Abstract
Circulating nitrate (NO3−), derived from dietary sources or endogenous nitric oxide production, is extracted from blood by the salivary glands, accumulates in saliva, and is then reduced to nitrite (NO2−) by the oral microflora. This process has historically been viewed as harmful, because nitrite can promote formation of potentially carcinogenic N-nitrosamines. More recent research, however, suggests that nitrite can also serve as a precursor for systemic generation of vasodilatory nitric oxide, and exogenous administration of nitrate reduces blood pressure in humans. However, whether oral nitrate-reducing bacteria participate in “setting” blood pressure is unknown. We investigated whether suppression of the oral microflora affects systemic nitrite levels and hence blood pressure in healthy individuals. We measured blood pressure (clinic, home, and 24-h ambulatory) in 19 healthy volunteers during an initial 7-day control period followed by a 7-day treatment period with a chlorhexidine-based antiseptic mouthwash. Oral nitrate-reducing capacity and nitrite levels were measured after each study period. Antiseptic mouthwash treatment reduced oral nitrite production by 90% (p < 0.001) and plasma nitrite levels by 25% (p = 0.001) compared to the control period. Systolic and diastolic blood pressure increased by 2–3 .5 mm Hg, increases correlated to a decrease in circulating nitrite concentrations (r2 = 0.56, p = 0.002). The blood pressure effect appeared within 1 day of disruption of the oral microflora and was sustained during the 7-day mouthwash intervention. These results suggest that the recycling of endogenous nitrate by oral bacteria plays an important role in determination of plasma nitrite levels and thereby in the physiological control of blood pressure.
Keywords: Blood pressure, Nitric oxide, Nitrite, Nitrate, Bacteria, Free radicals
Highlights
► Antiseptic mouthwash use abrogates oral bacterial conversion of nitrate to nitrite. ► Interruption of the enterosalivary circulation reduces plasma nitrite levels. ► The reduction in plasma nitrite levels is associated with elevations in blood pressure. ► The results indicate the importance of oral nitrate-reducing bacteria in blood pressure control. ► Our findings intimate adverse effects of mouthwash by disturbing NO/nitrite homeostasis.
The metabolic activity of oral bacteria has traditionally been associated with negative health effects ranging from halitosis and caries to more serious conditions such as periodontitis [1] and even cardiovascular disease [2,3]. This negative view is clearly reflected in the widespread use (30–45% of adults in the UK and USA) of antiseptic mouthwashes in the general population [4–6]. The conversion of dietary-derived inorganic nitrate (NO3−) to nitrite (NO2−) by oral bacteria [7] is a classical example of a proposed harmful process because salivary nitrite, when swallowed, can give rise to N-nitrosamines with potentially carcinogenic effects [8,9]. This has led to strict regulations of permissible levels of nitrate in food and drinking water. However, over the past decade the view of nitrate and nitrite as being only harmful has slowly started to shift.
A long-since established view has been that both nitrite and nitrate are generated endogenously as oxidation products of nitric oxide (NO) metabolism [10,11]: observations that underlie the dogma that these anions simply represent inert metabolites. However, more recent findings demonstrating significant vasodilator activity for nitrite in both isolated rat blood vessel studies [12] and consequently in healthy volunteers [13] have stimulated a reappraisal of the dogma. We now know that both nitrate and nitrite can be recycled back to NO under certain scenarios (particularly being favored by a hypoxic and/or acidic environment), thereby identifying a potential alternative to the l-arginine/NO synthase pathway for the in vivo generation of NO [14,15]. In support of the existence of a nitrate–nitrite –NO pathway, studies have demonstrated that inorganic nitrate or nitrite supplementation through either parenteral or oral administration exert functional effects that mimic NO bioactivity beyond vasodilatation [13,16,17], including protection against ischemia/reperfusion injury in animal models [18–21], improved mitochondrial function [22], and blood pressure (BP) reduction in humans [16,23–25].
The bioactivation of inorganic nitrate requires the formation of nitrite as an intermediate; a reaction that is facilitated by oral bacteria [26–29]. Ingested nitrate is rapidly absorbed and accumulates selectively in saliva [7,8,30] via active transport from blood to the salivary glands [31]. The nitrite formed in the oral cavity is swallowed and is consequently associated with elevations of circulating nitrite levels [24,25,32,33]. Exactly how nitrite, as a charged anion, might cross the gut wall into the circulation is unknown, although there have been suggestions that at least in erythrocytes the anion-exchange transporter-1 might have a role to play [34,35]. Alternatively, nitrite may be absorbed in the form of uncharged HNO2 after protonation in the acidic stomach. Nevertheless, several different groups have demonstrated that after elevation of nitrite levels within the blood and tissues, this nitrite can then undergo further reduction to NO and other bioactive nitrogen oxides, a reaction facilitated by one or more possible enzymatic and nonenzymatic pathways (for review see [36,37]). Because it is now evident that oral bacteria are crucial in the initial step of a nitrate–nitrite –NO pathway, it is important to clarify their role in physiological processes related to NO bioactivity. In this study we investigated whether reduction of endogenously generated nitrate to nitrite by oral bacteria under basal conditions (i.e., without exogenous provision of inorganic nitrate) contributes to circulating nitrite levels and BP regulation in healthy subjects. For this, we used an antiseptic mouthwash to disrupt oral nitrate reduction and measured plasma nitrite concentration and BP over a 1-week observation period.
Methods
Volunteers
The studies were peer reviewed by the institutional review board and were granted full ethics approval by the Local Research Ethics Committee. Informed, written consent was taken after satisfying the inclusion criteria of 18–45 years of age, body mass index of 18–40 kg/m2, no systemic medication (other than the oral contraceptive pill), nonsmoker, no self-reported use of mouthwash or tongue scrapes, no recent or current antibiotic use (within 3 months), and no history, or recent treatment, of (within past 3 months) any oral condition (excluding caries), including gingivitis, periodontitis, and halitosis. Volunteers were recruited with stratification for sex according to the power calculation performed before the start of the study.
Study design
A crossover study was performed in 19 healthy nonsmoking volunteers, who reported no prior oral mouthwash use. For all clinic visits individuals were requested to arrive fasted for 12 h and having adhered to a low-nitrate diet for the previous 24 h to study the effect of endogenously derived nitrate only. To support volunteers in adherence to this request a dietary sheet identifying low-nitrate foods and options for low-nitrate-containing meals was provided. For each volunteer repeat visits were scheduled at the same time of day as the first visit. Measurements were taken on each visit in the same order of clinic BP measurement, followed by collection of blood, urine, and whole saliva samples. Oral nitrate reduction capacity was examined (see below) and then a 24-h ambulatory BP monitor (ABPM) attached, to be returned the following day. At the end of the first visit volunteers were then instructed to measure their own BP on a daily basis, at the same time each day, while at home (home BP), for 1 week. Volunteers were instructed to make no other changes to their lifestyle or diet and to record their daily nutritional intake using self-reported dietary diaries. On day 7 after measurement of their home BP reading, volunteers were instructed to rinse their oral cavities with 10 ml Corsodyl (0.2% chlorhexidine, GlaxoSmithKline, UK) antiseptic mouthwash twice daily [38] and to continue monitoring home BP on a daily basis for a further 1 week.
After this entire 2-week period of home BP monitoring, volunteers returned for a repeat assessment of clinic BP measurement and blood, urine, and whole saliva sample collection and oral nitrate reduction. A further 24-h ABPM was attached and returned the following day. The volunteers were unaware of the hypothesis of the study.
BP measurements
Clinic BP
BP was measured using an Omron 715IT (Omron Corp., Tokyo, Japan) according to British Hypertension Society guidelines [39]. Seated BP readings were taken in triplicate every 15 min for 1 h using a validated semiautomated oscillometric machine (Omron 715IT; Omron Corp.). The second and third readings at each time point were averaged to determine mean clinic BP and heart rate (HR) and then these readings over the 1-h period were averaged to provide a clinic BP and HR reading. Both the operator and the volunteer were blind to these measurements.
Home BP
Volunteers were instructed during screening on the correct use of the Omron 715IT machine and were provided with a machine and appropriately sized cuff to perform home measurements in triplicate at the same time daily. The second and third readings at each time point were averaged to determine the daily mean home BP and HR.
ABPM
Twenty-four-hour ABPM was performed using a Spacelabs 90207 machine (Spacelabs Healthcare Ltd., Issaquah, WA, USA). The following protocol for measurements was used: 0700–2300 hours—one reading every 20 min; 2300–0700 hours—one reading every 1 h. Proprietary software was used to download readings and produce 24-h, daytime (0700–2300 hours) and nighttime (2300–0700 hours) mean BP and HR readings (90256 ABP Report Management System; Spacelabs Healthcare).
Blood sampling
Blood samples were taken directly after clinic BP measurement via standard venipuncture into prechilled lithium–heparin Vacutainers as previously described [24]. Plasma was separated and deproteinated by filtration (centrifugation 14,000 g, 4 °C, 60 min) using Vivaspin 500, 3-kDa filters (Sartorius Biotech, Aubagne, France) and the filtrate stored at −80 °C until analysis by ozone chemiluminescence. In addition, we determined the levels of nitrate and nitrite contamination as a consequence of use of the commercially available blood tubes and ascertained a nitrite contamination of 102.0±10.3 nmol/L and nitrate contamination of 1.7±0.1 μmol/L (n = 20 tubes). These levels of contamination were subtracted from all plasma measures of nitrite and nitrate concentration, respectively.
Urine sampling
Midstream urine samples were collected into sterile containers and an aliquot was stored at −80 °C until analysis at a later date for assessment of nitrate and nitrite concentration by ozone chemiluminescence.
Saliva sampling
Unstimulated, whole saliva samples were collected into sterile Eppendorfs and stored at −80 °C until analysis at a later date for assessment of nitrate and nitrite concentration by ozone chemiluminescence.
Oral nitrate-reducing capacity
Volunteers were instructed to hold 10 ml of nitrate- and nitrite-free water for 5 min in the oral cavity after which time the mouth rinse was collected into sterile ice-chilled Falcon tubes and centrifuged (5500 g, 4 °C, 10 min) and the supernatants were collected and stored at −80 °C until nitrite and nitrate determination by ozone chemiluminescence. After this, matched volumes of potassium nitrate (Martindale Pharmaceuticals, Ipswich, UK) solutions of 800 μmol/L (delivering 0.8 μmol in total), reflecting approximate near-physiological salivary nitrate levels, and 8 mmol/L (delivering 80 μmol in total), reflecting a 10-fold higher salivary nitrate level that corresponds to levels achieved after a nitrate-rich meal, were held in the oral cavity for 5 min in a randomized order. The mouth rinses were collected and stored as above.
Ozone chemiluminescence
Levels of nitrate and nitrite in deproteinated plasma and in urine and saliva samples were measured using ozone chemiluminescence as previously described [10]. Because all plasma samples were filtered using 3-kDa cutoff filters any potential other NO adducts that might interfere with the assessment of nitrite concentrations were absent for analysis. In brief, total nitrate and nitrite concentration (termed “NOx”) was determined by adding samples to 0.1 mol/L vanadium(III) chloride in 1 M hydrochloric acid refluxing at 95 °C under nitrogen. Nitrite concentration was determined by addition of samples to 0.09 mol/L potassium iodide in glacial acetic acid under nitrogen at room temperature. Nitrate concentration was calculated by subtraction of [nitrite] from [NOx]. All measurements of all samples were conducted by an individual blinded to the intervention.
Statistical analysis
All data are expressed as means ± SEM, unless otherwise specified, and all statistical analyses were performed using GraphPad Prism software version 5. For comparison of before and after BP measurements; salivary, urinary, and plasma nitrite and nitrate concentrations; and food group intake, paired Student’s t tests were used. For oral nitrate reduction and repeated daily home BP measurements the data were analyzed by repeated-measures two-way ANOVA. Correlations between plasma nitrite concentration and BP were determined using Pearson’s correlation coefficient analysis. The level for statistical significance was taken as α=0.05 for all analyses.
Results
The baseline demographic details are shown in Table 1. All subjects recorded their dietary habits during the two study periods. Examination of these data revealed no major variations in diet during the study periods. Estimated daily portion intake of fruit, high-nitrate-containing vegetables, low-nitrate-containing vegetables, and processed meat were determined from dietary diaries and did not differ between the two study periods (Table 2). Importantly baseline plasma nitrite concentration in these individuals was correlated with salivary nitrite levels (Supplementary Fig. S1A).
Table 1.
Baseline characteristics.
| Baseline characteristic | |
|---|---|
| Subjects (n) | 19 |
| Age (years) | 23.8±0.5 |
| Body mass index (kg/m2) | 23.0±0.6 |
| Clinic SBP (mm Hg) | 110.4±1.8 |
| Clinic DBP (mm Hg) | 66.2±1.6 |
| Home SBP (mm Hg) | 115.3±1.7 |
| Home DBP (mm Hg) | 67.3±1.1 |
| Ambulatory SBP (mm Hg) | 119.2±1.6 |
| Ambulatory DBP (mm Hg) | 70.3±1.3 |
| Plasma nitrate (μmol/L) | 26.7±2.4 |
| Plasma nitrite (nmol/L) | 284.2±16.9 |
| Salivary nitrate (μmol/L) | 441.8±68.6 |
| Salivary nitrite (μmol/L) | 316.1±30.7 |
| Urinary nitrate (μmol/L) | 1462.0±64.9 |
| Urinary nitrite (nmol/L) | 203.1±8.8 |
Baseline anthropometric and hemodynamic characteristics and baseline plasma, urinary, and salivary [nitrate]/[nitrite]. Data are expressed as means ± SEM.
Table 2.
Self-reported daily dietary intake.
| Food group | Mean portions/day |
Significance (p) | |
| Baseline | Post-mouthwash | ||
| Fruit | 0.5±0.2 | 0.5±0.2 | 0.205 |
| Low-nitrate vegetables | 0.6±0.1 | 0.6±0.1 | 0.928 |
| High-nitrate vegetables | 0.2±0.1 | 0.2±0.1 | 0.245 |
| Processed meat | 0.7±0.1 | 0.7±0.1 | 0.793 |
Self-reported daily dietary intake of specific food groups in the 7-day study periods. Data are expressed as means ± SEM (n = 19). Significance shown in last column for paired Student’s t test.
Effects of an antiseptic mouthwash on nitrite levels in saliva and plasma and oral nitrate-reducing capacity
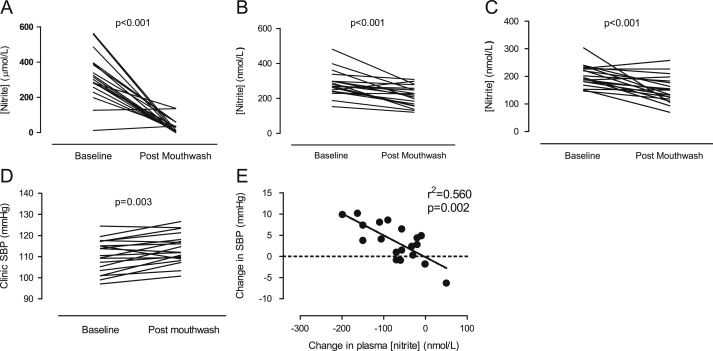
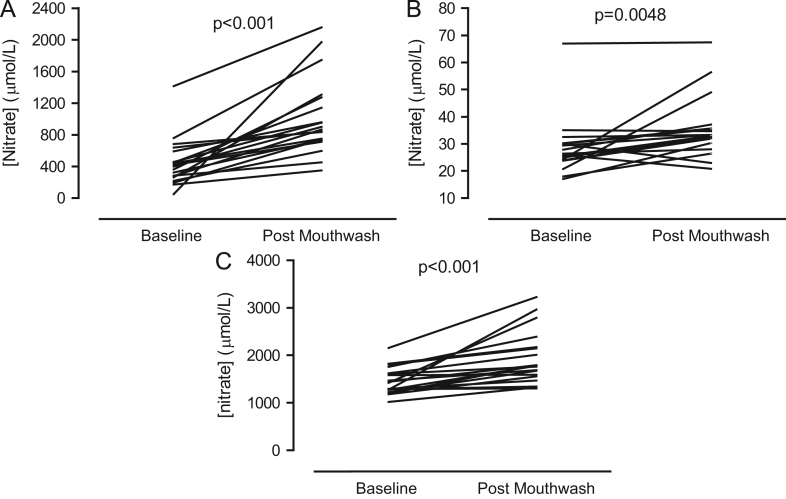
After 7 days of use of a twice-daily antiseptic mouthwash salivary, plasma, and urinary nitrite levels were significantly attenuated (Figs. 1A–C ). Salivary nitrite concentration was reduced by ∼90% (Δ 282 ± 35 μmol/L, p < 0.001) and plasma nitrite concentration by ∼25% (Δ 71±15 nmol/L, p = 0.001). In contrast, salivary, plasma, and urinary nitrate concentrations were significantly elevated after mouthwash treatment compared to baseline levels (Figs. 2A–C ).
Fig. 1.
Effects of a 7-day twice-daily use of antiseptic mouthwash on (A) salivary nitrite concentration, (B) plasma nitrite concentration, (C) urinary nitrite concentration, (D) clinic systolic blood pressure, and (E) the relationship between plasma nitrite concentration and clinic systolic blood pressure. Statistical significance was determined using paired Student t test for n = 19. Correlations were determined using Pearson’s correlation coefficient determination.
Fig. 2.
Effects of a 7-day, twice-daily use of antiseptic mouthwash on (A) salivary nitrate concentration, (B) plasma nitrate concentration, and (C) urinary nitrate concentration in healthy volunteers. Statistical significance was determined using paired Student t test for n = 19.
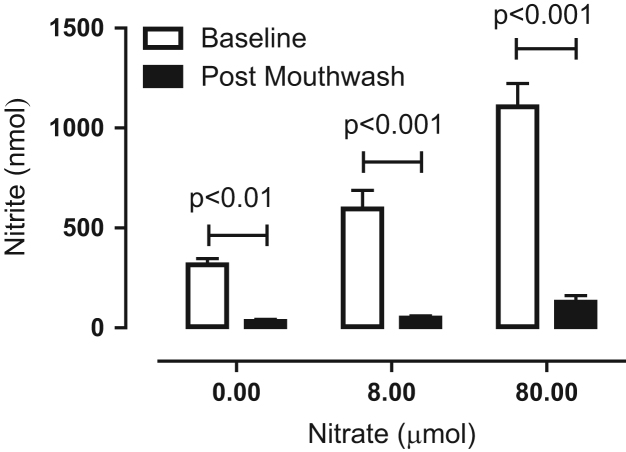
Before mouthwash treatment oral nitrate-reducing capacity was found to be dose-dependent (Fig. 3), with a calculated maximal rate of reduction after administration of 0.8 μmol of KNO3 of 119±19 nmol/min and after 80 μmol 221±23 nmol/min. After mouthwash, oral nitrate-reducing capacity was nearly abolished (∼90% reduction, p < 0.001 for both nitrate doses compared to pretreatment rates, Fig. 3).
Fig. 3.
Effects of a 7-day, twice-daily use of antiseptic mouthwash on oral nitrate-reducing capacity. The amount of nitrite in the expelled contents of the oral cavity, after instillation of 10 ml of 0, 800 μmol/L, or 8 mmol/L KNO3 solution delivering 0, 0.8, or 80 μmol of nitrate, respectively, which was held in the mouth for 5 min, of healthy volunteers was measured. Data are expressed as means ± SEM (n = 19) and statistical significance was determined using repeated-measures two-way ANOVA followed by Bonferroni posttests.
Antiseptic mouthwash and BP
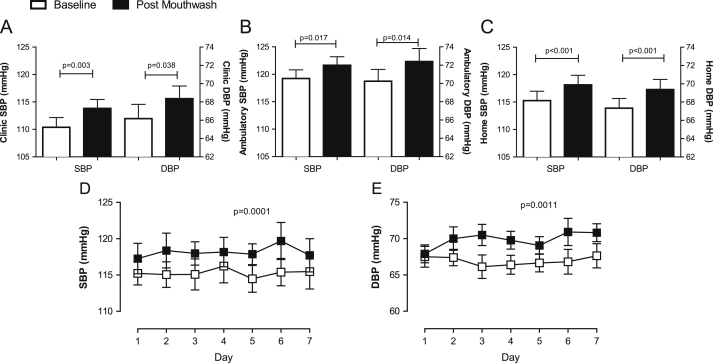
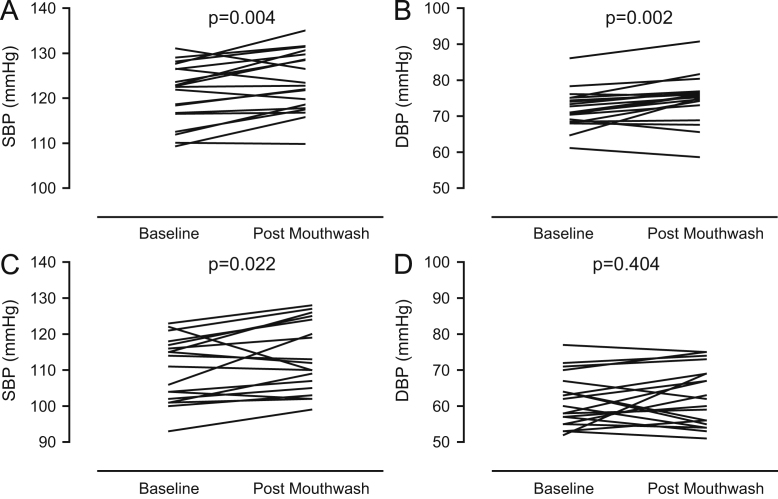
Seven days’ use of antiseptic mouthwash caused a rise in clinic systolic (SBP) and diastolic BP (DBP) (Figs. 1D and 4A) that was associated with a significant decrease in plasma nitrite concentration (r2 = 0.56, p = 0.002, Fig. 1E), and a similar trend for association between SBP and salivary nitrite concentration was evident, although this did not reach statistical significance (r2 = 0.203, p = 0.052, Supplementary Fig. S1B). The changes in BP were similar irrespective of the method of measurement, i.e., clinic BP (ΔSBP 3.5±1.0 mm Hg, p = 0.003; ΔDBP 2.2±1.0 mm Hg, p = 0.038), home (ΔSBP 2.9 ± 0.4 mm Hg, p < 0.001; ΔDBP 2.0 ± 0.5 mm Hg, p < 0.001), and ambulatory (ΔSBP 2.4 ± 0.9 mm Hg, p = 0.017; ΔDBP 2.2 ± 0.8 mm Hg, p = 0.014l Figs. 4A–4C). There were no changes in HR as estimated using all three distinct techniques: clinic ΔHR 2.0 ± 1.7 bpm, p = 0.25; home ΔHR 1.3 ± 1.0 bpm, p = 0.18; and ambulatory ΔHR 2.0 ± 1.4 bpm, p = 0.17 (data not shown).
Fig. 4.
Effects of a 7-day, twice-daily use of antiseptic mouthwash on systolic and diastolic blood pressure in healthy volunteers measured (A) in the clinic, (B) by 24-h ambulatory monitoring, and (C) at home and the daily profiles of home (D) systolic and (E) diastolic blood pressure during the 7-day study periods in healthy volunteers. Data are expressed as means ± SEM (n = 19) and statistical significance was determined using repeated-measures two-way ANOVA.
Home BP measurement over the course of the first week did not change significantly from day to day (Figs. 4D and E). However, after initiation of a single day’s use of mouthwash, both home SBP and home DBP were raised compared to before mouthwash use (Figs. 4D and 4E).
Separation of ABPM data into daytime and nighttime means demonstrates that daytime ambulatory SBP and DBP were increased post-mouthwash (ΔSBP 2.9 ± 0.9 mm Hg, p = 0.004, and ΔDBP 3.0 ± 0.8 mm Hg, p = 0.002, respectively, Figs. 5A and B), whereas mouthwash increased only nighttime SBP (ΔSBP 2.2 ± 0.9, p = 0.022) and not DBP (Figs. 5C and D).
Fig. 5.
Effects of a 7-day, twice-daily use of antiseptic mouthwash on 24-h ambulatory blood pressure, separated into daytime (A) systolic and (B) diastolic blood pressure and nighttime (C) systolic and (D) diastolic blood pressure. Data are expressed as means ± SEM (n = 19) and statistical significance was determined using paired Student t test.
Discussion
The colonization by micro-organisms of most surfaces of the human body, including the gut, provides us with a number of beneficial functions that we do not possess innately. For example, the symbiotic relationship that humans have with gut microflora endows the host with a number of qualities, not least the capacity to digest components of plants, host defense, and the development of tolerance to a number of pathogens [40]. Whereas a symbiotic relationship has been demonstrated for a number of host–microbial interactions in the lower gastrointestinal tract, the oral microflora remains virtually unexplored in this aspect. In fact, the general view is of an overall detrimental role for this microflora [1–3]. In contrast, our results presented herein support the notion that oral nitrate-reducing bacteria are beneficial to the host and participate in the control of cardiovascular NO homeostasis to modulate BP.
We have shown that twice-daily use of an antiseptic mouthwash for 1 week nearly abolished oral conversion of nitrate to nitrite. More importantly, this was accompanied by a decrease in plasma nitrite concentration (∼25%) and a concomitant increase in BP. Furthermore, as evidenced by the home BP measurements, the persistence of the effect of mouthwash on BP after the first day of instigation suggests that there is no immediate tachyphylaxis, at least over a 1-week period, in response to the mouthwash. Our data demonstrating a robust correlation between changes in plasma nitrite levels and changes in BP support our contention that prevention of the conversion of nitrate to nitrite by antiseptic mouthwash underlies the increases in BP. This thesis is further supported by evidence of the trends toward significant associations between changes in salivary nitrite concentration and change in SBP, although a prospective study designed to directly test this relationship is warranted. It is unlikely that these effects relate to other mechanisms, such as increased stress due to instillation of mouthwash, because the effects on systolic BP were present on every occasion throughout the 7-day home BP measurement protocol and also evident in the nighttime SBP.
All volunteers were fasted on clinic visits and had been on a low-nitrate-containing diet for the preceding 24 h; therefore, the majority of the circulating nitrate measured in their plasma originates from the endogenous source of the NO synthase pathway. The nitrate levels that we measured in our volunteers at baseline correlate well with previous estimates of the relative contributions of exogenous vs endogenous nitrate [41]. In light of this, the present data suggest the existence of a salvage pathway in which nitrate, derived from oxidized NO, is partly recycled back to NO with the help of oral nitrate-reducing bacteria. The significant correlation between baseline plasma nitrite levels and salivary nitrite levels supports the notion of a dependence of circulating levels of nitrite on the enterosalivary circuit. Our observations represent the first demonstration of the functional activity of endogenously generated nitrate. Nitrate is a very stable molecule and, unlike bacteria, mammalian cells cannot efficiently metabolize this anion. This fact together with our observations suggests that the process we describe herein fulfills the criteria for a true symbiotic relationship whereby the host provides the oral bacteria with nitrate via active secretion to the site where they reside. This nitrate is then used by the bacteria as a terminal electron acceptor to allow respiration in the absence of oxygen. In return, the bacteria provide the host with nitrite, a substrate used for further generation of the biological messenger NO [42].
That NO is the likely mediator of the effects seen is supported by previous observations demonstrating that the BP-reducing effects of exogenously administered inorganic nitrate are correlated with levels of the signaling nucleotide cyclic guanosine monophosphate [25], measurement of which has been recently shown to provide an “exquisitely” sensitive indicator of NO bioactivity [43] and to underlie many of the beneficial effects of NO.
What is still unclear is exactly how NO-like bioactivity is obtained from the nitrite formed and where this occurs. A number of potential pathways for nitrite reduction to NO within the blood and tissues have been described in preclinical models. However, to date, confirmation of many of these pathways in humans is limited. Previous studies in healthy volunteers support a role for deoxygenated hemoglobin in red blood cells as the mammalian nitrite reductase [13] and studies with human blood vessels ex vivo implicate xanthine oxidoreductase [44], although in these studies the blood vessels used were excess segments of left internal mammary artery removed for coronary artery bypass grafting and therefore may represent a pathophysiological scenario. It is also possible that the effects seen may relate to the activity of newly formed S-nitrosothiols in blood and tissue: compounds known to be potent transducers of NO bioactivity and of particular relevance to vasodilatation [45]. Indeed, interactions between heme-containing proteins, such as hemoglobin, and nitrite have been shown to promote the formation of S-nitrosothiols [45]. In this study we did not measure any NO species other than nitrite and nitrate, as we utilized low-molecular-weight cutoff filtration to enable specific estimation of plasma nitrite levels. However, previous observations have demonstrated that in healthy volunteers subject to exogenous oral nitrate treatment there were no measureable changes in S-nitrosothiol levels, whereas plasma nitrite levels were elevated four- to five-fold [32].
The increase in BP was reflected in all three of the methods that we employed to accurately measure BP. Indeed, home BP and 24-h ambulatory BP are being increasingly recognized as important BP measures for diagnosing and monitoring BP. Although the increases in BP may seem small (∼ΔSBP 2–3 .5 mm Hg), it is estimated each 2 mm Hg increase in SBP increases mortality due to ischemic heart disease and stroke by 7 and 10%, respectively [46]. Our study also suggests that oral nitrate reduction may play an important role in decreasing cardiovascular disease morbidity and mortality and intimates possible adverse cardiovascular effects of antiseptic mouthwash use in healthy individuals. This may have population-level significance, because it is estimated that 30–45 % of U.K. and U.S. adults regularly use antiseptic mouthwash [4–6].
This view contrasts with the clear association of periodontal infection with cardiovascular diseases [2,3] and the vascular benefits of periodontitis treatment [47], but this may reflect different health status and pathogenic bacterial colonization or possible translocation of bacteria. Whether a similar pathway operates in individuals with hypertension or in individuals with increased risk of developing clinical hypertension (i.e., prehypertension), or indeed whether dysfunction of this pathway might contribute to cardiovascular disease progression, is unknown, although recent evidence clearly demonstrates the presence of nitrate-reducing bacterial species, including Veilonella spp. and Pseudomonas luteola, in the oral cavity of individuals with atherosclerotic disease [48]. However, this possibility will be addressed, at least in part, by clinical trials that are currently under way to test whether inorganic nitrate supplementation in hypertensive populations might lower BP in a way similar to that demonstrated to occur in healthy individuals.
It is important to appreciate the limitations of this work. In this study the application of the intervention was not randomized and not placebo controlled. The former was because of uncertainty regarding the time taken for the recovery of oral bacterial function after mouthwash use. However, baseline BP was measured at home daily for 1 week and remained unchanged throughout this period and the rise in SBP was evident in nighttime ABPM measurements; this suggests that the increase in BP after the first day of mouthwash use was not due simply to an order effect. With respect to the second issue, in this study, we used a particular type of mouthwash that contains chlorhexidine as the active ingredient. Chlorhexidine has a very distinctive taste, complicating development of a “true” placebo. Further studies utilizing an appropriate placebo control would be of value. In addition, determination of whether other antiseptic mouthwash products would affect nitrite and NO homeostasis in a similar manner is uncertain. It is also possible that interfering with the oral bacteria resulted in changes in pathways independent of the nitrate-reductive pathway that might underlie the changes in BP. However, given the wealth of data demonstrating that elevations in plasma nitrite levels through exogenous provision of nitrite, or by supplementation with oral nitrate, are associated with reductions in BP [13,16,23–25], and the strong correlations evident in this study, it is likely that the resulting alterations in nitrite levels are responsible for the physiological results demonstrated herein. Finally our study included 19 young healthy volunteers based upon power calculations to determine the n value required to observe significant changes in plasma nitrite concentrations. Clearly larger scale clinical investigations are warranted to assess the general applicability of our findings across the wider population.
Conclusions
Our studies provide evidence of a role for oral nitrate-reducing bacteria in modulation of vascular nitrite and NO homeostasis and, thereby, a physiological role for nitrite, derived from the oral reduction of endogenously generated nitrate, in BP regulation. Our data further suggest that disturbances in nitrite homeostasis (herein achieved via interruption of nitrate reduction in the oral cavity by commercial mouthwash use) have small, yet potentially important, implications for cardiovascular health. Apart from vasodilator actions, nitrite-derived NO has a number of other potentially beneficial effects in humans, including inhibition of platelet aggregation, preservation of endothelial function, and improvement of mitochondrial efficiency [22,24]. Future studies to evaluate the importance of the enterosalivary circulation of nitrate to nitrite will reveal if the disturbances in circulating nitrite homeostasis might affect these processes too, especially in patient groups at higher overall cardiovascular risk. The recent discovery, using second-generation sequencing technology, that subtle regional differences in both species and number of oral microflora exist between populations [49] further support the possibility that differences in nitrate-reducing oral bacteria may play an important role in regional differences in physiological processes, including BP.
Author contributions
V.K. and A.A. designed the research. V.K., S.M.A.H., and V.P. performed the research. V.K., J.O.L., E.W., and A.A. analyzed data. V.K., J.O.L., E.W., and A.A. wrote the manuscript.
Acknowledgments
This work forms part of the research themes contributing to the translational research portfolio of the National Institute for Health Research Cardiovascular Biomedical Research Unit at Barts and the London School of Medicine and Dentistry. V.K., V.P., and this work are supported by the British Heart Foundation. J.O.L. and E.W. have received grants from the Swedish Heart–Lung Foundation, Torsten and Ragnar Söderberg Foundation, Vinnova (CIDaT), and EU’s 6th Framework Program (Flaviola). A.A., J.O.L., and E.W. are all directors of Heartbeet Ltd.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.freeradbiomed.2012.11.013.
Appendix A. Supplementary materials
Supplementary Material
References
- 1.Ratcliff P.A., Johnson P.W. The relationship between oral malodor, gingivitis, and periodontitis: a review. J. Periodontol. 1999;70:485–489. doi: 10.1902/jop.1999.70.5.485. [DOI] [PubMed] [Google Scholar]
- 2.Desvarieux M., Demmer R.T., Rundek T., Boden-Albala B., Jacobs D.R., Papapanou P.N., Sacco R.L. Relationship between periodontal disease, tooth loss, and carotid artery plaque: the Oral Infections and Vascular Disease Epidemiology Study (INVEST) Stroke. 2003;34:2120–2125. doi: 10.1161/01.STR.0000085086.50957.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desvarieux M., Demmer R.T., Rundek T., Boden-Albala B., Jacobs D.R., Sacco R.L., Papapanou P.N. Periodontal microbiota and carotid intima-media thickness: the Oral Infections and Vascular Disease Epidemiology Study (INVEST) Circulation. 2005;111:576–582. doi: 10.1161/01.CIR.0000154582.37101.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elmore J.G., Horwitz R.I. Oral cancer and mouthwash use: evaluation of the epidemiologic evidence. Otolaryngol. Head Neck Surg. 1995;113:253–261. doi: 10.1016/S0194-5998(95)70114-1. [DOI] [PubMed] [Google Scholar]
- 5.Fedorowicz, Z.; Aljufairi, H.; Nasser, M.; Outhouse, T.L.; Pedrazzi, V. Mouthrinses for the treatment of halitosis. Cochrane Database Syst. Rev. CD006701; 2008. [DOI] [PubMed]
- 6.Chadwick B., White D., Lader D., Pitts N. Preventative behaviour and risks to oral health. In: O'Sullivan I., editor. Adult Dental Health Survey 2009. Health and Social Care Information Centre; Leeds: 2011. pp. 1–44. [Google Scholar]
- 7.Tannenbaum S.R., Weisman M., Fett D. The effect of nitrate intake on nitrite formation in human saliva. Food Cosmet. Toxicol. 1976;14:549–552. doi: 10.1016/s0015-6264(76)80006-5. [DOI] [PubMed] [Google Scholar]
- 8.Spiegelhalder B., Eisenbrand G., Preussmann R. Influence of dietary nitrate on nitrite content of human saliva: possible relevance to in vivo formation of N-nitroso compounds. Food Cosmet. Toxicol. 1976;14:545–548. doi: 10.1016/s0015-6264(76)80005-3. [DOI] [PubMed] [Google Scholar]
- 9.Tannenbaum S.R., Correa P. Nitrate and gastric cancer risks. Nature. 1985;317:675–676. doi: 10.1038/317675b0. [DOI] [PubMed] [Google Scholar]
- 10.Ignarro L.J., Fukuto J.M., Griscavage J.M., Rogers N.E., Byrns R.E. Oxidation of nitric oxide in aqueous solution to nitrite but not nitrate: comparison with enzymatically formed nitric oxide from L-arginine. Proc. Natl. Acad. Sci. USA. 1993;90:8103–8107. doi: 10.1073/pnas.90.17.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukuto J.M., Cho J.Y., Switzer C.H. The chemical properties of nitric oxide and related nitrogen oxides. In: Ignarro L., editor. Nitric Oxide: Biology and Pathobiology. Academic Press; London: 2000. pp. 23–40. [Google Scholar]
- 12.Modin A., Björne H., Herulf M., Alving K., Weitzberg E., Lundberg J.O. Nitrite-derived nitric oxide: a possible mediator of 'acidic-metabolic' vasodilation. Acta Physiol. Scand. 2001;171:9–16. doi: 10.1046/j.1365-201X.2001.00771.x. [DOI] [PubMed] [Google Scholar]
- 13.Cosby K., Partovi K.S., Crawford J.H., Patel R.P., Reiter C.D., Martyr S., Yang B.K., Waclawiw M.A., Zalos G., Xu X., Huang K.T., Shields H., Kim-Shapiro D.B., Schechter A.N., Cannon R.O., Gladwin M.T. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 14.Lundberg J.O., Weitzberg E., Gladwin M.T. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discovery. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 15.Lundberg J.O., Gladwin M.T., Ahluwalia A., Benjamin N., Bryan N.S., Butler A., Cabrales P., Fago A., Feelisch M., Ford P.C., Freeman B.A., Frenneaux M., Friedman J., Kelm M., Kevil C.G., Kim-Shapiro D.B., Kozlov A.V., Lancaster J.R., Lefer D.J., McColl K., McCurry K., Patel R.P., Petersson J., Rassaf T., Reutov V.P., Richter-Addo G.B., Schechter A., Shiva S., Tsuchiya K., van Faassen E.E., Webb A.J., Zuckerbraun B.S., Zweier J.L., Weitzberg E. Nitrate and nitrite in biology, nutrition and therapeutics. Nat. Chem. Biol. 2009;5:865–869. doi: 10.1038/nchembio.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dejam A., Hunter C.J., Tremonti C., Pluta R.M., Hon Y.Y., Grimes G., Partovi K., Pelletier M.M., Oldfield E.H., Cannon R.O., Schechter A.N., Gladwin M.T. Nitrite infusion in humans and nonhuman primates: endocrine effects, pharmacokinetics, and tolerance formation. Circulation. 2007;116:1821–1831. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- 17.Maher A.R., Milsom A.B., Gunaruwan P., Abozguia K., Ahmed I., Weaver R.A., Thomas P., Ashrafian H., Born G.V., James P.E., Frenneaux M.P. Hypoxic modulation of exogenous nitrite-induced vasodilation in humans. Circulation. 2008;117:670–677. doi: 10.1161/CIRCULATIONAHA.107.719591. [DOI] [PubMed] [Google Scholar]
- 18.Webb A., Bond R., McLean P., Uppal R., Benjamin N., Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia–reperfusion damage. Proc. Natl. Acad. Sci. USA. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duranski M.R., Greer J.J., Dejam A., Jaganmohan S., Hogg N., Langston W., Patel R.P., Yet S.F., Wang X., Kevil C.G., Gladwin M.T., Lefer D.J. Cytoprotective effects of nitrite during in vivo ischemia–reperfusion of the heart and liver. J. Clin. Invest. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pluta R.M., Dejam A., Grimes G., Gladwin M.T., Oldfield E.H. Nitrite infusions to prevent delayed cerebral vasospasm in a primate model of subarachnoid hemorrhage. JAMA. 2005;293:1477–1484. doi: 10.1001/jama.293.12.1477. [DOI] [PubMed] [Google Scholar]
- 21.Tripatara P., Patel N.S., Webb A., Rathod K., Lecomte F.M., Mazzon E., Cuzzocrea S., Yaqoob M.M., Ahluwalia A., Thiemermann C. Nitrite-derived nitric oxide protects the rat kidney against ischemia/reperfusion injury in vivo: role for xanthine oxidoreductase. J. Am. Soc. Nephrol. 2007;18:570–580. doi: 10.1681/ASN.2006050450. [DOI] [PubMed] [Google Scholar]
- 22.Larsen F.J., Schiffer T.A., Borniquel S., Sahlin K., Ekblom B., Lundberg J.O., Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011;13:149–159. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Larsen F.J., Ekblom B., Sahlin K., Lundberg J.O., Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N. Engl. J. Med. 2006;355:2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 24.Webb A.J., Patel N., Loukogeorgakis S., Okorie M., Aboud Z., Misra S., Rashid R., Miall P., Deanfield J., Benjamin N., MacAllister R., Hobbs A.J., Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapil V., Milsom A.B., Okorie M., Maleki-Toyserkani S., Akram F., Rehman F., Arghandawi S., Pearl V., Benjamin N., Loukogeorgakis S., MacAllister R., Hobbs A.J., Webb A.J., Ahluwalia A. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension. 2010;56:274–281. doi: 10.1161/HYPERTENSIONAHA.110.153536. [DOI] [PubMed] [Google Scholar]
- 26.Tannenbaum S.R., Sinskey A.J., Weisman M., Bishop W. Nitrite in human saliva: its possible relationship to nitrosamine formation. J. Natl. Cancer Inst. 1974;53:79–84. [PubMed] [Google Scholar]
- 27.Ishiwata H., Tanimura A., Ishidate M. Studies on in vivo formation of nitroso compounds (III) Food Hyg. Saf. Sci. 1975;16:89–92. [Google Scholar]
- 28.Duncan C., Dougall H., Johnston P., Green S., Brogan R., Leifert C., Smith L., Golden M., Benjamin N. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat. Med. 1995;1:546–551. doi: 10.1038/nm0695-546. [DOI] [PubMed] [Google Scholar]
- 29.Doel J.J., Benjamin N., Hector M.P., Rogers M., Allaker R.P. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur. J. Oral Sci. 2005;113:14–19. doi: 10.1111/j.1600-0722.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- 30.Kortboyer, J. M.; Colbers, E. P. H.; Vaessen, H. A. M. G.; Groen, K.; Zeilmaker, M. J.; Slob, W.; Speilers, G. J. A.; Meulenbelt, J. A pilot-study to investigate nitrate and nitrite kinetics in healthy volunteers with normal and artificially increased gastric pH after sodium nitrate ingestion. In: International Workshop on Health Aspects of Nitrate and Its Metabolites (Particularly Nitrite). Bilthoven: Council of Europe Press; 1994:269-284.
- 31.Qin L., Liu X., Sun Q., Fan Z., Xia D., Ding G., Ong H.L., Adams D., Gahl W.A., Zheng C., Qi S., Jin L., Zhang C., Gu L., He J., Deng D., Ambudkar I.S., Wang S. Sialin (SLC17A5) functions as a nitrate transporter in the plasma membrane. Proc. Natl. Acad. Sci. USA. 2012;109:13434–13439. doi: 10.1073/pnas.1116633109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lundberg J.O., Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic. Biol. Med. 2004;37:395–400. doi: 10.1016/j.freeradbiomed.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 33.Bahra M., Kapil V., Pearl V., Ghosh S., Ahluwalia A. Inorganic nitrate ingestion improves vascular compliance but does not alter flow-mediated dilatation in healthy volunteers. Nitric Oxide. 2012;26:197–202. doi: 10.1016/j.niox.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shingles R., Roh M.H., McCarty R.E. Direct measurement of nitrite transport across erythrocyte membrane vesicles using the fluorescent probe, 6-methoxy-N-(3-sulfopropyl) quinolinium. J. Bioenerg. Biomembr. 1997;29:611–616. doi: 10.1023/a:1022491220299. [DOI] [PubMed] [Google Scholar]
- 35.Vitturi D.A., Teng X., Toledo J.C., Matalon S., Lancaster J.R., Patel R.P. Regulation of nitrite transport in red blood cells by hemoglobin oxygen fractional saturation. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H1398–407. doi: 10.1152/ajpheart.01303.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shiva S., Frizzell S., Gladwin M.T. Nitrite and heme globins: reaction mechanisms and physiological targets. In: Ignarro L., editor. Nitric Oxide: Biology and Pathobiology. Elsevier; San Diego: 2010. pp. 605–626. [Google Scholar]
- 37.Webb A.J., Ahluwalia A. Mechanisms of nitrite reduction in ischemia in the cardiovascular system. In: Ignarro L., editor. Nitric Oxide: Biology and Pathobiology. Elsevier; San Diego: 2010. pp. 555–586. [Google Scholar]
- 38.Govoni M., Jansson E.A., Weitzberg E., Lundberg J.O. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide. 2008;19:333–337. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Williams, B.; Poulter, N. R.; Brown, M. J.; Davis, M.; McInnes, G. T.; Potter, J. F.; Sever, P. S.; Thom, S. M. British Hypertension Society guidelines for hypertension management 2004 (BHS-IV): summary. BMJ 328:634-640; 2004. [DOI] [PMC free article] [PubMed]
- 40.Bäckhed F., Ley R.E., Sonnenburg J.L., Peterson D.A., Gordon J.I. Host–bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 41.Packer P.J., Leach S.A., Duncan S.N., Thompson M.H., Hill M.J. The effect of different sources of nitrate exposure on urinary nitrate recovery in humans and its relevance to the methods of estimating nitrate exposure in epidemiological studies. Carcinogenesis. 1989;10:1989–1996. doi: 10.1093/carcin/10.11.1989. [DOI] [PubMed] [Google Scholar]
- 42.Lundberg J.O., Weitzberg E., Cole J.A., Benjamin N. Nitrate, bacteria and human health. Nat. Rev. Microbiol. 2004;2:593–602. doi: 10.1038/nrmicro929. [DOI] [PubMed] [Google Scholar]
- 43.Batchelor A.M., Bartus K., Reynell C., Constantinou S., Halvey E.J., Held K.F., Dostmann W.R., Vernon J., Garthwaite J. Exquisite sensitivity to subsecond, picomolar nitric oxide transients conferred on cells by guanylyl cyclase-coupled receptors. Proc. Natl. Acad. Sci. USA. 2010;107:22060–22065. doi: 10.1073/pnas.1013147107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Webb A.J., Milsom A.B., Rathod K.S., Chu W.L., Qureshi S., Lovell M.J., Lecomte F.M., Perrett D., Raimondo C., Khoshbin E., Ahmed Z., Uppal R., Benjamin N., Hobbs A.J., Ahluwalia A. Mechanisms underlying erythrocyte and endothelial nitrite reduction to nitric oxide in hypoxia: role for xanthine oxidoreductase and endothelial nitric oxide synthase. Circ. Res. 2008;103:957–964. doi: 10.1161/CIRCRESAHA.108.175810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stamler J.S., Jia L., Eu J.P., McMahon T.J., Demchenko I.T., Bonaventura J., Gernert K., Piantadosi C.A. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science. 1997;276:2034–2037. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- 46.Lewington S., Clarke R., Qizilbash N., Peto R., Collins R., Collaboration P.S. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 47.Tonetti M.S., D'Aiuto F., Nibali L., Donald A., Storry C., Parkar M., Suvan J., Hingorani A.D., Vallance P., Deanfield J. Treatment of periodontitis and endothelial function. N. Engl. J. Med. 2007;356:911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- 48.Koren O., Spor A., Felin J., Fåk F., Stombaugh J., Tremaroli V., Behre C.J., Knight R., Fagerberg B., Ley R.E., Bäckhed F. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc. Natl. Acad. Sci. USA. 2011;108(Suppl. 1):4592–4598. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 486:207-214; 2012. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material