- Douglass J., Civelli O., Herbert E. Polyprotein gene expression: generation of diversity of neuroendocrine peptides. Annu Rev Biochem. 1984;53:665–715. doi: 10.1146/annurev.bi.53.070184.003313. [DOI] [PubMed] [Google Scholar]
- Gentry L. E., Webb N. R., Lim G. J., Brunner A. M., Ranchalis J. E., Twardzik D. R., Lioubin M. N., Marquardt H., Purchio A. F. Type 1 transforming growth factor beta: amplified expression and secretion of mature and precursor polypeptides in Chinese hamster ovary cells. Mol Cell Biol. 1987 Oct;7(10):3418–3427. doi: 10.1128/mcb.7.10.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Hazuda D., Webb R. L., Simon P., Young P. Purification and characterization of human recombinant precursor interleukin 1 beta. J Biol Chem. 1989 Jan 25;264(3):1689–1693. [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Kohase M., May L. T., Tamm I., Vilcek J., Sehgal P. B. A cytokine network in human diploid fibroblasts: interactions of beta-interferons, tumor necrosis factor, platelet-derived growth factor, and interleukin-1. Mol Cell Biol. 1987 Jan;7(1):273–280. doi: 10.1128/mcb.7.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegler M., Perez C., DeFay K., Albert I., Lu S. D. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988 Apr 8;53(1):45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linsley P. S., Bolton-Hanson M., Horn D., Malik N., Kallestad J. C., Ochs V., Zarling J. M., Shoyab M. Identification and characterization of cellular receptors for the growth regulator, oncostatin M. J Biol Chem. 1989 Mar 15;264(8):4282–4289. [PubMed] [Google Scholar]
- Linsley P. S., Hargreaves W. R., Twardzik D. R., Todaro G. J. Detection of larger polypeptides structurally and functionally related to type I transforming growth factor. Proc Natl Acad Sci U S A. 1985 Jan;82(2):356–360. doi: 10.1073/pnas.82.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh Y. P., Brownstein M. J., Gainer H. Proteolysis in neuropeptide processing and other neural functions. Annu Rev Neurosci. 1984;7:189–222. doi: 10.1146/annurev.ne.07.030184.001201. [DOI] [PubMed] [Google Scholar]
- Malik N., Kallestad J. C., Gunderson N. L., Austin S. D., Neubauer M. G., Ochs V., Marquardt H., Zarling J. M., Shoyab M., Wei C. M. Molecular cloning, sequence analysis, and functional expression of a novel growth regulator, oncostatin M. Mol Cell Biol. 1989 Jul;9(7):2847–2853. doi: 10.1128/mcb.9.7.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. A., Mizel S. B., Cohen D., Green I. Interleukin 1, a potential regulator of fibroblast proliferation. J Immunol. 1982 May;128(5):2177–2182. [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Clark E. A., Seed B. A B-lymphocyte activation molecule related to the nerve growth factor receptor and induced by cytokines in carcinomas. EMBO J. 1989 May;8(5):1403–1410. doi: 10.1002/j.1460-2075.1989.tb03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner D. F., Kemmler W., Tager H. S., Peterson J. D. Proteolytic processing in the biosynthesis of insulin and other proteins. Fed Proc. 1974 Oct;33(10):2105–2115. [PubMed] [Google Scholar]
- Sugarman B. J., Aggarwal B. B., Hass P. E., Figari I. S., Palladino M. A., Jr, Shepard H. M. Recombinant human tumor necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science. 1985 Nov 22;230(4728):943–945. doi: 10.1126/science.3933111. [DOI] [PubMed] [Google Scholar]
- Taniguchi T. Regulation of cytokine gene expression. Annu Rev Immunol. 1988;6:439–464. doi: 10.1146/annurev.iy.06.040188.002255. [DOI] [PubMed] [Google Scholar]
- Wong S. T., Winchell L. F., McCune B. K., Earp H. S., Teixidó J., Massagué J., Herman B., Lee D. C. The TGF-alpha precursor expressed on the cell surface binds to the EGF receptor on adjacent cells, leading to signal transduction. Cell. 1989 Feb 10;56(3):495–506. doi: 10.1016/0092-8674(89)90252-3. [DOI] [PubMed] [Google Scholar]
- Zarling J. M., Shoyab M., Marquardt H., Hanson M. B., Lioubin M. N., Todaro G. J. Oncostatin M: a growth regulator produced by differentiated histiocytic lymphoma cells. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9739–9743. doi: 10.1073/pnas.83.24.9739. [DOI] [PMC free article] [PubMed] [Google Scholar]