Background: Mechanical stimulation prevents osteocyte apoptosis and activates Wnt signaling.
Results: ERK-mediated anti-apoptosis is abolished by antagonists of Wnt signaling, and conversely, β-catenin accumulation is blocked by inhibiting the caveolin-1/ERK pathway.
Conclusion: Caveolin-1/ERK and Wnt/β-catenin signaling pathways cooperate in transducing mechanical cues into osteocyte survival.
Significance: This novel bidirectional crosstalk might be targeted to increase bone strength by preserving osteocyte viability.
Keywords: β-Catenin, Caveolin, ERK, Mechanotransduction, Osteocyte, Apoptosis
Abstract
Osteocyte viability is a critical determinant of bone strength and is promoted by both mechanical stimulation and activation of the Wnt signaling pathway. Earlier studies demonstrated that both stimuli promote survival of osteocytes by activating the ERKs. Here, we show that there is interaction between the caveolin-1/ERK and Wnt/β-catenin signaling pathways in the transduction of mechanical cues into osteocyte survival. Thus, ERK nuclear translocation and anti-apoptosis induced by mechanical stimulation are abolished by the Wnt antagonist Dkk1 and the β-catenin degradation stimulator Axin2. Conversely, GSK3β phosphorylation and β-catenin accumulation induced by mechanical stimulation are abolished by either pharmacologic inhibition of ERKs or silencing caveolin-1. In contrast, the canonical Wnt signaling inhibitor dominant-negative T cell factor does not alter ERK nuclear translocation or survival induced by mechanical stimulation. These findings demonstrate that β-catenin accumulation is an essential component of the mechanotransduction machinery in osteocytes, albeit β-catenin/T cell factor-mediated transcription is not required. The simultaneous requirement of β-catenin for ERK activation and of ERK activation for β-catenin accumulation suggests a bidirectional crosstalk between the caveolin-1/ERK and Wnt/β-catenin pathways in mechanotransduction leading to osteocyte survival.
Introduction
Mechanical force is an important regulator of bone mass, shape, and microarchitecture (1). The skeleton is able to continually adapt by increasing bone formation by osteoblasts in response to increased load or by increasing bone resorption by osteoclasts in response to either excessive loading or skeletal disuse. Recent evidence demonstrates that osteocytes, the long-proposed mechanosensor cells of bone, regulate osteoblast and osteoclast activity through changes in the expression of genes that affect osteoblast and osteoclast generation. The strategic location of osteocytes permits the detection of minimal variations in the level of strain and the transduction of mechanical cues into bone gain or loss by sending signals to osteoblasts and osteoclasts through the lacunar-canalicular network (2). In particular, osteocytes secrete sclerostin, an inhibitor of Wnt signaling that, when absent, leads to high bone mass (3–5). Moreover, osteocytes are the main source of the pro-osteoclastogenic cytokine RANKL (receptor activator of nuclear factor-κB ligand) in bone (6), and they also secrete the RANKL decoy receptor osteoprotegerin, which inhibits bone resorption (7).
Wnt proteins are a family of secreted glycoproteins involved in multiple cellular processes, including proliferation, differentiation, and viability (8, 9), and they play a major role in bone homeostasis (10, 11). The Wnt canonical pathway is activated by binding of Wnt proteins to the Fzd (frizzled) receptors and the co-receptors LRP5 and LRP6 (LDL receptor-related protein) (12, 13). This binding leads to the inactivation of GSK3β (glycogen synthase kinase 3β), stabilization of β-catenin, and its accumulation. In turn, β-catenin translocates to the nucleus, where it binds to the T cell factor (TCF)2/lymphoid enhancer factor family of transcription factors and induces gene transcription (14, 15). The Wnt/β-catenin pathway is essential for bone formation in response to mechanical loading (16, 17). Loading inhibits the expression of the Wnt antagonists Sost and Dkk1 (18), and down-regulation of Sost/sclerostin is required to achieve bone anabolism in response to ulna loading (19). In addition, mice lacking the LRP5 receptor (16) or lacking one copy of β-catenin in osteocytes (20) exhibit defective response to loading.
Mechanical stimuli also control the life span of osteocytes. Physiological levels of load are required to maintain osteocyte viability, as demonstrated by increased prevalence of osteocyte apoptosis with disuse, whereas mechanical stimulation decreases osteocyte death (21–23). However, how mechanical forces are transduced into cellular responses is only partially known. Earlier work demonstrated that the anti-apoptotic effect of mechanical stimulation on osteocytes requires integrin signaling, the kinase activities of Src and focal adhesion kinase, and downstream phosphorylation and nuclear translocation of the ERKs (24). ERK activation and osteocyte survival induced by mechanical stimulation are abolished by β-cyclodextrin, a cholesterol chelator that disrupts membrane microdomains called caveolae. Caveolin-1, the structural component of caveolae, interacts with ERKs and integrin β1 in MLO-Y4 osteocytic cells (24), suggesting the involvement of caveolin-1 in mechanotransduction in osteocytes.
Activation of Wnt signaling also leads to survival of osteoblastic cells, as evidenced by the reduction in osteoblast and osteocyte apoptosis in mice lacking the Wnt inhibitor sFRP1 (soluble frizzled-related protein 1) (25). Wnt proteins prevent osteoblast and osteocyte apoptosis by a mechanism that requires activation of the Src/ERK signaling pathway (26). β-Catenin accumulation was recently shown to be involved in fluid flow-induced anti-apoptosis in osteocytic cells (27). In addition, ERKs can activate Wnt signaling by phosphorylating both LRP6 and β-catenin (28, 29), suggesting the existence of a feed-forward loop that amplifies the prosurvival effect of the ERK pathway through Wnt/β-catenin signaling.
We investigated the crosstalk between the caveolin-1/ERK and Wnt/β-catenin signaling pathways in mechanotransduction leading to osteocyte survival. We report that caveolin-1 and ERK activation are required for β-catenin accumulation induced by mechanical stimulation, which conversely is essential for ERK nuclear translocation and osteocyte survival induced by mechanical signals, even though TCF-mediated transcription is not required.
EXPERIMENTAL PROCEDURES
Materials
The synthetic glucocorticoid dexamethasone was purchased from Sigma. PD98059 was from New England Biolabs (Beverly, MA), and Wnt3a recombinant protein was from R&D Systems (Minneapolis, MN).
Cell Culture
MLO-Y4 osteocytic cells derived from murine long bones were cultured as described previously (30, 31).
Plasmids and Transient Transfections
A reporter plasmid containing three TCF-binding sites upstream of a minimal c-fos promoter driving the firefly luciferase gene (TOPflash) was provided by B. Vogelstein (John Hopkins University Medical Institutions, Baltimore, MD). The plasmids expressing Axin2 and Dkk1 were provided by F. Costantini (Department of Genetics and Development, College of Physicians and Surgeons, Columbia University, New York, NY) and by C. Niehrs (Division of Molecular Embryology, Deutsches Krebsforschungszentrum, Heidelberg, Germany), respectively. Dominant-negative TCF was provided by G. Rawadi (ProSkelia, Paris, France). Wild-type ERK2 fused to red fluorescent protein (RFP) and wild-type MEK were kindly provided by L. Luttrell (Medical University of South Carolina, Charleston, SC) (32) and N. G. Ahn (University of Colorado, Boulder, CO) (33), respectively. The plasmid encoding nuclear targeted green fluorescent protein (nGFP) was described previously (31). Cells were transiently transfected with 0.1 μg/cm2 DNA using Lipofectamine Plus (Invitrogen) as described previously (34). The efficiency of transfection was 60–80%.
TCF-mediated Transcription
Cells were transiently transfected with TCF-firefly luciferase and Renilla luciferase. To test the efficiency of the effect of the Wnt inhibitors, cells were cotransfected with Dkk1, Axin2, or dominant-negative TCF together with empty vector or a Wnt3a-expressing construct and cultured for 24 h. In the experiments testing the effect of mechanical stimulation on TCF-mediated transcription, cells were either cultured in the presence of Wnt3a or mechanically stimulated for various times. Cell lysates were prepared, and luciferase activity was determined using the Dual-Luciferase® reporter assay system (Promega, Madison, WI) according to the manufacturer's instructions. Light intensity was measured with a luminometer, and firefly luciferase activity was divided by Renilla luciferase activity to normalize for transfection efficiency.
Mechanical Stimulation
Cells were plated on flexible bottom wells coated with collagen type I. 16–24 h later, cells were stretched at 5% elongation for 10 min using a 20-s stretching and 0.1-s resting regimen of biaxial stretching in a Flexercell FX-4000 strain unit (Flexcell International Corp., Hillsborough, NC) (24). For the experiments testing the effect of pulsatile fluid flow shear stress, cells were plated on glass slides coated with collagen type I. 24 h later, cells were stimulated by pulsatile fluid flow with a shear stress of 10 dynes/cm2 at 8 Hz for 10 min in a Flexcell® Streamer® shear stress device (Flexcell International Corp.) (35).
Gene Silencing
The expression of murine caveolin-1 or protein lamin A/C (used as a control) was silenced by treating MLO-Y4 cells with the corresponding siRNA (200 or 400 nm; Custom SMARTpool, Dharmacon, Lafayette, CO) for 3 h as described (36). 2 days after silencing, cells were replated and transfected with empty vector as a control or with human caveolin-1 (Invitrogen) to rescue caveolin-1 expression.
Quantification of Apoptotic Cells
Apoptosis was induced in semiconfluent cultures (<75% confluence) by addition of the glucocorticoid dexamethasone (1 μm) immediately after stretching. Cells were cultured for 6 h, and apoptosis was assessed by enumerating MLO-Y4 cells expressing nGFP exhibiting chromatin condensation and nuclear fragmentation under a fluorescence microscope as reported previously (31).
Subcellular Localization of ERK2 and β-Catenin
MLO-Y4 cells were transiently transfected using Lipofectamine Plus with wild-type MEK along with ERK2-RFP to allow the visualization of ERK and with nGFP to allow the localization of the cell nuclei (37). After stretching, cells were fixed in 10% neutral buffered formalin for 8 min. The percentage of cells showing nuclear accumulation of ERK2 was quantified by enumerating those cells exhibiting increased RFP in the nucleus compared with the cytoplasm using a fluorescence microscope. At least 250 cells from random fields were examined for each experimental condition. For the experiments in which the effect of fluid flow on β-catenin subcellular localization was assessed, MLO-Y4 cells were fixed immediately after stimulation with 2% paraformaldehyde for 5 min and incubated with rabbit anti-β-catenin polyclonal antibody (1:200; Abcam, Cambridge, United Kingdom), followed by Alexa Fluor 546-labeled anti-rabbit IgG antibody (1:200; Invitrogen). β-Catenin localization was visualized under a fluorescence microscope.
Western Blot Analysis
Cell lysates were prepared immediately after stimulation, and proteins were separated on 10% SDS-polyacrylamide gels and electrotransferred to PVDF membranes as reported previously (31). The phosphorylation status of GSK3β was analyzed using a rabbit polyclonal antibody recognizing Ser9-phosphorylated GSK3β (Cell Signaling Technology, Inc., Danvers, MA). β-Catenin, caveolin-1, and β-actin protein levels were assessed using mouse monoclonal antibodies recognizing β-catenin or caveolin-1 (BD Biosciences) and a mouse monoclonal antibody recognizing β-actin (Sigma). After incubation with primary antibodies, blots were exposed to anti-rabbit or anti-mouse antibody conjugated with horseradish peroxidase (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and developed using a chemiluminescent substrate (Pierce). Samples from each experiment were run on the same gel, transferred to the same membrane, and scanned at the same time and with same level of resolution using a VersaDoc imaging system (Bio-Rad). Background and contrast were uniformly adjusted. In some cases, images were cut and reordered to facilitate description of the data. Cuts are outlined by dashed lines.
Real-time PCR
Total RNA was isolated using Ultraspec (Biotecx Laboratories, Inc., Houston, TX). Reverse transcription was performed using a high-capacity cDNA archive kit. Primers and probes for the housekeeping gene choB (probe, 5′-TCCAGAGCAGGATCC-3′; forward primer, CCCAGGATGGCGACGAT; and reverse primer, CCGAATGCTGTAATGGCGTAT; assays-by-design service) and TaqMan gene expression assay for murine Axin2 were used. PCR was performed using 20 μl of Gene Expression Assay Mix TaqMan Universal Master Mix containing 80 ng of each cDNA template in triplicates using an ABI 7300 real-time PCR system. The -fold change in expression was calculated using the ΔΔCt comparative threshold cycle method. All of the reagents were from Applied Biosystems (Foster City, CA).
Statistical Analysis
Data were analyzed by one-way analysis of variance (ANOVA), and the Student-Newman-Keuls method was used to estimate the level of significance of differences between means.
RESULTS
Disruption of Caveolin-1 Abolishes ERK Nuclear Translocation and Osteocyte Survival Induced by Mechanical Stimulation
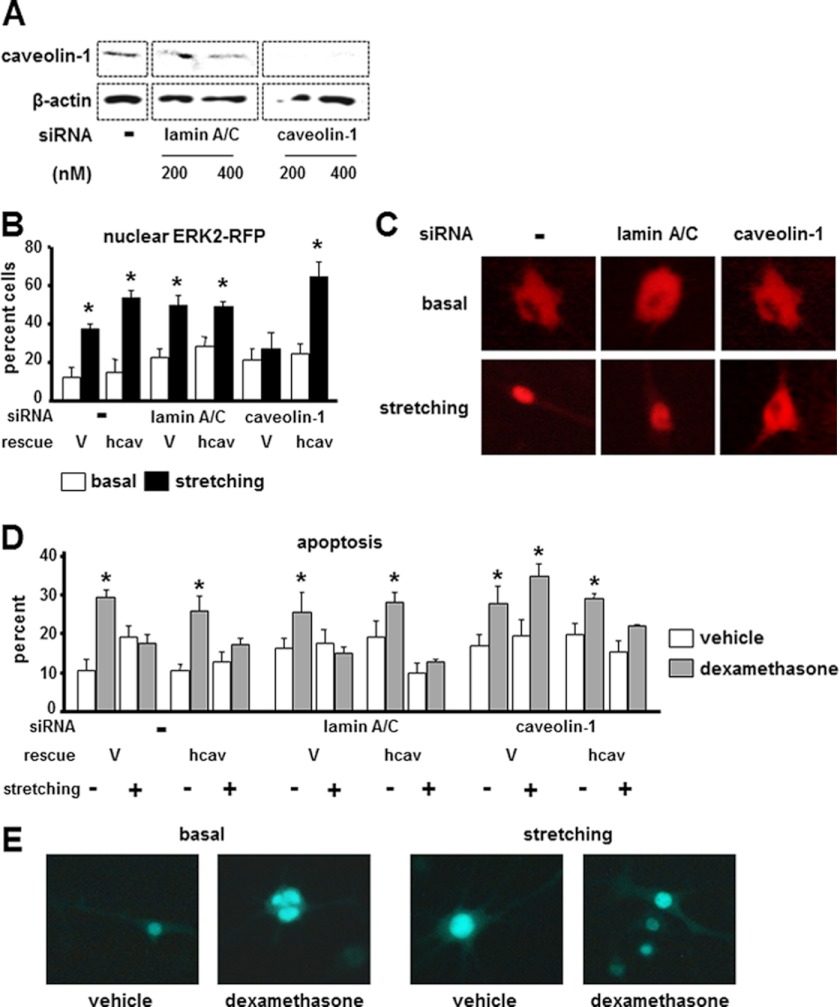
To determine the role of caveolin-1 in ERK activation and anti-apoptosis induced by mechanical stimulation, we examined the response to stretching of MLO-Y4 osteocytic cells in which the expression of caveolin-1 was knocked down using siRNA. Efficient silencing was obtained with 200 or 400 nm specific oligonucleotides (Fig. 1A); therefore, in subsequent experiments, oligonucleotides were used at 200 nm. Cells in which lamin A/C was silenced were used as negative controls.
FIGURE 1.
ERK nuclear translocation and anti-apoptosis induced by stretching are abolished by knocking down caveolin-1. A, caveolin-1 protein expression was determined by Western blot analysis in MLO-Y4 cells treated 200 or 400 nm siRNA oligonucleotides. β-Actin levels show equal loading. B–E, caveolin-1 expression was silenced in MLO-Y4 cells using 200 nm siRNA. Additional cultures were left untreated (−) or were silenced for lamin A/C (as controls), followed by transfection with empty vector (V) or human caveolin-1 (hcav) constructs together with ERK2-RFP and MEK to allow quantification of ERK nuclear translocation and with nGFP to allow quantification of apoptosis. 24 h later, cells were mechanically stimulated by stretching at 5% elongation for 10 min, and ERK nuclear translocation (B and C) and apoptosis (D and E) were quantified as described under “Experimental Procedures.” Error bars indicate means ± S.D. of triplicate determinations. *, p < 0.05 versus basal or vehicle for each construct in B and D, respectively, by one-way ANOVA. Representative images are shown exemplifying cells with cytoplasmic and nuclear ERK2-RFP (C) or live and apoptotic nGFP-expressing cells (E).
Stretching at 5% elongation for 10 min increased by ∼100–150% the number of cells exhibiting ERK nuclear accumulation in non-silenced cells and in control cells silenced for lamin A/C compared with non-stretched cells (Fig. 1B) as determined by quantifying cells exhibiting nuclear accumulation of ERK2-RFP (Fig. 1C). In contrast, stretching failed to induce ERK nuclear translocation in cells in which caveolin-1 was silenced, and full responsiveness to stretching was restored by transfecting human caveolin.
Consistent with the previously demonstrated role of ERK activation in stretching-induced anti-apoptosis (24), mechanical stimulation prevented osteocyte apoptosis induced by dexamethasone in non-silenced cells and in control cells silenced for lamin A/C, but not in cells silenced for caveolin-1 (Fig. 1D). Apoptosis was quantified by evaluating the nuclear morphology of cells transfected with nGFP (Fig. 1E). In addition, similar to ERK nuclear translocation, transfection of human caveolin restored the anti-apoptotic effect of stretching on caveolin-silenced cells.
Effects of Mechanical Stimulation in Osteocytes Are Blocked by Inhibiting the Early Steps of Wnt Signaling, but Not by Inhibiting TCF-mediated Transcription
To establish the potential role of Wnt signaling in ERK nuclear translocation and survival induced by stretching, Wnt signaling was inhibited at different stages of the pathway. First, Wnt activation at the pre-receptor level was inhibited by transfecting the Wnt antagonist Dkk1, which binds to the Wnt co-receptors LRP5 and LRP6. Second, accumulation of the Wnt signaling canonical mediator β-catenin was inhibited by transfecting Axin2, which induces its proteasomal degradation. Finally, canonical Wnt signaling-mediated gene transcription was inhibited by transfecting dominant-negative TCF. Overexpression of Dkk1 or Axin2 abolished both ERK nuclear translocation (Fig. 2A) and the anti-apoptotic effect of stretching (Fig. 2B). However, overexpression of dominant-negative TCF did not alter the response to mechanical stimulation (Fig. 2, A and B). As expected, however, the expression of these three inhibitors antagonized Wnt3a-induced TCF-mediated transcription as assessed by transfecting each one of the constructs along with a Wnt3a plasmid and a TCF-luciferase reporter plasmid (Fig. 2C).
FIGURE 2.
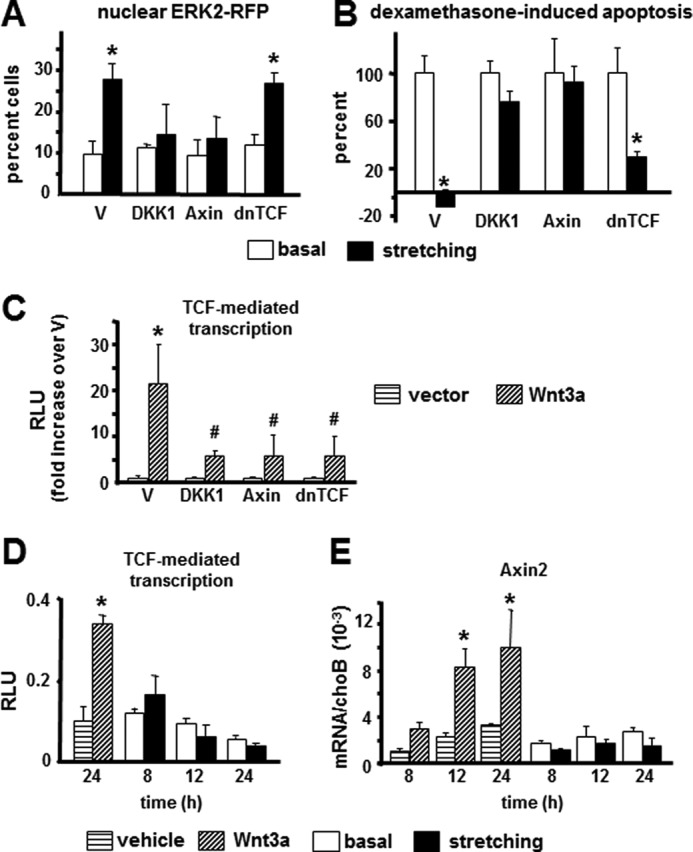
ERK nuclear translocation and osteocyte survival induced by stretching require β-catenin accumulation, but not TCF-mediated transcription. A and B, MLO-Y4 cells were transiently transfected with the indicated constructs together with nGFP, ERK2-RFP, and MEK. 24 h later, cells were stretched at 5% elongation for 10 min. Apoptosis and ERK translocation were quantified in transfected cells as described under “Experimental Procedures.” V, empty vector; dnTCF, dominant-negative TCF. Error bars indicate means ± S.D. of triplicate determinations. *, p < 0.05 versus basal for each construct by one-way ANOVA. C, TCF-mediated transcription was measured in cells transfected with the indicated constructs together with vector or a Wnt3a-expressing construct and TCF-luciferase/Renilla luciferase. TCF promoter activity was quantified as described under “Experimental Procedures.” Error bars indicate means ± S.D. of triplicate determinations. *, p < 0.05 versus vector; #, p < 0.05 versus Wnt3a-transfected cultures by one-way ANOVA. RLU, relative luminescence units. D, TCF-mediated transcription was measured in MLO-Y4 cells transfected with TCF-luciferase/Renilla luciferase and treated with 25 ng/ml Wnt3a as a positive control or stretched at 5% elongation for 10 min. Measurements were performed at the indicated times after addition of Wnt3a or after mechanical stimulation. Error bars indicate means ± S.D. of triplicate determinations. *, p < 0.05 versus vehicle by one-way ANOVA. E, the expression of the Wnt/β-catenin-dependent gene Axin2 was determined by quantitative PCR in MLO-Y4 cells treated with 25 ng/ml Wnt3a as a positive control or stimulated by stretching at 5% elongation for 10 min. Measurements were performed at the indicated times after addition of Wnt3a or after mechanical stimulation. Error bars indicate means ± S.D. of triplicate determinations. *, p < 0.05 versus vehicle or basal for each time point by one-way ANOVA.
Moreover, recombinant Wnt3a increased TCF-mediated transcription and the expression of the Wnt target gene Axin2 (Fig. 2, D and E). In contrast, 10-min stretching did not change these parameters measured at 8, 12, and 24 h after stimulation.
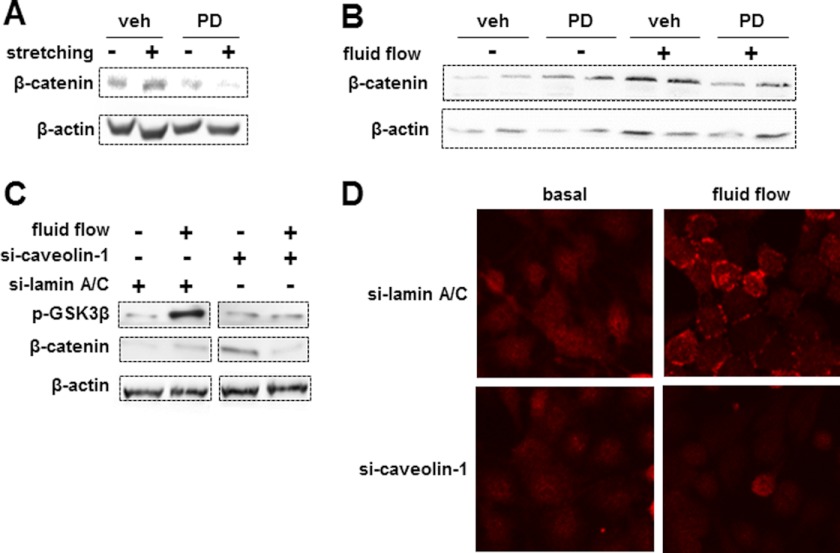
Mechanical Loading Induces β-Catenin Accumulation through an ERK-dependent Mechanism
We next evaluated the effect of mechanical stimulation on β-catenin accumulation and whether it was affected by the caveolin-1/ERK pathway. Stretching at 5% elongation for 10 min (Fig. 3, A and C) or fluid flow stimulation at 10 dynes/cm2 for 10 min (Fig. 3, B and D) increased the levels of phosphorylated GSK3β (Fig. 3, A and B). Phosphorylation of GSK3β inhibits its ability to phosphorylate β-catenin, which is required for β-catenin proteasomal degradation. Consistent with this, stretching and fluid flow also increased the levels of β-catenin (Fig. 3, C and D).
FIGURE 3.
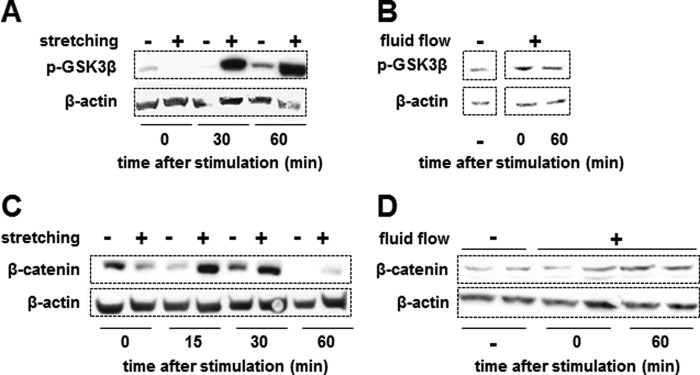
Mechanical stimulation induces inactivation of GSK3β and β-catenin accumulation. A–D, MLO-Y4 osteocytic cells were subjected to mechanical stimulation by stretching (A and C) or fluid flow (B and D) for 10 min, and cell lysates were prepared immediately (time 0) or at the indicated times after mechanical stimulation. Phosphorylated GSK3β (p-GSK3β; A and B) and β-catenin (C and D) were analyzed by Western blotting. β-Actin was used to demonstrate equal loading.
Moreover, β-catenin accumulation induced by either stretching or fluid flow was blocked by the ERK inhibitor PD98059 (Fig. 4, A and B). In addition, silencing caveolin-1 abolished GSK3β and β-catenin accumulation (Fig. 4C) as well as β-catenin translocation to the cell membrane (Fig. 4D) induced by fluid flow.
FIGURE 4.
ERK activation and caveolin-1 expression are required for mechanical loading-induced β-catenin accumulation. A and B, cells were treated with vehicle (veh) or 50 μm PD98059 (PD) for 25 min prior to mechanical stimulation by stretching at 5% elongation (A) or fluid flow at 10 dynes/cm2 (B) for 10 min. C and D, caveolin-1 expression was silenced in MLO-Y4 cells using siRNA (si). Control cultures were silenced for lamin A/C. 24 h later, cells were mechanically stimulated by fluid flow at 10 dynes/cm2 for 10 min. C, cells were lysed immediately after mechanical stimulation, and the levels of phosphorylated GSK3β (p-GSK3β), β-catenin, and β-actin were determined by Western blot analysis. D, cells were fixed immediately after stimulation, and the subcellular localization of β-catenin was evaluated by immunocytochemistry.
DISCUSSION
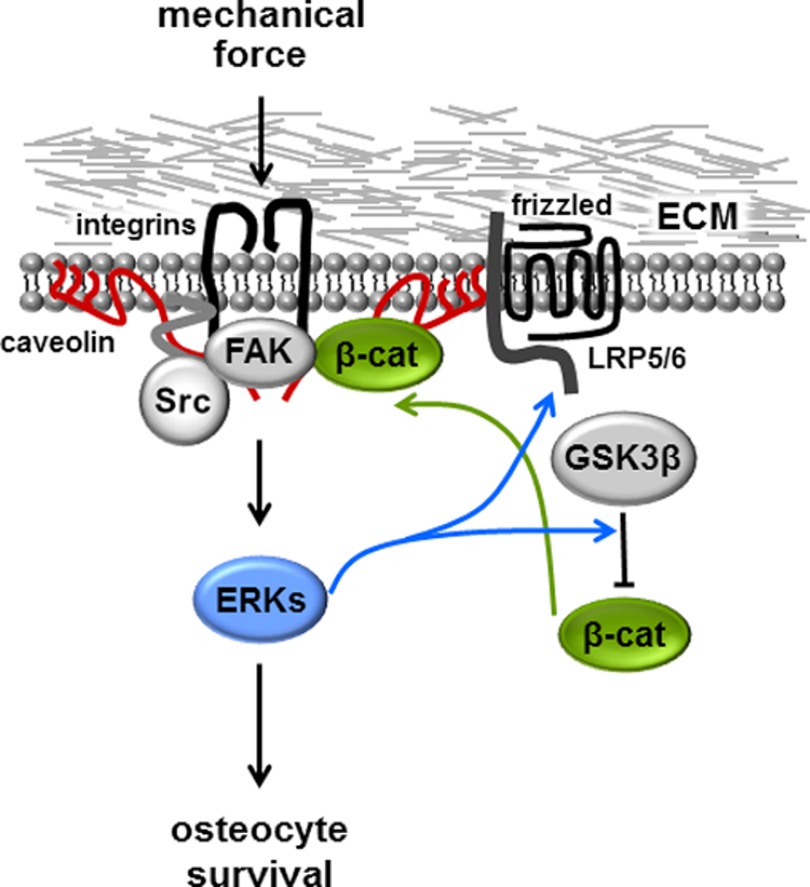
Osteocyte viability is a determinant of bone strength, and it is diminished in several pathologies associated with bone loss and bone fragility, including aging, loss of sex steroids, and glucocorticoid excess (38). Physiological levels of mechanical force, such as those provided by normal ambulatory activity or moderate physical exercise, are critical to maintain osteocyte viability by mechanisms still unclear. We have shown here that mechanotransduction leading to osteocyte survival is controlled by bidirectional crosstalk between the caveolin-1/ERK and Wnt/β-catenin signaling pathways (Fig. 5). Our findings provide evidence for a cause-effect relationship between these pathways. Specifically, blockade of the caveolin-1/ERK pathway, either genetically by silencing caveolin-1 or pharmacologically using an inhibitor of ERK activation, obliterates phosphorylation of GSK3β and accumulation of β-catenin induced by mechanical stimulation. Conversely, inhibition of the early steps of Wnt signaling, either at the pre-receptor level with Dkk1 or by inducing β-catenin degradation with Axin2, abolishes ERK nuclear translocation. Importantly, interfering with these molecular pathways translates into a change in the biological response. Thus, mechanical stimulation fails to promote osteocyte survival in the absence of activation of either the caveolin-1/ERK or Wnt/β-catenin pathway.
FIGURE 5.
Working model. Caveolin-1/ERK and Wnt/β-catenin signaling pathways cooperate in transducing mechanical cues into osteocyte survival (see text for details). FAK, focal adhesion kinase; β-cat, β-catenin; ECM, extracellular matrix.
Mechanical stimuli activate ERKs and stabilize β-catenin in an interdependent fashion. The fact that ERK activation and anti-apoptosis require β-catenin accumulation but not β-catenin/TCF-mediated transcription is consistent with our findings that mechanical stimulation induces translocation of β-catenin to the cell membrane, potentially decreasing its ability to move into the nucleus to activate transcription. These results also suggest that interaction of β-catenin with other structural and signaling molecules in the plasma membrane has permissive effects on events upstream of ERK activation. This notion is consistent with the evidence that silencing caveolin-1 not only abolishes ERK activation but also blocks β-catenin localization in the plasma membrane of osteocytic cells.
Caveolin-1 is the principal component of caveolae, specialized plasma membrane microdomains that act as reservoirs of catalytic and structural molecules. Integrin β1 interacts with caveolin-1, and in turn, caveolin-1 is required for the association of integrin β1 with Src kinases and phosphorylation of downstream substrates (39). Consistent with this earlier work, we showed previously that caveolin-1 physically interacts with integrin β1 and ERKs in osteocytic cells and that pharmacologic disruption of caveolae abolishes ERK activation and the anti-apoptotic effect of mechanical stimulation (24). Moreover, we found that the caveolin-interacting domain of estrogen receptor α is required for these mechanoresponses (40). Our current findings suggest that caveolin-1 also interacts with β-catenin in osteocytic cells, as expression of caveolin-1 is required for β-catenin membrane localization upon mechanical stimulation. Previous evidence has shown that caveolin-1 interacts with β-catenin through its scaffolding domain in zebrafish (41). Moreover, the interaction between these proteins controls the intracellular localization of β-catenin, as overexpression of caveolin-1 in caveolin-1-null U251 human glioma cells is sufficient to recruit β-catenin to the plasma membrane, thereby inhibiting β-catenin/TCF-mediated transcription (42). These findings are consistent with our results demonstrating that the ERK-dependent survival effect of mechanical stimulation in osteocytes requires accumulation of β-catenin, but not its transcriptional effects.
In contrast to our results, mechanical stimulation induced β-catenin translocation to the nucleus and transcription of Wnt target genes in pre-osteoblastic CIMC-4 cells (43) and in rat osteosarcoma UMR106 and ROS17/28 cells (44, 45). Moreover, the effects of mechanical stimulation in pre-osteoblastic cells were not altered by either silencing caveolin-1 or treatment with Dkk1 (43). The reason for the discrepancy between these studies and our current study is not known; however, it could be related to the different stage of differentiation of the osteoblastic cells. Nevertheless, our finding that Dkk1 overexpression abolishes the effect of mechanical signals on ERK activation and osteocyte survival suggests that events downstream of the LRP co-receptors are involved in mechanotransduction. This possibility is supported by in vivo studies in which it was shown that mice lacking LRP5 do not exhibit an anabolic effect upon loading (16).
Similar to our previous report (24) and our current study, Kitase et al. (27) showed that mechanical stimulation prevented glucocorticoid-induced apoptosis in MLO-Y4 osteocytic cells. However, these authors found that mechanical stimulation induced β-catenin accumulation in the cell nucleus instead of in the plasma membrane, as we now report. However, in their study, cells were stimulated for a longer time (2 h versus 10 min) and at a higher strain (16 versus 10 dynes/cm2) than in our study. This evidence suggests that translocation of β-catenin to the cell membrane temporally precedes its accumulation in the cell nucleus. In addition, higher mechanical strains might be required to induce β-catenin nuclear translocation. Nevertheless, our evidence suggests that mechanical stimulation for just 10 min at 10 dynes/cm2 is sufficient to prevent β-catenin degradation and to trigger survival signaling in osteocytic cells.
Similar to mechanical stimulation, activation of the Wnt signaling pathway promotes survival of osteocytes as well as osteoblasts. This has been demonstrated in cultured cells treated with Wnt3a, Wnt1, and Wnt5a (26). This in vitro evidence is supported by the finding that mice with deletion of the Wnt inhibitor sFRP1 or overexpressing an activated LRP5 Wnt co-receptor high bone mass mutant exhibit reduced osteoblast and osteocyte apoptosis (10, 25). In contrast to the anti-apoptotic effect of mechanical stimulation shown here, which involves stabilization of β-catenin but does not require its nuclear function, the survival effect of Wnt proteins depends on Wnt/β-catenin-induced TCF-mediated gene transcription (26).
In summary, on the basis of our findings, we propose that accumulation of β-catenin is essential for mechanotransduction and that its localization within caveolae is required for full activation of the caveolin-1/ERK signaling pathway leading to osteocyte survival. The evidence presented herein also suggests the existence of a bidirectional regulation loop by which, in turn, ERK activation induced by mechanical force is a prerequisite for β-catenin accumulation (Fig. 5).
Acknowledgments
We thank Dr. Ana Carolina Ronda, Laura Alonso, Sonia Moraleja, and Iraj Hassan for technical support.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 AR059357 (to T. B.). This work was also supported by Fundación Universitaria San Pablo CEU Grant USPPPCO07/09 and a fellowship from the Conchita Rabago Foundation (to A. R. G.).
- TCF
- T cell factor
- RFP
- red fluorescent protein
- nGFP
- nuclear targeted green fluorescent protein
- ANOVA
- analysis of variance.
REFERENCES
- 1. Turner C. H., Warden S. J., Bellido T., Plotkin L. I., Kumar N., Jasiuk I., Danzig J., Robling A. G. (2009) Mechanobiology of the skeleton. Sci. Signal. 2, pt3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bonewald L. F. (2011) The amazing osteocyte. J. Bone Miner. Res. 26, 229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Balemans W., Ebeling M., Patel N., Van Hul E., Olson P., Dioszegi M., Lacza C., Wuyts W., Van Den Ende J., Willems P., Paes-Alves A. F., Hill S., Bueno M., Ramos F. J., Tacconi P., Dikkers F. G., Stratakis C., Lindpaintner K., Vickery B., Foernzler D., Van Hul W. (2001) Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum. Mol. Genet. 10, 537–543 [DOI] [PubMed] [Google Scholar]
- 4. Loots G. G., Kneissel M., Keller H., Baptist M., Chang J., Collette N. M., Ovcharenko D., Plajzer-Frick I., Rubin E. M. (2005) Genomic deletion of a long-range bone enhancer misregulates sclerostin in Van Buchem disease. Genome Res. 15, 928–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paszty C., Turner C. H., Robinson M. K. (2010) Sclerostin: a gem from the genome leads to bone-building antibodies. J. Bone Miner. Res. 25, 1897–1904 [DOI] [PubMed] [Google Scholar]
- 6. Xiong J., O'Brien C. A. (2012) Osteocyte RANKL: new insights into the control of bone remodeling. J. Bone Miner. Res. 27, 499–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kramer I., Halleux C., Keller H., Pegurri M., Gooi J. H., Weber P. B., Feng J. Q., Bonewald L. F., Kneissel M. (2010) Osteocyte Wnt/β-catenin signaling is required for normal bone homeostasis. Mol. Cell. Biol. 30, 3071–3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moon R. T., Bowerman B., Boutros M., Perrimon N. (2002) The promise and perils of Wnt signaling through β-catenin. Science 296, 1644–1646 [DOI] [PubMed] [Google Scholar]
- 9. Willert K., Brown J. D., Danenberg E., Duncan A. W., Weissman I. L., Reya T., Yates J. R., 3rd, Nusse R. (2003) Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423, 448–452 [DOI] [PubMed] [Google Scholar]
- 10. Babij P., Zhao W., Small C., Kharode Y., Yaworsky P. J., Bouxsein M. L., Reddy P. S., Bodine P. V., Robinson J. A., Bhat B., Marzolf J., Moran R. A., Bex F. (2003) High bone mass in mice expressing a mutant LRP5 gene. J. Bone Miner. Res. 18, 960–974 [DOI] [PubMed] [Google Scholar]
- 11. Bodine P. V., Billiard J., Moran R. A., Ponce-de-Leon H., McLarney S., Mangine A., Scrimo M. J., Bhat R. A., Stauffer B., Green J., Stein G. S., Lian J. B., Komm B. S. (2005) The Wnt antagonist secreted frizzled-related protein-1 controls osteoblast and osteocyte apoptosis. J. Cell. Biochem. 96, 1212–1230 [DOI] [PubMed] [Google Scholar]
- 12. Tamai K., Semenov M., Kato Y., Spokony R., Liu C., Katsuyama Y., Hess F., Saint-Jeannet J. P., He X. (2000) LDL-receptor-related proteins in Wnt signal transduction. Nature 407, 530–535 [DOI] [PubMed] [Google Scholar]
- 13. He X., Semenov M., Tamai K., Zeng X. (2004) LDL receptor-related proteins 5 and 6 in Wnt/β-catenin signaling: arrows point the way. Development 131, 1663–1677 [DOI] [PubMed] [Google Scholar]
- 14. Hinoi T., Yamamoto H., Kishida M., Takada S., Kishida S., Kikuchi A. (2000) Complex formation of adenomatous polyposis coli gene product and Axin facilitates glycogen synthase kinase-3β-dependent phosphorylation of β-catenin and down-regulates β-catenin. J. Biol. Chem. 275, 34399–34406 [DOI] [PubMed] [Google Scholar]
- 15. Mao J., Wang J., Liu B., Pan W., Farr G. H., 3rd, Flynn C., Yuan H., Takada S., Kimelman D., Li L., Wu D. (2001) Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol. Cell 7, 801–809 [DOI] [PubMed] [Google Scholar]
- 16. Sawakami K., Robling A. G., Ai M., Pitner N. D., Liu D., Warden S. J., Li J., Maye P., Rowe D. W., Duncan R. L., Warman M. L., Turner C. H. (2006) The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J. Biol. Chem. 281, 23698–23711 [DOI] [PubMed] [Google Scholar]
- 17. Robinson J. A., Chatterjee-Kishore M., Yaworsky P. J., Cullen D. M., Zhao W., Li C., Kharode Y., Sauter L., Babij P., Brown E. L., Hill A. A., Akhter M. P., Johnson M. L., Recker R. R., Komm B. S., Bex F. J. (2006) Wnt/β-catenin signaling is a normal physiological response to mechanical loading in bone. J. Biol. Chem. 281, 31720–31728 [DOI] [PubMed] [Google Scholar]
- 18. Robling A. G., Niziolek P. J., Baldridge L. A., Condon K. W., Allen M. R., Alam I., Mantila S. M., Gluhak-Heinrich J., Bellido T. M., Harris S. E., Turner C. H. (2008) Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J. Biol. Chem. 283, 5866–5875 [DOI] [PubMed] [Google Scholar]
- 19. Tu X., Rhee Y., Condon K. W., Bivi N., Allen M. R., Dwyer D., Stolina M., Turner C. H., Robling A. G., Plotkin L. I., Bellido T. (2012) Sost down-regulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone 50, 209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Javaheri B., Dallas M., Zhao H., Bonewald L., Johnson M. (2011) β-Catenin haploinsufficiency in osteocytes abolishes the osteogenic effect of mechanical loading in vivo. J. Bone Miner. Res. 26, S24 [Google Scholar]
- 21. Aguirre J. I., Plotkin L. I., Stewart S. A., Weinstein R. S., Parfitt A. M., Manolagas S. C., Bellido T. (2006) Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J. Bone Min. Res. 21, 605–615 [DOI] [PubMed] [Google Scholar]
- 22. Dufour C., Holy X., Marie P. J. (2007) Skeletal unloading induces osteoblast apoptosis and targets α5β1-PI3K-Bcl-2 signaling in rat bone. Exp. Cell Res. 313, 394–403 [DOI] [PubMed] [Google Scholar]
- 23. Noble B. S., Peet N., Stevens H. Y., Brabbs A., Mosley J. R., Reilly G. C., Reeve J., Skerry T. M., Lanyon L. E. (2003) Mechanical loading: biphasic osteocyte survival and targeting of osteoclasts for bone destruction in rat cortical bone. Am. J. Physiol. Cell Physiol. 284, C934–C943 [DOI] [PubMed] [Google Scholar]
- 24. Plotkin L. I., Mathov I., Aguirre J. I., Parfitt A. M., Manolagas S. C., Bellido T. (2005) Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases, and ERKs. Am. J. Physiol. Cell Physiol. 289, C633–C643 [DOI] [PubMed] [Google Scholar]
- 25. Bodine P. V., Zhao W., Kharode Y. P., Bex F. J., Lambert A. J., Goad M. B., Gaur T., Stein G. S., Lian J. B., Komm B. S. (2004) The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol. Endocrinol. 18, 1222–1237 [DOI] [PubMed] [Google Scholar]
- 26. Almeida M., Han L., Bellido T., Manolagas S. C., Kousteni S. (2005) Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by β-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J. Biol. Chem. 280, 41342–41351 [DOI] [PubMed] [Google Scholar]
- 27. Kitase Y., Barragan L., Jiang J. X., Johnson M. L., Bonewald L. F. (2010) Mechanical induction of PGE2 in osteocytes blocks glucocorticoid-induced apoptosis through both the β-catenin and PKA pathways. J. Bone Miner. Res. 25, 2657–2668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ng S. S., Mahmoudi T., Danenberg E., Bejaoui I., de Lau W., Korswagen H. C., Schutte M., Clevers H. (2009) Phosphatidylinositol 3-kinase signaling does not activate the Wnt cascade. J. Biol. Chem. 284, 35308–35313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krejci P., Aklian A., Kaucka M., Sevcikova E., Prochazkova J., Masek J. K., Mikolka P., Pospisilova T., Spoustova T., Weis M., Paznekas W. A., Wolf J. H., Gutkind J. S., Wilcox W. R., Kozubik A., Jabs E. W., Bryja V., Salazar L., Vesela I., Balek L. (2012) Receptor tyrosine kinases activate canonical Wnt/β-catenin signaling via MAP kinase/LRP6 pathway and direct β-catenin phosphorylation. PLoS ONE 7, e35826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kato Y., Windle J. J., Koop B. A., Mundy G. R., Bonewald L. F. (1997) Establishment of an osteocyte-like cell line, MLO-Y4. J. Bone Miner. Res. 12, 2014–2023 [DOI] [PubMed] [Google Scholar]
- 31. Plotkin L. I., Weinstein R. S., Parfitt A. M., Roberson P. K., Manolagas S. C., Bellido T. (1999) Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J. Clin. Invest. 104, 1363–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Luttrell L. M., Roudabush F. L., Choy E. W., Miller W. E., Field M. E., Pierce K. L., Lefkowitz R. J. (2001) Activation and targeting of extracellular signal-regulated kinases by β-arrestin scaffolds. Proc. Natl. Acad. Sci. U.S.A. 98, 2449–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mansour S. J., Matten W. T., Hermann A. S., Candia J. M., Rong S., Fukasawa K., Vande Woude G. F., Ahn N. G. (1994) Transformation of mammalian cells by constitutively active MAP kinase kinase. Science 265, 966–970 [DOI] [PubMed] [Google Scholar]
- 34. Plotkin L. I., Manolagas S. C., Bellido T. (2002) Transduction of cell survival signals by connexin-43 hemichannels. J. Biol. Chem. 277, 8648–8657 [DOI] [PubMed] [Google Scholar]
- 35. Kamel M. A., Picconi J. L., Lara-Castillo N., Johnson M. L. (2010) Activation of β-catenin signaling in MLO-Y4 osteocytic cells versus 2T3 osteoblastic cells by fluid flow shear stress and PGE2: implications for the study of mechanosensation in bone. Bone 47, 872–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Plotkin L. I., Manolagas S. C., Bellido T. (2007) Glucocorticoids induce osteocyte apoptosis by blocking focal adhesion kinase-mediated survival. Evidence for inside-out signaling leading to anoikis. J. Biol. Chem. 282, 24120–24130 [DOI] [PubMed] [Google Scholar]
- 37. Plotkin L. I., Aguirre J. I., Kousteni S., Manolagas S. C., Bellido T. (2005) Bisphosphonates and estrogens inhibit osteocyte apoptosis via distinct molecular mechanisms downstream of extracellular signal-regulated kinase activation. J. Biol. Chem. 280, 7317–7325 [DOI] [PubMed] [Google Scholar]
- 38. Jilka R. L., Bellido T., Almeida M., Plotkin L. I., O'Brien C. A., Weinstein R. S., Manolagas S. C. (2008) Apoptosis in bone cells. in Principles of Bone Biology (Bilezikian J. P., Raisz L. G., Martin T. J., eds) pp. 237–261, Academic Press, San Diego, CA [Google Scholar]
- 39. Wei Y., Yang X., Liu Q., Wilkins J. A., Chapman H. A. (1999) A role for caveolin and the urokinase receptor in integrin-mediated adhesion and signaling. J. Cell Biol. 144, 1285–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aguirre J. I., Plotkin L. I., Gortazar A. R., Millan M. M., O'Brien C. A., Manolagas S. C., Bellido T. (2007) A novel ligand-independent function of the estrogen receptor is essential for osteocyte and osteoblast mechanotransduction. J. Biol. Chem. 282, 25501–25508 [DOI] [PubMed] [Google Scholar]
- 41. Mo S., Wang L., Li Q., Li J., Li Y., Thannickal V. J., Cui Z. (2010) Caveolin-1 regulates dorsoventral patterning through direct interaction with β-catenin in zebrafish. Dev. Biol. 344, 210–223 [DOI] [PubMed] [Google Scholar]
- 42. Galbiati F., Volonte D., Brown A. M., Weinstein D. E., Ben-Ze'ev A., Pestell R. G., Lisanti M. P. (2000) Caveolin-1 expression inhibits Wnt/β-catenin/Lef-1 signaling by recruiting β-catenin to caveolae membrane domains. J. Biol. Chem. 275, 23368–23377 [DOI] [PubMed] [Google Scholar]
- 43. Case N., Ma M., Sen B., Xie Z., Gross T. S., Rubin J. (2008) β-Catenin levels influence rapid mechanical responses in osteoblasts. J. Biol. Chem. 283, 29196–29205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Armstrong V. J., Muzylak M., Sunters A., Zaman G., Saxon L. K., Price J. S., Lanyon L. E. (2007) Wnt/β-catenin signaling is a component of osteoblastic bone cell early responses to load-bearing and requires estrogen receptor α. J. Biol. Chem. 282, 20715–20727 [DOI] [PubMed] [Google Scholar]
- 45. Sunters A., Armstrong V. J., Zaman G., Kypta R. M., Kawano Y., Lanyon L. E., Price J. S. (2010) Mechano-transduction in osteoblastic cells involves strain-regulated estrogen receptor α-mediated control of insulin-like growth factor (IGF) I receptor sensitivity to ambient IGF, leading to phosphatidylinositol 3-kinase/AKT-dependent Wnt/LRP5 receptor-independent activation of β-catenin signaling. J. Biol. Chem. 285, 8743–8758 [DOI] [PMC free article] [PubMed] [Google Scholar]