Background: The mechanisms of insect immune peptide recognition and evasion by T. cruzi are unknown.
Results: Secreted parasite cyclophilin neutralizes lytic peptide, causes activation of parasite calcineurin, and enhances parasite infection.
Conclusion: Cyclophilin is a key mediator of antimicrobial peptide evasion and triggers parasite calcineurin signaling.
Significance: The cyclophilin-calcineurin pathway is an extracellular peptide microbial-host sensing mechanism.
Keywords: Antimicrobial Peptides, Calcineurin, Immunophilin, Infectious Diseases, Trypanosome, Cationic Antimicrobial Peptide, Cyclophilin, Infection
Abstract
The mechanisms by which Trypanosoma cruzi survives antimicrobial peptides and differentiates during its transit through the gastrointestinal tract of the reduviid vector are unknown. We show that cyclophilin, a peptidyl-prolyl isomerase secreted from T. cruzi epimastigotes, binds to and neutralizes the reduviid antimicrobial peptide trialysin promoting parasite survival. This is dependent on a singular proline residue in trialysin and is inhibited by the cyclophilin inhibitor cyclosporine A. In addition, cyclophilin-trialysin complexes enhance the production of ATP and reductase responses of parasites, which are inhibited by both calcineurin-specific inhibitors cyclosporine A and FK506. Calcineurin phosphatase activity of cyclophilin-trialysin-treated parasites was higher than in controls and was inhibited by preincubation by either inhibitor. Parasites exposed to cyclophilin-trialysin have enhanced binding and invasion of host cells leading to higher infectivity. Leishmanial cyclophilin also mediates trialysin protection and metabolic stimulation by T. cruzi, indicating that extracellular cyclophilin may be critical to adaptation in other insect-borne protozoa. This work demonstrates that cyclophilin serves as molecular sensor leading to the evasion and adaptive metabolic response to insect defense peptides.
Introduction
Trypanosoma cruzi transits through the alimentary canal of the hematophagous reduviid bug vector wherein it replicates as non-infectious epimastigotes that differentiate into infective metacyclic forms. Parasite differentiation into infective forms is critical to human transmission, but the interplay between external signals that lead to differentiation is not entirely clear. Exposure of parasites to digested hemoglobin fragments can trigger adenylate cyclase activation and cyclic adenosine mono-phosphate production leading to enhanced infectivity (1), as can changes in ionic and nutritional conditions (2). These disparate stimuli trigger metabolic activation and ATP production leading to enhanced parasite invasion (3), but the intracellular signaling pathway(s) involved in these adaptive changes is not known.
Innate immune cationic antimicrobial peptides (CAMPs)2 are expressed by a wide variety of insects to prevent microbial colonization and infection (4). Several CAMPs have been identified from the saliva, hemolymph, and intestinal tract of reduviids (5); however, the biologic functions of these peptides in this vector are not known. The best-studied of these is an α-helical antimicrobial salivary peptide, from Triatoma infestans, termed trialysin (6, 7). Structure-activity studies have shown that the N-terminal 32-amino acid region, termed P6, contains a singular proline residue and has the greatest parasiticidal toxicity (6, 7). A number of other CAMPs from mammals, insects, and amphibians are also anti-trypanosomal and -leishmanial (8–12) in phosphate-buffered saline (PBS) at concentrations ranging from 1 to 50 μm (physiologic concentrations at which antimicrobial peptides are often found and active in host tissue) (11, 12). Despite the expression of a diverse array of host CAMPs, trypanosomatid parasites are still able to alternately infect insects and mammals successfully, implying that these parasites have evolved mechanisms to overcome antimicrobial peptide toxicities (12), such as the CAMP-resistance of Leishmania through proteolytic degradation of peptide by surface metalloprotease (8, 13).
Here we report our work on the interaction of CAMP with T. cruzi, which reveals a unique pathway of parasite-driven modification of host CAMP. In this pathway we have found that secreted parasite cyclophilin, a peptidyl-prolyl isomerase, binds to and inactivates trialysin via action at its proline residue. Cyclophilin-trialysin synergistically acts on parasites to activate calcineurin phosphatase signaling, which drives metabolic activation and ATP production leading to enhanced infectivity. This novel parasite pathway describes a mechanism of CAMP recognition, evasion, and adaptation mediated through calcineurin intracellular signaling.
EXPERIMENTAL PROCEDURES
Parasite Cultivation
T. cruzi strains (Brazil, CL, Y, Sylvio, and DM28c) were used in this study. Routine cultivation of epimastigotes was performed using liver-digested neutralized Tryptone medium supplemented with 10% heat-inactivated fetal bovine serum and 20 μg ml−1 hemin. Liver-digested neutralized Tryptone or defined non-protein containing Medium 199 (Invitrogen) was used to generate parasite-conditioned medium. Leishmania major (NHOM/SN/74/Seidman) and Leishmania amazonensis LV78 (MPRO/BR/72/M1845) were routinely cultivated as insect forms in M199 containing 10% heat-inactivated fetal bovine serum. Infection studies were done using the H9C2 line of rat heart myoblasts that were routinely grown in DMEM supplemented with 10% heat-inactivated fetal bovine serum.
Parasite Reductase and Viability Assays, ATP Measurements, and Calcineurin Phosphatase Assays and Intracellular Calcium Measurements
A standard parasite reductase assay was used as described previously for Leishmania (8). Routinely, 107 parasites were incubated overnight in 25 μg of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent followed by treatment with 10% SDS for 6–8 h followed by spectrophotometric analysis at 570 nm. Treated parasites were compared with parasites incubated in the same conditions in non-conditioned medium or PBS buffer alone. All CAMP-treated cells were incubated for 2 h with indicated amounts of peptide before analysis with the MTT assay. Propidium iodide flow cytometric analysis was done as described previously (8) and tested over a 15-min to 12-h time course. Cyclosporine A (Sigma) was reconstituted in sterile water at 1 mg/ml as a stock solution and added at the indicated final concentrations in reactions and incubated for 30 min with recombinant trypanosome cyclophilin 19 (14) or parasite-conditioned medium (CM) before the addition of CAMPs. In vitro Cyp19 assays were developed with stationary phase parasites, recombinant trypanosomal cyclophilin, and synthetic insect CAMPs in non-conditioned M199 according to the details provided in the figure legends. ATP measurement was done using a bioluminescent assay kit (Sigma) using 107 parasites lysed in releasing buffer and compared with a standard curve of known ATP concentration. For the contribution of mitochondrial function to ATP production, parasites were incubated under the same conditions with 10 μm carbonyl cyanide p-trifluoromethoxyphenylhydrazone (8, 13). For calcineurin assays, cell-free protein extracts were prepared by freeze (−20 °C)-thaw disruption of Cyp19/CAMP pretreated or control parasites followed by removal of insoluble material at by centrifugation at 100,000 × g for 1 h. Calcineurin phosphatase activity of extracts was performed using 160 mm p-nitrophenyl phosphate substrate (15–17) for 1 h at 25 °C using 250 μg of the protein extracts. This was compared with control reactions of purified bovine calcineurin (Sigma) under the same conditions. Intracellular calcium was measured in T. cruzi epimastigotes preloaded with the Ca2+-sensitive dye Fura 2AM before treatment with the indicated proteins in the in vitro Cyp19 assay. Fluorescence measurements were performed for 6 h with a plate reader (excitation, 340 nm; emission, 510 nm) as described previously (13). Parasites incubated with 10 mm CaCl2 were used as the positive control for maximum fluorescence.
Western Blotting, Immunoprecipitation, Anti-cyclophilin Antibodies, Recombinant Trypanosomal Cyclophilin, and Antimicrobial Peptides
For Western blotting, parasite protein lysates and parasite-conditioned medium boiled in SDS-PAGE buffer were fractionated by SDS-PAGE on 10–15% gels. Resolved proteins were electrophoretically transferred to nitrocellulose membranes (Schleicher and Schuell) and probed using primary antisera (at 1:10,000 dilution). Antisera used in these studies were human polyclonal anti-cyclophilin A and anti-recombinant Trypanosoma brucei cyclophilin A (14). Recombinant trypanosomal cyclophilin 19 was made as described previously (14). The secondary antibodies used were horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse IgG. Blots were developed with ECL reagent (Amersham Biosciences). The CAMEL, WT, and mutant P6-trialysin used were CAMEL (KWKLFKKIGIGAVLKVLTTGLPALIS), P6-trialysin, (FKIKPGKVLDKFGKIVGKVLKQLKKVSAVAKV), and the Mut-T-trialysin (FKIKAGKVLDKFGKIVGKVLKQLKKVSAVAKV) and were synthesized on an Applied Biosystems model 433A synthesizer; other peptides were described previously (8, 13). Protein biotinylation was performed by incubation of lyophilized-conditioned medium proteins in EZ-link Sulfo-NH-biotin reagent (Pierce) as described previously (18). Magnetic beads (Dynabeads-Carboxylic acid, Invitrogen) were covalently linked to synthetic insect CAMPs according the Manufacturer's specifications. Coupling efficiency was determined by protein quantitation of CAMP bound after coupling, blocking and washing reactions. Equal amounts of bead-bound protein were used in reactions with 5 μg of rCyp19 in PBS for 2 h at 25 °C followed by three washings in PBS and solubilization in SDS-PAGE. Samples were fractionated by SDS-PAGE on 12% gels, and Western blotting was performed using α-tCyp19 antibodies. Immunodepletion of each medium was done twice to ensure removal of Cyp19 before MTT reactions. Media were checked for complete removal of Cyp19 by Western blotting. Densitometry analysis of blots was done using a Bio-Rad Gel Doc system. Blocked beads alone were used for control reactions to gauge nonspecific protein binding, which was negligible in all cases.
Fractionation and Mass Spectrometric Analysis of Secreted Proteins from Parasite-conditioned M199
Parasite-conditioned M199 was harvested from late stationary phase cultures of epimastigotes and concentrated 10-fold by lyophilization. Concentrated medium was fractionated by reverse-phase C-18 HPLC using a continuous acetonitrile gradient from 0–30%. Aliquots of individual fractions were tested for parasite reductase activity in the presence and absence of insect AMP as described above, and fractions containing activity were pooled, digested in trypsin, and subjected to capillary-liquid chromatography-nanospray tandem LTQ orbitrap mass spectrometry (LC-MS/MS). Protein identifications were checked manually, and those with Mascot scores (19) of 50 or higher with a minimum of 2 unique peptides from 1 protein having a sequence tag of 5 residues or better were accepted.
Flow Cytometric Analysis
Flow cytometry analysis was performed using a FACSCalibur flow cytometer and CellQuestPro software (BD Biosciences). Parasites were treated as indicated above, fixed, and permeabilized in 10% ice-cold methanol and then stained with anti-metacyclin III (MET-III) (20, 21) at a 1:1000 dilution in PBS, washed, and incubated with FITC-conjugated anti-rabbit IgG and then analyzed. For carboxyfluorescein succinimidyl ester (CFSE) dilutional analysis of cell division parasites were incubated for 5 min at 37 °C with 5 μm CFSE in PBS followed by three washings. Parasites were harvested at the indicated times and subjected to fluorescence quantitation by flow cytometry as indicated above.
Parasite Microscopy and Myoblast Infections
Parasites from peptide reactions were visualized by light microscopy in 96-well plates or by wet mount using an Olympus Bx41 microscope and images were captured using the Olympus DP Manager software Version 3.1.1.208. Parasites were used to infect myoblasts pre-grown on coverslips at a multiplicity of infection of 5:1 at 37 °C. At various times indicated in the figure legends post-infection, coverslips were rinsed three times in fresh medium, fixed in methanol, and stained with either Giemsa or phalloidin-red to visualize host cell actin. The percentage of infected myoblasts and number of associated parasites per infected cell were enumerated upon visualization by light microscopy.
RESULTS
Insect CAMPs Selectively Induce Parasite Mitochondrial Bio-energetic Activation
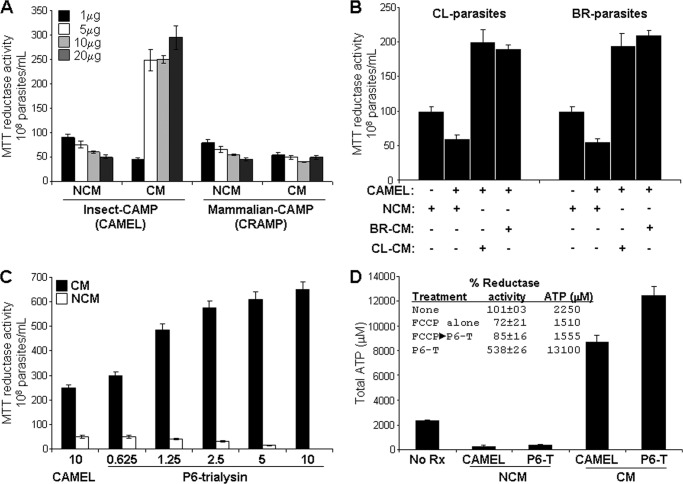
The anti-trypanosomal activities of a panel of CAMPs were compared in fresh parasite growth medium or added to conditioned-culture medium using standardized parasite viability assay based on measurement of reductase activity using the tetrazolium-based compound MTT (8, 9, 13). The CAMPs used in these assays included: a synthetic hybrid peptide containing insect-derived sequences from cecropin A and melittin (termed CAMEL), mammalian cathelicidin (murine CRAMP), α-defensin (cryptdin-4), β-defensin-3 and amphibian-pexiganan (8). Remarkably, of these peptides only CAMEL enhanced parasite reductase activity >2-fold in conditioned medium compared with control reactions in non-conditioned medium, which was not found with mammalian cathelicidin (Fig. 1A) or defensin peptides. CAMEL activated the reductase activity in the presence of the conditioned medium of several T. cruzi strains. Furthermore, the conditioned medium from epimastigotes from one parasite strain stimulated the reductase activity of the epimastigotes of another parasite strain, indicating that the CAMP response pathway functions in more than one T. cruzi isolate (Fig. 1B). We have found this pathway in CL, Y, Tulahuen, and Brazil strains of T. cruzi epimastigotes. To test whether the response observed with CAMEL occurs with an antimicrobial peptide from the natural vector of T. cruzi, we tested the N-terminal region (termed P6) of the reduviid peptide trialysin (6, 7) (Fig. 1, C and D). We observed a dose-dependent increase in the reductase activity to P6-trialysin that was more robust (∼2.5-fold greater) than that of CAMEL at the same concentration. The conversion of MTT to formazan in the reductase assay is due to dehydrogenases and cofactors, NADH, and succinate from endoplasmic reticulum and/or mitochondria (22, 23). Enhancement of reductase activity is related to increased production of these co-factors, which is also crucial to ATP production, both of which occur only in viable parasites. Washing and reconstitution in fresh medium of parasite remnants from reactions with CAMPs alone did not result in their resumption of mobility or growth further, indicating that they were not viable. The residual MTT reductase activity in these reactions was present within or released from these nonviable parasites. To understand if the reductase response was part of an overall increase in parasite metabolic activity, we measured the total intracellular ATP produced by epimastigotes treated with these insect-derived CAMPs in the presence of conditioned medium. Both peptides stimulated the production of intracellular ATP from ∼4 (with CAMEL)- to ∼6-fold (with trialysin) compared with non-stimulated epimastigotes, reflecting overall “bio-energetic activation” (Fig. 1D). The preincubation of parasites with the oxidative phosphorylation uncoupler, carbonyl cyanide p-trifluoromethoxyphenylhydrazone, inhibited peptide-induced MTT-reductase response and ATP production, indicating that these are predominantly derived from mitochondrial activation (Fig. 1D, inset). The base-line bioenergetic activity of non-activated epimastigotes was partially affected by the inhibitor, whereas that from CAMP activation was completely ablated, indicating that parasites switched from mixed cytosolic and mitochondrial ATP production in resting parasites to predominately mitochondrial ATP production during CAMP activation.
FIGURE 1.
Insect CAMPs selectively induce parasite reductase activity and ATP production in conditioned medium. A, trypanosomal viability assay based on MTT reductase activity (9) in the presence of parasite-conditioned medium (CM), fresh non-conditioned parasite growth medium (NCM) at indicated amounts of insect-derived (CAMEL), and mammalian-CAMP (the cathelicidin, CRAMP) (60) in 100 μl. The effects of α-defensin (cryptdin-4) and β-defensin-3, the cathelicidin, protegrin-1, and amphibian-pexiganan (8) were similar to CRAMP and are not shown. B, cross-stimulation of CAMEL-induced reductase activity among different parasite strains (CL and Brazil (BR)) as indicated. Control reactions of MTT reductase activities of parasites in NCM or CM or alone were not significantly different (not shown). C, the effect of a P6 subfragment of trialysin (6) (P6-trialysin) (shown in μg) compared with CAMEL on parasite reductase activity. D, P6-trialysin and CAMEL treatment (3.5 μg each) in CM of the Brazil strain T. cruzi increased the ATP content ∼3.5–5-fold over that of cells in medium alone (no Rx); untreated cells in CM and NCM alone had similar ATP content (not shown). The inset table shows MTT reductase activity and ATP content of stationary-phase parasites in in vitro reactions with 3.5 μg of P6-trialysin in CM from Brazil strain compared with those in which parasites were preincubated for 30 min with the mitochondrial poison carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) (10 μm) before the assay. Parasites were treated under the indicated conditions for 2 h and then subjected to the MTT reductase assay, which is calculated based on % MTT conversion to formazan over 24 h compared with the untreated stationary-phase parasites in control reactions that retain full viability (taken as 100%). Activity is expressed as the mean % of untreated controls ± S.D. (n = 9 for experiments in panels A–C, n = 6 for those on panel D).
Secreted Cyclophilin in Conditioned Medium Is Responsible for Evasion of Trialysin Anti-parasitic Activity and Induction of Parasite Reductase Activity
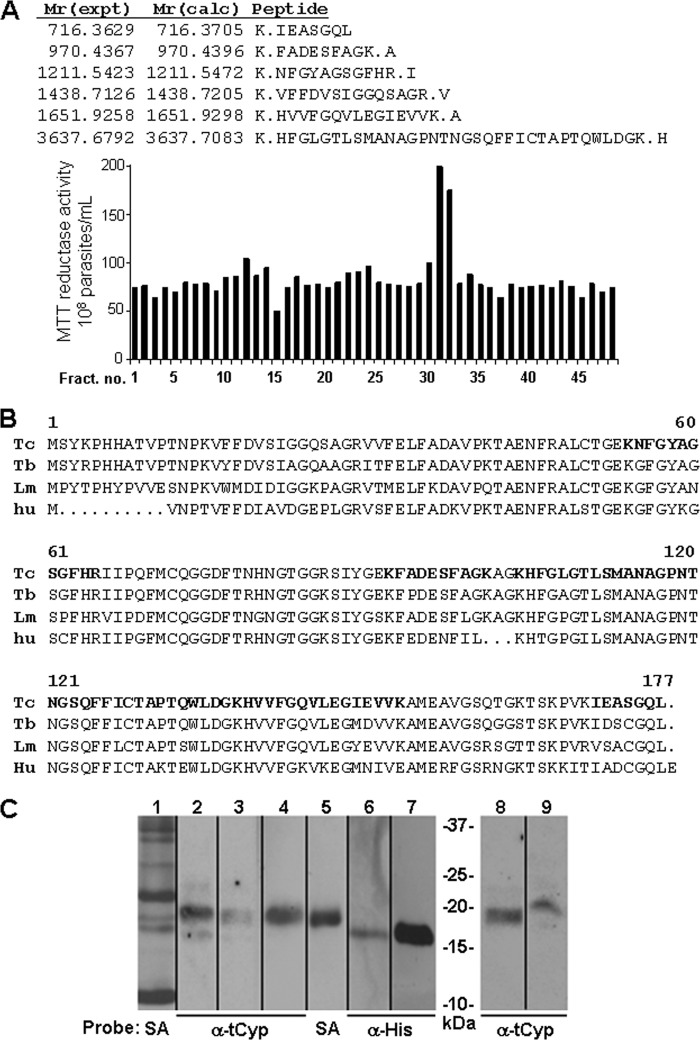
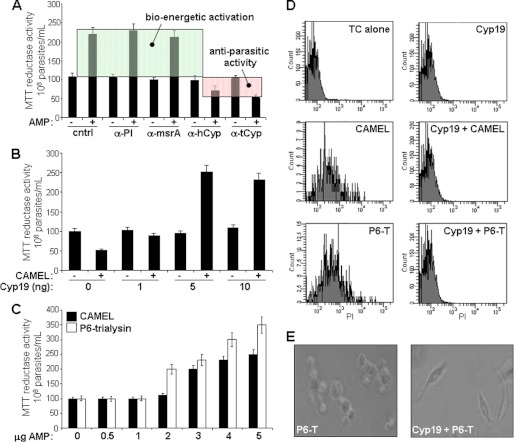
The stimulation of reductase activity and ATP production by viable epimastigotes exposed to trialysin only occurred in parasite-conditioned medium, suggesting that secreted products from parasites worked in synergy with the peptide. Analysis of conditioned-medium fractions from C-18 reverse-phase HPLC (Fig. 2A) showed that CAMP induced bioenergetic activity in clustered individual fractions that were susceptible to proteinase K-inactivation (not shown), indicating that secreted parasite proteins are critical to this effect. Mass spectrometric (MS) analysis of un-fractionated and active fractions showed that both contained T. cruzi cyclophilin 19 (Cyp19) (24), whereas inactive HPLC fractions did not. T. cruzi Cyp19 has orthologs in both T. brucei (14) and Leishmania (25) (Fig. 2B). Immunoblot analysis of cell lysates and conditioned medium from T. cruzi and L. amazonensis with anti-recombinant trypanosomal Cyp19 detected cyclophilin 19 (Fig. 2C). To test the physical interaction of CAMP and Cyp19, we prepared magnetic beads covalently linked with P6-trialysin for use in a Cyp19 pulldown analysis. Western blot analysis of pulldown reactions from T. cruzi-conditioned medium with P6-trialysin-containing beads detected Cyp19, as did control pulldown reactions using recombinant trypanosomal Cyp19 (shown for P6-trialysin beads in Figs. 2C and 5A). Blots of control pulldown reactions of beads alone or those containing bovine serum albumin (not shown) or a mutant P6-trialysin peptide (see Fig. 5A) did not identify Cyp19. Although MS analysis suggested that Cyp19 was important for T. cruzi bioenergetic activation and CAMP protection, the fractionation experiments did not result in fully purified protein. To further confirm the role of Cyp19 we performed immunodepletion experiments of conditioned medium using either anti-human-CypA or anti-trypanosomal cyclophilin 19 antibodies, which ablated both protection from CAMP parasiticidal activity and CAMP-induced MTT reductase activation (Fig. 3A). Immunoprecipitation with control anti-sera (preimmune and that to methionine sulfoxide reductase (26), a protein found co-isolated with Cyp19 in the MS experiments), did not change the MTT activity or CAMP protection. Last, an in vitro assay was developed using recombinant trypanosomal cyclophilin 19 and trialysin in non-conditioned defined Medium 199, which showed that this protein worked synergistically with either CAMP to induce parasite bio-energetic activation (Fig. 3, B and C) and protected epimastigotes from their cytolytic effects (Fig. 3, D and E). Dose-response analysis indicated that the molar ratio of trialysin to Cyp19 leading to bio-energetic activation was ∼2200:1.
FIGURE 2.
Identification of secreted cyclophilin 19 in T. cruzi-conditioned medium. A, reverse-phase C-18 HPLC fractionation of conditioned medium identified fractions 32 and 33 containing proteins leading to enhanced parasite reductase activity. Mass spectrometry of unfractionated CM and the active HPLC fractions contained trypanosomal cyclophilin 19. B, shown is alignment of the cyclophilin 19 proteins from T. cruzi (Tc) (61), T. brucei (Tb) (14), and L. major (Lm) (25) with human cyclophilin A (hu). Bold residues correspond to the peptide sequences from the mass spectrometric data in panel A. C, Western blot analysis of trypanosomal and leishmanial cyclophilin 19. Lane 1, total secreted proteins of T. cruzi labeled with biotin and detected with streptavidin-horseradish peroxidase (HRP) (SA); Lanes 2 and 3, cell lysate and conditioned medium of T. cruzi (107 cells), respectively; lanes 4 and 5, pulldown of T. cruzi-conditioned medium-biotinylated proteins using immobilized-SA beads probed with trypanosomal cyclophilin 19 antibodies (α-tCyp) and streptavidin-HRP, respectively; lanes 6 and 7, immobilized magnetic P6-trialysin bead pulldown of recombinant tCyp19 and tCyp19 protein alone (1 μg used as control) probed with anti-His-tag antibodies (α-His), respectively; lanes 8 and 9, cell lysate and conditioned medium of L. amazonensis promastigotes (from 107 cells) probed with α-tCyp19 antibodies. Comparative densitometry analysis of signals from lanes 2 and 3 and of 8 and 9 indicate that the conditioned medium contains 25–30% of total cellular Cyp19.
FIGURE 5.
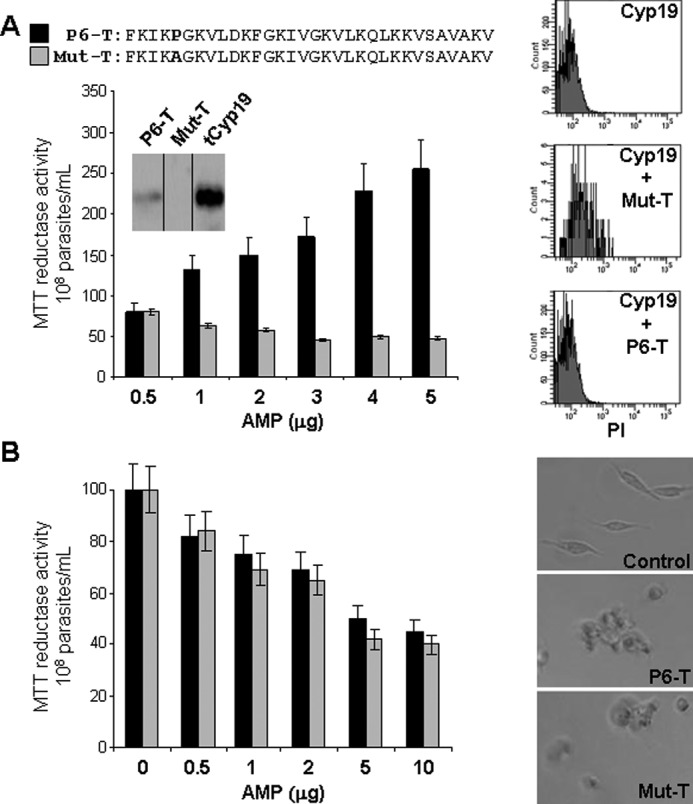
Cyclophilin evasion of antimicrobial peptide killing and bioenergetic activation is dependent on the proline residue within trialysin. A, left panel, a wild type and the proline replacement mutant of P6-trialysin and Mut-T (black and gray bars, respectively, as indicated); a comparison for parasite reductase activity shows that the presence of proline is crucial for induction of Cyp19-mediated parasite reductase activity and survival. Inset, anti-trypanosomal Cyp19 Western blots of recombinant trypanosomal Cyp19 pulldown reactions of Dynabeads containing either WT-P6-trialysin or Mut-P6-trialysin (Mut-T). The results indicate that only the WT-P6-trialysin, but not the mutant, is able to bind Cyp19 protein. Right panel, propidium iodide (PI) flow cytometric viability assay of parasites treated with P6-T or Mut-T in the presence of Cyp19 (as indicated) shows that only parasites treated with the Mut-T but not P6-T leads to decreased viability. B, Mut-T and P6-trialysin are equally trypanocidal in non-conditioned medium. Treatment of stationary phase epimastigotes (107 in 100 μl of fresh M199 for 2 h) with the indicated amounts of either P6-T or Mut-T analyzed by MTT reductase activity (left panel) or light microscopy (right panel). Both peptides cause parasites to lose their trypanosome morphology and clump under non-Cyp19-containing conditions. Reductase activity is expressed as % MTT reductase activity of CAMP-untreated (0 peptide control) parasites ± S.D., in M199 which was 100%.
FIGURE 3.
Secreted cyclophilin 19 mediates insect CAMP-dependent activation of parasite reductase activity and evasion of anti-microbial activity. A, parasite reductase assays with T. cruzi-conditioned M199 after treatment with the indicated antisera: preimmune (PI), methionine sulfoxide reductase (msrA), and human-CypA and trypanosomal Cyp19. − and + indicate in the presence or absence of CAMEL, respectively. The shaded green area indicates induction of parasite reductase activity (termed bioenergetic activation), which correlates with the increase in ATP production (see Fig. 1); the red shaded area reflects the reduced reductase activity attributable to the presence of non-viable parasites. B, standardization of CAMP-induced reductase assay using recombinant trypanosomal Cyp19 (0–10 ng) with 5 μg of CAMEL in M199 (see Fig. 1 for conditions). C, optimization of in vitro parasite reductase assays with CAMEL and trialysin P-6 with constant rCyp19 concentration (5 ng). Values are the mean % ± S.D. (n = 9 for experiments in panels A–C). D, propidium fluorescence flow cytometric analysis of parasites treated with the indicated conditions for 30 min in the presence of propidium iodide and analyzed using flow cytometry for the penetration of propidium iodide into non-viable cells. Analysis of cells at up to 12 h shows no appreciable difference in propidium iodide penetration. E, light microscopy of parasites treated with insect CAMP with and without Cyp19 protection; CAMP alone induces loss of motility and morphologic changes including loss of typical elongated cell shape and causes clumping of nonviable parasites.
Cyclophilin 19-trialysin Bio-energetic Activation of T. cruzi Occurs through Induction of Calcineurin Phosphatase
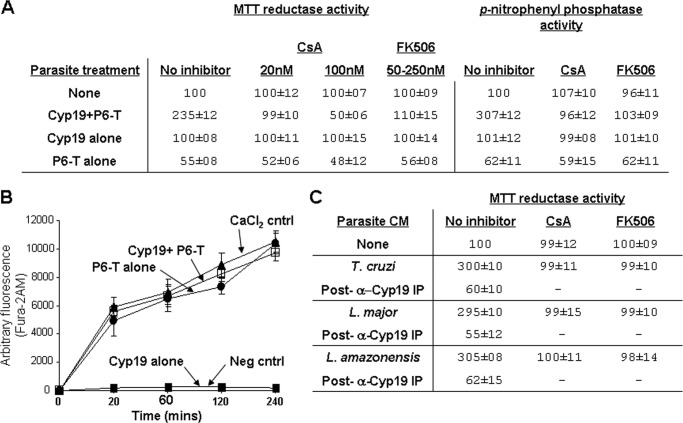
Cyclophilin 19 is a peptidyl-prolyl isomerase that, like other cyclophilins, is inhibited by the immunosuppressant cyclosporine A (CsA) (27). Preincubation of Cyp19 with 20 nm CsA before trialysin addition in our in vitro assay inhibited Cyp19-trialysin-mediated bio-energetic parasite activation, whereas 100 nm CsA potentiated trialysin parasite toxicity (Fig. 4A). Neither concentration of CsA was toxic to parasites, allowing them retain their bio-energetic activity in control reactions. CsA did not affect the parasiticidal activity of trialysin in the absence of Cyp19. The differential effects of CsA at different concentrations in our assay suggested that the action of CsA occurs at more than one target. This is consistent with the dual actions of CsA as an inhibitor of cyclophilin peptidyl-prolyl isomerase activity and as an inhibitor of calcineurin (CaN) phosphatase within a CsA-cyclophilin complex. To test whether CaN is involved in our pathway, we used another CaN inhibitor, FK506, which when bound to its cognate immunophilin, FK-binding protein (FKBP), also inhibits CaN phosphatase. Preincubation of epimastigotes with as little as 50 nm, but as high as 250 nm FK506, completely inhibited Cyp19-trialysin MTT reductase activation (Fig. 4A). Increasing the FK506 concentration as much as 5-fold was not toxic to parasites either in the presence or absence of trialysin. The inhibition of the MTT reductase activation with FK506 and CsA indicated that CaN is a crucial mediator of the bioenergetic pathway induced by Cyp19-trialysin. To further show that CaN activation was important in this pathway, we measured the phosphatase activity of soluble lysates prepared from parasites treated with Cyp19-trialysin using p-nitrophenyl phosphate (a well known substrate for CaN phosphatase). The phosphatase activity from these lysates was ∼3-fold over base-line activity of untreated epimastigotes. Pretreatment of epimastigotes with either CsA or FK506, both highly specific inhibitors of CaN, before exposure to Cyp-19-trialysin completely ablated p-nitrophenyl phosphate hydrolysis, indicating that the phosphatase activity induced in our assay was specifically due to CaN activation. Because CaN activation can be related to increases in intracellular calcium, we tested whether Cyp19-trialysin treatment of epimastigotes led to a difference in the extent, or kinetics, of intracellular Ca2+ levels compared with that of Cyp19 or trialysin alone (Fig. 4B). Analysis of Ca2+ increase in Fura-2AM-loaded parasites over a 4-h period indicated that treatment with either Cyp19-trialysin or trialysin alone led to a nearly identical increase in both the extent and kinetics of intracellular Ca2+, but Cyp19 treatment alone did not. This was similar in assays using M199 (which contains 1.8 mm CaCl2) as well as in PBS (not containing supplemental Ca2+), indicating that the increase is from the release of Ca2+ from intracellular stores. Controls in which we added additional CaCl2 (10 mm) to parasites led to an increase similar to that induced by Cyp19-trialysin or trialysin alone. Because CaN activation only occurs in parasites treated with Cyp19-trialysin (Fig. 4A), this indicates that an increase in Ca2+ alone is not the sole activator of CaN in our assays.
FIGURE 4.
Cyclophilin 19 induces parasite calcineurin phosphatase activation that is dependent on the proline residues within trialysin. A, MTT reductase activity is inhibited by calcineurin inhibitors CsA and FK506. This correlates with the induction of p-nitrophenyl phosphate phosphatase activity in parasite extracts that is also susceptible to inhibition with both inhibitors. B, analysis of intracellular Ca2+ flux induced using Fura-2AM-loaded epimastigotes indicates that Cyp19+P6-trialysin and P6-trialysin alone cause the same kinetics of intracellular Ca2+ increase, whereas Cyp19 treatment does not. Assays were done in non-conditioned M199. For the positive control, medium was supplemented with 10 mm CaCl2. Assays done in PBS in place of M199 showed similar results. Assays were done over 6 h; no significant increase was observed after 4 h. The mean values of arbitrary fluorescence ± S.D. of assays done in triplicate are shown. C, CM from T. cruzi, L. major, and L. amazonensis (as indicated) was used for reductase assays of T. cruzi using WT P6-trialysin. Cyclophilin 19 immunoprecipitation of each medium depleted the reductase activation of parasites and provided a control for Cyp19 specificity. Preimmune serum (PI) had no effect on the ATP content of activated parasites, which correlated with the reductase activity similar to that shown in Fig. 1. CsA (10 nm) and FK506 (250 nm) preincubation of parasites for 30 min inhibits parasite reductase activity induced by all CM. CsA and FK506 alone is not toxic to parasites (as indicated). Dashes indicate that reactions were not done. MTT reductase activity is expressed as the mean % of untreated controls ± S.D. (n = 9 in panels A and C, n = 6 in panel B).
Because the secretome of Leishmania contains cyclophilin (28), we tested whether this protein in conditioned medium of L. major or L. amazonensis could act similarly to that of T. cruzi Cyp19. The conditioned medium from cultures of L. major and L. amazonensis promastigotes both protected T. cruzi from killing by P6-trialysin and induced the T. cruzi reductase activity (Fig. 4C). Immunodepletion of each medium with anti-Cyp19 antibodies ablated both activities, indicating the specificity of these for cyclophilin. In addition, these effects were inhibited by pretreatment with either CsA or FK506 (Fig. 4C), further indicating that CaN activation is crucial.
Cyclophilin 19-mediated Antimicrobial Peptide Evasion and Bio-energetic Activation Is Dependent on Proline Residues in Trialysin
Since cyclophilins are enzymes that bind to peptides containing proline residues, we tested the specificity of the proline in P6-trialysin for binding cyclophilin 19 and evasion of parasiticidal killing and downstream bio-energetic activation. For this, we utilized the in vitro assay system with recombinant Cyp19 (shown in Fig. 3, B and C) to compare wild type P6-trialysin to that of a mutant peptide in which the proline residue was changed to alanine (designated Mut-T in Fig. 5A). Unlike the wild type peptide, the mutant peptide remained highly parasiticidal and did not induce bioenergetic activity in the presence of Cyp19 as measured using propidium iodide flow cytometry (Fig. 5A, right panel). This correlated with the inability of the cyclophilin 19 to bind to the mutant peptide in our magnetic bead pulldown assay (Fig. 5A, inset), indicating that the proline is crucial for direct interaction of cyclophilin 19 with trialysin and for triggering downstream bioenergetic activation. Secondary structure modeling of both peptides showed that mutation at proline in the mutant peptide does not change the extent of α-helicity (not shown). In addition, the mutant peptide was as equally parasiticidal as the wild type peptide (Fig. 5B) in non-Cyp19 containing conditions, indicating the mutation had no effect on the parasiticidal activity. This was also the case for the mutant peptide in presence of L. major and L. amazonensis-conditioned medium (Fig. 6A), indicating that the proline residue in trialysin is also critical for the interaction with leishmanial Cyp19 and that the cyclophilins secreted from these parasites function similarly in the context of T. cruzi epimastigotes (Figs. 4C and 6A). Interestingly, the secreted cyclophilins from T. cruzi and Leishmania protected leishmanial promastigotes from P6-trialysin killing but did not induce bioenergetic activity (Fig. 6B), indicating that the metabolic effects on T. cruzi by Cyp19-trialysin may be ligand- and/or parasite-specific.
FIGURE 6.
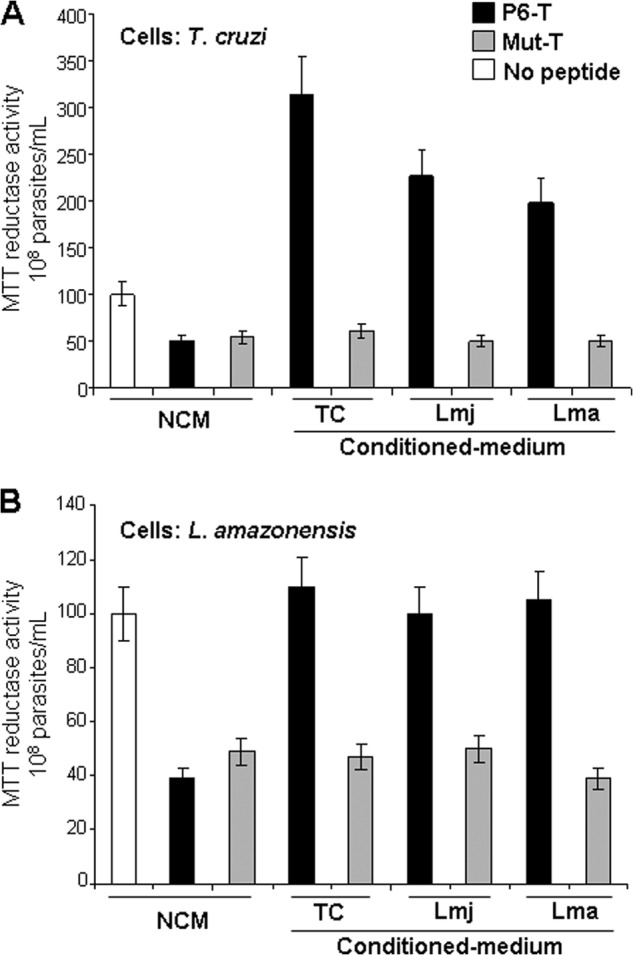
Leishmanial-conditioned medium neutralizes trialysin-mediated killing of T. cruzi and Leishmania but shows selective bio-energetic activation for T. cruzi. A, the conditioned medium from T. cruzi (TC) epimastigotes, L. amazonensis (Lma) and L. major (Lmj) promastigotes (as indicated) protect T. cruzi parasites from the parasiticidal effects of P6-trialysin and induce reductase activity (also shown in Fig. 4C), yet they have no effect on the neutralization of the Pro → Ala mutant of trialysin (Mut-T). Black and gray bars indicate WT and mutant trialysin peptides, respectively, compared with untreated control cells (white). B, the effect of P6-T on L. amazonensis with different conditioned-M199 shows that conditioned medium protects parasites from P6-T (black)- but not Mut-T (gray)-induced killing but does not induce reductase activation. The same trends were found using recombinant tCyp19 (not shown). Non-conditioned M199 (NCM) was used as a control (white).
Cyclophilin-trialysin Enhances T. cruzi Binding and Intracellular Growth in Host Cells
Metabolic activation of T. cruzi with a variety of stimuli can increase infectivity in vitro and drive metacyclogenesis (3, 29). We tested whether the effects of cyclophilin-trialysin could serve as a trigger for parasite infectivity. Epimastigotes treated with P6-trialysin and cyclophilin 19 or conditioned medium had increased expression of the metacyclic-specific protein, MET-III (Fig. 7A) (20, 21). CFSE dilution flow cytometric analysis of Cyp19-containing medium and trialysin indicated that these epimastigotes ceased replication, whereas controls of epimastigotes treated with Cyp19-containing conditioned medium alone did not (Fig. 7B). The lack of replication of these treated parasites is reminiscent of epimastigote differentiation into metacyclic trypomastigotes (29). Epimastigotes pretreated with either conditioned medium-P6-trialysin or Cyp19-P6-trialysin remained morphologically as epimastigotes yet had enhanced binding and invasion of rat heart myoblast, resulting in more robust intracellular infection (shown for P6-trialysin in Fig. 7C). Parasite-host cell binding assays tested more than 120 min showed that those treated with conditioned medium or Cyp19 together with P6-trialysin had 3–5-fold higher binding to fixed host cells than controls with the CAMP or Cyp19/conditioned medium alone (Fig. 7C, top panel). Fluorescence microscopic analysis of host cell entry using rhodamine-phalloidin showed actin commonly overlying intracellular parasites treated with Cyp19-trialysin to a greater degree than controls (Fig. 7C, lower panel), confirming that these parasites bound to and also entered host cells. We did not observe parasites housed in actin-surrounded vacuoles. Infection was allowed to progress over 10 days followed by Giemsa staining to visualize the development of intracellular infection (Fig. 7D). The development of amastigotes was more robust for those treated with conditioned medium and trialysin, showing a higher percentage of infected host cells and a higher average number of intracellular parasites than in controls. Thus the pretreatment of epimastigotes with trialysin in the presence of Cyp19 or that contained in conditioned medium leads to their enhanced binding to and invasion of host cells and to more robust intracellular infection.
FIGURE 7.
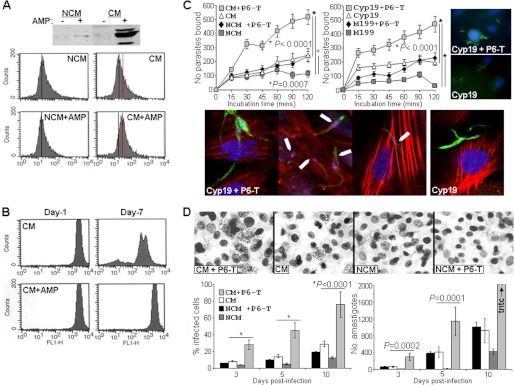
Insect AMP induces metacyclic-specific MET-III expression, halts parasite replication, and enhances parasite binding and intracellular growth in host cells. A, anti-MET-III Western blot analysis (upper panel) and flow cytometric analysis of methanol-permeabilized Brazil strain parasites (lower panel) treated with secreted proteins in CM or NCM with (+) and without (-) insect AMP (shown for CAMEL; P6-trialysin gave similar results). Black vertical lines in panel b represent base-line fluorescence, and the red line denotes an increase in fluorescence. B, CFSE-loaded epimastigotes treated with CM or CM and insect AMP (as indicated) show diminished fluorescence, indicating cell replication in CM-treated cells. Parasites in NCM replicate, whereas those in NCM and insect AMP have reduced viable parasites (not shown). C, for parasite binding assays CFSE-labeled T. cruzi epimastigotes were treated with proteins for 72 h before being applied for the indicated times (up to 120 min) to paraformaldehyde-fixed rat heart myoblasts pre-grown on coverslips, washed three times, and then fixed. Bound parasites were enumerated microscopically throughout coverslips (graphs in the upper panel). To the right are representative images using CFSE-labeled parasites treated under the indicated conditions. To analyze invasion, T. cruzi epimastigotes were treated as indicated and used to infect rat heart myoblasts pre-grown on coverslips for 2 h, then non-cell-associated parasites were removed by washing, and preparations were fixed, permeabilized, and stained with rhodamine labeled phalloidin. Enhanced invasion of CFSE-labeled parasites (green) treated with Cyp19 and trialysin underneath intracellular host cell actin (red) (indicated with white arrows in some panels) is shown. D, intracellular infection was assessed by light microscopy of fixed, Giemsa-stained preparations (the upper panel shows a representative field from day 5 post-infection). At least 20 high powered fields of each treatment were analyzed for % of infected cells (lower left graph) and total number of amastigotes per culture (lower right graph) at 3, 5, and 10 days post-infection with Brazil strain T. cruzi. Similar results were also seen using CL and Dm28 strains. Each experiment was performed three times with comparable results. Each value represents the mean ± S.D. (n = 9 for panel C, n = 6 for panel D). Amastigotes differentiated into trypomastigotes and exited the cells normally (not shown).
DISCUSSION
Our results describe a novel strategy in which secreted T. cruzi cyclophilin neutralizes insect CAMP, enhancing parasite survival and driving bioenergetic mitochondrial-driven ATP production and enhanced infectivity. In our model (Fig. 8) we envision that replicating insect-stage parasites secrete cyclophilin 19 as they migrate through the reduviid gastrointestinal tract. As parasites encounter CAMP, extracellular cyclophilin 19 binds to and isomerizes peptide neutralizing its anti-parasitic activity. The cyclophilin 19-CAMP complex may associate with the parasite surface or be trans-located intracellularly to trigger calcineurin phosphatase activity. This leads to downstream events, which increase intracellular ATP levels leading to enhanced infectivity. These findings represent one of the few descriptions of specific stimuli that enhance infectivity of T. cruzi and describe a defined host molecule-based environmental sensing paradigm in this group of organisms.
FIGURE 8.
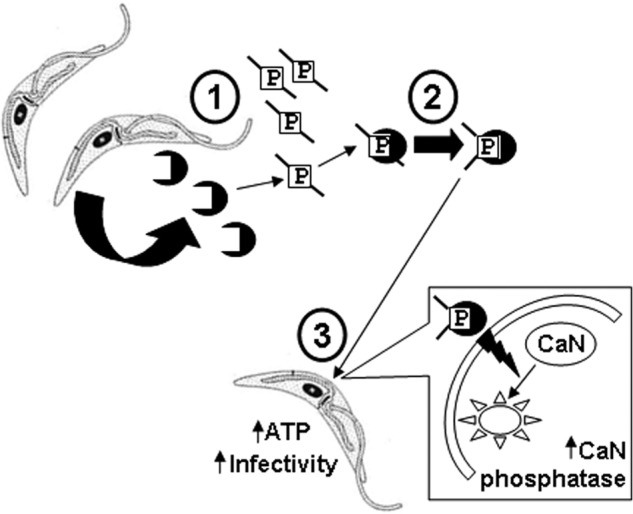
Model of secreted cyclophilin-mediated sensing and adaptation to insect CAMP. Step 1, replicating T. cruzi epimastigotes secrete cyclophilin 19 extracellularly, which binds to insect host proline-containing CAMP, such as trialysin (indicated with P surrounded by a box) (see Figs. 1 and 2). Step 2, the binding and proline isomerization of trialysin inactivates the CAMP and protects parasites from trypanocidal action. Step 3, cyclophilin 19-trialysin complexes feed back on parasites by action on the membrane to trigger intracellular calcineurin phosphatase activation (large box with lightening bolt) (see Fig. 3). This leads to the downstream metabolic responses of increased ATP and reductase activity, stoppage of cell division, and MET-III expression (see Fig. 4), which contributes to enhanced parasite infectivity. We hypothesize that the interaction of cyclophilin 19 with other protein ligands, such as proline-containing defensin-like peptides (5, 62, 63) or α-globin (1, 29, 51), may also contribute to aspects of in vivo parasite differentiation.
Cyclophilins are highly conserved cis/trans isomerases of peptidyl-prolyl bonds, which facilitate protein-folding in many subcellular locations and can also modulate protein functionality (30). The molar ratio of trialysin to cyclophilin in our in vitro assays at which bioenergetic activation occurs is ∼2200:1 (Fig. 3E), which is reasonable to describe an substrate-enzyme relationship. The effects of cyclophilin on trialysin are specific to the proline residue, as replacement of this residue ablated both the ability of the enzyme to neutralize the parasiticidal effect of the peptide and the bioenergetic effect of the Cyp19-peptide complex. Secreted T. cruzi cyclophilin alters the microbicidal effect of trialysin, akin to the effect of amphibian peptidyl-prolyl isomerase on proline-containing CAMPs (31). The lower energy trans conformation of prolyl residues is found in the majority of peptides and proteins (32). The cis conformation, when found, is dependent on the identity of flanking amino acid residues, with aromatic residues tryptophan and tyrosine and small nonpolar residues at the −1 position favoring the increase possibility of cis prolyl conformation (33, 34). The sequences surrounding the proline in P6-trialysin predict that the proline residue adopts a trans conformation, and isomerization of this peptide would increase the proportion of cis forms of the peptide. Because the conversion of trans to cis forms can influence peptide functionality, this may account for diminishment of parasite killing we observe and may also be important for stimulation of calcineurin signaling and bioenergetic response by the Cyp19-trialysin complex.
The T. cruzi genome contains genes encoding 15 cyclophilin orthologs at least 4 of which bind to and are inhibited by cyclosporine A, including cyclophilin 19 (24, 35), yet until now the function of individual parasite cyclophilins has not been determined. Numerous cyclophilin genes are also present in the related parasites Leishmania (36) and T. brucei (14, 24, 37), and cyclophilin 19 homologs of that secreted from T. cruzi are also secreted from these parasites (Fig. 2B) (14, 28). The closet mammalian homolog to cyclophilin 19 is human cyclophilin A (Fig. 2B), which is secreted extracellularly in response to oxidative stress and inflammation, where it acts as a growth factor for vascular smooth muscle and macrophages acting through ERK1/2 intracellular signaling both in an autocrine and paracrine fashion (38). Several cyclophilin and cyclophilin-like proteins of Plasmodium falciparum have been expressed recombinantly (39). Heat treatment of these proteins appears to ablate their ability to bind to CsA, indicative of changes in protein conformation. In our case the heating conditioned medium containing Cyp19 to 65 °C for 15 min did not affect its ability to increase MTT activity in association with CAMP (not shown). Whether this is related to differences in the properties of native T. cruzi Cyp19 and those of recombinant Plasmodium Cyps or a dissociation of CsA binding and peptidyl-prolyl isomerase activity is unknown. The mechanism of CypA secretion is largely unknown, although it may involve vesicular transport (40). The related cyclophilin B can also be secreted via targeting through the endoplasmic reticulum with an N-terminal signal sequence (41, 42). It is unknown if Cyp19 is destined for secretion using an N-terminal signal sequence, although it possesses a 10-residue N-terminal region not found in human CypA (Fig. 2B). This region does not resemble a classical signal peptide, but may be important for regulation of cyclophilin 19 function.
We speculate that secreted trypanosomal and leishmanial cyclophilin 19 may modify host proteins at various lifecycle steps of these parasites. Our data indicate that secreted leishmanial cyclophilin protects Leishmania from trialysin killing in a proline-dependent manner (Fig. 6) and can synergize with trialysin to metabolically activate T. cruzi. It is unclear whether cyclophilin may be secreted from other stages of T. cruzi such as intracellular amastigotes or bloodstream trypomastigotes. In these locations there are numerous potential host protein substrates that cyclophilin could bind and modify. Secretion of cyclophilin by amastigotes within parasitophorous vacuoles or those replicating within the host cytoplasm may act on host proteins to trigger the escape of parasites from parasitophorous vacoules or their differentiation into trypomastigotes before host cell escape.
Our report is the first to document calcineurin activation in trypanosomatid protozoa and shows that calcineurin intracellular signaling is necessary for the downstream bioenergetic activation and implicates this signaling pathway in environmental adaptation, similar to that recently found in Leishmania (43) and infectivity of T. cruzi (15). In addition, activation of calcineurin has been linked with growth rate responses due to antimicrobial peptide treatment of Candida albicans (44). How cyclophilin-trialysin complexes trigger parasite calcineurin is unknown. Calcium binding of regulatory proteins, calmodulin and calcineurin B, are known activators of the phosphatase domain of calcineurin A (45–47). We found both the cyclophilin-trialysin complexes and trialysin alone increase intracellular calcium, whereas cyclophilin treatment does not. It may be that despite the calcium increase caused by trialysin alone, calcineurin activation is dysfunctional in these non-viable parasites. However, control reactions of parasites treated with calcium or either protein alone did not result in calcineurin activation, indicating that an increase in intracellular calcium alone is not sufficient for calcineurin activation but that calcium increase together with a second trigger is necessary. The association of cyclophilin with trialysin may result in the exposure or creation of cryptic domains in either protein or a gain-of-function by isomerized trialysin, resulting in receptor engagement. Alternatively, cyclophilin-trialysin complexes or isomerized trialysin may penetrate parasites acting on intracellular targets such as calcineurin subunits where intracellular calcium might serve as an additional co-factor. T. cruzi has genes encoding A and B subunits of calcineurin; the A subunit contains the phosphatase domain, whereas the B subunit functions in regulation of the A subunit (15, 48). Interestingly, the A subunit of T. cruzi calcineurin lacks the calmodulin and autoinhibition domains found in mammalian counterparts, supporting the idea that interaction with the regulatory B subunit may lead to subunit A activation, similar to that in Neurospora where calcineurin activation is part of stress adaptation and stage differentiation (49). We predict that the membrane action of cyclophilin-trialysin leads to the activation of the calcineurin A subunit (Fig. 8) perhaps by inducing its association with subunit B.
The increase in MTT reductase activity and ATP production reflective of heightened metabolic activity of stress adaptation of parasites to CAMP is reminiscent of previous findings of the effect of cecropin on Leishmania (50). CAMP enhances epimastigote infectivity and may be one of several environmental signals parasites encounter during transit through the insect alimentary tract, which may additively or synergistically affect infectivity. Past work has shown that certain proline-containing subfragments of α-globin stimulate T. cruzi adenylate cyclase activity, contributing to metacyclogenesis and increased parasite infectivity (1, 29, 51). We hypothesize that recognition of the active α-globin peptides with secreted Cyp19 may have been crucial to parasite signaling and infectivity in that system. Other reduviid defensin-like CAMPs, which are less well characterized than trialysin, may also interact with cyclophilin. These defensins in both Triatoma and Rhodnius species have a singular conserved proline reside at position 15 of the predicted mature peptides that is not found within related defensins from other species such as Drosophila, Aedes aegypti (vector for malaria), or Anopheles gambiae (vector for the African trypanosome). Difficulty in the production of these complex intra-disulfide-bonded insect defensins has so far precluded us from testing the hypothesis of whether cyclophilin 19 can bind to these peptides in a proline-specific manner.
In our system, CAMP-Cyp19 increases MET-III expression and stops parasite replication, both features of metacyclic-stage parasites, yet they remain morphologically as epimastigotes. They have enhanced binding to host cells, which implies they may have increased expression of ligands for binding host receptors, such as gp82 (3, 52) or the down-regulation of anti-infective proteins such as gp90 (53) or may enhance the host signaling pathways important for infection (54–56). Within the natural environment of the reduviid intestinal tract, parasites encounter several disparate stressors such as changes in ionic conditions, α-globin, and CAMP exposure, which probably culminate in full differentiation of parasites. Epimastigotes represent the predominant parasite population in the hindgut and rectum of the insect (57) and can infect non-phagocytic cells in vitro, which has implications for the role of this life stage in natural infection (58). We observed that epimastigotes treated with Cyp19-trialysin bound to and entered cells and created a productive infection which supports the idea that transition forms in parasite differentiation may contribute to the natural infection of mammalian cells (59). T. cruzi cyclophilin 19 is both cell-associated and secreted as it is Leishmania (28), and African trypanosomes (14) and the secreted forms neutralize insect antimicrobial peptide killing and can induce bioenergetic stimulation in T. cruzi. This supports the hypothesis that it may act as an extracellular sensor of other protein ligands in these related insect-borne protozoan parasites.
Acknowledgments
We thank Prof. John Kelly (London Tropical School of Medicine and Hygiene) for antibodies to MET-III and Drs. S. Dhandayuthapani (University of Texas San Antonio) and J. Moskovitz (University of Kansas) for α-MSR antisera.
This work was supported by the American Heart Association (to B. S. M) and The Ohio State University.
- CAMP
- cationic antimicrobial peptide
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- CM
- conditioned medium
- NCM
- non-conditioned medium
- Cyp19
- cyclophilin 19
- CsA
- cyclosporine A
- CaN
- calcineurin
- CAMEL
- cecropin-melittin hybrid peptide
- P6-T
- trialysin P6 subfragment
- Mut-T
- proline-alanine trialysin P6 mutant peptide
- MET-III
- Metacyclin III
- CFSE
- carboxyfluorescein succinimidyl ester.
REFERENCES
- 1. Garcia E. S., Gonzalez M. S., de Azambuja P., Baralle F. E., Fraidenraich D., Torres H. N., Flawiá M. M. (1995) Induction of Trypanosoma cruzi metacyclogenesis in the gut of the hematophagous insect vector, Rhodnius prolixus, by hemoglobin and peptides carrying α d-globin sequences. Exp. Parasitol. 81, 255–261 [DOI] [PubMed] [Google Scholar]
- 2. Krassner S. M., Granger B., Phermsangngnam P., Le T., Linden V. (1990) Further studies on substrates inducing metacyclogenesis in Trypanosoma cruzi. J. Protozool. 37, 128–132 [DOI] [PubMed] [Google Scholar]
- 3. Martins R. M., Covarrubias C., Rojas R. G., Silber A. M., Yoshida N. (2009) Use of l-proline and ATP production by Trypanosoma cruzi metacyclic forms as requirements for host cell invasion. Infect. Immun. 77, 3023–3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lehrer R. I., Barton A., Daher K. A., Harwig S. S., Ganz T., Selsted M. E. (1989) Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J. Clin. Invest. 84, 553–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lopez L., Morales G., Ursic R., Wolff M., Lowenberger C. (2003) Isolation and characterization of a novel insect defensin from Rhodnius prolixus, a vector of Chagas disease. Insect Biochem. Mol. Biol. 33, 439–447 [DOI] [PubMed] [Google Scholar]
- 6. Martins R. M., Sforça M. L., Amino R., Juliano M. A., Oyama S., Jr., Juliano L., Pertinhez T. A., Spisni A., Schenkman S. (2006) Lytic activity and structural differences of amphipathic peptides derived from trialysin. Biochemistry 45, 1765–1774 [DOI] [PubMed] [Google Scholar]
- 7. Amino R., Martins R. M., Procopio J., Hirata I. Y., Juliano M. A., Schenkman S. (2002) Trialysin, a novel pore-forming protein from saliva of hematophagous insects activated by limited proteolysis. J. Biol. Chem. 277, 6207–6213 [DOI] [PubMed] [Google Scholar]
- 8. Kulkarni M. M., McMaster W. R., Kamysz E., Kamysz W., Engman D. M., McGwire B. S. (2006) The major surface-metalloprotease of the parasitic protozoan, Leishmania, protects against antimicrobial peptide-induced apoptotic killing. Mol. Microbiol. 62, 1484–1497 [DOI] [PubMed] [Google Scholar]
- 9. McGwire B. S., Olson C. L., Tack B. F., Engman D. M. (2003) Killing of African trypanosomes by antimicrobial peptides. J. Infect. Dis. 188, 146–152 [DOI] [PubMed] [Google Scholar]
- 10. Díaz-Achirica P., Ubach J., Guinea A., Andreu D., Rivas L. (1998) The plasma membrane of Leishmania donovani promastigotes is the main target for CA(1–8)M(1–18), a synthetic cecropin A-melittin hybrid peptide. Biochem. J. 330, 453–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu A. Y., Destoumieux D., Wong A. V., Park C. H., Valore E. V., Liu L., Ganz T. (2002) Human β-defensin-2 production in keratinocytes is regulated by interleukin-1, bacteria, and the state of differentiation. J. Invest. Dermatol. 118, 275–281 [DOI] [PubMed] [Google Scholar]
- 12. McGwire B. S., Kulkarni M. M. (2010) Interactions of antimicrobial peptides with Leishmania and trypanosomes and their functional role in host parasitism. Exp. Parasitol. 126, 397–405 [DOI] [PubMed] [Google Scholar]
- 13. Kulkarni M. M., McMaster W. R., Kamysz W., McGwire B. S. (2009) Antimicrobial peptide-induced apoptotic death of Leishmania results from calcium-dependent, caspase-independent mitochondrial toxicity. J. Biol. Chem. 284, 15496–15504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pellé R., McOdimba F., Chuma F., Wasawo D., Pearson T. W., Murphy N. B. (2002) The African trypanosome cyclophilin A homologue contains unusual conserved central and N-terminal domains and is developmentally regulated. Gene 290, 181–191 [DOI] [PubMed] [Google Scholar]
- 15. Araya J. E., Cornejo A., Orrego P. R., Cordero E. M., Cortéz M., Olivares H., Neira I., Sagua H., da Silveira J. F., Yoshida N., González J. (2008) Calcineurin B of the human protozoan parasite Trypanosoma cruzi is involved in cell invasion. Microbes Infect. 10, 892–900 [DOI] [PubMed] [Google Scholar]
- 16. Pallen C. J., Wang J. H. (1983) Calmodulin-stimulated dephosphorylation of p-nitrophenyl phosphate and free phosphotyrosine by calcineurin. J. Biol. Chem. 258, 8550–8553 [PubMed] [Google Scholar]
- 17. Pallen C. J., Wang J. H. (1984) Regulation of calcineurin by metal ions. Mechanism of activation by Ni2+ and an enhanced response to Ca2+/calmodulin. J. Biol. Chem. 259, 6134–6141 [PubMed] [Google Scholar]
- 18. McGwire B. S., O'Connell W. A., Chang K. P., Engman D. M. (2002) Extracellular release of the glycosylphosphatidylinositol (GPI)-linked Leishmania surface metalloprotease, gp63, is independent of GPI phospholipolysis. Implications for parasite virulence. J. Biol. Chem. 277, 8802–8809 [DOI] [PubMed] [Google Scholar]
- 19. Perkins D. N., Pappin D. J., Creasy D. M., Cottrell J. S. (1999) Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20, 3551–3567 [DOI] [PubMed] [Google Scholar]
- 20. Gluenz E., Taylor M. C., Kelly J. M. (2007) The Trypanosoma cruzi metacyclic-specific protein Met-III associates with the nucleolus and contains independent amino and carboxyl terminal targeting elements. Int. J. Parasitol. 37, 617–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yamada-Ogatta S. F., Motta M. C., Toma H. K., Monteiro-Goes V., Avila A. R., Muniz B. D., Nakamura C., Fragoso S. P., Goldenberg S., Krieger M. A. (2004) Trypanosoma cruzi. Cloning and characterization of two genes whose expression is up-regulated in metacyclic trypomastigotes. Acta Trop. 90, 171–179 [DOI] [PubMed] [Google Scholar]
- 22. Berridge M. V., Herst P. M., Tan A. S. (2005) Tetrazolium dyes as tools in cell biology. New insights into their cellular reduction. Biotechnol. Annu. Rev. 11, 127–152 [DOI] [PubMed] [Google Scholar]
- 23. Berridge M. V., Tan A. S. (1993) Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). Subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch. Biochem. Biophys. 303, 474–482 [DOI] [PubMed] [Google Scholar]
- 24. Búa J., Aslund L., Pereyra N., García G. A., Bontempi E. J., Ruiz A. M. (2001) Characterization of a cyclophilin isoform in Trypanosoma cruzi. FEMS Microbiol. Lett. 200, 43–47 [DOI] [PubMed] [Google Scholar]
- 25. Rascher C., Pahl A., Pecht A., Brune K., Solbach W., Bang H. (1998) Leishmania major parasites express cyclophilin isoforms with an unusual interaction with calcineurin. Biochem. J. 334, 659–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arias D. G., Cabeza M. S., Erben E. D., Carranza P. G., Lujan H. D., Téllez Iñón M. T., Iglesias A. A., Guerrero S. A. (2011) Functional characterization of methionine sulfoxide reductase A from Trypanosoma spp. Free Radic. Biol. Med. 50, 37–46 [DOI] [PubMed] [Google Scholar]
- 27. Búa J., Ruiz A. M., Potenza M., Fichera L. E. (2004) In vitro anti-parasitic activity of cyclosporin A analogs on Trypanosoma cruzi. Bioorg. Med. Chem. Lett. 14, 4633–4637 [DOI] [PubMed] [Google Scholar]
- 28. Silverman J. M., Chan S. K., Robinson D. P., Dwyer D. M., Nandan D., Foster L. J., Reiner N. E. (2008) Proteomic analysis of the secretome of Leishmania donovani. Genome Biol. 9, R35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fraidenraich D., Peña C., Isola E. L., Lammel E. M., Coso O., Añel A. D., Pongor S., Baralle F., Torres H. N., Flawia M. M. (1993) Stimulation of Trypanosoma cruzi adenylyl cyclase by an α-d-globin fragment from Triatoma hindgut. Effect on differentiation of epimastigote to trypomastigote forms. Proc. Natl. Acad. Sci. U.S.A. 90, 10140–10144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lu K. P., Finn G., Lee T. H., Nicholson L. K. (2007) Prolyl cis-trans isomerization as a molecular timer. Nat. Chem. Biol. 3, 619–629 [DOI] [PubMed] [Google Scholar]
- 31. Miele R., Borro M., Mangoni M. L., Simmaco M., Barra D. (2003) A peptidylprolyl cis/trans isomerase from Xenopus laevis skin. Cloning, biochemical characterization and putative role in the secretion. Peptides 24, 1713–1721 [DOI] [PubMed] [Google Scholar]
- 32. Stewart D. E., Sarkar A., Wampler J. E. (1990) Occurrence and role of cis peptide bonds in protein structures. J. Mol. Biol. 214, 253–260 [DOI] [PubMed] [Google Scholar]
- 33. Lorenzen S., Peters B., Goede A., Preissner R., Frömmel C. (2005) Conservation of cis prolyl bonds in proteins during evolution. Proteins 58, 589–595 [DOI] [PubMed] [Google Scholar]
- 34. Pal D., Chakrabarti P. (1999) Cis peptide bonds in proteins. Residues involved, their conformations, interactions, and locations. J. Mol. Biol. 294, 271–288 [DOI] [PubMed] [Google Scholar]
- 35. Búa J., Fichera L. E., Fuchs A. G., Potenza M., Dubin M., Wenger R. O., Moretti G., Scabone C. M., Ruiz A. M. (2008) Anti-Trypanosoma cruzi effects of cyclosporin A derivatives. Possible role of a P-glycoprotein and parasite cyclophilins. Parasitology 135, 217–228 [DOI] [PubMed] [Google Scholar]
- 36. Yau W. L., Blisnick T., Taly J. F., Helmer-Citterich M., Schiene-Fischer C., Leclercq O., Li J., Schmidt-Arras D., Morales M. A., Notredame C., Romo D., Bastin P., Späth G. F. (2010) Cyclosporin A treatment of Leishmania donovani reveals stage-specific functions of cyclophilins in parasite proliferation and viability. PLoS Negl. Trop. Dis. 4, e729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dao-Thi M. H., Transue T. R., Pellé R., Murphy N. B., Poortmans F., Steyaert J. (1998) Expression, purification, crystallization, and preliminary X-ray analysis of cyclophilin A from the bovine parasite Trypanosoma brucei brucei. Acta Crystallogr. D. Biol. Crystallogr. 54, 1046–1048 [DOI] [PubMed] [Google Scholar]
- 38. Satoh K., Nigro P., Berk B. C. (2010) Oxidative stress and vascular smooth muscle cell growth. A mechanistic linkage by cyclophilin A. Antioxid. Redox Signal. 12, 675–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marín-Menéndez A., Bell A. (2011) Overexpression, purification, and assessment of cyclosporin binding of a family of cyclophilins and cyclophilin-like proteins of the human malarial parasite Plasmodium falciparum. Protein Expr. Purif. 78, 225–234 [DOI] [PubMed] [Google Scholar]
- 40. Suzuki J., Jin Z. G., Meoli D. F., Matoba T., Berk B. C. (2006) Cyclophilin A is secreted by a vesicular pathway in vascular smooth muscle cells. Circ. Res. 98, 811–817 [DOI] [PubMed] [Google Scholar]
- 41. Schumacher A., Westermann B., Osborn M., Nordheim A. (1994) The N-terminal signal peptide of the murine cyclophilin mCyP-S1 is required in vivo for ER localization. Eur. J. Cell Biol. 63, 182–191 [PubMed] [Google Scholar]
- 42. Fearon P., Lonsdale-Eccles A. A., Ross O. K., Todd C., Sinha A., Allain F., Reynolds N. J. (2011) Keratinocyte secretion of cyclophilin B via the constitutive pathway is regulated through its cyclosporin-binding site. J. Invest Dermatol. 131, 1085–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Naderer T., Dandash O., McConville M. J. (2011) Calcineurin is required for Leishmania major stress response pathways and for virulence in the mammalian host. Mol. Microbiol. 80, 471–480 [DOI] [PubMed] [Google Scholar]
- 44. Lis M., Liu T. T., Barker K. S., Rogers P. D., Bobek L. A. (2010) Antimicrobial peptide MUC7 12-mer activates the calcium/calcineurin pathway in Candida albicans. FEMS Yeast Res. 10, 579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Watanabe Y., Perrino B. A., Soderling T. R. (1996) Activation of calcineurin A subunit phosphatase activity by its calcium binding B subunit. Biochemistry 35, 562–566 [DOI] [PubMed] [Google Scholar]
- 46. Perrino B. A., Ng L. Y., Soderling T. R. (1995) Calcium regulation of calcineurin phosphatase activity by its B subunit and calmodulin. Role of the autoinhibitory domain. J. Biol. Chem. 270, 7012. [DOI] [PubMed] [Google Scholar]
- 47. Feng B., Stemmer P. M. (1999) Interactions of calcineurin A, calcineurin B, and Ca2+. Biochemistry 38, 12481–12489 [DOI] [PubMed] [Google Scholar]
- 48. Moreno V. R., Agüero F., Tekiel V., Sánchez D. O. (2007) The calcineurin A homologue from Trypanosoma cruzi lacks two important regulatory domains. Acta Trop. 101, 80–89 [DOI] [PubMed] [Google Scholar]
- 49. Ueki K., Kincaid R. L. (1993) Interchangeable associations of calcineurin regulatory subunit isoforms with mammalian and fungal catalytic subunits. J. Biol. Chem. 268, 6554–6559 [PubMed] [Google Scholar]
- 50. Akuffo H., Hultmark D., Engstöm A., Frohlich D., Kimbrell D. (1998) Drosophila antibacterial protein, cecropin A, differentially affects non-bacterial organisms such as Leishmania in a manner different from other amphipathic peptides. Int. J. Mol. Med. 1, 77–82 [DOI] [PubMed] [Google Scholar]
- 51. Fraidenraich D., Peña C., Isola E. L., Lammel E. M., Coso O., Añel A. D., Baralle F., Torres H. N., Flawia M. M. (1993) An α d-globin fragment from Triatoma infestans hindgut stimulates Trypanosoma cruzi adenylyl cyclase and promotes metacyclogenesis. Biol. Res. 26, 279–283 [PubMed] [Google Scholar]
- 52. Manque P. M., Eichinger D., Juliano M. A., Juliano L., Araya J. E., Yoshida N. (2000) Characterization of the cell adhesion site of Trypanosoma cruzi metacyclic stage surface glycoprotein gp82. Infect. Immun. 68, 478–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Málaga S., Yoshida N. (2001) Targeted reduction in expression of Trypanosoma cruzi surface glycoprotein gp90 increases parasite infectivity. Infect. Immun. 69, 353–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Burleigh B. A. (2005) Host cell signaling and Trypanosoma cruzi invasion. Do all roads lead to lysosomes? Sci. STKE 2005, pe36. [DOI] [PubMed] [Google Scholar]
- 55. Woolsey A. M., Burleigh B. A. (2004) Host cell actin polymerization is required for cellular retention of Trypanosoma cruzi and early association with endosomal/lysosomal compartments. Cell. Microbiol. 6, 829–838 [DOI] [PubMed] [Google Scholar]
- 56. Burleigh B. A., Woolsey A. M. (2002) Cell signalling and Trypanosoma cruzi invasion. Cell. Microbiol. 4, 701–711 [DOI] [PubMed] [Google Scholar]
- 57. Asin S., Catalá S. (1995) Development of Trypanosoma cruzi in Triatoma infestans. Influence of temperature and blood consumption. J. Parasitol. 81, 1–7 [PubMed] [Google Scholar]
- 58. Florencio-Martínez L., Márquez-Dueñas C., Ballesteros-Rodea G., Martínez-Calvillo S., Manning-Cela R. (2010) Cellular analysis of host cell infection by different developmental stages of Trypanosoma cruzi. Exp. Parasitol. 126, 332–336 [DOI] [PubMed] [Google Scholar]
- 59. Nogueira N., Cohn Z. (1977) Trypanosoma cruzi. Uptake and intracellular fate in normal and activated cells. Am. J. Trop. Med. Hyg. 26, 194–203 [DOI] [PubMed] [Google Scholar]
- 60. Kulkarni M. M., Barbi J., McMaster W. R., Gallo R. L., Satoskar A. R., McGwire B. S. (2011) Mammalian antimicrobial peptide influences control of cutaneous Leishmania infection. Cell. Microbiol. 13, 913–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Potenza M., Galat A., Minning T. A., Ruiz A. M., Duran R., Tarleton R. L., Marín M., Fichera L. E., Búa J. (2006) Analysis of the Trypanosoma cruzi cyclophilin gene family and identification of cyclosporin A binding proteins. Parasitology 132, 867–882 [DOI] [PubMed] [Google Scholar]
- 62. Waniek P. J., Castro H. C., Sathler P. C., Miceli L., Jansen A. M., Araújo C. A. (2009) Two novel defensin-encoding genes of the Chagas disease vector Triatoma brasiliensis (Reduviidae, Triatominae). Gene expression and peptide-structure modeling. J. Insect Physiol. 55, 840–848 [DOI] [PubMed] [Google Scholar]
- 63. Araújo C. A., Waniek P. J., Stock P., Mayer C., Jansen A. M., Schaub G. A. (2006) Sequence characterization and expression patterns of defensin and lysozyme encoding genes from the gut of the reduviid bug Triatoma brasiliensis. Insect Biochem. Mol. Biol. 36, 547–560 [DOI] [PubMed] [Google Scholar]