Background: Beta cell VEGF-A is critical for islet vascularization and Insulin secretion.
Results: VEGF-A release and synthesis in beta cells are regulated separately. Sustained hypoglycemia reduces beta cell mass through a decrease in Vegf-A signaling.
Conclusion: Beta cell mass can be regulated via modulated Vegf-A signaling.
Significance: Our data reveal a novel pathway for regulating beta cell mass physiologically.
Keywords: Beta Cell, Pancreas, Pancreatic Islets, Vascular, Vascular Endothelial Growth Factor (VEGF), Beta Cell Mass, Hypoglycemia
Abstract
VEGF-A expression in beta cells is critical for pancreatic development, formation of islet-specific vasculature, and Insulin secretion. However, two key questions remain. First, is VEGF-A release from beta cells coupled to VEGF-A production in beta cells? Second, how is the VEGF-A response by beta cells affected by metabolic signals? Here, we show that VEGF-A secretion, but not gene transcription, in either cultured islets or purified pancreatic beta cells, was significantly reduced early on during low glucose conditions. In vivo, a sustained hypoglycemia in mice was induced with Insulin pellets, resulting in a significant reduction in beta cell mass. This loss of beta cell mass could be significantly rescued with continuous delivery of exogenous VEGF-A, which had no effect on beta cell mass in normoglycemic mice. In addition, an increase in apoptotic endothelial cells during hypoglycemia preceded an increase in apoptotic beta cells. Both endothelial and beta cell apoptosis were prevented by exogenous VEGF-A, suggesting a possible causative relationship between reduced VEGF-A and the loss of islet vasculature and beta cells. Furthermore, in none of these experimental groups did beta cell proliferation and islet vessel density change, suggesting a tightly regulated balance between these two cellular compartments. The average islet size decreased in hypoglycemia, which was also prevented by exogenous VEGF-A. Taken together, our data suggest that VEGF-A release in beta cells is independent of VEGF-A synthesis. Beta cell mass can be regulated through modulated release of VEGF-A from beta cells based on physiological need.
Introduction
The vascular endothelial growth factor (VEGF)2 family is composed of six secreted proteins: VEGF-A, -B, -C, -D, -E, and placental growth factor. VEGF-A plays an important role in the reciprocal interaction between endothelial cells and surrounding tissues during development, regeneration, and carcinogenesis (1–3). By differential mRNA splicing, the murine Vegf-A gene can give rise to three protein isoforms, VEGF120, VEGF164, and VEGF188. Whereas VEGF188 is heparin-binding and mainly associated with the cell surface and with the extracellular matrix, VEGF120 is freely diffusible due to the lack of exons 6 and 7 that encode heparan-sulfate proteoglycan binding domains. The predominant isoform, VEGF164, appears to have the highest bioavailability and biological potency, and exhibits only partial binding to the cell surface and extracellular matrix (2, 4, 5). VEGF-A has two tyrosine kinase receptors, VEGF receptors 1 and 2 (VEGFR1 and VEGFR2) (2, 3). VEGFR2 is expressed mainly by endothelial cells and mediates most of the biological effects of VEGF-A, including blood vessel growth and branching, endothelial cell survival, and vessel permeability. VEGFR1 is expressed by endothelial cells and many other cell types and its functions and signaling properties are developmental stage- and cell type-dependent (2). VEGFR1 binds VEGF-A with very high affinity, but only induces weak tyrosine autophosphorylation, suggesting a possible competitive inhibitor role in attenuating the biological activity of VEGF-A. VEGFR1 also binds placental growth factor and VEGF-B, which further complicates our understanding of the regulation of vascular networks (2, 3). Although both VEGFR1 and VEGFR2 are expressed by islet endothelial cells (6–8), VEGFR1 may play a more important role than VEGFR2 in the intra-islet microvasculature (9). Because VEGF-A mRNA and protein levels have been shown to be closely correlated with each other in many biological systems (10–12), VEGF-A transcription levels have frequently been used to represent the levels of VEGF-A synthesis. The most well known and extensively studied regulator for VEGF-A is oxygen tension, in which hypoxia strongly increases Vegf-A transcription via up-regulation of hypoxia-inducible factor 1 (2, 3, 13, 14).
Pancreatic islets contain a 5-fold denser capillary network than the exocrine pancreas, and have specialized capillary fenestrations. There is an intimate association between beta cells and the islet vasculature, with one cell domain abutting an afferent capillary, whereas another abuts an efferent capillary (9, 15–17). Although VEGF-A, -B, -C, -D, and placental growth factor are all expressed in pancreatic islets (8), VEGF-A, which is predominantly produced by beta cells, had been shown to play a critical role in mediating signaling from beta cells to islet endothelial cells for proper pancreatic organogenesis, islet-specific capillary formation, and beta cell function (6–8). Beta cells promote endothelial cell recruitment, proliferation, growth, and extensive islet vascularization through angiogenic factors like VEGF-A, whereas endothelial cells also appear to signal back to beta cells to promote islet development and maintain beta cell homeostasis (1, 18–20).
VEGF-A has been reported to be essential for islet revascularization following islet transplantation (7, 21, 22). Gene deletion studies have shown that VEGF-A produced by beta cells is necessary for the maintenance of intra-islet endothelial cells and islet-specific capillary fenestrations, which are necessary for normal beta cell function and insulin secretion (7, 8, 19, 23). Interestingly, genetic overexpression of Vegf-A in beta cells resulted in islet hypervascularization, but the effect on beta cell mass and beta cell function differed among studies (18, 24–26). In general, the physiological effects of VEGF-A are known to be dosage-dependent over a fairly narrow physiologic range (2, 3). It was shown that a 2-fold deviation (increase or decrease) in Vegf-A levels could lead to significant defects in some developmental systems (27, 28). In addition, absence or overexpression of Vegf-A may change the expression of other VEGF family members, or activate other compensatory pathways (2, 3, 8, 13). These epiphenomena can diminish the power of VEGF-A gene deletion or overexpression models because the relatively extreme changes in VEGF-A levels in such studies do not normally occur physiologically, which may explain the discrepancies between the previous studies (18, 24–26).
As a secreted peptide, VEGF-A has a surprisingly intense intracellular immunohistochemical signal in beta cells, suggesting that its secretion may be regulated (6–8). However, although previous studies in beta cells have reported that VEGF-A production can be affected by glucose levels (29, 30), a possible separate regulation of VEGF-A release and VEGF-A synthesis in beta cells has not been examined.
In the current study, we show a reduction of VEGF-A release, but not production, by islets or purified beta cells in low glucose culture. To mimic in vitro low glucose culture, insulin pellets were given to mice to induce sustained hypoglycemia for 1 month, resulting in a 22% reduction in beta cell mass. Importantly, exogenous physiologic doses of VEGF-A could partially rescue the loss of beta cell mass in these hypoglycemic mice. Further dissection of cell apoptosis, proliferation, and vessel area in this study allow us to propose a model in which beta cell mass is regulated by the glucose level, via modulating VEGF-A in beta cells.
EXPERIMENTAL PROCEDURES
Mouse Manipulation
All mouse experiments were performed in accordance with the guidelines from the Animal Research and Care Committee at the Children's Hospital of Pittsburgh and the University of Pittsburgh IACUC. Both C57/6 and mouse insulin promoter GFP reporter (MIP-GFP) (C57/6 background) mice (31) were purchased from the Jackson Laboratory. For both strains, only 8-week-old males were used for experiments. To induce sustained hypoglycemia, each mouse received subcutaneous implantation of 2 mouse insulin pellets (LINβIT) at the back side of the neck, according to the manufacturer's instruction. To provide continuous exogenous VEGF-A to the mice, each mini-osmotic pump (ALZET, model 2004; 4 weeks content release) was filled with 1.5 μg of recombinant mouse VEGF-A (R&D) 40 h before implantation into the abdomen of mice by surgery. Non-fasting blood glucose measurements of mice were performed at 8 a.m.
Isolation and Culture of Islets and Beta Cells
For islet isolation, pancreatic duct perfusion and subsequent digestion of pancreas was performed with 0.3 mg/ml of collagenase. Islets were hand-picked three times to avoid contamination of non-islet cells (32). Purity of the islets was confirmed by absence of Amylase and Ck19 transcript in the RNA samples extracted from the isolated islets.
For isolation of beta cells by fluorescence-activated cell sorting (FACS), islets that were isolated from MIP-GFP mice were further dissociated into single cells with DNase (Roche Applied Science, 10 μg/ml) and trypsin (20 μg/ml) (Sigma), filtrated at 30 μm, and sorted for beta cells with a FACSAria (BD Biosciences) based on green fluorescent protein, as described (33). Flow cytometry data were shown by FlowJo (Tree Star Inc.).
For isolation of beta cells by laser-capture microdissection (LCM), 1 week after various treatments, MIP-GFP mouse pancreas was harvested, treated with RNAlaterTM (Qiagen), and snap frozen in Tissue-Tek OCT (Sakura) under RNase-free conditions. Frozen pancreas block was sectioned 10-μm thick and mounted on RNase-free membrane-coated microscopy slides (Molecular Machines and Industries, MMI). The sections were air dried and processed with MMI CellCut Plus as described (34, 35). LCM was performed by melting thermoplastic films mounted on transparent LCM MMI isolation caps on beta cells, visualized with their direct green fluorescence. The system was set to the following parameters: 70% for the laser power, 42% laser focus, and 28% laser speed. Purity of the beta cells by FACS and LCM was confirmed by absence of Amylase (acinar cell marker), Ck19 (duct cell marker), Vimentin (mesenchymal marker), CD31 (endothelial cell marker), Glucagon (α cell marker), Somatostain (δ cell marker), and Pancreatic polypeptide (PP cell marker) transcripts, and enrichment of Insulin transcript in the extracted RNA samples, as described (33).
Isolated GFP+ beta cells by FACS were suspended in a 24-well plate with Ham's F-10 medium (Invitrogen) supplemented with 0.5% BSA (Sigma), 2 mm glutamine, 2 mm calcium, and 5 mm glucose and re-aggregated for 2 h before overnight culture (37 °C, 95% air, 5% CO2). Isolated islets were kept in the same medium overnight. Thereafter, islets or beta cell aggregates were cultured in 2, 5, and 20 mm glucose, respectively. At 0.5, 1, and 25 h (fresh medium with corresponding glucose is supplied at the 24th h), cells or islets were harvested for RNA extraction to examine the levels of Vegf-A gene expression after conditioned medium was collected for VEGF-A enzyme-linked immunosorbent assay (ELISA). The number of cells in the wells was manually counted, followed by total DNA extraction (Qiagen). DNA content was determined by Nanodrop1000 (Thermo Scientific) correlated with manual counts, and was used as an objective way to normalize released VEGF-A levels.
Quantitative Polymerase Chain Reaction
RNA was extracted from harvested cultured cells or islets with RNAeasy (Qiagen) and quantified with Nanodrop 1000 (Thermo Scientific), followed by cDNA synthesis (Qiagen) (32). Quantitative PCR primers were all purchased from Qiagen. They are Cyclophilin A (QT00247709), Vegf-A (QT00160769), Ck19 (QT00156667), Amylase (QT00179242), Vimentin (QT00159670), CD31 (QT01052044), Synaptophysin (QT01042314), Insulin (QT00114289), Glucagon (QT00124033), Somatostatin (QT01046528), and Pancreatic polypeptide (QT00103999). Quantitative PCR were performed as described (32, 33) and values of genes were normalized against Cyclophilin A, which proved to be stable across the samples.
Nuclear Run-on Assay
The nuclear run-on protocol is performed according to previous publications (36, 37). Briefly, crude nuclei were prepared by detergent lysis, homogenization, and re-suspension in 100 μl of nuclear run-on buffer (50 mm Tris-HCl, pH 7.5, 5 mm MgCl2, 150 mm KCl, 0.1% Sarkosyl, and 10 mm DTT), 1 μl each of 10 mm ATP, GTP, and CTP, 1 μl of 10 mm Br-UTP (Invitrogen), and 1 μl of RNaseOUT (Invitrogen). Reaction mixtures were preincubated on ice for 3 min and subsequently at 28 ºC for 5 min. Run-on reactions were stopped by addition of 350 μl of RLT buffer (Qiagen). RNAs were isolated with RNAeasy kit (Qiagen) and incubated with 2 μl of anti-BrU antibody (Sigma) in the presence of 2 μl of RNaseOUT at 4 ºC for 2 h, immunoprecipitated with 10 μl of protein G-agarose beads (Santa Cruz). Precipitated RNA was converted to cDNA and quantified by quantitative PCR. Nascent Vegf-A transcripts were normalized to cyclophilin A.
ELISA for VEGF-A
Cell culture media was analyzed using a VEGF-ELISA kit (Raybio) according to the manufacturer's instructions, which detects both 120 and 164 isoforms of mouse VEGF-A. Each sample was assayed in duplicates and the mean value was taken for statistical analysis.
Immunohistochemistry and Quantification
All pancreas samples were fixed for 4 h in 4% formaldehyde, then cryoprotected in 30% sucrose overnight before freezing. Primary antibodies for immunostaining are: guinea pig polyclonal insulin-specific (Dako); rat polyclonal Ki-67-specific (Dako) and CD31-specific (BD Biosciences); rabbit polyclonal synaptophysin-specific (Invitrogen), and caspase 3-specific (Cell Signaling). No antigen retrieval was necessary for these antigens, except for caspase 3, which needs microwave treatment, and for Ki-67, which needs pretreatment with protease for 5 min followed by a 45-min incubation with 2 n HCl (neutralized with Tris borate-EDTA buffer (Sigma)) as described (32, 33). Secondary antibodies for indirect fluorescent staining were Cy2-, Cy3-, or Cy5-conjugated donkey anti-rabbit, anti-rat, anti-goat, and anti-guinea pig (Jackson ImmunoResearch Laboratories). Nuclear staining was performed with Hoechst (BD Biosciences). Imaging of cryosections was performed as described (32, 33). The quantification of apoptotic islet endothelial cells was done by counting vessels (based on CD31 staining) that contained caspase 3+ cells. The quantification of apoptotic beta cells was done by counting the beta cells (based on insulin staining) that were caspase 3+. Endocrine vascular density was determined with ImageJ (NIH) software by measuring the percentage of CD31+ area to the total islet area (based on synaptophysin staining). Average islet size from each pancreas was determined based on 200 islets. For all these quantifications, at least five pancreatic sections that were 100 μm apart from each other were analyzed and five animals were used for each experimental group. The beta cell mass was quantified on the basis of 10 sections that were 100 μm apart from each other, as described before (32).
Data Analysis
All values are depicted as mean ± S.E. Each in vitro experimental condition contains 5 repeats. Each in vivo experimental group used 5 mice. Significance is considered when p < 0.05. All data were statistically analyzed by 2-tailed Student's t test.
RESULTS
VEGF-A Release, but Not Transcription, in Beta Cells Is Reduced in Low Glucose
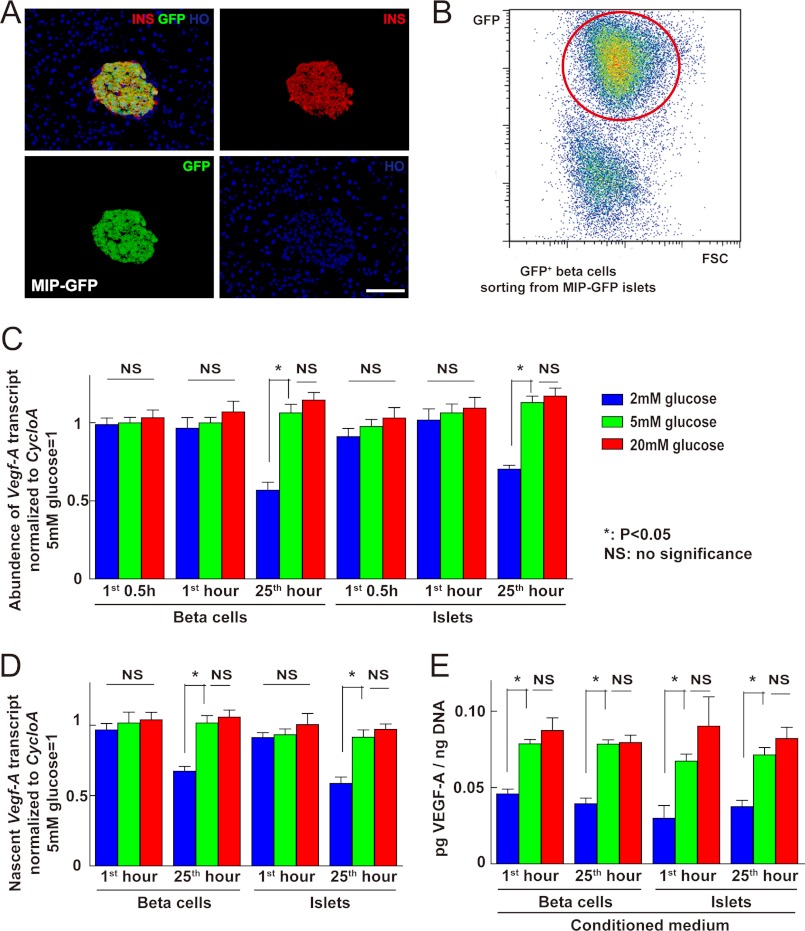
We examined the expression of VEGF-A in normal mouse pancreas and found that VEGF-A immunoreactivity was extremely strong in beta cells. Because VEGF-A is a secreted peptide, this finding encouraged us to test whether VEGF-A secretion is independent of VEGF-A synthesis, and if so, how its secretion is regulated in beta cells. Because glucose is a key regulator of beta cell function and insulin release, we first tested the effect of altered glucose levels on the release and production of VEGF-A. We isolated islets from C57/6 mice and purified beta cells from MIP-GFP mice by FACS (Fig. 1, A and B). After overnight culture in 24-well plates, C57/6 mouse islets or re-aggregated MIP-GFP beta cells were fed with fresh serum-free medium supplemented with 2, 5, or 20 mm glucose, and analyzed at 0.5, 1, or 25 h (the latter was fed with fresh medium at 24 h). After the conditioned media were taken for VEGF-A ELISA, the remaining islets or cells were harvested for RNA extraction and DNA content measurement. Our data showed that neither low glucose nor high glucose affected the levels of Vegf-A transcripts within 1 h (Fig. 1C), but low glucose inhibited Vegf-A transcript levels after 24 h. Nuclear run-on assay further confirmed that this constant level of Vegf-A transcript after 1 h of low glucose culture was due to unchanged Vegf-A transcription, rather than altered mRNA degradation (Fig. 1D). Interestingly, on the other hand, VEGF-A release into the culture medium was significantly reduced at 0.5 and 1 h under low glucose, although not significantly increased under high glucose (Fig. 1E). These data suggest that VEGF-A release, rather than transcription, may be a control point for the early response of beta cells to low glucose.
FIGURE 1.
VEGF-A release, but not transcription, from islets or beta cells was reduced in early low glucose culture. A, representative image of a histologic section from a MIP-GFP mouse pancreas showing co-localization of insulin (INS, red) and GFP. B, FACS of MIP-GFP mouse islets: beta cells were isolated based on green fluorescence. C-E, to examine whether VEGF-A release in beta cells is separate from VEGF-A synthesis, we analyzed the release and gene transcription of Vegf-A by either isolated islets or re-aggregated beta cells at 0.5, 1, and 25 h (for the latter, fresh medium was added at 24 h) in serum-free medium supplemented with 2, 5, or 20 mm glucose. C, quantitative RT-PCR was performed to check Vegf-A transcripts in cultured islets or beta cells. Cyclophilin A (cycloA) was used as a housekeeping gene to normalize Vegf-A values. Exposure to high glucose did not change the levels of Vegf-A transcript, whereas exposure to low glucose did not change the levels of Vegf-A transcript within 1 h, but did so at 25 h. D, nuclear run-on assay was performed on cultured islets or beta cells and showed that nascent Vegf-A transcription did not change within the 1-h exposure to low glucose. E, total DNA content of the cells was used to normalize the quantity of released VEGF-A into culture medium. VEGF-A release by either islets or beta cells was significantly reduced in 2 mm glucose (p < 0.05) at both 1 and 25 h. However, 20 mm glucose did not significantly increase VEGF-A release. *, p < 0.05; **, p < 0.01; NS, no significance; INS, insulin; HO, Hoechst. Scale bars are 30 μm.
Sustained Hypoglycemia Reduces Beta Cell Mass in Vivo
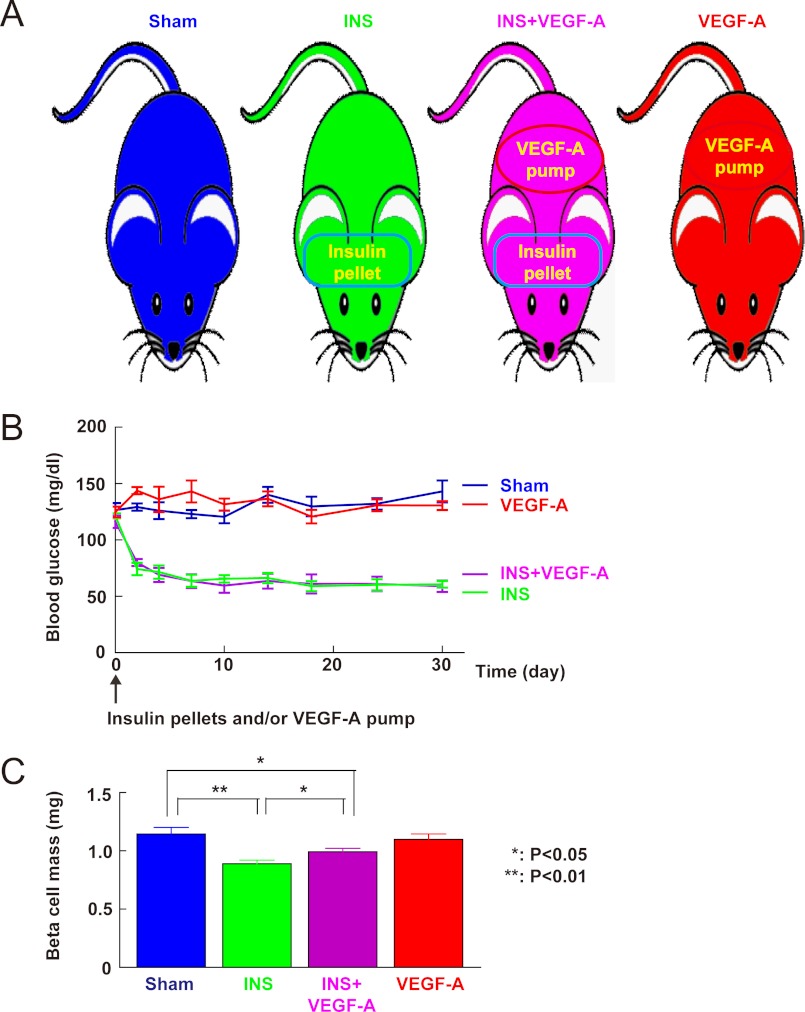
We tried to determine the biological relevance of reduced VEGF-A in low glucose. Because VEGF-A production and release by beta cells is important for islet endothelial cell survival and proper islet vasculature maintenance, we hypothesized that reduced VEGF-A release from beta cells under low glucose may decrease survival signals to islet endothelial cells, a loss of which might in turn have a direct feedback effect on beta cell mass. To test this hypothesis, insulin pellets were implanted subcutaneously to induce sustained hypoglycemia in mice, which should mimic the in vitro low glucose culture environment. To test whether any effect on beta cell mass during sustained hypoglycemia may be due to a reduced release of VEGF-A by beta cells, we “rescued” some insulin-treated mice with exogenous VEGF-A through continuous releasing pumps. To exclude an independent beneficial effect of VEGF-A on beta cell growth under normal glucose, we also included a control with only exogenous VEGF-A pump treatment (no insulin pellet, Fig. 2A). Both untreated control mice and insulin-treated mice received sham operations. Non-fasting blood glucose was monitored and showed that all mice that received insulin pellet treatment, regardless of implantation of VEGF-A releasing pumps, developed sustained hypoglycemia during the 1 month experiment (Fig. 2B). Exogenous VEGF-A did not affect the glycemia of the mice. After 1 month the mice were sacrificed and the pancreases were analyzed and quantified for beta cell mass. Our data showed a 22% reduction (Fig. 2C; p < 0.01) in beta cell mass in insulin-treated mice (0.89 ± 0.03 mg) compared with sham-treated mice (1.14 ± 0.06 mg). The body weights between the two groups had no significant difference. These data suggest that sustained hypoglycemia is adequate to reduce beta cell mass in vivo.
FIGURE 2.
Reduction of beta cell mass during sustained hypoglycemia can be partially rescued by exogenous VEGF-A. A, experimental design. To evaluate whether hypoglycemia has an effect on beta cell mass, insulin pellets were implanted subcutaneously in mice to induce sustained hypoglycemia (INS, green). To check whether any effect of hypoglycemia on beta cell mass is due to the reduced VEGF-A, insulin pellet-treated mice were rescued with an additionally implanted VEGF-A-releasing pump (INS + VEGF-A, purple). To exclude an independent effect of VEGF-A on beta cell mass, treatment with exogenous VEGF-A alone (VEGF-A, red) was included as a control. The mice from both sham (Sham, blue) and INS groups also received a sham operation to control for the effect of surgery. B, non-fasting blood glucose showed sustained hypoglycemia in mice that received either insulin only (INS, green) or a combination of insulin and VEGF-A releasing pumps (INS + VEGF-A, purple). Mice that received VEGF-A pumps only (VEGF-A, red) were normoglycemic like sham-treated mice (Sham, blue). C, beta cell mass analysis showed a reduction (p < 0.01) in insulin-treated mice (INS, green) compared with sham-treated mice (Sham, blue). This reduction of beta cell mass was significantly attenuated (p < 0.05) in the insulin-treated mice that also received VEGF-A pumps (INS + VEGF-A, purple). VEGF-A pump (VEGF-A, red) itself did not affect (no significance) beta cell mass in normoglycemic mouse controls. *, p < 0.05; **, p < 0.01.
Exogenous VEGF-A Partially Rescued the Loss of Beta Cell Mass during Sustained Hypoglycemia
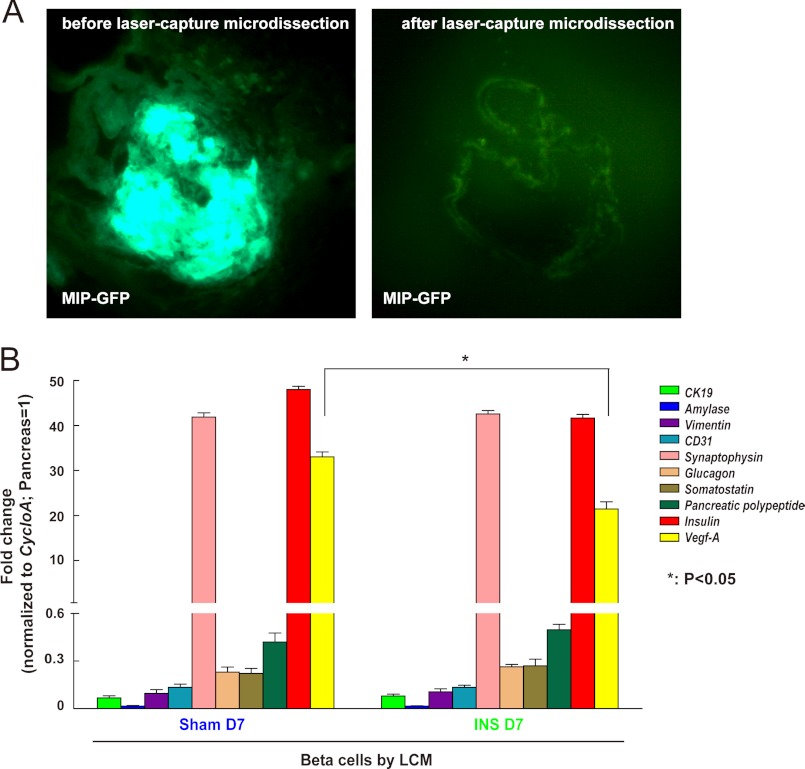
Even though our in vitro data clearly showed that low glucose significantly reduces VEGF-A release by beta cells, and can further reduce Vegf-A transcription in beta cells when the low glucose exposure persists for greater than 24 h, we tried to confirm that VEGF-A synthesis by beta cells is indeed reduced in our in vivo hypoglycemia model. We used LCM (Fig. 3A), rather than FACS, to isolate beta cells from MIP-GFP mouse pancreas sections after the mice were treated for 1 week with insulin-pellets. We used LCM because of the concern that the Vegf-A gene is highly hypoxia-sensitive, and its transcription may quickly change during the pancreas digestion process necessary for FACS. On the other hand, LCM allows prompt fixation of the cells and may better reflect the actual Vegf-A mRNA levels in beta cells. Beta cells isolated by LCM were checked for transcription levels of Amalyse, CK19, Vimentin, CD31, Glucagon, Somatostain, and Pancreatic polypeptide to confirm the absence of contamination, and for insulin transcripts to confirm enrichment (Fig. 3B). Our data showed that Vegf-A transcript levels were significantly reduced in beta cells from insulin pellet-treated mice compared with controls (Fig. 3B), confirming that insulin-induced hypoglycemia indeed reduced VEGF-A synthesis in beta cells, similar to what we found in vitro.
FIGURE 3.
Vegf-A transcripts decrease in beta cells during sustained hypoglycemia. A, representative images before and after beta cell isolation from MIP-GFP mice by LCM. B, levels of mRNA for CK19, Amylase, Vimentin, CD31, Synaptophysin, Glucagon, Somatostatin, Pancreatic polypeptide, and Insulin were evaluated in the isolated beta cells by LCM to assure the purity of the beta cells. Gene values are normalized with cyclophilin A and compared with levels from total pancreas. The Vegf-A transcripts significantly decreased in the beta cells from the hypoglycemic mice. *, p < 0.05.
Next, we examined whether the reduction of beta cell mass in hypoglycemic mice is due to reduced release of VEGF-A. We found that the beta cell mass of insulin-treated mice that also received VEGF-A pumps (Fig. 2C, 0.99 ± 0.03 mg) was significantly greater (p < 0.05) than that of mice that only received insulin pellets (0.89 ± 0.03 mg), rescuing roughly half of the lost beta cell mass, but still lower (p < 0.05) than sham-treated mice (1.14 ± 0.06 mg). Importantly, exogenous VEGF-A pumps alone did not affect beta cell mass in normoglycemic mouse controls (1.10 ± 0.05 mg, no significance). This partial rescue of the reduction of the beta cell mass by exogenous VEGF-A suggests that the reduction of endogenous VEGF-A release directly leads to loss of beta cell mass during sustained hypoglycemia. Partial rescue, rather than complete rescue, may reflect either an inadequate supply of VEGF-A, or that other factors may also play a role in the beta cell mass adaptation during sustained hypoglycemia.
Beta Cell Proliferation Was Not Affected by Hypoglycemia, Regardless of Exogenous VEGF-A Supply
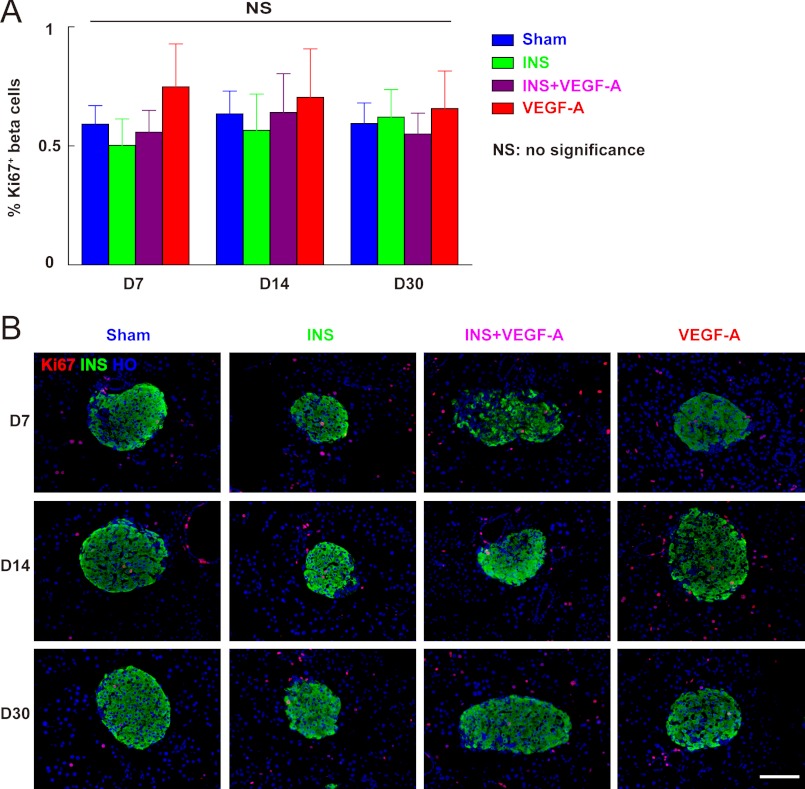
As adult beta cell mass is determined by a balance of beta cell proliferation and apoptosis, we first examined whether beta cell proliferation was altered in our experimental groups. Therefore, the percentage of the pancreatic beta cells that were positive for Ki-67 (32), a cellular proliferation marker, was quantified at 7, 14, and 30 days. Our data show that Ki-67+ beta cell percentages did not change across all groups during the experiment (Fig. 4, A and B). These data suggest that reduced beta cell mass under sustained hypoglycemia is not due to decreased beta cell proliferation.
FIGURE 4.
Beta cell proliferation was not affected by hypoglycemia regardless of exogenous VEGF-A supply. The percentage of pancreatic beta cells that are positive for Ki-67, a cellular marker for proliferation, was quantified at 7, 14, and 30 days. A, Ki-67+ beta cell percentages across all four experimental conditions were consistent (no difference). B, representative immunofluorescent images of mouse pancreas at day 7 (D7), 14 (D14), and 30 (D30) are shown: insulin (INS) in green; Ki-67 in red, and Hoechst (HO) in blue. NS, no significance. Scale bars are 50 μm.
Apoptosis of Islet Endothelial Cells Precedes Beta Cell Loss in Sustained Hypoglycemia
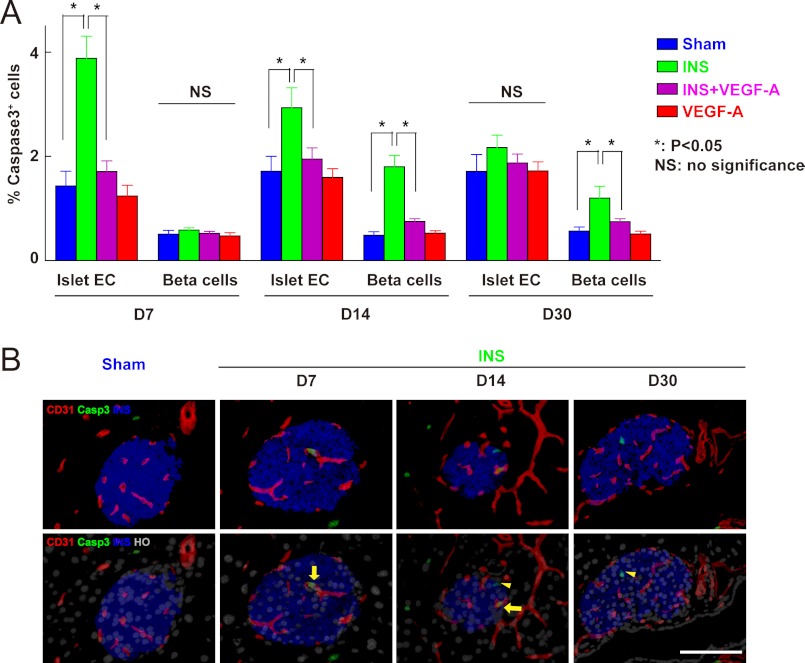
Because beta cell proliferation was not affected by hypoglycemia, the reduction of beta cell mass would likely be due to increased apoptosis of beta cells. Because the major target of VEGF-A released by beta cells is islet endothelial cells, the adaption of beta cell mass under hypoglycemia may be secondary to the effect of reduced VEGF-A release on endothelial cells, e.g. reduced endothelial cell survival. Thus, we checked apoptosis of endothelial cells and beta cells under all experiment conditions at 7, 14, and 30 days. The percentage of islet vessels that contained apoptotic caspase 3+ endothelial cells and the percentage of apoptotic beta cells were quantified. Our data show that apoptotic islet endothelial cells increased significantly at day 7, persisted at day 14, but reverted to normal by day 30 (Fig. 5, A and B). On the other hand, the increase in apoptotic beta cells could only be detected by day 14 (Fig. 5, B and C). This time window for the presence of apoptotic cells suggests a causative link between apoptosis of endothelial cells and apoptosis of beta cells, which is strengthened by the fact that exogenous VEGF-A prevented apoptosis of endothelial cells and also reduced apoptosis of beta cells (Fig. 5A).
FIGURE 5.
Apoptosis of islet endothelial cells precedes apoptosis of beta cells during sustained hypoglycemia, and can be prevented with exogenous VEGF-A. A-C, pancreases from mice treated with insulin pellets (INS, green), combined insulin pellets and VEGF-A pump (INS + VEGF-A, purple), untreated sham controls (Sham, blue), and VEGF-A only controls (VEGF-A, red) were analyzed at 7, 14, and 30 days for apoptotic islet endothelial cells and beta cells. A, the percentage of islet vessels that contained caspase 3+ endothelial cells (CD31+) was quantified and showed an increase (p < 0.05) at days 7 and 14, but reverted to normal at day 30 after insulin pellet treatment. This apoptosis of endothelial cells can be completely prevented by exogenous VEGF-A. The percentage of caspase 3+ beta cells was also quantified and showed no change at day 7 after insulin pellet treatment, compared with controls. However, this percentage increased by day 14. Similarly, beta cell apoptosis was significantly reduced by exogenous VEGF-A. B, representative immunofluorescent images of mouse pancreas from different time points (D7, D14, and D30) after insulin pellet treatments are shown: CD31 in red, insulin (INS) in blue, caspase 3 in green, and nuclei staining (Hoechst, HO) in gray. Arrows point to caspase 3+ CD31+ cells and arrowheads point to caspase 3+ INS+ cells. Casp3, caspase 3; EC, endothelial cell. *, p < 0.05; NS, no significance. Scale bars are 50 μm.
Islet Vessel Density Was Not Affected by Hypoglycemia Regardless of Exogenous VEGF-A Supply
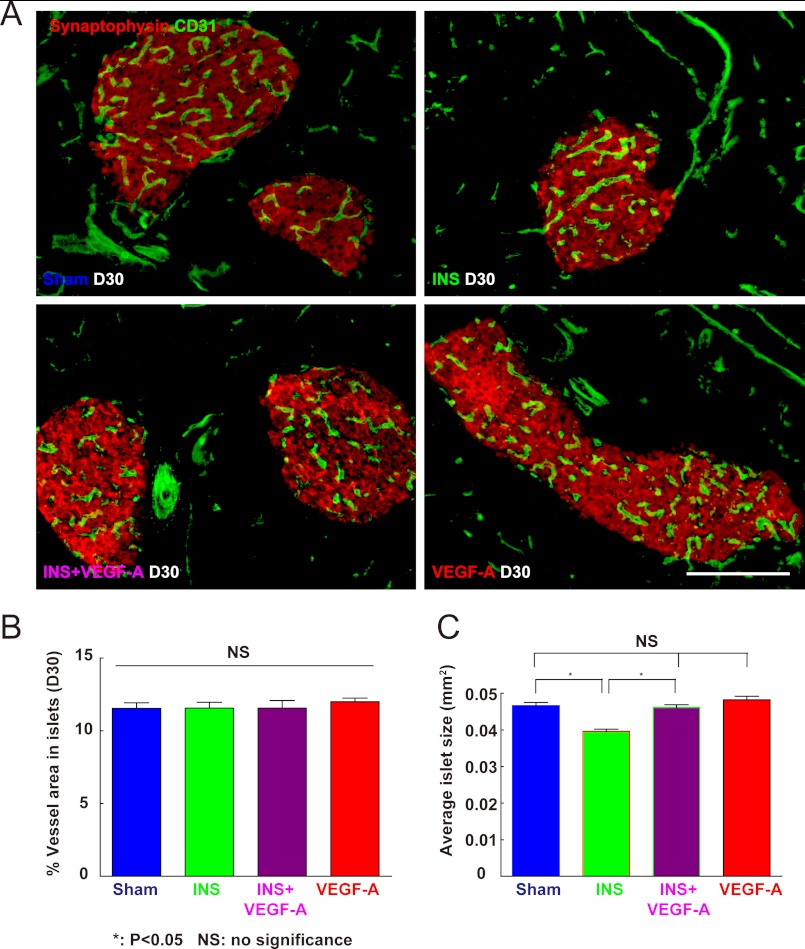
Next, we evaluated islet vessel density. If our hypothesis is correct that loss of endothelial cells affects the survival of beta cells, then the vessel density in islets should be relatively stable across all experimental groups. If, however, islet vessel density decreases under hypoglycemia, it might suggest that beta cells can survive after the loss of nearby islet endothelial cells, and thus beta cell mass is not strictly regulated by feedback from endothelial cells in this model. We found consistent islet vessel density among all four experimental conditions (Fig. 6, A and B), supporting our hypothesis that a reduction in VEGF-A release by beta cells leads to a loss of islet endothelial cells which, in turn, leads to a proportionate loss of beta cells.
FIGURE 6.
Quantification of islet vessel density and average islet size. A and B, islet vessel densities were measured under all experimental conditions and evaluated with the ratio of islet CD31+ cell area to synaptophysin+ cell area. Synaptophysin is a pan-endocrine cell marker. A, representative immunofluorescent images of mouse pancreas at day 30 were shown: CD31 in green and synaptophysin in red. B, the islet vessel densities were consistent (no difference) across all four experimental conditions. C, average islet size was measured among all four experimental conditions, showing a significant decrease in insulin-treated mice, which was prevented with exogenous VEGF-A. *, p < 0.05; NS, no significance. Scale bars are 50 μm.
Average Islet Size Was Reduced by Hypoglycemia and Preserved by Exogenous VEGF-A
Finally, we evaluated islet number and size. We did not find a change in islet number across all experiment conditions, but saw an approximate 22% reduction in average islet size with hypoglycemia, consistent with the measured reduction of beta cell mass. Importantly, this decrease in average islet size was prevented by exogenous VEGF-A (Fig. 6C). These data support our previous finding that loss of beta cell mass by hypoglycemia results from loss of some beta cells in the islets. Exogenous VEGF-A itself did not significantly change islet size (Fig. 6C) in normoglycemic mice, consistent with our previous data that VEGF-A does not significantly increase beta cell proliferation (Fig. 5).
DISCUSSION
VEGF-A expression by beta cells has been shown to be important for proper pancreatic organogenesis and proper insulin secretion (1, 2, 6, 19, 20). However, most rodent studies on VEGF-A in beta cells were based on either gene deletion or on overexpression under a strong promoter in beta cells (7, 19, 23). However, the physiological effects of VEGF-A are dosage-dependent over a narrow physiologic range (2, 3, 27, 28). Moreover, inactivation of only one allele of Vegf-A results in embryonic lethality at mid-gestation (38, 39), whereas a 2-fold increase in Vegf-A levels also leads to embryonic lethality (40). Therefore, it appears that the nonphysiological Vegf-A levels in these transgenic models may considerably diminish the power of these studies. Indeed, we found that the promoter activity of insulin is much stronger than Pdx1 in beta cells, and is more than 2000-fold stronger than the Vegf-A promoter in beta cells (not shown), which suggests that expression of Vegf-A under either the insulin promoter or the Pdx1 promoter may produce supraphysiologic, and potentially biologically irrelevant levels of Vegf-A in beta cells, possibly explaining some of the discrepancies among these studies (18, 23–25). In addition, such deletion or extreme overexpression of Vegf-A may activate compensatory pathways or change the expression of other VEGF family members, with the occurrence of the latter having been clearly demonstrated (8). In the current study, we chose to study only beta cells from wild-type or reporter mice without interfering genetic modifications, hopefully providing more physiologically relevant information for understanding VEGF-A regulation in beta cells. Our dosage of exogenous VEGF-A was close to normal physiological replacement values, and should not lead to significant side effects (2, 3).
We first found a reduction in VEGF-A release from cultured islets or beta cells specifically early on during exposure to low glucose culture conditions, and thus we hypothesized that reduced VEGF-A release under hypoglycemia from beta cells may affect beta cell mass, as a feedback of an influence on the survival of islet endothelial cells. To test this hypothesis, we gave mice insulin pellets to induce sustained hypoglycemia. The environment of beta cells in this insulin-induced hypoglycemia model should mimic the low glucose culture conditions of beta cells we used in vitro. To further confirm our findings, we isolated beta cells by LCM and found that Vegf-A mRNA levels in beta cells indeed were decreased in hypoglycemia. Furthermore, we showed that beta cell mass during hypoglycemia was reduced by 22% at 1 month. This reduction may be particularly significant in that it did not involve any genetic modification, and beta cell mass is normally tightly regulated (41). Importantly, this reduction was largely due to reduced VEGF-A signaling because the loss of beta cell mass was partially rescued by exogenous VEGF-A. Of note, apoptotic endothelial cells were seen well before apoptotic beta cells during hypoglycemia, and exogenous VEGF-A reduced the apoptosis of both endothelial cells and beta cells, suggesting that the loss of endothelial cells could result from reduced VEGF-A release from beta cells, and the loss of endothelial cells may contribute to the subsequent reduction in beta cell mass. Moreover, no change in beta cell proliferation was detected in any experimental conditions, suggesting that down-regulation of beta cell mass under sustained hypoglycemia is primarily due to increased beta cell apoptosis rather than deceased beta cell proliferation. A strong relationship between the amount of islet vasculature and beta cell mass was further manifested by the fact that vessel density remained constant and that the reduction in islet size during hypoglycemia was prevented by VEGF-A replacement.
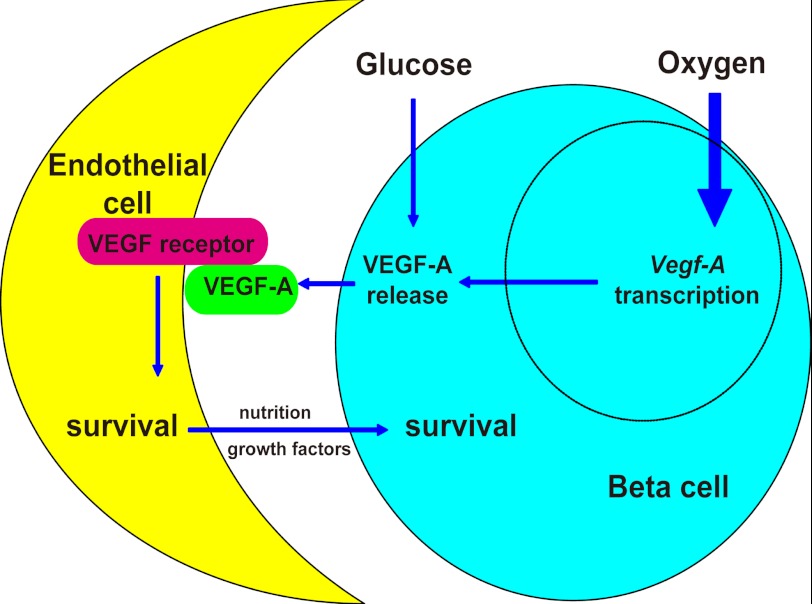
Reduced beta cell mass as a result of a hypoglycemia-induced reduction in VEGF-A release may have implications for both beta cell development and beta cell biology. Beta cell mass is well known to change with body weight, insulin demand, and other physiological parameters (42), including blood glucose levels. The molecular basis for this adaptation is not completely understood. From our study it appears that the cross-talk between islet endothelial cells and beta cells is an important regulator of beta cell mass, with VEGF-A being a key component of the cross-talk. Our study thus suggests a novel pathway for beta cell mass adaptation to metabolic changes (Fig. 7). Future studies might focus on the control of VEGF-A release during islet development and under other physiological conditions when changes in beta cell mass occur, such as pregnancy. Our study improves the understanding of cross-talk between endothelial cells and beta cells, and thus provides new insights into the regulation of functional beta cell mass in diabetic patients.
FIGURE 7.
Hypoglycemia regulates beta cell mass via modulated VEGF-A release. A model of how beta cell mass is regulated by hypoglycemia via VEGF-A is proposed. Oxygen tension is the major regulator for Vegf-A transcription in pancreatic beta cells. Hypoxia can greatly increase Vegf-A transcription. However, glucose can regulate VEGF-A release from beta cells before the adaptation of Vegf-A transcription occurs. Hypoglycemia can reduce VEGF-A release, resulting in a decrease in survival of neighboring islet endothelial cells, which subsequently leads to a secondary loss of beta cells.
Acknowledgments
Special thanks to Alexis J. Styche, Robert J. Lakomy, and Lauren Brink for technical assistance in flow cytometry and mouse genotyping. We also thank Christine Kalinyak, Anne L. Meinert, and JoAnn Stiles for administrative assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1 DK064952 and R01 DK083541 (to G. K. G.) and the Children's Hospital of Pittsburgh.
- VEGF
- vascular endothelial growth factor
- LCM
- laser capture microdissection
- VEGFR1
- receptor 1 for vascular endothelial growth factor
- VEGFR2
- receptor 2 for vascular endothelial growth factor.
REFERENCES
- 1. Cleaver O., Melton D. A. (2003) Endothelial signaling during development. Nat. Med. 9, 661–668 [DOI] [PubMed] [Google Scholar]
- 2. Ferrara N., Gerber H. P., LeCouter J. (2003) The biology of VEGF and its receptors. Nat. Med. 9, 669–676 [DOI] [PubMed] [Google Scholar]
- 3. Carmeliet P., Jain R. K. (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473, 298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee S., Jilani S. M., Nikolova G. V., Carpizo D., Iruela-Arispe M. L. (2005) Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J. Cell Biol. 169, 681–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stalmans I., Ng Y. S., Rohan R., Fruttiger M., Bouché A., Yuce A., Fujisawa H., Hermans B., Shani M., Jansen S., Hicklin D., Anderson D. J., Gardiner T., Hammes H. P., Moons L., Dewerchin M., Collen D., Carmeliet P., D'Amore P. A. (2002) Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J. Clin. Invest. 109, 327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Christofori G., Naik P., Hanahan D. (1995) Vascular endothelial growth factor and its receptors, flt-1 and flk-1, are expressed in normal pancreatic islets and throughout islet cell tumorigenesis. Mol. Endocrinol. 9, 1760–1770 [DOI] [PubMed] [Google Scholar]
- 7. Brissova M., Shostak A., Shiota M., Wiebe P. O., Poffenberger G., Kantz J., Chen Z., Carr C., Jerome W. G., Chen J., Baldwin H. S., Nicholson W., Bader D. M., Jetton T., Gannon M., Powers A. C. (2006) Pancreatic islet production of vascular endothelial growth factor-A is essential for islet vascularization, revascularization, and function. Diabetes 55, 2974–2985 [DOI] [PubMed] [Google Scholar]
- 8. Inoue M., Hager J. H., Ferrara N., Gerber H. P., Hanahan D. (2002) VEGF-A has a critical, nonredundant role in angiogenic switching and pancreatic beta cell carcinogenesis. Cancer Cell 1, 193–202 [DOI] [PubMed] [Google Scholar]
- 9. Kamba T., Tam B. Y., Hashizume H., Haskell A., Sennino B., Mancuso M. R., Norberg S. M., O'Brien S. M., Davis R. B., Gowen L. C., Anderson K. D., Thurston G., Joho S., Springer M. L., Kuo C. J., McDonald D. M. (2006) VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am. J. Physiol. Heart Circ. Physiol. 290, H560–576 [DOI] [PubMed] [Google Scholar]
- 10. Shima D. T., Adamis A. P., Ferrara N., Yeo K. T., Yeo T. K., Allende R., Folkman J., D'Amore P. A. (1995) Hypoxic induction of endothelial cell growth factors in retinal cells. Identification and characterization of vascular endothelial growth factor (VEGF) as the mitogen. Mol. Med. 1, 182–193 [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng S. Y., Nagane M., Huang H. S., Cavenee W. K. (1997) Intracerebral tumor-associated hemorrhage caused by overexpression of the vascular endothelial growth factor isoforms VEGF121 and VEGF165 but not VEGF189. Proc. Natl. Acad. Sci. U.S.A. 94, 12081–12087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huez I., Créancier L., Audigier S., Gensac M. C., Prats A. C., Prats H. (1998) Two independent internal ribosome entry sites are involved in translation initiation of vascular endothelial growth factor mRNA. Mol. Cell. Biol. 18, 6178–6190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pugh C. W., Ratcliffe P. J. (2003) Regulation of angiogenesis by hypoxia. Role of the HIF system. Nat. Med. 9, 677–684 [DOI] [PubMed] [Google Scholar]
- 14. Vasir B., Aiello L. P., Yoon K. H., Quickel R. R., Bonner-Weir S., Weir G. C. (1998) Hypoxia induces vascular endothelial growth factor gene and protein expression in cultured rat islet cells. Diabetes 47, 1894–1903 [DOI] [PubMed] [Google Scholar]
- 15. Bonner-Weir S. (1988) Morphological evidence for pancreatic polarity of beta-cell within islets of Langerhans. Diabetes 37, 616–621 [DOI] [PubMed] [Google Scholar]
- 16. Nikolova G., Jabs N., Konstantinova I., Domogatskaya A., Tryggvason K., Sorokin L., Fässler R., Gu G., Gerber H. P., Ferrara N., Melton D. A., Lammert E. (2006) The vascular basement membrane. A niche for insulin gene expression and Beta cell proliferation. Dev. Cell 10, 397–405 [DOI] [PubMed] [Google Scholar]
- 17. El-Gohary Y., Sims-Lucas S., Lath N., Tulachan S., Guo P., Xiao X., Welsh C., Paredes J., Wiersch J., Prasadan K., Shiota C., Gittes G. K. (2012) Anat. Rec. 295, 1473–1481 [DOI] [PubMed] [Google Scholar]
- 18. Lammert E., Cleaver O., Melton D. (2001) Induction of pancreatic differentiation by signals from blood vessels. Science 294, 564–567 [DOI] [PubMed] [Google Scholar]
- 19. Lammert E., Gu G., McLaughlin M., Brown D., Brekken R., Murtaugh L. C., Gerber H. P., Ferrara N., Melton D. A. (2003) Role of VEGF-A in vascularization of pancreatic islets. Curr. Biol. 13, 1070–1074 [DOI] [PubMed] [Google Scholar]
- 20. Cleaver O., Dor Y. (2012) Vascular instruction of pancreas development. Development 139, 2833–2843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brissova M., Fowler M., Wiebe P., Shostak A., Shiota M., Radhika A., Lin P. C., Gannon M., Powers A. C. (2004) Intraislet endothelial cells contribute to revascularization of transplanted pancreatic islets. Diabetes 53, 1318–1325 [DOI] [PubMed] [Google Scholar]
- 22. Nyqvist D., Köhler M., Wahlstedt H., Berggren P. O. (2005) Donor islet endothelial cells participate in formation of functional vessels within pancreatic islet grafts. Diabetes 54, 2287–2293 [DOI] [PubMed] [Google Scholar]
- 23. Iwashita N., Uchida T., Choi J. B., Azuma K., Ogihara T., Ferrara N., Gerber H., Kawamori R., Inoue M., Watada H. (2007) Impaired insulin secretion in vivo but enhanced insulin secretion from isolated islets in pancreatic beta cell-specific vascular endothelial growth factor-A knock-out mice. Diabetologia 50, 380–389 [DOI] [PubMed] [Google Scholar]
- 24. Magenheim J., Ilovich O., Lazarus A., Klochendler A., Ziv O., Werman R., Hija A., Cleaver O., Mishani E., Keshet E., Dor Y. (2011) Blood vessels restrain pancreas branching, differentiation and growth. Development 138, 4743–4752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cai Q., Brissova M., Reinert R. B., Pan F. C., Brahmachary P., Jeansson M., Shostak A., Radhika A., Poffenberger G., Quaggin S. E., Jerome W. G., Dumont D. J., Powers A. C. (2012) Enhanced expression of VEGF-A in β cells increases endothelial cell number but impairs islet morphogenesis and β cell proliferation. Dev. Biol. 367, 40–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Agudo J., Ayuso E., Jimenez V., Casellas A., Mallol C., Salavert A., Tafuro S., Obach M., Ruzo A., Moya M., Pujol A., Bosch F. (2012) Vascular endothelial growth factor-mediated islet hypervascularization and inflammation contribute to progressive reduction of beta-cell mass. Diabetes 61, 2851–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Darland D. C., Cain J. T., Berosik M. A., Saint-Geniez M., Odens P. W., Schaubhut G. J., Frisch S., Stemmer-Rachamimov A., Darland T., D'Amore P. A. (2011) Vascular endothelial growth factor (VEGF) isoform regulation of early forebrain development. Dev. Biol. 358, 9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wada T., Haigh J. J., Ema M., Hitoshi S., Chaddah R., Rossant J., Nagy A., van der Kooy D. (2006) Vascular endothelial growth factor directly inhibits primitive neural stem cell survival but promotes definitive neural stem cell survival. J. Neurosci. 26, 6803–6812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Akirav E. M., Baquero M. T., Opare-Addo L. W., Akirav M., Galvan E., Kushner J. A., Rimm D. L., Herold K. C. (2011) Glucose and inflammation control islet vascular density and beta-cell function in NOD mice. Control of islet vasculature and vascular endothelial growth factor by glucose. Diabetes 60, 876–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eichler W., Kuhrt H., Hoffmann S., Wiedemann P., Reichenbach A. (2000) VEGF release by retinal glia depends on both oxygen and glucose supply. Neuroreport 11, 3533–3537 [DOI] [PubMed] [Google Scholar]
- 31. Hara M., Wang X., Kawamura T., Bindokas V. P., Dizon R. F., Alcoser S. Y., Magnuson M. A., Bell G. I. (2003) Transgenic mice with green fluorescent protein-labeled pancreatic beta-cells. Am. J. Physiol. Endocrinol. Metab. 284, E177–183 [DOI] [PubMed] [Google Scholar]
- 32. Xiao X., Wiersch J., El-Gohary Y., Guo P., Prasadan K., Paredes J., Welsh C., Shiota C., Gittes G. K. (Jan. 15, 2013) TGFβ receptor signaling is essential for inflammation-induced but not β-cell workload-induced β-cell proliferation. Diabetes, DOI: 10.2337/db12-1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xiao X., Chen Z., Shiota C., Prasadan K., Guo P., El-Gohary Y., Wiersch J., Paredes J., Welsh C., Gittes G. K. (2013) No evidence for beta cell neogenesis in the adult pancreas. J. Clin. Invest., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bonner R. F., Emmert-Buck M., Cole K., Pohida T., Chuaqui R., Goldstein S., Liotta L. A. (1997) Laser capture microdissection. Molecular analysis of tissue. Science 278, 1481–1483 [DOI] [PubMed] [Google Scholar]
- 35. Marselli L., Sgroi D. C., Bonner-Weir S., Weir G. C. (2009) Laser capture microdissection of human pancreatic beta-cells and RNA preparation for gene expression profiling. Methods Mol. Biol. 560, 87–98 [DOI] [PubMed] [Google Scholar]
- 36. Guang S., Bochner A. F., Burkhart K. B., Burton N., Pavelec D. M., Kennedy S. (2010) Small regulatory RNAs inhibit RNA polymerase II during the elongation phase of transcription. Nature 465, 1097–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lin S., Coutinho-Mansfield G., Wang D., Pandit S., Fu X. D. (2008) The splicing factor SC35 has an active role in transcriptional elongation. Nat. Struct. Mol. Biol. 15, 819–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carmeliet P., Ferreira V., Breier G., Pollefeyt S., Kieckens L., Gertsenstein M., Fahrig M., Vandenhoeck A., Harpal K., Eberhardt C., Declercq C., Pawling J., Moons L., Collen D., Risau W., Nagy A. (1996) Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380, 435–439 [DOI] [PubMed] [Google Scholar]
- 39. Ferrara N., Carver-Moore K., Chen H., Dowd M., Lu L., O'Shea K. S., Powell-Braxton L., Hillan K. J., Moore M. W. (1996) Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380, 439–442 [DOI] [PubMed] [Google Scholar]
- 40. Miquerol L., Langille B. L., Nagy A. (2000) Embryonic development is disrupted by modest increases in vascular endothelial growth factor gene expression. Development 127, 3941–3946 [DOI] [PubMed] [Google Scholar]
- 41. Ackermann A. M., Gannon M. (2007) Molecular regulation of pancreatic beta-cell mass development, maintenance, and expansion. J. Mol. Endocrinol. 38, 193–206 [DOI] [PubMed] [Google Scholar]
- 42. Bouwens L., Rooman I. (2005) Regulation of pancreatic beta-cell mass. Physiol. Rev. 85, 1255–1270 [DOI] [PubMed] [Google Scholar]