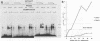Abstract
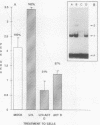
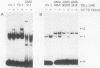
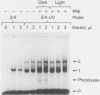
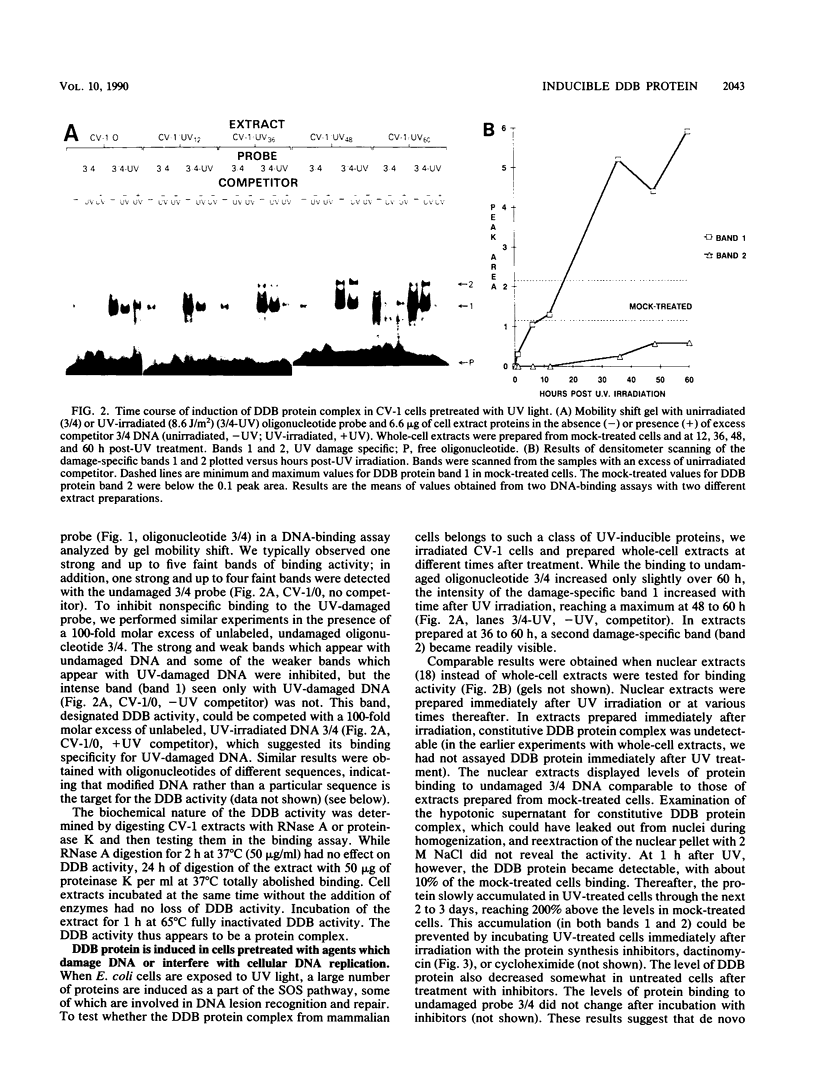
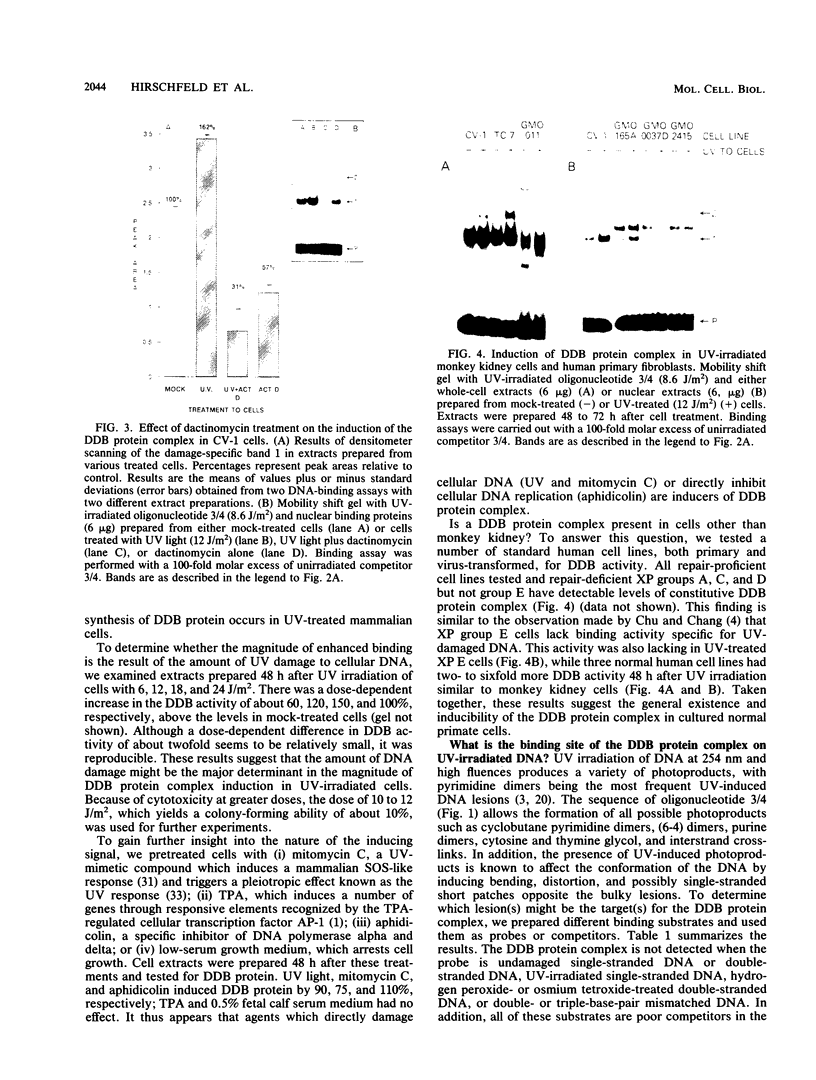
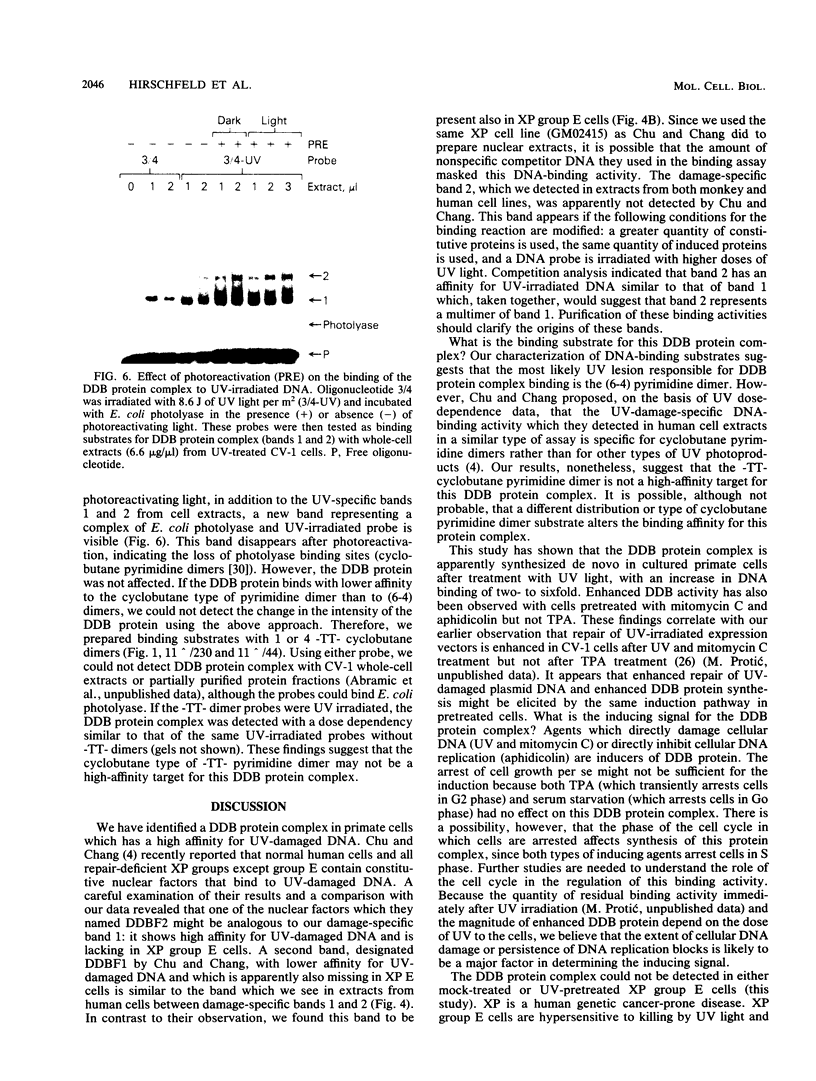
Using a DNA band shift assay, we have identified a DNA-binding protein complex in primate cells which is present constitutively and has a high affinity for UV-irradiated, double-stranded DNA. Cells pretreated with UV light, mitomycin C, or aphidicolin have higher levels of this damage-specific DNA-binding protein complex, suggesting that the signal for induction can either be damage to the DNA or interference with cellular DNA replication. Physiochemical modifications of the DNA and competition analysis with defined substrates suggest that the most probable target site for the damage-specific DNA-binding protein complex is a 6-4'-(pyrimidine-2'-one)-pyrimidine dimer: specific binding could not be detected with probes which contain -TT- cyclobutane dimers, and damage-specific DNA binding did not decrease after photoreactivation of UV-irradiated DNA. This damage-specific DNA-binding protein complex is the first such inducible protein complex identified in primate cells. Cells from patients with the sun-sensitive cancer-prone disease, xeroderma pigmentosum (group E), are lacking both the constitutive and the induced damage-specific DNA-binding activities. These findings suggest a possible role for this DNA-binding protein complex in lesion recognition and DNA repair of UV-light-induced photoproducts.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Banerjee S. K., Christensen R. B., Lawrence C. W., LeClerc J. E. Frequency and spectrum of mutations produced by a single cis-syn thymine-thymine cyclobutane dimer in a single-stranded vector. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8141–8145. doi: 10.1073/pnas.85.21.8141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourre F., Renault G., Sarasin A. Sequence effect on alkali-sensitive sites in UV-irradiated SV40 DNA. Nucleic Acids Res. 1987 Nov 11;15(21):8861–8875. doi: 10.1093/nar/15.21.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Chang E. Xeroderma pigmentosum group E cells lack a nuclear factor that binds to damaged DNA. Science. 1988 Oct 28;242(4878):564–567. doi: 10.1126/science.3175673. [DOI] [PubMed] [Google Scholar]
- Doetsch P. W., Chan G. L., Haseltine W. A. T4 DNA polymerase (3'-5') exonuclease, an enzyme for the detection and quantitation of stable DNA lesions: the ultraviolet light example. Nucleic Acids Res. 1985 May 10;13(9):3285–3304. doi: 10.1093/nar/13.9.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elespuru R. K. Inducible responses to DNA damage in bacteria and mammalian cells. Environ Mol Mutagen. 1987;10(1):97–116. doi: 10.1002/em.2850100111. [DOI] [PubMed] [Google Scholar]
- Feldberg R. S., Grossman L. A DNA binding protein from human placenta specific for ultraviolet damaged DNA. Biochemistry. 1976 Jun 1;15(11):2402–2408. doi: 10.1021/bi00656a024. [DOI] [PubMed] [Google Scholar]
- Feldberg R. S., Lucas J. L., Dannenberg A. A damage-specific DNA binding protein. Large scale purification from human placenta and characterization. J Biol Chem. 1982 Jun 10;257(11):6394–6401. [PubMed] [Google Scholar]
- Feldberg R. S. On the substrate specificity of a damage-specific DNA binding protein from human cells. Nucleic Acids Res. 1980 Mar 11;8(5):1133–1143. doi: 10.1093/nar/8.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Zmudzka B., Hollander M. C., Wilson S. H. Induction of beta-polymerase mRNA by DNA-damaging agents in Chinese hamster ovary cells. Mol Cell Biol. 1989 Feb;9(2):851–853. doi: 10.1128/mcb.9.2.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer P. M., Greggio N. A., Metherall J. E., Summers W. C. UV-induced DNA-binding proteins in human cells. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1163–1167. doi: 10.1073/pnas.86.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helland D. E., Doetsch P. W., Haseltine W. A. Substrate specificity of a mammalian DNA repair endonuclease that recognizes oxidative base damage. Mol Cell Biol. 1986 Jun;6(6):1983–1990. doi: 10.1128/mcb.6.6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiricny J., Hughes M., Corman N., Rudkin B. B. A human 200-kDa protein binds selectively to DNA fragments containing G.T mismatches. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8860–8864. doi: 10.1073/pnas.85.23.8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. A., Bindereif A., Green M. R. A small-scale procedure for preparation of nuclear extracts that support efficient transcription and pre-mRNA splicing. Gene Anal Tech. 1988 Mar-Apr;5(2):22–31. doi: 10.1016/0735-0651(88)90023-4. [DOI] [PubMed] [Google Scholar]
- Lefebvre P., Laval F. Enhancement of O6-methylguanine-DNA-methyltransferase activity induced by various treatments in mammalian cells. Cancer Res. 1986 Nov;46(11):5701–5705. [PubMed] [Google Scholar]
- Lippke J. A., Gordon L. K., Brash D. E., Haseltine W. A. Distribution of UV light-induced damage in a defined sequence of human DNA: detection of alkaline-sensitive lesions at pyrimidine nucleoside-cytidine sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3388–3392. doi: 10.1073/pnas.78.6.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzina M., Nocentini S. DNA ligase activity in UV-irradiated monkey kidney cells. Nucleic Acids Res. 1978 Nov;5(11):4317–4328. doi: 10.1093/nar/5.11.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. L., Humphrey R. M., Adair G. M., Thompson L. H., Clarkson J. M. Repair of (6-4)photoproducts correlates with split-dose recovery in UV-irradiated normal and hypersensitive rodent cells. Mutat Res. 1988 Jan;193(1):53–63. doi: 10.1016/0167-8817(88)90007-7. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L. The relative cytotoxicity of (6-4) photoproducts and cyclobutane dimers in mammalian cells. Photochem Photobiol. 1988 Jul;48(1):51–57. doi: 10.1111/j.1751-1097.1988.tb02785.x. [DOI] [PubMed] [Google Scholar]
- Moranelli F., Lieberman M. W. Recognition of chemical carcinogen-modified DNA by a DNA-binding protein. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3201–3205. doi: 10.1073/pnas.77.6.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protić-Sabljić M., Kraemer K. H. One pyrimidine dimer inactivates expression of a transfected gene in xeroderma pigmentosum cells. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6622–6626. doi: 10.1073/pnas.82.19.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protić-Sabljić M., Kraemer K. H. Reduced repair of non-dimer photoproducts in a gene transfected into xeroderma pigmentosum cells. Photochem Photobiol. 1986 May;43(5):509–513. doi: 10.1111/j.1751-1097.1986.tb09528.x. [DOI] [PubMed] [Google Scholar]
- Protić M., Roilides E., Levine A. S., Dixon K. Enhancement of DNA repair capacity of mammalian cells by carcinogen treatment. Somat Cell Mol Genet. 1988 Jul;14(4):351–357. doi: 10.1007/BF01534643. [DOI] [PubMed] [Google Scholar]
- Sancar A., Smith F. W., Sancar G. B. Purification of Escherichia coli DNA photolyase. J Biol Chem. 1984 May 10;259(9):6028–6032. [PubMed] [Google Scholar]
- Sarasin A., Benoit A. Enhanced mutagenesis of UV-irradiated simian virus 40 occurs in mitomycin C-treated host cells only at a low multiplicity of infection. Mol Cell Biol. 1986 Apr;6(4):1102–1107. doi: 10.1128/mcb.6.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleif R. DNA binding by proteins. Science. 1988 Sep 2;241(4870):1182–1187. doi: 10.1126/science.2842864. [DOI] [PubMed] [Google Scholar]
- Schorpp M., Mallick U., Rahmsdorf H. J., Herrlich P. UV-induced extracellular factor from human fibroblasts communicates the UV response to nonirradiated cells. Cell. 1984 Jul;37(3):861–868. doi: 10.1016/0092-8674(84)90421-5. [DOI] [PubMed] [Google Scholar]
- Tsang S. S., Kuhnlein U. DNA-binding protein from HeLa cells that binds preferentially to supercoiled DNA damaged by ultraviolet light or N-acetoxy-N-acetyl-2-aminofluorene. Biochim Biophys Acta. 1982 May 31;697(2):202–212. doi: 10.1016/0167-4781(82)90078-1. [DOI] [PubMed] [Google Scholar]
- Valerie K., Delers A., Bruck C., Thiriart C., Rosenberg H., Debouck C., Rosenberg M. Activation of human immunodeficiency virus type 1 by DNA damage in human cells. Nature. 1988 May 5;333(6168):78–81. doi: 10.1038/333078a0. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Inducible DNA repair systems. Annu Rev Biochem. 1985;54:425–457. doi: 10.1146/annurev.bi.54.070185.002233. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R. D., Robins P., Lindahl T. Complementation of the xeroderma pigmentosum DNA repair defect in cell-free extracts. Cell. 1988 Apr 8;53(1):97–106. doi: 10.1016/0092-8674(88)90491-6. [DOI] [PubMed] [Google Scholar]