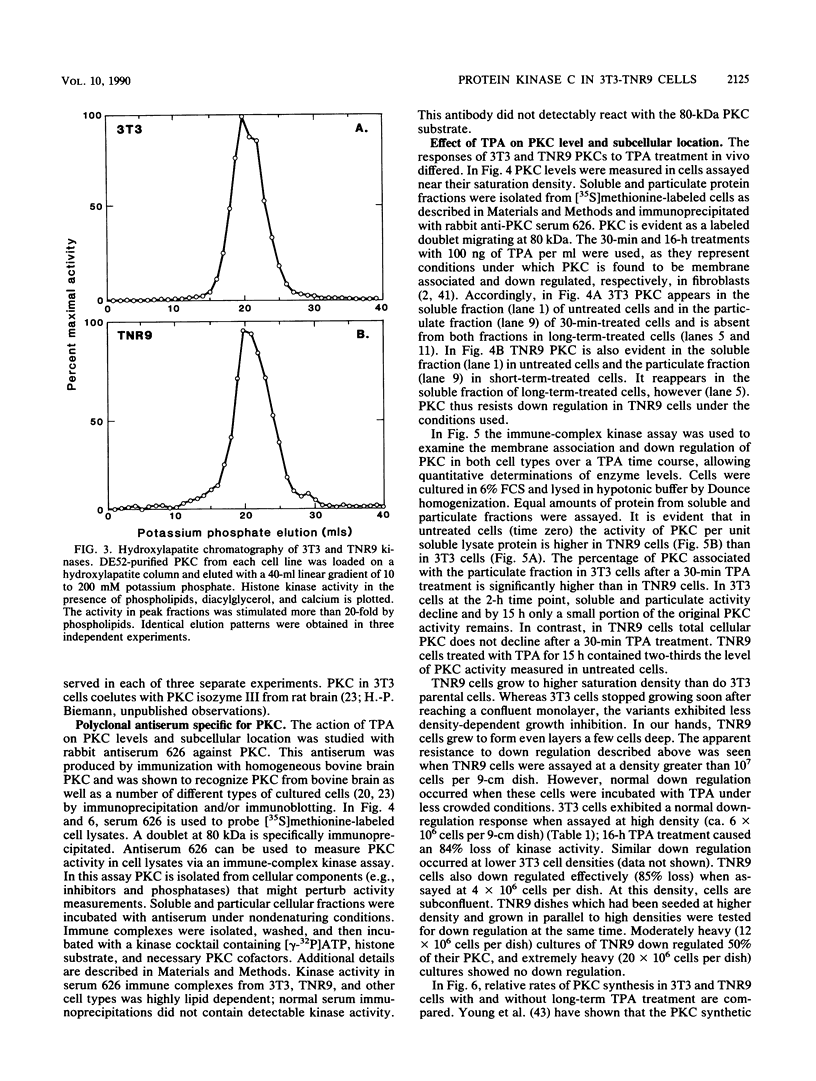
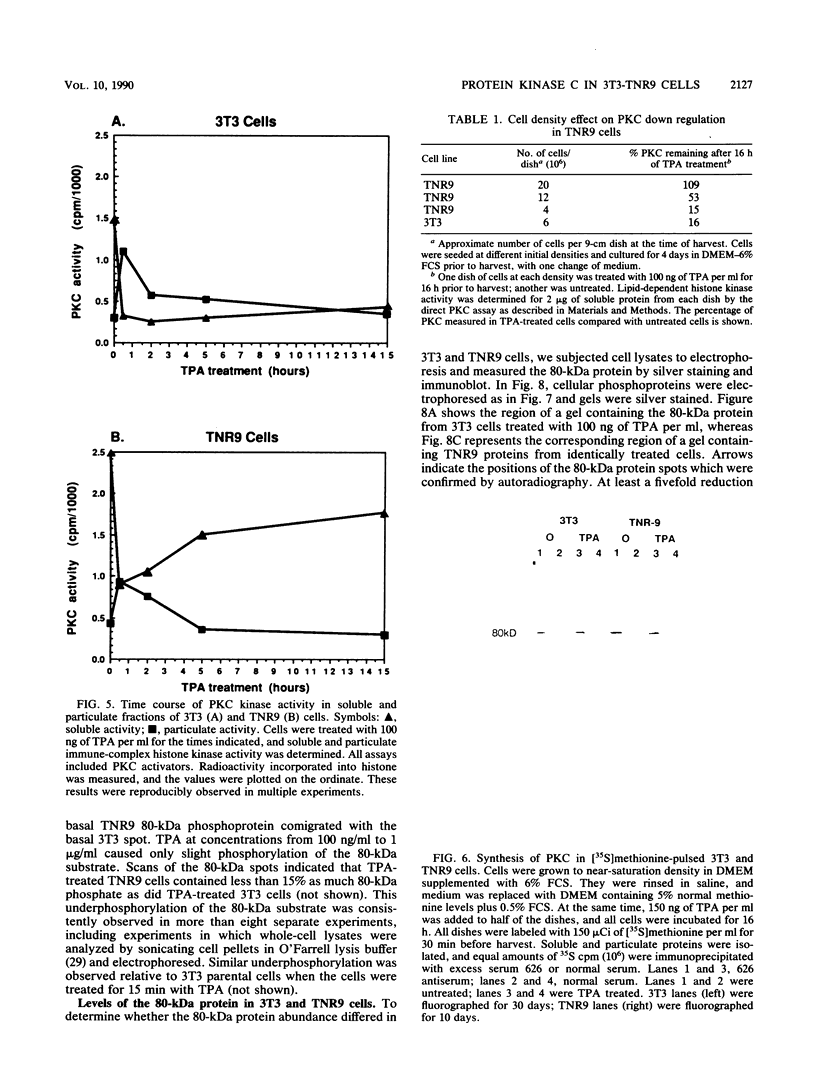
Abstract
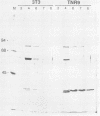
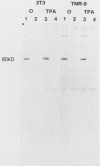
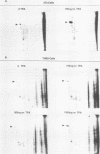
The cell line TNR9 (E. Butler-Gralla and H. R. Herschman, J. Cell. Physiol. 107:59-67, 1981) in a Swiss 3T3 cell variant that expresses protein kinase C (PKC) but is mitogenically nonresponsive to the phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA). We have found that PKCs purified from variant and parental cells are identical as judged by kinase activity, protease mapping, and column chromatography. We analyzed cellular levels and subcellular location of PKC in TPA-treated 3T3 and TNR9 cells via immunoprecipitation of [35S]methionine-labeled protein and assay of immune-complex PKC kinase activity. TNR9 cells grew to higher densities than parental 3T3 cells. TNR9 cells at maximal density did not down regulate PKC in response to long-term TPA treatment. We compared the 80-kilodalton (kDa) PKC substrate phosphorylation in 3T3 and TNR9 cells by using two-dimensional gels and found that TNR9 cells treated with TPA for 30 min contained only 10 to 15% as much 32Pi associated with the 80-kDa as did parental cells. The TNR9 80-kDa substrate was present at reduced levels compared with the parental-cell 80-kDa substrate as judged by immunoblot and silver staining. Thus, the loss of mitogenic responsiveness to TPA in TNR9 cells is accompanied by resistance to TPA-mediated down regulation of PKC and reduced phosphosubstrate levels.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert K. A., Nairn A. C., Greengard P. The 87-kDa protein, a major specific substrate for protein kinase C: purification from bovine brain and characterization. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7046–7050. doi: 10.1073/pnas.84.20.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester R., Rosen O. M. Fate of immunoprecipitable protein kinase C in GH3 cells treated with phorbol 12-myristate 13-acetate. J Biol Chem. 1985 Dec 5;260(28):15194–15199. [PubMed] [Google Scholar]
- Bishop R., Martinez R., Weber M. J., Blackshear P. J., Beatty S., Lim R., Herschman H. R. Protein phosphorylation in a tetradecanoyl phorbol acetate-nonproliferative variant of 3T3 cells. Mol Cell Biol. 1985 Sep;5(9):2231–2237. doi: 10.1128/mcb.5.9.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshear P. J., Wen L., Glynn B. P., Witters L. A. Protein kinase C-stimulated phosphorylation in vitro of a Mr 80,000 protein phosphorylated in response to phorbol esters and growth factors in intact fibroblasts. Distinction from protein kinase C and prominence in brain. J Biol Chem. 1986 Jan 25;261(3):1459–1469. [PubMed] [Google Scholar]
- Blumberg P. M., Butler-Gralla E., Herschman H. R. Analysis of phorbol ester receptors in phorbol ester unresponsive 3T3 cell variants. Biochem Biophys Res Commun. 1981 Oct 15;102(3):818–823. doi: 10.1016/0006-291x(81)91611-9. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Blakeley D. M., Hamon M. H., Laurie M. S., Corps A. N. Protein kinase C-mediated negative-feedback inhibition of unstimulated and bombesin-stimulated polyphosphoinositide hydrolysis in Swiss-mouse 3T3 cells. Biochem J. 1987 Aug 1;245(3):631–639. doi: 10.1042/bj2450631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Butler-Gralla E., Herschman H. R. Glucose uptake and ornithine decarboxylase activity in tetradecanoyl phorbol acetate non-proliferative variants. J Cell Physiol. 1983 Mar;114(3):317–320. doi: 10.1002/jcp.1041140310. [DOI] [PubMed] [Google Scholar]
- Butler-Gralla E., Herschman H. R. Variants of 3T3 cells lacking mitogenic response to the tumor promoter tetradecanoyl-phorbol-acetate. J Cell Physiol. 1981 Apr;107(1):59–67. doi: 10.1002/jcp.1041070108. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Stimulation of DNA synthesis by transient exposure of cell cultures to TPA or polypeptide mitogens: induction of competence or incomplete removal? J Cell Physiol. 1981 Oct;109(1):99–109. doi: 10.1002/jcp.1041090112. [DOI] [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Stimulation of DNA synthesis by tumour promoter and pure mitogenic factors. Nature. 1978 Dec 14;276(5689):723–726. doi: 10.1038/276723a0. [DOI] [PubMed] [Google Scholar]
- Erikson R. L., Alcorta D., Bedard P. A., Blenis J., Biemann H. P., Erikson E., Jones S. W., Maller J. L., Martins T. J., Simmons D. L. Molecular analyses of gene products associated with the response of cells to mitogenic stimulation. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 1):143–151. doi: 10.1101/sqb.1988.053.01.020. [DOI] [PubMed] [Google Scholar]
- Hepler J. R., Earp H. S., Harden T. K. Long-term phorbol ester treatment down-regulates protein kinase C and sensitizes the phosphoinositide signaling pathway to hormone and growth factor stimulation. Evidence for a role of protein kinase C in agonist-induced desensitization. J Biol Chem. 1988 Jun 5;263(16):7610–7619. [PubMed] [Google Scholar]
- Herschman H. R. A 12-O-tetradecanoylphorbol-13-acetate (TPA)-nonproliferative variant of 3T3 cells is resistant to TPA-enhanced gene amplification. Mol Cell Biol. 1985 May;5(5):1130–1135. doi: 10.1128/mcb.5.5.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housey G. M., Johnson M. D., Hsiao W. L., O'Brian C. A., Murphy J. P., Kirschmeier P., Weinstein I. B. Overproduction of protein kinase C causes disordered growth control in rat fibroblasts. Cell. 1988 Feb 12;52(3):343–354. doi: 10.1016/s0092-8674(88)80027-8. [DOI] [PubMed] [Google Scholar]
- Kamata T., Sullivan N. F., Wooten M. W. Reduced protein kinase C activity in a ras-resistant cell line derived from Ki-MSV transformed cells. Oncogene. 1987 Mar;1(1):37–46. [PubMed] [Google Scholar]
- Kikkawa U., Ono Y., Ogita K., Fujii T., Asaoka Y., Sekiguchi K., Kosaka Y., Igarashi K., Nishizuka Y. Identification of the structures of multiple subspecies of protein kinase C expressed in rat brain. FEBS Lett. 1987 Jun 15;217(2):227–231. doi: 10.1016/0014-5793(87)80668-3. [DOI] [PubMed] [Google Scholar]
- Krug E., Biemann H. P., Tashjian A. H., Jr Evidence for increased synthesis as well as increased degradation of protein kinase C after treatment of human osteosarcoma cells with phorbol ester. J Biol Chem. 1987 Aug 25;262(24):11852–11856. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lim R. W., Varnum B. C., O'Brien T. G., Herschman H. R. Induction of tumor promotor-inducible genes in murine 3T3 cell lines and tetradecanoyl phorbol acetate-nonproliferative 3T3 variants can occur through protein kinase C-dependent and -independent pathways. Mol Cell Biol. 1989 Apr;9(4):1790–1793. doi: 10.1128/mcb.9.4.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey P. G., Rosner M. R., Kikkawa U., Sekiguchi K., Ogita K., Ase K., Nishizuka Y. Characterization of protein kinase C from normal and transformed cultured murine fibroblasts. Biochem Biophys Res Commun. 1987 Jul 15;146(1):140–146. doi: 10.1016/0006-291x(87)90702-9. [DOI] [PubMed] [Google Scholar]
- Metcalfe J. C., Hesketh T. R., Smith G. A., Morris J. D., Corps A. N., Moore J. P. Early response pattern analysis of the mitogenic pathway in lymphocytes and fibroblasts. J Cell Sci Suppl. 1985;3:199–228. doi: 10.1242/jcs.1985.supplement_3.19. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Patel J., Kligman D. Purification and characterization of an Mr 87,000 protein kinase C substrate from rat brain. J Biol Chem. 1987 Dec 5;262(34):16686–16691. [PubMed] [Google Scholar]
- Persons D. A., Wilkison W. O., Bell R. M., Finn O. J. Altered growth regulation and enhanced tumorigenicity of NIH 3T3 fibroblasts transfected with protein kinase C-I cDNA. Cell. 1988 Feb 12;52(3):447–458. doi: 10.1016/s0092-8674(88)80037-0. [DOI] [PubMed] [Google Scholar]
- Resh M. D., Erikson R. L. Development and characterization of antisera specific for amino- and carboxy-terminal regions of pp60src. J Virol. 1985 Jul;55(1):242–245. doi: 10.1128/jvi.55.1.242-245.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Disappearance of Ca2+-sensitive, phospholipid-dependent protein kinase activity in phorbol ester-treated 3T3 cells. Biochem Biophys Res Commun. 1984 May 16;120(3):1053–1059. doi: 10.1016/s0006-291x(84)80213-2. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena M., Smith K. A. Phorbol esters, phospholipase C, and growth factors rapidly stimulate the phosphorylation of a Mr 80,000 protein in intact quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7244–7248. doi: 10.1073/pnas.80.23.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanki V., Slaga T. J., Callaham M., Huberman E. Down regulation of specific binding of [20-3H]phorbol 12,13-dibutyrate and phorbol ester-induced differentiation of human promyelocytic leukemia cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1722–1725. doi: 10.1073/pnas.78.3.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpo D. J., Graff J. M., Albert K. A., Greengard P., Blackshear P. J. Molecular cloning, characterization, and expression of a cDNA encoding the "80- to 87-kDa" myristoylated alanine-rich C kinase substrate: a major cellular substrate for protein kinase C. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4012–4016. doi: 10.1073/pnas.86.11.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada K., Amino N., Tetsumoto T., Miyai K. Phorbol esters have a dual action through protein kinase C in regulation of proliferation of FRTL-5 cells. FEBS Lett. 1988 Jul 4;234(1):13–16. doi: 10.1016/0014-5793(88)81292-4. [DOI] [PubMed] [Google Scholar]
- Uchida T., Filburn C. R. Affinity chromatography of protein kinase C-phorbol ester receptor on polyacrylamide-immobilized phosphatidylserine. J Biol Chem. 1984 Oct 25;259(20):12311–12314. [PubMed] [Google Scholar]
- Wahl M., Carpenter G. Regulation of epidermal growth factor-stimulated formation of inositol phosphates in A-431 cells by calcium and protein kinase C. J Biol Chem. 1988 Jun 5;263(16):7581–7590. [PubMed] [Google Scholar]
- Wolfman A., Wingrove T. G., Blackshear P. J., Macara I. G. Down-regulation of protein kinase C and of an endogenous 80-kDa substrate in transformed fibroblasts. J Biol Chem. 1987 Dec 5;262(34):16546–16552. [PubMed] [Google Scholar]
- Woodgett J. R., Hunter T. Immunological evidence for two physiological forms of protein kinase C. Mol Cell Biol. 1987 Jan;7(1):85–96. doi: 10.1128/mcb.7.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Phorbol-ester-induced alterations of free calcium ion transients in single rat hepatocytes. Biochem J. 1987 Sep 15;246(3):619–623. doi: 10.1042/bj2460619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S., Parker P. J., Ullrich A., Stabel S. Down-regulation of protein kinase C is due to an increased rate of degradation. Biochem J. 1987 Jun 15;244(3):775–779. doi: 10.1042/bj2440775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary I., Sinnett-Smith J. W., Rozengurt E. Early events elicited by bombesin and structurally related peptides in quiescent Swiss 3T3 cells. I. Activation of protein kinase C and inhibition of epidermal growth factor binding. J Cell Biol. 1986 Jun;102(6):2211–2222. doi: 10.1083/jcb.102.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]