Background: INSIG proteins are central to the control of lipids in mammals.
Results: Sterols regulate Nsg1-Hmg2 interactions, imparting a distinct level of HMGR stability control.
Conclusion: INSIG sterol dependence is conserved between mammals and yeast.
Significance: The yeast INSIGs can provide a genetically tractable platform to study the action of these medically important regulators of sterol homeostasis.
Keywords: Cholesterol, Endoplasmic Reticulum (ER), Protein Degradation, Sterol, Ubiquitin, ERAD, GGPP, HMGR, INSIG, Lanosterol
Abstract
Insulin-induced gene proteins (INSIGs) function in control of cellular cholesterol. Mammalian INSIGs exert control by directly interacting with proteins containing sterol-sensing domains (SSDs) when sterol levels are elevated. Mammalian 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase (HMGR) undergoes sterol-dependent, endoplasmic-reticulum (ER)-associated degradation (ERAD) that is mediated by INSIG interaction with the HMGR SSD. The yeast HMGR isozyme Hmg2 also undergoes feedback-regulated ERAD in response to the early pathway-derived isoprene gernanylgeranyl pyrophosphate (GGPP). Hmg2 has an SSD, and its degradation is controlled by the INSIG homologue Nsg1. However, yeast Nsg1 promotes Hmg2 stabilization by inhibiting GGPP-stimulated ERAD. We have proposed that the seemingly disparate INSIG functions can be unified by viewing INSIGs as sterol-dependent chaperones of SSD clients. Accordingly, we tested the role of sterols in the Nsg1 regulation of Hmg2. We found that both Nsg1-mediated stabilization of Hmg2 and the Nsg1-Hmg2 interaction required the early sterol lanosterol. Lowering lanosterol in the cell allowed GGPP-stimulated Hmg2 ERAD. Thus, Hmg2-regulated degradation is controlled by a two-signal logic; GGPP promotes degradation, and lanosterol inhibits degradation. These data reveal that the sterol dependence of INSIG-client interaction has been preserved for over 1 billion years. We propose that the INSIGs are a class of sterol-dependent chaperones that bind to SSD clients, thus harnessing ER quality control in the homeostasis of sterols.
Introduction
Regulation of the sterol pathway is critical for maintaining physiological homeostasis and the function of cellular membranes. Lessons learned from the natural mechanisms of sterol regulation are directly applicable to clinical management of cholesterol. A rate-limiting step in the highly conserved cholesterol synthesis pathway is catalyzed by HMGR2 that produces mevalonate from HMG-CoA (1). As such, HMGR is subject to multiple forms of feedback control by the sterol pathway; high flux through the pathway results in lower levels of HMGR, whereas lower flux results in higher HMGR (2, 3). Understanding feedback regulation of HMGR has implications for the clinic because HMGR is the target of cholesterol-lowering statin drugs, and sterol pathway feedback impacts the efficacy of these drugs.
The most selective mechanism controlling HMGR is the feedback regulation of its stability. Lipid signals generated by the sterol pathway feed back to regulate HMGR degradation (3, 4). When the synthesis of sterol pathway products is high, degradation is fast and HMGR protein levels fall. When synthesis is low, degradation is slow and HMGR protein levels rise (2, 5). HMGR resides in the ER and is thus subject to ER-associated degradation (ERAD). HMGR consists of an N-terminal membrane-spanning domain and a C-terminal cytosolic catalytic domain; the N-terminal domain is necessary and sufficient for HMGR-regulated degradation (3).
HMGR-regulated degradation is conserved between mammals and yeast; accordingly, we have exploited Saccharomyces cerevisiae to discover the mechanisms of degradation and its control by the sterol pathway (4). There are two HMGR isozymes in yeast, Hmg1 and Hmg2. Hmg1 is a stable isoform, whereas Hmg2 undergoes ubiquitin-mediated degradation by the ER-localized HRD pathway (4). In response to high signal, Hmg2 undergoes HRD-dependent ubiquitination, extraction from the ER membrane, and degradation by the 26 S proteasomes (6, 4).
The HRD pathway primarily functions in protein quality control to target misfolded or damaged proteins in the ER lumen or membrane for ERAD. When sterol pathway activity is high, Hmg2 appears to adopt a structure recognized by the HRD quality control pathway, allowing feedback-regulated destruction of Hmg2 (7, 8). Hmg2 entry into the HRD pathway is controlled by a signal derived from farnesyl pyrophosphate (FPP), a 15-carbon early pathway isoprene. High FPP results in more degradation and lower HMGR levels, and low FPP results in less degradation and higher HMGR protein levels (9) (Fig. 1A). In more recent work, we identified the 20-carbon FPP derivative gernaylgeranyl pyrophosphate (GGPP) as the likely endogenous FPP-derived signal (8). In addition, an oxysterol signal augments the isoprene signal to promote Hmg2-regulated degradation by the HRD pathway (10). Response to either of these signals depends on the highly conserved sterol-sensing domain (SSD) in the Hmg2 transmembrane domain, such that the SSD is required for the degradation-enhancing effects of each class of signal (11).
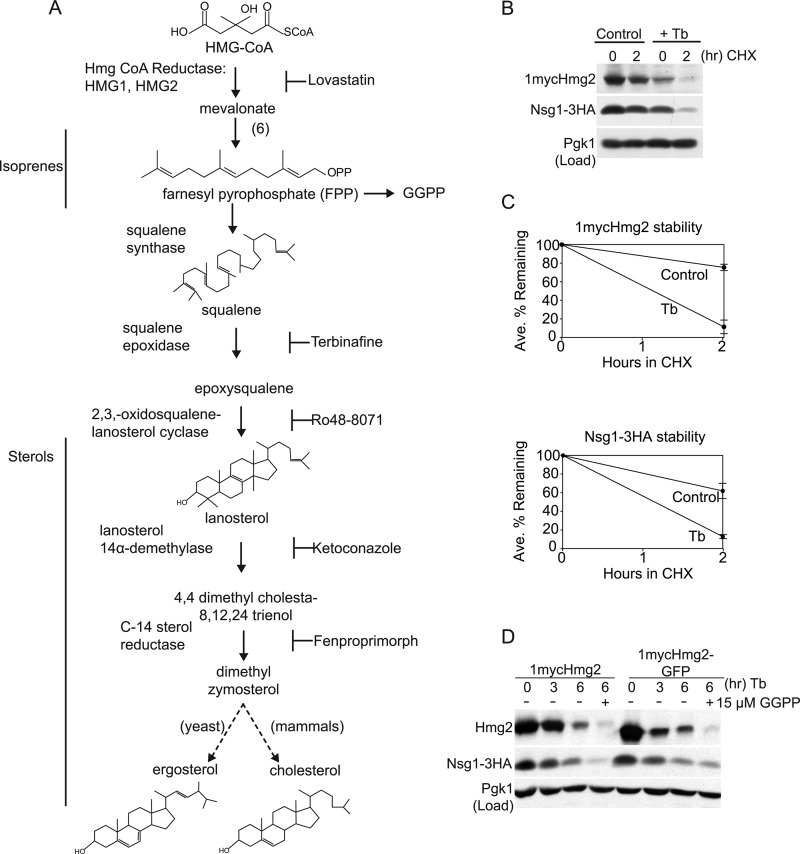
FIGURE 1.
Inhibition of sterol synthesis destabilized Hmg2 and Nsg1. A, conserved superpathway of mevalonate and sterol synthesis. The early portion produces non-sterol isoprenoid molecules, including FPP. FPP is the source of the isoprene signal GGPP (also see Fig. 7) that promotes Hmg2-regulated degradation independently of Nsg proteins. Squalene production is the first committed step in the late portion of the sterol synthesis pathway. Blocking arrows beside pharmacological inhibitors indicate their target enzyme. For example, Tb is a pharmacological inhibitor of squalene epoxidase and thus blocks synthesis of all downstream sterol molecules. B, CHX protein stability assay of natively expressed 1mycHmg2 and Nsg1–3HA. Wild-type cells were grown to log phase in minimal medium and were control-treated or treated with Tb (10 μg/ml) for 2 h at 30 °C, and then 5 μg/ml CHX was added for 2 h to assay protein stability (CHX chase assay). Following SDS-PAGE, 1mycHmg2 and Nsg1–3HA were detected by anti-Myc and anti-HA immunoblotting. Protein levels were equal in all lanes, as indicated by blotting for phosphoglycerate kinase (Pgk1; load control) in the bottom panel. C, quantitation of 1mycHmg2 and Nsg1–3HA stability in two separate experiments. Average percentages remaining and S.E. (bars) were calculated from raw integrated density values obtained with ImageJ (National Institutes of Health). Unprocessed scanned images were used, and background correction was applied to each band. Corresponding results were obtained in three more experiments when the length of Tb incubation or CHX chase was varied. D, the TMD of Hmg2 is sufficient for sterol regulation. Cells expressing 1mycHmg2 (full-length as in B) or 1mycHmg2-GFP (with GFP replacing the reductase catalytic domain) were treated with 20 μg/ml Tb at 30 °C, and samples were taken at the indicated time points. After harvesting samples at t = 3 h, 15 μm GGPP was added to half of the remaining culture to further drive Hmg2 degradation. Three hours later, (t = 6 h), samples were harvested, lysed, and subjected to SDS-PAGE followed by immunoblotting. Similar experiments were performed three times.
Regulated degradation of mammalian HMGR also occurs by ERAD, and a homologue of the Hrd1 E3 ligase called gp78 is responsible for ubiquitination (12). In the presence of pathway signals, HMGR degradation is accelerated. The primary signal for mammalian HMGR degradation is a methylated derivative of lanosterol, called 24,25-dihydrolanosterol (13, 14). The use of a lanosterol derivative ensures that the regulation of the primary enzyme of sterol synthesis is keyed to the synthesis of this class of molecules (13). In addition, degradation of mammalian HMGR by gp78 is also increased by a 20-carbon isoprene, but the nature and action of this second signal is not clear (15).
Sterol-regulated degradation of mammalian HMGR requires the highly conserved ER membrane-resident INSIG proteins (12). INSIG promotes HMGR degradation when sterol signal is high by recruiting the E3 ubiquitin ligase gp78 to HMGR. gp78, a HRD1-related ligase, promotes ubiquitination of both HMGR and INSIG that ultimately leads to their destruction by cytosolic 26 S proteasomes (3). This mechanism, a sterol-dependent association between HMGR and INSIG followed by HRD pathway destruction, functions to lower HMGR activity when sterol intermediates are high. Similarly, INSIG binds to the regulatory protein SCAP, to allow sterol-dependent control of the SREBP transcription factor's activation by cleavage. In the case of SCAP, cholesterol promotes the interaction, rather than lanosterol. Cholesterol induces INSIG binding to SCAP and traps it in the ER, blocking transfer of SCAP-bound SREBP to its site of activation in the Golgi. Both mammalian targets of INSIG, HMGR and SCAP, have SSDs, and these are required for the sterol-dependent interaction of each with INSIG. In fact, it has been suggested that the primary function of the SSD is interaction with INSIG (16), although our data and other recent data clearly show that the SSD has INSIG-independent functions as well (11, 17).
Two conserved INSIGs are expressed in yeast (18), called Nsg1 and Nsg2, and they function to control regulation of Hmg2. However, rather than promote HMGR degradation, Nsgs stabilize Hmg2, and Nsg1 functions at its natural levels to block the regulated degradation of Hmg2 by GGPP (18). As expected from INSIG action in mammals, Nsg1 stabilizes Hmg2 by binding to its SSD-containing transmembrane domain. Thus, considering all the examples, INSIGs appear to have unrelated functions: retaining SCAP in the ER, recruiting an E3 for degradation of HMGR, or causing stabilization of Hmg2. However, these apparently distinct functions represent documented activities of chaperones, and we have proposed the idea that INSIGs are chaperones dedicated to SSD-containing proteins (18).
Do these different INSIG functions toward SSD-containing proteins use the same mechanism? In mammals, INSIG function requires sterol-induced binding to the membrane anchors of HMGR and SCAP. If yeast INSIG, Nsg1, functions in a similar manner, we would expect the association between Hmg2 and Nsg1 to require a sterol molecule. Thus, we tested this idea.
In this work, we demonstrate that the action of INSIGs toward HMGR is mechanistically conserved across a billion years of evolution between yeast and mammals. Nsg1 binding and stabilization of Hmg2 required lanosterol. INSIGs in yeast thus prohibit Hmg2 entry into the HRD ERAD when sterol synthesis is active. In the absence of sterols, when Hmg2 is freed from Nsg1, high GGPP caused Hmg2 to be degraded by the HRD pathway. We propose that this regulatory logic for Hmg2-regulated degradation (“GGPP yes, sterols no”) may be appropriate when the role of anaerobiosis is considered in the natural biology of yeast. When Hmg2 and Nsg1 were disengaged, Nsg1 was also subject to ER-associated degradation. Surprisingly, Nsg1 degradation appeared to use a novel ERAD pathway. The remarkable consistency between mammalian and yeast INSIG function and mechanism will be a valuable asset in understanding this key lipid regulatory axis and the interplay between sterol biology and ER protein quality control.
EXPERIMENTAL PROCEDURES
Plasmids and DNA Methods
All strains (Table 1) and plasmids (Table 2) were constructed with standard molecular biology techniques. To construct pRH2566 (pMET3-ERG11::URA3), 850 nucleotides of the 5′ portion of the ERG11 ORF were amplified from a tiling array plasmid (19) with oligonucleotides oRH4219 and oRH4247, cut with PstI and ClaI, and cloned into pRH1205 cut with the same enzymes. pRH2566 was cut with BmgBI for integration at ERG11. pRH1206 (pMET3-ERG7::URA3) was cut with BamHI for integration at ERG7. DNA primers are listed in Table 3.
TABLE 1.
Yeast strains
| Strain | Genotype | Reference/Source | Figure |
|---|---|---|---|
| RHY8204 | MATα ade2-101 met2 lys2-801 ura3-52 hmg2Δ::1mycHMG2 trp1::hisG leu2Δ his3Δ200 pdr5Δ::KanMX4loxP pNSG1-3HA::TRP1MX6 | This work | Figs. 1 (B–D), 2A, 3C, 4A, 5 (A and B), and 6 (A and B) |
| RHY8490 | MATa ade2-101 met2 lys2-801 ura3-52 hmg2Δ::1mycHMG2 trp1::hisG leu2Δ his3Δ200 pdr5Δ::KanMX4loxP TRP1empty vector nsg1Δ::KanMX4 | This work, from RHY8299 non-parental ditype tetrad | Figs. 4 (A and B) and 6B |
| RHY8525 | MATα ade2-101 met2 lys2-801 ura3-52 hmg2Δ::1mycHMG2 trp1::hisG leu2Δ his3Δ200 pNSG1-3HA::TRP1MX6 pdr5Δ::KanMX4loxP hrd1Δ::LEU2 | This work, from RHY8204 | Fig. 6 (A and B) |
| RHY8608 | MATa ade2-101 met2 lys2-801 ura3-52 hmg2Δ::1mycHMG2 trp1::hisG leu2Δ his3Δ200 pdr5Δ::KanMX4loxP TRP1empty vector nsg1Δ::KanMX4 nsg2Δ::NatMX4 | This work, from RHY8490 | Figs. 3C and 5 (B and C) |
| RHY3425 | MATα ade2-101 met2 lys2-801 ura3-52 hmg2Δ::1mycHMG2 trp1::hisG leu2Δ his3Δ200 pNSG1-3HA::TRP1MX6 | Ref. 18 | Fig. 3 (A and B) |
| RHY8689 | MATα ade2-101 met2 lys2-801 ura3-52 hmg2Δ::NatMX4 trp1::hisG leu2Δ his3Δ200 pNSG1-3HA::TRP1MX6 | This work, from RHY3425 | Fig. 3 (A and B) |
| RHY8723 | MATα ade2-101 met2 lys2-801 ura3-52 hmg2Δ::1mycHMG2 trp1::hisG leu2Δ his3Δ200 pNSG1-3HA::TRP1MX6 hmg1Δ::NatMX4 | This work, from RHY3425 | Fig. 3 (A and B) |
| RHY3388 | MATa ade2-101 met2 lys2-801 ura3-52 hmg2Δ::1mycHMG2 trp1::hisG leu2Δ his3Δ200 nsg1Δ::KanMX4 | Ref. 18 | |
| RHY7480 | MATα ade2-101::TDH3-NSG1–3HA::ADE2 met2 lys2-801 ura3-52 his3Δ200 | This work | Fig. 6C |
| RHY7479 | MATα ade2-101::TDH3-NSG1-3HA::ADE2 met2 lys2-801 ura3-52 his3Δ200 hrd1Δ::KanMX4 | This work | Fig. 6C |
| RHY7532 | MATα ade2-101::TDH3-NSG1-3HA::ADE2 met2 lys2-801 ura3-52 his3Δ200 doa10Δ::KanMX4 | This work | Fig. 6C |
| RHY7536 | MATα ade2-101::TDH3-NSG1-3HA::ADE2 met2 lys2-801 ura3-52 his3Δ200 hrd1ΔKanMX4 doa10Δ::cloNAT | This work | Fig. 6C |
| RHY8299 | RHY8204 × RHY3388 | This work | |
| RHY8767 | MATα ade2-101 met2 lys2-801 ura3-52 hmg2Δ::1mycHMG2-GFP::HIS3MX6 trp1::hisG leu2Δ his3Δ200 pdr5Δ::KanMX4loxP pNSG1–3HA::TRP1MX6 | This work | Fig. 1D |
| RHY8928 | MAT ade2-101 MET2 lys2-801 ura3-52 hmg2Δ::1mycHMG2 trp1::hisG leu2Δ his3Δ200 pNSG1-3HA::TRP1MX6 URA3 empty vector | This work | Fig. 2 (B–D) |
| RHY8896 | MAT ade2-101 MET2 lys2-801 ura3-52 hmg2Δ::1mycHMG2 trp1::hisG leu2Δ his3Δ200 pNSG1-3HA::TRP1MX6 pMET3-ERG7::URA3 | This work | Fig. 2 (B–D) |
| RHY8899 | MAT ade2-101 MET2 lys2-801 ura3-52 hmg2Δ::1mycHMG2 trp1::hisG leu2Δ his3Δ200 pNSG1-3HA::TRP1MX6 pMET3-ERG11::URA3 | This work | Fig. 2 (B–D) |
TABLE 2.
Plasmids
| Plasmid | Genotype | Reference |
|---|---|---|
| pRH311 | pRS404 TRP1 empty vector | Ref. 30 |
| pRH313 | pRS406 URA3 empty vector | Ref. 30 |
| pRH728 | pUG6 kanMX knockout cassette | Ref. 31 |
| pRH1658 | pAG25/Euroscarf 30104 natMX knockout cassette | Ref. 32 |
| pRH1812 | pFA6a-3HA-TRP1 | Ref. 33 |
| pRH1184 | hrd1Δ::LEU2 | Ref. 34 |
| pRH1415 | MET2 | Ref. 10 |
| pRH1206 | pMET3-ERG7::URA3 | Ref. 10 |
| pRH1805 | pTDH3-NSG1-3HA::ADE2 | Ref. 18 |
| pRH2560 | pFA6a-GFP(S65T)-HIS3MX6 | Ref. 33 |
| pRH2566 | pMET3-ERG11::URA3 | This work |
TABLE 3.
Oligonucleotides
| Oligonucleotide | Sequence | Purpose, reference/source |
|---|---|---|
| oRH1975 | GACCCTTTTAAGTTTTCGTATCCGCTCGTTCGAAAGACTTTAGACAAAACAGCTGAAGCTTCGTACGC | KO PDR5, Ref. 35 |
| oRH1976 | AAAAGTCCATCTTGGTAAGTTTCTTTTCTTAACCAAATTCAAAATTCTACATAGGCCACTAGTGGATCTG | KO PDR5, Ref. 35 |
| oRH1979 | CGCCGTGGTACGATATCTGT | Check pdr5Δ, Ref. 35 |
| oRH1980 | AAGACGGTTCGCCATTCGG | Check pdr5Δ, Ref. 35 |
| oRH4160 | CGAAGATAAACGACAAAGTATTTCTCAAAGAAAACAGCATACAGACAGCTGAAGCTTCGTACGC | KO NSG2, this work |
| oRH4161 | TCTTGTACTTCTAATTAATAATATTTACTCGTCAGAATTTCGACTGCATAGGCCACTAGTGGATCTG | KO NSG2, this work |
| oRH4155 | GTATATACGAACGTCGCTGG | Check nsg2Δ, this work |
| oRH4156 | GAAATGTCAAGTGTTACAGCC | Check nsg2Δ, This work |
| oRH4171 | AAACAACAGCAATTTTATTTTGGACCTGCATAATATTTACCGAAATATGCAGCTGAAGCTTCGTACGC | KO HMG2, this work |
| oRH4172 | TAGAGTCAAAATATACCGTGTTTAGTATTGTAGCATTTAACTTATCTGTGCATAGGCCACTAGTGGATCTG | KO HMG2, This work |
| oRH4173 | ACAGTGTTGACCATACCAGG | Check hmg2Δ, this work |
| oRH4174 | GGCAACACGGAAATGATCAC | Check hmg2Δ, this work |
| oRH1716 | GACTGCTGAAGTGCTGGAGTT | Check hrd1Δ Ref. 18 |
| oRH1717 | CATTTAGTCATGAACGCTTCTC | Check hrd1Δ, Ref. 18 |
| oRH4219 | CGG ATc tgc agg gat ctA TGT CTG CTA CCA AGT CAA TCG TTG GAG AGG C | Clone MET3-ERG11 |
| oRH4247 | CAATTCTTGTTGGACATCTGG | Clone MET3-ERG11 |
| oRH4182 | GAATCGCCAATTTTAGTTTCCGAAAAATTGCCCTTCAGAAATTATGATTATcggatccccgggttaattaa | Make 1mycHmg2-GFP |
| oRH4183 | TATTGGCATATAGCCGATGACATTTTCACAGCAAGCTCCAAAAACGCGATCgaattcgagctcgtttaaac | Make 1mycHmg2-GFP |
Reagents
All stocks were stored at −20 °C unless stated. Cycloheximide (CHX; 1001B3, ICN Biomedicals, Inc.) stock was 50 mg/ml in DMSO. Lovastatin (a gift from Merck) stock was 25 mg/ml in methanol ammonia at 4 °C. Ro48-8071 (Ro48; a gift from Johannes Aebi at Hoffmann-La Roche) stock was 40 mg/ml in DMSO. Terbinafine was prepared as an ethanolic solution (10 mg/ml). Ketoconazole (K1003, Sigma) was 10 mg/ml in DMSO. Fenpropimorph (36772, Sigma) was 100 mg/ml in 95% ethanol. MG132 (benzyloxycarbonyl-Leu-Leu-aldehyde; C2211, Sigma) was 25 mg/ml in DMSO. GGPP (G6025, Sigma) was 200 μg/vial in methanol/ammonia (7:3). Digitonin (5628, Sigma) was recrystallized three times in ethanol and dried in aliquots using a SpeedVac. The following immunological reagents were used: anti-HA monoclonal antibody ascites (1:2500; Covance); anti-Myc 9E10.2 monoclonal antibody from hybridoma supernatants prepared in the laboratory; anti-HA affinity matrix (AFC-101P, Covance); goat anti-mouse-HRP (1:10,000; Jackson Immunochemicals); anti-Pgk1 (1:2500; A6457, Molecular Probes); and anti-ubiquitin monoclonal antibody (gift from Richard Gardner, University of Washington).
Yeast Strains, Growth Conditions
S. cerevisiae strains (Table 1) were derived from S288C background and are isogenic. Yeasts were grown at 30 °C in minimal medium with 2% glucose. Experiments were performed on cultures in log phase between 0.2 and 0.6 OD/ml (A600 nm). Standard yeast techniques were used to introduce plasmids and prepare gene deletions. The hrd1Δ, nsg1Δ, nsg2Δ, and hmg2Δ null strains were made by PCR-mediated homologous recombination of the KanMX4 or nourseothricin gene (NatMX4) cassettes flanked by 50 base pairs of homology to the coding regions (Table 3, Oligonucleotide). Nulls were confirmed by PCR and associated deletion phenotypes. To make 1mycHmg2-GFP expressed from the HMG2 promoter, a PCR fragment was generated using pFA6a-GFP(S65T)-HIS3MX6 as a template. The primers contained 50 base pairs of homology to the HMG2 gene, such that GFP is inserted after amino acid 668, the same position as in pRH469.
Preparation of Whole Cell Lysates, Microsomes, and Immunoblotting
For analysis of protein levels, 4 OD equivalents of cells were harvested and lysed on multivortexer for 3 min in 100 μl of SUME (1% SDS, 8 m urea, 10 mm MOPS, pH 6.8, 10 mm EDTA) plus protease inhibitors (PI; 62.5 mg/ml 4-(2-aminoethyl)-benzenesulfonyl fluoride, 870 mg/ml PMSF, 5 mg/ml leupeptin, 5 mg/ml pepstatin, 5 mg/ml tosylphenylalanyl chloromethyl ketone) and glass beads. Samples were heated at 50 °C for 2 min. For Nsg1–3HA analysis, 0.4 OD equivalents were loaded in each lane of 10% gel. Following a 1.3-h transfer in Western Transfer Buffer (6.6 mm Tris, 50 mm glycine, 0.01% SDS) with 15% methanol, monoclonal anti-HA antibody was used to detect Nsg1–3HA. For 1mycHmg2 analysis, 0.7 OD equivalents were loaded in each lane of an 8% gel. Following a 2.3-h transfer in Western Transfer Buffer plus 12% methanol, monoclonal anti-Myc 9E10 antibody was used to detect 1mycHmg2.
Analysis of Ubiquitination
Nsg1–3HA was immunoprecipitated from detergent-solubilized lysates. Briefly, 20 OD units (Abs600) of log phase cells were pelleted and lysed in 300 μl of SUME buffer with PI and 5 mm N-ethylmaleimide (Sigma). 1.4 ml of IP buffer (15 mm NaHPO4, pH 7.5, 150 mm NaCl, 2% Triton X-100, 0.1% SDS, 0.5% deoxycholate, 10 mm EDTA, 0.02% NaN3) and 30 μl (packed bead volume) of anti-HA affinity beads (Covance, AFC-101P) were added to cleared lysates. Following overnight precipitation at 4 °C, beads were washed four times with 1 ml of IP Wash (50 mm NaCl, 10 mm Tris, pH 7.5, 0.02% NaN3). 75% of the IP was loaded on 10% SDS-PAGE gel. Protein was transferred to nitrocellulose in transfer buffer containing 15% methanol for 2.25 h. For ubiquitin immunoblotting, the blot was autoclaved wet for 20 min and then dry for 15 min. The blot was blocked for 30 min and incubated with primary antibody overnight (TBST, 20% FCS with 1:20 anti-ubiquitin mouse monoclonal antibody. The secondary antibody, goat anti-mouse-HRP antibody (Jackson Immunochemicals), was used at 1:10,000.
Degradation Assays
CHX chase assays were performed by the addition of CHX to log phase cultures followed by SUME lysis at the indicated times, as described previously (5, 20). In experiments using strains with wild-type PDR5, 50 μg/ml CHX and 20 μg/ml terbinafine (Tb) were used. For pdr5Δ strains, 5 μg/ml CHX and 10 μg/ml Tb were used due to pdr5Δ-associated drug sensitivities.
Co-immunoprecipitations between Nsg1 and Hmg2
10 OD equivalents (A600) of cells were harvested and lysed in non-denaturing TMM buffer (50 mm Tris-HCl, pH 7.4, 1 mm MgCl2, 1 mm MnCl2) with PI. Cleared lysates were spun at 14,000 × g for 45 min at 4 °C to prepare microsomes. Microsomes were washed one time with TMM + PI and solubilized with 1% digitonin on ice for 10–30 min. Following vigorous pipetting, IP buffer with protease inhibitors was added. Following more vigorous pipetting, the lysate was cleared by pulse spins with a bench top microcentrifuge. The cleared lysate was transferred to a new tube, and Nsg1–3HA was immunoprecipitated with anti-HA affinity matrix overnight at 4 °C. Beads were washed four times with IP Wash. 55 μl of sample buffer was added to dried beads, and sample was heated at 50 °C for 3 min. 80% of each sample was loaded on an 8% gel. Transfer to nitrocellulose and immunoblotting were done as described above.
RESULTS
Nsg1-mediated Hmg2 Stabilization Required a Sterol Molecule
We tested whether Nsg1-depdendent stabilization of Hmg2 required a sterol molecule. Hmg2 is unstable when expressed in excess of Nsg1 (5, 8). However, at native levels of both Hmg2 and Nsg1, Hmg2 is subject to Nsg1-mediated stabilization (18). If a sterol molecule were required for the Hmg2-Nsg1 physical interaction, we predicted that Nsg1-stabilized Hmg2 would become unstable in sterol-depleted cells. Accordingly, we examined the behavior of Hmg2 and Nsg1 in cells treated with the well characterized sterol inhibitor terbinafine. Terbinafine blocks production of all sterols but allows production of isoprenes, including the GGPP degradation signal. Specifically, terbinafine inhibits squalene epoxidase, which produces the direct precursor of lanosterol, the first sterol in the pathway (Fig. 1A). A wild-type strain carrying functional epitope-tagged versions of Hmg2 (1mycHmg2) and Nsg1 (Nsg1–3HA) (see also supplemental Fig. S1) expressed from their native promoters was treated with terbinafine or vehicle (DMSO) for 2 h, and then cells were harvested and prepared for SDS-PAGE and immunoblotting. A concentration of terbinafine was chosen that slightly inhibited growth and that we have previously demonstrated blocks production of sterols in our strain background (10). This treatment resulted in lower 1mycHmg2 and Nsg1–3HA levels compared with untreated cells (Fig. 1B, 0 h time point). The effect of terbinafine on 1mycHmg2 and Nsg1–3HA protein stability was directly assayed by a CHX chase assay, adding the protein synthesis inhibitor after the 2-h terbinafine incubation. 1mycHmg2 was markedly unstable in terbinafine-treated cells compared with control cells (Fig. 1, B and C). These data implied that sterols are required for Nsg1-mediated stabilization of Hmg2.
In mammals, the SSD in the N-terminal transmembrane region of HMGR is responsible for sterol regulation of stability. The Hmg2 N-terminal transmembrane region has homology to mammalian reductase and SCAP, and is important for regulation of Hmg2 stability by the isoprene degradation signal. We tested if the Hmg2 transmembrane domain was sufficient for sterol regulation in yeast by fusing it to GFP and assaying the stability of this protein following inhibition of sterol synthesis. To do this, we substituted GFP (S65T) for the catalytic domain of the reductase at the native HMG2 locus (driven by the natural HMG2 promoter) by homologous recombination. In this construct, the GFP coding sequence is fused in frame at the 3′ end of the HMG2 transmembrane domain. The protein, 1mycHmg2-GFP, was expressed at similar levels to the full-length 1mycHmg2 (Fig. 1D, t = 0). Nsg1–3HA could bind to 1mycHmg2-GFP and stabilize it (18) (data not shown). To assay regulation by sterols, cells expressing 1mycHmg2-GFP or full-length 1mycHmg2 were treated with terbinafine, and samples were taken at the indicated time points. 1mycHmg2-GFP levels decreased (Fig. 1D, right side). This paralleled the decrease in full-length 1mycHmg2 in the control strain (Fig. 1D, left side). GGPP is added to samples to drive Hmg2 degradation (see Fig. 5 and supplemental Fig. S1). The result implied that the N-terminal transmembrane domain of Hmg2, containing the SSD, is sufficient to confer sterol regulation on Hmg2 and that the catalytic domain of reductase is dispensable.
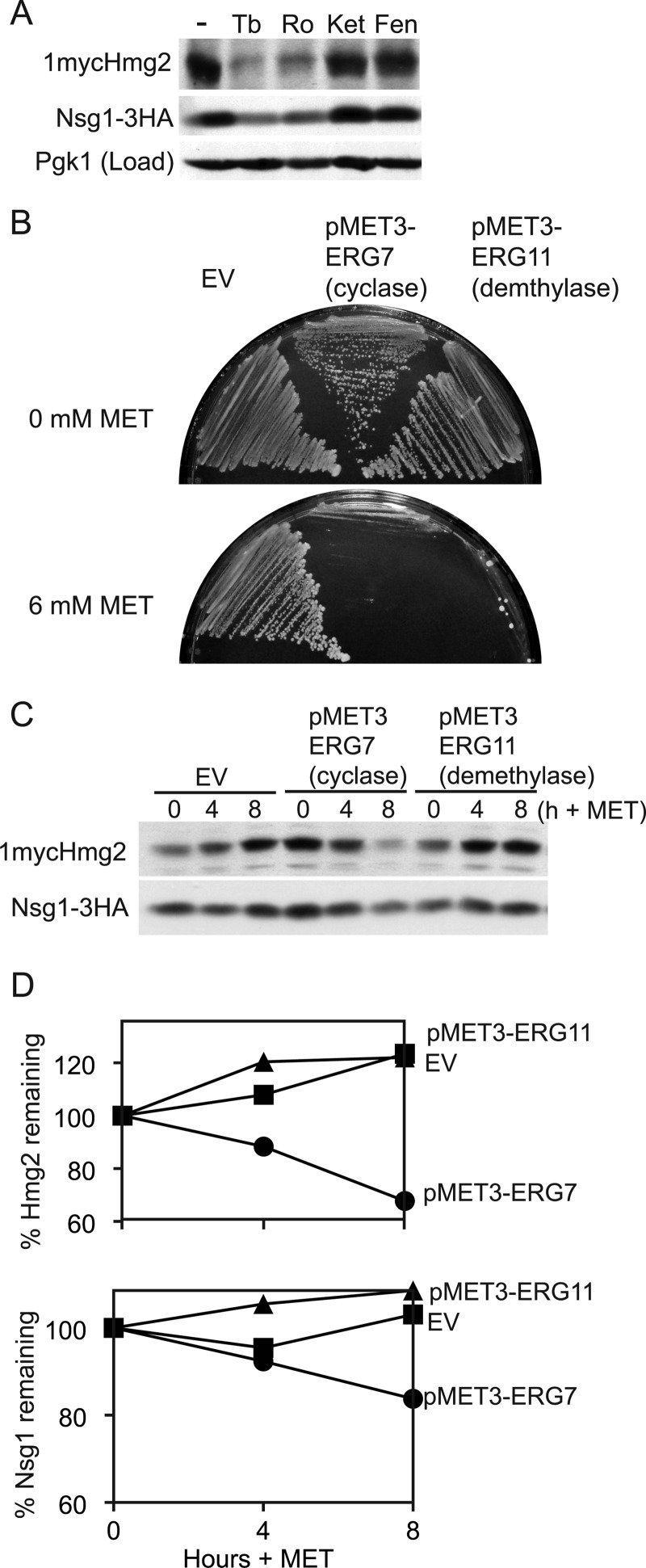
FIGURE 5.
Interplay of sterol and isoprenoid signals on Hmg2 and Nsg1. A, effect of blocking the sterol pathway before or after GGPP synthesis on 1mycHmg2 and Nsg1–3HA protein levels. Cells were treated with lovastatin (Lova) (25 μg/ml) or Tb (10 μg/ml) for 4 h at 30 °C. At the indicated time points, 4 OD equivalents of cells were harvested and lysed in SUME. Following SDS-PAGE, 1mycHmg2 and Nsg1–3HA were detected by immunoblotting. Similar experiments were performed at least four times with similar results. B, GGPP reversed the stabilization of Hmg2 by lovastatin treatment. WT and nsg1Δnsg2Δ cells were treated with vehicle or lovastatin (6.25 μg/ml). After 1 h at 30 °C, each culture was split in half and treated with methanol ammonia (vehicle) or with 20 μm GGPP for 1 h. After lysis and SDS-PAGE, 1mycHmg2, Nsg1–3HA, and Pgk1 (load control) were detected by immunoblotting. Similar experiments were performed at least four times with similar results. C, Hmg2 and Nsg1 protein levels in a GGPP dose-response experiment. WT and nsg1Δnsg2Δ cells were treated with 0, 5, or 20 μm GGPP at 30 °C for 1 h. Samples were harvested, and immunoblotting was performed as in B. In B and C, a darker exposure is displayed for the nsg1Δnsg2Δ strain because Hmg2 levels are so low. The vertical bar marks where the image was cut. In the graph showing the amount of Hmg2 remaining, the value is compared with the 0 sample and normalized to the load control (Pgk1). Similar experiments were performed at least three times.
Nsg1–3HA was also unstable in terbinafine-treated cells (Fig. 1, B–D), implying that sterols impact Nsg1 stability as well. Below we explore the basis of this finding.
We confirmed that each untagged version of the two interacting proteins could function in this new regulatory capacity. Because we do not have antibodies for untagged versions, we ran two parallel types of experiments. We prepared strains co-expressing epitope-tagged Nsg1 along with untagged Hmg2 from its genomic promoter, and analogous strains expressing epitope-tagged Hmg2 in the presence of untagged Nsg1. In each strain, the expected effects of altering the sterol pathway on the still-tagged protein were observed, indicating that the untagged partner protein (Hmg2 or Nsg1, respectively) could participate in the response to the drugs further studied below. Thus, the tagged versions employed function like the native versions (supplemental Fig. S1).
Lanosterol Promoted Hmg2 and Nsg1 Stability
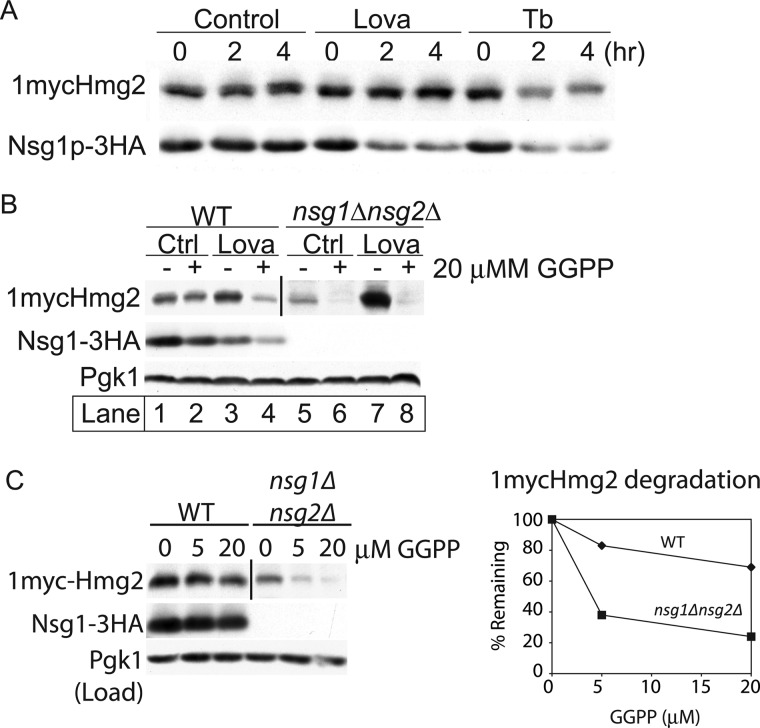
To ascertain which sterol is involved in Nsg1-mediated stabilization of Hmg2, we used a series of inhibitors to block distinct sterol pathway enzymes (Fig. 1A). The rationale behind this approach is that a block upstream of the required sterol will cause Hmg2 and Nsg1 to be unstable, whereas a block downstream of that sterol will cause Hmg2 and Nsg1 to remain stable. For each inhibitor, we used concentrations that similarly and only slightly affected growth during the treatment period and that we and others have shown to effectively inhibit sterol formation (10, 21). We treated wild-type cells with each inhibitor for 4 h and monitored steady state levels of 1mycHmg2 and Nsg1–3HA. Both were lowered by terbinafine or Ro48 (Ro) treatment, whereas ketoconazole (Ket) or fenpropimorph (Fen) had no effect (Fig. 2A). Terbinafine and Ro48 block upstream of lanosterol production. Ketoconazole and fenpropimorph block downstream of lanosterol production. Thus, blocking production of lanosterol with terbinafine or Ro48 led to lower 1mycHmg2 and Nsg1–3HA levels, whereas blocking downstream of lanosterol production did not perturb protein levels. This experiment indicated that lanosterol or a signal derived from lanosterol, but not downstream sterol molecules, is important for Nsg1-mediated stabilization of Hmg2.
FIGURE 2.
Lanosterol synthesis promotes Hmg2-Nsg1 stability. A, inhibition of lanosterol synthesis destabilized Hmg2 and Nsg1. Wild-type cells grown to log phase in minimal medium were treated with inhibitors for 4 h at 30 °C, lysed with SUME, and subjected to SDS-PAGE followed by immunoblotting for 1mycHmg2, Nsg1–3HA, and Pgk1 (load control). The following concentrations of inhibitors were used: Tb, 10 μg/ml; Ro48–8071 (Ro), 40 μg/ml; ketoconazole (Ket), 10 μg/ml; fenpropimorph (Fen), 10 μg/ml. The experiment was performed at least three times. A typical result is shown. B, methionine repression of ERG7 (lanosterol cyclase) or ERG11 (lanosterol demethylase) genes efficiently kills yeast cells. Strains prototrophic for methionine production were transformed with URA3 empty vector (EV) or MET3-ERG7 or MET3-ERG11 plasmids, and growth was assayed in the absence (top) or presence (bottom) of 6 mm methionine. ERG7 and ERG11 are essential genes, and their growth is accordingly prohibited by the addition of methionine, demonstrating the effectiveness of the repression system. C, the same strains as in B were grown to log phase in minimal medium lacking methionine, and then 6 mm methionine was added to half of each culture. Samples were taken at the indicated time points, lysed and subjected to SDS-PAGE followed by immunoblotting. D, quantitations of protein levels in C were performed with ImageJ. Raw integrated density values were used to calculate the percentage remaining as for Fig. 1. Similar experiments were performed at least three times. Protein levels in strains carrying empty vector (squares), pMET3-ERG7 (circles), and pMET3-ERG11 (triangles) are plotted.
Both terbinafine and Ro48 caused destabilization of Hmg2 and Nsg1. Previously, we employed Ro48 to show that an oxysterol signal generated by an alternate sterol pathway, in conjunction with the FPP-derived signal, promotes the destruction of Hmg2 independently of Nsgs (10). Ro48 targets squalene epoxidase, and partial inhibition of the enzyme causes the formation of oxysterols via an alternative sterol synthesis pathway; complete inhibition does not affect Hmg2 degradation (10). The use of Ro48 allowed the possibility that an off-pathway oxysterol molecule promotes the destruction of Hmg2, even with Nsg1 present. However, the similar effectiveness of terbinafine (Fig. 1A), which blocks the production of molecules needed to make any sterol, indicated that loss of sterols was causing the effect on Hmg2 and Nsg1 stability. In addition, in these studies, we used a concentration of Ro48 expected to completely inhibit squalene epoxidase. Effectively lower doses of Ro48, as used in our previous work (10, 11), to assay direct effects of oxysterols on the degradation of Hmg2-GFP did not affect the degradation of 1mycHmg2 or Nsg1–3HA in these experiments.
We used an alternative genetic approach to identify the sterol molecule involved in Nsg1-Hmg2 interaction. This technique was developed by our laboratory to successfully pinpoint the source of the Hmg2 prodegradation signals, FPP, synthesized early in the sterol pathway (9, 10). In this assay, an essential sterol pathway gene of interest is placed under the control of the MET3 gene promoter, which is stringently controlled by methionine in the growth medium. Methionine addition to the culture medium efficiently turns off the expression of the sterol pathway gene, allowing shutoff of essential genes when a null mutation is not viable. Extended growth of these strains in methionine leads to cell death because the production of pathway molecules is halted (9). For example, MET3-regulated expression of ERG7 (2,3-oxidosqualene lanosterol cyclase) or ERG11 (lanosterol demethylase) allowed robust growth of cells in the absence of methionine. Cells died when 6 mm methionine was present, but growth of control cells was not altered by methionine addition (Fig. 2B, top versus bottom). Lanosterol cyclase makes lanosterol and lanosterol demethylase consumes lanosterol to make a partially demethylated version. We asked whether turning off the expression of either of these genes affected Hmg2 and Nsg1 protein expression. Treatment of cells expressing pMET3-ERG7, but not pMET3-ERG11, with 6 mm methionine led to the reduced expression of 1mycHmg2 and Nsg1–3HA (Fig. 2, C and D). Thus, blocking expression of the enzyme that makes lanosterol reduced the expression of Hmg2 and Nsg1. These results implied that lanosterol synthesis was required for stabilization of 1mycHmg2 and Nsg1–3HA. These data corroborated our findings with the inhibitors and indicated that lanosterol synthesis promotes the stabilization of 1mycHmg2 and Nsg1–3HA.
The DeBose-Boyd laboratory (22) demonstrated that INSIG-dependent accelerated degradation of mammalian HMGR uses a derivative of lanosterol, 24,25-dihydrolanosterol. We wished to examine if a derivative of lanosterol was the sterol molecule governing Hmg2-Nsg1 regulation in S. cerevisiae. 24,25-Dihydrolanosterol, specifically, is not likely to occur in S. cerevisiae because the enzyme that catalyzes the desaturation of the C24=C25 double bond (DHCR24, EC 1.3.1.72) is absent from the genome. A somewhat related C24 desaturase, Erg4 (EC 1.3.1.71), catalyzes the final step in ergosterol synthesis by reducing the C24=C28 double bond (23). We tested whether this enzyme might have a cryptic role in creating a lanosterol derivative important for Hmg2-Nsg1 sterol regulation by examining 1mycHmg2 and Nsg1–3HA stability in an erg4Δ mutant strain. The levels and stability of each were unaffected in the erg4Δ mutant (data not shown), ruling out the possibility that an Erg4-dependent product, either a heretofore unknown lanosterol or the end product of sterol synthesis, ergosterol, is involved in Hmg2-Nsg1 sterol regulation. The experiments presented are consistent with the idea that Nsg1-dependent Hmg2 stabilization is mediated by lanosterol. Thus, the same step of sterol biosynthesis in mammals and yeast serves as the source of a sterol signal to promote INSIG function toward HMGR.
Sterol-dependent Stabilization Required both Hmg2 and Nsgs
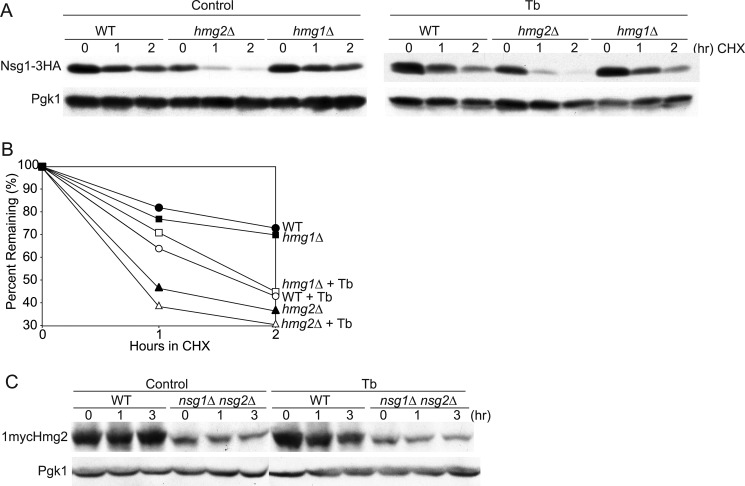
Because Hmg2 and Nsg1 co-stabilize one another, the increased degradation of Hmg2 and Nsg1 observed upon sterol depletion could reflect the loss of a sterol-dependent interaction between these two proteins. This model predicts that Hmg2 and Nsg1 will each be unstable in the absence of their binding partner and that sterol depletion will no longer have any effect on the degradation rate of either lone protein. A second possibility is that Hmg2 and Nsg1 are regulated by lanosterol in distinct ways, independent of the interaction with each other. If so, then in a cell lacking Hmg2, depleting sterols should still destabilize Nsg1. We tested if Hmg2 or Nsg1 protein stability changed in sterol-depleted cells when each binding partner was lacking.
We compared Nsg1 stability in wild-type and Hmg2 mutant cells when sterol synthesis was inhibited as for Fig. 1. Wild-type and hmg2Δ cells were grown to log phase and preincubated with terbinafine or vehicle for 2 h, and then a cycloheximide chase assay was performed to assess protein stability. Terbinafine treatment of wild-type cells yielded the usual increased degradation of Nsg1–3HA compared with control-treated cells (Fig. 3A). In hmg2Δ cells, Nsg1 was unstable in control-treated cells and did not become more unstable in terbinafine-treated cells (Fig. 3A, Tb). In fact, the stability of Nsg1 in hmg2Δ cells was identical in unperturbed or sterol-depleted cells (Fig. 3B), consistent with the idea that Nsg1 stabilization occurs exclusively through its lanosterol-dependent interaction with Hmg2. This result was somewhat surprising because we have observed that Nsg1–3HA can interact with the other HMGR isozyme in yeast, Hmg1.3 We had supposed that interaction would also be sterol-dependent and observable in the hmg2Δ background where Hmg1 is present. We explicitly tested this by comparing Nsg1 sterol-dependent stability in wild-type and hmg1Δ cells. Nsg1–3HA had the same stability in both strains and the same increased degradation rate in terbinafine-treated cells (Fig. 3, A and B). We conclude that Hmg1 does not interact with Nsg1 and thus does not participate in sterol-dependent Nsg1 stability control at native levels of each protein.
FIGURE 3.
Sterol regulation of Hmg2 and Nsg1 required both proteins. A, CHX stability assay of Nsg1–3HA protein levels in WT, hmg2Δ, and hmg1Δ cells. Cultures were grown to log phase at 30 °C and preincubated with Tb (20 μg/ml) or vehicle for 2 h, and then CHX (50 μg/ml) was added to control or Tb-treated cells. These strains have wild type PDR5 and require higher concentrations of CHX and Tb, as determined by dose-response experiments. B, quantitation of data in A. Closed symbols, control-treated cultures; open symbols, Tb-treated cultures. C, effect of inhibiting sterol synthesis on 1mycHmg2 levels in WT and nsg1Δnsg2Δ cells. Log phase cultures were control-treated or Tb-treated (10 μg/ml) for the indicated times at 30 °C. 1mycHmg2 in WT appears less affected by Tb in this experiment because dark exposures are presented to illuminate the quite low levels of 1mycHmg2 in the nsg1Δnsg2Δ cells. Experiments were repeated at least three times with similar results.
We next tested if lanosterol-dependent stabilization of Hmg2 required Nsg1 or could occur independently. We observed 1mycHmg2 levels in wild-type and nsg1Δ cells treated with vehicle or terbinafine. In both strains, 1mycHmg2 levels went down with terbinafine treatment, and an increase in degradation was observed by a CHX chase assay (data not shown). We reasoned that the Hmg2 remaining in nsg1Δ cells was being stabilized by Nsg2, the Nsg1 homolog. Nsg2 was the first INSIG identified in our laboratory on the basis of its ability to stabilize Hmg2. We examined Hmg2 response to sterol depletion in double mutant nsg1Δnsg2Δ cells. Untreated nsg1Δnsg2Δ cells have very low levels of Hmg2 because Hmg2 is extremely unstable (18) (data not shown). For this reason, we loaded more sample on the gel for this strain and increased exposure times to facilitate comparison of protein dynamics between the strains. The rapid degradation of Hmg2 in nsg1Δnsg2Δ cells was not further accelerated when treated with terbinafine, although degradation in wild-type cells is accelerated by terbinafine treatment (Fig. 3C). We also checked to see if Nsg2 was destabilized by sterol regulation, and it was not (data not shown). Therefore, in cells lacking Nsg proteins, Hmg2 is unstable, and decreasing the sterol signal does not further destabilize the protein. These results support the notion that lanosterol increased Hmg2 stability through its interaction with both Nsg proteins and imply that Nsg2-Hmg2 interaction can be regulated by sterols, although Nsg2 stability is not regulated.
Lanosterol Promoted Hmg2-Nsg1 Interaction
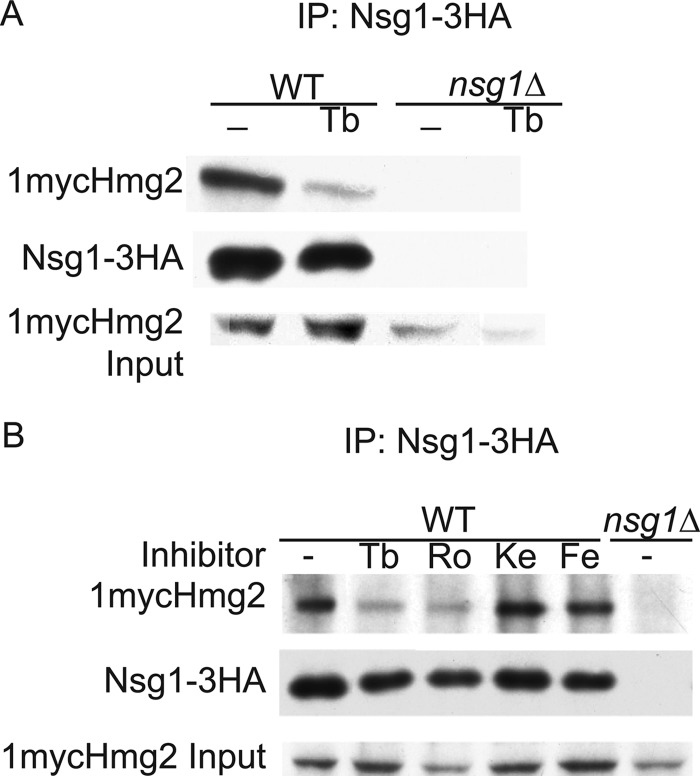
The above results suggest a model where Hmg2 and Nsg1 co-stabilize each other by a lanosterol-dependent interaction. We directly tested if the sterol molecule promotes the Hmg2-Nsg1 interaction. To do this, we performed co-immunoprecipitation assays between 1mycHmg2 and Nsg1–3HA in normal and sterol-depleted cells. Because sterol depletion causes Hmg2 and Nsg1 degradation, we also treated the cells with MG132 to inhibit the proteasome and keep protein levels up. Wild-type cells were treated with terbinafine and MG132 or MG132 alone for 2 h and then lysed in non-denaturing buffer. Microsomes were prepared, and following solubilization with digitonin, Nsg1–3HA was immunoprecipitated, and 1mycHmg2 binding was assessed by SDS-PAGE and immunoblotting. In untreated and inhibitor-treated cells, the amounts of Hmg2 and Nsg1 were similar, demonstrating that the MG132 is preventing their degradation. The amount of 1mycHmg2 co-immunoprecipitated with Nsg1–3HA in terbinafine-treated cells was lower than in untreated cells (Fig. 4A), demonstrating that sterol depletion reduces the Hmg2-Nsg1 interaction. To evaluate which sterol is required, we performed co-immunoprecipitation assays after treatment with the series of sterol pathway inhibitors used to map the stability signal. As expected, the Hmg2-Nsg1 interaction was disrupted with terbinafine or Ro48 treatment but not in cells treated with the downstream inhibitors ketoconazole or fenpropimorph (Fig. 4B). Thus, the interaction is compromised if lanosterol synthesis is blocked but not when the production of downstream sterols is blocked. These data are consistent with the idea that a lanosterol molecule promotes the physical association between Hmg2 and Nsg1.
FIGURE 4.
Blocking lanosterol production reduced Hmg2-Nsg1 interaction. A, the Hmg2-Nsg1 interaction was disrupted when sterol synthesis was blocked. WT and nsg1Δ cells were treated with Tb (10 μg/ml) and MG132 (25 μg/ml) or with MG132 alone for 2 h at 30 °C. 10 OD equivalents of cells for each sample were lysed in non-denaturing TMM, microsomes were prepared and then solubilized with 1% digitonin, and then Nsg1–3HA was immunoprecipitated (IP) by anti-HA affinity matrix. 1mycHmg2 and Nsg1–3HA were detected by immunoblotting. Similar experiments were repeated at least five times with similar results. B, specifically blocking lanosterol production disrupted the Hmg2-Nsg1 interaction. WT and nsg1Δ cells were treated with the series of pathway inhibitors and MG132 (25 μg/ml) or untreated (MG132 only) for 2 h at 30 °C. The co-immunoprecipitation assay was performed as in A. Inhibitors were used at the same concentration as in Fig. 2A. The experiment was repeated at least three times with similar results.
INSIG Allowed Control of Hmg2 Stability by Two Signals: GGPP and Lanosterol
The lanosterol signal couples Hmg2 stability to the level of sterol synthesis. There is a sizable body of work characterizing feedback-regulated degradation of mammalian HMGR and yeast Hmg2 mediated by isoprenes, lipid molecules made early in the sterol pathway. In yeast, our studies using genetic and pharmacological means to manipulate isoprene synthesis demonstrated that Hmg2 degradation is directly related to the amount of FPP being synthesized, which determines the amount of GGPP being synthesized (8, 9). For instance, the drug lovastatin inhibits HMGR catalytic activity, lowers isoprene levels, and stabilizes Hmg2 (5). Conversely, genetic elevation or the direct addition of GGPP to cells results in fast degradation (8). This feedback-regulated degradation by an early pathway isoprene signal only occurs when Hmg2 is not stabilized by Nsg1, either when Hmg2 is in excess (8, 9) or when Nsg1 is absent (18). The data presented in this paper suggest that this is due to the sterol-mediated stabilization of Hmg2 by Nsg1, thus illuminating a role for a sterol molecule as a second signal in controlling Hmg2 stability. Our model is that Hmg2 and Nsg1 are bound in a sterol-dependent manner when expressed at native levels. If Nsg1-mediated stability is lifted by lowering lanosterol, we would predict from this model and our earlier studies on Nsg1 (18) that the GGPP signal should then prevail. We directly tested this model below.
If this two-signal model is correct, then a block to sterol synthesis that precludes both the synthesis of GGPP and sterols should render Hmg2 stable. To test this, we compared the effects on Hmg2 and Nsg1 protein levels of blocking sterol synthesis before or after GGPP synthesis. Lovastatin blocks HMGR activity and the synthesis of all downstream molecules (isoprenes and sterols), whereas terbinafine blocks the synthesis of late sterols but still allows GGPP production (Fig. 1A). Wild-type cells were treated with no drug, terbinafine, or lovastatin, and 1mycHmg2 and Nsg1–3HA levels were monitored over time. Untreated wild-type cells showed no change in protein levels over the time course (Fig. 5A, Control). In terbinafine-treated cells, 1mycHmg2 and Nsg1–3HA levels both go down, indicating that the proteins are unstable as shown above (Fig. 5, Tb). However, the lovastatin treatment only reduced Nsg1–3HA (Fig. 5, Lova). These results are consistent with the idea that Nsg1 was unstable because it was no longer bound to Hmg2, whereas the Hmg2, now capable of responding to GGPP, remained stable because the lovastatin also blocked GGPP synthesis. To test if the stable Hmg2 in lovastatin-treated cells could respond to GGPP, we added GGPP to similarly treated cultures (Fig. 5B). The addition of GGPP potently caused the degradation of Hmg2 in lovastatin-treated cells but not in cells with unperturbed sterol synthesis (Fig. 5B, compare lanes 1 and 3 with lanes 2 and 4). This action of GGPP on Hmg2 was independent of Nsgs as expected, because GGPP addition to lovastatin-treated nsg1Δnsg2Δ cells caused Hmg2 degradation (Fig. 5B, lanes 7 and 8). Furthermore, altered sterol synthesis was not required for the effect of GGPP on Hmg2, because even without lovastatin pretreatment, GGPP caused Hmg2 degradation in nsg1Δnsg2Δ cells (Fig. 5B, lanes 5 and 6). The data are consistent with the model that two separate signals impact natively expressed Hmg2; the isoprene signal directly stimulates Hmg2 ERAD, whereas lanosterol mediates a stabilizing association with Nsg1. We infer that the sterol-dependent stabilization of Hmg2 by Nsg1 controls the entry of Hmg2 into ERAD by blocking response to the GGPP degradation signal.
Degradation Pathways Employed in Hmg2 and Nsg1 Degradation
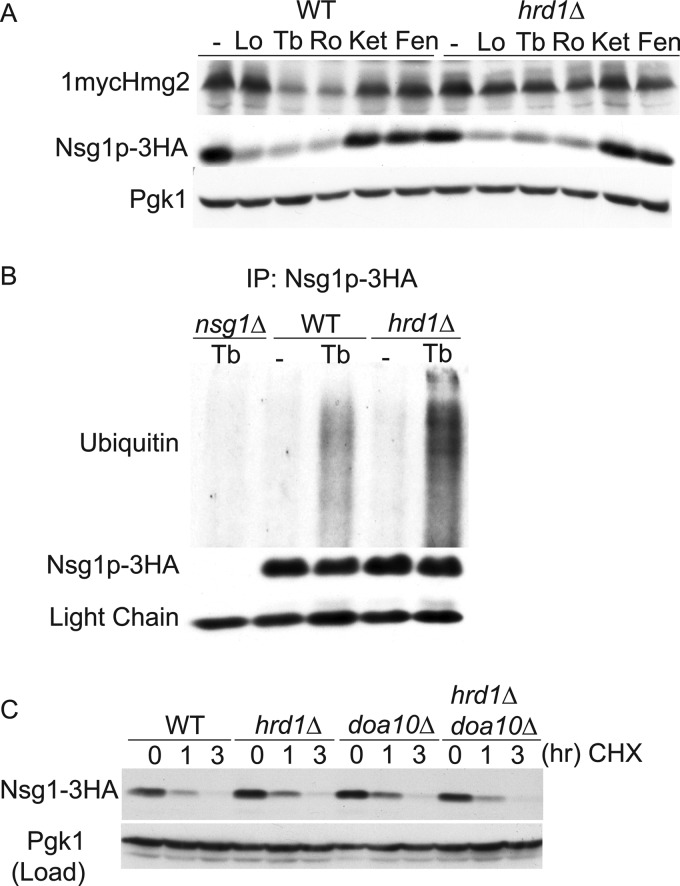
In mammals, degradation of INSIGs and HMGR proceeds by the same ERAD pathway; the Hrd1-like E3 ubiquitin ligase gp78 brokers their ubiquitination before delivery to the proteasome (12, 24). Accordingly, we directly tested if Nsg1, like Hmg2, was targeted for degradation by the HRD pathway. We monitored 1mycHmg2 and Nsg1–3HA levels in wild-type and hrd1Δ cells incubated without or with terbinafine or Ro48 for 4 h. As expected, in terbinafine and Ro48-treated cells, 1mycHmg2 was stabilized in hrd1Δ cells compared with the 1mycHmg2 in wild-type cells similarly treated (Fig. 6A). Surprisingly, in hrd1Δ cells treated with lovastatin, terbinafine, or Ro48, Nsg1–3HA levels decreased to the same extent as in wild-type cells. During the co-immunoprecipitation experiments above, we observed that the proteasome inhibitor MG132 specifically blocked the decline in Nsg1 levels when lanosterol synthesis was blocked, implying a role for proteasome-mediated degradation (Fig. 4, A and B) (data not shown). Thus, it appeared that the ER-localized Nsg1 is degraded by an ERAD pathway distinct from the HRD pathway used for Hmg2. Proteasome substrates are targeted for degradation by multiubiquitination. We examined Nsg1–3HA ubiquitination in control or terbinafine-treated cells. Lysates were prepared from log phase cultures of wild-type cells expressing native Nsg1–3HA treated with terbinafine or vehicle alone for 2 h. MG132 was present throughout the incubation to block degradation of Nsg1–3HA by the proteasome. Nsg1–3HA was immunoprecipitated from detergent-solubilized lysates followed by SDS-PAGE and anti-ubiquitin immunoblotting. Nsg1–3HA displayed a low basal level of ubiquitination that was significantly increased in terbinafine-treated cells, demonstrating that Nsg1–3HA is polyubiquitinated in cells inhibited for sterol synthesis (Fig. 6B). This pattern was unchanged in hrd1Δ cells, confirming that Nsg1 ubiquitin-dependent degradation in sterol-depleted cells is independent of the Hrd1 E3 ligase. The other characterized ERAD E3 ligase, Doa10, was also dispensable for Nsg1 degradation (Fig. 6C). Hrd1 and Doa10 do not compensate for each other in Nsg1 degradation, because Nsg1–3HA is unstable in a hrd1Δdoa10Δ mutant. Proteins known to be ubiquitinated by Hrd1 and Doa10 (Hmg2-GFP and Ste6–166) were stabilized in the appropriate strains (data not shown). Together, the data indicate that a distinct ERAD mechanism is employed for Nsg1, which we are pursuing.
FIGURE 6.
Degradation pathways of Hmg2 and Nsg1. A, steady-state 1mycHmg2 and Nsg1–3HA protein levels in WT and hrd1Δ cells treated with the series of sterol pathway inhibitors. Log phase cultures of WT and hrd1Δ cells were treated with inhibitors for 4 h at 30 °C as in Fig. 2A, lysed, and subjected to SDS-PAGE followed by immunoblotting for 1mycHmg2, Nsg1–3HA, and Pgk1 (load control). The experiment was performed at least three times. B, in vivo ubiquitination of Nsg1 in untreated and terbinafine-treated cells. 20 OD units of wild-type, hrd1Δ, or nsg1Δ cells from log phase cultures were treated with Tb (10 μg/ml) and MG132 (25 μg/ml) or control-treated (ethanol is the control vehicle for Tb, and DMSO is the control vehicle for MG132) for 2 h at 30 °C. Cells were lysed with SUME, and Nsg1–3HA was immunoprecipitated (IP) from clarified lysates with anti-HA affinity matrix. Following SDS-PAGE, ubiquitinated Nsg1–3HA was detected by anti-ubiquitin immunoblotting. The bottom panel shows the amount of Nsg1–3HA pulled down in each sample. The experiment was performed at least three times with similar results. C, degradation of TDH3-Nsg1–3HA occurs in hrd1Δ and doa10Δ mutants. Cells expressing Nsg1–3HA in excess of Hmg2 (by virtue of TDH3 promoter) were grown to log phase, and then the CHX chase assay was performed. At the indicated time points, samples were harvested and lysed, and then SDS-PAGE immunoblotting was performed. Similar experiments were performed at least five times. Lo, lovastatin; Ro, Ro48-8071; Ket, ketoconazole; Fen, fenpropimorph.
DISCUSSION
Our data demonstrate the molecular underpinnings of INSIG function are highly conserved between mammals and S. cerevisiae. A lanosterol-derived signal promotes the association between mammalian HMGR and INISG, and lanosterol mediates the association between Hmg2 and yeast INSIG. The outcome of sterol-dependent control by INSIGs varies according to the client. Interaction of INSIG with SCAP (mediated by cholesterol) anchors the SCAP-SREBP complex in the ER, thus blocking activation of the SREBP transcription factor. INSIG promotes HMGR degradation in mammals and, conversely, stabilizes Hmg2 in S. cerevisiae. In our previous work, we suggested that the diverse functions for INSIGs can be unified from the perspective of molecular chaperones that perform these same functions (18). From the above studies, it is clear that sterol dependence is a general feature of INSIG-SSD client interactions. We suggest that in general, INSIGs function as sterol-dependent chaperones of SSD client proteins (although see below).
A possible concern in these studies is that the effects of the late sterol inhibitors are simply due to them raising GGPP levels in the cell and thus stimulating Hmg2 degradation when Nsg1 is present in stabilizing concentrations. A number of lines of evidence indicate that this is not the case. First, direct addition of GGPP to cells at concentrations that maximally stimulate Hmg2 degradation in the absence of both Nsgs (Fig. 5 (B and C), right column, nsg1Δnsg2Δ strain) had no effect on Hmg2 when the Nsgs were present (left column, WT strain). Furthermore, the addition of Ro48 (data not shown) or Tb (Fig. 3C) to the GGPP-responsive nsg1Δnsg2Δ strains has no effect on Hmg2 stability, indicating that GGPP is not being generated. Finally, we have been able to observe the sterol-sensitive Nsg1-stabilizing effect on a mutant version of Hmg2-GFP, called NR1 (7), that is completely unresponsive to GGPP. Critically, Hmg2 (NR1)-GFP still undergoes Nsg1-dependent stabilization that is removed by treatment with Tb or Ro48 (supplemental Fig. S2). Taken together, these studies indicate that elevation of GGPP alone is not sufficient for relief from Nsg-mediated stabilization of Hmg2, that alteration of GGPP is not occurring when the late sterol pathway inhibitors are applied, and that GGPP responsiveness is independent of sterol-mediated stabilization of Hmg2 by Nsg1.
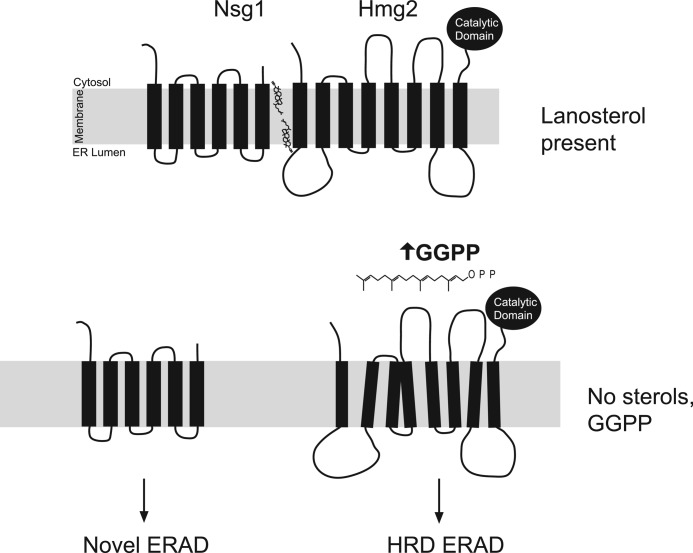
We have shown that Hmg2 stability is controlled by two sterol pathway signals: 1) FPP-derived GGPP that strongly stimulates Hmg2 degradation and 2) lanosterol-dependent inhibition of the GGPP signal mediated by INSIG. Why have two signals that work against each other in Hmg2 regulation? The answer could lie in the natural biology of yeast, which allows this organism to thrive in both O2-rich and anoxic conditions. The sterol pathway is cleanly divided into two segments by oxygen dependence; the early reactions of the pathway, including synthesis of FPP and GGPP, are O2-independent and occur in all conditions. Conversely, sterol synthesis is strongly oxygen-dependent. Therefore, in low or absent oxygen, sterols cannot be produced, whereas isoprenes, such as FPP and GGPP, are still made with no route for use or removal by further sterol synthesis. This physiological state is indicated by low lanosterol and high GGPP, precisely the circumstances where INSIG-mediated inhibition of GGPP-dependent degradation of Hmg2 is lifted (Fig. 7). Thus, the logic of INSIG-controlled Hmg2 degradation (“GGPP yes, lanosterol no”) may be geared toward allowing feedback-mediated control of Hmg2 by isoprenes in those conditions where it is needed: when sterols cannot be made. Consistent with this idea, Hmg2 is reported to be the predominant form of HMGR in anaerobiosis, and Nsg1 is also expressed in these conditions (25, 26). We have preliminary experiments indicating that indeed, in low oxygen, HRD-dependent degradation of Hmg2 is enhanced (data not shown). We are currently exploring this model in more detail, but whatever the natural role of INSIG-mediated sterol sensing in Hmg2 regulation, its conservation between yeast and mammals allows meaningful study of INSIG action using the powerful technical advantages of yeast.
FIGURE 7.
Model of two-input control for Hmg2-regulated degradation. Lanosterol promotes the association of Nsg1 and Hmg2. When lanosterol is absent, the interaction between Hmg2 and Nsg1 is compromised, and both undergo ubiquitination and degradation by ERAD pathways. In these circumstances, Hmg2 degradation is keyed to the level of FPP-derived isoprene degradation signal, GGPP.
Lanosterol and oxysterol signals affect Hmg2 degradation differently. Lanosterol promotes the Nsg-dependent stabilization of Hmg2 (Fig. 1) and has no direct effect on Hmg2 (or Nsg1) stability (Fig. 3). Conversely, oxysterols promote degradation of Hmg2 independently of Nsgs, and the SSD is directly implicated in their action (10, 11). In mammals, oxysterols directly interact with INSIGs and can induce INSIG function without cholesterol or lanosterol (27). Thus, it will be interesting to test if yeast Nsgs sense oxysterols and what effect this might have on HMGR or other cellular processes.
The mechanism of sterol and isoprene signaling and how INSIGs interface with these signals remain open questions. Originally, it was proposed that the SSD was directly involved in sensing signals (28). More recently, a lumenal loop proximal to the SSD of SCAP was implicated in direct binding to sterol (16), indicating that the SSD may not be the sterol binding site but rather an effector region for structural changes that trigger INSIG binding. Yeast Hmg2 has a lumenal loop in a similar position upstream of the SSD, and this loop is important for regulated degradation of the protein (29). In contrast to SCAP, we have recently shown that the SSD in Hmg2 functions independently of INSIGs in responding to both the primary GGPP and ancillary oxysterol degradation signals (11). Perhaps in both mammalian HMGR and Hmg2, sterol binding occurs in an SSD-proximal lumenal region, whereas the common 20-carbon isoprene degradation signal is directly detected by the SSD. By this model for Hmg2 regulation, the lanosterol-mediated binding of INSIG would block or antagonize the degradation-promoting effects of GGPP through direct interaction with the Hmg2 SSD. Consistent with this, we show in this paper that the SSD-containing TMD is sufficient to confer Nsg1 and sterol-mediated regulation. Alternatively, it is possible that GGPP binds to a site distinct from the SSD proper and causes changes in conformation to present SSD-located cues for misfolding that are recognized by the HRD machinery for degradation or recognized by INSIG in its capacity as a chaperone to block degradation. Clearly, the details of the interplay between sterol pathway signals, HRD degradation, and INSIG binding need to be unraveled. Whatever the answer, the deep similarities of the pathway signals, domains, and effectors involved ensure that the results will be generally interesting and relevant to both basic and biomedical imperatives for understanding them.
In the mammal as well as yeast, disengagement of SSD proteins causes destabilization of INSIG. The degradation of INSIG in mammals has some unique features, such as dependence on UbxD8 for postubiquitination steps (24). However, mammalian INSIG ubiquitination is performed by the same Hrd1 homologue, gp78, responsible for HMGR ubiquitination (12, 24). In striking contrast, we found that the degradation of unengaged yeast Nsg1 appears to be mediated by a novel ERAD pathway. Neither canonical ERAD E3 ligase (Hrd1 or Doa10) was required for Nsg1 degradation. Thus, despite the activity of the yeast ERAD field, it appears that the Nsg1 may be undergoing ERAD by an undescribed pathway. Discovering the responsible E3 is a priority, because its identity will have implications for understanding both sterol regulation and cellular quality control.
The co-stabilization of Hmg2 and Nsg1 highlights a new role for the Hmg2 isozyme. It has long been thought that Hmg1 and Hmg2 are functionally redundant, with Hmg1 providing a majority of catalytic activity in aerobic, logarithmically growing cultures (36). This is reflected by an increased sensitivity to lovastatin in hmg1Δ cells versus hmg2Δ cells. Both isoforms of yeast HMGR can interact with Nsg1 at sufficient expression levels,3 and thus we had presumed that Hmg1 would participate in Nsg1 regulation. Contrary to this, Nsg1 remains stable in hmg1Δ cells, whereas Nsg1 is rapidly degraded in hmg2Δ cells, despite the much lower levels of this isoform of HMGR (Fig. 2). Increasing catalytic activity in hmg2Δ cells does not stabilize Nsg1 (data not shown). Thus, beyond the common function of supplying catalytic activity, the two HMGR isoforms have distinct roles toward Nsg1. Hmg2 has a unique function, mediated by its transmembrane anchor, in stabilizing Nsg1.
Might INSIGs have other, as yet undefined, functions? We think so. We have observed interactions between Nsg1 and other yeast SSD-containing proteins, but the functional implication of the interactions is completely opaque. In our previous studies, Nsg1 expressed in excess of Hmg2 can be seen in many different complexes by blue native PAGE (18). The array of complexes in this experiment suggests that INSIGs may have binding partners that are not SSD-containing proteins. The identification of these binding partners, the possible involvement of sterols, and the significance of binding INSIGs await discovery.
Acknowledgments
We thank Dr. Michael David (University of California San Diego) for use of the FACSCalibur flow cytometer. C. L. T. thanks Drs. Renee Garza and Amy Gladfelter for help in avoiding unwanted degradation pathways. R. Y. H. thanks Jim Balmer for advice on top-down analyses of fundamental issues.
This work was supported, in whole or in part, by National Institutes of Health, NIGMS, National Research Service Award 5F32GM82195 (to C. L. T.) and Grant 5R01DK051996 (to R. Y. H.).

This article contains supplemental Figs. S1 and S2.
C. L. Theesfeld and R. Y. Hampton, unpublished observations.
- HMGR
- HMG-CoA reductase
- SSD
- sterol-sensing domain
- ER
- endoplasmic reticulum
- ERAD
- endoplasmic reticulum-associated degradation
- SREBP
- sterol regulatory element-binding protein
- SCAP
- SREBP cleavage-activating protein
- INSIG
- insulin-induced gene protein
- HRD
- HMG-CoA reductase degradation
- FPP
- farnesyl pyrophosphate
- GGPP
- geranylgeranyl pyrophosphate
- CHX
- cycloheximide
- Ro48
- Ro48-8071
- Tb
- terbinafine
- PI
- protease inhibitors.
REFERENCES
- 1. Goldstein J. L., Brown M. S. (1990) Regulation of the mevalonate pathway. Nature 343, 425–430 [DOI] [PubMed] [Google Scholar]
- 2. Brown M. S., Goldstein J. L. (1980) Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J. Lipid Res. 21, 505–517 [PubMed] [Google Scholar]
- 3. Jo Y., Debose-Boyd R. A. (2010) Control of cholesterol synthesis through regulated ER-associated degradation of HMG CoA reductase. Crit. Rev. Biochem. Mol. Biol. 45, 185–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hampton R. Y., Garza R. M. (2009) Protein quality control as a strategy for cellular regulation. Lessons from ubiquitin-mediated regulation of the sterol pathway. Chem. Rev. 109, 1561–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hampton R. Y., Rine J. (1994) Regulated degradation of HMG-CoA reductase, an integral membrane protein of the endoplasmic reticulum, in yeast. J. Cell Biol. 125, 299–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garza R. M., Sato B. K., Hampton R. Y. (2009) In vitro analysis of Hrd1p-mediated retrotranslocation of its multispanning membrane substrate 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase. J. Biol. Chem. 284, 14710–14722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shearer A. G., Hampton R. Y. (2005) Lipid-mediated, reversible misfolding of a sterol-sensing domain protein. EMBO J. 24, 149–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garza R. M., Tran P. N., Hampton R. Y. (2009) Geranylgeranyl pyrophosphate is a potent regulator of HRD-dependent 3-hydroxy-3-methylglutaryl-CoA reductase degradation in yeast. J. Biol. Chem. 284, 35368–35380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gardner R. G., Hampton R. Y. (1999) A highly conserved signal controls degradation of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase in eukaryotes. J. Biol. Chem. 274, 31671–31678 [DOI] [PubMed] [Google Scholar]
- 10. Gardner R. G., Shan H., Matsuda S. P., Hampton R. Y. (2001) An oxysterol-derived positive signal for 3-hydroxy-3-methylglutaryl-CoA reductase degradation in yeast. J. Biol. Chem. 276, 8681–8694 [DOI] [PubMed] [Google Scholar]
- 11. Theesfeld C. L., Pourmand D., Davis T., Garza R. M., Hampton R. Y. (2011) The sterol-sensing domain (SSD) directly mediates signal-regulated endoplasmic reticulum-associated degradation (ERAD) of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase isozyme Hmg2. J. Biol. Chem. 286, 26298–26307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Song B. L., Sever N., DeBose-Boyd R. A. (2005) Gp78, a membrane-anchored ubiquitin ligase, associates with Insig-1 and couples sterol-regulated ubiquitination to degradation of HMG CoA reductase. Mol. Cell 19, 829–840 [DOI] [PubMed] [Google Scholar]
- 13. Song B. L., Javitt N. B., DeBose-Boyd R. A. (2005) Insig-mediated degradation of HMG CoA reductase stimulated by lanosterol, an intermediate in the synthesis of cholesterol. Cell Metab. 1, 179–189 [DOI] [PubMed] [Google Scholar]
- 14. Nguyen A. D., McDonald J. G., Bruick R. K., DeBose-Boyd R. A. (2007) Hypoxia stimulates degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase through accumulation of lanosterol and hypoxia-inducible factor-mediated induction of insigs. J. Biol. Chem. 282, 27436–27446 [DOI] [PubMed] [Google Scholar]
- 15. Sever N., Song B. L., Yabe D., Goldstein J. L., Brown M. S., DeBose-Boyd R. A. (2003) Insig-dependent ubiquitination and degradation of mammalian 3-hydroxy-3-methylglutaryl-CoA reductase stimulated by sterols and geranylgeraniol. J. Biol. Chem. 278, 52479–52490 [DOI] [PubMed] [Google Scholar]
- 16. Motamed M., Zhang Y., Wang M. L., Seemann J., Kwon H. J., Goldstein J. L., Brown M. S. (2011) Identification of luminal Loop 1 of Scap protein as the sterol sensor that maintains cholesterol homeostasis. J. Biol. Chem. 286, 18002–18012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hughes A. L., Stewart E. V., Espenshade P. J. (2008) Identification of twenty-three mutations in fission yeast Scap that constitutively activate SREBP. J. Lipid Res. 49, 2001–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Flury I., Garza R., Shearer A., Rosen J., Cronin S., Hampton R. Y. (2005) INSIG. A broadly conserved transmembrane chaperone for sterol-sensing domain proteins. EMBO J. 24, 3917–3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jones G. M., Stalker J., Humphray S., West A., Cox T., Rogers J., Dunham I., Prelich G. (2008) A systematic library for comprehensive overexpression screens in Saccharomyces cerevisiae. Nat. Methods 5, 239–241 [DOI] [PubMed] [Google Scholar]
- 20. Gardner R., Cronin S., Leader B., Rine J., Hampton R. (1998) Sequence determinants for regulated degradation of yeast 3-hydroxy-3-methylglutaryl-CoA reductase, an integral endoplasmic reticulum membrane protein. Mol. Biol. Cell 9, 2611–2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Crowley J. H., Tove S., Parks L. W. (1998) A calcium-dependent ergosterol mutant of Saccharomyces cerevisiae. Curr. Genet. 34, 93–99 [DOI] [PubMed] [Google Scholar]
- 22. Nguyen A. D., Lee S. H., DeBose-Boyd R. A. (2009) Insig-mediated, sterol-accelerated degradation of the membrane domain of hamster 3-hydroxy-3-methylglutaryl-coenzyme A reductase in insect cells. J. Biol. Chem. 284, 26778–26788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Neal W. D., Parks L. W. (1977) Sterol 24(28) methylene reductase in Saccharomyces cerevisiae. J. Bacteriol. 129, 1375–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee J. N., Song B., DeBose-Boyd R. A., Ye J. (2006) Sterol-regulated degradation of Insig-1 mediated by the membrane-bound ubiquitin ligase gp78. J. Biol. Chem. 281, 39308–39315 [DOI] [PubMed] [Google Scholar]
- 25. Lai L.-C., Kosorukoff A. L., Burke P. V., Kwast K. E. (2006) Metabolic-state-dependent remodeling of the transcriptome in response to anoxia and subsequent reoxygenation in Saccharomyces cerevisiae. Eukaryotic Cell 5, 1468–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ter Linde J. J., Steensma H. Y. (2002) A microarray-assisted screen for potential Hap1 and Rox1 target genes in Saccharomyces cerevisiae. Yeast 19, 825–840 [DOI] [PubMed] [Google Scholar]
- 27. Radhakrishnan A., Ikeda Y., Kwon H. J., Brown M. S., Goldstein J. L. (2007) Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi. Oxysterols block transport by binding to Insig. Proc. Natl. Acad. Sci. U.S.A. 104, 6511–6518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goldstein J. L., DeBose-Boyd R. A., Brown M. S. (2006) Protein sensors for membrane sterols. Cell 124, 35–46 [DOI] [PubMed] [Google Scholar]
- 29. Hampton R. Y., Gardner R. G., Rine J. (1996) Role of 26S proteasome and HRD genes in the degradation of 3-hydroxy-3-methylglutaryl-CoA reductase, an integral endoplasmic reticulum membrane protein. Mol. Biol. Cell 7, 2029–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sikorski R. S., Hieter P. (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Güldener U., Heck S., Fielder T., Beinhauer J., Hegemann J. H. (1996) A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 24, 2519–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goldstein A. L., McCusker J. H. (1999) Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15, 1541–1553 [DOI] [PubMed] [Google Scholar]
- 33. Longtine M. S., McKenzie A., 3rd, Demarini D. J., Shah N. G., Wach A., Brachat A., Philippsen P., Pringle J. R. (1998) Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953–961 [DOI] [PubMed] [Google Scholar]
- 34. Wilhovsky S., Gardner R., Hampton R. (2000) HRD gene dependence of endoplasmic reticulum-associated degradation. Mol. Biol. Cell 11, 1697–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carroll S. M., Hampton R. Y. (2010) Usa1p is required for optimal function and regulation of the Hrd1p endoplasmic reticulum-associated degradation ubiquitin ligase. J. Biol. Chem. 285, 5146–5156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Basson M. E., Thorsness M., Rine J. (1986) Saccharomyces cerevisiae contains two functional genes encoding 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Proc. Natl. Acad. Sci. U.S.A. 83, 5563–5567 [DOI] [PMC free article] [PubMed] [Google Scholar]