Abstract
Polypeptone is widely excluded from Schizosaccharomyces pombe growth medium. However, the reasons why polypeptone should be avoided have not been documented. Polypeptone dramatically induced cell lysis in the ura4 deletion mutant when cells approached the stationary growth phase, and this phenotype was suppressed by supplementation of uracil. To determine the specificity of this cell lysis phenotype, we created deletion mutants of other genes involved in de novo biosynthesis of uridine monophosphate (ura1, ura2, ura3, and ura5). Cell lysis was not observed in these gene deletion mutants. In addition, concomitant disruption of ura1, ura2, ura3, or ura5 in the ura4 deletion mutant suppressed cell lysis, indicating that cell lysis induced by polypeptone is specific to the ura4 deletion mutant. Furthermore, cell lysis was also suppressed when the gene involved in coenzyme Q biosynthesis was deleted. This is likely because Ura3 requires coenzyme Q for its activity. The ura4 deletion mutant was sensitive to zymolyase, which mainly degrades (1,3)-beta-D glucan, when grown in the presence of polypeptone, and cell lysis was suppressed by the osmotic stabiliser, sorbitol. Finally, the induction of cell lysis in the ura4 deletion mutant was due to the accumulation of orotidine-5-monophosphate. Cell wall integrity was dramatically impaired in the ura4 deletion mutant when grown in the presence of polypeptone. Because ura4 is widely used as a selection marker in S. pombe, caution needs to be taken when evaluating phenotypes of ura4 mutants.
Introduction
The fission yeast, Schizosaccharomyces pombe, is a eukaryotic model organism used to study a wide range of molecular and cellular biological processes, including cell cycle regulation, signal transduction, cell polarity control, and chromatin structure [1]–[3]. S. pombe is also used to study the mechanisms responsible for controlling cell wall synthesis and cellular morphogenesis [4].
The composition of the media on which S. pombe is grown is an important factor that needs to be considered during phenotypic analysis. S. pombe is commonly grown on the minimum medium, EMM, and the rich medium, YE [5]. The sensitivity of S. pombe to drugs or temperature often depends on the growth media used; for example, S. pombe is less sensitive to G418 when grown on EMM than when grown on YE. Growth media commonly used for S. cerevisiae, such as YPD that contains polypeptone, and SD that contains a nitrogen base, are not widely used to grow S. pombe. This is because many researchers have observed that S. pombe grown on these media can exhibit unexpected and unwanted alterations in the phenotypes of interest. Although polypeptone in YPD media is generally considered to be responsible for these effects, the reasons for this have not been thoroughly investigated.
The leu1, ade6, and ura4 genes are genetic markers commonly used for selection in S. pombe. However, these selectable marker genes can affect the phenotype of interest; for example, specific amino acids affect sexual differentiation [6].
We observed dramatic cell lysis of the ura4 deletion mutant grown in the presence of polypeptone. This was specific to the ura4 gene and was not observed in the other uracil auxotrophs, ura1, ura2, ura3, and ura5. Cell lysis was also observed when the ura4 strain was grown on YE media, although to a lesser extent. These results indicate that caution must be taken when interpreting phenotypes of ura4 deletion mutants of S. pombe. Our analysis indicates that ura4 specifically, rather than other genes involved in de novo biosynthesis of uridine monophosphate (UMP), affects cell wall integrity.
Materials and Methods
Strains, Media, and Genetic Manipulation
The S. pombe, S. japonicas, and S. cerevisiae strains used in this study are listed in Table 1. Standard yeast culture media and genetic manipulations were used [5]. S. pombe strains were grown in complete YES medium (0.5% yeast extract, 3% glucose, 225 mg/L each of adenine, leucine, uracil, histidine, and lysine hydrochloride), in YPD medium (1.0% yeast extract, 2% glucose, and 2% polypeptone), or in EMM medium (0.3% potassium hydrogen phthalate, 0.56% sodium phosphate, 0.5% ammonium chloride, 2% glucose, vitamins, minerals, and salts). The appropriate auxotrophic supplements were added as necessary (75 mg/L of adenine, leucine, uracil, histidine, lysine, or cysteine). YE medium (0.5% yeast extract and 3% glucose), YTD medium (0.5% yeast extract, 3% glucose, and 2% tryptone) and YCD medium (0.5% yeast extract, 3% glucose, and 2% casamino acids) were also used. SPA medium was used to induce sporulation. Phloxin B was added to a final concentration of 5 mg/L. G418 disulphate (Sigma Co. Ltd), hygromycin B (Wako Co. Ltd), and 5-Fluoroorotic acid (5-FOA) (Wako Co. Ltd) were used in solid YES plates at a concentration of 100 mg/L, 150 mg/L, and 1 g/L, respectively. Calcofluor white M2R (Sigma chemical) was used to test the sensitivity of strains on YES or YPD plates.
Table 1. S. pombe, S. japonicus and S. cerevisiae strains used in this study.
| Schizosaccharomyces pombe | ||
| Strain | Genotype | Source |
| L972 | h− | Lab stock |
| L975 | h+ | Lab stock |
| L968 | h90 | C. Shimoda |
| PR109 | h− leu1-32 ura4-D18 | P. Russel |
| PR110 | h+ leu1-32 ura4-D18 | P. Russel |
| SP870 | h90 leu1-32 ura4-D18 ade6-M216 | [13] |
| TP4-1D | h+ leu1-32 ura4-D18 his2 ade6-M216 | [14] |
| TP4-5A | h− leu1-32 ura4-D18 ade6-M210 | [14] |
| RM1 | h+ leu1-32 ura4-D18 Δcoq7::kanMX6 | [23] |
| UMP31 | h− Δura4::kanMX6 | This study |
| UMP32 | h− Δura4::hphMX6 | This study |
| UMP33 | h+ Δura4::hphMX6 | This study |
| UMP34 | h− Δura1::kanMX6 | This study |
| UMP35 | h− Δura2::kanMX6 | This study |
| UMP36 | h− Δura3::kanMX6 | This study |
| UMP37 | h− Δura5::kanMX6 | This study |
| UMP38 | h− Δcoq8::kanMX6 | This study |
| UMP39 | h− Δura4::hphMX6 Δura1::kanMX6 | This study |
| UMP40 | h− Δura4::hphMX6 Δura2::kanMX6 | This study |
| UMP41 | h− Δura4::hphMX6 Δura3::kanMX6 | This study |
| UMP42 | h− Δura4::hphMX6 Δura5::kanMX6 | This study |
| UMP43 | h− Δura4::hphMX6 Δcoq8::kanMX6 | This study |
| UMP44 | h+ Δura4::hphMX6 esc1 | This study |
| UMP45 | h− esc1 leu1-32 | This study |
| UMP46 | h+ Δura1::kanMX6 | This study |
| Schizosaccharomyces japonicus | ||
| NIG2028 | h− | H. Niki |
| NIG5091 | h− ura4-D3 | H. Niki |
| Saccharomyces cerevisiae | ||
| SP1 | MATa leu2 ura3 trp1 his3 ade8 can1 | Lab stock |
| W303A | MATa ade2-1 trp1-1 leu2-3 112 his1-11 ura3 can1-100 | Lab stock |
Escherichia coli DH5α was the host strain for all plasmid manipulations, and was grown in LB medium (1% bactotryptone, 0.5% yeast extract, and 1% NaCl, pH 7.0).
DNA Manipulations and Plasmids
Standard molecular biology techniques were followed as previously described [7]. Restriction enzymes (BamHI and SalI) were used according to the supplier’s recommendation (TOYOBO). Nucleotide sequences were determined by the dideoxynucleotide chain-termination method using an Applied Biosystems 3500 Genetic Analyzer. The plasmids pREP1-ura1, pREP1-ura4, pREP1-URA3, pREP2-ura2, pREP2-ura3, and pREP2-ura5 were constructed using the pREP1 or pREP2 [8] vectors and the primers listed in Table S1 with the gap repair cloning method as previously described [9]. The plasmids pFA6a-kanMX6 [10] and pCR2.1-hphMX6 [11] were used as templates to amplify DNA fragments to construct the gene deletion mutants. A pAL-SK-based genomic DNA library (provided from Dr. T. Nakamura) was used to screen the responsible genes of the revertants derived from the ura4 deletion mutant.
Gene Disruption and Marker Switch
Chromosomal genes were disrupted using PCR generated fragments [10]. The 1.6 kb kanMX6 module was amplified with flanking homology sequences corresponding to the 5′ and 3′ ends of the target genes. G418-resistant colonies were selected on YES plates containing G418. Correct disruption of the gene of interest was verified by colony PCR. One-step marker switch from kanMX6 to hphMX6 was performed as previously described [11].
Zymolyase Assay to Assess Cell Wall Integrity
Fission yeast cells were pre-grown at 30°C in YES media and were then grown at 30°C to mid-log phase in YPD media. Cells were harvested by centrifugation and washed with water and TE (10 mM Tris-HCl, pH 8.0, and 1 mM EDTA). Cells were re-suspended in TE and incubated at 30°C for 180 min in the absence or presence of 0.1 mg/ml Zymolyase20T (Seikagaku Kogyo). The degree of cell lysis was evaluated by measuring the optical density at 595 nm.
Alkaline Phosphatase Assay to Assess Cell Lysis
Cells were grown on YES plates for 3 days at 30°C and were re-suspended in water to a density of 107 cells/ml. Cell suspensions were serially diluted (1∶10) and plated on YES or YPD plates and incubated for 3 days at 30°C. For the alkaline phosphatase assay, each plate was overlaid for 10, 30, and 60 min with a phosphatase assay solution containing 0.05 M glycine-NaOH (pH 9.8), 1% agar, and 2.5 mg/ml 5-bromo-4-chloro-3-indorylphosphate (BCIP).
Cell Cultivation and Sample Preparation for Liquid Chromatography-mass Spectrometry (LC-MS) Analysis
Extraction of metabolites from yeast cells was performed as previously described [12]. The S. pombe wild-type (WT) (L972) and Δura4 (UMP31) strains, and the SP1 and W303A ura3 mutant S. cerevisiae strains, were grown in YPD media. Cells (4×108) were collected by centrifugation, washed with H2O, immediately quenched in methanol at −80°C, and then collected again by centrifugation. Cells were disrupted using a Multi-Beads Shocker (Yasui Kikai Co.) in 800 µL of 50% methanol. Proteins were removed with an Amicon Ultra 10 kDa cut-off filter (Millipore), and the samples were concentrated by vacuum evaporation. Finally, each sample was re-suspended in 80 µL of 50% acetonitrile and 50% 20 mM ammonium formate. Samples were filtrated with YMC Duo-Filter QDUO 04 (pore size 0.2 µm) and 2 µL was used for injection.
LC-MS data were obtained using a MassLynx system (Waters) coupled to a Xexo-TQS mass spectrometer (Waters). LC separation was performed on an ACQUITY UPLC BEH Amide column (Merck SeQuant; 2.1×100 mm, 1.7 µm particle size). The mobile phase was 20 mM ammonium formate, pH 3.1 (buffer A) and acetonitrile buffer (buffer B). The chromatographic conditions were 30% buffer A and 70% buffer B at 2 min, which increased immediately to 50% buffer A at 3 min. This condition was maintained for 6 min and the initial condition was restored after 9 min. The flow rate was 0.4 ml/min. Matrix assisted laser desorption/ionisation-time of flight-of-mass spectrometry (MALDI-TOF MS) (SYNAPT G2-S; Waters Corps.) was used to determine the precise molecular masses of compounds.
Results
S. pombe ura4 Deletion Mutants Undergo Dramatic Cell Lysis when Grown in YPD Media
YPD media is widely used to grow S. cerevisiae, but not S. pombe. Although the exact reasons why S. pombe should not be grown in YPD media have not been reported, many researchers have observed that polypeptone in YPD media has undesirable effects on S. pombe growth. The PR110 strain (h+ leu1-32 ura4-D18) lysed upon reaching late-log phase in YPD liquid medium, whereas the WT strain L972 did not (data not shown).
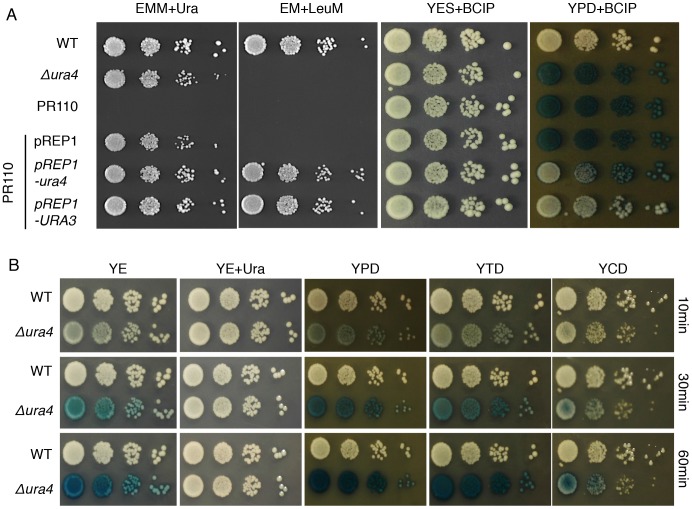
To determine whether the absence of leucine or uracil in the PR110 strain is responsible for this, we separated the two markers by crossing PR110 with L972 and isolated four siblings. Only strains that lacked uracil lysed in YPD media (Fig. S1). This phenotype was visualised in the form of blue colonies by staining with BCIP, which is an artificial chromogenic substrate that is converted to blue dye by endogenous alkaline phosphatase (Fig. 1A). The blue color became clear in Δura4 cells after incubation with BCIP for 30 min. Cell lysis of PR110 was suppressed in YES medium that contained uracil, or by introducing pREP2 containing the ura4 gene (pREP2-ura4) or the S. cerevisiae URA3 gene (pREP2-URA3), which is a ura4 ortholog. This indicates that uracil auxotrophy by deletion of ura4 is responsible for the cell lysis phenotype. Δura4 cells grown on YE medium also underwent cell lysis, although to a lesser extent, and this was enhanced by supplementation of polypeptone or tryptone, but not casamino acids (Fig. 1B). This suggests that components of polypeptone or tryptone, apart from amino acids, enhance cell lysis. Other ura4 deletion mutants, including the SP870 [13], TP4-1D, and TP4-5A strains [14], also underwent cell lysis (data not shown).
Figure 1. Lysis of Δura4 cells by polypeptone.
(A) L972 (WT), UMP31 (Δura4), PR110 strains, and PR110 containing empty vector (pREP1), pREP1-ura4 or pREP1-URA3 were grown on the indicted plates for 3 days at 30°C. Cells were serial diluted by 10-fold. For the alkaline phosphatase assay, YPD plates were overlaid for 10 min with a phosphatase assay solution containing 0.05 M glycine-NaOH (pH 9.8), 1% agar, and 2.5 mg/ml of BCIP. (B) L972 (WT) and UMP31 (Δura4) strains were serially diluted by 10-fold and spotted onto the indicated plates and incubated for 3 days at 30°C. For the alkaline phosphatase assay, each plate was overlaid with the phosphatase assay solution for the indicated time as described in (A).
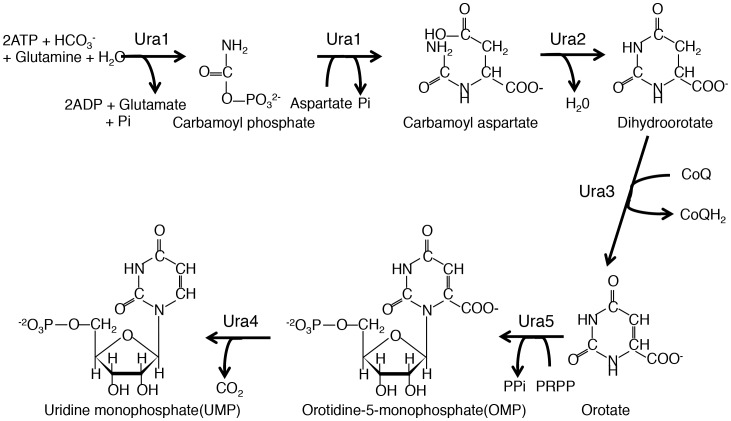
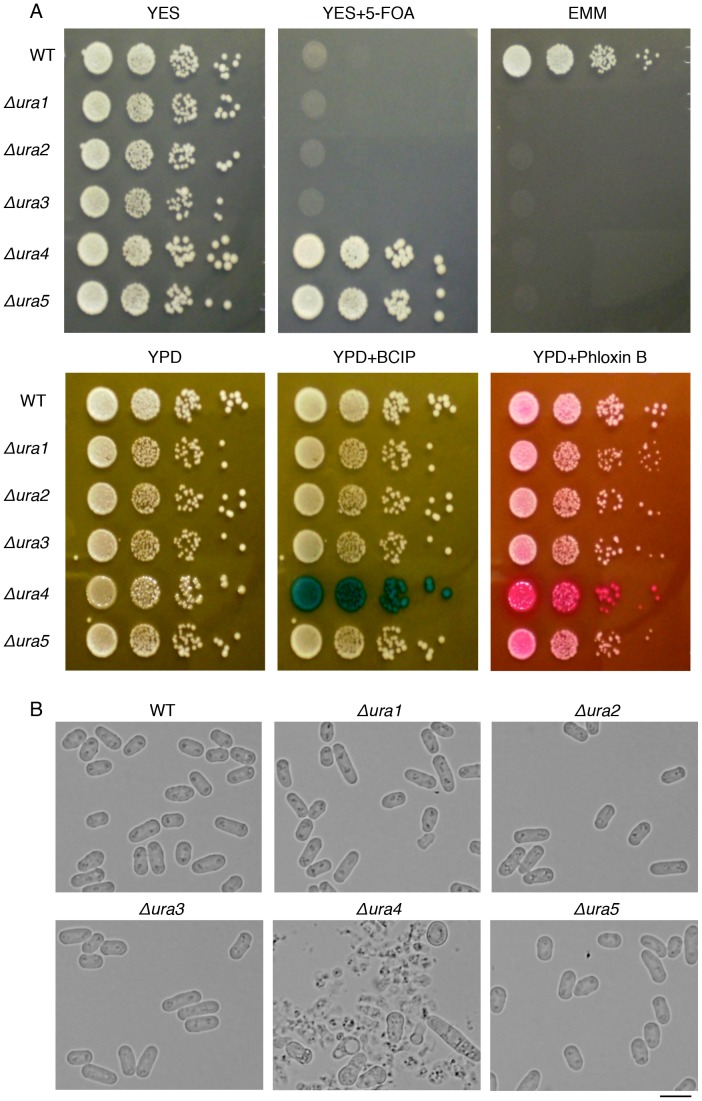
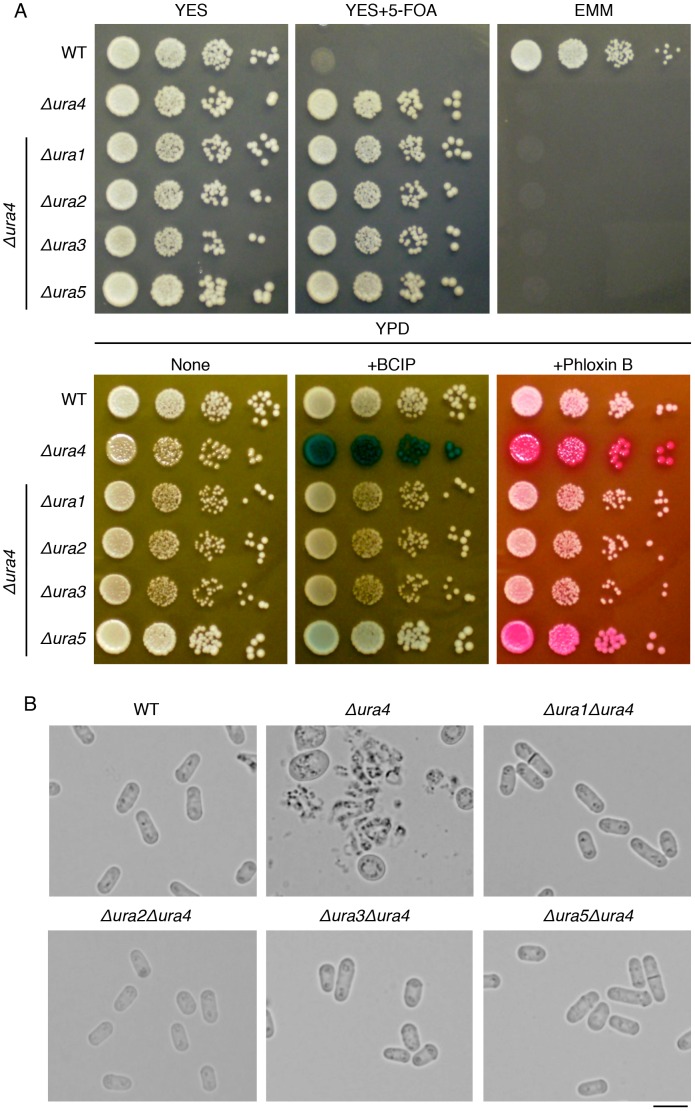
We generated deletion mutants of genes involved in de novo metabolism of UMP to further explore the relationship between uracil auxotrophy and cell lysis. The ura4 gene encodes orotidine-5-monophosphate (OMP) decarboxylase that mediates the formation of UMP from OMP. Four other genes (ura1, ura2, ura3, and ura5) are also involved in de novo synthesis of UMP in S. pombe (Fig. 2) [15]–[17]. We observed the phenotypes of strains in which these genes were disrupted. All five ura deletion mutants showed uracil auxotrophy, and the ura4 and ura5 deletion strains were resistant to 5-FOA as expected (Fig. 3A). When the ura4 deletion strain was grown on YPD plates in the presence of phloxin B, it formed darker red colonies than the other strains, and it formed dark blue colonies when grown in the presence of BCIP (Fig. 3A), indicating that cell lysis occurred specifically in the ura4 deletion mutant. Addition of uracil in YPD medium suppressed the cell lysis in the ura4 deletion strain (Fig. S2). Microscopic observation also indicated that cells of the ura4 deletion mutant lysed, whereas those of the other four mutants were normal (Fig. 3B).
Figure 2. De novo UMP synthesis in S. pombe involves six steps and five enzymes.
The de novo synthesis of UMP in S. pombe is outlined. Ura1 is a bi-functional enzyme consisting of carbamoyl phosphate synthetase I and aspartate transcarbamoylase, Ura2 is dihydroorotase, Ura3 is dihydroorotate dehydrogenase that requires quinone as a cofactor and localizes in mitochondria, Ura5 is orotate phosphoribosyltransferase, and Ura4 is OMP decarboxylase.
Figure 3. Cell lysis caused by polypeptone specifically occurs in the ura4 deletion mutant.
(A) Cell cultures of L972 (WT), UMP34 (Δura1), UMP35 (Δura2), UMP36 (Δura3), UMP31 (Δura4), and UMP37 (Δura5) strains were serially diluted by 10-fold, plated on the indicated plates and incubated for 3 days at 30°C. For the alkaline phosphatase assay, BCIP was used as described in Fig. 1. (B) Cellular morphologies of the indicated strains after being grown on YPD plates for 3 days at 30°C. Scale bar: 10 µm.
The ura4 Deletion Mutant has a Defect in Cell Wall Integrity when Grown in YPD Media
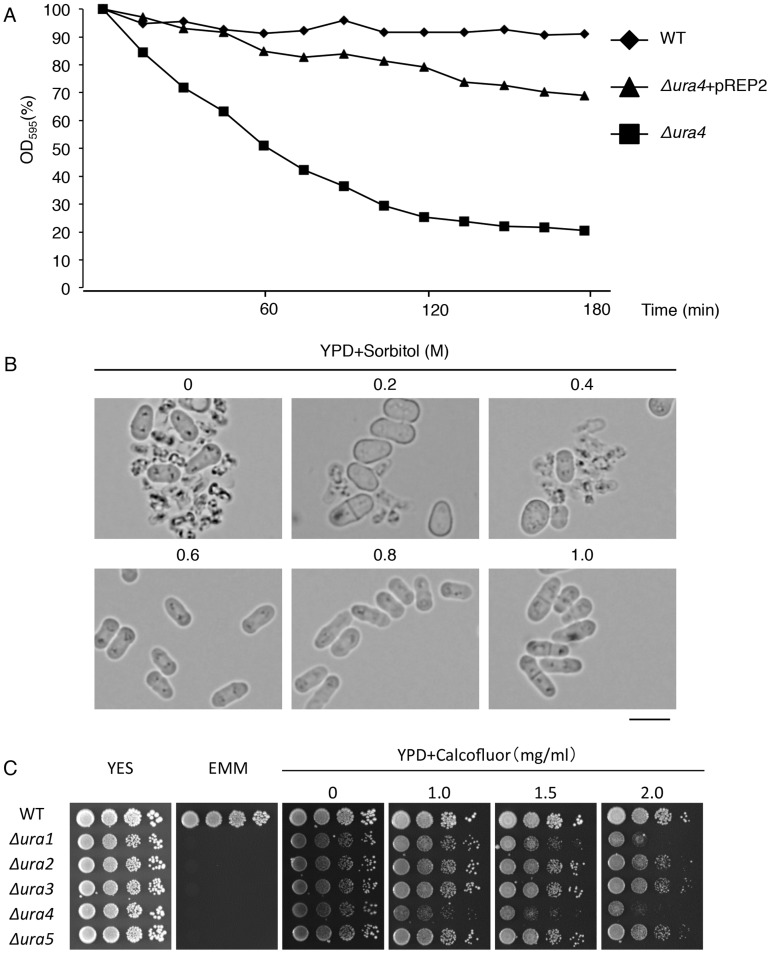
When the ura4 deletion mutant was grown in YPD media, cells had a round or a tadpole-like morphology before cell lysis began (Fig. 3B). Because this morphology is often found in mutants that a defect in cell wall integrity [18], we suspected that the morphology defect and cell lysis of the ura4 deletion mutant grown in YPD media was due to a defect in cell wall integrity. To test this, we examined the sensitivity of the ura4 deletion mutant to ß-glucanase (zymolyase20T), which breaks down cell walls. The ura4 deletion mutant was more sensitive to zymolyase than the WT strain and the ura4 deletion mutant containing pREP2 (Fig. 4A). We then tested whether the osmotic stabiliser, sorbitol, could rescue the cell lysis phenotype of the ura4 deletion mutant. Cell lysis was suppressed when sorbitol was added at a concentration of 0.6 M or higher (Fig. 4B). Consistent with these observations, the ura4 deletion mutant was sensitive to calcofluor white, whereas the ura2, ura3, and ura5 deletion mutants were not (Fig. 4C). Calcofluor white inhibits the growth of S. pombe mutants that have defects in the integrity of cell walls [19]. These results suggest that the morphological defects and cell lysis of the ura4 gene deletion mutant grown in YPD media is due to a defect in cell wall integrity. The ura1 deletion mutant was also sensitive to calcofluor white (Fig. 4C), but we do not have clear explanation of this phenotype.
Figure 4. Cell wall integrity is impaired in the ura4 deletion mutant when grown in the presence of polypeptone.
(A) L972 (WT) and Δura4 strains and Δura4 containing pREP2 were grown at 30°C in YPD media and treated with 0.1 mg/ml Zymolyase20T. The degree of cell lysis was evaluated by measuring the optical density at 595 nm (OD595nm). The OD595nm at various time-points relative to the value at 0 min is indicated. (B) The morphology of UMP31 (Δura4) cells grown in YPD media in the presence of various concentrations of sorbitol. Scale bar: 10 µm (C) The indicated strains were serially diluted 10-fold and grown on YES, EMM or YPD media containing calcofluor white for 4 days at 30°C.
Disruption of Genes Upstream of ura4 in UMP Synthesis Suppresses the Cell Lysis Phenotype of the ura4 Deletion Mutant
Ura4 catalyses the last step of UMP synthesis, and Ura1, Ura2, Ura3, and Ura5 catalyse the reactions upstream of Ura4 (Fig. 2) [17]. We created double deletion mutants to determine whether concomitant disruption of ura1, ura2, ura3, or ura5 in the ura4 deletion mutant affected the cell lysis phenotype. The cell lysis of the ura4 deletion mutant was markedly suppressed by concomitant disruption of ura1, ura2, ura3, or ura5 as indicated by BCIP and phloxin B staining (Fig. 5A) and cellular morphology (Fig. 5B). Thus, ura4 was predominantly responsible for the cell lysis induced by polypeptone.
Figure 5. Concomitant disruption of ura1, ura2, ura3, or ura5 in the ura4 deletion mutant suppresses cell lysis in the presence of polypeptone.
(A) Serially diluted cells (10-fold) of L972 (WT), UMP31 (Δura4), UMP39 (Δura1Δura4), UMP40 (Δura2Δura4), UMP41 (Δura3Δura4), and UMP42 (Δura5Δura4) strains were grown on the indicated plates and incubated for 3 days at 30°C. For the alkaline phosphatase assay, BCIP was used as described in Fig. 1. (B) Cellular morphologies of the indicated strains after being grown on YPD plates for 3 days at 30°C. Scale bar: 10 µm.
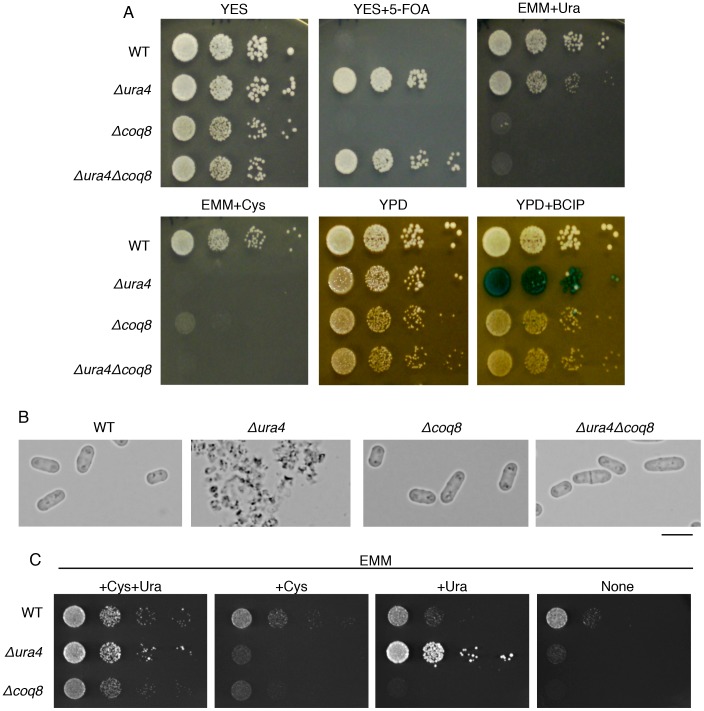
In S. pombe, Ura3 localizes in the inner membrane of mitochondria and requires quinone for its activity [20]. Because coenzyme Q is the only quinone that exists in the mitochondria, we hypothesised that genes involved in the biosynthesis of coenzyme Q might be required for de novo biosynthesis of UMP. To test this possibility, we disrupted the coq8 gene, which is required for coenzyme Q synthesis [21], [22]. Disruption of coq8 suppressed the cell lysis phenotype of the ura4 deletion mutant (Fig. 6A and B). The coq8 deletion mutant showed uracil auxotrophy similar to gene deletion mutants involved in de novo metabolism of UMP (Fig. 6C). This suggests that suppression of the cell lysis phenotype of the ura4 deletion mutant by deletion of coq8 is mediated by UMP metabolism. Cysteine was added to the medium, as this is required to grow the coq deletion mutant on minimum medium [14], [21], [22]. Another coq mutant, RM1 (coq7) [23], also suppressed the cell lysis phenotype of the ura4 deletion mutant (data not shown).
Figure 6. Deletion of coq8 suppresses the cell lysis phenotype of the ura4 deletion mutant.
(A) Serially diluted cells (10-fold) of L972 (WT), UMP31 (Δura4), UMP38 (Δcoq8), and UMP43 (Δcoq8Δura4) strains were grown on the indicated plates and incubated for 3 days at 30°C. For the alkaline phosphatase assay, BCIP was used as described in Fig. 1. (B) Cellular morphologies of the indicated strains after being grown on YPD plates for 3 days at 30°C. Scale bar: 10 µm. (C) Serially diluted cells (10-fold) of L972 (WT), UMP31 (Δura4), and UMP38 (Δcoq8) strains were grown on the indicated plates and incubated for 3 days at 30°C.
Search for Genes that Contribute to the Cell Lysis Phenotype of the ura4 Gene Deletion Mutant
To identify genes that might contribute to the cell lysis phenotype, we attempted to isolate multi-copy suppressors that suppressed the cell lysis of the ura4 deletion mutant when grown on YPD media. PR110 was transformed with a pAL-SK-based genomic DNA library and transformants were selected on EMM plates. All transformants (approximately 50,000 colonies) were replica-plated onto YPD plates containing phloxin B and incubated at 30°C for 2 days. Four colonies were identified that were more weakly stained than the ura4 deletion mutant by phoxin B, indicating that cell lysis was suppressed. Plasmids were recovered from these colonies and the plasmid genes were identified. All plasmids contained the ura4 gene (data not shown). Thus, no multi-copy suppressor genes were identified at least in our screening. This confirmed that ura4 is fully responsible for the cell lysis of the ura4 deletion mutant when grown in the presence of polypeptone.
To gain further insight into the mechanism by which cell lysis is induced in the ura4 deletion strain, we isolated spontaneous revertants that suppressed cell lysis of the ura4 deletion mutant. UMP33 cells (h+ Δura4::hphMX6) were incubated on a YPD plate containing phloxin B at 30°C for 1 week. Eight independent spontaneous revertants were screened by observing colony color and cell morphology. Tetrad analyses showed that extragenic suppression of revertants was caused by a single genetic locus and that this suppression was recessive. These were designated esc (extragenic suppressor of cell lysis) mutants. These revertants were back-crossed with the WT strains L972 and L975 to eliminate secondary mutations. Genetic analysis established that these eight mutant alleles were derived from one genetic locus and these mutants showed uracil auxotrophy. To isolate the responsible genes of the esc mutants, the UMP45 (h− esc1 leu1-32) strain was transformed with a pAL-SK-based genomic DNA library and selected on EMM plates. All transformants (approximately 50,000 colonies) were replica-plated onto EMM plates without uracil and incubated at 30°C for 5 days. The ura1 gene was identified by sequencing of the clones. The sequencing of the ura1 locus of UMP45 further identified an insertion mutation in the ura1 ORF between bases 135 and 136 (data not shown). These results reconfirmed that disruption of genes upstream of ura4 in UMP synthesis suppresses the cell lysis phenotype of the ura4 deletion mutant.
Accumulation of Precursors of OMP in the ura4 Deletion Mutant
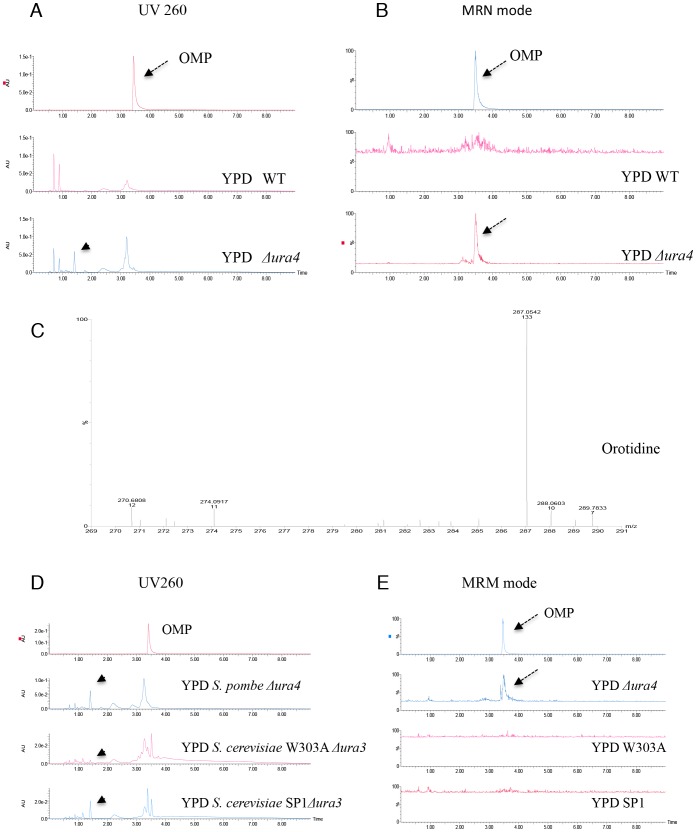
Ura4 is thought to catalyse the decarboxylation of OMP to UMP in S. pombe based on its amino acid sequence similarity with other orthologs and genetic complementation analyses [17]; however, metabolic analysis of Ura4 has not been conducted in fission yeast. We therefore studied accumulation of a precursor of OMP in the ura4 deletion mutant using mass spectrometry. We extracted metabolites according to the procedure described by Pluskal et al. [12]. The extract was separated by HPLC and absorbance at 260 nm was used to detect nucleic acids. A clear peak at 1.40 was detected when the ura4 deletion mutant was grown on YPD media, which was not observed when WT cells were grown on YPD media (Fig. 7A; arrow head). This peak disappeared when the ura4 deletion mutant was grown on uracil-containing medium (data not shown). Therefore this peak specifically appeared only in conditions in which cell lysis occurred. The peak did not merge with that of an OMP standard, ruling out the possibility that the peak was OMP (Fig. 7A; arrow).
Figure 7. Mass spectrometry analysis of OMP precursors in the ura4 deletion mutant.
(A) HPLC analysis of L972 (WT) and UMP31 (Δura4) cells grown in YPD media. Metabolites were extracted in 50% methanol from bead-disrupted cells and isolated by a 10 kDa cut-off filter. A peak corresponding to OMP was not observed in the samples, but a specific peak at 1.40 (arrow head) was observed in the UMP31 (Δura4) sample. (B) A peak at 367 m/z (the size of OMP) that was detected by mass spectrometry in negative ESI mode was fragmented in MRM mode (MS/MS). A peak at 3.51 (arrow) observed in metabolites from UMP31 (Δura4) coincides well with the peak of OMP. (C) The peak in the UMP31 (Δura4) sample at 1.40 detected by absorbance at 260 nm was identified as orotidine when analysed by TOF-MS (range 270–290 m/z) in ionization mode. (D) HPLC analysis of S. pombe UMP31 (Δura4) cells, and S. cerevisiae SP1 and W303A ura3 mutant cells grown in YPD media. Peaks corresponding to orotidine (arrow heads) were observed. (E) A peak at 367 m/z was fragmented by MS/MS in negative ESI mode. A peak corresponding to OMP (arrow) was observed in the UMP31 (Δura4) sample, but not in the S. cerevisiae ura3 mutants.
Even though OMP was not detected by HPLC, mass spectrometric analysis in MRM mode identified the accumulation of OMP in the ura4 deletion mutant when grown on YPD media, which was not observed in WT cells (Fig. 7B) or under conditions in which cell lysis did not occur, such as when Δura4 cells were grown on YES media (data not shown). The product at 1.40 had the molecular weight (m/Z 288) of orotidine when analysed by TOF MS (Fig. 7C). Thus, the accumulation of low levels of OMP as well as a dephosphorylated form of OMP, orotidine, was identified in the ura4 deletion mutant. Importantly, these products were only detected in samples of cells that had undergone cell lysis. A higher amount of uracil lowered the OMP production when the ura4 deletion mutant was grown in YPD liquid medium (Fig. S2). We further tested the accumulation of these compounds in ura3 deletion mutants of S. cerevisiae. Accumulation of orotidine was detected by HPLC in these mutants (Fig. 7D; arrow heads), but OMP was not detected by mass spectrometric analysis in MRM mode (Fig. 7E; arrows).
Cell Lysis in S. japonicus
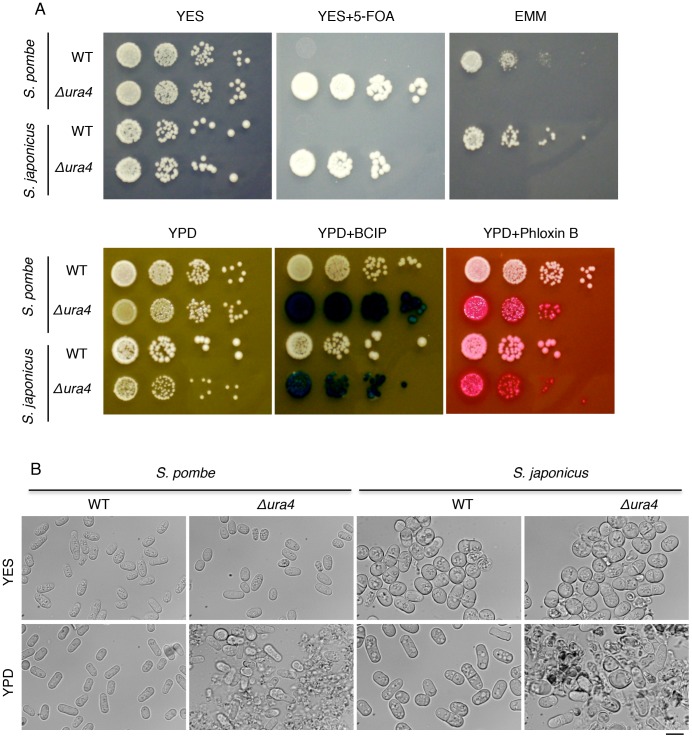
We next investigated whether cell lysis occurred in the ura4 deletion mutant of S. japonicas, which was derived from the WT strain [24]. S. japonicus is a fission yeast that forms eight spores, and is closely related to S. pombe. BCIP and phloxin B staining (Fig. 8A) and cellular morphology (Fig. 8B) indicated that cells of the S. japonicus ura4 deletion mutant underwent cell lysis when grown in the presence of polypeptone, whereas cells of the WT S. japonicus strain did not. This indicates that cell lysis of ura4 deletion mutants grown in the presence of polypeptone is conserved in two fission yeast species.
Figure 8. Cell lysis of the S. japonicus ura4 deletion mutant in the presence of polypeptone.
(A) Serially diluted cells (10-fold) of S. pombe L972 (WT) and UMP31 (Δura4), and S. japonicus NIG2028(h−) and NIG5091(h− ura4-D3) strains were grown on the indicated plates and incubated for 3 days at 30°C. For the alkaline phosphatase assay, BCIP was used as described in Fig. 1. (B) Cellular morphologies of the indicated strains after being grown on YPD plates for 3 days at 30°C. Scale bar: 10 µm.
Discussion
We report the ura4 deletion strains of S. pombe and S. japonicus undergo dramatic cell lysis when grown on media containing polypeptone or tryptone. The lysis phenotype was observed to some extent even when cells were grown in the commonly used YE medium, which contains a limited amount of adenine and uracil, and the phenotype was enhanced by the addition of polypeptone. The lysis phenotype of the S. pombe ura4 deletion mutant was suppressed by the expression of ura4 or S. cerevisiae URA3, or the addition of uracil to the media. The lysis phenotype is specific to ura4 deletion mutant strains and was not observed in other ura deletion strains (ura1, ura2, ura3, and ura5). In addition, the cell lysis phenotype of the ura4 deletion strains was suppressed by concomitant deletion of ura1, ura2, ura3, ura5, or coq8, which is required for Ura3 activity. These results indicate that the cell lysis phenotype was solely due to the deletion of ura4, and was not due to the general requirement of fission yeast for uracil. The lysis phenotype was not observed in the S. cerevisiae URA3 deletion mutant, indicating that the cell lysis phenotype linked to ura4 is specific to fission yeast.
The mutations of eight spontaneous revertants that overcame the cell lysis phenotype of the ura4 deletion mutant resided in the ura1 gene. It is reasonable that mutation of ura1 would overcome the cell lysis phenotype of the ura4 deletion mutant because Ura1 is upstream of Ura4 in UMP biosynthesis. However, it is unclear why all mutations in these revertants resided predominately in ura1 rather than ura2, ura3, or ura5. The ura1 gene (6 kb) is larger than the other ura genes, meaning that the rate of mutation in ura1 may be higher than that of the other ura genes.
Cells of the ura4 deletion strain dramatically lysed upon reaching stationary phase and this was suppressed by the addition of the osmotic stabiliser, sorbitol. The sensitivity of the ura4 deletion strain to zymolyase and calcofluor white indicated that the cell wall integrity of this mutant is impaired. The cell wall of fission yeast is composed predominantly of polysaccharides, including (1,3) ß-D-glucan; (1,6) ß-D-glucan; (1,3) α-D-glucan [4]; galactomannan; and small amounts of chitin. Chitin is essential for S. cerevisiae growth but does not play an important role in mitotic growth of fission yeast [25], [26]. α-D-glucan is essential for the growth of fission yeast [4], [27], but not S. cerevisiae. Differences in the cell wall components of S. pombe and S. cerevisiae may explain why the cell lysis phenotype was not observed in the S. cerevisiae URA3 deletion strain. If this is the case, α-glucan may be the cell wall component that is responsible for the perturbation of cell wall integrity in ura4 deletion strains, as this component is absent from S. cerevisiae. Indeed, the main α-glucan synthase in S. pombe (Mok1/Ags1) is essential for growth and its temperature-sensitive mutant exhibits a cell lysis phenotype when grown at a restrictive temperature [27]. However, even if α-glucan is the target of ura4-mediated cell lysis, this does not explain why deletion of other ura genes apart from ura4 did not cause cell lysis. If the level of uridine diphosphate (UDP)-glucose is solely responsible for α-glucan synthesis, cell lysis should be observed in all ura mutants.
Since the ura4 gene encodes OMP decarboxylase that mediates the formation of UMP from OMP, ura4 deletion strains were expected to accumulate OMP that might trigger cell lysis. Mass spectrometric analysis in MRM mode identified accumulation of OMP in the ura4 mutant and HPLC identified an apparent peak of orotidine. Importantly, these products were only detected when cells were grown under conditions in which cell lysis occurred. Since OMP and cell lysis were not detected in S. cerevisiae ura3 mutants when cells were grown on YPD, OMP may be a ‘cell lysis inducing factor’ in the S. pombe ura4 deletion strains. One possibility is that OMP inhibits cell wall synthesis by preventing α-glucan synthesis from UDP-glucose and thereby inducing cell lysis. However, further study is required to test this hypothesis.
It is unknown which components of polypeptone enhance cell lysis in the ura4 deletion strains. Casamino acids did not enhance the cell lysis phenotype. Since casamino acids are generated by complete hydrolysis of polypeptone, this suggests that amino acids do not trigger the cell lysis phenotype. As casein is converted to polypeptone by pepsin, specific peptides in polypeptone may trigger the cell lysis in the ura4 deletion strains, although identifying such peptides will not be easy.
Because ura4 is widely used as a selection marker in S. pombe, researchers need to be careful when interpreting phenotype of ura4 mutants. The cell lysis phenotype is observed to some extent when ura4 deletion mutants are grown in the commonly used YE medium, and this is enhanced by polypeptone. The phenotype should be compared to strains without any auxotrophic background.
Supporting Information
Cellular morphology of a tetrad, 5a (Δ ura4 ), 5b ( leu1-32 Δ ura4 ), 5c ( leu1-32 ) and 5d (wild type), derived by crossing L972 ( h −) with PR110 ( h + ura4-D18 leu1-32 ). Cells were grown on YES and YPD. Bar: 10 µm
(TIFF)
(A) Cell’s culture of the L972 (WT), UMP34 (Δ ura1 ), UMP35 (Δ ura2 ), UMP36 (Δ ura3 ), UMP31 (Δ ura4 ), and UMP37 (Δ ura5 ) strains were serially diluted 10-fold, plated on the indicated plates and incubated for 3 days at 30°C. For alkaline phosphatase assay, each plate was overlaid for 10, 30 and 60 min with a phosphatase assay solution as in Fig. 1. The amount of OMP was measured by mass spectrometry in UMP31 (Δura4) cells grown in YPD liquid medium containing an indicted amount of uracil. Under these conditions, a higher amount of uracil (900 mg/L) can only suppress the cell lysis phenotype.
(TIFF)
Oligonucleotide primers used in this study.
(DOCX)
Acknowledgments
We thank Drs. J. Bahler, H. Niki, T. Nakamura, C. Shimoda, and NBRP/YRC for providing materials and the strains used in this study. We also thank Drs. T. Nakagawa, K. Furuta and H. Tohda for helpful discussions and experimental support.
Funding Statement
This work was supported by a grant-in-aid (#24380056) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan to Makoto Kawamukai. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Egel R (2003) Fission yeast in general genetics; Egel R, editor. Berlin: Springer-Verlag. 1–12 p.
- 2. Nurse P (2000) A long twentieth century of the cell cycle and beyond. Cell 100: 71–78. [DOI] [PubMed] [Google Scholar]
- 3. Tanae K, Horiuchi T, Matsuo Y, Katayama S, Kawamukai M (2012) Histone chaperone Asf1 plays an essential role in maintaining genomic stability in fission yeast. PLoS One 7: e30472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Katayama S, Hirata D, Arellano M, Perez P, Toda T (1999) Fission yeast alpha-glucan synthase Mok1 requires the actin cytoskeleton to localize the sites of growth and plays an essential role in cell morphogenesis downstream of protein kinase C function. J Cell Biol 144: 1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moreno S, Klar A, Nurse P (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. . Methods Enzymol 194: 795–823. [DOI] [PubMed] [Google Scholar]
- 6. Paul SK, Goldar MM, Yakura M, Oowatari Y, Kawamukai M (2009) Glutamyl tRNA synthetases and glutamic acid induce sexual differentiation of Schizosaccharomyces pombe . Biosci Biotechnol Biochem 73: 1339–1347. [DOI] [PubMed] [Google Scholar]
- 7.Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a lablatory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 8. Maundrell K (1990) nmt1 of fission yeast: a highly transcribed gene completely repressed by thiamine. J Biol Chem 265: 10857–10864. [PubMed] [Google Scholar]
- 9. Matsuo Y, Kishimoto H, Horiuchi T, Tanae K, Kawamukai M (2010) Simple and effective gap-repair cloning using short tracts of flanking homology in fission yeast. Biosci Biotechnol Biochem 74: 685–689. [DOI] [PubMed] [Google Scholar]
- 10. Bahler J, Wu JQ, Longtine MS, Shah NG, McKenzie A 3rd, et al (1998) Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe . Yeast 14: 943–951. [DOI] [PubMed] [Google Scholar]
- 11. Sato M, Dhut S, Toda T (2005) New drug-resistant cassettes for gene disruption and epitope tagging in Schizosaccharomyces pombe . Yeast 22: 583–591. [DOI] [PubMed] [Google Scholar]
- 12. Pluskal T, Nakamura T, Villar-Briones A, Yanagida M (2010) Metabolic profiling of the fission yeast S. pombe: quantification of compounds under different temperatures and genetic perturbation. Mol Biosyst 6: 182–198. [DOI] [PubMed] [Google Scholar]
- 13. Kawamukai M, Gerst J, Field J, Riggs M, Rodgers L, et al. (1992) Genetic and biochemical analysis of the adenylyl cyclase-associated protein, cap, in Schizosaccharomyces pombe . Mol Biol Cell 3: 167–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Uchida N, Suzuki K, Saiki R, Kainou T, Tanaka K, et al. (2000) Phenotypes of fission yeast defective in ubiquinone production due to disruption of the gene for p-hydroxybenzoate polyprenyl diphosphate transferase. J Bacteriol 182: 6933–6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sakaguchi J, Yamamoto M (1982) Cloned ural locus of Schizosaccharomyces pombe propagates autonomously in this yeast assuming a polymeric form. Proc Natl Acad Sci U S A 79: 7819–7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lollier M, Jaquet L, Nedeva T, Lacroute F, Potier S, et al. (1995) As in Saccharomyces cerevisiae, aspartate transcarbamoylase is assembled on a multifunctional protein including a dihydroorotase-like cryptic domain in Schizosaccharomyces pombe . Curr Genet 28: 138–149. [DOI] [PubMed] [Google Scholar]
- 17. Bach ML (1987) Cloning and expression of the OMP decarboxylase gene URA4 from Schizosaccharomyces pombe . Curr Genet 12: 527–534. [DOI] [PubMed] [Google Scholar]
- 18. Godoy C, Arellano M, Diaz M, Duran A, Perez P (1996) Characterization of cwl1 +, a gene from Schizosaccharomyces pombe whose overexpression causes cell lysis. Yeast 12: 983–990. [DOI] [PubMed] [Google Scholar]
- 19. Arellano M, Valdivieso MH, Calonge TM, Coll PM, Duran A, et al. (1999) Schizosaccharomyces pombe protein kinase C homologues, pck1p and pck2p, are targets of rho1p and rho2p and differentially regulate cell integrity. J Cell Sci 112 (Pt 20): 3569–3578. [DOI] [PubMed] [Google Scholar]
- 20. Nagy M, Lacroute F, Thomas D (1992) Divergent evolution of pyrimidine biosynthesis between anaerobic and aerobic yeasts. Proc Natl Acad Sci U S A 89: 8966–8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saiki R, Ogiyama Y, Kainou T, Nishi T, Matsuda H, et al. (2003) Pleiotropic phenotypes of fission yeast defective in ubiquinone-10 production. A study from the abc1Sp (coq8Sp) mutant. Biofactors 18: 229–235. [DOI] [PubMed] [Google Scholar]
- 22. Kawamukai M (2009) Biosynthesis and bioproduction of coenzyme Q10 by yeasts and other organisms. Biotechnol Appl Biochem 53: 217–226. [DOI] [PubMed] [Google Scholar]
- 23. Miki R, Saiki R, Ozoe Y, Kawamukai M (2008) Comparison of a coq7 deletion mutant with other respiration-defective mutants in fission yeast. Febs J 275: 5309–5324. [DOI] [PubMed] [Google Scholar]
- 24. Furuya K, Niki H (2009) Isolation of heterothallic haploid and auxotrophic mutants of Schizosaccharomyces japonicus . Yeast 26: 221–233. [DOI] [PubMed] [Google Scholar]
- 25. Matsuo Y, Tanaka K, Nakagawa T, Matsuda H, Kawamukai M (2004) Genetic analysis of chs1 + and chs2 + encoding chitin synthases from Schizosaccharomyces pombe . Biosci Biotechnol Biochem 68: 1489–1499. [DOI] [PubMed] [Google Scholar]
- 26. Matsuo Y, Matsuura Y, Tanaka K, Matsuda H, Kawamukai M (2004) Chr4, a Schizosaccharomyces pombe homologue of the Saccharomyces cerevisiae Chs4p/Skt5p protein, is related to septum formation and is required for the proper localization of Chs2. Yeast 21: 1005–1019. [DOI] [PubMed] [Google Scholar]
- 27. Vos A, Dekker N, Distel B, Leunissen JA, Hochstenbach F (2007) Role of the synthase domain of Ags1p in cell wall alpha-glucan biosynthesis in fission yeast. J Biol Chem 282: 18969–18979. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cellular morphology of a tetrad, 5a (Δ ura4 ), 5b ( leu1-32 Δ ura4 ), 5c ( leu1-32 ) and 5d (wild type), derived by crossing L972 ( h −) with PR110 ( h + ura4-D18 leu1-32 ). Cells were grown on YES and YPD. Bar: 10 µm
(TIFF)
(A) Cell’s culture of the L972 (WT), UMP34 (Δ ura1 ), UMP35 (Δ ura2 ), UMP36 (Δ ura3 ), UMP31 (Δ ura4 ), and UMP37 (Δ ura5 ) strains were serially diluted 10-fold, plated on the indicated plates and incubated for 3 days at 30°C. For alkaline phosphatase assay, each plate was overlaid for 10, 30 and 60 min with a phosphatase assay solution as in Fig. 1. The amount of OMP was measured by mass spectrometry in UMP31 (Δura4) cells grown in YPD liquid medium containing an indicted amount of uracil. Under these conditions, a higher amount of uracil (900 mg/L) can only suppress the cell lysis phenotype.
(TIFF)
Oligonucleotide primers used in this study.
(DOCX)