Abstract
Eukaryotic replication origins are “licensed” for replication early in the cell cycle by loading Mcm(2-7). As chromatin replicates, Mcm(2-7) are removed, thus preventing origin re-firing. Here we report purification of the RLF-B component of the licensing system and show that it corresponds to Cdt1. RLF-B/Cdt1 was inhibited by geminin, a protein that is degraded during late mitosis. Immunodepletion of geminin from metaphase extracts allowed them to assemble licensed replication origins. Inhibition of CDKs in metaphase stimulated origin assembly only after geminin depletion. These experiments suggest that geminin-mediated inhibition of RLF-B/Cdt1 is essential for repressing origin assembly late in the cell cycle of higher eukaryotes.
Introduction
To ensure that chromosomal DNA is precisely duplicated and no sections of DNA are over-replicated, eukaryotic replication origins must fire no more than once in a single cell cycle1-3. Early in the cell cycle, origins are “licensed” for replication by loading a hetero-hexameric complex of Mcm(2-7) proteins (also termed RLF-M)4-9. Mcm(2-7) are probably responsible for the larger “pre-replicative complex” (pre-RC) on replication origins in the yeast Saccharomyces cerevisiae during late mitosis and G110. The Mcm(2-7) proteins are essential for DNA replication, possibly providing helicase activity required for the initiation and elongation of replication forks11-15. As DNA replicates, Mcm(2-7) are removed from it, thus ensuring that re-firing of replicated origins will not occur. To prevent re-replication of DNA it is therefore necessary to prevent re-licensing of DNA once S phase has begun.
A stepwise assembly reaction leads to the assembly of licensed origins, involving first the assembly of the Origin Recognition Complex (ORC), then Cdc6 and Cdt1, and finally Mcm(2-7) 16-22. Biochemical fractionation of Xenopus egg extracts has shown that at least five activities are required to assemble licensed origins on Xenopus sperm nuclei4,18,23,24. The chromatin remodelling protein nucleoplasmin first decondenses sperm chromatin to permit binding of ORC24, and then Cdc617. Once this has occurred another currently unidentified activity termed RLF-B promotes the loading of Mcm(2-7)4,23. RLF-B, which has not previously been purified, is known to be distinct from nucleoplasmin, ORC, Cdc6 and Mcm(2-7)4,23,24, but its relationship with Cdt1 is unknown.
Mechanisms that prevent re-licensing of replicated DNA in metazoans are currently unclear, though in yeast, cyclin-dependent kinases (CDKs) active during S, G2 and M phases of the cell cycle are ultimately responsible25-30. The ability of CDKs to block replication appears to be mediated by a number of redundant mechanisms, including exclusion of Mcm(2-7) from the nucleus31-33 and the degradation of Cdc634,35. In mammalian cells, Cdc6 is exported from the nucleus as a consequence of CDK activation in late G136-38. However, in the Xenopus cell-free system, inhibition of CDK activity in G2 does not cause re-licensing39. Instead, regulation of RLF-B appears to play a critical role in preventing re-licensing of replicated DNA. RLF-B activity is low in metaphase, is rapidly activated on exit into anaphase, and then declines during interphase4,40. These changes in RLF-B activity are not dependent on CDK activity40.
Here we report the purification of RLF-B and show that it corresponds to Xenopus Cdt120. RLF-B/Cdt1 was inhibited by geminin, a replication inhibitor found in higher eukaryotes that is degraded during late mitosis41. We show that immunodepletion of geminin from metaphase extracts rendered them competent to assemble licensed replication origins. In contrast, inhibition of CDK activity in metaphase extracts stimulated origin assembly only after geminin depletion. These experiments suggest that geminin-mediated inhibition of RLF-B/Cdt1 is essential for repressing origin assembly late in the cell cycle of higher eukaryotes.
Results
Purification of RLF-B and identification as Cdt1
Geminin was originally identified in a screen for Xenopus proteins specifically degraded at the metaphase-anaphase transition41. Addition of recombinant geminin to Xenopus extracts blocked the loading of Mcm(2-7) onto chromatin41. We first wanted to determine whether the inhibition of DNA replication by geminin was specifically due to inhibition of origin licensing. Metaphase Xenopus egg extracts treated with the protein kinase inhibitor 6-dimethyalaminopurine (6-DMAP) are specifically defective in origin licensing42. Following a brief incubation in untreated interphase egg extract or in fractions derived from it, chromatin becomes licensed for replication and gains the ability to replicate in 6-DMAP-treated extract42. Fig 1a shows that when geminin was added to the interphase extract before licensing had occurred, it abolished subsequent replication in 6-DMAP-treated extract (Fig 1a, “before”), whilst addition of geminin after licensing had occurred caused virtually no inhibition of replication (Fig 1a, “after”). This suggests that geminin inhibits DNA replication by specifically blocking replication licensing.
Figure 1.
Inhibition of DNA replication by geminin is mediated by inhibition of RLF-B.
a, Unlicensed chromatin isolated from 6-DMAP-treated extract (“6-DMAP chromatin”) was incubated in partially-purified licensing factor fractions (MQE and BPAS). 35 nM recombinant geminin was added either before or after incubation with licensing factor fractions, or not at all. After incubation for 20 mins, the extent of licensing was assayed by addition of 6-DMAP-treated extract containing [α-32P]dATP and incubation for a further 90 mins. b, Fractionation scheme for origin components from Xenopus extracts. c, d, Interphase egg extract was treated with geminin-coupled agarose beads or with control beads, and was supplemented with various fractions. After incubation for 20 mins with sperm nuclei, the extent of licensing was assayed. Inset: BPAS (1), BQE (2), and BQFT (3) immunoblotted for Orc1 and Cdc6.
We next wanted to determine which activity required for origin licensing is inhibited by geminin. We have been undertaking a systematic fractionation of Xenopus egg extract to identify all the proteins required for the assembly of licensed replication origins on Xenopus sperm nuclei4,18,23,24. Figure 1b shows a cartoon of the current fractionation scheme. In order to determine which of the licensing activities interact with geminin, we coupled recombinant geminin to agarose beads and incubated them with interphase Xenopus extract. Figures 1c and 1d show that this geminin bead-treated extract was unable to license origins on sperm nuclei and could be rescued only by fractions containing RLF-B, and not by other fractions containing nucleoplasmin, ORC, Mcm(2-7) or Cdc6. This suggests that geminin specifically binds RLF-B.
We next investigated whether we could use recombinant geminin to affinity-purify RLF-B from the “BPAS” fraction (Fig 1b), which contains RLF-B enriched ~220-fold by three purification steps18,23,24; BPAS also contains ORC, Cdc6 and Cdt1 (Fig 2a, inset). After application to a geminin column, BPAS lost all RLF-B activity, but retained ORC, Cdc6 and ~50% of the Cdt1 (Fig 2a, inset). Neither RLF-B nor the bound Cdt1 were eluted from the geminin beads by 1M KCl, but both were efficiently eluted in buffer containing 4 M urea. This eluate contained RLF-B activity enriched >20,000-fold. Further fractionation by gel filtration in 4M urea gave a peak of RLF-B activity at an apparent molecular weight of ~350 kDa (Fig 2b). SDS PAGE and silver staining showed a single major band of ~75 kDa (the size of Xenopus Cdt1) co-eluting with RLF-B (Fig 2c, arrowhead). Because of the low concentration of protein present, contaminating keratin bands were also evident (arrows). The ~75 kDa protein was recognized by anti-Cdt1 antibody (Fig 2c, bottom panel), suggesting that RLF-B is identical to Cdt1.
Figure 2.
Purification of RLF-B.
a, Geminin-coupled agarose beads were incubated with BPAS, washed with different buffers, and RLF-B was eluted with buffer containing 4 M urea. Fractions were desalted and assayed for RLF-B activity. >65% of the applied RLF-B activity was recovered in the eluate. Dashed line: buffer alone control. Inset: BPAS (1), geminin bead flow-through (2), and 4 M urea eluate (3) immunoblotted for Orc1, Cdc6 and Cdt1. b, Geminin bead eluate (fractions 33-37) were fractionated by Superose 6 gel filtration and assayed for RLF-B activity. Molecular weight markers (in kDa) are above. Dashed line, buffer alone control. c, 0.25 ml aliquots from the Superose 6 column were precipitated with deoxycholate/TCA and run on a 10% SDS acrylamide gel. Top panel: silver stain of the gel. Molecular weight markers (kDa) are to the left. Arrowhead: ~75 kDa band co-eluting with RLF-B. Small arrows: contaminating keratin bands. Bottom panel: fractions immunoblotted for Cdt1.
To confirm this identity, we showed that recombinant Cdt120 could substitute for purified RLF-B in replication licensing (Fig 3a). As we have previously shown for RLF-B4,23, licensing induced by recombinant Cdt1 also required the Mcm(2-7) proteins. Conversely, purified RLF-B was capable of fully restoring licensing activity to extracts immunodepleted of Cdt1 (Figure 3b). Extract affinity-depleted of RLF-B by geminin beads was also fully rescued by purified RLF-B (Figure 3b). Similarly, depletion of Cdt1 from RLF-B fractions abolished RLF-B activity (data not shown). Taken together, these results prove conclusively that RLF-B activity is provided by the Cdt1 protein.
Figure 3.
Recombinant Cdt1 can provide RLF-B activity.
a, Unlicensed “6-DMAP chromatin” was incubated in interphase extract, a crude fraction containing Mcm(2-7) activity (RLF-M) and 10 nM recombinant Cdt1. After 20 mins, the extent of licensing was assayed by addition of 6-DMAP-treated extract containing [α-32P]dATP and incubation for a further 90 mins. b, Unlicensed “6-DMAP chromatin” was incubated in interphase extract depleted with either non-immune antibody, anti-Cdt1 antibody, or with recombinant geminin beads, plus or minus purified RLF-B. After 20 mins, the extent of licensing was assayed by addition of 6-DMAP-treated extract containing [α-32P]dATP and incubation for a further 90 mins. c, d, Interphase extract containing [α-32P]dATP and sperm nuclei was supplemented with 35 nM recombinant geminin, 20 nM recombinant Cdt1 or 0.25 vols purified RLF-B. After 90 mins, total DNA synthesis was measured.
To show that geminin and RLF-B/Cdt1 specifically antagonize one another, we inhibited replication by adding recombinant geminin to interphase Xenopus extract41. This inhibition was completely overcome by addition of recombinant Cdt1 (Fig 3c) or by addition of purified RLF-B (Fig 3d). These results suggest that RLF-B/Cdt1 is the only essential replication activity inhibited by geminin.
Geminin is a phsyiological inhibitor of RLF-B/Cdt1
The above results show that recombinant geminin can specifically inhibit RLF-B/Cdt1, but do not indicate whether geminin normally performs this function in vivo. We next set out to determine whether endogenous geminin is a physiological inhibitor of RLF-B/Cdt1. First we investigated whether geminin could associate with endogenous RLF-B/Cdt1 in whole Xenopus egg extract. Figure 4a shows that when recombinant geminin was added to interphase extract and was then depleted again, almost all of the endogenous RLF-B/Cdt1 (~95%) was removed from the extract. This suggests that endogenous RLF-B/Cdt1 is capable of forming a complex with recombinant geminin in whole extract. The residual RLF-B activity remaining in the extract following geminin addition and removal is probably sufficient to account for the DNA replication reported by McGarry and Kirschner41 after this treatment. We next investigated whether endogenous geminin present during interphase of the cell cycle is associated with endogenous RLF-B/Cdt1. We have previously described the behaviour of RLF-B activity on phenyl Sepharose chromatography23. Figure 4b shows that whilst RLF-B activity eluted in a single peak23, Cdt1 eluted in two overlapping peaks. One peak corresponded to active RLF-B, whilst the other peak contained endogenous geminin. This co-purification of Cdt1 and geminin probably represents a physical interaction since immunodepletion of geminin from the inactive fractions co-depleted Cdt1 (data not shown). Interestingly, only fractions with RLF-B activity could rescue licensing in Cdt1-depleted extracts (Fig 4b, triangles). These results suggest that geminin is an important physiological inhibitor of RLF-B/Cdt1. We propose that Xenopus egg extracts contain two forms of Cdt1: an inactive form associated with geminin, and a form not associated with geminin that has RLF-B activity. The inactive geminin-associated form probably accounts for the Cdt1 that does not bind recombinant geminin beads (Fig 1a).
Figure 4.
RLF-B/Cdt1 can associate with geminin in interphase extracts.
a, Interphase extract was supplemented with 35 nM recombinant geminin and then immunodepleted with either non-immune (1) or anti-geminin (2) antibodies. Inset, samples were blotted for either Cdt1 or geminin. Unlicensed “6-DMAP chromatin” was incubated in these depleted extracts or interphase extract or the BPAS fraction, plus or minus a crude fraction containing Mcm(2-7) activity (RLF-M). After 20 mins, the extent of licensing was assayed by addition of 6-DMAP-treated extract containing [α-32P]dATP and incubation for a further 90 mins. b, The BPAS fraction containing partially-purified RLF-B/Cdt1 was fractionated on phenyl Sepharose. Fractions were assayed for RLF-B, for the ability to rescue licensing in Cdt1-depleted extracts, or were immunoblotted for Cdt1 and geminin.
Support for this idea comes from analysing proteins assembled onto chromatin under different conditions (Fig 5). In interphase extracts which support origin licensing, XOrc1, XCdc6, XCdt1 and XMcm7 are found associated with sperm chromatin. In contrast, metaphase extracts support the loading of much lower levels of these proteins. When metaphase extracts are treated with 3 mM 6-DMAP, they enter an interphase-like state where origin licensing is inhibited40,42 and so XMcm7 loading onto chromatin remains low. However, this “6-DMAP chromatin” contained high levels of XORC, XCdc6, XCdt1 and geminin (Fig 5). The Cdt1 loaded onto “6-DMAP chromatin” is probably in the inactive geminin-bound form, since this chromatin requires incubation with both RLF-B and Mcm(2-7) for licensing to occur4,23. Addition of recombinant geminin to interphase extracts also blocked assembly of XMcm7 onto chromatin (Fig 5), but had a different effect on the other origin proteins. Although levels of XCdc6 on the chromatin were increased, there was virtually no stimulation of XCdt1 loading, and only a modest stimulation of geminin loading. Addition of geminin or 6-DMAP to extracts at levels insufficient to block licensing (0.5 mM 6-DMAP or 7 nM geminin) produced chromatin similar to that assembled in untreated extract (Fig 5). We therefore conclude that although 6-DMAP and geminin can both block licensing in the Xenopus system, they do not do so in exactly the same way.
Figure 5.
Proteins associated with chromatin in different extracts.
Sperm nuclei were incubated for 20 mins in either metaphase extract, interphase extract, in metaphase extract supplemented with 0.5 or 3 mM 6-DMAP and 0.3mM CaCl2 (“metaphase+6-DMAP”), or in interphase extract supplemented with 7 or 35 nM geminin. Chromatin was isolated and immunoblotted for Orc1, Cdc6, Cdt1, Mcm7 and geminin.
Geminin is the major inhibitor of origin assembly in metaphase extracts
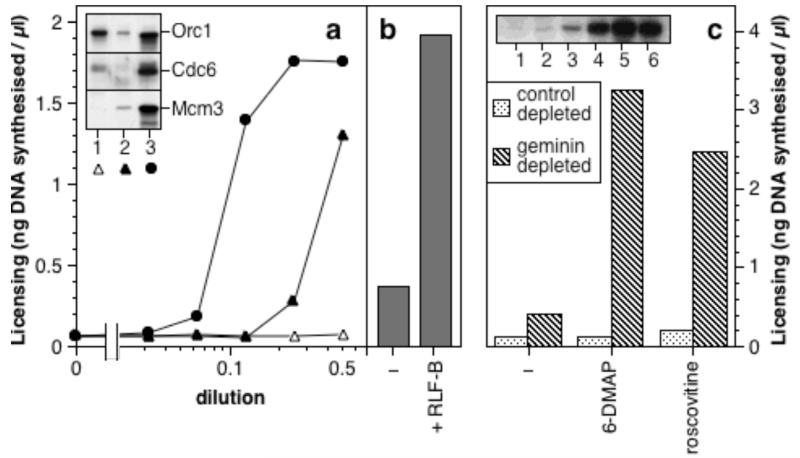
Metaphase Xenopus extracts contain an inhibitor of licensing that is lost on exit from mitosis40,43. This inhibitor does not appear to target Mcm(2-7), since Mcm(2-7) activity can be detected in metaphase extracts40. Figure 6a shows that when unlicensed “6DMAP chromatin” (which contains ORC, Cdc6, Cdt1 and geminin) was incubated in metaphase extract, no licensing occurred. However, addition of purified RLF-B/Cdt1 to the metaphase extract promoted licensing. This suggests that metaphase extract cannot license DNA because it contains an inhibitor of RLF-B/Cdt1. Since geminin is degraded on exit from metaphase just as RLF-B becomes active40,41 and we have shown above that geminin inhibits RLF-B/Cdt1, geminin is a good candidate for this inhibitor. We therefore immunodepleted metaphase extracts of geminin and tested its ability to inhibit licensing. Figure 6b shows that unlike control-depleted metaphase extract, high concentrations of geminin-depleted extract did not inhibit, but instead stimulated, licensing. Although there is an unavoidable ~2-fold dilution of extracts as a consequence of the immunodepletion protocol, geminin-depleted and control-depleted extracts had similar CDK activity as judged by H1 kinase levels (Fig 6c), and geminin-depleted extract still condensed sperm DNA into mitotic chromatids (Fig 6d). This suggests that the depleted extracts are still arrested in metaphase and that the loss of the licensing inhibitor following geminin depletion is not simply due to a loss of CDK activity. As well as having lost its licensing inhibitor, geminin-depleted metaphase extract was competent to license “6DMAP chromatin” by itself (Figure 6e). This suggests that metaphase extracts contain both RLF-B/Cdt1 and Mcm(2-7) activities, with RLF-B/Cdt1 normally being restrained by geminin.
Figure 6.
Characterization of the licensing inhibitor in metaphase extracts
a, Metaphase-arrested extract was mixed with either geminin bead-purified RLF-B or interphase extract. The mixture was incubated for 20 min with “6-DMAP chromatin”, and licensing subsequently assessed. b, Metaphase extract was immunodepleted with anti-geminin antibodies (filled triangles) or non-immune antibodies (open triangles). Serial dilutions in LFB2/50 buffer of depleted extract or untreated metaphase extract (open circles) were then assayed for their ability to inhibit licensing of “6-DMAP chromatin” by an equal volume of interphase extract. Inset: extract depleted with control antibodies (lane 1) or anti-geminin antibodies (lane 2) immunoblotted for geminin. c, Histone H1 kinase activity of interphase extract (1), metaphase extract (2), geminin-depleted metaphase extract (3) or control-depleted metaphase extract (4). d, Morphology of sperm nuclei incubated in metaphase extract (i, ii), geminin-depleted metaphase extract (iii, iv), or interphase extract (v, vi), stained with Hoechst 33258 and viewed under UV fluorescence (i, iii, v) or phase contrast optics (ii, iv, vi). Scale bar 10 μm. e, The indicated dilution of depleted metaphase extract, or untreated metaphase or interphase extract was incubated for 20 min with “6-DMAP chromatin” and licensing subsequently assessed.
In order for chromatin to become licensed by the combined activity of RLF-B/Cdt1 and Mcm(2-7), it must already contain bound ORC and Cdc616-19. Figure 7a shows that geminin-depletion of metaphase extract allowed it to license naive sperm nuclei containing none of these proteins. Geminin is therefore essential for blocking the assembly of licensed origins in metaphase. However, serial dilution revealed that the licensing activity of geminin-depleted metaphase extracts was less than that of untreated interphase extract (Figs 6e and 7a). Analysis of proteins assembled onto chromatin (Fig 7a, inset) showed that in geminin-depleted metaphase extract, the Mcm3 component of the Mcm(2-7) heterohexamer was assembled onto chromatin, though at levels lower than in interphase extract. Since 10-20 copies of Mcm3 are loaded onto each origin in interphase extracts but maximal replication requires only ~1 copy per origin40, the quantity of Mcm3 loaded onto chromatin correlates well with the activity titration. In control-depleted metaphase extract, levels of Orc1 and Cdc6 loaded onto chromatin were lower than in interphase extracts, consistent with previous reports44,45. We have previously shown that once licensing has occurred, the affinity of Orc1 for chromatin in metaphase extract is lowered45. Consistent with this, depletion of geminin from the metaphase extract permitted origins to become licensed and thus reduced the quantity of Orc1 on chromatin even further (Fig 7a, inset).
Figure 7.
Licensing of sperm nuclei in geminin-depleted metaphase extract.
a, The indicated dilution of geminin-depleted metaphase extract (filled triangles), control-depleted metaphase extract (open triangles), or untreated interphase extract (filled circles) was incubated for 20 min with Xenopus sperm nuclei and licensing subsequently assessed. Inset: sperm chromatin incubated for 20 mins in 0.5× control-depleted metaphase extract (lane 1), geminin-depleted metaphase extract (lane 2), or interphase extract (lane 3), was isolated and immunoblotted for Orc1, Cdc6 and Mcm3. b, A 0.25 dilution of geminin-depleted metaphase extract plus or minus RLF-B was incubated for 20 min with sperm nuclei and licensing subsequently assessed. c, A 0.25 dilution of geminin-depleted metaphase extract or control-depleted metaphase extract plus or minus 3mM 6-DMAP or 2mM roscovitine was incubated for 20 min with sperm nuclei; the chromatin was then isolated and licensing assessed. Inset: Sperm chromatin incubated in 0.5× non-immune depleted metaphase extract (lanes 1, 2), geminin-depleted metaphase extract (lanes 3, 4) or interphase extract (lanes 5, 6) minus (lanes 1, 3, 5) or plus (lanes 2, 4, 6) 2 mM roscovitine, isolated and immunoblotted for Mcm7.
We next explored possible explanations for the relatively low licensing activity of geminin-depleted metaphase extracts. The licensing activity of diluted geminin-depleted extract could be stimulated by addition of purified RLF-B/Cdt1 (Fig 7b), showing that the extract contained adequate nucleoplasmin, ORC, Cdc6 and Mcm(2-7). Figure 7c shows that when dilutions of geminin-depleted metaphase extract with only partial licensing activity were supplemented with the CDK inhibitors 6-DMAP or roscovitine46,47, licensing was strongly stimulated. At an equivalent dilution of control-depleted extract, addition of these inhibitors caused virtually no stimulation. A combination of geminin-depletion and CDK inhibition in metaphase extract was sufficient to load Mcm7 onto chromatin at levels approaching those seen in interphase extracts (Fig 7c, inset). These results suggest that CDKs provide an additional inhibitory effect on replication licensing, but that in the absence of geminin this is insufficient to block licensing.
Discussion
In this paper we have shown that the previously unidentified RLF-B activity is provided by Cdt1 protein. We have shown that RLF-B/Cdt1 is specifically inhibited by geminin and that the two proteins can interact tightly with one another. We also show that in Xenopus egg extracts, geminin is the major inhibitor of origin assembly in metaphase. When depleted of geminin, metaphase extracts become competent to assembly functional licensed replication origins. These results are in contrast with experiments in yeast which show that inhibition of cyclin dependent kinases late in the cell cycle is necessary and sufficient to permit origin re-assembly.
Geminin inhibits replication by antagonizing RLF-B/Cdt1 function
We have purified the RLF-B component of the replication licensing system by exploiting its high affinity for recombinant geminin protein. Purified RLF-B consisted of a single major polypeptide of ~75 kDa that was recognized by antibodies to the Xenopus Cdt1 protein. Recombinant Cdt1 could provide RLF-B activity, whilst depletion of Cdt1 from extracts also removed RLF-B activity. Recent work has shown that like RLF-B4,23, Cdt1 is required for loading Mcm(2-7) proteins onto DNA20,21. We also demonstrate that purified RLF-B can restore licensing activity to Cdt1-depleted extracts. Taken together, these results provide strong evidence for the identity of RLF-B and Cdt1.
We also provide evidence that RLF-B/Cdt1 is the major target of replication inhibition by geminin. Addition of recombinant geminin to Xenopus egg extracts blocks the loading of Mcm(2-7) onto chromatin and functional origin licensing. The inhibition of replication caused by recombinant geminin can be overcome by addition of recombinant Cdt1 or purified RLF-B, whilst endogenous RLF-B/Cdt1 binds specifically to recombinant geminin. Further, ~50% Cdt1 in interphase Xenopus egg extracts is in an inactive form that appears to be in a complex with endogenous geminin. These results suggest that geminin is a highly specific inhibitor of RLF-B/Cdt1.
The simplest interpretation for the inhibition of RLF-B/Cdt1 by geminin is that the two proteins interact via their coiled-coil domains, leaving Cdt1 unable to provide RLF-B activity. This is consistent with the strong interaction seen between purified RLF-B/Cdt1 and recombinant geminin, which required 4 M urea to release purified RLF-B/Cdt1. A similar sort of interaction between recombinant geminin and endogenous RLF-B/Cdt1 probably occurs in vivo since addition of recombinant geminin to interphase extract can be used to remove most endogenous RLF-B/Cdt1 (Figs 3b and 4a). However, residual RLF-B activity remained in these extracts following geminin removal, suggesting that there is something in whole extract that is capable of reducing the affinity of these proteins for one another. In metaphase extracts the affinity of endogenous geminin and endogenous RLF-B/Cdt1 appears to be even weaker, since immunodepletion of geminin from metaphase extract left a significant proportion of RLF-B/Cdt1 behind. We are currently investigating the possible existence of factors that can modulate the interaction between RLF-B/Cdt1 and geminin.
Geminin is the major inhibitor of origin assembly in metaphase-arrested Xenopus extracts
RLF-B activity is highly periodic in the Xenopus cell-free system, unlike other components of the replication licensing system40. RLF-B is absent during metaphase, but is abruptly activated on exit into anaphase, and then declines again during interphase. The absence of RLF-B activity in metaphase is due to the presence of an RLF-B inhibitor40, and the activation of RLF-B on progression into anaphase corresponds to the time when geminin undergoes cell-cycle specific degradation41. We show here that this inhibition is dependent on the presence of geminin, and that when geminin was immunodepleted from metaphase extracts, the extract became competent to assemble licensed replication origins on sperm chromatin. A similar effect was achieved by addition of RLF-B/Cdt1 to metaphase extract. This suggests that the main reason why origin assembly is inhibited in these metaphase extracts is because of geminin-mediated repression of RLF-B/Cdt1 (Fig 8a).
Figure 8.
Model for licensing control during mitosis.
a, In metaphase, RLF-B/Cdt1 is inhibited by geminin. CDKs play a minor role in preventing origin assembly by reducing the affinity of ORC and Cdc6 for replication origins, or possibly by direct inhibition of RLF-B/Cdt1. The Mcm(2-7) heterohexamer (“M”) probably remains active during metaphase. b, On exit from metaphase, geminin and the mitotic cyclins are degraded, thus allowing Mcm(2-7) to be assembled onto origins under the combined action of ORC, Cdc6 and RLF-B/Cdt1. Inhibition of CDK activity in metaphase prevents the degradation of geminin, and so stabilizes the inhibitory state into interphase.
This conclusion appears to contrast with results obtained in yeast. When CDK activity is abolished in G2 of the yeast cell cycle, chromosomal DNA is re-replicated25-28 presumably as a consequence of the rebinding of Mcm(2-7) to origins. Further, the formation of pre-RCs (licensed origins) in yeast can only occur in G1, when CDK activity is low29. Importantly, the ability to activate pre-RC re-assembly following CDK inactivation does not require the activity of the Anaphase Promoting Complex (APC/C) that is required for the cell-cycle-dependent degradation of proteins during anaphase30. This suggests that in yeast, re-licensing that occurs as a consequence of CDK inactivation does not depend on the degradation of (currently unidentified) geminin homologues.
Previous work in the Xenopus system has also shown that inhibition of CDK activity alone is not sufficient to induce re-licensing of replicated DNA. When extracts arrested in G2 phase were treated with the CDK inhibitor p21cip1/waf1, re-licensing of replicated DNA was not observed39. When CDK inhibitors (6-DMAP, staurosporine or olomoucine) are added to metaphase extracts, they spontaneously exit into an interphase-like state whilst maintaining their metaphase-specific RLF-B inhibition40,42,43,46. These observations are consistent with the conclusion of the current work, which shows that only when geminin is removed from metaphase extracts do they lose their RLF-B/Cdt1 inhibitory activity and become competent to assemble licensed replication origins. Addition of 6-DMAP to metaphase extracts is known to block the degradation of cyclins that normally occurs on exit from metaphase40,48. The lack of RLF-B activity in 6-DMAP-treated extracts is therefore likely to be in at least part due to a failure to degrade geminin, though other effects may also operate (Li and Blow, unpublished data).
Whilst geminin appears to be the major activity responsible for blocking origin assembly in metaphase Xenopus extracts, CDKs clearly play a role. Inhibition of CDK activity in geminin-depleted metaphase extracts strongly stimulated origin assembly. Further, addition of large quantities of recombinant CDKs to Xenopus extracts inhibited origin assembly and licensing40,49. High CDK levels reduce the affinity of XORC and XCdc6 for chromatin44,45, which is clearly one route by which they can inhibit origin assembly (Fig 8a). Whether CDKs also directly inhibit RLF-B/Cdt1 is currently unclear. Since immunodepletion of geminin from metaphase Xenopus extracts necessarily dilutes the extracts, we cannot rule out the possibility that in untreated metaphase extracts, CDK-dependent inhibition of XORC and Cdc6 chromatin binding is also sufficient to block origin licensing. Nevertheless, on exit from metaphase, both geminin and the mitotic cyclins are degraded, thus removing both inhibitory components of the origin assembly pathway (Fig 8b).
The experiments presented here have addressed the role of geminin during metaphase of meiosis II (unfertilized Xenopus eggs). However, a similar inhibition of RLF-B activity is seen in interphase of the embryonic (mitotic) cell cycle that is dependent on new protein synthesis but not on CDK activity40. Further, a human geminin homologue in HeLa cells accumulates during interphase and disappears abruptly on exit from metaphase41. We therefore propose that the interaction between geminin and RLF-B/Cdt1 will play a conserved role in the mitotic cell cycles of higher eukaryotes.
Methods
Preparation of Egg Extracts
Metaphase-arrested and interphase Xenopus egg extracts were prepared as described50. 6-DMAP-treated extracts were prepared by supplementing metaphase extracts with 100 μg ml−1 cycloheximide, 25 mM phosphocreatine, 15 μg ml−1 creatine phosphokinase, 3 mM 6-DMAP and [α-32P]dATP, and were then released into interphase with 0.3 mM CaCl2.
Licensing Factor Extract (LFE) which was used as a source of protein for fractionation studies was prepared as described50. Briefly, eggs were activated for 5 minutes by the calcium ionophore A23187, before being spin-crushed in buffer lacking EGTA. Recovered cytoplasm was diluted 1:5 with LFB1 (40 mM Hepes KOH pH 8.0, 20 mM K2HPO4/KH2PO4 pH 8.0, 2 mM MgCl2, 1 mM EGTA, 2 mM DTT, 10% (w/v) sucrose and 1 μg ml−1 each of leupeptin, pepstatin and aprotinin) supplemented with 50 mM KCl (i.e. LFB1/50) and re-centrifuged at 20,000 rpm for 30 mins in an SW41 swinging bucket rotor (Beckman). The clarified supernatant (LFE) was frozen and stored at −80°C until required.
Immunodepletion of was performed as described50. Briefly, protein A Sepharose beads were incubated with 2 volumes of serum for 1 hr, and the beads were extensively washed. 100 μl metaphase-arrested or interphase Xenopus egg extract were incubated with 30-50 μl protein A Sepharose beads for 45 min at 4°C. After removal of the beads by centrifuging through a 5 μm nylon filter, the process was repeated. This procedure leads to an 2-fold dilution of the extract, and is therefore designated “0.5×” in titration experiments. Geminin-bead depleted extract was prepared in a similar way, except that 100 μl extract was incubated twice with 25 μl of geminin-coupled beads (see below) for 15 mins.
Chromatin Templates
Demembranated Xenopus sperm nuclei were prepared as described50 and frozen in aliquots in liquid nitrogen. Unlicensed “6-DMAP chromatin” was prepared by incubating sperm nuclei for 15 minutes at 23°C in 6-DMAP treated extracts at 30,000 nuclei μl−1. The extract was then diluted 10 fold in nuclear isolation buffer50 (NIB: 50 mM KCl, 50 mM Hepes KOH pH 7.6, 5 mM MgCl2, 2 mM DTT, 0.5 mM spermidine 3HCl, 0.15M spermine 4HCl, 1 μg ml−1 each of leupeptin, pepstatin and aprotinin) supplemented with 0.01% Triton X-100, and then underlayered with the same buffer containing 15% sucrose. The chromatin was pelleted at 6,000g in a swinging bucket rotor at 4°C for 5 min. The diluted extract was then removed and the chromatin pellet was resuspended in NIB and frozen in aliquots in liquid nitrogen. Isolation of chromatin for immunoblotting was performed in a similar way, except that the Triton X-100 concentration was 0.1%.
Purification of RLF-B
All chromatographic procedures were performed at 4°C. After thawing, Licensing Factor Extract was supplemented with 4.25 % w:v PEG 6000 (BDH), incubated on ice for 30 min, and centrifuged at 12,000 × g for 10 min in a fixed-angle rotor. The pellet was resuspended in LFB1 + 150 mM KCl, and adsorbed in batch for 15 min at 4°C onto freshly prepared, precycled phosphocellulose (Whatman) equilibrated in LFB1 + 150 mM KCl. After packing into a column, activity was eluted by step in LFB1 + 500 mM KCl. The phosphocellulose eluate was then supplemented with saturated ammonium sulphate in 50 mM Tris-Cl, pH 8 to a final concentration of 40% and incubated on ice for 40 min. Precipitated material was pelleted by centrifugation (12,000 × g for 10 min in a fixed-angle rotor) and was resuspended in LFB1 at 1/35 the volume of neat extract.
Recombinant geminin-DEL was coupled to AffiGel 10 beads (Biorad) at 20 - 40 μg / ml, and 300 μl beads were packed into a column. The column was loaded with 100 μl BPAS (35× with respect to starting extract) and washed with LFB2 buffer50 plus 0.03% Triton X-100 and 0.2 M KCl, THED buffer (20 mM Hepes-KOH pH 8, 20% ethylene glycol, 1 mM dithiothreitol, 0.03% Triton X-100) plus 1 M KCl, THED buffer plus 0.4 M KCl, and THED buffer plus 0.4 M KCl, 4 M urea and 0.6% polyvinyl alcohol. 500 μl geminin bead eluate (fractions 33-37) were then further fractionated by Superose 6 gel filtration (Pharmacia) in THED buffer plus 0.4 M KCl, 4 M urea and 0.6% polyvinyl alcohol. For assay, samples were supplemented with 1/25 vol 10% BSA (which is essential to prevent precipitation) and were then desalted using spin columns into LFB1 + 200 mM KCl + 0.03% Triton X-100.
For phenyl Sepharose chromatography23, BPAS was made 1 M with respect to KCl in Hepes Buffer (20 mM Hepes KOH pH 8; 10% sucrose; 1 μg/ml each aprotinin, leupeptin and pepstatin) and applied to a 1ml HiTrap phenyl Sepharose column (Pharmacia) equilibrated in the same buffer. The column was developed with a gradient over 3 column volumes to Hepes Buffer without KCl. For assay, samples were desalted as above.
Recombinant proteins and antibodies
Recombinant Xenopus geminin-DEL41 was produced from an expression plasmid kindly provided by T. McGarry, and was purified on Ni-agarose by standard techniques. Recombinant protein was used to generate rabbit antiserum. Recombinant Xenopus Cdt1 (XCdt1) was produced as previously described20. Anti-XCdt1 antibodies were as described previously20.
Licensing Assay
To assay licensing, 2 μl fractions (typically containing 1 μl RLF-B and 1 μl RLF-M, or 2 μl whole extract) were incubated with 0.3 μl unlicensed “6-DMAP chromatin” (containing ORC and Cdc6) or 0.3 μl demembranated Xenopus sperm nuclei at 80 ng DNA / μl and incubated for 20 min at 23°C. 5.7 μl 6-DMAP-treated extract containing [α-32P]dATP was subsequently added and the total DNA synthesised over a further 90 min incubation was measured by TCA precipitation42,50.
Acknowledgments
We thank Jason Swedlow, Tom Owen-Hughes, Neil Perkins, Margret Michalski and members of the lab for comments on the manuscript. This work was supported by the Cancer Research Campaign [CRC] grant SP2385/0101 and by a Uehara Fellowship to ST.
References
- 1.Blow JJ, Laskey RA. A role for the nuclear envelope in controlling DNA replication within the cell cycle. Nature. 1988;332:546–548. doi: 10.1038/332546a0. [DOI] [PubMed] [Google Scholar]
- 2.Diffley JF. Once and only once upon a time: specifying and regulating origins of DNA replication in eukaryotic cells. Genes Dev. 1996;10:2819–2830. doi: 10.1101/gad.10.22.2819. [DOI] [PubMed] [Google Scholar]
- 3.Tada S, Blow JJ. The replication licensing system. Biol. Chem. 1998;379:941–949. [PMC free article] [PubMed] [Google Scholar]
- 4.Chong JPJ, Mahbubani MH, Khoo C-Y, Blow JJ. Purification of an Mcm-containing complex as a component of the DNA replication licensing system. Nature. 1995;375:418–421. doi: 10.1038/375418a0. [DOI] [PubMed] [Google Scholar]
- 5.Kubota Y, Mimura S, Nishimoto S, Takisawa H, Nojima H. Identification of the yeast MCM3-related protein as a component of Xenopus DNA Replication Licensing Factor. Cell. 1995;81:601–609. doi: 10.1016/0092-8674(95)90081-0. [DOI] [PubMed] [Google Scholar]
- 6.Madine MA, Khoo C-Y, Mills AD, Laskey RA. MCM3 complex required for cell cycle regulation of DNA replication in vertebrate cells. Nature. 1995;375:421–424. doi: 10.1038/375421a0. [DOI] [PubMed] [Google Scholar]
- 7.Kubota Y, et al. Licensing of DNA replication by a multi-protein complex of MCM/P1 proteins in Xenopus eggs. EMBO J. 1997;16:3320–3331. doi: 10.1093/emboj/16.11.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thömmes P, Kubota Y, Takisawa H, Blow JJ. The RLF-M component of the replication licensing system forms complexes containing all six MCM/P1 polypeptides. EMBO J. 1997;16:3312–3319. doi: 10.1093/emboj/16.11.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prokhorova TA, Blow JJ. Sequential MCM/P1 subcomplex assembly is required to form a heterohexamer with replication licensing activity. J. Biol. Chem. 2000;275:2491–2498. doi: 10.1074/jbc.275.4.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diffley JF, Cocker JH, Dowell SJ, Rowley A. Two steps in the assembly of complexes at yeast replication origins in vivo. Cell. 1994;78:303–316. doi: 10.1016/0092-8674(94)90299-2. [DOI] [PubMed] [Google Scholar]
- 11.Ishimi Y. A DNA helicase activity is associated with an MCM4, -6, and -7 protein complex. J. Biol. Chem. 1997;272:24508–24513. doi: 10.1074/jbc.272.39.24508. [DOI] [PubMed] [Google Scholar]
- 12.Kelman Z, Lee JK, Hurwitz J. The single minichromosome maintenance protein of Methanobacterium thermoautotrophicum Delta H contains DNA helicase activity. Proc. Natl. Acad. Sci. U. S. A. 1999;96:14783–14788. doi: 10.1073/pnas.96.26.14783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chong JPJ, Hayashi MK, Simon MN, Xu RM, Stillman B. A double-hexamer archaeal minichromosome maintenance protein is an ATP-dependent DNA helicase. Proc. Natl. Acad. Sci. U. S. A. 2000;97:1530–1535. doi: 10.1073/pnas.030539597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labib K, Tercero JA, Diffley JFX. Uninterrupted MCM2-7 function required for DNA replication fork progression. Science. 2000;288:1643–1647. doi: 10.1126/science.288.5471.1643. [DOI] [PubMed] [Google Scholar]
- 15.Shechter DF, Ying CY, Gautier J. The intrinsic DNA helicase activity of Methanobacterium thermoautotrophicum Delta H minichromosome maintenance protein. J. Biol. Chem. 2000;275:15049–15059. doi: 10.1074/jbc.M000398200. [DOI] [PubMed] [Google Scholar]
- 16.Cocker JH, Piatti S, Santocanale C, Nasmyth K, Diffley JF. An essential role for the Cdc6 protein in forming the pre-replicative complexes of budding yeast. Nature. 1996;379:180–182. doi: 10.1038/379180a0. [DOI] [PubMed] [Google Scholar]
- 17.Coleman TR, Carpenter PB, Dunphy WG. The Xenopus Cdc6 protein is essential for the initiation of a single round of DNA replication in cell-free extracts. Cell. 1996;87:53–63. doi: 10.1016/s0092-8674(00)81322-7. [DOI] [PubMed] [Google Scholar]
- 18.Rowles A, et al. Interaction between the origin recognition complex and the replication licensing system in Xenopus. Cell. 1996;87:287–296. doi: 10.1016/s0092-8674(00)81346-x. [DOI] [PubMed] [Google Scholar]
- 19.Romanowski P, Madine MA, Rowles A, Blow JJ, Laskey RA. The Xenopus origin recognition complex is essential for DNA replication and MCM binding to chromatin. Curr. Biol. 1996;6:1416–1425. doi: 10.1016/s0960-9822(96)00746-4. [DOI] [PubMed] [Google Scholar]
- 20.Maiorano D, Moreau J, Mechali M. XCDT1 is required for the assembly of pre-replicative complexes in Xenopus laevis. Nature. 2000;404:622–625. doi: 10.1038/35007104. [DOI] [PubMed] [Google Scholar]
- 21.Nishitani H, Lygerou Z, Nishimoto T, Nurse P. The Cdt1 protein is required to license DNA for replication in fission yeast. Nature. 2000;404:625–628. doi: 10.1038/35007110. [DOI] [PubMed] [Google Scholar]
- 22.Whittaker AJ, Royzman I, Orr-Weaver TL. Drosophila Double parked: a conserved, essential replication protein that colocalizes with the origin recognition complex and links DNA replication with mitosis and the down-regulation of S phase transcripts. Genes Dev. 2000;14:1765–1776. [PMC free article] [PubMed] [Google Scholar]
- 23.Tada S, Chong JPJ, Mahbubani HM, Blow JJ. The RLF-B component of the replication licensing system is distinct from Cdc6 and functions after Cdc6 binds to chromatin. Curr. Biol. 1999;9:211–214. doi: 10.1016/s0960-9822(99)80092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillespie PJ, Blow JJ. Nucleoplasmin-mediated chromatin remodelling is required for Xenopus sperm nuclei to become licensed for DNA replication. Nucleic Acids Res. 2000;28:472–480. doi: 10.1093/nar/28.2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broek D, Bartlett R, Crawford K, Nurse P. Involvement of p34cdc2 in establishing the dependency of S phase on mitosis. Nature. 1991;349:388–393. doi: 10.1038/349388a0. [DOI] [PubMed] [Google Scholar]
- 26.Hayles J, Fisher D, Woollard A, Nurse P. Temporal order of S phase and mitosis in fission yeast is determined by the state of the p34cdc2-mitotic B cyclin complex. Cell. 1994;78:813–822. doi: 10.1016/s0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 27.Correa-Bordes J, Nurse P. p25rum1 orders S phase and mitosis by acting as an inhibitor of the p34cdc2 mitotic kinase. Cell. 1995;83:1001–1009. doi: 10.1016/0092-8674(95)90215-5. [DOI] [PubMed] [Google Scholar]
- 28.Dahmann C, Diffley J, Nasmyth K. S-phase-promoting cyclin-dependent kinases prevent re-replication by inhibiting the transition of replication origins to a pre-replicative state. Curr. Biol. 1995;5:1257–1269. doi: 10.1016/s0960-9822(95)00252-1. [DOI] [PubMed] [Google Scholar]
- 29.Piatti S, Bohm T, Cocker JH, Diffley JF, Nasmyth K. Activation of S-phase-promoting CDKs in late G1 defines a “point of no return” after which Cdc6 synthesis cannot promote DNA replication in yeast. Genes Dev. 1996;10:1516–1531. doi: 10.1101/gad.10.12.1516. [DOI] [PubMed] [Google Scholar]
- 30.Noton E, Diffley JFX. CDK inactivation is the only essential function of the APC/C and the mitotic exit network proteins for origin resetting during mitosis. Mol. Cell. 2000;5:85–95. doi: 10.1016/s1097-2765(00)80405-0. [DOI] [PubMed] [Google Scholar]
- 31.Hennessy KM, Clark CD, Botstein D. Subcellular localization of yeast CDC46 varies with the cell cycle. Genes Dev. 1990;4:2252–2263. doi: 10.1101/gad.4.12b.2252. [DOI] [PubMed] [Google Scholar]
- 32.Labib K, Diffley JFX, Kearsey SE. G1-phase and B-type cyclins exclude the DNA-replication factor Mcm4 from the nucleus. Nat. Cell Biol. 1999;1:415–422. doi: 10.1038/15649. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen VQ, Co C, Irie K, Li JJ. Clb/Cdc28 kinases promote nuclear export of the replication initiator proteins Mcm2-7. Curr. Biol. 2000;10:195–205. doi: 10.1016/s0960-9822(00)00337-7. [DOI] [PubMed] [Google Scholar]
- 34.Drury LS, Perkins G, Diffley JFX. The Cdc4/34/53 pathway targets Cdc6p for proteolysis in budding yeast. EMBO J. 1997;16:5966–5976. doi: 10.1093/emboj/16.19.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elsasser S, Chi Y, Yang P, Campbell JL. Phosphorylation controls timing of Cdc6p destruction: A biochemical analysis. Mol. Biol. Cell. 1999;10:3263–3277. doi: 10.1091/mbc.10.10.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saha P, et al. Human CDC6/Cdc18 associates with Orc1 and cyclin-cdk and is selectively eliminated from the nucleus at the onset of S phase. Mol. Cell. Biol. 1998;18:2758–2767. doi: 10.1128/mcb.18.5.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang W, Wells NJ, Hunter T. Multistep regulation of DNA replication by Cdk phosphorylation of HsCdc6. Proc. Natl. Acad. Sci. U. S. A. 1999;96:6193–6198. doi: 10.1073/pnas.96.11.6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petersen BO, Lukas J, Sorensen CS, Bartek J, Helin K. Phosphorylation of mammalian CDC6 by Cyclin A/CDK2 regulates its subcellular localization. EMBO J. 1999;18:396–410. doi: 10.1093/emboj/18.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun W, et al. The replication capacity of intact mammalian nuclei in Xenopus egg extracts declines with quiescence, but the residual DNA synthesis is independent of Xenopus MCM proteins. J. Cell Sci. 2000;113:683–695. doi: 10.1242/jcs.113.4.683. [DOI] [PubMed] [Google Scholar]
- 40.Mahbubani HM, Chong JP, Chevalier S, Thömmes P, Blow JJ. Cell cycle regulation of the replication licensing system: involvement of a Cdk-dependent inhibitor. J. Cell Biol. 1997;136:125–135. doi: 10.1083/jcb.136.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGarry TJ, Kirschner MW. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell. 1998;93:1043–1053. doi: 10.1016/s0092-8674(00)81209-x. [DOI] [PubMed] [Google Scholar]
- 42.Blow JJ. Preventing re-replication of DNA in a single cell cycle: evidence for a replication licensing factor. J. Cell Biol. 1993;122:993–1002. doi: 10.1083/jcb.122.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kubota Y, Takisawa H. Determination of initiation of DNA replication before and after nuclear formation in Xenopus egg cell free extracts. J. Cell Biol. 1993;123:1321–1331. doi: 10.1083/jcb.123.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hua XH, Newport J. Identification of a preinitiation step in DNA replication that is independent of origin recognition complex and cdc6, but dependent on cdk2. J. Cell Biol. 1998;140:271–281. doi: 10.1083/jcb.140.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rowles A, Tada S, Blow JJ. Changes in association of the Xenopus origin recognition complex with chromatin on licensing of replication origins. J. Cell Sci. 1999;112:2011–2018. doi: 10.1242/jcs.112.12.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vesely J, et al. Inhibition of Cyclin-Dependent Kinases by Purine Analogs. Eur. J. Biochem. 1994;224:771–786. doi: 10.1111/j.1432-1033.1994.00771.x. [DOI] [PubMed] [Google Scholar]
- 47.Meijer L, et al. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 48.Luca FC, Ruderman JV. Control of programmed cyclin destruction in a cell-free system. J. Cell Biol. 1989;109:1895–1909. doi: 10.1083/jcb.109.5.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hua XH, Yan H, Newport J. A role for Cdk2 kinase in negatively regulating DNA replication during S phase of the cell cycle. J. Cell Biol. 1997;137:183–192. doi: 10.1083/jcb.137.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chong JP, Thömmes P, Rowles A, Mahbubani HM, Blow JJ. Characterization of the Xenopus replication licensing system. Methods Enzymol. 1997;283:549–564. doi: 10.1016/s0076-6879(97)83043-1. [DOI] [PubMed] [Google Scholar]