Abstract
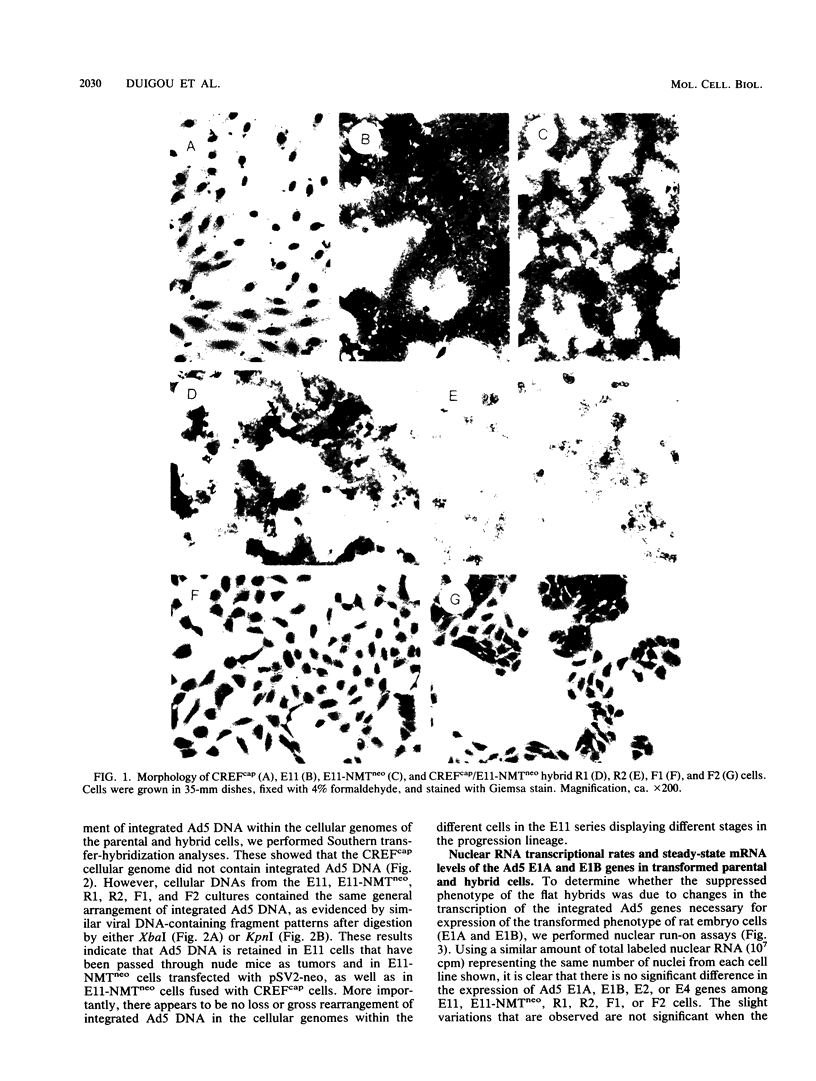
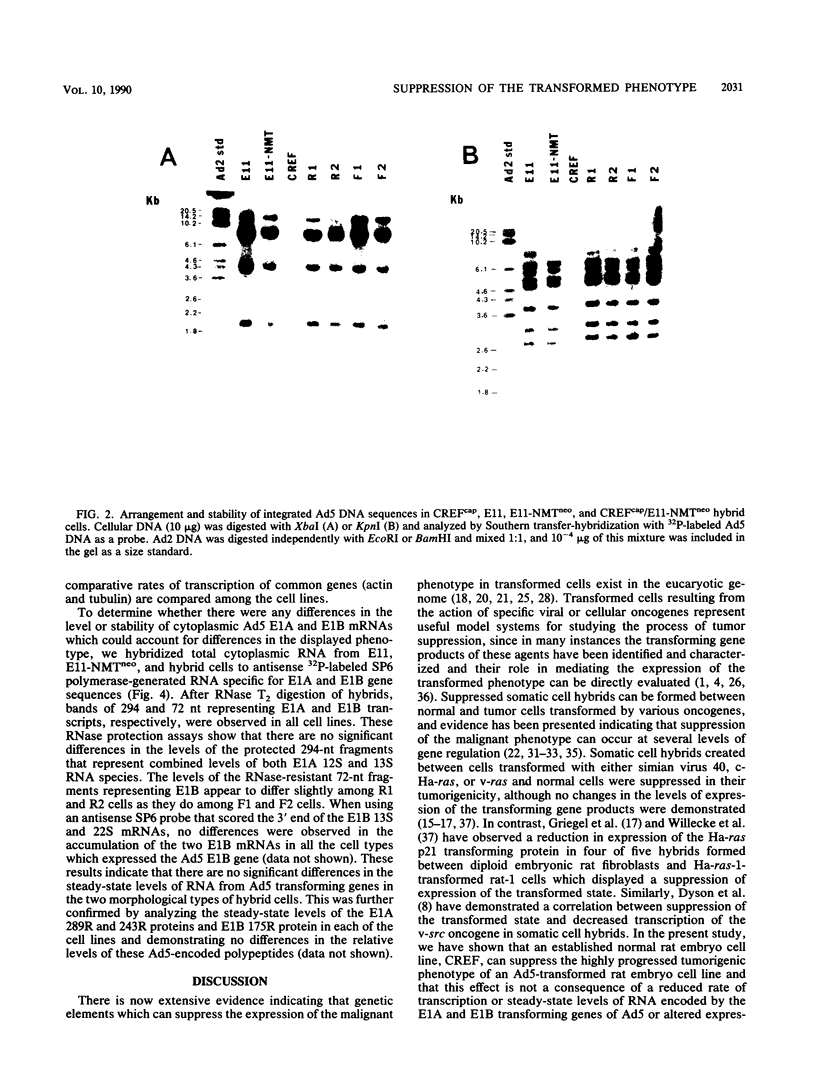
In the present study we have analyzed the genetic regulation of increased expression of transformation-associated traits, a process termed progression, in adenovirus type 5 (Ad5)-transformed secondary rat embryo cells. Somatic cell hybrids were formed between a highly progressed neomycin-resistant Ad5-transformed cloned cell line (E11-NMTneo) and an untransformed chloramphenicol-resistant rat embryo fibroblast cell line (CREFcap). Parental E11-NMTneo cells grew with high efficiency in agar, exhibited reduced 125I-epidermal growth factor (EGF) binding, and were tumorigenic in nude mice. Parental CREFcap cells exhibited phenotypes opposite to those of E11-NMTneo cells. A high proportion (84%) of the presumptive hybrid cell types obtained after fusion and genetic selection (G418 and chloramphenicol) displayed a flat morphological phenotype intermediate between CREFcap and E11-NMTneo cells, suggesting that a trans-dominant extinction phenomenon had occurred. Two hybrids with a round morphology (R), which still exhibited the progressed phenotype, and two hybrids with a flat morphology (F), which had lost the progressed phenotype, were chosen for detailed analysis. Both R hybrids grew efficiently in agar, exhibited low 125I-EGF binding, and were tumorigenic in nude mice, whereas both F hybrids grew poorly in agar, displayed increased 125I-EGF binding in comparison with E11-NMTneo and R hybrids, and were nontumorigenic in nude mice. An analysis of the viral DNA integration patterns and the rates of transcription, steady-state mRNA accumulation, and relative levels of the Ad5 E1A and E1B gene products revealed no differences among the parental and hybrid cells. These studies indicate that normal CREF cells may contain a suppressor gene(s) which can inhibit the expression of specific traits of the progression phenotype in Ad5-transformed cells and that this suppression is not associated with changes in the expression of Ad5 transforming genes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babiss L. E., Zimmer S. G., Fisher P. B. Reversibility of progression of the transformed phenotype in Ad5-transformed rat embryo cells. Science. 1985 May 31;228(4703):1099–1101. doi: 10.1126/science.2581317. [DOI] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Dorsch-Häsler K., Fisher P. B., Weinstein I. B., Ginsberg H. S. Patterns of viral DNA integration in cells transformed by wild type or DNA-binding protein mutants of adenovirus type 5 and effect of chemical carcinogens on integration. J Virol. 1980 May;34(2):305–314. doi: 10.1128/jvi.34.2.305-314.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson P. J., Quade K., Wyke J. A. Expression of the ASV src gene in hybrids between normal and virally transformed cells: specific suppression occurs in some hybrids but not others. Cell. 1982 Sep;30(2):491–498. doi: 10.1016/0092-8674(82)90246-x. [DOI] [PubMed] [Google Scholar]
- Fisher P. B., Babiss L. E., Weinstein I. B., Ginsberg H. S. Analysis of type 5 adenovirus transformation with a cloned rat embryo cell line (CREF). Proc Natl Acad Sci U S A. 1982 Jun;79(11):3527–3531. doi: 10.1073/pnas.79.11.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P. B., Boersig M. R., Graham G. M., Weinstein I. B. Production of growth factors by type 5 adenovirus transformed rat embryo cells. J Cell Physiol. 1983 Mar;114(3):365–370. doi: 10.1002/jcp.1041140315. [DOI] [PubMed] [Google Scholar]
- Fisher P. B., Bozzone J. H., Weinstein I. B. Tumor promoters and epidermal growth factor stimulate anchorage-independent growth of adenovirus-tranformed rat embryo cells. Cell. 1979 Nov;18(3):695–705. doi: 10.1016/0092-8674(79)90124-7. [DOI] [PubMed] [Google Scholar]
- Fisher P. B., Dorsch-Häsler K., Weinstein I. B., Ginsberg H. S. Tumour promoters enhance anchorage-independent growth of adenovirus-transformed cells without altering the integration pattern of viral sequences. Nature. 1979 Oct 18;281(5732):591–594. doi: 10.1038/281591a0. [DOI] [PubMed] [Google Scholar]
- Friedman J. M., Babiss L. E., Clayton D. F., Darnell J. E., Jr Cellular promoters incorporated into the adenovirus genome: cell specificity of albumin and immunoglobulin expression. Mol Cell Biol. 1986 Nov;6(11):3791–3797. doi: 10.1128/mcb.6.11.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser A. G., Anderson M. J., Stanbridge E. J. Suppression of tumorigenicity in human cell hybrids derived from cell lines expressing different activated ras oncogenes. Cancer Res. 1989 Mar 15;49(6):1572–1577. [PubMed] [Google Scholar]
- Geiser A. G., Der C. J., Marshall C. J., Stanbridge E. J. Suppression of tumorigenicity with continued expression of the c-Ha-ras oncogene in EJ bladder carcinoma-human fibroblast hybrid cells. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5209–5213. doi: 10.1073/pnas.83.14.5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griegel S., Traub O., Willecke K., Schäfer R. Suppression and re-expression of transformed phenotype in hybrids of HA-ras-1-transformed rat-1 cells and early-passage rat embryonic fibroblasts. Int J Cancer. 1986 Nov 15;38(5):697–705. doi: 10.1002/ijc.2910380513. [DOI] [PubMed] [Google Scholar]
- Hansen M. F., Cavenee W. K. Tumor suppressors: recessive mutations that lead to cancer. Cell. 1988 Apr 22;53(2):173–174. doi: 10.1016/0092-8674(88)90376-5. [DOI] [PubMed] [Google Scholar]
- Huang H. J., Yee J. K., Shew J. Y., Chen P. L., Bookstein R., Friedmann T., Lee E. Y., Lee W. H. Suppression of the neoplastic phenotype by replacement of the RB gene in human cancer cells. Science. 1988 Dec 16;242(4885):1563–1566. doi: 10.1126/science.3201247. [DOI] [PubMed] [Google Scholar]
- Kitayama H., Sugimoto Y., Matsuzaki T., Ikawa Y., Noda M. A ras-related gene with transformation suppressor activity. Cell. 1989 Jan 13;56(1):77–84. doi: 10.1016/0092-8674(89)90985-9. [DOI] [PubMed] [Google Scholar]
- Klinger H. P. Suppression of tumorigenicity in somatic cell hybrids. I. Suppression and reexpression of tumorigenicity in diploid human X D98AH2 hybrids and independent segregation of tumorigenicity from other cell phenotypes. Cytogenet Cell Genet. 1980;27(4):254–266. doi: 10.1159/000131494. [DOI] [PubMed] [Google Scholar]
- Koufos A., Hansen M. F., Lampkin B. C., Workman M. L., Copeland N. G., Jenkins N. A., Cavenee W. K. Loss of alleles at loci on human chromosome 11 during genesis of Wilms' tumour. Nature. 1984 May 10;309(5964):170–172. doi: 10.1038/309170a0. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Tumor cell instability, diversification, and progression to the metastatic phenotype: from oncogene to oncofetal expression. Cancer Res. 1987 Mar 15;47(6):1473–1487. [PubMed] [Google Scholar]
- Noda M., Kitayama H., Matsuzaki T., Sugimoto Y., Okayama H., Bassin R. H., Ikawa Y. Detection of genes with a potential for suppressing the transformed phenotype associated with activated ras genes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):162–166. doi: 10.1073/pnas.86.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Selinger Z., Scolnick E. M., Bassin R. H. Flat revertants isolated from Kirsten sarcoma virus-transformed cells are resistant to the action of specific oncogenes. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5602–5606. doi: 10.1073/pnas.80.18.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell P. C. Mechanisms of tumor progression. Cancer Res. 1986 May;46(5):2203–2207. [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stanbridge E. J., Der C. J., Doersen C. J., Nishimi R. Y., Peehl D. M., Weissman B. E., Wilkinson J. E. Human cell hybrids: analysis of transformation and tumorigenicity. Science. 1982 Jan 15;215(4530):252–259. doi: 10.1126/science.7053574. [DOI] [PubMed] [Google Scholar]
- Stanbridge E. J., Flandermeyer R. R., Daniels D. W., Nelson-Rees W. A. Specific chromosome loss associated with the expression of tumorigenicity in human cell hybrids. Somatic Cell Genet. 1981 Nov;7(6):699–712. doi: 10.1007/BF01538758. [DOI] [PubMed] [Google Scholar]
- Stoler A., Bouck N. Identification of a single chromosome in the normal human genome essential for suppression of hamster cell transformation. Proc Natl Acad Sci U S A. 1985 Jan;82(2):570–574. doi: 10.1073/pnas.82.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein I. B. Growth factors, oncogenes, and multistage carcinogenesis. J Cell Biochem. 1987 Mar;33(3):213–224. doi: 10.1002/jcb.240330308. [DOI] [PubMed] [Google Scholar]
- Weissman B. E., Saxon P. J., Pasquale S. R., Jones G. R., Geiser A. G., Stanbridge E. J. Introduction of a normal human chromosome 11 into a Wilms' tumor cell line controls its tumorigenic expression. Science. 1987 Apr 10;236(4798):175–180. doi: 10.1126/science.3031816. [DOI] [PubMed] [Google Scholar]
- Whyte P., Buchkovich K. J., Horowitz J. M., Friend S. H., Raybuck M., Weinberg R. A., Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988 Jul 14;334(6178):124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- Willecke K., Griegel S., Martin W., Traub O., Schäfer R. The Ha-ras-induced transformed phenotype of rat-1 cells can be suppressed in hybrids with rat embryonic fibroblasts. J Cell Biochem. 1987 May;34(1):23–30. doi: 10.1002/jcb.240340104. [DOI] [PubMed] [Google Scholar]