Background: Dishevelled is a critical component of Wnt signaling; however, its stability control is not fully understood.
Results: NEDD4L regulates Wnt signaling through Dishevelled2 degradation, and Wnt5a-induced NEDD4L phosphorylation by JNK1 is required for this process.
Conclusion: NEDD4L modulates Wnt signaling through a negative feedback mechanism.
Significance: Our findings shed light on the understanding of Dishevelled2 stability control and NEDD4L-associated diseases.
Keywords: Jun N-terminal Kinase (JNK), Protein Stability, Ubiquitin Ligase, Ubiquitination, Wnt Signaling, Dishevelled, Nedd4L
Abstract
Wnt signaling plays a pivotal role in embryogenesis and tissue homeostasis. Dishevelled (Dvl) is a central mediator for both Wnt/β-catenin and Wnt/planar cell polarity pathways. NEDD4L, an E3 ubiquitin ligase, has been shown to regulate ion channel activity, cell signaling, and cell polarity. Here, we report a novel role of NEDD4L in the regulation of Wnt signaling. NEDD4L induces Dvl2 polyubiquitination and targets Dvl2 for proteasomal degradation. Interestingly, the NEDD4L-mediated ubiquitination of Dvl2 is Lys-6, Lys-27, and Lys-29 linked but not typical Lys-48-linked ubiquitination. Consistent with the role of Dvl in both Wnt/β-catenin and Wnt/planar cell polarity signaling, NEDD4L regulates the cellular β-catenin level and Rac1, RhoA, and JNK activities. We have further identified a hierarchical regulation that Wnt5a induces JNK-mediated phosphorylation of NEDD4L, which in turn promotes its ability to degrade Dvl2. Finally, we show that NEDD4L inhibits Dvl2-induced axis duplication in Xenopus embryos. Our work thus demonstrates that NEDD4L is a negative feedback regulator of Wnt signaling.
Introduction
Wnt Signaling directs cell proliferation, polarity, and fate determination during embryonic development and tissue homeostasis. Mutations in the Wnt pathway components are often linked to various human diseases (1). Wnt signaling is initiated by binding of extracellular Wnt ligands to Frizzled transmembrane receptors, leading to membrane recruitment and activation of Dishevelled (Dvl).4 Downstream of Dvl, Wnt signals diverge into the canonical Wnt/β-catenin and noncanonical Wnt pathways including the Wnt/planar cell polarity (PCP) pathway. Wnt/β-catenin signaling prevents β-catenin from ubiquitination and degradation by destruction of a complex composed of Axin, adenomatous polyposis coli, glycogen synthase kinase 3β, and casein kinase 1, leading to the nuclear accumulation of β-catenin, and eventually β-catenin/T cell factor-mediated transcription. In contrast, Wnt/PCP signaling is involved in cytoskeleton remodeling and coordinates dynamic cell-cell adhesion and cell migration via the small GTPases RhoA and Rac1 and c-Jun N-terminal kinases (JNK) (2–6).
As Dvl is a pivotal component of Wnt signaling, regulation of its stability is of great significance for proper signal transduction. Dvl undergoes degradation through two distinct pathways: the ubiquitin-proteasomal pathway and the autophagy-lysosomal pathway (7–9). Several ubiquitin ligases have been suggested to regulate Dvl stability, including KLHL12-Cullin-3 ubiquitin ligase (10), NEDL1 (11), adenomatous polyposis coli/C (12), pVHL (8), Malin (13), and ITCH (14). These E3 ubiquitin ligases were reported to promote ubiquitination and degradation of Dvl under various physiological conditions. However, the mechanisms underlying Dvl stability control is still not fully understood.
NEDD4 family ubiquitin ligases contain nine members with distinct physiological functions (15). NEDD4L (neural precursor cell expressed, developmentally down-regulated 4-like, also called NEDD4-2) is a member of the NEDD4 family ubiquitin ligases (16, 17). It consists of an N-terminal calcium/lipid-binding C2 domain, four WW domains, and a C-terminal HECT (homologous to E6-AP C terminus of the human papilloma virus) ubiquitin-ligase domain. The WW domains of NEDD4L have been shown to mediate its binding to substrates containing PPXY (PY) motifs. By far, several proteins have been described as the substrates of NEDD4L, including ion and neurotransmitter channels, growth factor receptors, signaling intermediates, and tight junction molecules (17–20). NEDD4L knock-out mice die perinatally due to impaired lung function (21, 22).
In this study, we reported that NEDD4L could directly bind Dvl2 and target Dvl2 for proteasomal degradation through Lys-6-, Lys-27-, and Lys-29-linked atypical ubiquitination. The regulation of Dvl2 by NEDD4L was required for both Wnt/β-catenin and Wnt/PCP signaling. We further demonstrated that Wnt5a-induced phosphorylation of NEDD4L by JNK1 was required for it to regulate Dvl stability. NEDD4L also negatively regulated Dvl2-induced axis duplication in Xenopus embryos. Thus NEDD4L plays an essential role in Wnt signaling through a negative feedback mechanism.
EXPERIMENTAL PROCEDURES
Plasmids and RNA Interference
Human NEDD4L isoform 2 (NM_001144964.1) was cloned into ClaI and XbaI sites of pCS2+-HA or pCS2+-FLAG vectors or into BglII and SalI sites of pEGFP-C3 vector or into XbaI and SalI sites of the pGEX-4T1 vector. pCS2+-HA-Dvl2, pCS2+-Flag-Dvl2, and pDsRed-Dvl2 plasmids were described previously (8). Various point mutants were generated using the QuikChange Site-directed Mutagenesis Kit (Stratagene). Nonspecific and NEDD4L shRNAs were described previously (8, 18). To generate a NEDD4L shRNA1-resistant construct, the target sequence of NEDD4L shRNA1 was mutated to 5′-gctaggctatggatcgagt-3′ (sense); for generation of the NEDD4L shRNA2-resistant construct, the target sequence of NEDD4L shRNA2 was mutated to 5′-tgaggaccacttatcatac-3′ (sense).
Cell Culture and Transfection
HEK293T and HeLa cells were maintained in DMEM supplemented with 10% FBS (Hyclone) in a 37 °C humidified incubator containing 5% CO2. Transient transfection was performed with VigoFect (Vigorous).
Reagents and Antibodies
Chloroquine, bafilomycin A1, ALLN, lactacystin, JNK inhibitor SP600125, and Hanks' balanced salt solution were purchased from Sigma. Wnt5a protein was from Millipore. Antibodies were from various sources: Cell Signaling (anti-NEDD4L, anti-Dvl2, and anti-phospho-JNK), BD Biosciences (anti-Rac1), Sigma (anti-FLAG M2, anti-Ser(P), and anti-Thr(P)), Santa Cruz (anti-RhoA, anti-total JNK, anti-tubulin, anti-HA, and anti-Myc), and Jackson ImmunoResearch (Aminomethylcoumarin Acetate-conjugated anti-rabbit, Aminomethylcoumarin Acetate-conjugated anti-mouse, fluorescein isothiocyanate-conjugated anti-mouse, TRITC-conjugated anti-goat, TRITC-conjugated anti-rabbit).
Real-time RT-PCR
Quantitative RT-PCR analysis was carried out as described previously (8). The primers used for quantitative RT-PCR in cultured cells were as follows: human β-ACTIN (5′-gtaccactggcatcgtgatggact-3′ and 5′-ccgctcattgccaatggtgat-3′), human DVL2 (5′-gcttccacatggccatgggc-3′ and 5′-tggcactgctggtgagagtcacag-3′), human NEDD4L (5′-tccaatggtcctcagctgttta-3′ and 5′-attttccacggccatgaga-3′), human AXIN2 (5′-agtgtgaggtccacggaaac-3′ and 5′-cttcacactgcgatgcattt-3′), human c-MYC (5′-tctccttgcagctgcttag-3′ and 5′-gtcgtagtcgaggtcatag-3′), and human DKK1 (5′-tcccctgtgattgcagtaaa-3′ and 5′-tccaagagatccttgcgttc-3′).
Reporter Assay, Immunoblotting, Immunofluorescence, and Immunoprecipitation
They were performed as described previously (8).
In Vitro Pulldown Assay
GST-NEDD4L was purified from Escherichia coli and immobilized for 1 h on glutathione-Sepharose beads (Amersham Biosciences) at 4 °C. The beads were washed extensively with binding buffer (50 mm Tris-HCl, pH 8.0, 250 mm NaCl) and subsequently incubated for 90 min with FLAG-tagged Dvl2, which was in vitro translated by a reticulocyte lysate system (Promega). Bound proteins were extracted with loading buffer and analyzed by immunoblotting.
Ubiquitination Assay
In vivo ubiquitination was performed as follows. HEK293T cells were transfected with pCMV5-His-Myc-ubiquitin along with other expression vectors as indicated. At 40 h post-transfection, HEK293T cells were treated with ALLN for 4 h. The cells were lysed and cell lysates were boiled for 5 min in 1% SDS. After 10-fold dilution of the lysate with lysis buffer (20 mm Tris-HCl, pH 7.4, 2 mm EDTA, 25 mm NaF, 1% Triton X-100) plus protease inhibitors (Roche Applied Science) for 30 min at 4 °C, Dvl2 was immunoprecipitated and followed by immunoblotting. The in vitro ubiquitination assay was performed as described previously with modifications that 500 ng of recombinant human UbcH5b (E2; Enzo Life Science) and 2 μg of purified GST-NEDD4L were used in the reaction (8).
Embryo Microinjections
Xenopus laevis embryos were obtained and maintained as described (23). Embryos were microinjected in 2% Ficoll solution with mRNA at the required stages and cultured in 0.1 × MMR (Marc's Modified Ringers). Capped synthetic RNAs were generated by in vitro transcription using the mMessage mMachine kit (Ambion).
Statistical Analysis
Statistical analyses were performed with a two-tailed unpaired t test. p < 0.05 was considered statistically significant.
RESULTS
NEDD4L Interacts and Colocalizes with Dvl2
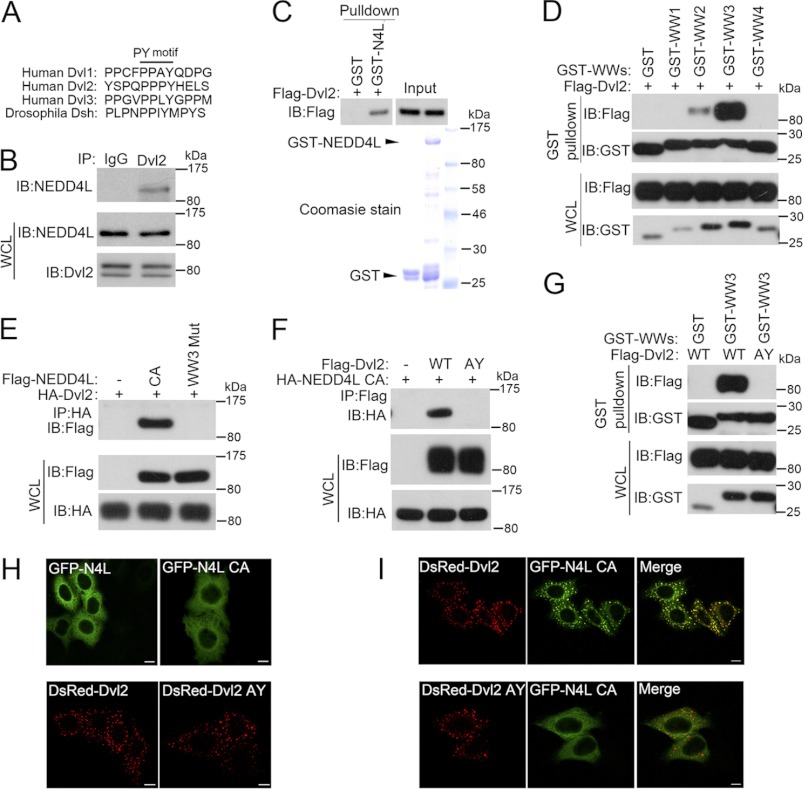
As the C-terminal of Dvl2 harbors a PY motif (PPXY), which is highly conserved among three human Dvl proteins and Drosophila Dsh (Fig. 1A); we asked whether this PY motif (PPXY) could be recognized by WW domain-containing NEDD4L through a physical interaction. Coimmunoprecipitation revealed the interaction between endogenous NEDD4L and endogenous Dvl2 in HEK293T cells (Fig. 1B). Purified GST-NEDD4L could interact with in vitro translated Dvl2, indicating the interaction is direct (Fig. 1C). The interaction was also confirmed when Dvl2 was overexpressed together with the NEDD4L C821A mutant (CA mutant) (Fig. 1E), which lacked ubiquitin ligase activity (17). Domain mapping revealed that the WW3 domain of NEDD4L alone had a strong interaction with Dvl2, and the WW2 domain had a weaker binding affinity, whereas both the WW1 and WW4 domains failed to bind to Dvl2 (Fig. 1D). Furthermore, the interaction between Dvl2 and NEDD4L was abrogated when the conserved residues (WW … P) in the WW3 domain were substituted (AA … F) (Fig. 1E). Consistent with the reported interaction between WW domains of NEDD4L and PY motifs of its substrates (15, 17), mutation of the PY motif (PPGY to AAGY, AY mutant) of Dvl2 abolished its interaction with full-length NEDD4L (Fig. 1F) or the WW3 domain (Fig. 1G). These data indicate that NEDD4L directly interacts with Dvl2 through the WW3 domain of NEDD4L and the PY motif of Dvl2.
FIGURE 1.
NEDD4L interacts with Dvl2. A, the PY motif (PPXY) is highly conserved among three human Dvl proteins and the Drosophila Dishevelled protein. B, endogenous NEDD4L interacts with Dvl2. HEK293T cell lysates were subjected to immunoprecipitation (IP) with control IgG or anti-Dvl2 antibodies followed by anti-NEDD4L immunoblotting (IB). C, NEDD4L interacts with Dvl2 in vitro. GST pulldown assay using in vitro translated FLAG-Dvl2 and immobilized GST or GST-NEDD4L fusion protein is shown. Precipitated proteins were detected with anti-FLAG antibody. Loading of GST and GST-NEDD4L protein was shown by Coomassie staining. D, WW3 and WW2 domains of NEDD4L bind to Dvl2. After transfection with different GST-NEDD4L WW domains together with FLAG-Dvl2 for 48 h, HEK293T cells were harvested for GST pulldown followed by anti-FLAG immunoblotting. Total protein expression was confirmed by immunoblotting with whole cell lysates (WCL). E, the WW3 domain of NEDD4L is required for its interaction with Dvl2. After transfection with FLAG-NEDD4L CA or WW3 mutant together with HA-Dvl2 for 48 h, HEK293T cells were harvested for anti-HA immunoprecipitation followed by anti-FLAG immunoblotting. Total protein expression was confirmed by immunoblotting with whole cell lysates (WCL) (lower panels). F, the PY motif of Dvl2 is essential for its interaction with NEDD4L. The experiment was performed similarly as in D. G, the WW3 domain binds to wild-type, but not the AY mutant Dvl2. After transfection with GST-NEDD4L (WW3) together with wild-type or AY mutant FLAG-Dvl2 for 48 h, HEK293T cells were harvested for GST pulldown followed by anti-FLAG immunoblotting. Total protein expression was confirmed by immunoblotting with whole cell lysates. H, Cellular distribution of wild-type, CA mutant NEDD4L, wild-type and AY mutant Dvl2. HeLa cells transfected with wild-type or mutant GFP-NEDD4L or DsRed-Dvl2 individually were processed for immunofluorescence. Scale bar: 10 μm. I, wild-type, but not AY mutant Dvl2, recruits NEDD4L to puncta. HeLa cells transfected with wild-type or AY mutant DsRed-Dvl2 together with EGFP-NEDD4L CA were processed for immunofluorescence. Scale bar, 10 μm.
We then analyzed the subcellular localization of NEDD4L and Dvl2. When expressed alone in HeLa cells, both wild-type and AY mutant Dvl2 appeared as puncta in the cytoplasm, whereas wild-type and the NEDD4L CA mutant were diffused throughout the cytoplasm (Fig. 1H). Strikingly, when co-expressed with wild-type Dvl2, NEDD4L (CA) formed puncta and colocalized well with Dvl2 (Fig. 1I). However, coexpression with the Dvl2 (AY) mutant did not induce punctate distribution of NEDD4L (Fig. 1I), consistent with that Dvl2 (AY) did not interact with NEDD4L. Together, these results demonstrate that NEDD4L and Dvl2 interact with each other.
NEDD4L Ubiquitinates Dvl2 and Induces Its Degradation
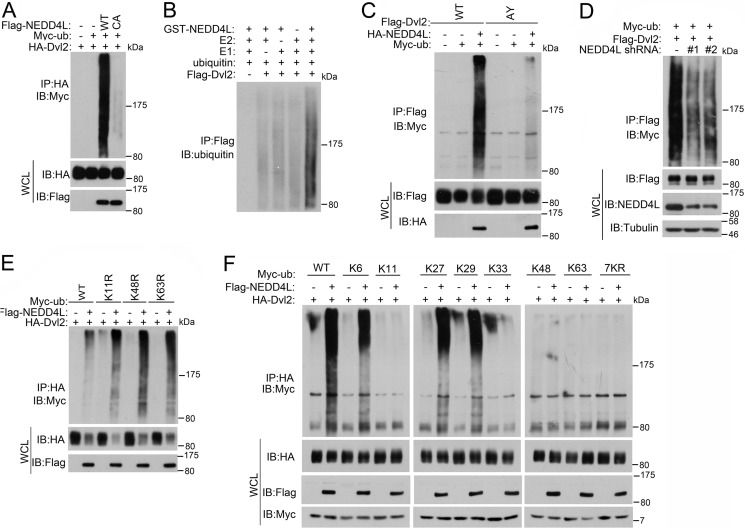
As NEDD4L is a HECT-domain E3 ubiquitin ligase, we then tested whether NEDD4L could ubiquitinate Dvl2. Indeed, wild-type NEDD4L, but not the CA mutant, efficiently promoted Dvl2 ubiqutination in vivo (Fig. 2A). In vitro ubiquitination reconstitution with recombinant proteins also confirmed that Dvl2 is a direct target of NEDD4L (Fig. 2B). Consistently, Dvl2 (AY) could not be ubiquitinated by NEDD4L (Fig. 2C), and knockdown of NEDD4L decreased Dvl2 ubiqutination (Fig. 2D).
FIGURE 2.
NEDD4L ubiquitinates Dvl2. A, NEDD4L promotes Dvl2 ubiquitination in vivo. After HEK293T cells were transfected with the plasmids as indicated for 40 h, cells were treated with ALLN (30 μm) for 4 h. Cells were harvested for anti-HA immunoprecipitation (IP) followed by anti-Myc immunoblotting (IB). Total protein expression was confirmed by immunoblotting with whole cell lysates (WCL). B, in vitro ubiquitination of Dvl2 by NEDD4L. In vitro translated FLAG-Dvl2 was incubated with or without purified E1, E2, and E3 ligase NEDD4L as indicated. The reaction was subjected to anti-FLAG immunoprecipitation, followed by anti-ubiquitin immunoblotting. C, NEDD4L enhances ubiquitination of wild-type but not AY mutant Dvl2. The experiment was performed similarly as in A. D, NEDD4L knockdown decreases Dvl2 ubiquitination. After HEK293T cells were transfected with FLAG-Dvl2, Myc-ubiquitin, and NEDD4L shRNAs for 60 h, cells were treated with ALLN (30 μm) for 4 h. Cell extracts were then subjected to anti-FLAG immunoprecipitation followed by anti-Myc immunoblotting. Total protein expression was confirmed by immunoblotting with whole cell lysates. E, ubiquitin K11R, K48R, and K63R mutants have no effect on Dvl2 ubiquitination induced by NEDD4L. The experiment was performed similarly as in A. F, NEDD4L promotes Lys-6, Lys-27, and Lys-29-linked ubiquitination of Dvl2. The experiment was performed similarly as in A.
Lys-48-linked ubiquitination is mainly to target the substrates for proteasomal degradation, whereas Lys-63-linked ubiquitination is involved in regulation of protein activity (24). It has been reported that NEDD4L promotes Lys-11-, Lys-48-, and Lys-63-linked ubiquitination (25). However, overexpression of K11R, K48R, and K63R ubiquitin mutants did not abolish the NEDD4L-mediated ubiquitination of Dvl2 (Fig. 2E). This raised the intriguing question, what type of ubiquitination NEDD4L induces on Dvl2? To address this, we created a series of ubiquitin mutants possessing a single lysine and found that NEDD4L promoted Lys-6-, Lys-27-, and Lys-29-linked polyubiquitination of Dvl2 (Fig. 2F). These results suggest that NEDD4L is an ubiquitin ligase for Dvl2 and catalyzes Lys-6-, Lys-27-, and Lys-29-linked ubiquitination.
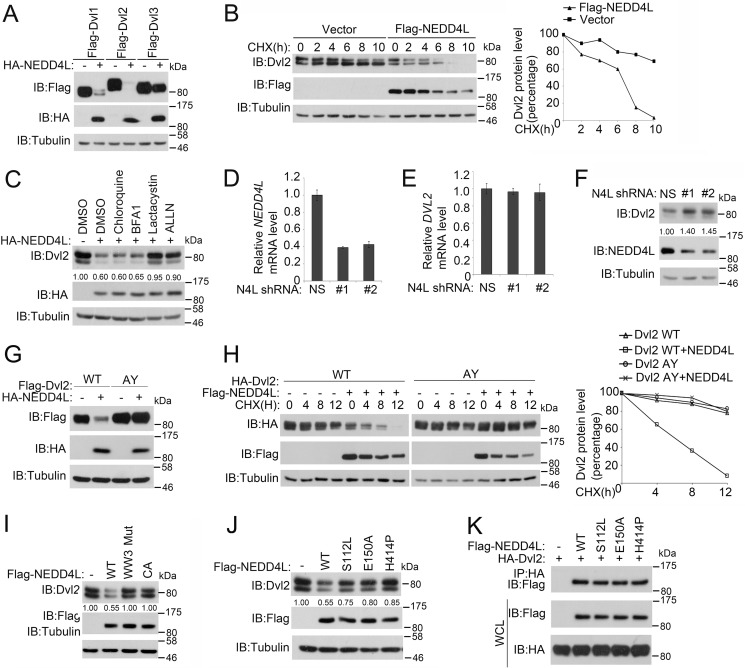
We then examined whether NEDD4L regulated Dvl stability via its ubiquitin ligase activity. Overexpression of NEDD4L down-regulated the protein levels of all three Dvl members albeit less efficiently on Dvl3 in HEK293T cells (Fig. 3A). Consistently, the half-life of Dvl2 was greatly reduced by NEDD4L (Fig. 3B). Moreover, down-regulation of the Dvl2 protein by NEDD4L was blocked by proteasome inhibitors lactacystin and ALLN, but not by lysosome inhibitors bafilomycin A1 and chloroquine (Fig. 3C), indicating that NEDD4L promoted Dvl2 degradation through the proteasomal pathway. Conversely, knockdown of NEDD4L significantly increased the Dvl2 protein level without affecting the Dvl2 mRNA levels (Fig. 3, D–F). The intact PY motif, which was required for the association of Dvl2 with NEDD4L, was necessary for NEDD4L-promoted Dvl2 degradation (Fig. 3, G and H). In line with this, both the WW3 mutant NEDD4L that was incapable of binding Dvl2 and CA NEDD4L that was unable to ubiquitinate Dvl2 failed to degrade Dvl2 (Fig. 3I).
FIGURE 3.
NEDD4L promotes Dvl2 degradation. A, NEDD4L induces degradation of three Dvl members. After transfection with human FLAG-Dvl1, -2, and -3 together with or without HA-NEDD4L for 48 h, HEK293T cells were harvested for immunoblotting (IB). Tubulin served as a loading control. B, NEDD4L decreases Dvl2 half-life. After HEK293T cells were transfected with FLAG-NEDD4L for 36 h, cells were treated with cycloheximide (CHX) (20 μg/ml) for the indicated times and harvested for immunoblotting. Tubulin served as a loading control. Dvl2 levels were quantified. C, NEDD4L degrades Dvl2 through the ubiquitin-proteasome pathway. HEK293T cells transfected with HA-NEDD4L were treated with lysosome inhibitors bafilomycin A1 (0.2 μm) and chloroquine (50 μm) or proteasome inhibitors lactacystin (10 μm) and ALLN (30 μm) for 4 h before harvesting for immunoblotting. Tubulin served as a loading control. The lower panel is the quantitation of Dvl2 band density. D and E, NEDD4L knockdown has no effect on the Dvl2 mRNA level. After HEK293T cells were transfected with nonspecific (NS) or two independent NEDD4L shRNAs for 72 h, total RNA was extracted and subjected to quantitative RT-PCR for examine NEDD4L (D) or Dvl2 (E) mRNA expression. The results show mean ± S.D. (n = 3). F, NEDD4L knockdown increases the Dvl2 level. After HEK293T cells were transfected with NS or two independent NEDD4L shRNAs for 72 h, cells were harvested for immunoblotting. Tubulin served as a loading control. G, the PY motif of Dvl2 is necessary for NEDD4L-induced degradation. After wild-type or AY mutant FLAG-Dvl2 was transfected with or without HA-NEDD4L into HEK293T cells for 48 h, cells were harvested for immunoblotting. Tubulin served as a loading control. H, NEDD4L decreases the half-life of wild-type Dvl2, but not Dvl2 (AY). The experiment was performed similarly as in B. I, the WW3 domain and ubiquitin ligase activity of NEDD4L are required for its induction of Dvl2 degradation. After wild-type or WW3 mutant or CA FLAG-NEDD4L was transfected into HEK293T cells for 48 h, cells were harvested for immunoblotting. Tubulin served as a loading control. J, point mutations of NEDD4L associated with epilepsy attenuate its ability to degrade Dvl2. The experiment was preformed similarly as in I. K, mutations of NEDD4L associated with epilepsy do not affect the interaction with Dvl2. HEK293T cells were transfected with the indicated constructs. After 36 h, the cells were treated with 30 μm ALLN for 4 h before harvesting for anti-HA immunoprecipitation (IP) followed by anti-FLAG immunoblotting. Total protein expression was confirmed by immunoblotting with whole cell lysates (WCL) (lower panels).
Several point mutations of NEDD4L have been indicated to be associated with epilepsy (26). We then assessed if these mutations would affect its ability to regulate Dvl2 turnover and found that these mutations indeed attenuated the ability of NEDD4L to promote Dvl2 degradation (Fig. 3J). Interestingly, these mutations did not affect the interaction between NEDD4L and Dvl2 (Fig. 3K). Collectively, our results indicate that NEDD4L promotes Dvl2 degradation through the proteasomal pathway and the interaction between these two proteins is needed for this process.
NEDD4L Negatively Regulates Wnt/β-Catenin Signaling
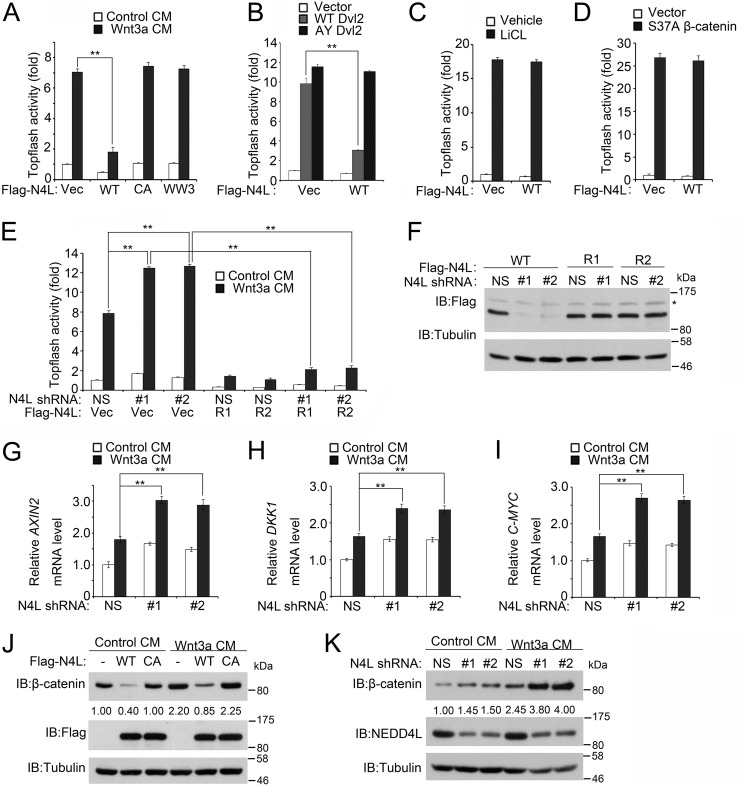
Given that NEDD4L targets Dvl2 for proteasomal degradation and Dvl2 is a critical component of both Wnt/β-catenin and Wnt/PCP pathways, we assessed whether NEDD4L modulated the Wnt/β-catenin signaling. Indeed, overexpression of wild-type NEDD4L, but not the CA or WW3 mutant NEDD4L, decreased Wnt3a-induced Topflash reporter expression (Fig. 4A). Moreover, NEDD4L inhibited wild-type, but not the AY mutant, Dvl2 induced Topflash reporter expression (Fig. 4B). However, NEDD4L had no effect on reporter expression induced by the glycogen synthase kinase 3β inhibitor LiCl and a constitutively active (S37A) β-catenin (Fig. 4, C and D). Conversely, knockdown of NEDD4L enhanced Wnt3a-induced Topflash reporter expression, which was blocked by expression of shRNA-resistant NEDD4L (Fig. 4, E and F). Consistently, knockdown of NEDD4L increased the expression of Wnt/β-catenin signaling target genes AXIN2, DKK1, and C-MYC (Fig. 4, G–I). Accordingly, overexpression and knockdown of NEDD4L decreased and increased cellular β-catenin levels, respectively (Fig. 4, J and K). Taken together, these results indicate that NEDD4L is a negative regulator of Wnt/β-catenin signaling, functioning at the level of Dvl.
FIGURE 4.
NEDD4L attenuates Wnt/β-catenin signaling. A, wild-type NEDD4L, but not CA or WW3 NEDD4L mutants, attenuates Wnt3a-induced Topflash reporter expression. After transfection with the Topflash luciferase reporter with or without FLAG-NEDD4L variants for 36 h, HEK293T cells were treated with control medium or Wnt3a conditioned medium (CM) overnight and then luciferase activity was measured. The pRL-TK Renilla reporter was co-transfected to normalize transfection efficiency. The experiment was performed in triplicate, and data were represented as the mean ± S.D. after normalized to Renilla activity (**, p < 0.01). B, NEDD4L decreases wild-type Dvl2, but not AY mutant Dvl2, induced Topflash reporter activity. The experiment was performed similarly as in A except that Dvl2 was transfected to activate the reporter activity. C, NEDD4L has no effect on LiCl-induced Topflash reporter activity. The experiment was performed similarly as in A, except that 20 mm LiCl was used to activate the reporter activity. D, NEDD4L has no effect on β-catenin (S37A)-induced Topflash reporter activity. The experiment was performed similarly as in A, except that β-catenin (S37A) was transfected to activate reporter activity. E, NEDD4L knockdown increases Topflash reporter activity. The experiment was performed as in A, except that various plasmids and nonspecific (NS) or NEDD4L shRNAs were transfected for 60 h. FLAG-NEDD4L R1 and R2 were resistant to NEDD4L shRNA1 and shRNA2, respectively. F, NEDD4L shRNAs have no effect on expression of shRNA-resistant NEDD4L. HEK293T cells transfected in E were harvested for immunoblotting (IB) with anti-FLAG antibody. Tubulin served as a loading control. The asterisk indicates a nonspecific band. G–I, NEDD4L knockdown increases the expression of AXIN2, DKK1, and C-MYC. The experiments were performed similarly as in Fig. 3D. J, NEDD4L overexpression decreases the β-catenin level. After transfection with wild-type or CA FLAG-NEDD4L for 40 h, HEK293T cells were treated with control medium or Wnt3a condition medium (CM) for 6 h and then harvested for immunoblotting. Tubulin served as a loading control. K, NEDD4L knockdown increases the β-catenin level. The experiment was performed as in J, except that nonspecific or NEDD4L shRNAs were transfected for 60 h.
NEDD4L Regulates Activities of the Wnt/PCP Pathway Components RhoA, Rac1, and JNK
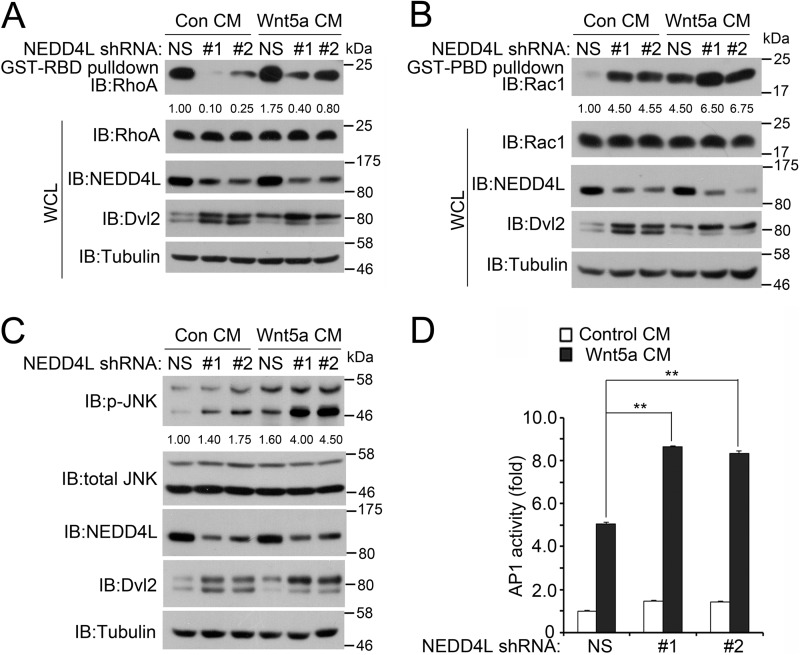
To explore the function of NEDD4L in Wnt/PCP signaling, we tested whether the activities of Rac1 and RhoA, which can be activated by Wnt5a and act downstream of Dvl2, were altered in response to NEDD4L depletion. Using GST-PBD (p21 binding domain) and GST-RBD (Rho binding domain) fusion proteins to pull down the active form of Rac1 and RhoA, respectively, we found that NEDD4L depletion dramatically increased the Rac1-GTP level and decreased the RhoA-GTP level both in the basal level and upon Wnt5a stimulation (Fig. 5, A and B). Rac1 activation resulted from NEDD4L depletion was further corroborated by enhanced phosphorylation of JNK, an effector of Rac1-GTP (Fig. 5C) and elevated expression of the JNK-responsive AP1 reporter (Fig. 5D). These results are in agreement with the observation that the increased Dvl protein level stimulated JNK activity, whereas impairing Rho activity (27). These data together indicate that NEDD4L could regulate PCP signaling via Dvl.
FIGURE 5.
NEDD4L regulates the activities of RhoA, Rac1, and JNK. A, NEDD4L knockdown decreases RhoA activity. HEK293T cells were transfected with nonspecific (NS) shRNA or NEDD4L shRNAs. At 40 h post-transfection, the cells were serum-starved for 20 h and then treated with control medium or Wnt5a medium for 4 h. The cells were then lysed, and active GTP-bound RhoA was immunoprecipitated using purified GST-RBD protein and detected by anti-RhoA antibody. Total protein expression was confirmed by immunoblotting with whole cell lysates (WCL). B, NEDD4L knockdown increases Rac1 activity. As in A, except that active GTP-bound Rac1 was immunoprecipitated using purified GST-PBD protein and detected by anti-Rac1 antibody. C, NEDD4L knockdown increases JNK activity. HEK293T cells were transfected, serum starved, and treated with control medium or Wnt5a medium as in A. Then cells were harvested for immunoblotting (IB). Tubulin serves as a loading control. D, NEDD4L knockdown increases AP1 luciferase-reporter activity. HEK293T cells were transfected with NS or NEDD4L shRNA together with AP-1 reporter and Renilla. The cells were then serum-starved and treated with control medium or Wnt5a medium as in A and harvested for luciferase assay. The experiment was performed in triplicate, and data represented as the mean ± S.D. after normalized to Renilla activity (**, p < 0.01).
JNK-mediated Phosphorylation Is Required for NEDD4L to Down-regulate Dvl2
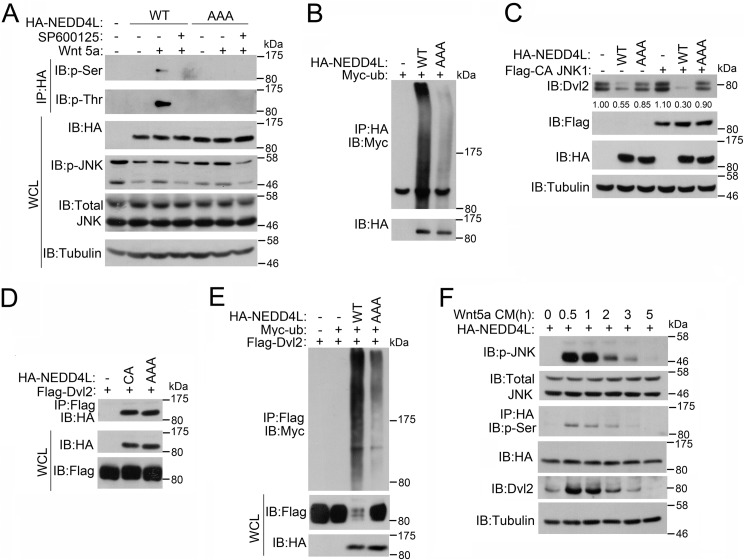
Several kinases have been reported to regulate NEDD4L activity. For instance, JNK1 can phosphorylate NEDD4L at three conserved serine and threonine residues (Ser-176, Thr-291, and Thr-882), leading to stimulation of its ubiquitin ligase activity (28, 29). As JNK1 activity is regulated by Wnt5a, we then asked whether Wnt5a could affect NEDD4L phosphorylation. Indeed, Wnt5a treatment induced NEDD4L phosphorylation, which was abolished by the JNK inhibitor SP600125, indicative of JNK involvement. Consistently, mutation of the three JNK1-phosphorylated serine and threonine residues to alanines (AAA mutant) abolished Wnt5a-induced phosphorylation (Fig. 6A). In agreement with the previous report (28), NEDD4L(AAA) showed impaired autoubiquitination, suggesting that JNK1-mediated phosphorylation is required for its ubiquitin ligase activity (Fig. 6B). We then assessed whether NEDD4L phosphorylation affected its ability to induce Dvl2 degradation. Compared with the wild-type, NEDD4L(AAA) was less effective in promoting Dvl2 turnover (Fig. 6C). This was not due to impaired interaction between NEDD4L and Dvl2, as Dvl2 showed similar binding affinity with wild-type and the AAA mutant NEDD4L (Fig. 6D). Moreover, NEDD4L(AAA) did not promote Dvl2 ubiquitination as efficiently as wild-type NEDD4L (Fig. 6E). Consistently, co-expression of constitutively active JNK1 (30, 31) facilitated Dvl2 degradation by wild-type NEDD4L, but not the AAA mutant NEDD4L (Fig. 6C). Finally, we assessed the temporal order of JNK activation and NEDD4L phosphorylation. Upon Wnt5a treatment, JNK was fast activated and then gradually inactivated. The phosphorylation of NEDD4L correlated well with JNK activity, suggesting that JNK may act as an upstream kinase for NEDD4L. Activated NEDD4L then facilitated Dvl2 degradation (Fig. 6F). Collectively, these results indicate that Wnt5a-induced JNK activation and subsequent NEDD4L phosphorylation were critical for NEDD4L to promote Dvl2 ubiquitination and degradation.
FIGURE 6.
JNK1-mediated phosphorylation is required for NEDD4L to promote Dvl2 degradation. A, Wnt5a induces NEDD4L phosphorylation in a JNK-dependent manner. After HEK293T cells were transfected wild-type or AAA mutant HA-NEDD4L for 40 h, cells were treated with Wnt5a (100 ng/ml) and JNK inhibitor (20 μm) for 4 h. Cells were then harvested for anti-HA immunoprecipitation (IP) followed by anti-Ser(P) or anti-Thr(P) immunoblotting (IB). Total protein expression was confirmed by immunoblotting with whole cell lysates (WCL). B, mutation of JNK1 phosphorylation sites disrupts ubiquitin ligase activity of NEDD4L. The experiment was performed similarly as in Fig. 3A. C, JNK1 phosphorylation of NEDD4L triggers its degradation of Dvl2. HEK293T cells were transfected with the indicated plasmids. After 48 h, cells were harvested for immunoblotting. Tubulin served as a loading control. D, mutation of JNK1 phosphorylation sites of NEDD4L has no effect on its interaction with Dvl2. The experiment was performed similarly as in Fig. 1E. E, JNK1 phosphorylation of NEDD4L is requisite for its ubiquitination of Dvl2. The experiment was performed similarly as in Fig. 2A. F, NEDD4L phosphorylation is correlated with JNK activation. HEK293T cells were transfected with wild-type NEDD4L. At 40 h post-transfection, the cells were nutrient starved in Hanks' balanced salt solution for 6 h, treated with Wnt5a conditional medium, and then harvested at the indicated time points for immunoblotting. Tubulin served as a loading control.
NEDD4L Inhibits Dvl2-induced Axis Duplication in Xenopus
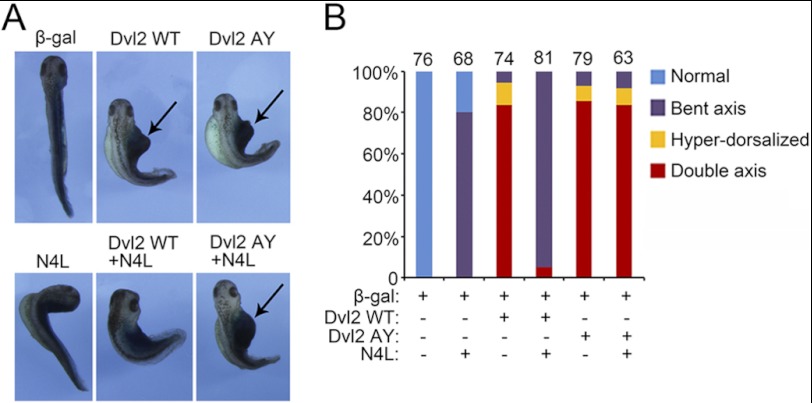
Finally, we assessed the physiological significance of NEDD4L-regulated Wnt/β-catenin signaling in vivo. Wnt/β-catenin signaling activation induces dorsal axis duplication in Xenopus embryos (5, 9, 12). Consistent with previous reports (12), injection of wild-type or AY mutant Dvl2 mRNA could induce a second axis. However, co-injection of NEDD4L mRNA repressed axis duplication induced by wild-type Dvl2, but not by the AY mutant Dvl2 (Fig. 7). Interestingly, injection of NEDD4L mRNA alone also resulted in bent axis, indicating a role of NEDD4L in axis formation.
FIGURE 7.
NEDD4L antagonizes Dvl2-induced axis duplication in Xenopus embryos. A, NEDD4L blocks axis duplication induced by wild-type Dvl2, but not by AY mutant. mRNA (1 ng) encoding wild-type or AY mutant human Dvl2 was injected alone or together with 500 pg of human NEDD4L mRNA into the ventral marginal zone at the 4-cell stage Xenopus embryos, and β-galactosidase mRNA (500 pg) was coinjected as a lineage marker. The phenotype was scored at the tadpole stage. B, quantitative analysis of the phenotype shown in A. The total number of injected embryos of each group was indicated.
DISCUSSION
NEDD4L is an ubiquitin ligase implicated in several cellular or physiological processes (16, 17). Here we provide multiple lines of evidence to show a novel function of NEDD4L to regulate Wnt signaling. NEDD4L ubiquitinates Dvl and promotes its degradation and then controls the cellular β-catenin level and the activities of Rac1, RhoA, and JNK. We further demonstrated that JNK1 could potentiate NEDD4L ubiquitin ligase activity through phosphorylation, implicating a possible negative feedback regulation of Wnt signaling. Finally we showed that NEDD4L antagonized Dvl2-induced axis duplication in Xenopus embryos.
The activity and stability of Dvl proteins are tightly regulated by many cellular proteins. They can undergo either proteasomal degradation or autophagy-lysosomal degradation upon ubiquitination induced by various E3 ligases (7–9). Recent studies have suggested a role of NEDD4 family ubiquitin ligases in regulation of Dvl stability. NEDL1 has been reported to target Dvl1 for degradation (11, 32). NEDD4 was shown to degrade Dvl1, but not Dvl2 and Dvl3 (32). However, we observed that, like NEDD4L, NEDD4 could interact with Dvl2 and promote its degradation through ubiquitination (data not shown). ITCH specifically regulates turnover of phosphorylated Dvl (14). In this study, we provide compelling evidence that NEDD4L is a genuine ubiquitin ligase for Dvl2 and regulates both Wnt/β-catenin and Wnt/PCP pathways. In vivo studies using mouse models suggest that these NEDD4 family members have distinct functions (15). NEDL1 transgenic mice mainly show muscle atrophy and motor neuron degeneration (33). Disruption of NEDD4 in mice causes embryonic lethality at midgestation and severe heart defects and vasculature abnormalities due to up-regulation of the Tsp-1 protein (34). It has also been reported that NEDD4 knock-out mice show delayed embryonic development and growth retardation resulting from reduced insulin-like growth factor 1 and insulin signaling (35). ITCH knock-out mice primarily show defects in the immune system through regulation of diverse substrates, including c-Jun, JunB, and PLCγ1 (36, 37). Recently it was shown that ITCH knock-out mice also showed age-dependent alterations in spermatogenesis (38). NEDD4L knock-out mice died perinatally due to disrupted lung function (21, 22). Therefore, it remains to clarify whether NEDD4 family ubiquitin ligases play a unique or redundant role in regulating stability of Dvl family proteins and thus examine their relevant contributions to the related physiological and pathological processes.
E3 ligases can promote the formation of polyubiquitin chains on substrates through any of the seven lysines present on ubiquitin molecules (24, 39). NEDD4L activity has been associated with chain formation mainly via Lys-11, Lys-48, and Lys-63 linkages (25). However, none of the linkages was used in the ubiquitination of Dvl2 by NEDD4L. In contrast, NEDD4L promoted Dvl2 ubiquitination via atypical ubiquitin chain formation involving residues Lys-6, Lys-27, and Lys-29, leading to its proteasomal degradation. Our study uncovered a novel role of NEDD4L to catalyze Lys-6, Lys-27, and Lys-29 ubiquitin chain formation and showed that these atypical ubiquitin chains played an important role in the regulation of Dvl stability.
NEDD4L gene mutations have been associated with neurological disorders. It was reported that NEDD4L has been implicated to be a susceptibility locus for bipolar affective disorder (40). NEDD4L was also identified as a candidate gene for dyslexia susceptibility and a modifier of age at neurological onset in Huntington disease (41). In addition, point mutations of NEDD4L have also been identified in idiopathic generalized epilepsies in patients (26). However, the role of NEDD4L in the pathogenesis and progression of these disorders is unknown. We show here that its point mutations associated with epilepsy, which did not affect its regulation of ion channels (26), impaired its ability to degrade Dvl2. Taken together, given the critical role of Wnt signaling in neural development and diseases, our findings may shed light on the role of NEDD4L in neurological disorders. Furthermore, down-regulation or loss of function of NEDD4L has been proposed to be associated with the malignancy of prostate cancer, non-small cell lung cancer, and glioma (42–44). Interestingly, Dvl protein levels have been reported to be elevated in these cancers (45–47). Therefore, the NEDD4L/Dvl axis may also have an important function in tumorigenesis.
Acknowledgments
We thank Dr. Lingqiang Zhang for providing human NEDD4 plasmid, Dr. Long Yu for providing NEDD4 antibody, Dr. Wei Wu for providing Rac1 antibody, and Dr. He Li for help in purifying NEDD4L protein.
This work was supported by National Key Basic Research Program of China (973 Program) Grants 2011CB943800 (to Y. G. C. and Q. H. T.) and National Natural Science Foundation of China Grants 30930050 and 30921004 (to Y. G. C.) and 30930012 (to Q. H. T.).
- Dvl
- Dishevelled
- PCP
- planar cell polarity
- JNK
- c-Jun N-terminal kinases
- GST
- glutathione S-transferase
- TRITC
- tetramethylrhodamine isothiocyanate.
REFERENCES
- 1. Clevers H., Nusse R. (2012) Wnt/β-catenin signaling and disease. Cell 149, 1192–1205 [DOI] [PubMed] [Google Scholar]
- 2. Wallingford J. B. (2012) Planar cell polarity and the developmental control of cell behavior in vertebrate embryos. Annu. Rev. Cell Dev. Biol. 28, 627–653 [DOI] [PubMed] [Google Scholar]
- 3. Gray R. S., Roszko I., Solnica-Krezel L. (2011) Planar cell polarity. Coordinating morphogenetic cell behaviors with embryonic polarity. Dev. Cell 21, 120–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goodrich L. V., Strutt D. (2011) Principles of planar polarity in animal development. Development 138, 1877–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. MacDonald B. T., Tamai K., He X. (2009) Wnt/β-catenin signaling. Components, mechanisms, and diseases. Dev. Cell 17, 9–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simons M., Mlodzik M. (2008) Planar cell polarity signaling. From fly development to human disease. Annu. Rev. Genet. 42, 517–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gao C., Chen Y. G. (2010) Dishevelled. The hub of Wnt signaling. Cell. Signal. 22, 717–727 [DOI] [PubMed] [Google Scholar]
- 8. Gao C., Cao W., Bao L., Zuo W., Xie G., Cai T., Fu W., Zhang J., Wu W., Zhang X., Chen Y. G. (2010) Autophagy negatively regulates Wnt signalling by promoting Dishevelled degradation. Nat. Cell Biol. 12, 781–790 [DOI] [PubMed] [Google Scholar]
- 9. Wallingford J. B., Habas R. (2005) The developmental biology of Dishevelled. An enigmatic protein governing cell fate and cell polarity. Development 132, 4421–4436 [DOI] [PubMed] [Google Scholar]
- 10. Angers S., Thorpe C. J., Biechele T. L., Goldenberg S. J., Zheng N., MacCoss M. J., Moon R. T. (2006) The KLHL12-Cullin-3 ubiquitin ligase negatively regulates the Wnt-β-catenin pathway by targeting Dishevelled for degradation. Nat. Cell Biol. 8, 348–357 [DOI] [PubMed] [Google Scholar]
- 11. Miyazaki K., Fujita T., Ozaki T., Kato C., Kurose Y., Sakamoto M., Kato S., Goto T., Itoyama Y., Aoki M., Nakagawara A. (2004) NEDL1, a novel ubiquitin-protein isopeptide ligase for dishevelled-1, targets mutant superoxide dismutase-1. J. Biol. Chem. 279, 11327–11335 [DOI] [PubMed] [Google Scholar]
- 12. Ganner A., Lienkamp S., Schäfer T., Romaker D., Wegierski T., Park T. J., Spreitzer S., Simons M., Gloy J., Kim E., Wallingford J. B., Walz G. (2009) Regulation of ciliary polarity by the APC/C. Proc. Natl. Acad. Sci. U.S.A. 106, 17799–17804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sharma J., Mulherkar S., Mukherjee D., Jana N. R. (2012) Malin regulates Wnt signaling pathway through degradation of dishevelled2. J. Biol. Chem. 287, 6830–6839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wei W., Li M., Wang J., Nie F., Li L. (2012) The E3 ubiquitin ligase ITCH negatively regulates canonical Wnt signaling by targeting dishevelled protein. Mol. Cell. Biol. 32, 3903–3912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rotin D., Kumar S. (2009) Physiological functions of the HECT family of ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 10, 398–409 [DOI] [PubMed] [Google Scholar]
- 16. Bernassola F., Karin M., Ciechanover A., Melino G. (2008) The HECT family of E3 ubiquitin ligases. Multiple players in cancer development. Cancer Cell 14, 10–21 [DOI] [PubMed] [Google Scholar]
- 17. Yang B., Kumar S. (2010) Nedd4 and Nedd4–2. Closely related ubiquitin-protein ligases with distinct physiological functions. Cell Death Differ. 17, 68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gao S., Alarcón C., Sapkota G., Rahman S., Chen P. Y., Goerner N., Macias M. J., Erdjument-Bromage H., Tempst P., Massagué J. (2009) Ubiquitin ligase Nedd4L targets activated Smad2/3 to limit TGF-β signaling. Mol. Cell 36, 457–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Campenhout C. A., Eitelhuber A., Gloeckner C. J., Giallonardo P., Gegg M., Oller H., Grant S. G., Krappmann D., Ueffing M., Lickert H. (2011) Dlg3 trafficking and apical tight junction formation is regulated by nedd4 and nedd4–2 E3 ubiquitin ligases. Dev. Cell 21, 479–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang C., An J., Zhang P., Xu C., Gao K., Wu D., Wang D., Yu H., Liu J. O., Yu L. (2012) The Nedd4-like ubiquitin E3 ligases target angiomotin/p130 to ubiquitin-dependent degradation. Biochem. J. 444, 279–289 [DOI] [PubMed] [Google Scholar]
- 21. Boase N. A., Rychkov G. Y., Townley S. L., Dinudom A., Candi E., Voss A. K., Tsoutsman T., Semsarian C., Melino G., Koentgen F., Cook D. I., Kumar S. (2011) Respiratory distress and perinatal lethality in Nedd4–2-deficient mice. Nat. Commun. 2, 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kimura T., Kawabe H., Jiang C., Zhang W., Xiang Y. Y., Lu C., Salter M. W., Brose N., Lu W. Y., Rotin D. (2011) Deletion of the ubiquitin ligase Nedd4L in lung epithelia causes cystic fibrosis-like disease. Proc. Natl. Acad. Sci. U.S.A. 108, 3216–3221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nandadasa S., Tao Q., Menon N. R., Heasman J., Wylie C. (2009) N- and E-cadherins in Xenopus are specifically required in the neural and non-neural ectoderm, respectively, for F-actin assembly and morphogenetic movements. Development 136, 1327–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kravtsova-Ivantsiv Y., Ciechanover A. (2012) Non-canonical ubiquitin-based signals for proteasomal degradation. J. Cell Sci. 125, 539–548 [DOI] [PubMed] [Google Scholar]
- 25. Fotia A. B., Cook D. I., Kumar S. (2006) The ubiquitin-protein ligases Nedd4 and Nedd4–2 show similar ubiquitin-conjugating enzyme specificities. Int. J. Biochem. Cell Biol. 38, 472–479 [DOI] [PubMed] [Google Scholar]
- 26. Dibbens L. M., Ekberg J., Taylor I., Hodgson B. L., Conroy S. J., Lensink I. L., Kumar S., Zielinski M. A., Harkin L. A., Sutherland G. R., Adams D. J., Berkovic S. F., Scheffer I. E., Mulley J. C., Poronnik P. (2007) NEDD4–2 as a potential candidate susceptibility gene for epileptic photosensitivity. Genes Brain Behav. 6, 750–755 [DOI] [PubMed] [Google Scholar]
- 27. Wen J., Chiang Y. J., Gao C., Xue H., Xu J., Ning Y., Hodes R. J., Gao X., Chen Y. G. (2010) Loss of Dact1 disrupts planar cell polarity signaling by altering dishevelled activity and leads to posterior malformation in mice. J. Biol. Chem. 285, 11023–11030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hallows K. R., Bhalla V., Oyster N. M., Wijngaarden M. A., Lee J. K., Li H., Chandran S., Xia X., Huang Z., Chalkley R. J., Burlingame A. L., Pearce D. (2010) Phosphopeptide screen uncovers novel phosphorylation sites of Nedd4–2 that potentiate its inhibition of the epithelial Na+ channel. J. Biol. Chem. 285, 21671–21678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Snyder P. M. (2009) Down-regulating destruction. Phosphorylation regulates the E3 ubiquitin ligase Nedd4–2. Sci. Signal 2, pe41. [DOI] [PubMed] [Google Scholar]
- 30. Lei K., Nimnual A., Zong W. X., Kennedy N. J., Flavell R. A., Thompson C. B., Bar-Sagi D., Davis R. J. (2002) The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun NH2-terminal kinase. Mol. Cell Biol. 22, 4929–4942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liao G., Tao Q., Kofron M., Chen J. S., Schloemer A., Davis R. J., Hsieh J. C., Wylie C., Heasman J., Kuan C. Y. (2006) Jun NH2-terminal kinase (JNK) prevents nuclear β-catenin accumulation and regulates axis formation in Xenopus embryos. Proc. Natl. Acad. Sci. U.S.A. 103, 16313–16318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nethe M., de Kreuk B. J., Tauriello D. V., Anthony E. C., Snoek B., Stumpel T., Salinas P. C., Maurice M. M., Geerts D., Deelder A. M., Hensbergen P. J., Hordijk P. L. (2012) Rac1 acts in conjunction with Nedd4 and Dishevelled-1 to promote maturation of cell-cell contacts. J. Cell Sci., 125, 3430–3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang L., Haraguchi S., Koda T., Hashimoto K., Nakagawara A. (2011) Muscle atrophy and motor neuron degeneration in human NEDL1 transgenic mice. J. Biomed. Biotechnol. 83, 1092–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fouladkou F., Lu C., Jiang C., Zhou L., She Y., Walls J. R., Kawabe H., Brose N., Henkelman R. M., Huang A., Bruneau B. G., Rotin D. (2010) The ubiquitin ligase Nedd4–1 is required for heart development and is a suppressor of thrombospondin-1. J. Biol. Chem. 285, 6770–6780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cao X. R., Lill N. L., Boase N., Shi P. P., Croucher D. R., Shan H., Qu J., Sweezer E. M., Place T., Kirby P. A., Daly R. J., Kumar S., Yang B. (2008) Nedd4 controls animal growth by regulating IGF-1 signaling. Sci. Signal. 1, ra5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Melino G., Gallagher E., Aqeilan R. I., Knight R., Peschiaroli A., Rossi M., Scialpi F., Malatesta M., Zocchi L., Browne G., Ciechanover A., Bernassola F. (2008) Itch. A HECT-type E3 ligase regulating immunity, skin and cancer. Cell Death Differ. 15, 1103–1112 [DOI] [PubMed] [Google Scholar]
- 37. Perry W. L., Hustad C. M., Swing D. A., O'Sullivan T. N., Jenkins N. A., Copeland N. G. (1998) The itchy locus encodes a novel ubiquitin protein ligase that is disrupted in a18H mice. Nat. Genet. 18, 143–146 [DOI] [PubMed] [Google Scholar]
- 38. Dwyer J. L., Richburg J. H. (2012) Age-dependent alterations in spermatogenesis in itchy mice. Spermatogenesis 2, 104–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Metzger M. B., Hristova V. A., Weissman A. M. (2012) HECT and RING finger families of E3 ubiquitin ligases at a glance. J. Cell Sci. 125, 531–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rao L., Kanakavalli M., Padmalatha V., Nallari P., Singh L. (2010) Paternally derived translocation t(8;18)(q22.1;q22)pat associated in a patient with developmental delay. Case report and review. J. Pediatr. Neurosci. 5, 64–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kamnasaran D. (2003) Genetic analysis of psychiatric disorders associated with human chromosome 18. Clin. Invest. Med. 26, 285–302 [PubMed] [Google Scholar]
- 42. He S., Deng J., Li G., Wang B., Cao Y., Tu Y. (2012) Down-regulation of Nedd4L is associated with the aggressive progression and worse prognosis of malignant glioma. Jpn. J. Clin. Oncol. 42, 196–201 [DOI] [PubMed] [Google Scholar]
- 43. Hu X. Y., Xu Y. M., Fu Q., Yu J. J., Huang J. (2009) Nedd4L expression is down-regulated in prostate cancer compared to benign prostatic hyperplasia. Eur. J. Surg. Oncol. 35, 527–531 [DOI] [PubMed] [Google Scholar]
- 44. Jiang F., Yin Z., Caraway N. P., Li R., Katz R. L. (2004) Genomic profiles in stage I primary non small cell lung cancer using comparative genomic hybridization analysis of cDNA microarrays. Neoplasia 6, 623–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mizutani K., Miyamoto S., Nagahata T., Konishi N., Emi M., Onda M. (2005) Up-regulation and overexpression of DVL1, the human counterpart of the Drosophila dishevelled gene, in prostate cancer. Tumori 91, 546–551 [DOI] [PubMed] [Google Scholar]
- 46. Pulvirenti T., Van Der Heijden M., Droms L. A., Huse J. T., Tabar V., Hall A. (2011) Dishevelled 2 signaling promotes self-renewal and tumorigenicity in human gliomas. Cancer Res. 71, 7280–7290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Uematsu K., He B., You L., Xu Z., McCormick F., Jablons D. M. (2003) Activation of the Wnt pathway in non-small cell lung cancer. Evidence of dishevelled overexpression. Oncogene 22, 7218–7221 [DOI] [PubMed] [Google Scholar]