Background: Induced pluripotent stem cells (iPSCs) are a novel technology for modeling neurodegeneration.
Results: We established spinal and bulbar muscular atrophy (SBMA)-derived iPSCs and confirmed motor neuron differentiation. Aggregation of androgen receptor in SBMA-iPSC-derived neurons is enhanced by DHT and inhibited by 17-AAG.
Conclusion: SBMA-iPSCs show disease-specific biochemical features.
Significance: Using SBMA-iPSCs is an innovative strategy for studying polyglutamine diseases.
Keywords: Androgen Receptor, Induced Pluripotent Stem Cells, Neurodegeneration, Neurodegenerative Diseases, Polyglutamine Disease, Dentatorubral-Pallidoluysian Atrophy, Spinal and Bulbar Muscular Atrophy
Abstract
Spinal and bulbar muscular atrophy (SBMA) is an X-linked motor neuron disease caused by a CAG repeat expansion in the androgen receptor (AR) gene. Ligand-dependent nuclear accumulation of mutant AR protein is a critical characteristic of the pathogenesis of SBMA. SBMA has been modeled in AR-overexpressing animals, but precisely how the polyglutamine (polyQ) expansion leads to neurodegeneration is unclear. Induced pluripotent stem cells (iPSCs) are a new technology that can be used to model human diseases, study pathogenic mechanisms, and develop novel drugs. We established SBMA patient-derived iPSCs, investigated their cellular biochemical characteristics, and found that SBMA-iPSCs can differentiate into motor neurons. The CAG repeat numbers in the AR gene of SBMA-iPSCs and also in the atrophin-1 gene of iPSCs derived from another polyQ disease, dentato-rubro-pallido-luysian atrophy (DRPLA), remain unchanged during reprogramming, long term passage, and differentiation, indicating that polyQ disease-associated CAG repeats are stable during maintenance of iPSCs. The level of AR expression is up-regulated by neuronal differentiation and treatment with the AR ligand dihydrotestosterone. Filter retardation assays indicated that aggregation of ARs following dihydrotestosterone treatment in neurons derived from SBMA-iPSCs increases significantly compared with neurological control iPSCs, easily recapitulating the pathological feature of mutant ARs in SBMA-iPSCs. This phenomenon was not observed in iPSCs and fibroblasts, thereby showing the neuron-dominant phenotype of this disease. Furthermore, the HSP90 inhibitor 17-allylaminogeldanamycin sharply decreased the level of aggregated AR in neurons derived from SBMA-iPSCs, indicating a potential for discovery and validation of candidate drugs. We found that SBMA-iPSCs possess disease-specific biochemical features and could thus open new avenues of research into not only SBMA, but also other polyglutamine diseases.
Introduction
Spinal and bulbar muscular atrophy (SBMA),2 also known as Kennedy-Alter-Sung syndrome (1), is an X-linked recessive motor neuron disease characterized by progressive bulbar and proximal limb weakness and atrophy, as well as signs of mild androgen insensitivity, such as gynecomastia, testicular atrophy, and reduced fertility. This disease was the first neurodegenerative disorder caused by trinucleotide (CAG) repeat expansions to be identified. SBMA is characterized by polyglutamine (polyQ) expansions in exon 1 of the androgen receptor (AR) gene (2).
The polymorphic glutamine region normally ranges between 9 and 37 glutamine residues, with an average of approximately 22 residues. The AR of SBMA patients typically includes more than 38 contiguous glutamines (3–6). Repeat expansions have the property of genetic instability, meaning that their lengths often change during paternal transmission. PolyQ disorders such as SBMA share clinical features such as anticipation and somatic mosaicism. A correlation exists between the CAG repeat length and disease onset, and there is selective neuronal involvement despite the ubiquitous expression of the causative genes in polyQ diseases (7, 8).
The defining pathology of SBMA is intranuclear AR inclusions in the affected neuronal population. Misfolding and altered degradation of the mutant AR may also be involved in the pathogenesis of SBMA. Among the polyQ diseases, SBMA is unique in that the mutant AR has a specific ligand, testosterone. Results obtained from Drosophila and mouse AR overexpression models clearly indicate that translocation of mutant AR to the nucleus in response to ligand binding is critical to pathogenesis (9–12). However, there is no direct biochemical evidence demonstrating translocation of mutant AR in response to ligand binding in living human neurons derived from SBMA patients, and therefore precisely how the polyQ expansion leads to neurodegeneration remains unclear.
Recently, human induced pluripotent stem cells (iPSCs) have emerged as a useful tool for research into the pathogenesis of many diseases, including neurodegeneration (13–15). Previous information regarding the molecular pathogenesis of neurodegenerative diseases has come from investigations utilizing postmortem brain tissues or transgenic animals because of the invasive nature of accessing the living human central nervous system. The advent of iPSC technology has made it possible to analyze living disease-specific human neurons in vitro. An increasing number of studies have employed iPSCs derived from patients with neurological diseases (15), with many focusing on their potential for use in rejection tolerance personalized cell replacement therapy. In recent years, patient-derived iPSCs have been used to recapitulate the phenotypes of a number of neurological diseases, thereby broadening our understanding of the pathogenesis of many neurological diseases, including those of late onset (16–27). Generation of polyQ disease-specific iPSCs from patients with Huntington disease and spinocerebellar ataxia type 3 (SCA3) has also been reported (17, 27–29). Using SCA3-specific iPSC-derived neurons, Koch et al. (27) recently revealed that glutamate-induced excitation (but not the patient's fibroblasts, iPSCs, or differentiated glia) is critical for proteolysis and aberrant insoluble aggregation of ataxin 3, thereby explaining the reason for neuron-specific degeneration.
Although SBMA was the first polyQ disease described, it has yet to be modeled using iPSC technology. Here, we report the generation of iPSCs from fibroblasts obtained at the autopsy of an SBMA patient and their differentiation into motor neurons. We also show that the CAG repeat is stable during reprogramming, long term passage, and differentiation. In addition, we examined expression of the AR and its aggregated state in the differentiated neuronal cells derived from SBMA-iPSCs. Our findings demonstrate that patient-derived neurons represent a novel model for use in studying polyQ diseases and that they can provide important clues for the identification and validation of candidate drugs.
EXPERIMENTAL PROCEDURES
Cell Culture and iPS Generation
Dermal fibroblasts were collected at autopsy from an SBMA patient and healthy control using protocols approved by Keio University (approval number 20-97(3)) after informed consent was obtained from the decedent's family. Fibroblasts from a dentato-rubro-pallido-luysian dystrophy (DRPLA) patient (GM13716) were purchased from Coriell Cell Repositories (Camden, NJ). Fibroblasts were cultured in DMEM (Invitrogen) containing 10% fetal bovine serum, 50 units/ml penicillin, 50 mg/ml streptomycin, and 1 mm l-glutamine. SBMA-iPSCs (KAS01 #2 and #3) were generated using the human iPS cell generation vector set (Takara) as described previously (25). The generation of sporadic Parkinson disease (PD)-derived iPSCs and the methods used for RT-PCR, in vitro differentiation, and teratoma formation are described elsewhere (25). The original human iPSC line 201B7 was kindly provided by Dr. Shinya Yamanaka of the Center for iPS Cell Research and Application (13).
DNA Preparation and Determination of the Size of CAG Repeats in Genomic DNA
DNA was extracted using DNeasy Blood & Tissue kits (Qiagen). PCR amplification of the CAG repeat in the AR gene was performed using a fluorescent-labeled forward primer (5′-TCCAGAATCTGTTCCAGAGCGTGC-3′) and an unlabeled reverse primer (5′-TGGCCTCGCTCAGGATGTCTTTAAG-3′). Detailed PCR conditions were described previously (30). Similarly, PCR amplification of the CAG repeat in the atrophin-1 gene was performed using a fluorescently labeled forward primer (5′-CACCAGTCTCAACACATCACCATC-3′) and an unlabeled reverse primer (5′-CCTCCAGTGGGTGGGGAAATGCTC-3′). Detailed PCR conditions were also described previously (31). The CAG repeat number of each PCR product was determined using GeneScan analysis and big dye DNA sequencing reactions according to the manufacturer's instructions (Applied Biosystems).
Immunofluorescence Staining of iPSCs and iPSC-derived Differentiated Neurons
Immunofluorescence staining was performed using the following primary antibodies: anti-SSEA 3 (Abcam; 1:200), anti-SSEA 4 (Abcam; 1:200), anti-Tra-1–60 (Millipore; 1:200), anti-Tra-1–81 (Millipore; 1:200), anti-SSEA1 (Abcam; 1:200), anti-MAP-2 (Chemicon; 1:200), anti-tubulin βIII, clone 2G10 (Millipore 05-559; 1:200), anti-AR N-20 (Santa Cruz sc-816; 1:200), and anti-islet-1 (39.4D5-c; Developmental Studies Hybridoma Bank; 1:100). DAPI (Molecular Probes) was used for nuclear staining. The secondary antibodies used were: anti-rat IgG, anti-mouse IgG, and IgM conjugated with Alexa Fluor 488 or Alexa Fluor 568 (Molecular Probes).
Neural Induction
Neural induction of hiPSCs was performed as described previously, with slight modifications (32–34).3 For terminal differentiation, induced neural cells were plated onto Matrigel-coated coverslips and cultured for 2 weeks in N2 medium (DMEM/Ham's F-12 medium (Invitrogen) containing 50 units/ml penicillin, 50 mg/ml streptomycin, 1 mm l-glutamine, and 1% N2 supplement (Invitrogen, 17502-048)). To identify Hb9-positive neurons, we transduced the Hb9::GFP promoter into the neural progenitor cells using a lentivirus system (31), and the resulting neural progenitor cells were plated onto Matrigel-coated coverslips and cultured for 2 weeks in motor neuron medium (50% DMEM/Ham's F-12 medium and 50% neurobasal medium (Invitrogen, 21103) containing 50 units/ml penicillin, 50 mg/ml streptomycin, 1 mm l-glutamine, 1% N2 supplement, 2% B27 supplement (Invitrogen, 17504-044), 1 μm retinoic acid (Sigma, R2625), and 1 μm recombinant human Sonic Hedgehog N-Terminus (R&D, 1314-SH)).
Immunoblot Analysis and Filter Retardation Assay
The cells were sonicated briefly in cold lysis buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 0.5% Nonidet P-40, 0.5% sodium deoxycholate, 0.25% sodium dodecyl sulfate, 5 mm EDTA, and protease inhibitor mixture (Sigma)). The concentration of total protein in the supernatant was determined using a Bio-Rad protein assay kit. Proteins were separated using reducing SDS-PAGE on a 4–20% Tris-glycine gradient gel (Invitrogen) and then transferred onto a polyvinylidene difluoride membrane (Millipore). The membrane was incubated with primary antibody followed by horseradish peroxidase-conjugated secondary antibody. Enhanced chemiluminescence reagents were used according to the manufacturer's instructions (PerkinElmer Life Sciences). Membranes processed as described above were subsequently stripped for 10 min at 60 °C in Restore Western blot stripping buffer (Thermo Scientific) and analyzed using different antibodies to detect the levels of different proteins in the same gel samples. Anti-AR N-20 (Santa Cruz; 1:500) and anti-β-actin (#4970, Cell Signaling; 1:1000) primary monoclonal antibodies were used in this portion of the study.
For filter retardation assays, samples were sequentially filtered through a 0.45-μm nitrocellulose membrane (Bio-Rad) and a 0.2-μm cellulose acetate membrane (Sartorius Stedim Biotech) filters using a slot blot apparatus (Bio-Rad). A total of 3 μg of protein was used for immunoblot analysis of both aggregated AR and total AR. The blots were probed as described above.
The optical intensity of samples assayed using the filter retardation assay was determined and analyzed using Image J software. The intensity of each sample was determined three times, and the data were statistically evaluated using JMP® version 9.0 software.
RESULTS
Generation of iPSCs from an SBMA Patient
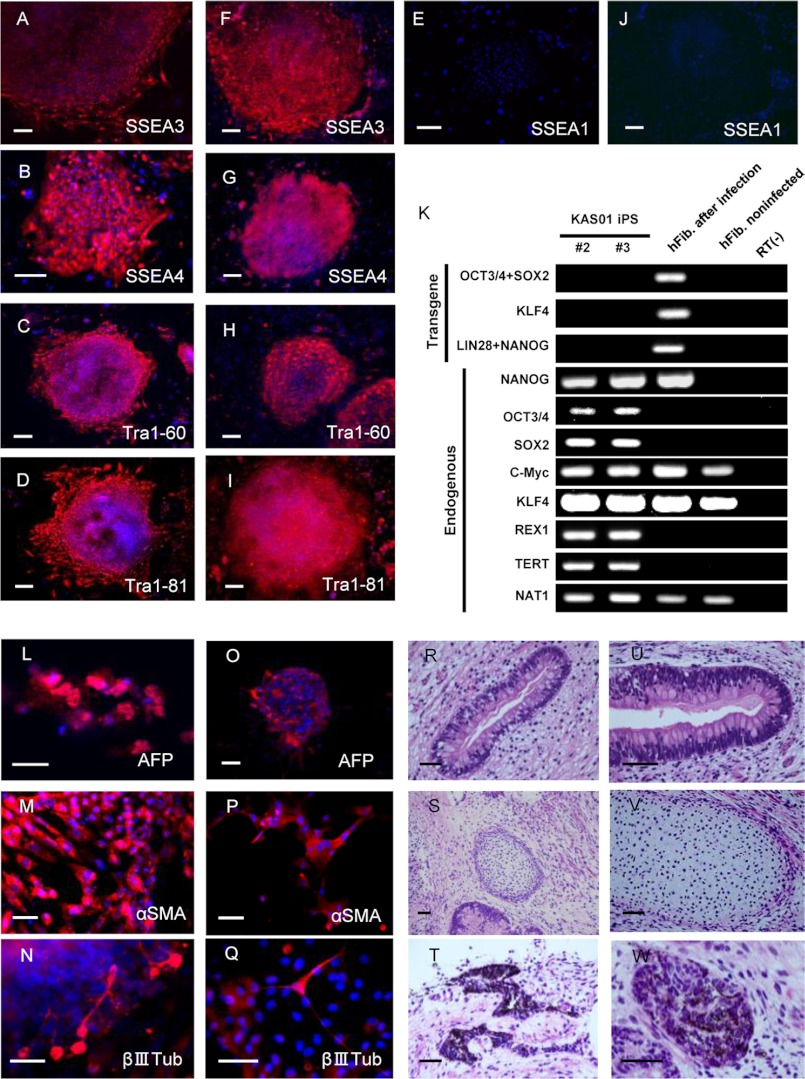
Using retroviral transduction with the five factors OCT4, SOX2, KLF4, LIN28, and NANOG, we established two iPSC clones (KAS01 #2 and #3) from primary human fibroblasts obtained from an 80-year-old male SBMA patient. The patient's first symptom was fasciculation of the upper limbs at age 58, at which time he was diagnosed with SBMA by a genetic analysis that revealed repeat expansion of the AR gene. Limb weakness and dysphagia slowly progressed, and the donor died of respiratory failure resulting from respiratory muscle palsy. Dermal fibroblasts were obtained postmortem using protocols approved by Keio University after informed consent was obtained from the family of the donor. Two previously described iPSC lines (PD01 #24 and #26) derived from male sporadic PD patients served as neurological controls (25). Both the KAS01 #2 and #3 iPSC clones demonstrated characteristics typical of pluripotent stem cells: similar morphology to embryonic stem cells, expression of pluripotency markers, including Tra-1–60, Tra-1–81, SSEA3, and SSEA4 (Fig. 1, A–J), silencing of retroviral transgenes, and reactivation of endogenous genes indicative of pluripotency (Fig. 1K). The differentiation capacity of clones KAS01 #2 and #3 was confirmed in vitro by the generation of three germ layers via embryoid body formation as determined using fluorescent immunostaining (Fig. 1, L–Q) and in vivo by teratoma formation as determined using HE staining (Fig. 1, R–W) and fluorescent immunostaining (data not shown).
FIGURE 1.
Generation of KAS01 #2 and #3 iPSCs and determination of their pluripotency. A–J, both KAS01 #2 and #3 iPSC lines exhibit markers of pluripotency (A–E, KAS01 #2; F–J, KAS01 #3). All iPSCs expressed the pluripotency markers Tra-1–60, Tra-1–81, SSEA3, and SSEA4. SSEA1 is used as a negative surface marker for undifferentiated stem cells. The nuclei were stained with DAPI. Scale bar, 200 μm. K, RT-PCR analysis of the transgenes OCT3/4, SOX2, KLF4, and the endogenous human embryonic stem cell marker genes. Patient fibroblasts 6 days after retroviral transduction are positive for the transgenes. hFib, healthy control fibroblast; RT(−), healthy control samples without RT-PCR. L–Q, embryoid bodies derived from KAS01 #2 and KAS01 #3 iPSCs express germ layer-specific markers, including α-fetoprotein (AFP, endoderm), α-smooth muscle actin (αSMA, mesoderm), and βIII-tubulin (βIIITub, ectoderm). L–N, KAS01 #2, O–Q: KAS01 #3. Scale bar, 100 μm. R–W, teratomas derived from SCID mice injected with KAS01 #2 and KAS01 #3 iPSCs. Representative images of hematoxylin and eosin staining of teratoma sections are shown (R–T, KAS01 #2; U–W, KAS01 #3). Tissues representing all three embryonic germ layers, including glandular structure (endoderm), cartilage (mesoderm), and pigmented epithelium (ectoderm), are visible in cells of both iPSC lines. Scale bar, 50 μm.
Differentiation of SBMA-iPSCs into Neurons
Neuronal differentiation of SBMA patient-specific iPSCs enables in vitro modeling of the disease pathogenesis. To determine whether neurons derived from SBMA-iPSCs show the SBMA phenotype, the KAS01 #2 and #3 iPSC lines, as well as the control PD-iPSC lines, were induced to differentiate into neural cells (32, 33) and cultured for 2 weeks on Matrigel-coated dishes with N2 medium. We confirmed expression of the neuronal markers βIII-tubulin and MAP2 (Fig. 2). Approximately 80% of the neural cells that differentiated from SBMA-iPSCs were positive for βIII-tubulin (201B7, 81.0 ± 5.8%; KAS01 #2, 78.1 ± 5.8%; KAS01 #3, 79.6 ± 5.0%, (n = 3)), and we detected no obvious differences with human iPSCs we described in a previous report with respect to generation of neurons from the SBMA-iPSCs (25, 35).
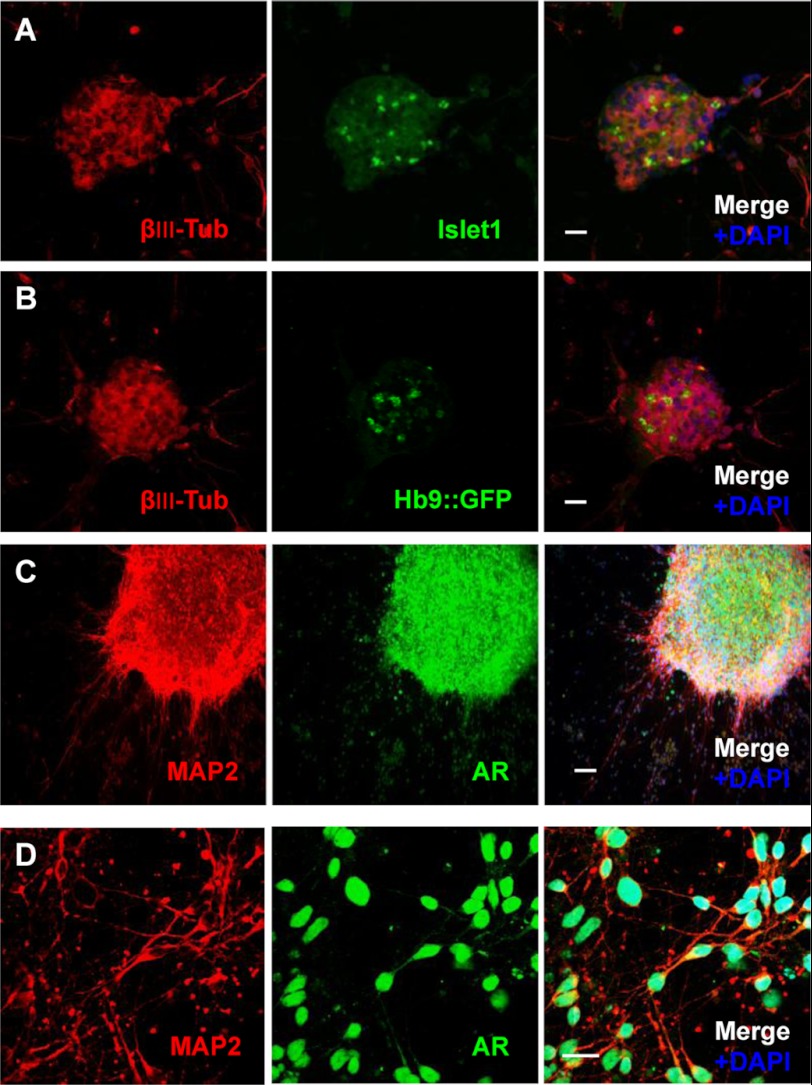
FIGURE 2.
Motor neuron differentiation of SBMA-iPSCs and AR expression in differentiated neurons. A and B, expression of the motor neuron progenitor marker islet-1 (A, green) and GFP under the control of the HB9 promoter for the mature neuron marker (green) in βIII-tubulin-positive (βIII-Tub, red) cells (B). Scale bar, 100 μm. C and D, differentiated neurons were double-stained with anti-MAP2 and anti-AR antibodies. ARs are localized primarily in the nuclei. Scale bar in C, 200 μm. Scale bar in D, 20 μm.
Neural cells derived from SBMA-iPSCs were cultured in motor neuron medium for 2 weeks, after which their capacity to differentiate into motor neurons was assessed by examining expression of the motor neuron progenitor marker islet-1 and a reporter specific for activity of the homeo box Hb9 (also called Mnx1 or Hlxb9) gene, which encodes a transcription factor specifically expressed by mature motor neurons (36). As shown in Fig. 2 (A and B), both the islet-1 protein and Hb9 reporter gene were expressed in differentiated neurons derived from the SBMA-iPSCs.
Stability of the CAG Repeat Number in SBMA-derived iPSCs
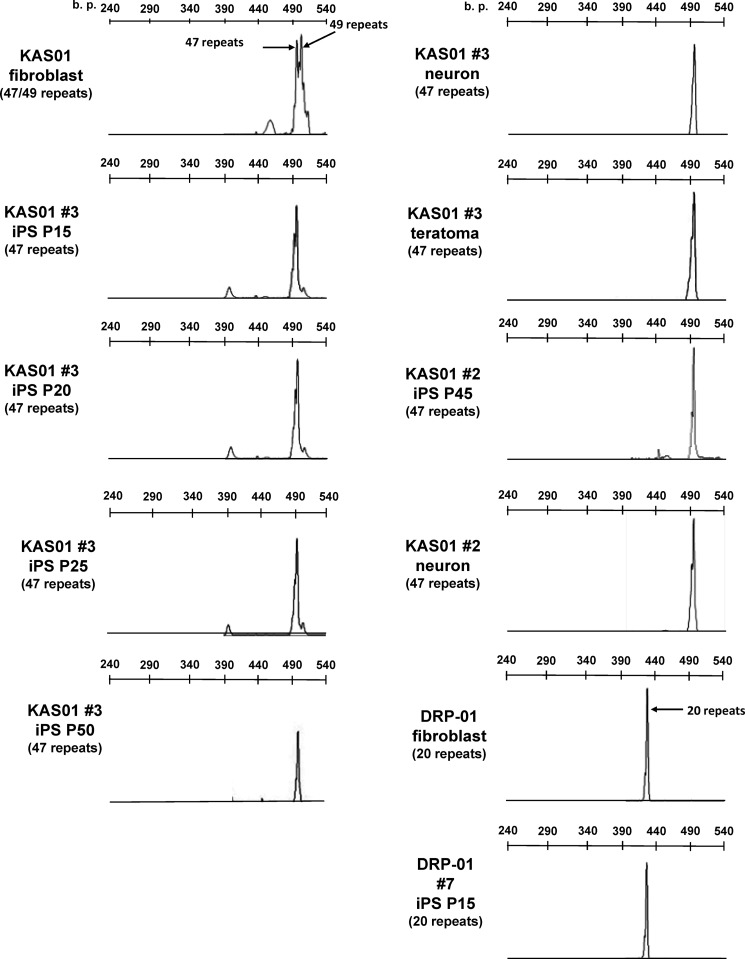
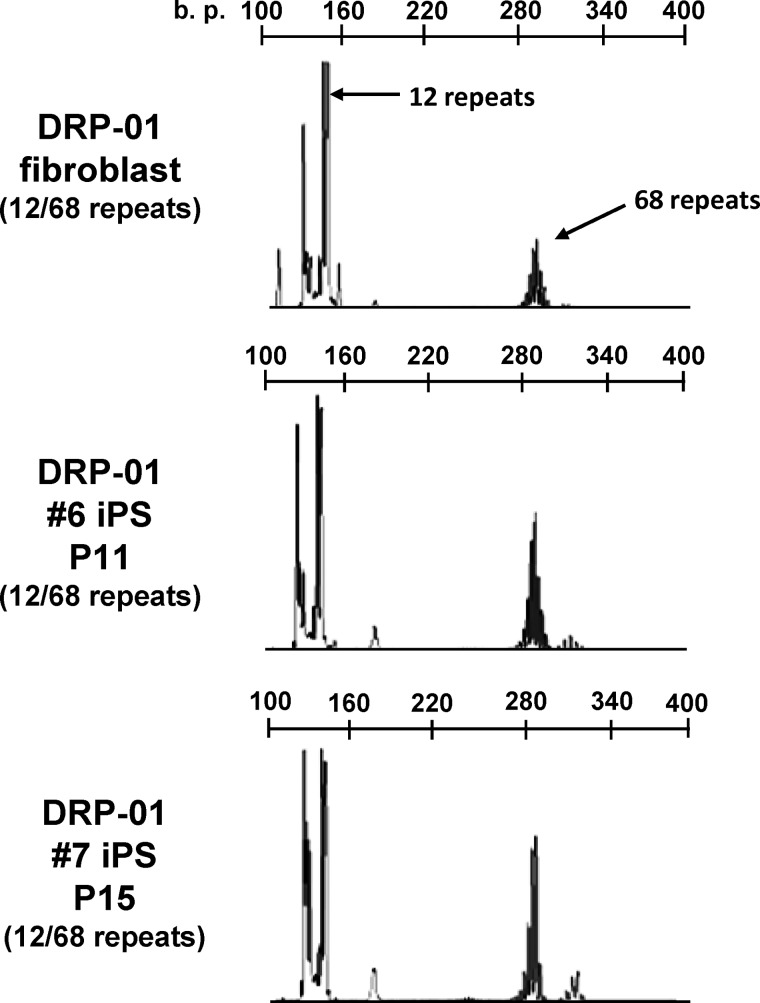
It has been reported that the number of GAA·TTC repeats in Friedreich ataxia-derived iPSCs and the number of CTG repeats in myotonic dystrophy-derived human embryonic stem cells are unstable over time in culture (26, 28). To determine the stability of the CAG repeat number in SBMA-derived iPSCs, we measured the length of the repeats in the AR gene during the reprogramming and differentiated stages in fibroblasts, iPSCs subjected to long term passage, differentiated neurons, and teratomas (Fig. 3). The results of GeneScan fragment analysis and direct sequencing revealed repeat numbers of 47 and 49 in the patient-derived fibroblasts, which is indicative of somatic mosaicism. Both the KAS01 #2 and #3 iPSCs had only 47 repeats, and long term passage of these cell lines did not affect the repeat number. In addition, the differentiated neurons and teratomas had the same number of CAG repeats. The stability of the CAG repeat number was also assessed in another polyQ disease-specific line of iPSCs by examining the number of repeats in the atrophin-1 gene in fibroblasts and iPSCs derived from a patient with DRPLA, which is characterized by marked repeat expansion during paternal transmission, resulting in prominent anticipation (Fig. 4). The CAG repeat numbers of 12 and 68 in the atrophin-1 gene of DRPLA-iPSCs were also quite stable, remaining unchanged after long term culture. These findings indicated that the number of CAG repeats in the causative gene of polyQ diseases is stable during reprogramming, maintenance, and differentiation of iPSCs.
FIGURE 3.
The CAG repeat number in the AR gene is stable during reprogramming and differentiation. GeneScan analyses indicating that fibroblasts have 47 and 49 CAG repeats in the AR gene are shown. Both KAS01 #2 and #3 iPSCs had only 47 repeats, even after long term passage. Differentiation (differentiated neurons and teratomas) does not influence the number of CAG repeats. DRP01 #7, which contains the normal 20 CAG repeats in the AR gene, was used as a control iPSC line. P indicates passage number.
FIGURE 4.
The number of CAG repeats in the atrophin-1 gene is stable during reprogramming. Shown are GeneScan analyses indicating that DRP-01 fibroblasts and DRP-01 #6 and DRP-01 #7 iPSCs had the same number of CAG repeats: 12 (normal allele) and 68 (mutant allele).
AR Expression Is Up-regulated by Neuronal Differentiation and Dihydrotestosterone
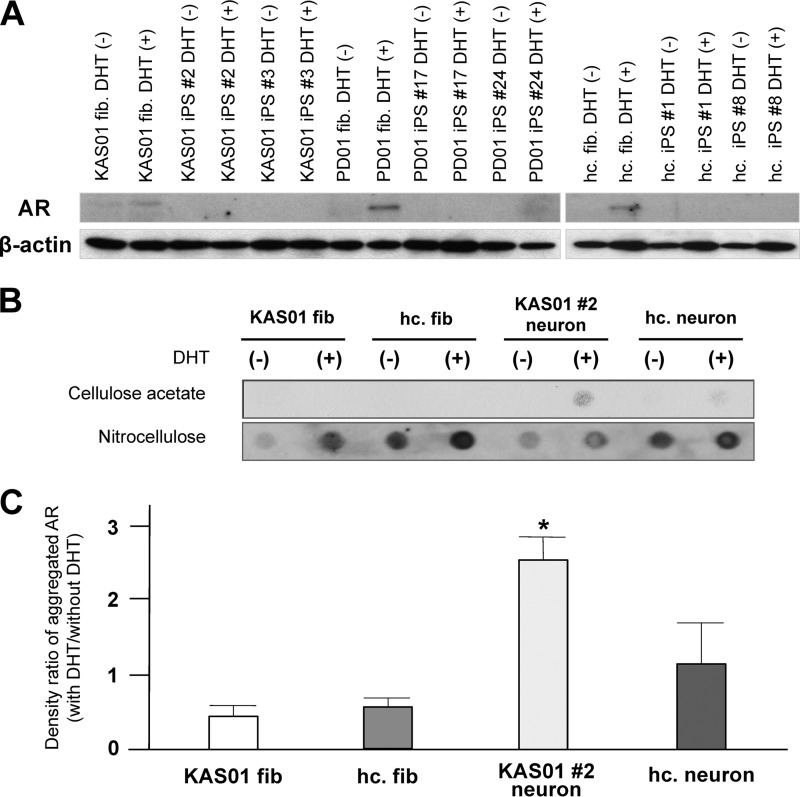
The AR agonist testosterone and the testosterone derivative dihydrotestosterone (DHT) lead to nuclear translocation of the AR and also regulate stabilization of the AR in neuronal tissue (10, 37–41). Several lines of evidence indicate that activation of mutant AR by its agonist is a critical step in the pathogenesis of SBMA. To biochemically characterize the dynamics of AR expression in SBMA-iPSCs, we investigated AR expression in fibroblasts, iPSCs, and differentiated neurons. Although expression of the AR was detectable by Western blotting in fibroblasts, it could not be detected in SBMA-iPSCs or control sporadic PD-iPSCs (PD01 #24 and PD01 #26) (Fig. 5A). However, expression of the AR reappeared in iPSC-derived neurons, indicating that AR expression is suppressed during the pluripotent state. Although there was some clonal variation with respect to the level of AR expression, the addition of DHT to the culture medium resulted in a sharp up-regulation of AR expression in differentiated neurons derived from all the iPSCs examined. There were no significant differences among the disease-specific iPSCs with respect to the relative fold increase in the total AR level following DHT addition (data not shown).
FIGURE 5.
DHT enhances aggregation of AR in SBMA-iPSC-derived neurons. A, analysis of AR expression in KAS01 fibroblasts, iPSCs, and differentiated neurons. Differentiated neurons were cultured with/without 50 nm DHT (DHT (+) or (−)) for 7 days. iPSC lines derived from an idiopathic PD patient (PD01 #24 or #26) were used as a control. AR expression was up-regulated by neuronal differentiation and DHT treatment in all iPSCs. B and C, results of filter retardation assays. The cellulose acetate membrane traps insoluble aggregated AR, whereas the nitrocellulose membrane traps total AR. Levels of aggregated AR (upper membrane) were determined using densitometry and normalized to the levels of total AR (lower membrane). The histogram shows the ratio of the level of aggregated AR in neurons treated with DHT to that in untreated neurons (means ± S.D.). Note that the level of aggregated AR in both KAS01 #2 and #3 neurons subjected to DHT treatment was significantly higher than the level of aggregated AR in PD01 #24 and PD01 #26 neurons. Differences were assessed using the Student's t test. *, versus KAS01 #2; p < 0.05. †, versus KAS01 #3, p < 0.05.
Aggregation of mutant AR was examined using a well established filter retardation assay procedure. The level of aggregated AR (which is retained on the cellulose acetate membrane) is determined using densitometry and normalized by the level of total AR (which is retained on the nitrocellulose membrane). Fig. 5 (B and C) show that the ratio of aggregated AR in neurons treated with DHT to that in untreated neurons was significantly higher in neurons derived from SBMA-iPSCs than in control PD iPSC-derived neurons, indicating that the androgen agonist enhances abnormal aggregation of the polyQ-expanded mutant AR in SBMA-iPSCs.
Pathological degeneration in SBMA patients is predominant in the nervous system. We thus asked whether aggregation of AR is restricted to neurons or whether it also occurs in other cell types, such as iPSCs and fibroblasts. KAS01 and PD01 iPSCs and healthy control fibroblasts were treated with DHT, after which Western blotting and filter retardation assays were performed (Fig. 6). Total AR expression was up-regulated by DHT in all fibroblasts but was not detectable in iPSCs. Remarkably, the level of aggregated AR in SBMA fibroblasts was quite a bit lower than that in SBMA-iPSC neurons (Fig. 6, B and C). Neurons derived from healthy control iPSCs showed a slight but insignificant aggregation of AR following DHT treatment. These data indicate that abnormal AR aggregation is prominent in neurons derived from SBMA-iPSCs.
FIGURE 6.
DHT does not enhance AR aggregation in SBMA-fibroblasts and iPSCs. A, analysis of AR expression in KAS01, PD01, healthy control fibroblasts, and iPSCs. Differentiated neurons were cultured with/without 50 nm DHT (DHT (+) or (−)) for 7 days. iPSC lines derived from an idiopathic PD patient (PD01 #17 or #24) and an iPSC line derived from a healthy volunteer were used as controls. Total AR expression was up-regulated by DHT treatment in all fibroblasts, but not in all iPSCs. B and C, results of filter retardation assays. The cellulose acetate membrane traps insoluble aggregated AR, whereas the nitrocellulose membrane traps total AR. Levels of aggregated AR (upper membrane) were determined using densitometry and normalized to the levels of total AR (lower membrane). The histogram shows the ratio of the level of aggregated AR in neurons treated with DHT to that in untreated neurons (means ± S.D.). Significant up-regulation of aggregated AR by addition of DHT was not observed with any fibroblasts. hc., healthy control. *, versus healthy control fibroblast (hc. fib.), p < 0.05.
Next, to investigate the key pathological events in SBMA, we sought to determine whether SBMA-iPSC-derived differentiated neurons exhibit AR inclusion bodies in the nucleus. As shown in Fig. 2 (C and D), ARs were localized primarily in the nucleus even in the absence of AR agonist, but no abnormal AR accumulation was detected in SBMA-iPSC-derived differentiated neurons under the described culture conditions.
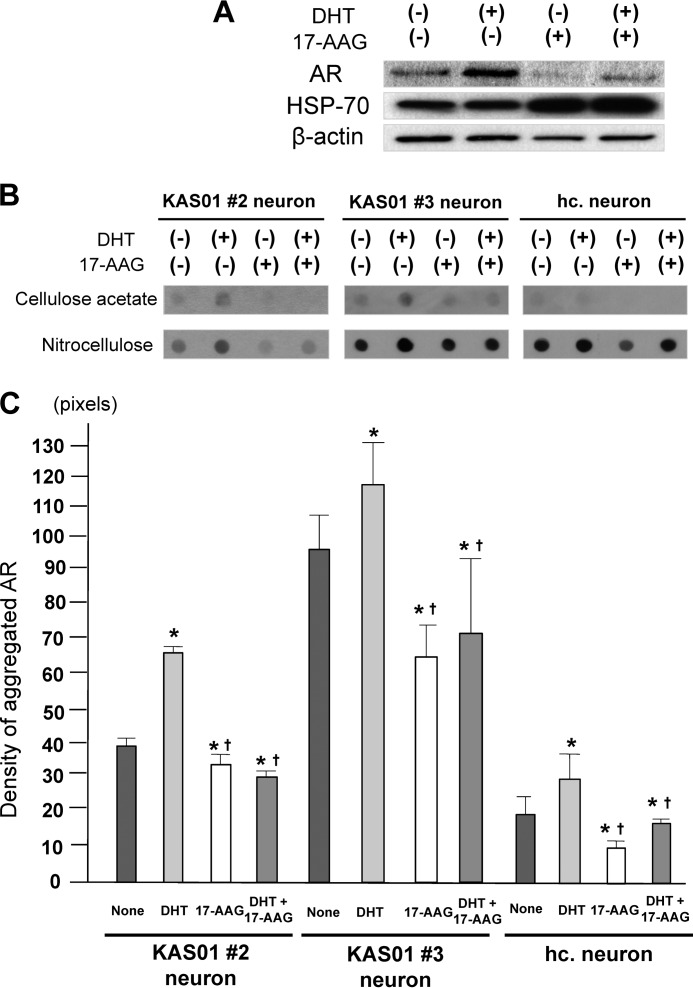
17-Allylaminogeldanamycin (17-AAG) Down-regulates the AR Level and Inhibits the Effect of DHT
To evaluate the potential for using SBMA-iPSCs in pharmacological drug screening studies, we assessed the level of mutant AR expression in SBMA-iPSC-derived neurons using the HSP90 inhibitor 17-AAG, which enhances degradation of the polyglutamine-expanded mutant AR (42, 43). Treatment of KAS01 #2 neurons with 165 nm 17-AAG for 24 h resulted in an expected increase in the expression of HSP70, which is known to be up-regulated by 17-AAG (Fig. 7A), whereas the levels of both total and aggregated mutant AR declined markedly. Similar results were found with KAS01 #3 neurons and healthy control neurons (Fig. 7, B and C). Although DHT treatment enhanced AR expression, treatment with 17-AAG resulted in down-regulation of total AR expression and a significant reduction in aggregated AR (Fig. 7, B and C). These results indicate that SBMA iPSC-derived neurons respond to candidate drug treatment in an expected manner (43).
FIGURE 7.
Effect of 17-AAG on the mutant AR in SBMA-iPSCs. A, KAS01 #2 neurons were cultured with/without 50 nm DHT and/or 165 nm 17-AAG. Note that treatment with 17-AAG resulted in a decrease in AR expression, regardless of the presence of DHT. B and C, filter retardation assay results demonstrated that 17-AAG effectively reduces the level of aggregated AR, even in the presence of DHT. The histogram in C shows the level of aggregated AR as determined by densitometric analysis in DHT- and/or 17-AAG-treated neurons and untreated neurons (means ± S.D.). Differences were assessed using the Student's t test. *, versus none, p < 0.05. †, versus DHT, p < 0.05.
We also treated KAS01, PD01, and healthy control fibroblasts with 17-AAG. As was the case with the neurons, total AR expression in each fibroblast line was down-regulated by 17-AAG; however, aggregated AR expression was too low to assess the effect of 17-AAG (data not shown).
DISCUSSION
To the best of our knowledge, this study is the first to demonstrate a model of SBMA using iPSC technology. We found that the number of CAG repeats is stable during reprogramming, long term passage, and differentiation in the mutant AR gene of SBMA-IPSCs and also in the DRPLA gene of DRPLA-iPSCs (Figs. 3 and 4). A recent study demonstrated that the GAA·TTC repeat observed in Friedreich ataxia and the CTG repeat characteristic of myotonic dystrophy are unstable during passage of human iPSCs or human embryonic stem cells (26, 28). Both repeat expansions are very large (∼100 to ∼1000 bp) in the noncoding regions, and instability with maternal transmission of the expanded allele has been described (44, 45). The mismatch repair enzyme MSH2, which is up-regulated during reprogramming, has been implicated in GAA·TTC repeat instability. In contrast, in polyQ diseases the expanded CAG trinucleotide coding region repeats (less than 100 bp), which increase in size during paternal transmission, are quite stable, even over long term passage. Similar stability of CAG repeats has also been described in Huntington disease and SCA3-iPSCs (27–29), suggesting that different processes contribute to expansion and/or contraction of repeats in between large trinucleotide repeats in noncoding regions and CAG coding repeat expansions.
The present study also demonstrated that neuronal differentiation and treatment with AR agonists induce dynamic changes in AR expression in iPSCs. As shown in Figs. 5A and 6A, AR could not be detected after fibroblasts were reprogrammed to iPSCs but was detectable after these cells differentiated into neuronal cells. We also found that treatment with DHT led to a significant increase in the level of AR protein in differentiated neurons, which is consistent with in vitro and in vivo studies showing that agonists increase the level of AR protein via its stabilization (46–48). It has been well established that AR activation by androgen hormones is a key event in the pathogenesis of SBMA (10, 39). Release from heat shock protein complexes allows the AR molecule with an expanded polyQ region to translocate to the nucleus and interact physically with other mutant AR molecules with expanded polyQ regions, producing aberrant conformational changes that lead to aggregation (49, 50). Our results demonstrated that the fold increase in the amount of aggregated mutant AR protein produced in SBMA neurons in response to DHT is significantly higher than in PD01 neurons and healthy control neurons (Figs. 5 and 6), indicating that AR aggregation is enhanced in SBMA-derived neurons treated with DHT, recapitulating the biochemical feature of SBMA (10–12).
Another important finding of the present study is that enhanced AR aggregation is the prominent neuron phenotype of this disease. Although total AR expression was markedly up-regulated by DHT treatment in both KAS01 fibroblasts and neuron lines #2 and #3 derived from SBMA-iPSCs, AR aggregation mediated by DHT was significantly higher in SBMA-iPSC-derived neurons compared with fibroblasts, indicating that neuron-specific factor(s) lead AR aggregation. A similar observation has been reported in a study of Machado-Joseph disease, in which SDS-insoluble aggregates of ATXN3 are restricted to patient-specific iPSC-derived neurons (27). This neuron-specific phenotype is dependent on calpain proteolysis activated by excitation-mediated Ca2+ influx. The results of our study indicate that activation of mutant AR by agonist is the critical step leading to abnormal aggregation, but aggregation of AR is substantially low in fibroblasts, in which total AR is strongly up-regulated by DHT (Fig. 6). Therefore, additional unknown factor(s) for aggregation must be involved in determining neuronal specificity, in addition to total AR expression.
Finally, to evaluate the potential of iPSC technology for use in drug screening, we examined the pharmacological response to the HSP90 inhibitor 17-AAG, which is a candidate drug for treating SBMA. The drug 17-AAG specifically binds to the ATP-binding site of HSP90, shifting the HSP90 complex to the proteasomal-targeting form, thus resulting in coordinated degradation of HSP90 clients, such as the AR (53). As expected, the level of aggregated AR decreased sharply upon addition of 17-AAG to cultures of SBMA-iPSCs (Fig. 7), confirming the usefulness of these cells for screening new drug compounds. Because SBMA was the first polyQ disease identified and the biochemical properties of the AR have been well characterized, we propose that living human neurons derived from SBMA-iPSCs are quite suitable for use in experiments aimed at elucidating the toxicity of polyQ expanded proteins and for use in development of new therapies.
The design of large scale drug screening studies using SBMA-iPSCs should take into account several limitations of the present study. First, obtaining a high yield of differentiated motor neurons from human iPSCs requires multi-step procedures, prolonged culture periods, and cell sorting techniques. A high degree of heterogeneity of differentiated neuronal cells and a dependence of the differentiation efficiency on clonal variability and passage number are also characteristics of the currently available differentiation methods (33, 54, 55). Although a recently described differentiation strategy reportedly allows for rapid generation of induced motor neurons from human pluripotent stem cells using gene-delivered transcription factors (11 days after gene delivery, with >60–70% efficiency) (56), the development of reliable protocols for more efficient neuronal differentiation with minimal clonal variation, as well as new cell sorting techniques, is necessary.
The second limitation of the present study is that we were unable to demonstrate one of the key pathological phenotypes of SBMA, nuclear inclusions, by immunocytochemistry. This result was not unexpected, however, because a recent study using SCA3-iPSCs also failed to detect formation of inclusion bodies in patient-derived neurons, despite the clearly observable formation of insoluble aggregates of ATXN3 upon l-glutamate excitation, indicating that age-dependent processes play a critical role in recapitulation of pathological features (27). Recently, Sánchez-Danés et al. (57) demonstrated that over extended culture periods (75 days), neurons cocultured with astrocytes can recapitulate disease-specific morphological phenotypes, such as reduced neurite formation and accumulation of autophagic vacuoles in dopamine neurons from iPSC-based models of sporadic PD. Because the donor from whom we obtained fibroblasts to generate SBMA-iPSCs suffered from a late-onset and slowly progressing disease, the appropriate period to culture iPSC-derived neurons to show the pathological phenotype of SBMA must be determined in a future study.
Although this study was limited to a single patient line with subclones, we clearly recapitulated disease-specific biochemical features and demonstrated the potential for identification and validation of candidate drugs using our SBMA-iPSC lines. Although drugs such as leuprorelin have recently emerged as promising candidates for treating SBMA, unfortunately it has been difficult to verify their clinical benefits because SBMA is a slowly progressing disease (58). Although additional advances in iPSC methods are needed before iPSCs can be used in clinical applications to treat neurodegeneration, we hope that the results of our study will contribute to the identification and validation of novel candidate medicines for the treatment of polyQ diseases, including SBMA.
Acknowledgments
We also thank Dr. Fred H. Gage for providing the Lenti-Hb9::GFP plasmid (Laboratory of Genetics, Salk Institute for Biological Studies, CA), and Dr. Shinya Yamanaka for providing the 201B7 iPSC line (Center for iPS Cell Research and Application).
This work was supported by grants from Eisai Co., Ltd. (to D. I. and N. S.), the ALS Foundation, the Japan ALS Association (to Y. N.), the Nakabayashi Trust for ALS Research (to Y. N.), and a grant from the Project for the Realization of Regenerative Medicine of the Ministry of Education, Culture, Sports, Science and Technology of Japan (to H. O.).
Y. Okada, F. Miya, M. Koike, S. Tomisato, T. Tokura, Y. Ishihara, D. Shimojo, C. Hattori, D. Kanematsu, Y. Kanemura, K. Kohda, G. Sobue, S. Yamanaka, M. Yuzaki, Y. Uchiyama, E. Ikeda, T. Tsunoda, and H. Okano, submitted.
- SBMA
- spinal and bulbar muscular atrophy
- polyQ
- polyglutamine
- AR
- androgen receptor
- iPSC
- induced pluripotent stem cell
- SCA
- spinocerebellar ataxia
- PD
- Parkinson disease
- DRPLA
- dentato-rubro-pallido-luysian dystrophy
- DHT
- dihydrotestosterone
- HSP
- heat shock protein
- 17-AAG
- 17-allylaminogeldanamycin.
REFERENCES
- 1. Kennedy W. R., Alter M., Sung J. H. (1968) Progressive proximal spinal and bulbar muscular atrophy of late onset. A sex-linked recessive trait. Neurology 18, 671–680 [DOI] [PubMed] [Google Scholar]
- 2. La Spada A. R., Wilson E. M., Lubahn D. B., Harding A. E., Fischbeck K. H. (1991) Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature 352, 77–79 [DOI] [PubMed] [Google Scholar]
- 3. Brooks B. P., Fischbeck K. H. (1995) Spinal and bulbar muscular atrophy. A trinucleotide repeat expansion neurodegenerative disease. Trends Neurosci., 18, 459–461 [DOI] [PubMed] [Google Scholar]
- 4. Fischbeck K. H. (1997) Kennedy disease. J. Inher. Metab. Dis. 20, 152–158 [DOI] [PubMed] [Google Scholar]
- 5. Fischbeck K. H. (2001) PolyQ expansion neurodegenerative disease. Brain Res. Bull. 56, 161–163 [DOI] [PubMed] [Google Scholar]
- 6. Kim T. W., Tanzi R. E. (1998) Neuronal intranuclear inclusions in polyQ diseases. Nuclear weapons or nuclear fallout? Neuron 21, 657–659 [DOI] [PubMed] [Google Scholar]
- 7. Zoghbi H. Y., Orr H. T. (2000) Glutamine repeats and neurodegeneration. Annu. Rev. Neurosci. 23, 217–247 [DOI] [PubMed] [Google Scholar]
- 8. Paulson H. L. (2000) Toward an understanding of polyQ neurodegeneration. Brain Pathol. 10, 293–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takeyama K., Ito S., Yamamoto A., Tanimoto H., Furutani T., Kanuka H., Miura M., Tabata T., Kato S. (2002) Androgen-dependent neurodegeneration by polyglutamine-expanded human androgen receptor in Drosophila. Neuron 35, 855–864 [DOI] [PubMed] [Google Scholar]
- 10. Katsuno M., Adachi H., Kume A., Li M., Nakagomi Y., Niwa H., Sang C., Kobayashi Y., Doyu M., Sobue G. (2002) Testosterone reduction prevents phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Neuron 35, 843–854 [DOI] [PubMed] [Google Scholar]
- 11. Montie H. L., Cho M. S., Holder L., Liu Y., Tsvetkov A. S., Finkbeiner S., Merry D. E. (2009) Cytoplasmic retention of polyQ-expanded androgen receptor ameliorates disease via autophagy in a mouse model of spinal and bulbar muscular atrophy. Hum. Mol. Genet. 18, 1937–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nedelsky N. B., Pennuto M., Smith R. B., Palazzolo I., Moore J., Nie Z., Neale G., Taylor J. P. (2010) Native functions of the androgen receptor are essential to pathogenesis in a Drosophila model of spinobulbar muscular atrophy. Neuron 67, 936–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 [DOI] [PubMed] [Google Scholar]
- 14. Yu J., Vodyanik M. A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J. L., Tian S., Nie J., Jonsdottir G. A., Ruotti V., Stewart R., Slukvin I. I., Thomson J. A. (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 [DOI] [PubMed] [Google Scholar]
- 15. Ito D., Okano H., Suzuki N. (2012) Accelerating progress in iPS cell research for neurological diseases. Ann. Neurol. 72, 167–174 [DOI] [PubMed] [Google Scholar]
- 16. Dimos J. T., Rodolfa K. T., Niakan K. K., Weisenthal L. M., Mitsumoto H., Chung W., Croft G. F., Saphier G., Leibel R., Goland R., Wichterle H., Henderson C. E., Eggan K. (2008) Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science 321, 1218–1221 [DOI] [PubMed] [Google Scholar]
- 17. Park I. H., Arora N., Huo H., Maherali N., Ahfeldt T., Shimamura A., Lensch M. W., Cowan C., Hochedlinger K., Daley G. Q. (2008) Disease-specific induced pluripotent stem cells. Cell 134, 877–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Soldner F., Hockemeyer D., Beard C., Gao Q., Bell G. W., Cook E. G., Hargus G., Blak A., Cooper O., Mitalipova M., Isacson O., Jaenisch R. (2009) Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell 136, 964–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ebert A. D., Yu J., Rose F. F., Jr., Mattis V. B., Lorson C. L., Thomson J. A., Svendsen C. N. (2009) Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature 457, 277–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee G., Papapetrou E. P., Kim H., Chambers S. M., Tomishima M. J., Fasano C. A., Ganat Y. M., Menon J., Shimizu F., Viale A., Tabar V., Sadelain M., Studer L. (2009) Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature 461, 402–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chamberlain S. J., Chen P. F., Ng K. Y., Bourgois-Rocha F., Lemtiri-Chlieh F., Levine E. S., Lalande M. (2010) Induced pluripotent stem cell models of the genomic imprinting disorders Angelman and Prader-Willi syndromes. Proc. Natl. Acad. Sci. U.S.A. 107, 17668–17673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nguyen H. N., Byers B., Cord B., Shcheglovitov A., Byrne J., Gujar P., Kee K., Schüle B., Dolmetsch R. E., Langston W., Palmer T. D., Pera R. R. (2011) LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell 8, 267–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marchetto M. C., Carromeu C., Acab A., Yu D., Yeo G. W., Mu Y., Chen G., Gage F. H., Muotri A. R. (2010) A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell 143, 527–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mitne-Neto M., Machado-Costa M., Marchetto M. C., Bengtson M. H., Joazeiro C. A., Tsuda H., Bellen H. J., Silva H. C., Oliveira A. S., Lazar M., Muotri A. R., Zatz M. (2011) Downregulation of VAPB expression in motor neurons derived from induced pluripotent stem cells of ALS8 patients. Hum. Mol. Genet. 20, 3642–3652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yagi T., Ito D., Okada Y., Akamatsu W., Nihei Y., Yoshizaki T., Yamanaka S., Okano H., Suzuki N. (2011) Modeling familial Alzheimer's disease with induced pluripotent stem cells. Hum. Mol. Genet. 20, 4530–4539 [DOI] [PubMed] [Google Scholar]
- 26. Ku S., Soragni E., Campau E., Thomas E. A., Altun G., Laurent L. C., Loring J. F., Napierala M., Gottesfeld J. M. (2010) Friedreich's ataxia induced pluripotent stem cells model intergenerational GAA TTC triplet repeat instability. Cell Stem Cell 7, 631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koch P., Breuer P., Peitz M., Jungverdorben J., Kesavan J., Poppe D., Doerr J., Ladewig J., Mertens J., Tüting T., Hoffmann P., Klockgether T., Evert B. O., Wüllner U., Brüstle O. (2011) Excitation-induced ataxin-3 aggregation in neurons from patients with Machado-Joseph disease. Nature 480, 543–546 [DOI] [PubMed] [Google Scholar]
- 28. Seriola A., Spits C., Simard J. P., Hilven P., Haentjens P., Pearson C. E., Sermon K. (2011) Huntington's and myotonic dystrophy hESCs. Down-regulated trinucleotide repeat instability and mismatch repair machinery expression upon differentiation. Hum. Mol. Genet. 20, 176–185 [DOI] [PubMed] [Google Scholar]
- 29. Zhang N., An M. C., Montoro D., Ellerby L. M. (2010) Characterization of human Huntington's disease cell model from induced pluripotent stem cells. PLoS Curr. 2, RRN1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tanaka F., Doyu M., Ito Y., Matsumoto M., Mitsuma T., Abe K., Aoki M., Itoyama Y., Fischbeck K. H., Sobue G. (1996) Founder effect in spinal and bulbar muscular atrophy (SBMA). Hum. Mol. Genet. 5, 1253–1257 [DOI] [PubMed] [Google Scholar]
- 31. Takiyama Y., Sakoe K., Amaike M., Soutome M., Ogawa T., Nakano I., Nishizawa M. (1999) Single sperm analysis of the CAG repeats in the gene for dentatorubral-pallidoluysian atrophy (DRPLA). The instability of the CAG repeats in the DRPLA gene is prominent among the CAG repeat diseases. Hum. Mol. Genet. 8, 453–457 [DOI] [PubMed] [Google Scholar]
- 32. Okada Y., Matsumoto A., Shimazaki T., Enoki R., Koizumi A., Ishii S., Itoyama Y., Sobue G., Okano H. (2008) Spatiotemporal recapitulation of central nervous system development by murine embryonic stem cell-derived neural stem/progenitor cells. Stem Cells 26, 3086–3098 [DOI] [PubMed] [Google Scholar]
- 33. Miura K., Okada Y., Aoi T., Okada A., Takahashi K., Okita K., Nakagawa M., Koyanagi M., Tanabe K., Ohnuki M., Ogawa D., Ikeda E., Okano H., Yamanaka S. (2009) Variation in the safety of induced pluripotent stem cell lines. Nat. Biotechnol. 27, 743–745 [DOI] [PubMed] [Google Scholar]
- 34. Nori S., Okada Y., Yasuda A., Tsuji O., Takahashi Y., Kobayashi Y., Fujiyoshi K., Koike M., Uchiyama Y., Ikeda E., Toyama Y., Yamanaka S., Nakamura M., Okano H. (2011) Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc. Natl. Acad. Sci. U.S.A. 108, 16825–16830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yagi T., Kosakai A., Ito D., Okada Y., Akamatsu W., Nihei Y., Nabetani A., Ishikawa F., Arai Y., Hirose H., Okano H., Suzuki N. (July 25, 2012) Establishment of induced pluripotent stem cells from centenarians for neurodegenerative disease research. PLoS One 7, e41572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marchetto M. C., Muotri A. R., Mu Y., Smith A. M., Cezar G. G., Gage F. H. (2008) Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell 3, 649–657 [DOI] [PubMed] [Google Scholar]
- 37. Schmidt B. J., Greenberg C. R., Allingham-Hawkins D. J., Spriggs E. L. (2002) Expression of X-linked bulbospinal muscular atrophy (Kennedy disease) in two homozygous women. Neurology 59, 770–772 [DOI] [PubMed] [Google Scholar]
- 38. Katsuno M., Adachi H., Doyu M., Minamiyama M., Sang C., Kobayashi Y., Inukai A., Sobue G. (2003) Leuprorelin rescues polyQ-dependent phenotypes in a transgenic mouse model of spinal and bulbar muscular atrophy. Nat. Med. 9, 768–773 [DOI] [PubMed] [Google Scholar]
- 39. Chevalier-Larsen E. S., O'Brien C. J., Wang H., Jenkins S. C., Holder L., Lieberman A. P., Merry D. E. (2004) Castration restores function and neurofilament alterations of aged symptomatic males in a transgenic mouse model of spinal and bulbar muscular atrophy. J. Neurosci. 24, 4778–4786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lu S., Simon N. G., Wang Y., Hu S. (1999) Neural androgen receptor regulation. Effects of androgen and antiandrogen. J. Neurobiol. 41, 505–512 [DOI] [PubMed] [Google Scholar]
- 41. Palazzolo I., Gliozzi A., Rusmini P., Sau D., Crippa V., Simonini F., Onesto E., Bolzoni E., Poletti A. (2008) The role of the polyQ tract in androgen receptor. J. Steroid Biochem. Mol. Biol. 108, 245–253 [DOI] [PubMed] [Google Scholar]
- 42. Rusmini P., Simonini F., Crippa V., Bolzoni E., Onesto E., Cagnin M., Sau D., Ferri N., Poletti A. (2011) 17-AAG increases autophagic removal of mutant androgen receptor in spinal and bulbar muscular atrophy. Neurobiol. Dis. 41, 83–95 [DOI] [PubMed] [Google Scholar]
- 43. Waza M., Adachi H., Katsuno M., Minamiyama M., Sang C., Tanaka F., Inukai A., Doyu M., Sobue G. (2005) 17-AAG, an Hsp90 inhibitor, ameliorates polyQ-mediated motor neuron degeneration. Nat. Med. 11, 1088–1095 [DOI] [PubMed] [Google Scholar]
- 44. De Michele G., Cavalcanti F., Criscuolo C., Pianese L., Monticelli A., Filla A., Cocozza S. (1998) Parental gender, age at birth and expansion length influence GAA repeat intergenerational instability in the X25 gene. Pedigree studies and analysis of sperm from patients with Friedreich's ataxia. Hum. Mol. Genet. 7, 1901–1906 [DOI] [PubMed] [Google Scholar]
- 45. Martorell L., Cobo A. M., Baiget M., Naudó M., Poza J. J., Parra J. (2007) Prenatal diagnosis in myotonic dystrophy type 1. Thirteen years of experience. Implications for reproductive counselling in DM1 families. Prenat. Diagn. 27, 68–72 [DOI] [PubMed] [Google Scholar]
- 46. Chang C. Y., Hsuuw Y. D., Huang F. J., Shyr C. R., Chang S. Y., Huang C. K., Kang H. Y., Huang K. E. (2006) Androgenic and antiandrogenic effects and expression of androgen receptor in mouse embryonic stem cells. Fertil. Steril. 85, 1195–1203 [DOI] [PubMed] [Google Scholar]
- 47. Kemppainen J. A., Lane M. V., Sar M., Wilson E. M. (1992) Androgen receptor phosphorylation, turnover, nuclear transport, and transcriptional activation. Specificity for steroids and antihormones. J. Biol. Chem. 267, 968–974 [PubMed] [Google Scholar]
- 48. Burnstein K. L., Maiorino C. A., Dai J. L., Cameron D. J. (1995) Androgen and glucocorticoid regulation of androgen receptor cDNA expression. Mol. Cell. Endocrinol. 115, 177–186 [DOI] [PubMed] [Google Scholar]
- 49. Perutz M. F., Johnson T., Suzuki M., Finch J. T. (1994) Glutamine repeats as polar zippers. Their possible role in inherited neurodegenerative diseases. Proc. Natl. Acad. Sci. U.S.A. 91, 5355–5358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rusmini P., Sau D., Crippa V., Palazzolo I., Simonini F., Onesto E., Martini L., Poletti A. (2007) Aggregation and proteasome. The case of elongated polyQ aggregation in spinal and bulbar muscular atrophy. Neurobiol. Aging 28, 1099–1111 [DOI] [PubMed] [Google Scholar]
- 51. Deleted in proof.
- 52. Deleted in proof.
- 53. Neckers L. (2002) Heat shock protein 90 inhibition by 17-allylamino-17-demethoxygeldanamycin. A novel therapeutic approach for treating hormone-refractory prostate cancer. Clin. Cancer Res. 8, 962–966 [PubMed] [Google Scholar]
- 54. Hu B. Y., Weick J. P., Yu J., Ma L. X., Zhang X. Q., Thomson J. A., Zhang S. C. (2010) Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc. Natl. Acad. Sci. U.S.A. 107, 4335–4340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Boulting G. L., Kiskinis E., Croft G. F., Amoroso M. W., Oakley D. H., Wainger B. J., Williams D. J., Kahler D. J., Yamaki M., Davidow L., Rodolfa C. T., Dimos J. T., Mikkilineni S., MacDermott A. B., Woolf C. J., Henderson C. E., Wichterle H., Eggan K. (2011) A functionally characterized test set of human induced pluripotent stem cells. Nat. Biotechnol. 29, 279–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hester M. E., Murtha M. J., Song S., Rao M., Miranda C. J., Meyer K., Tian J., Boulting G., Schaffer D. V., Zhu M. X., Pfaff S. L., Gage F. H., Kaspar B. K. (2011) Rapid and efficient generation of functional motor neurons from human pluripotent stem cells using gene delivered transcription factor codes. Mol. Ther. 19, 1905–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sánchez-Danés A., Richaud-Patin Y., Carballo-Carbajal I., Jiménez-Delgado S., Caig C., Mora S., Di Guglielmo C., Ezquerra M., Patel B., Giralt A., Canals J. M., Memo M., Alberch J., López-Barneo J., Vila M., Cuervo A. M., Tolosa E., Consiglio A., Raya A. (2012) Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson's disease. EMBO Mol. Med. 4, 380–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Katsuno M., Banno H., Suzuki K., Takeuchi Y., Kawashima M., Yabe I., Sasaki H., Aoki M., Morita M., Nakano I., Kanai K., Ito S., Ishikawa K., Mizusawa H., Yamamoto T., Tsuji S., Hasegawa K., Shimohata T., Nishizawa M., Miyajima H., Kanda F., Watanabe Y., Nakashima K., Tsujino A., Yamashita T., Uchino M., Fujimoto Y., Tanaka F., Sobue G. Japan SBMA Interventional Trial for TAP-144-SR (JASMITT) study group (2010) Efficacy and safety of leuprorelin in patients with spinal and bulbar muscular atrophy (JASMITT study). A multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 9, 875–884 [DOI] [PubMed] [Google Scholar]