Highlights
► Our study provides new insights into the pre-regressional development of RTT. ► The pre-regression period should not be considered asymptomatic. ► Peculiarities in speech-language development are potential red flags for RTT.
Keywords: Rett syndrome, Preserved speech variant, Speech-language development, Milestones, Video analysis, Regression
Abstract
We delineated the achievement of early speech-language milestones in 15 young children with Rett syndrome (MECP2 positive) in the first two years of life using retrospective video analysis. By contrast to the commonly accepted concept that these children are normal in the pre-regression period, we found markedly atypical development of speech-language capacities, suggesting a paradigm shift in the pathogenesis of Rett syndrome and a possible approach to its early detection.
1. Introduction
Mutations in the X-linked gene encoding Methyl-CpG-binding protein 2 (MeCP2) account for 95–97% of cases of Rett syndrome (RTT, MIM 312750), a neurodevelopmental disorder affecting predominantly females (Amir et al., 1999; Hagberg, Aicardi, Dias, & Ramos, 1983; Neul et al., 2010). Key features of typical RTT, and the relatively milder preserved speech variant (PSV), are stereotyped hand movements coinciding with a regression in purposeful hand use and expressive language (Hagberg et al., 1983; Kerr, Archer, Evans, & Gibbon, 2006; Matson, Fodstad, & Boisjoli, 2008; Neul et al., 2010; Renieri et al., 2009). The dynamic course of RTT is said to involve a period of apparently normal early development followed by a profound neurologic regression and subsequent stabilization or partial recovery. However, this view contrasts with growing knowledge about MeCP2 expression in the developing brain and its role in terminal neuronal differentiation (Kaufmann, Johnston, & Blue, 2005; Zoghbi, 2003). Over the last two decades there has been a shift from some initial thoughts about atypical pre-regressional development in individuals with RTT to the growing evidence of early behavioral abnormalities reflecting a poor integration of the young nervous system (Einspieler, Kerr, & Prechtl, 2005a, 2005b; Leonard & Bower, 1998; Marschik, Einspieler, Oberle, Laccone, & Prechtl, 2009; Marschik, Einspieler, Prechtl, Oberle, & Laccone, 2010; Marschik, Einspieler, & Sigafoos, 2012; Marschik, Kaufmann, et al., 2012; Tams-Little & Holdgrafer, 1996). The redefined clinical diagnostic criteria by the RettSearch consortium (Neul et al., 2010) highlight the age-dependency of clinical changes and suggest the existence of initial signs in RTT, which might appear earlier than, for example, the (non obligatory) deceleration of head growth. Nonetheless, the nature of these early signs and their potential for early and reliable identification of RTT are still unclear. Consequently, the present study aimed at shedding light into pre-regressional development in RTT by focusing on early speech-language development using a video database.
2. Materials and methods
Audio-video recordings of sufficient length and quality standards during the first two years of life were available for 15 RTT participants with MECP2 mutations (10 typical RTT, 5 PSV; Neul et al., 2010; Renieri et al., 2009) comprising a total footage of 2431 min (i.e., 40.5 h; typical RTT 2041 min, median = 68; PSV 390 min, median = 78). All RTT participants were females, born as a singleton after uneventful pregnancy and delivery; birth weights, birth lengths, occipitofrontal circumferences, and Apgar scores were within the normal ranges. The recordings of play situations, daily routines and special events were made by the parents at a time when they were not aware that their daughters had typical RTT or PSV. The recordings were copied, prepared for analyses by unifying codecs, sampled across the age range and analyzed retrospectively.
We focused on and coded the achievement and realization of age-specific speech-language milestones. These included: protophones such as cooing and babbling vocalizations, proto-words, word combinations, and the communicative milestone of intentional gestures (Fig. 1). In addition to the achievement of these milestones, we also focused on the detection of atypical vocalizations during the cooing and babbling period. Fixed vocal signals (e.g., crying, laughing), vegetative sounds (e.g., sneezing, coughing), and utterances unintelligible to the coders were excluded. All remaining vocalizations were transcribed in chronological order and analyses were carried out using the Noldus Observer-XT (Versions 9.0 and 11.0, Noldus Information Technology, http://www.noldus.com). Two PhD students (TW, KDBP; blind to the background and purpose of the study) and two of the authors (PBM, CE) coded the data in parallel with an inter-rater agreement of 97%. The study was approved by the relevant Institutional Review Boards and all parents gave their informed consent, including consenting to the publication of the results.
Fig. 1.
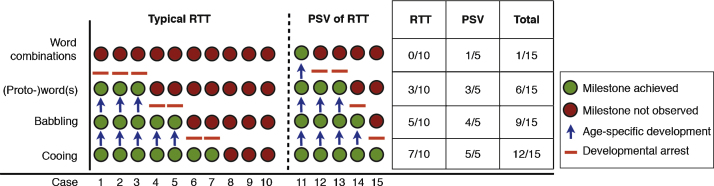
Step by step: Trajectories of reaching developmental speech-language milestones in typical RTT (n = 10) or PSV (n = 5) during the first two years of life.
3. Results
We focused on the extent to which children reached developmental milestones in the speech-language domain and found that, with each sequential milestone, more participants failed to reach the next level of complexity. Whereas 13/15 participants, including all of those with PSV, showed cooing vocalizations only 9 had canonical babbling (half of the females with typical RTT and all but one with PSV; Fig. 1). Proto-words were largely absent in participants with typical RTT (only 3/10), although they were observed in three participants with PSV; furthermore, none of the participants with typical RTT and only one of those with PSV produced any word combinations (Fig. 1). Only 5/10 typical RTT and 3/5 PSV participants actively used communicative gestures.
Qualitative analyses of verbal behaviors revealed frequent abnormalities, even when the milestones of cooing and babbling were reached. Vocalizations on inspiratory airstream were among the first atypical features observed during the cooing and babbling period in 7/10 typical RTT and 2/5 PSV participants. Normal cooing vocalizations were observed in 5/10 typical RTT participants whereas 2/10 exhibited an intermittent character of normal and abnormal cooing (i.e., typical age-specific vocalizations interspersed with atypical ones such as inspiratory vocalizations, pressed or high-pitched crying-like vocalizations), and 3/10 did not display any cooing vocalizations. For PSV, 3/5 participants had normal and 2/5 had intermittently abnormal vocalizations in the cooing stage. In the babbling period, in which half of the typical RTT participants used babbling vocalizations, only one of them produced exclusively typical vocalizations; the others, again, demonstrated a combination of typical and atypical vocalizations, such as inspiratory ones. Of the four toddlers with PSV uttering babbling sounds, two showed these interspersed vocalization patterns of normal and atypical quality.
4. Discussion
The majority of individuals with typical RTT or PSV are believed to experience a partial or complete loss of acquired speech-language abilities (Kerr et al., 2006; Neul et al., 2010; Renieri et al., 2009; Uchino, Suzuki, Hoshino, Nomura, & Segawa, 2001). As diagnosis usually follows the onset of regression, the systematic knowledge about complexity levels and pathways of various developmental domains before the loss of previously acquired functions is limited. Retrospective video analysis has proven to be a valuable tool to delineate various developmental aspects in dynamic neurodevelopmental disorders such as RTT and autism spectrum disorders (e.g., Einspieler et al., 2005a, 2005b; Maestro et al., 2001; Marschik and Einspieler, 2011; Marschik et al., 2012a; Marschik, Pini, 2012; Marschik, Sigafoos, 2012; Palomo et al., 2006; Saint-Georges et al., 2010). In this study, we present new evidence for clearly quantitatively and qualitatively deviating development in the speech-language domain among typical RTT and PSV prior to the developmental regression characteristic of these conditions. Our systematic characterization of speech-language milestones and their deviation, including both lack of achievement and peculiar features, before the onset of regression opens a new chapter on early abnormalities in RTT.
However, these findings should be interpreted in the context of the methodological limitations associated with retrospective video analysis. Among this method's limitations are missing data and non-standardized data acquisition (e.g., duration of the recording, high versus low communicative settings), which might influence the conclusions to be drawn (Einspieler et al., 2005a, 2005b; Maestro et al., 2001; Marschik & Einspieler, 2011; Marschik, Kaufmann, et al., 2012; Palomo et al., 2006; Saint-Georges et al., 2010). However, within the limits of these methodological caveats, the results presented here do allow for new insights into the pre-regressional speech-language development in RTT and may, in turn, lead to the application of novel approaches to studying infants and toddlers with this neurodevelopmental disorder.
The observed developmental abnormalities could also be interpreted with caution as antecedents of later cardinal features of RTT. For example, vocalizations produced on ingressive airstream might be viewed as precursors to the breathing irregularities that characterize the post-regression RTT phenotype (Budden, 2012; Kaufmann et al., 2012; Marschik, Pini, 2012; Marschik, Sigafoos, 2012; Neul et al., 2010). In addition, the absence or markedly restricted repertoire and functionality of gestures, a delayed or absent response to interactive stimuli and impairments in joint attention are all antecedents of later developing marked pragmatic difficulties and peculiarities in social reciprocity (Kaufmann et al., 2012; Marschik et al., 2012a). These features, in turn, precede or mirror some autistic symptoms and would seem to refute the assumption that autistic behaviors are a transient or late appearing phenomenon in RTT (Kaufmann et al., 2012).
5. Conclusions
Typical RTT and PSV involve a period of regression followed by recovery or stabilization (Neul et al., 2010). This trajectory is consistent with the present findings, but our data suggest that the pre-regression period should not be considered asymptomatic. Instead, our findings indicate an increasing divergence between expected typical age-related and atypical behaviors. A qualitatively deviant pattern and a failure to develop more complex patterns in terms of communicative forms and functions are potential red flags for RTT. They become greater warning signals with every non-adequately solidified developmental step. Translating these findings to other neurodevelopmental disorders, researchers should consider the robustness of ontogenetic development in the speech-language domain for delineating early behavioral abnormalities.
Conflicts of interest
None.
Acknowledgements
We are grateful to the participants’ parents and colleagues for sharing their audio-video archives; We thank A.M. Kerr for her groundbreaking thoughts on the early Rett phenotype, the members of the RettSearch consortium and all colleagues in the related fields for their constant input in discussing this topic, sharing their expertise from molecular to the clinical level that enabled these insights, the understanding of this phenomenon and some of its underlying mechanisms. Parts of the study supported by the Austrian Science Fund FWF P19581 & FWF P25241.
References
- Amir R.E., Van den Veyver I.B., Wan M., Tran C.Q., Francke U., Zoghbi H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nature Genetics. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Budden S. Clinical variability in early speech-language development in females with Rett syndrome. Developmental Medicine and Child Neurology. 2012;54:339–392. doi: 10.1111/j.1469-8749.2012.04246.x. [DOI] [PubMed] [Google Scholar]
- Einspieler C., Kerr A.M., Prechtl H.F. Is the early development of girls with Rett disorder really normal? Pediatric Research. 2005;57:696–700. doi: 10.1203/01.PDR.0000155945.94249.0A. [DOI] [PubMed] [Google Scholar]
- Einspieler C., Kerr A.M., Prechtl H.F. Abnormal general movements in girls with Rett disorder: The first four months of life. Brain and Development. 2005;27:8–13. doi: 10.1016/j.braindev.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Hagberg B., Aicardi J., Dias K., Ramos O. A progressive syndrome of autism, dementia, and loss of purposeful hand use in girls: Rett's syndrome: Report of 35 cases. Annals of Neurology. 1983;14:471–479. doi: 10.1002/ana.410140412. [DOI] [PubMed] [Google Scholar]
- Kaufmann W.E., Johnston M.V., Blue M.E. MeCP2 expression and function during brain development: Implications for Rett syndrome's pathogenesis and clinical evolution. Brain and Development. 2005;27:S77–S87. doi: 10.1016/j.braindev.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Kaufmann W.E., Tierney E., Rohde C.A., Suarez-Pedraza M.C., Clarke M.A., Salorio C.F. Social impairments in Rett syndrome: Characteristics and relationship with clinical severity. Journal of Intellectual Disability Research. 2012;56:233–247. doi: 10.1111/j.1365-2788.2011.01404.x. [DOI] [PubMed] [Google Scholar]
- Kerr A.M., Archer H.L., Evans J.C., Gibbon F. People with mutation positive Rett disorder who converse. Journal of Intellectual Disability Research. 2006;50:386–394. doi: 10.1111/j.1365-2788.2005.00786.x. [DOI] [PubMed] [Google Scholar]
- Leonard H., Bower C. Is the girl with Rett syndrome normal at birth? Developmental Medicine and Child Neurology. 1998;40:115–121. [PubMed] [Google Scholar]
- Maestro S., Muratori F., Barbieri F., Casella C., Cattaneo V., Cavallaro M.C. Early behavioral development in autistic children: The first 2 years of life through home movies. Psychopathology. 2001;34:147–152. doi: 10.1159/000049298. [DOI] [PubMed] [Google Scholar]
- Marschik P.B., Einspieler C. Methodological note: Video analysis of the early development of Rett syndrome – One method for many disciplines. Developmental Neurorehabilitation. 2011;14:355–357. doi: 10.3109/17518423.2011.604355. [DOI] [PubMed] [Google Scholar]
- Marschik P.B., Einspieler C., Oberle A., Laccone F., Prechtl H.F. Case report: Retracing atypical development: A preserved speech variant of Rett syndrome. Journal of Autism and Developmental Disorders. 2009;39:958–961. doi: 10.1007/s10803-009-0703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschik P.B., Einspieler C., Prechtl H.F., Oberle A., Laccone F. Relabelling the preserved speech variant of Rett syndrome. Developmental Medicine and Child Neurology. 2010;52:218. doi: 10.1111/j.1469-8749.2009.03531.x. [DOI] [PubMed] [Google Scholar]
- Marschik P.B., Einspieler C., Sigafoos J. Contributing to the early detection of Rett syndrome: The potential role of auditory Gestalt perception. Research in Developmental Disabilities. 2012;33:461–466. doi: 10.1016/j.ridd.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschik P.B., Kaufmann W.E., Einspieler C., Bartl-Pokorny K.D., Wolin T., Pini G. Profiling early socio-communicative development in five young girls with the preserved speech variant of Rett syndrome. Research in Developmental Disabilities. 2012;33:1749–1756. doi: 10.1016/j.ridd.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschik P.B., Pini G., Bartl-Pokorny K.D., Duckworth M., Gugatschka M., Vollmann R. Early speech-language development in females with Rett syndrome: Focusing on the preserved speech variant. Developmental Medicine and Child Neurology. 2012;54:451–456. doi: 10.1111/j.1469-8749.2012.04123.x. [DOI] [PubMed] [Google Scholar]
- Marschik P.B., Sigafoos J., Kaufmann W.E., Wolin T., Talisa V.B., Bartl-Pokorny K.D. Peculiarities in the gestural repertoire: An early marker for Rett syndrome? Research in Developmental Disabilities. 2012;33:1715–1721. doi: 10.1016/j.ridd.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson J.L., Fodstad J.C., Boisjoli J.A. Nosology and diagnosis of Rett syndrome. Research in Autism Spectrum Disorders. 2008;2:601–611. [Google Scholar]
- Neul J.L., Kaufmann W.E., Glaze D.G., Christodolou J., Clarke A.J., Bahi-Buisson N. Rett syndrome: Revised diagnostic criteria and nomenclature. Annals of Neurology. 2010;68:944–950. doi: 10.1002/ana.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomo R., Belinchón M., Ozonoff S. Autism and family home movies: A comprehensive review. Developmental and Behavioral Pediatrics. 2006;27:59–68. doi: 10.1097/00004703-200604002-00003. [DOI] [PubMed] [Google Scholar]
- Renieri A., Mari F., Mencarelli M.A., Scala E., Ariani F., Longo I. Diagnostic criteria for the Zappella variant of Rett syndrome (the preserved speech variant) Brain and Development. 2009;31:208–216. doi: 10.1016/j.braindev.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Saint-Georges C., Cassel R.S., Cohen D., Chetouani M., Laznik M.-C., Maestro S. What studies of family home movies can teach us about autistic infants: A literature review. Research in Autism Spectrum Disorders. 2010;4:355–366. [Google Scholar]
- Tams-Little S., Holdgrafer G. Early communication development in children with Rett syndrome. Brain and Development. 1996;18:376–378. doi: 10.1016/0387-7604(96)00023-x. [DOI] [PubMed] [Google Scholar]
- Uchino J., Suzuki M., Hoshino K., Nomura Y., Segawa M. Development of language in Rett syndrome. Brain and Development. 2001;23:S233–S235. doi: 10.1016/s0387-7604(01)00367-9. [DOI] [PubMed] [Google Scholar]
- Zoghbi H.Y. Postnatal neurodevelopmental disorders: Meeting at the synapse? Science. 2003;31:826–830. doi: 10.1126/science.1089071. [DOI] [PubMed] [Google Scholar]


