Abstract
Recently, a mammalian tRNA which was previously designated as an opal suppressor seryl-tRNA and phosphoseryl-tRNA was shown to be a selenocysteyl-tRNA (B. J. Lee, P. J. Worland, J. N. Davis, T. C. Stadtman, and D. Hatfield, J. Biol. Chem. 264:9724-9727, 1989). Hence, this tRNA is now designated as selenocysteyl-tRNA[Ser]Sec, and its function is twofold, to serve as (i) a carrier molecule upon which selenocysteine is biosynthesized and (ii) as a donor of selenocysteine, which is the 21st naturally occurring amino acid of protein, to the nascent polypeptide chain in response to specific UGA codons. In the present study, the selenocysteine tRNA gene was sequenced from Xenopus laevis, Drosophila melanogaster, and Caenorhabditis elegans. The tRNA product of this gene was also identified within the seryl-tRNA population of a number of higher and lower animals, and the human tRNA[Ser]Sec gene was used as a probe to identify homologous sequences within genomic DNAs of organisms throughout the animal kingdom. The studies showed that the tRNA[Ser]Sec gene has undergone evolutionary change and that it is ubiquitous in the animal kingdom. Further, we conclude that selenocysteine-containing proteins, as well as the use of UGA as a codon for selenocysteine, are far more widespread in nature than previously thought.
Full text
PDF
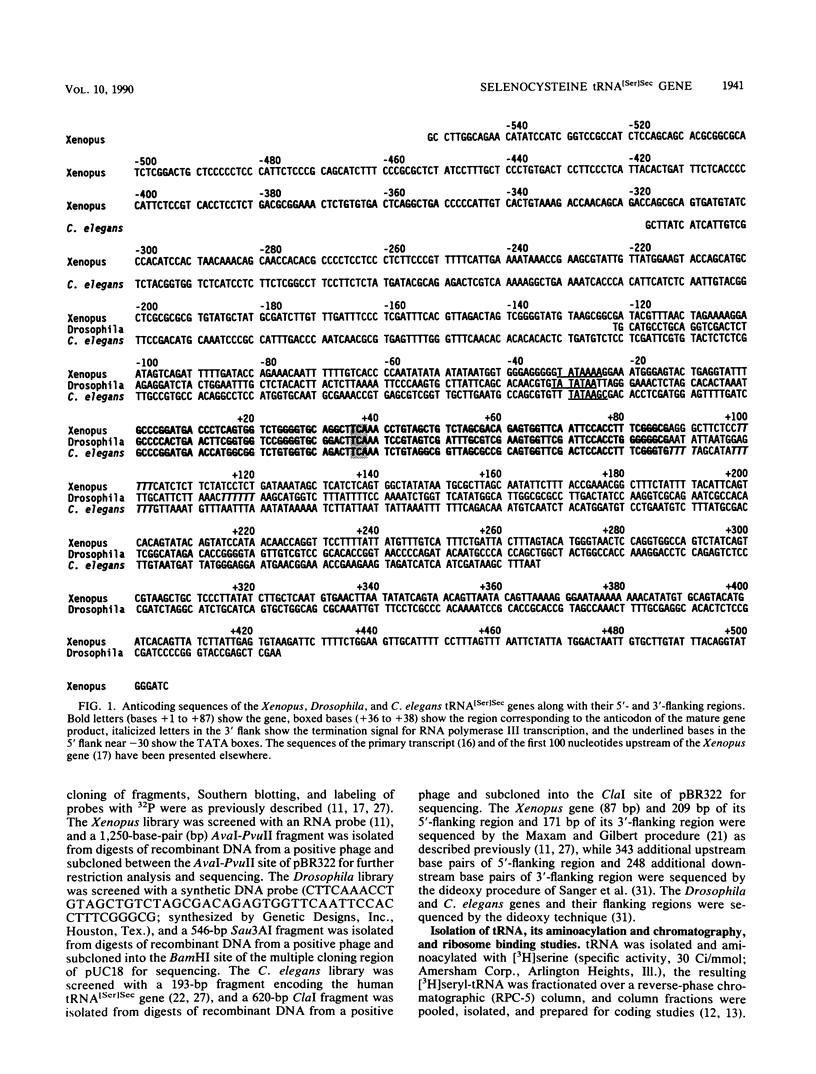
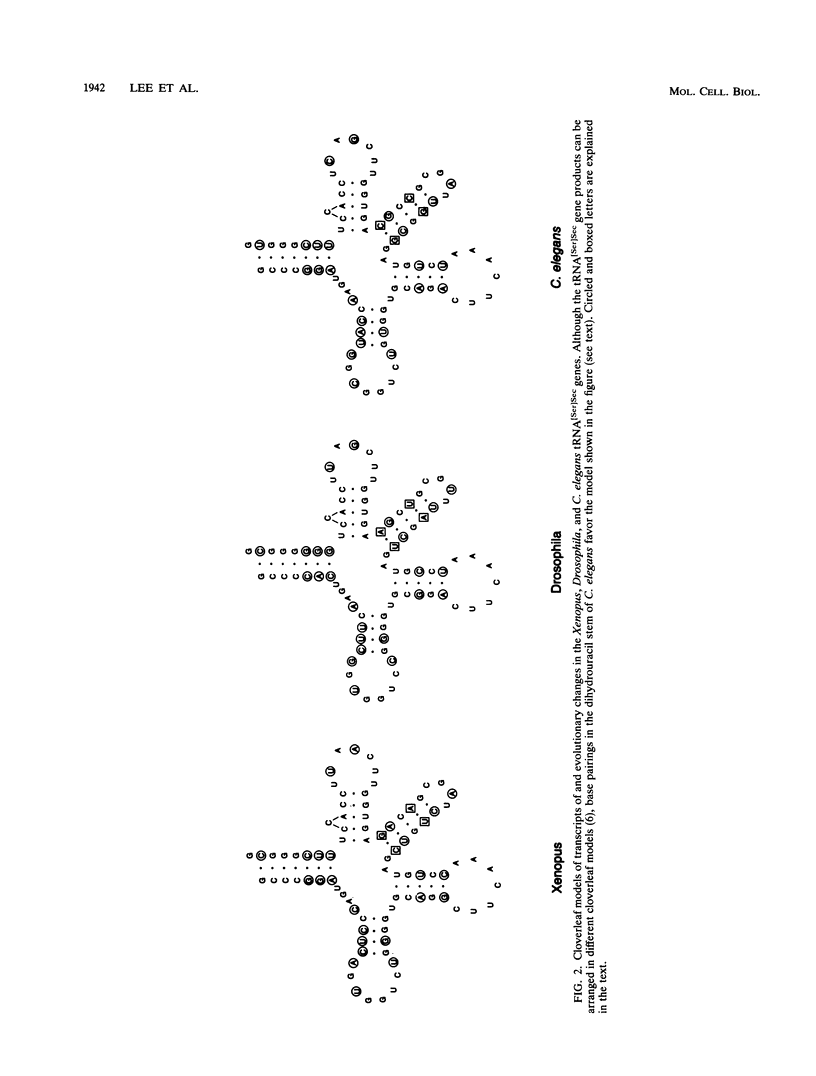
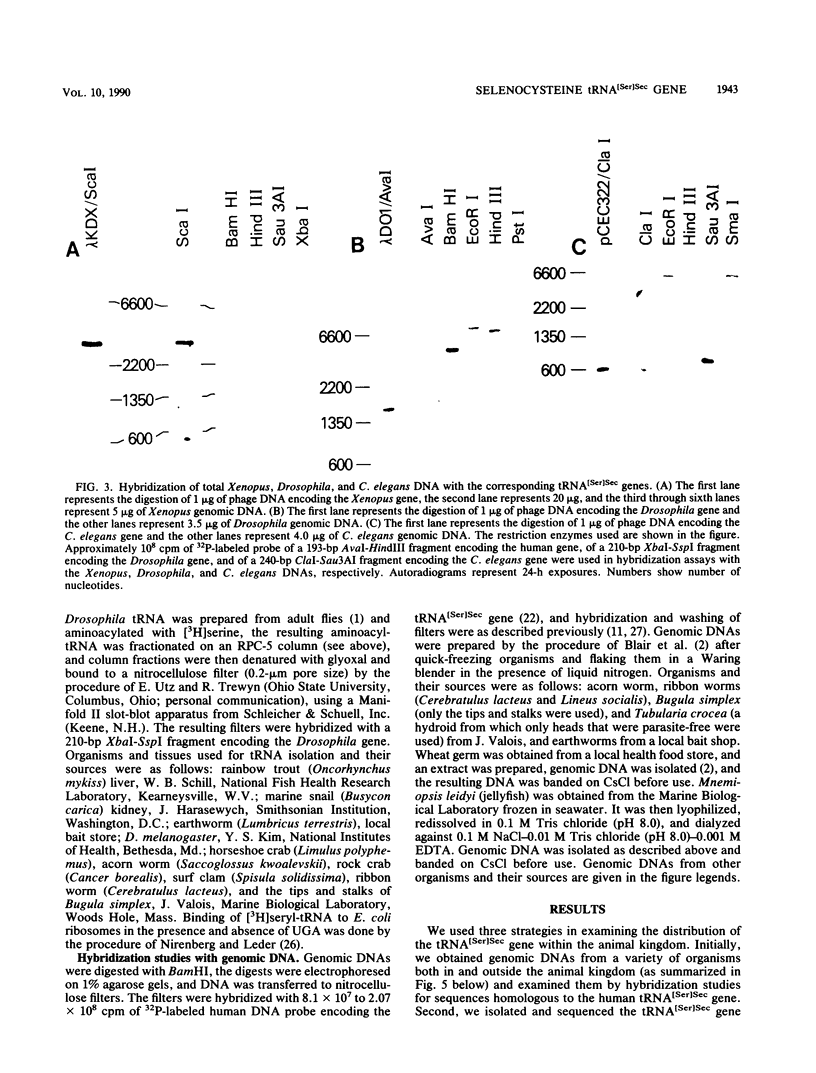
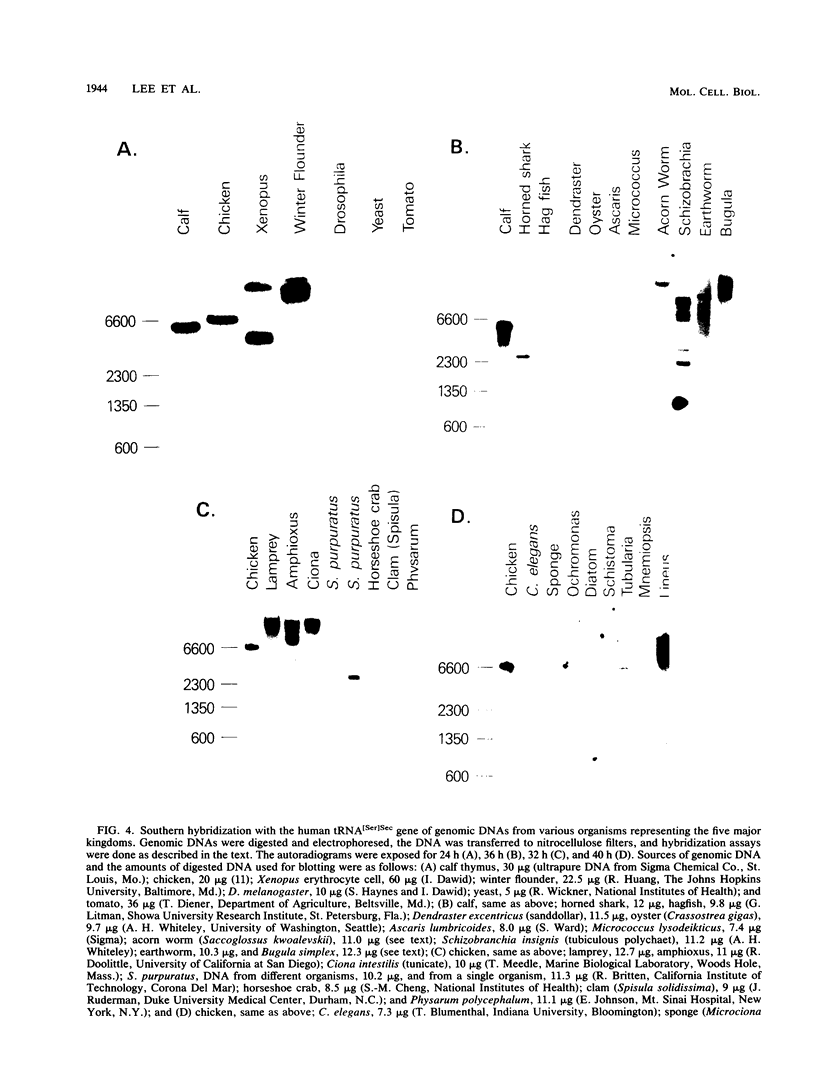




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birchler J. A., Owenby R. K., Jacobson K. B. Dosage compensation of serine-4 transfer RNA in Drosophila melanogaster. Genetics. 1982 Nov;102(3):525–537. doi: 10.1093/genetics/102.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair D. G., Cooper C. S., Oskarsson M. K., Eader L. A., Vande Woude G. F. New method for detecting cellular transforming genes. Science. 1982 Dec 10;218(4577):1122–1125. doi: 10.1126/science.6293052. [DOI] [PubMed] [Google Scholar]
- Chambers I., Frampton J., Goldfarb P., Affara N., McBain W., Harrison P. R. The structure of the mouse glutathione peroxidase gene: the selenocysteine in the active site is encoded by the 'termination' codon, TGA. EMBO J. 1986 Jun;5(6):1221–1227. doi: 10.1002/j.1460-2075.1986.tb04350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien Y. H., Dawid I. B. Isolation and characterization of calmodulin genes from Xenopus laevis. Mol Cell Biol. 1984 Mar;4(3):507–513. doi: 10.1128/mcb.4.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das G., Henning D., Wright D., Reddy R. Upstream regulatory elements are necessary and sufficient for transcription of a U6 RNA gene by RNA polymerase III. EMBO J. 1988 Feb;7(2):503–512. doi: 10.1002/j.1460-2075.1988.tb02838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A., Dudock B., Hatfield D. Structure and properties of a bovine liver UGA suppressor serine tRNA with a tryptophan anticodon. Cell. 1981 Aug;25(2):497–506. doi: 10.1016/0092-8674(81)90068-4. [DOI] [PubMed] [Google Scholar]
- Garcia A. D., O'Connell A. M., Sharp S. J. Formation of an active transcription complex in the Drosophila melanogaster 5S RNA gene is dependent on an upstream region. Mol Cell Biol. 1987 Jun;7(6):2046–2051. doi: 10.1128/mcb.7.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield D. L., Dudock B. S., Eden F. C. Characterization and nucleotide sequence of a chicken gene encoding an opal suppressor tRNA and its flanking DNA segments. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4940–4944. doi: 10.1073/pnas.80.16.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield D., Diamond A., Dudock B. Opal suppressor serine tRNAs from bovine liver form phosphoseryl-tRNA. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6215–6219. doi: 10.1073/pnas.79.20.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield D., Matthews C. R., Rice M. Aminoacyl-transfer RNA populations in mammalian cells chromatographic profiles and patterns of codon recognition. Biochim Biophys Acta. 1979 Oct 25;564(3):414–423. doi: 10.1016/0005-2787(79)90032-7. [DOI] [PubMed] [Google Scholar]
- Hatfield D., Varricchio F., Rice M., Forget B. G. The aminoacyl-tRNA population of human reticulocytes. J Biol Chem. 1982 Mar 25;257(6):3183–3188. [PubMed] [Google Scholar]
- Heider J., Leinfelder W., Böck A. Occurrence and functional compatibility within Enterobacteriaceae of a tRNA species which inserts selenocysteine into protein. Nucleic Acids Res. 1989 Apr 11;17(7):2529–2540. doi: 10.1093/nar/17.7.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. J., Kang S. G., Hatfield D. Transcription of Xenopus selenocysteine tRNA Ser (formerly designated opal suppressor phosphoserine tRNA) gene is directed by multiple 5'-extragenic regulatory elements. J Biol Chem. 1989 Jun 5;264(16):9696–9702. [PubMed] [Google Scholar]
- Lee B. J., Worland P. J., Davis J. N., Stadtman T. C., Hatfield D. L. Identification of a selenocysteyl-tRNA(Ser) in mammalian cells that recognizes the nonsense codon, UGA. J Biol Chem. 1989 Jun 15;264(17):9724–9727. [PubMed] [Google Scholar]
- Lee B. J., de la Peña P., Tobian J. A., Zasloff M., Hatfield D. Unique pathway of expression of an opal suppressor phosphoserine tRNA. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6384–6388. doi: 10.1073/pnas.84.18.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinfelder W., Stadtman T. C., Böck A. Occurrence in vivo of selenocysteyl-tRNA(SERUCA) in Escherichia coli. Effect of sel mutations. J Biol Chem. 1989 Jun 15;264(17):9720–9723. [PubMed] [Google Scholar]
- Leinfelder W., Zehelein E., Mandrand-Berthelot M. A., Böck A. Gene for a novel tRNA species that accepts L-serine and cotranslationally inserts selenocysteine. Nature. 1988 Feb 25;331(6158):723–725. doi: 10.1038/331723a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McBride O. W., Rajagopalan M., Hatfield D. Opal suppressor phosphoserine tRNA gene and pseudogene are located on human chromosomes 19 and 22, respectively. J Biol Chem. 1987 Aug 15;262(23):11163–11166. [PubMed] [Google Scholar]
- Mizutani T., Hashimoto A. Purification and properties of suppressor seryl-tRNA: ATP phosphotransferase from bovine liver. FEBS Lett. 1984 Apr 24;169(2):319–322. doi: 10.1016/0014-5793(84)80342-7. [DOI] [PubMed] [Google Scholar]
- Mullenbach G. T., Tabrizi A., Irvine B. D., Bell G. I., Tainer J. A., Hallewell R. A. Selenocysteine's mechanism of incorporation and evolution revealed in cDNAs of three glutathione peroxidases. Protein Eng. 1988 Sep;2(3):239–246. doi: 10.1093/protein/2.3.239. [DOI] [PubMed] [Google Scholar]
- Murphy S., Di Liegro C., Melli M. The in vitro transcription of the 7SK RNA gene by RNA polymerase III is dependent only on the presence of an upstream promoter. Cell. 1987 Oct 9;51(1):81–87. doi: 10.1016/0092-8674(87)90012-2. [DOI] [PubMed] [Google Scholar]
- NIRENBERG M., LEDER P. RNA CODEWORDS AND PROTEIN SYNTHESIS. THE EFFECT OF TRINUCLEOTIDES UPON THE BINDING OF SRNA TO RIBOSOMES. Science. 1964 Sep 25;145(3639):1399–1407. doi: 10.1126/science.145.3639.1399. [DOI] [PubMed] [Google Scholar]
- O'Neill V. A., Eden F. C., Pratt K., Hatfield D. L. A human opal suppressor tRNA gene and pseudogene. J Biol Chem. 1985 Feb 25;260(4):2501–2508. [PubMed] [Google Scholar]
- Pratt K., Eden F. C., You K. H., O'Neill V. A., Hatfield D. Conserved sequences in both coding and 5' flanking regions of mammalian opal suppressor tRNA genes. Nucleic Acids Res. 1985 Jul 11;13(13):4765–4775. doi: 10.1093/nar/13.13.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price N. M., Harrison P. J. Specific Selenium-Containing Macromolecules in the Marine Diatom Thalassiosira pseudonana. Plant Physiol. 1988 Jan;86(1):192–199. doi: 10.1104/pp.86.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy A. P., Hsu B. L., Reddy P. S., Li N. Q., Thyagaraju K., Reddy C. C., Tam M. F., Tu C. P. Expression of glutathione peroxidase I gene in selenium-deficient rats. Nucleic Acids Res. 1988 Jun 24;16(12):5557–5568. doi: 10.1093/nar/16.12.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speier C., Baker S. S., Newburger P. E. Relationships between in vitro selenium supply, glutathione peroxidase activity, and phagocytic function in the HL-60 human myeloid cell line. J Biol Chem. 1985 Jul 25;260(15):8951–8955. [PubMed] [Google Scholar]
- Sprinzl M., Hartmann T., Weber J., Blank J., Zeidler R. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1989;17 (Suppl):r1–172. doi: 10.1093/nar/17.suppl.r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman T. C. Specific occurrence of selenium in enzymes and amino acid tRNAs. FASEB J. 1987 Nov;1(5):375–379. doi: 10.1096/fasebj.1.5.2445614. [DOI] [PubMed] [Google Scholar]
- Yokota A., Shigeoka S., Onishi T., Kitaoka S. Selenium as Inducer of Glutathione Peroxidase in low-CO(2)-Grown Chlamydomonas reinhardtii. Plant Physiol. 1988 Mar;86(3):649–651. doi: 10.1104/pp.86.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. S., Takahashi N., Sprague K. U. Upstream sequences confer distinctive transcriptional properties on genes encoding silkgland-specific tRNAAla. Proc Natl Acad Sci U S A. 1986 Jan;83(2):374–378. doi: 10.1073/pnas.83.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinoni F., Birkmann A., Leinfelder W., Böck A. Cotranslational insertion of selenocysteine into formate dehydrogenase from Escherichia coli directed by a UGA codon. Proc Natl Acad Sci U S A. 1987 May;84(10):3156–3160. doi: 10.1073/pnas.84.10.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]





