Abstract
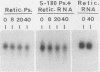
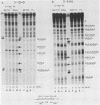
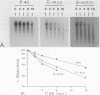
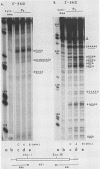
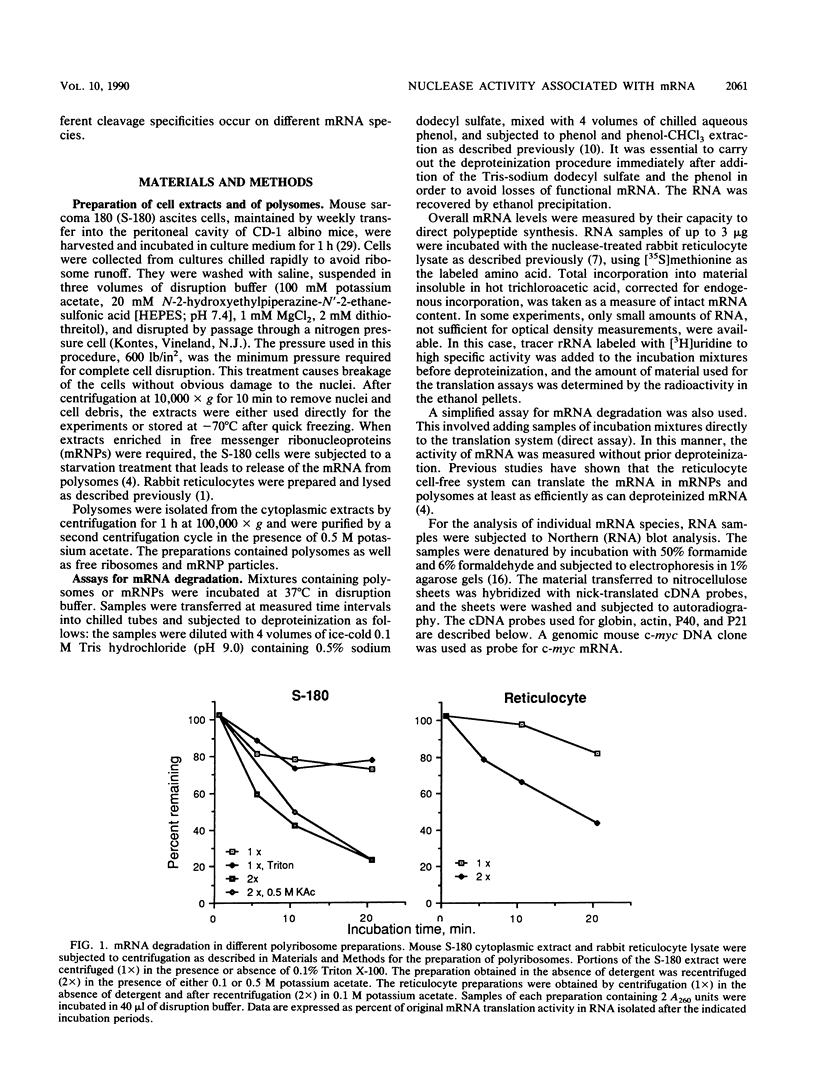
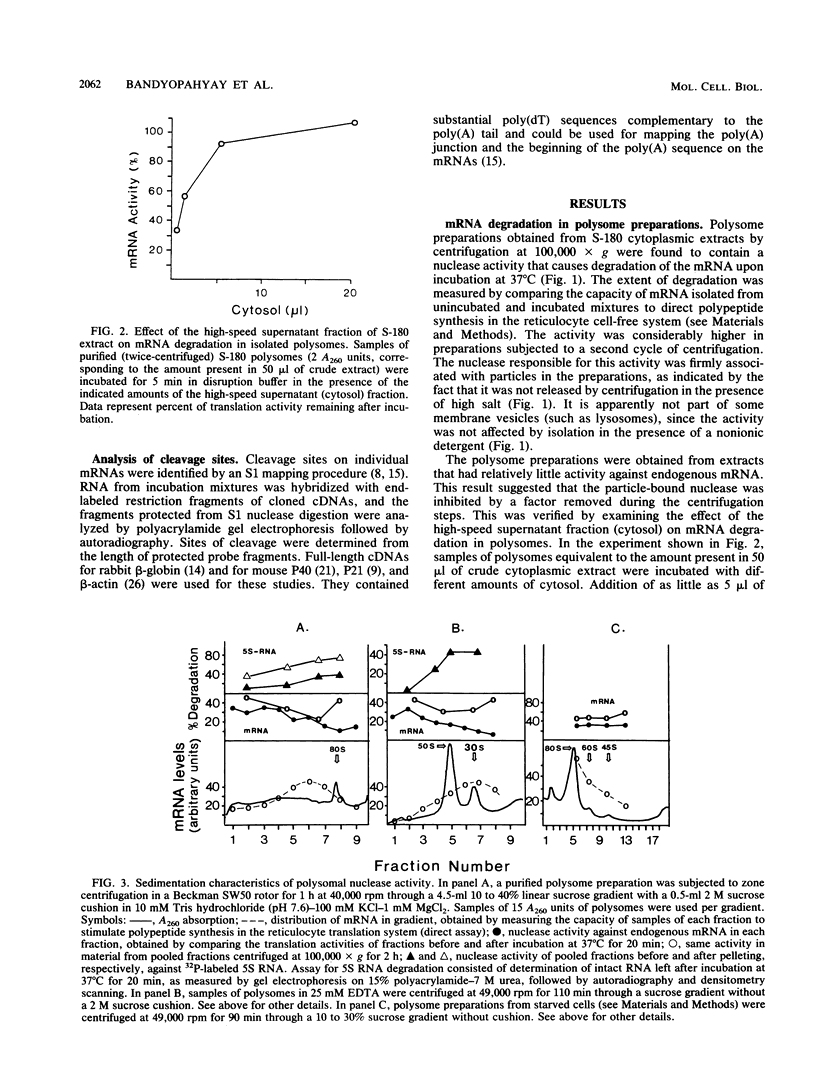
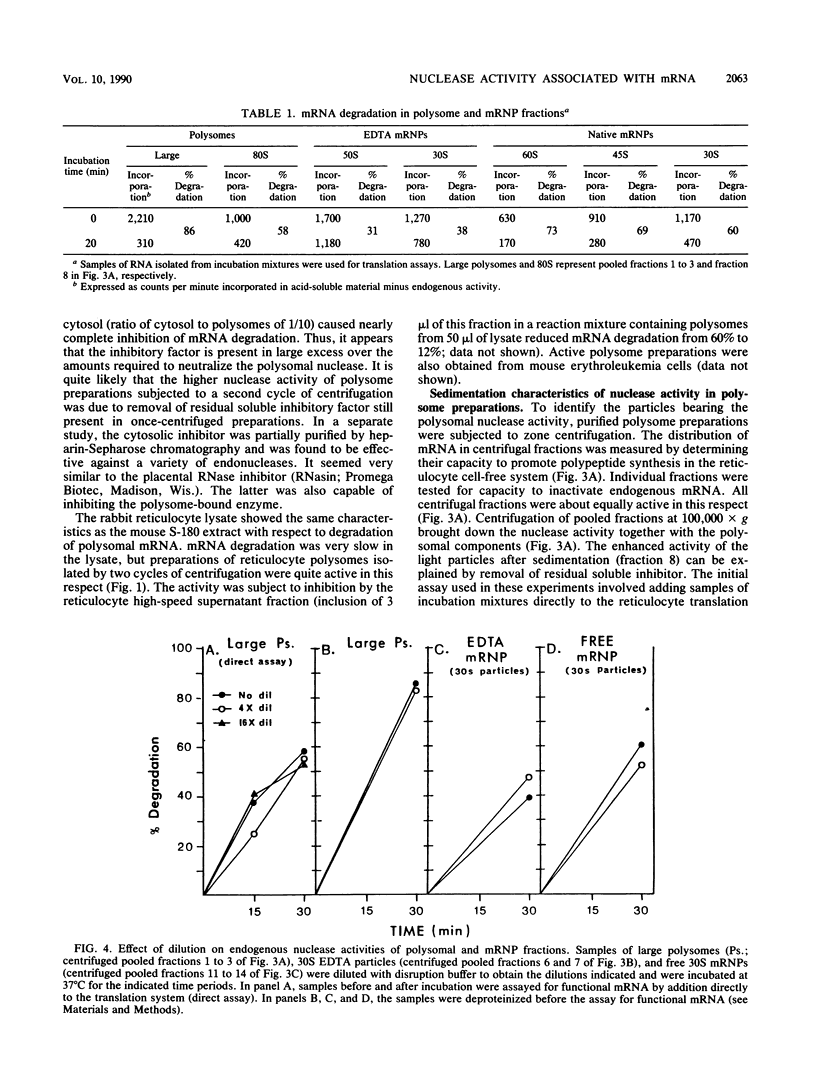
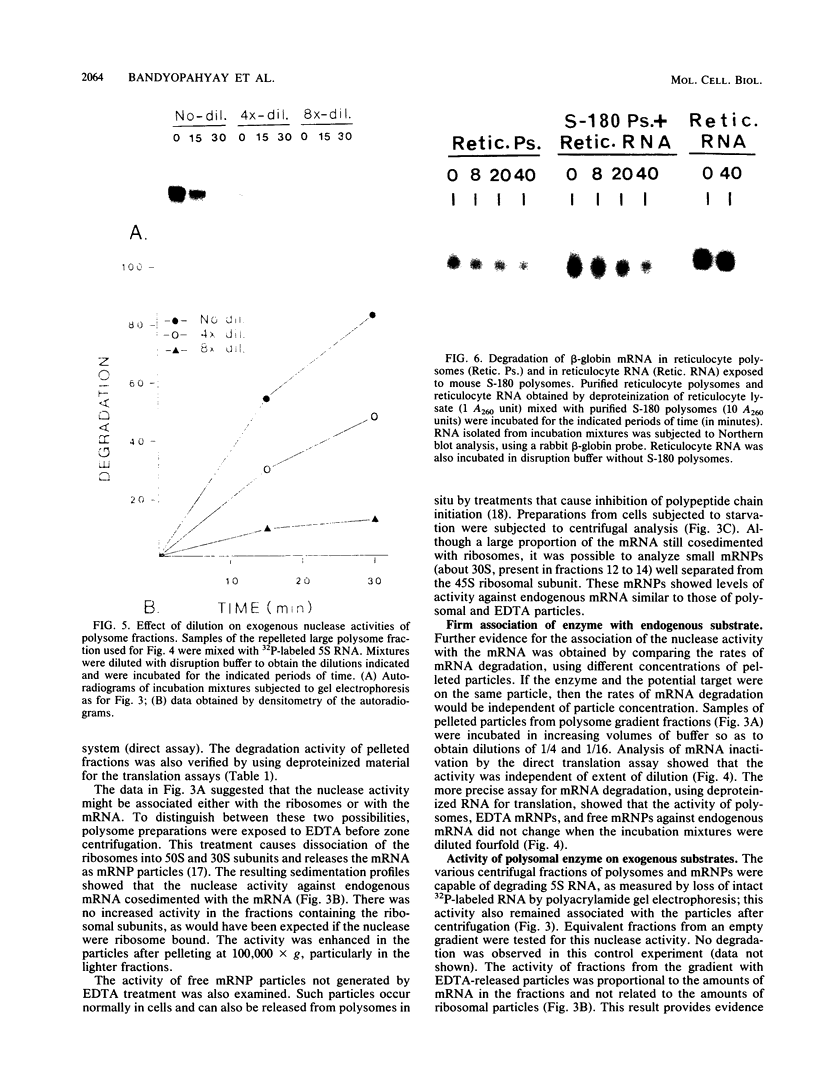
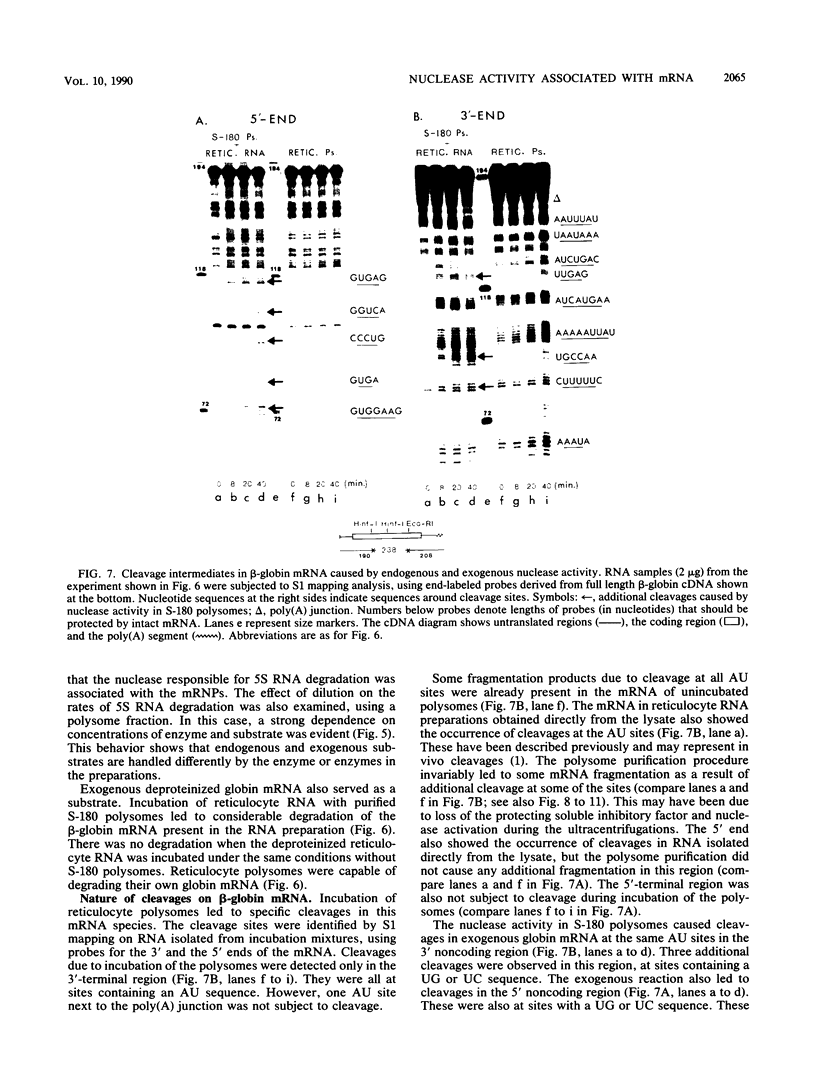
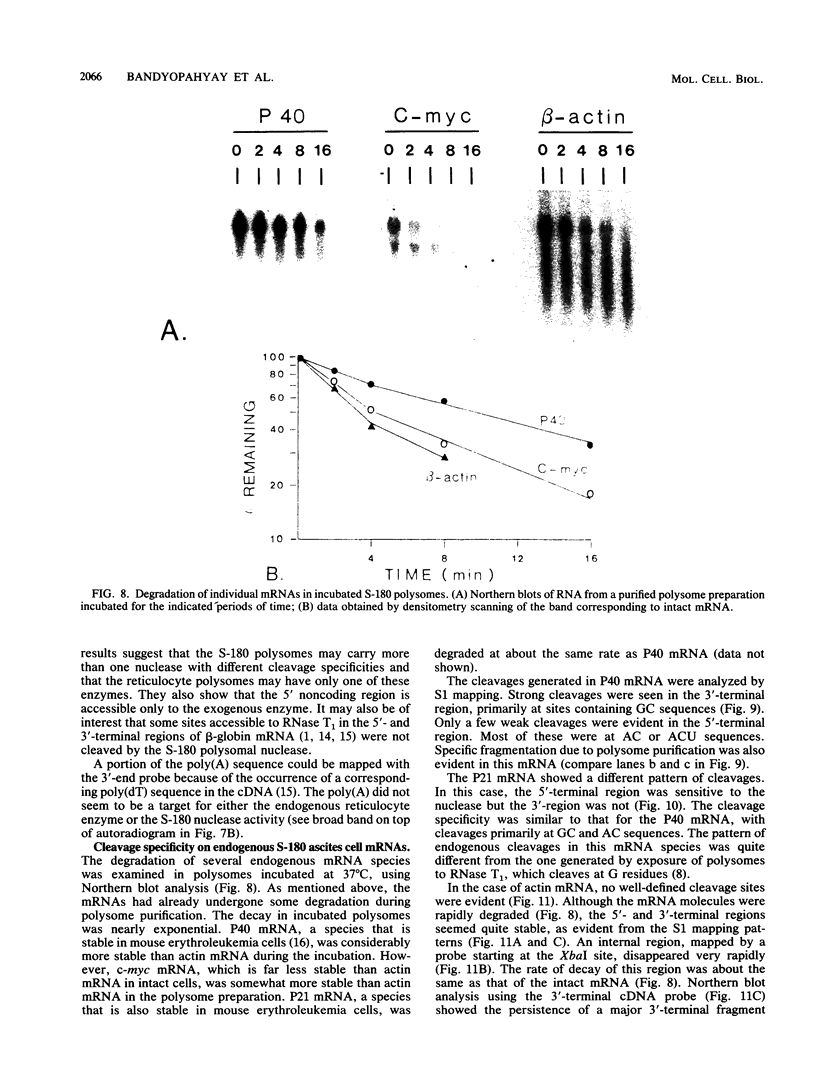
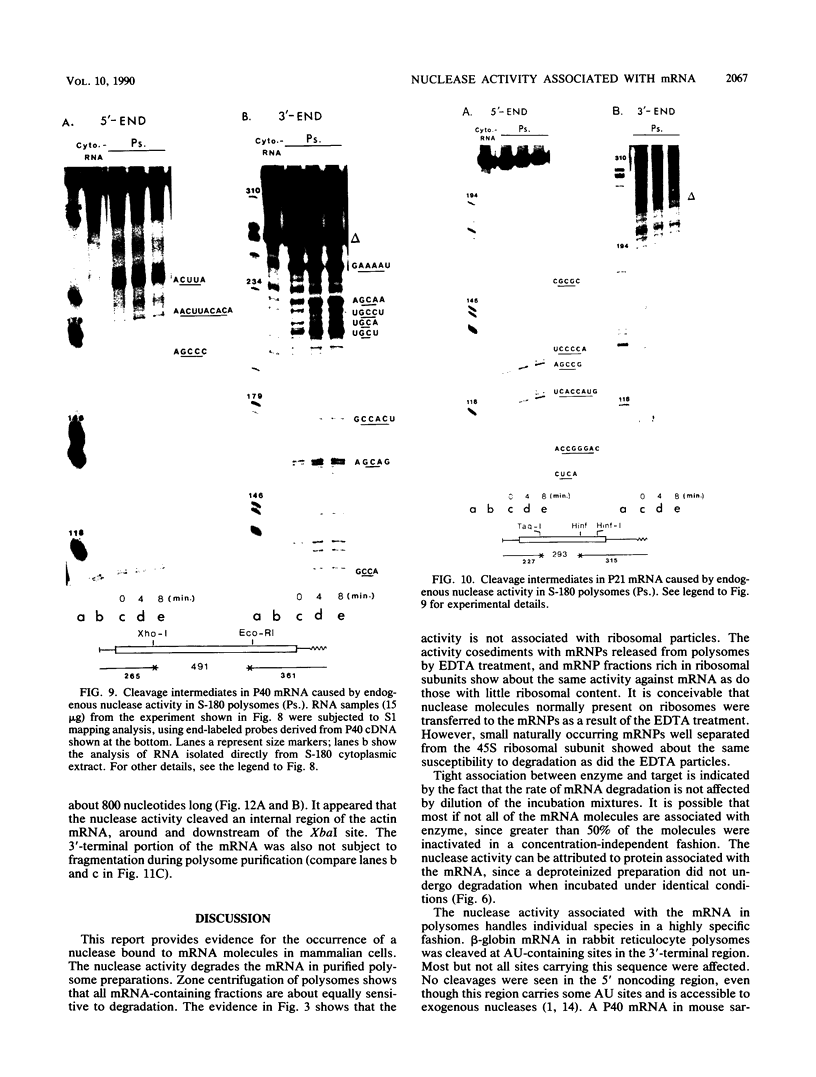
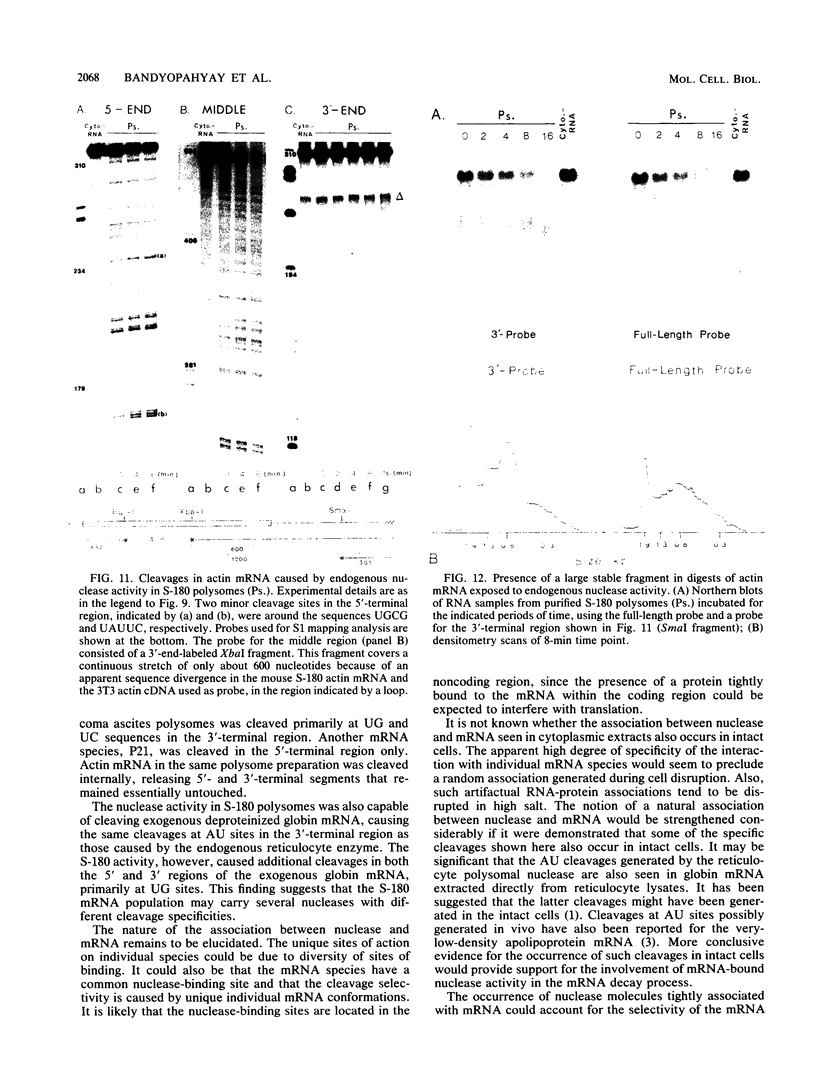
Polysome and messenger ribonucleoprotein (mRNP) preparations from various mammalian cells contain tightly bound nuclease activity that causes degradation of the mRNA in the preparations. This activity was found to cosediment with all polysome size classes as well as with free mRNPs and to remain associated with the mRNPs released from polysomes by treatment with EDTA. No association with ribosomal subunits was evident. The rates of mRNA degradation were not affected by serial dilution, an indication that enzyme and substrate are tightly associated. beta-Globin mRNA in purified reticulocyte polysomes was cleaved at AU sequences in the 3'-terminal region. Cleavages at the same sites occurred when deproteinized reticulocyte RNA was incubated with mouse sarcoma 180 (S-180) polysomes. The S-180 preparations caused additional cleavages, primarily at UG sequences. A P40 mRNA in S-180 polysomes was cleaved primarily in the 3' noncoding region, but the cleavages in a P21 mRNA were seen in the 5' noncoding region only. Actin mRNA was cleaved in an internal region, yielding large relatively stable 3'- and 5'-terminal fragments. These data suggest the occurrence of highly specific interactions between one or more mRNA-bound nucleases and individual mRNA species.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht G., Krowczynska A., Brawerman G. Configuration of beta-globin messenger RNA in rabbit reticulocytes. Identification of sites exposed to endogenous and exogenous nucleases. J Mol Biol. 1984 Oct 5;178(4):881–896. doi: 10.1016/0022-2836(84)90317-6. [DOI] [PubMed] [Google Scholar]
- Aviv H., Voloch Z., Bastos R., Levy S. Biosynthesis and stability of globin mRNA in cultured erythroleukemic Friend cells. Cell. 1976 Aug;8(4):495–503. doi: 10.1016/0092-8674(76)90217-8. [DOI] [PubMed] [Google Scholar]
- Bakker O., Arnberg A. C., Noteborn M. H., Winter A. J., Ab G. Turnover products of the apo very low density lipoprotein II messenger RNA from chicken liver. Nucleic Acids Res. 1988 Nov 11;16(21):10109–10118. doi: 10.1093/nar/16.21.10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock M. L., Shapiro D. J. Estrogen stabilizes vitellogenin mRNA against cytoplasmic degradation. Cell. 1983 Aug;34(1):207–214. doi: 10.1016/0092-8674(83)90151-4. [DOI] [PubMed] [Google Scholar]
- Casey J. L., Hentze M. W., Koeller D. M., Caughman S. W., Rouault T. A., Klausner R. D., Harford J. B. Iron-responsive elements: regulatory RNA sequences that control mRNA levels and translation. Science. 1988 May 13;240(4854):924–928. doi: 10.1126/science.2452485. [DOI] [PubMed] [Google Scholar]
- Cereghini S., Geoghegan T., Bergmann I., Brawerman G. Studies on the efficiency of translation and on the stability of actin messenger ribonucleic acid in mouse sarcoma ascites cells. Biochemistry. 1979 Jul 10;18(14):3153–3159. doi: 10.1021/bi00581a037. [DOI] [PubMed] [Google Scholar]
- Chitpatima S. T., Brawerman G. Shifts in configuration of the 5'-noncoding region of a mouse messenger RNA under translational control. J Biol Chem. 1988 May 25;263(15):7164–7169. [PubMed] [Google Scholar]
- Chitpatima S. T., Makrides S., Bandyopadhyay R., Brawerman G. Nucleotide sequence of a major messenger RNA for a 21 kilodalton polypeptide that is under translational control in mouse tumor cells. Nucleic Acids Res. 1988 Mar 25;16(5):2350–2350. doi: 10.1093/nar/16.5.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoghegan T. E., Sonenshein G. E., Brawerman G. Characteristics and polyadenylate content of the actin messenger RNA of mouse sarcoma-180 ascites cells. Biochemistry. 1978 Oct 3;17(20):4200–4207. doi: 10.1021/bi00613a014. [DOI] [PubMed] [Google Scholar]
- Graves R. A., Pandey N. B., Chodchoy N., Marzluff W. F. Translation is required for regulation of histone mRNA degradation. Cell. 1987 Feb 27;48(4):615–626. doi: 10.1016/0092-8674(87)90240-6. [DOI] [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Hereford L. M., Osley M. A., Ludwig T. R., 2nd, McLaughlin C. S. Cell-cycle regulation of yeast histone mRNA. Cell. 1981 May;24(2):367–375. doi: 10.1016/0092-8674(81)90326-3. [DOI] [PubMed] [Google Scholar]
- Krowczynska A., Brawerman G. Structural features in the 3'-terminal region of polyribosome-bound rabbit globin messenger RNAs. J Biol Chem. 1986 Jan 5;261(1):397–402. [PubMed] [Google Scholar]
- Krowczynska A., Brawerman G. Structural features of the 5' noncoding region of the rabbit globin messenger RNAs engaged in translation. Proc Natl Acad Sci U S A. 1986 Feb;83(4):902–906. doi: 10.1073/pnas.83.4.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krowczynska A., Yenofsky R., Brawerman G. Regulation of messenger RNA stability in mouse erythroleukemia cells. J Mol Biol. 1985 Jan 20;181(2):231–239. doi: 10.1016/0022-2836(85)90087-7. [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Brawerman G. Pulse-labeled ribonucleic acid complexes released by dissociation of rat liver polysomes. Biochemistry. 1971 Feb 2;10(3):510–516. doi: 10.1021/bi00779a025. [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Krsmanovic V., Brawerman G. Initiation of polysome formation in mouse sarcoma 180 ascites cells. Utilization of cytoplasmic messenger ribonucleic acid. Biochemistry. 1971 Mar 2;10(5):895–900. doi: 10.1021/bi00781a026. [DOI] [PubMed] [Google Scholar]
- Levine B. J., Chodchoy N., Marzluff W. F., Skoultchi A. I. Coupling of replication type histone mRNA levels to DNA synthesis requires the stem-loop sequence at the 3' end of the mRNA. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6189–6193. doi: 10.1073/pnas.84.17.6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenhaupt K., Lingrel J. B. A change in the stability of globin mRNA during the induction of murine erythroleukemia cells. Cell. 1978 Jun;14(2):337–344. doi: 10.1016/0092-8674(78)90119-8. [DOI] [PubMed] [Google Scholar]
- Makrides S., Chitpatima S. T., Bandyopadhyay R., Brawerman G. Nucleotide sequence for a major messenger RNA for a 40 kilodalton polypeptide that is under translational control in mouse tumor cells. Nucleic Acids Res. 1988 Mar 25;16(5):2349–2349. doi: 10.1093/nar/16.5.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müllner E. W., Kühn L. C. A stem-loop in the 3' untranslated region mediates iron-dependent regulation of transferrin receptor mRNA stability in the cytoplasm. Cell. 1988 Jun 3;53(5):815–825. doi: 10.1016/0092-8674(88)90098-0. [DOI] [PubMed] [Google Scholar]
- Ross J., Kobs G. H4 histone messenger RNA decay in cell-free extracts initiates at or near the 3' terminus and proceeds 3' to 5'. J Mol Biol. 1986 Apr 20;188(4):579–593. doi: 10.1016/s0022-2836(86)80008-0. [DOI] [PubMed] [Google Scholar]
- Ross J., Peltz S. W., Kobs G., Brewer G. Histone mRNA degradation in vivo: the first detectable step occurs at or near the 3' terminus. Mol Cell Biol. 1986 Dec;6(12):4362–4371. doi: 10.1128/mcb.6.12.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Tokunaga K., Taniguchi H., Yoda K., Shimizu M., Sakiyama S. Nucleotide sequence of a full-length cDNA for mouse cytoskeletal beta-actin mRNA. Nucleic Acids Res. 1986 Mar 25;14(6):2829–2829. doi: 10.1093/nar/14.6.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen T. J., Machlin P. S., Cleveland D. W. Autoregulated instability of beta-tubulin mRNAs by recognition of the nascent amino terminus of beta-tubulin. Nature. 1988 Aug 18;334(6183):580–585. doi: 10.1038/334580a0. [DOI] [PubMed] [Google Scholar]
- Yenofsky R., Bergmann I., Brawerman G. Messenger RNA species partially in a repressed state in mouse sarcoma ascites cells. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5876–5880. doi: 10.1073/pnas.79.19.5876. [DOI] [PMC free article] [PubMed] [Google Scholar]