Summary
During late mitosis and early interphase, origins of replication become ‘licensed’ for DNA replication by loading Mcm2-7 complexes [1-5]. Mcm2-7 are removed from origins as replication forks initiate replication, thus preventing re-replication of DNA in a single cell cycle. Premature origin licensing is prevented in metaphase by the action of geminin, which binds and inhibits Cdt1/RLF-B, a protein that is required for the loading of Mcm2-7 [6-9]. Recombinant geminin that is added to Xenopus egg extracts is efficiently degraded on exit from metaphase [10]. Here we show that recombinant and endogenous forms of Xenopus geminin behave differently from one another such that a significant proportion of endogenous geminin escapes proteolysis on exit from metaphase. During late mitosis and early G1 the surviving population of endogenous geminin does not associate with Cdt1/RLF-B and does not inhibit licensing. Following nuclear assembly, geminin is imported into nuclei and becomes reactivated to bind Cdt1/RLF-B. This reactivated geminin provides the major nucleoplasmic inhibitor of origin re-licensing during late interphase. Since the initiation of replication at licensed origins depends on nuclear assembly [11], our results suggest an elegant and novel mechanism for preventing re-replication of DNA in a single cell cycle.
Results
DNA-dependent generation of an inhibitor of replication licensing
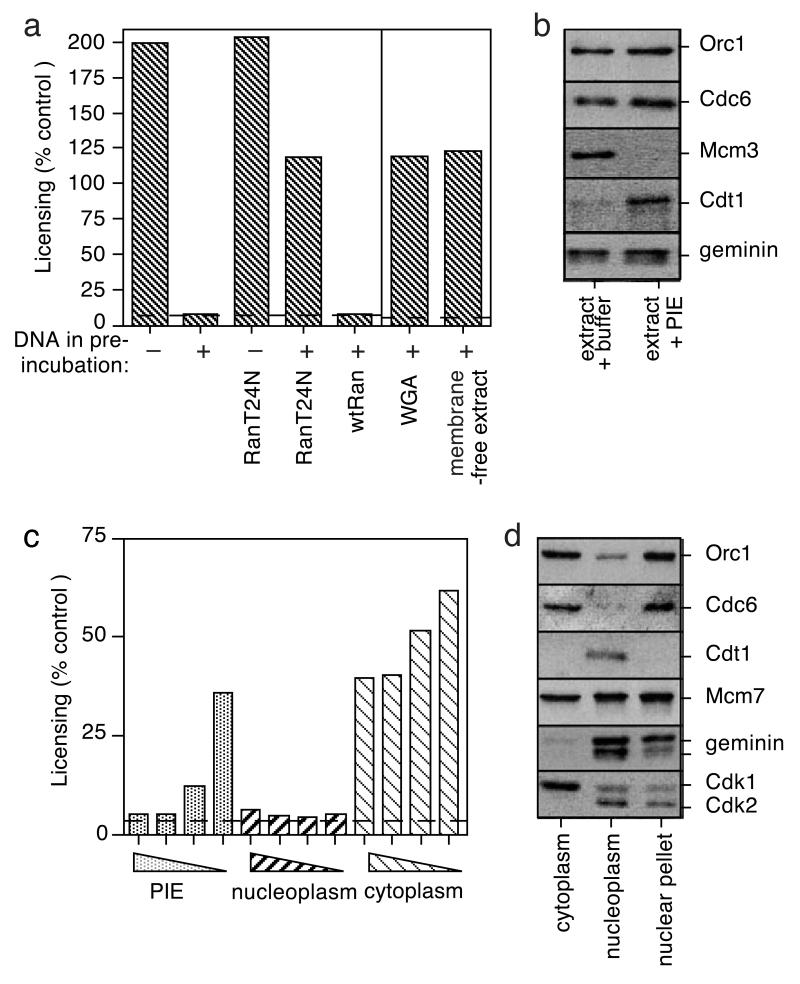
As part of our studies into how re-licensing of replicated DNA is prevented in G2, we investigated whether the presence of DNA in Xenopus extract affected the replication licensing system. Extract was supplemented with cycloheximide (to arrest extracts in G2 phase) plus or minus sperm nuclei and incubated for 90 min. Extract was then diluted and the chromatin removed by centrifugation, to generate a “pre-incubated extract” (PIE). In this protocol almost all of the soluble protein is released from the nuclei, so the PIEs contain a mixture of both cytoplasmic and nucleoplasmic proteins (data not shown). When DNA was present during the pre-incubation, the resultant PIE contained a potent inhibitor of replication licensing (Fig 1a) and blocked the loading of Mcm3 onto chromatin (Fig 1b). In contrast, when no DNA was present in the pre-incubation, no licensing inhibitor was generated. The PIE had no inhibitory effect on the replication of licensed DNA templates, suggesting that the inhibitory activity is specific for licensing (data not shown).
Figure 1.
DNA-dependent generation of an inhibitor of replication licensing.
a, Whole extract or membrane-free extract was pre-incubated with or without sperm nuclei (10 ng DNA/μl) plus or minus 2.5 μM wild-type or T24N mutant Ran, or 200 μg/ml wheat germ agglutinin. The ability of the PIEs to inhibit licensing in untreated interphase extracts was measured. Dashed lines, background licensing of sperm nuclei incubated in buffer alone. b, Sperm nuclei were incubated for 30 min in interphase extract supplemented with either buffer or PIE. Chromatin was isolated and immunoblotted for Orc1, Cdc6, Mcm3, Cdt1 and geminin. c, PIE, nucleoplasmic extract and cytoplasmic extract were subject to serial 3-fold dilutions, then assayed for their ability to inhibit licensing of interphase extract. Dashed line, background licensing of sperm nuclei incubated in buffer alone. d, Cytoplasmic extract, nucleoplasmic extract and insoluble nuclear pellet were immunoblotted for Orc1, Cdc6, Cdt1, Mcm7, geminin and CDKs (PSTAIR).
Since the inhibitory activity first appeared around the time nuclear assembly was completed (data not shown), we investigated whether nuclear assembly is involved in its generation. Ran T24N, a mutant Ran protein stabilised predominantly in the GDP-bound form, inhibits nuclear assembly in Xenopus extract [12, 13]. Fig 1a shows that when extract was pre-incubated for 90 min with sperm nuclei and Ran T24N protein, no licensing inhibitor was generated. In contrast, wild-type Ran (which does not block nuclear assembly) did not block the appearance of the inhibitor. Wheat germ agglutinin, a lectin which binds to nuclear pores, inhibits nuclear protein transport and nuclear envelope assembly [14]. No licensing inhibitor was generated when DNA was pre-incubated in extract supplemented with wheat germ agglutinin (Fig 1a). The concentration of wheat germ agglutinin used in this experiment was sufficient to inhibit DNA replication and gross nuclear swelling, but did not block complete nuclear envelope assembly (data not shown), suggesting that inhibition of nuclear transport was responsible for the effect. Similarly, when sperm nuclei were incubated in extract lacking nuclear envelope precursors (membrane-free extract), no licensing inhibitor was generated (Fig 1a). Taken together, these results suggest that nuclear assembly and nuclear transport are required for generation of the inhibitory activity in pre-incubated extracts.
We next investigated whether the inhibitory activity is localised in the nucleus. Nucleoplasmic and cytoplasmic fractions of Xenopus egg extracts can be prepared by floating intact nuclei through the cytoplasm by gentle centrifugation [15]. Hard centrifugation of the nuclear fraction then separates it into a soluble nucleoplasmic extract and an insoluble pellet. When pre-incubated extract was fractionated in this way most of the licensing inhibitor present in pre-incubated extracts was seen to be concentrated in the nucleoplasmic extract (Fig 1c). It was of interest that Cdk2 and geminin (both potential licensing inhibitors) were also concentrated within the nucleoplasmic extract (Fig 1d).
The licensing inhibitor is geminin
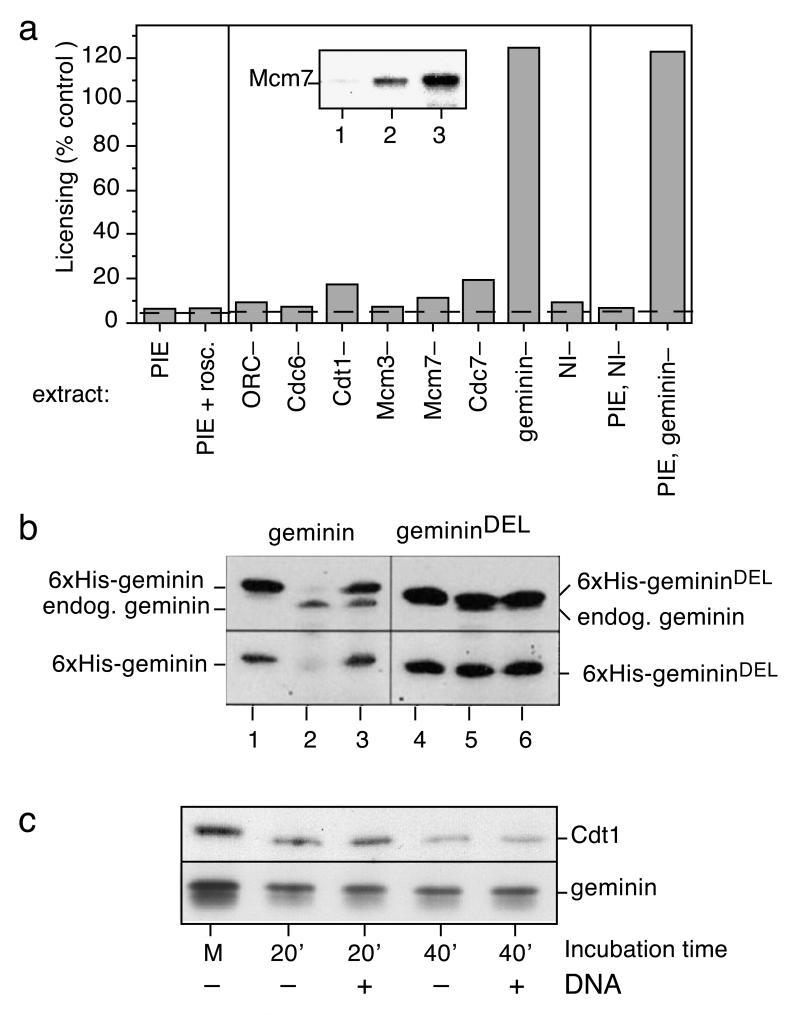
Studies in yeast have shown that high cyclin-dependent kinase (CDK) activity is important for preventing re-licensing of DNA and re-assembly of pre-replicative complexes (pre-RCs) late in the cell cycle [2, 3]. To investigate whether our licensing inhibitor depended on high CDK activity, we used the specific CDK inhibitor roscovitine [16]. Although 2.5mM roscovitine is sufficient to inhibit the initiation of DNA replication [16], it did not block the appearance of the licensing inhibitor (Fig 2a, PIE+rosc.). Similar results were obtained with the CDK inhibitor p21Cip1 (data not shown). Interphase extracts were depleted of ORC, Cdc6, Cdt1, Mcm3, Mcm7, Cdc7, or mock-depleted with non-immune serum. When DNA was pre-incubated in each of these extracts, a potent licensing inhibitor was still generated (Fig 2a), suggesting that generation of the licensing inhibitor is not dependent on any known aspect of pre-RC assembly.
Figure 2.
The licensing inhibitor is geminin.
a, The ability of various pre-incubated extracts to inhibit replication licensing was assayed. sperm nuclei were pre-incubated in interphase extract minus or plus 2.5 mM roscovitine (PIE, PIE+rosc.). Alternatively, sperm nuclei were pre-incubated in interphase extract previously depleted of with antibodies against Orc1, Cdc6, Mcm3, Mcm7, Cdc7 or geminin, or mock-depleted with non-immune antibodies (NI–), or were depleted of Cdt1 using geminin-coupled beads. Alternatively, PIE was prepared in normal interphase extract, and then depleted with either anti-geminin antibodies (PIE, geminin–) or non-immune antibodies (PIE, NI–). Inset: Immunoblot of chromatin-bound Mcm7 following 30 min incubation of sperm nuclei in PIE (lane 1), PIE + recombinant Cdt1 (lane 2) or PIE prepared without DNA (lane 3). b, Wild type and geminin-DEL (which lacks a functional destruction box) were incubated at 1.3 ng/μl for 45 min in metaphase extract released into interphase with CaCl2 (lanes 2 and 5) or interphase extract (lanes 3 and 6). Input levels of recombinant geminin are shown in lanes 1 and 4. Samples were analysed by SDS-PAGE and immun0blotted with anti-geminin antibodies (top panel) or anti-6xHis antibodies (bottom panel). c, Metaphase extract (M) plus or minus sperm nuclei (10 ng DNA/μl) was released into interphase by addition of CaCl2 and at the indicated times (in min) aliquots were taken and immunoblotted for endogenous geminin and Cdt1.
The major inhibitor of origin licensing present in metaphase Xenopus egg extract is geminin [9]. Figure 2a shows that interphase extracts immunodepleted of geminin failed to generate the licensing inhibitor after pre-incubation with DNA. In addition, when inhibitory PIEs prepared from untreated extract were subsequently depleted of geminin, the inhibitory activity was lost (Fig 2a ‘PIE, geminin–’). Addition of recombinant Cdt1 (the target of geminin inhibition) to PIE reactivated its ability to assemble Mcm7 onto sperm chromatin (Fig 2a, inset). When similar experiments were performed on nucleoplasmic fractions, immunodepletion of geminin removed the licensing inhibitor, whilst inhibition of CDKs did not (see supplementary data 1). These results therefore show that geminin provides the major inhibitory activity generated in G2 as a result of nuclear assembly and nuclear transport.
We were surprised to find geminin in our extracts, since geminin was originally identified in a screen for proteins specifically degraded at the metaphase-to-anaphase transition [10] and our extracts were supplemented with cycloheximide to prevent any new protein synthesis. We noted, however, that the assays performed by McGarry and Kirschner involved the degradation of recombinant geminin, while the degradation of endogenous geminin was not reported. To confirm that our extracts could efficiently degrade recombinant geminin on exit from metaphase, two forms of 6xhis-tagged geminin protein were produced in E. coli: one wild-type (Fig 2b, lane 1), and one containing a mutation in its destruction box to make it resistant to degradation (geminin-DEL, Fig 2b, lane 4) [10]. When incubated in extracts exiting from metaphase, the wild-type recombinant protein was almost completely degraded, whilst the DEL mutant remained stable (Fig 2b, lanes 2 and 5). In contrast, both proteins remained stable when incubated for an equivalent period of time in interphase extract (Fig 2b, lanes 3 and 6). These results are consistent with the efficient cell-cycle specific degradation of recombinant geminin reported by McGarry and Kirschner [10].
However, in these experiments, significant quantities of endogenous geminin remained in the extract (Fig 2b, upper panel, lanes 2 and 3). The quantity of endogenous geminin and Cdt1/RLF-B present in extracts was therefore measured at different times after exit from metaphase arrest in vitro (Fig 2c). Although some geminin degradation was clearly observed on exit from metaphase, 30 - 60% of endogenous geminin resisted degradation. We also noticed a slight decline in the amount of Cdt1/RLF-B, consistent with the loss of RLF-B activity seen when pre-incubations were performed without added DNA [17]. The presence of sperm nuclei in the extract had no significant effect on the degree of degradation observed (Fig 2c). We conclude that a significant quantity of endogenous geminin escapes degradation, and that this geminin is incapable of inhibiting licensing until nuclear assembly occurs.
Geminin’s inhibitory activity correlates with its ability to interact with Cdt1/RLF-B
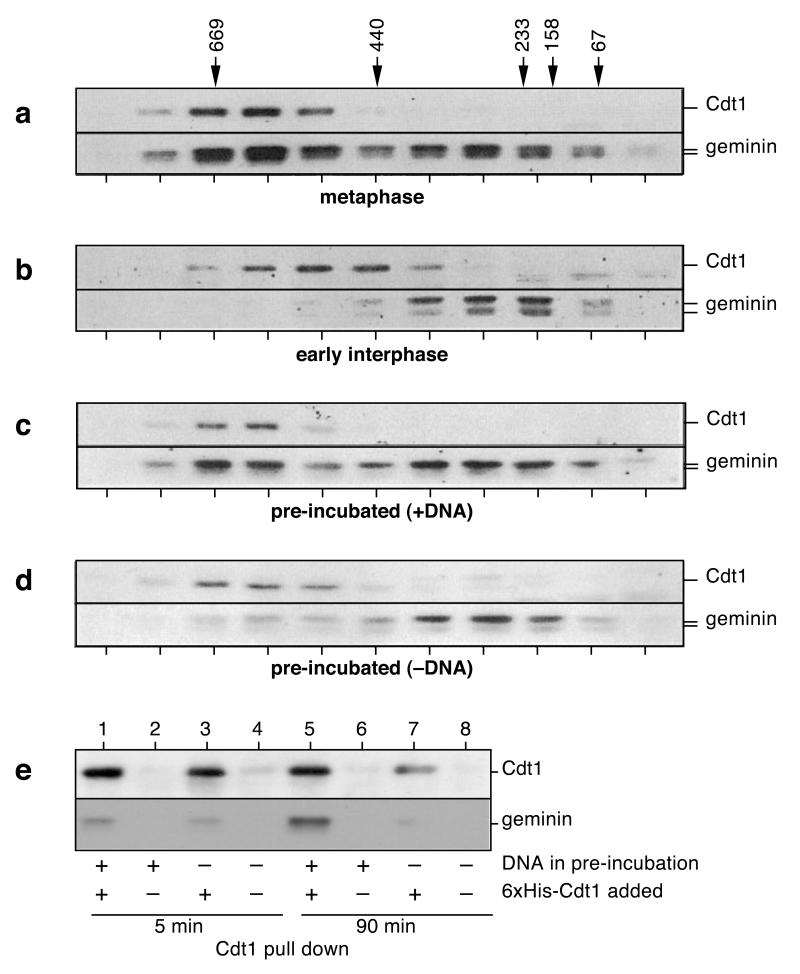
If some endogenous geminin escapes degradation in anaphase, why does it not inhibit licensing? A change in its ability to interact with Cdt1/RLF-B could provide a potential explanation. We therefore examined the behavior of geminin and Cdt1/RLF-B by gel filtration. In metaphase-arrested extracts, Cdt1 migrated in a single peak of ~500kDa. Geminin was observed in two peaks, ~61% in a large peak co-fractionating with Cdt1/RLF-B and the rest in a smaller peak without Cdt1/RLF-B (Fig 3a). The smaller peak migrated in the same position as free recombinant geminin (data not shown). This suggests that in metaphase, Cdt1/RLF-B is fully bound and inhibited by geminin, and that there is also excess geminin activity present [9]. On exit from metaphase into interphase, some but not all geminin was degraded, but >95% of the remaining geminin was found in the smaller complex (Fig 3b). Further, the endogenous Cdt1/RLF-B migrated as a slightly smaller complex, consistent with it no longer binding geminin. When extract was pre-incubated with DNA, so that geminin once again inhibited Cdt1/RLF-B, the fractionation pattern again resembled that seen in metaphase, with all the Cdt1/RLF-B and ~45% geminin co-fractionating in the larger complex (Fig 3c). Immunodepletion experiments showed that under these conditions, a complex between Cdt1/RLF-B and geminin had reformed (supplementary data 2). In contrast, when extract was pre-incubated in the absence of DNA, most geminin and Cdt1/RLF-B did not co-fractionate, though some complex (~24% of the geminin) was formed (Fig 3d). Co-precipitation also showed a significant quantity of free Cdt1 in the absence of DNA (supplementary data 2). We therefore see a good correspondence between the presence of inhibitory activity and the co-fractionation of Cdt1/RLF-B and geminin.
Figure 3.
Geminin’s affinity for Cdt-1 changes during the cell cycle.
a-d, Gel filtration of different extracts immunoblotted for geminin and Cdt1: metaphase extract (a), interphase extract (10 min after metaphase release) (b), pre-incubated extract plus sperm (10 ng DNA/μl, 90 min) (c) and pre-incubated extract minus sperm (no DNA, 90 min) (d). Migration of molecular weight markers (in kDa) is shown above. e, Pre-incubated extracts were prepared plus or minus 10ng DNA/μl at the indicate times; 6xhis-Cdt1 was added and incubated for 30 min, then pulled out on Ni-NTA agarose. Bead-bound Cdt1 and geminin were analysed by SDS-PAGE and immunoblotting.
Since recombinant geminin can bind Cdt1/RLF-B and inhibit licensing when added to early interphase Xenopus extracts [9, 10], we reasoned that endogenous geminin might become altered during late mitosis and early interphase so that it is unable to bind Cdt1/RLF-B. To address this extract was pre-incubated plus or minus DNA for either 5 or 90 min. Recombinant 6xhis-Cdt1 was added to these PIEs and was then collected on Ni-agarose bead. Bead-bound material was immunoblotted to determine whether endogenous geminin had bound to the recombinant Cdt1. Figure 3e shows that when nuclear assembly did not occur (either because no DNA was present or because insufficient time had elapsed to allow nuclei to assemble) endogenous geminin did not bind significantly to the recombinant Cdt1. However, when nuclear assembly occurred and the inhibitor was activated, a significant quantity of endogenous geminin was bound to the recombinant Cdt1 (Fig 3e, lane 5). This suggests that as a consequence of nuclear assembly, endogenous geminin becomes competent for inhibition of Cdt1/RLF-B.
Discussion
Replication licensing requires ORC to bind origin DNA, which in turn permits the recruitment of Cdc6 and Cdt1/RLF-B, and eventually the loading of Mcm2-7 [1-5]. While each of the different components can potentially be regulated [5], previous studies have indicated that origin licensing is controlled within the Xenopus embryonic cell cycle predominantly by the regulation of Cdt1/RLF-B activity [9, 17, 18]. Cdt1/RLF-B is specifically bound and inhibited by geminin, and this provides the major mechanism by which origin licensing is regulated during metaphase [9]. Geminin was originally identified in a screen for recombinant proteins degraded upon exit from mitosis in Xenopus egg extracts [10]. However, this earlier study did not investigate the degradation of endogenous geminin in the Xenopus system. We demonstrate here that, although recombinant geminin is efficiently degraded by Xenopus egg extracts on exit from metaphase, a significant proportion of endogenous geminin escapes degradation. This is different from what is observed in somatic cells, where geminin appears to be virtually absent throughout G1 [10, 19].
Despite the continued presence of endogenous geminin, Cdt1/RLF-B activity appears abruptly on exit from metaphase [17, 20]. We show here that as Cdt1/RLF-B becomes activated on exit from metaphase, it changes from being in a complex with geminin to a form where it is not. When recombinant geminin is added to early interphase Xenopus egg extracts, it forms a complex with endogenous Cdt1/RLF-B and strongly inhibits licensing [9, 10]. In contrast, we show here that when recombinant Cdt1/RLF-B is added to the extract, it binds endogenous geminin only very poorly. This suggests that on exit from metaphase, geminin is altered in some way that prevents it binding to and inhibiting Cdt1/RLF-B. This change leads to the abrupt activation of origin licensing on exit from metaphase (Fig 4a and b). It is possible that this geminin alteration is also responsible for protecting it from degradation. Although we have detected different forms of geminin on 2-dimensional gels, we have so far been unable to identify a covalent modification that strictly correlates with the changes in activity (AL, unpublished). Alternatively there may be another protein that is activated on exit from metaphase and that modulates the activity of geminin in a non-covalent way.
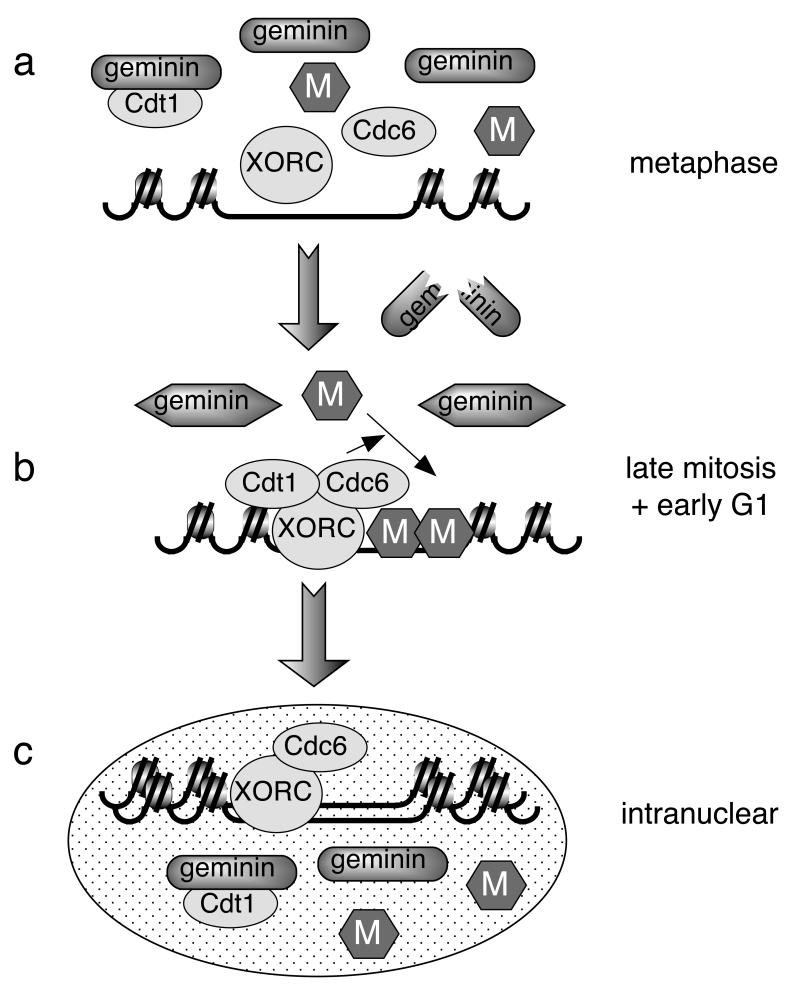
Figure 4.
Model for geminin regulation during the Xenopus embryonic cell cycle.
Cartoon showing a small segment of chromatin containing a replication origin as it passes through the cell cycle. Hexagons labelled ‘M’ represent hexameric complexes of Mcm2-7. a, In metaphase geminin is both abundant and active, so is able to inhibit origin licensing by formation of a tight complex with Cdt1/RLF-B. ORC and Cdc6 do not bind tightly to DNA, probably due to high CDK levels. b, Upon metaphase release some of the geminin is degraded, but the remaining geminin is altered in some way that prevents it binding and inhibiting Cdt1/RLF-B. The loss of CDK activity allows ORC and Cdc6 to associate tightly with DNA. With all components of the licensing system active, Mcm2-7 is loaded onto origins. c, Following nuclear assembly, geminin is imported into a functional nucleus and becomes reactivated. This allows it to bind and inhibit Cdt1/RLF-B, thus preventing the relicensing of replicated origins.
We also show that endogenous geminin regains the capacity to bind recombinant Cdt1 and inhibit further origin licensing when it has been imported into an interphase nucleus (Fig 4c). Possibly the factor(s) required to inactivate geminin are excluded from interphase nuclei, thus allowing the alteration that had occurred on metaphase exit to become reversed inside the nucleus. The reactivation of geminin did not depend on CDK activity, distinguishing this mechanism from what has been reported in yeast, where high nuclear CDK activity is required to preventing re-assembly of pre-RCs and re-licensing of replicated DNA [2, 3, 5]. This parallels earlier work showing that geminin is the major inhibitor of origin licensing in metaphase-arrested Xenopus egg extracts [9]. Since the initiation of DNA replication only takes place within the nucleus [11], the reactivation of geminin as a licensing inhibitor in the nucleus provides an elegant mechanism for preventing re-replication of DNA.
Experimental Procedures
Preparation of egg extracts and sperm nuclei
Metaphase-arrested and interphase low speed supernatant Xenopus egg extracts were prepared as described [21]. Extracts were supplemented with 250 μg/ml cycloheximide, 25mM phosphocreatine, 10 μg/ml creatine phosphokinase before use, and incubations were performed at 23°. 6-DMAP-treated extract was prepared by supplementing metaphase-arrested extracts with 3 mM 6-DMAP, [α-32P]dATP and 0.3 mM CaCl2 [20]. Xenopus sperm nuclei were demembranated with lysolecithin as described [21] and frozen in aliquots in liquid nitrogen. Pre-incubated extracts (PIEs) were prepared by incubating interphase extract with sperm nuclei at 10 ng DNA/μl for 90 min. Extracts were then diluted by addition of 3 volumes ice cold LFB2/50 (40 mM Hepes KOH pH 8.0, 50 mM KCl, 20 mM K2HPO4/KH2PO4 pH 8.0, 2 mM MgCl2, 1 mM EGTA, 2 mM DTT, 2.5mM Mg-ATP, 10% sucrose and 1 μg/ml each of leupeptin, pepstatin and aprotinin). The diluted extracts were then transferred to pre-cooled polycarbonate centrifuge tubes (Beckman #343775) and centrifuged at 72,000rpm (~230,000 × g), using a TLA-100 rotor (Beckman) for 20 min at 4°C. The supernatant was recovered and snap frozen in 10μl beads in liquid nitrogen.
Membrane-free extracts were prepared by 3-fold dilution of extract in LFB2/50 followed by centrifugation at 72,000rpm (~230,000 × g), using a TLA-100 rotor (Beckman) for 20 min at 4°C. Nucleoplasmic extract and cytoplasmic extract were prepared as previously described [15]. Immunodepletion of interphase extracts, PIE and nucleoplasmic extract with antibodies raised against XOrc1 [22], XCdc6 [18], XMcm3 [23], XMcm7 [24], geminin [9] or with antibodies from non-immune rabbit serum, was performed as described [21]. Extract was depleted of Cdt1 using geminin-coupled beads as described [9].
Licensing assays
Licensing inhibition assays were performed essentially as described [21]. Briefly, 2 μl fractions of interest were incubated with 0.4 μl interphase extract and 0.3 μl of sperm nuclei (80 ng DNA/μl) for 30 min. The degree of licensing was then assessed by addition of 6 μl 6-DMAP-treated extract containing [α-32P]dATP and incubation for 90 min; total DNA synthesis was measured by scintillation counting of acid-insoluble material.
To investigate chromatin binding, licensing assays were scaled up 10-fold to a total volume of 24 μl. After 30 min incubation, each reaction was diluted in 300 μl NIBA (50 mM KCl, 50 mM Hepes KOH pH 7.6, 5 mM MgCl2, 2 mM DTT, 0.5 mM spermidine, 0.15mM spermine, 2.5mM Mg-ATP, 1 μg/ml each of leupeptin, pepstatin and aprotinin) supplemented with 0.1% Triton X-100 and underlayered with 100 μl of the same buffer containing 15% sucrose. The chromatin was pelleted at 3,500g in a swinging bucket rotor at 4°C. The diluted extract was removed and the chromatin pellet was resuspended in SDS-PAGE loading buffer.
Recombinant proteins, immunoblotting and pull-down assays
Recombinant 6his-tagged Xenopus geminin and geminin-DEL [10] were produced from expression plasmids (kindly provided by Tom McGarry), and were purified on Ni-agarose by standard techniques. Recombinant 6xHis-tagged Xenopus ΔCdt-1 was produced as described [4, 6]. To investigate the interaction between geminin and Cdt1/RLF-B, recombinant Cdt1 was incubated in mock and pre-incubated extracts at room temperature for 30 min. Ni-agarose beads (Qiagen), washed extensively in PDB + 66mM KCl (PDB: 40mM Hepes KOH pH 8.0, 20mM K2HPO4/KH2PO4 pH 8.0, 2 mM β-mercaptoethanol, 10% (w/v) sucrose, 10 μg/ml each of leupeptin, pepstatin and aprotinin, 20 mM imidazole and 0.01% Triton X-100), were then added to extracts and incubated for a further 30 min at 4°. Beads were removed by centrifugation through a 25μm nylon filter, washed twice with 10 vol. PDB + 500 mM KCl, and once with 10 vol. PDB + 10 mM KCl. Beads were boiled in SDS loading buffer, centrifuged and supernatant immunoblotted for geminin or Cdt1.
For immunoblotting, samples were run on 4-12% gradient Novex pre-cast gels and analysed by immuno-blotting by ECL detection (Amersham). Antibodies against XOrc1 [22], XCdc6 [18], XMcm3 [23], XMcm7 [24], XCdt1 [9], geminin [9], 6xHis (Santa Cruz Biotechnology, Santa Cruz) and PSTAIR (Zymed, San Francisco) were used. Film was scanned and imported into Adobe Photoshop for printing.
Gel filtration
20 μl metaphase extract or interphase extract and 50μl PIE (90 min, plus or minus 10 ng DNA/μl) were applied to a 2.4 ml Superose 6 column (Amersham-Pharmacia) equilibrated in T’LFB1/200 (40 mM Hepes KOH pH 8.0, 200 mM KCl, 20 mM K2HPO4/KH2PO4 pH 8.0, 2 mM MgCl2, 1 mM EGTA, 2 mM DTT, 10% sucrose 1μg/ml each of leupeptin, pepstatin and aprotinin and 0.01% Triton-X 100). The column was run at a flow rate of 25 μl/min on a SMART chromatography system (Amersham-Pharmacia) at 4°C and 50 μl fractions were collected.
Supplementary Material
Acknowledgements
We would like to thank Paul Clarke for recombinant Ran protein; Pedro Jares and Peter Gillespie for helpful discussions; and Anne Donaldson, Christine Gosden, Tom Owen-Hughes and Jason Swedlow for comments on the manuscript. BJH is the recipient of a UBI (Upstate Biotechnology Inc.) studentship. This work was supported by the Cancer Research UK [CRC] grant SP2385/0101.
References
- 1.Blow JJ, Laskey RA. A role for the nuclear envelope in controlling DNA replication within the cell cycle. Nature. 1988;332:546–548. doi: 10.1038/332546a0. [DOI] [PubMed] [Google Scholar]
- 2.Diffley JFX. Building the perfect switch. Curr. Biol. 2001;11:R367–370. doi: 10.1016/s0960-9822(01)00196-8. [DOI] [PubMed] [Google Scholar]
- 3.Lei M, Tye BK. Initiating DNA synthesis: from recruiting to activating the MCM complex. J. Cell Sci. 2001;114:1447–1454. doi: 10.1242/jcs.114.8.1447. [DOI] [PubMed] [Google Scholar]
- 4.Gillespie PJ, Li A, Blow JJ. Reconstitution of licensed replication origins on Xenopus sperm nuclei using purified proteins. BioMed Central Biochem. 2001;2:15. doi: 10.1186/1471-2091-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blow JJ, Hodgson B. Replication licensing - defining the proliferative state? Trends Cell Biol. 2002;12:72–76. doi: 10.1016/s0962-8924(01)02203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maiorano D, Moreau J, Mechali M. XCDT1 is required for the assembly of pre-replicative complexes in Xenopus laevis. Nature. 2000;404:622–625. doi: 10.1038/35007104. [DOI] [PubMed] [Google Scholar]
- 7.Nishitani H, Lygerou Z, Nishimoto T, Nurse P. The Cdt1 protein is required to license DNA for replication in fission yeast. Nature. 2000;404:625–628. doi: 10.1038/35007110. [DOI] [PubMed] [Google Scholar]
- 8.Wohlschlegel JA, Dwyer BT, Dhar SK, Cvetic C, Walter JC, Dutta A. Inhibition of eukaryotic replication by geminin binding to Cdt1. Science. 2000;290:2309–2312. doi: 10.1126/science.290.5500.2309. [DOI] [PubMed] [Google Scholar]
- 9.Tada S, Li A, Maiorano D, Mechali M, Blow JJ. Repression of origin assembly in metaphase depends on inhibition of RLF-B/Cdt1 by geminin. Nat. Cell Biol. 2001;3:107–113. doi: 10.1038/35055000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGarry TJ, Kirschner MW. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell. 1998;93:1043–1053. doi: 10.1016/s0092-8674(00)81209-x. [DOI] [PubMed] [Google Scholar]
- 11.Blow JJ. Control of chromosomal DNA replication in the early Xenopus embryo. EMBO J. 2001;20:3293–3297. doi: 10.1093/emboj/20.13.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dasso M, Seki T, Azuma Y, Ohba T, Nishimoto T. A mutant form of the Ran/TC4 protein disrupts nuclear function in Xenopus-laevis egg extracts by inhibiting the RCC1 protein, a regulator of chromosome condensation. EMBO J. 1994;13:5732–5744. doi: 10.1002/j.1460-2075.1994.tb06911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes M, Zhang CM, Avis JM, Hutchison CJ, Clarke PR. The role of the Ran GTPase in nuclear assembly and DNA replication: characterisation of the effects of Ran mutants. J. Cell Sci. 1998;111:3017–3026. doi: 10.1242/jcs.111.20.3017. [DOI] [PubMed] [Google Scholar]
- 14.Finlay DR, Newmeyer DD, Price TM, Forbes DJ. Inhibition of in vitro nuclear transport by a lectin that binds to nuclear-pores. J. Cell Biol. 1987;104:189–200. doi: 10.1083/jcb.104.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walter J, Sun L, Newport J. Regulated chromosomal DNA replication in the absence of a nucleus. Mol. Cell. 1998;1:519–529. doi: 10.1016/s1097-2765(00)80052-0. [DOI] [PubMed] [Google Scholar]
- 16.Meijer L, Borgne A, Mulner O, Chong JP, Blow JJ, Inagaki N, Inagaki M, Delcros JG, Moulinoux JP. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 17.Mahbubani HM, Chong JP, Chevalier S, Thömmes P, Blow JJ. Cell cycle regulation of the replication licensing system: involvement of a Cdk-dependent inhibitor. J. Cell Biol. 1997;136:125–135. doi: 10.1083/jcb.136.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tada S, Chong JPJ, Mahbubani HM, Blow JJ. The RLF-B component of the replication licensing system is distinct from Cdc6 and functions after Cdc6 binds to chromatin. Curr. Biol. 1999;9:211–214. doi: 10.1016/s0960-9822(99)80092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quinn LM, Herr A, McGarry TJ, Richardson H. The Drosophila Geminin homolog: roles for Geminin in limiting DNA replication, in anaphase and in neurogenesis. Genes Dev. 2001;15:2741–2754. doi: 10.1101/gad.916201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blow JJ. Preventing re-replication of DNA in a single cell cycle: evidence for a replication licensing factor. J. Cell Biol. 1993;122:993–1002. doi: 10.1083/jcb.122.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chong JP, Thömmes P, Rowles A, Mahbubani HM, Blow JJ. Characterization of the Xenopus replication licensing system. Methods Enzymol. 1997;283:549–564. doi: 10.1016/s0076-6879(97)83043-1. [DOI] [PubMed] [Google Scholar]
- 22.Rowles A, Chong JP, Brown L, Howell M, Evan GI, Blow JJ. Interaction between the origin recognition complex and the replication licensing system in Xenopus. Cell. 1996;87:287–296. doi: 10.1016/s0092-8674(00)81346-x. [DOI] [PubMed] [Google Scholar]
- 23.Thömmes P, Kubota Y, Takisawa H, Blow JJ. The RLF-M component of the replication licensing system forms complexes containing all six MCM/P1 polypeptides. EMBO J. 1997;16:3312–3319. doi: 10.1093/emboj/16.11.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prokhorova TA, Blow JJ. Sequential MCM/P1 subcomplex assembly is required to form a heterohexamer with replication licensing activity. J. Biol. Chem. 2000;275:2491–2498. doi: 10.1074/jbc.275.4.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.