Abstract
Purpose
Compare the clinical characteristics, rate of recurrent venous thromboembolism (VTE) and outcome of suspected and unsuspected pulmonary embolism (PE) detected on computed tomography in patients with lung cancer.
Methods
In this IRB-approved retrospective study, 77 patients [38 men, 39 women; mean age 64 (range, 35-90)] with lung cancer who developed PE between January 2004 and December 2009 were identified using research patient data registry and medical records. Patients with suspected (45/77,58%) and unsuspected (32/77,42%) PE were compared for the characteristics, treatment of PE, and rate of recurrent VTE using Fisher's exact test. The survival was compared using log-rank test, and Cox proportional hazards regression models were applied for univariate and multivariable analyses.
Results
Most cases of PE were found in patients undergoing chemotherapy (79%) and with metastatic disease (70%). Suspected PE more commonly involved main/lobar pulmonary arteries (33/45,73% vs. 9/32, 28%), while unsuspected PE more frequently involved of segmental/subsegmental arteries (p = 0.0001). All 11 cases of squamous cell carcinoma had suspected PE. Suspected and unsuspected PE did not differ in terms of age, gender, presence of metastatic disease at the time of PE or treatment for PE. 44/45 (98%) patients with suspected PE and 30/32 (94%) patients with unsuspected PE were treated for PE, mostly with anticoagulation (68/74,92%). Recurrent VTE was seen in 20% (9/45) of suspected PE and 19% (6/32) of unsuspected PE (p = 1.00). Median survival after PE was 5.6 months in suspected group and 6.2 month in unsuspected group, without significant difference by univariate or multivariate analyses.
Conclusion
Although unsuspected PE more frequently involved peripheral pulmonary arteries, the treatments of PE, bleeding complications, rates of recurrent VTE, and survival after PE were similar for clinically suspected and unsuspected PE.
Keywords: Lung cancer, Pulmonary embolism, Venous thromboembolism
1. Introduction
It is well-known that cancer patients have a higher incidence of venous thromboembolism (VTE), including pulmonary embolism (PE) and deep venous thrombosis (DVT), compared to the general population [1]. With the advances in multidetector-row computed tomography (MDCT) technology and its increasing use for routine staging and follow-up imaging in cancer patients, clinically unsuspected PE are commonly detected as incidental findings on routine oncology CT scans [2]. Unsuspected PE on routine CT scans is noted in 1.5% of the cases in general population, with 5% prevalence in inpatient and 0.6% prevalence in outpatient [2]. In addition, recognition of PE as an important complication in cancer patients has led to consideration of primary antithrombotic prophylaxis, even in ambulatory patients with cancer [3–5]. Low molecular weight heparin (LMWH) is considered to be the initial treatment of choice for VTE [6,7], while LMWH or low-dose unfractionated heparin have been recommended for prophylaxis of VTE in high risk patients [8].
Given the increasing number of patients with cancer treated and followed as outpatients, this population of cancer patients is of considerable interest. We have previously investigated the incidence of suspected and unsuspected PE in a population of 13,783 oncologic outpatients who underwent imaging studies at our institution over a six year period, and reported that the overall incidence of PE was 2.87% during a 6-year period, of which 51.1% had clinically unsuspected PE noted on routine staging or follow-up CT scans [9]. When the risk of PE was compared across 16 predefined cancer types, lung/pleural malignancy was one of the four cancer types which had significantly higher risk for PE compared to others, along with central nervous system, pancreatic and upper gastrointestinal malignancies [9]. During the course of the analysis of the data, it came to our attention that the patients with lung cancer consist of the largest subcohort of the population that developed PE. The clinical question was raised as to whether there is any difference in patient outcomes and clinical characteristics between those with unsuspected vs. suspected PE, which led to further analysis conducted in the present study.
Lung cancer is the leading cause of cancer death in the United States for both men and women, with over 156,000 deaths in 2011 in the United States [10]. It is also one of the malignancies that are commonly associated with VTE, including PE, with reported incidence of VTE 3–13.8% and that of PE up to 3.8% [9,11–13]. While high incidence of PE in lung cancer patients is known, to our knowledge, the characteristics and clinical outcome of clinically suspected and unsuspected PE in patients with lung cancer has not yet been systematically studied. Recently, den Exter et al. evaluated the outcome of incidental PE in 51 cancer patients and reported the similar high rate of recurrent VTE, bleeding complications and mortality as in the symptomatic group. However, lung cancer was one of the eight different cancer types included in this study, and only eight patients with unsuspected PE had lung cancer [14]. Studies on larger patient population are lacking, and currently there is no consensus on the optimal management of PE in this patient population.
The purpose of the present study was to investigate the clinical characteristics, rate of recurrent VTE and survival of lung cancer patients who developed unsuspected PE in comparison with patients with suspected PE.
2. Materials and methods
2.1. Subjects
In this institutional review board-approved, Health Insurance Portability and Accountability Act (HIPAA) compliant retrospective study, the previously reported original cohort included a total of 13,783 cancer patients with 16 different cancer types who had imaging studies at our institution as outpatients from January 2004 to December 2009, of which 395 developed PE based on radiology reports [9]. In the original cohort of 13,783, there were 2262 patients with lung cancer, of which 77 patients developed PE, including 32 patients who developed unsuspected PE and 45 patients with suspected PE. Therefore, the study population of the present study included 77 patients with pathologically proven lung cancer who developed PE.
2.2. Clinical data
For each patient, the clinical data including age, gender, tumor histology, metastatic status and anti-cancer therapy status at the time of PE were collected using the electronic medical records. Whether PE was clinically suspected or not was also recorded for each patient. The PE was considered as suspected if a dedicated CT pulmonary angiography study was ordered by the referring physician, and considered unsuspected if the PE was incidentally detected on routine staging or follow-up CT scans as described previously [5].
The most proximal location of PE was recorded based on the radiology reports. The location of PE was categorized as main, lobar, segmental or subsegmental [9]. When PE involved multiple locations, the most proximal location was recorded. PE involving main or lobar pulmonary arteries was considered to be proximal, and that involving segmental and subsegmental arteries was considered to be distal [9]. Involvement of bilateral pulmonary arteries was also recorded.
Management of PE, including types of anti-coagulation therapy, the occurrence of bleeding complications, recurrent VTE events and their management, as well as the date of death were also obtained. Recurrent PE was defined as a new intraluminal filling defect in a pulmonary artery branch. Documentation of a single episode of PE on multiple subsequent scans was not considered a recurrence. Each episode of DVT was confirmed by compression duplex ultrasonography or contrast venography [15].
2.3. Comparison of suspected and unsuspected PE
Demographic and clinical characteristics of the patients, the location and treatment for PE, and the rate of recurrent VTE were compared between suspected and unsuspected PE using Fisher's exact test. For the patients with recurrent PE, the demographic characteristics at the time of the first episode of PE were used for this comparison. The median time between the initial diagnosis of lung cancer and the first episode of PE was compared between the suspected and unsuspected groups, between those with and without recurrent VTE, and between those with proximal and distal PE, using the Wilcoxon rank-sum test. The survival after the diagnosis of PE was compared between the suspected and unsuspected groups, between patients with recurrent VTE and those without, as well as proximal and distal PE using the log-rank test assuming the Cox proportional hazard model. Kaplan–Meier plots were created to illustrate the difference in survival times.
To further study the survival between two groups adjusting for potential confounders, multivariate analysis was performed. The association of suspected and unsuspected PE with overall survival, as well as the association of proximal and distal PE with overall survival, was assessed by applying the Cox proportional hazards regression model. Propensity scores were used to adjust for potential confounders in the multivariate model, reducing them to a single composite variable, because of the limited sample size. The propensity scores were obtained by creating binary logistic regression models, and included age, sex, histology, location of PE, and the presence of metastasis for unsuspected and suspected PE groups. The propensity scores for proximal and distal PE groups included age, sex, histology, and the presence of metastasis. The linear assumption was checked by including quadratic terms of continuous variables in each model. The proportional hazards assumption for covariates was examined with cumulative sums of Martingale-based residuals. No violation of assumption was observed. All the statistical analyses were performed with Statistical Analysis Software version 9.3 (SAS Institute Inc., Cary, NC). A p value less than 0.05 was considered statistically significant.
3. Results
The study population consisted of 77 lung cancer patients (39 women and 38 men) with mean age of 64 years (range, 35–90 years). The median follow-up time after diagnosis of PE was 5.7 months (range, 0.1–79.0 months). Of 77 patients, 43 (56%) had adenocarcinoma, 11 (14%) had squamous cell carcinoma, 9 (12%) had poorly differentiated tumor, 3 (4%) had small cell cancer, one (1%) had large cell cancer, and 10 (13%) patients had subtype that was not specified [16]. Total 54 (70%) patients had metastatic disease and 23 (30%) had no evidence of metastases at the time of first episode of PE. Sixty-one (79%) patients were being treated with systemic anti-cancer therapeutic agents at the time of diagnosis of PE. Of 77 patients, 7 patients (9%) had indwelling catheters, which could predispose to fibrin sheath development and subsequent emboli.
In a total population of 77 patients, clinically suspected PE was noted in 45 (58%) patients, and 32 (42%) patients had unsuspected PE. Table 1 summarizes the clinical characteristics of patients with clinically suspected and unsuspected PE. Adenocarcinoma was more common in the clinically unsuspected group (20/45, 44% in suspected group vs. 23/32, 72% in unsuspected group, p = 0.02). All 11 cases of squamous cell carcinoma were in the clinically suspected group (11/45, 24% in suspected group vs. 0/32, 0% in unsuspected group, p = 0.002).There was no difference in the extent of disease in terms of presence or absence of metastatic disease between the clinically suspected and unsuspected groups (29/45, 64% vs. 25/32, 78%, respectively, p = 0.22). Among 77 patients who developed PE, 10 (13%) had liver metastasis at the time of PE. The prevalence of liver metastasis at the time of PE was not different between suspected (7/45) and unsuspected (3/32) groups (Fisher p = 0.51).
Table 1.
Clinical characteristics, treatment and outcome of the patients with suspected and unsuspected PE.
| Total (n = 77) | Suspected PE (n = 45) | Unsuspected PE(n=32) | p value | |
|---|---|---|---|---|
| Mean age, years (range) | 64(range,35–90) | 65 (range, 43–90) | 64(range,35–83) | 0.83a |
| Male gender | 38(49%) | 20(44%) | 18(56%) | 0.36b |
| Histology | ||||
| Adeno | 43(56%) | 20(44%) | 23(72%) | 0.02b |
| Squamous | 11 (14%) | 11(24%) | 0(0%) | 0.002b |
| Large cell | 1 (1%) | 0(0%) | 1 (3%) | 0.42b |
| Small cell | 3(4%) | 1 (2%) | 2(6%) | 0.57b |
| NSCLC, subtype not specified | 10(13%) | 6(13%) | 4(13%) | 1.00b |
| Poorly diff | 9(12%) | 7(16%) | 2(6%) | 0.29b |
| Metastatic disease | 54(70%) | 29(64%) | 25(78%) | 0.22b |
| On anti-cancer therapy | 61(79%) | 31 (69%) | 30(94%) | 0.01b |
| Treated for PE | 74(96%) | 44(98%)c | 30(94%) | 1.00 |
| Recurrent PE or DVT | 15(19%) | 9(20%) | 6(19%) | 1.00 |
Values in bold denote statistically significant differences.
Mann–Whitney test.
Fisher's exact probability test.
Information about treatment was not available in one patient.
Patients with unsuspected PE were more frequently on anti-cancer therapeutic agents compared to those with suspected PE (31/45,69% in suspected group vs. 30/32,94% in unsuspected group, p = 0.01). In 4 out of 45 patients (9%) with suspected PE, lung cancer was diagnosed on the CT pulmonary angiography performed for PE.
Table 2 summarizes the location of suspected and unsuspected PE. Clinically suspected PE more frequently involved the main pulmonary arteries (10/45, 22% in suspected group vs. 1/32, 3% in unsuspected group, p = 0.02) and lobar arteries (23/45, 51% in suspected group vs. 8/32, 25% in unsuspected group, p = 0.03) arteries. Segmental PE was more common in unsuspected group than in suspected group (5/45, 11% in suspected group vs. 12/32, 38% in unsuspected group, p = 0.01). While subsegmental PE had a tendency to be more common in the unsuspected group, this did not meet the criteria for statistical significance (p = 0.06). Suspected PE was more likely to be proximal, involving main and/or lobar pulmonary arteries than unsuspected PE (33/45, 73% vs. 9/32, 28%, respectively, p = 0.0001).
Table 2.
Location of pulmonary embolism.
| Suspected PE no. (%) (n = 45) | Unsuspected PE no. (%) (n = 32) | p valuea | |
|---|---|---|---|
| Location of pulmonary embolism | |||
| Main | 10(22%) | 1 (3%) | 0.02 |
| Lobar | 23 (51%) | 8(25%) | 0.03 |
| Segmental | 5 (11%) | 12 (38%) | 0.01 |
| Subsegmental | 7 (16%) | 11 (34%) | 0.06 |
| Bilateral PE | 19 (42%) | 10 (31%) | 0.35 |
Values in bold denote statistically significant differences.
Fisher's exact probability test.
74 of 77 patients (96%) were treated for PE, with no difference in number of treated patients between the suspected and unsuspected groups (44/45, 98% vs. 30/32, 94%, respectively, p = 1.00). Of 45 patients with suspected PE, 37 patients were treated with anticoagulation (27 with LMWH, four with heparin, three with warfarin and details not available in three), four patients were treated with both anticoagulation using LMWH and inferior vena cava (IVC) filter placement, two patients had IVC filter placement only, one patient was already receiving warfarin when PE was diagnosed; in one patient the information about treatment of PE was not available. Of 32 patients with unsuspected PE, 25 patients were treated with anticoagulation (20 with LMWH, three with Fondaparinux, one with heparin and details not available in one patient), four patients received IVC filter, one was already on anticoagulation (receiving warfarin) at diagnosis of PE, and two remained untreated. One of the two untreated patients had disseminated and progressive lung cancer and therefore was not anticoagulated, and the other patient was not treated with anticoagulation because of a history of hemoptysis and the patient refused IVC filter. A total 6 patients had contraindications to anticoagulant treatment due to recent history of hemoptysis (n = 4), known hemorrhagic brain metastases (n = 1) and gastrointestinal hemorrhage (n = 1). Of these 6 patients, all except for one untreated patient received IVC filter.
Two out of a total of 68 patients (3%) with anticoagulation developed major bleeding (intracranial hemorrhage in one patient with suspected PE and life-threatening hemoptysis in one patient with unsuspected PE), which resulted in discontinuation of anticoagulation and subsequent insertion of IVC filter. Another patient with suspected PE, who was initially treated with anticoagulation alone was, diagnosed with new hemorrhagic brain metastases and was subsequently treated with IVC filter.
The median follow-up time after diagnosis of PE was 5.6 months (range, 0.2–79.0 months) for patients with suspected PE and 6.0 months (range, 0.1–65.8 months) for those with unsuspected PE. Recurrent VTE was noted in 9/45 (20%) patients with suspected PE and 6/32 (19%) patients with unsuspected PE, without any statistical difference between the two groups (p = 1.00). There was no significant difference in metastatic status in patients with recurrent VTE vs. those without (11/15, 73% vs. 43/62, 69%, respectively, Fisher p = 1.00). Of the total 15 patients with recurrent VTE, 12 patients developed new episodes of PE, two developed subsequent DVT, and one patient developed thrombosis of the internal jugular vein. Among 9 patients with recurrent VTE after suspected PE, 3 patients were on anticoagulation using LMWH at the time of recurrent VTE, while 6 patients were not on anticoagulation. Of these 6 patients, 3 patients were on LMWH less than 6 months, 2 patients were on LMWH for more than 6 months, and 1 patient developed recurrent VTE in the form of internal jugular venous thrombus while on IVC filter without anticoagulation. Of 6 patients with recurrent VTE after unsuspected PE, 3 patients developed recurrent PE while on anticoagulation (warfarin in 2 and LMWH in 1), and 3 patients were off anticoagulation at the time of recurrent VTE. Of these 3 patients, one patient was off anticoagulation after continuing LMWH for more than 6 months; one patient was on IVC filter without anticoagulation; and the exact duration of anticoagulation was not known in one patient.
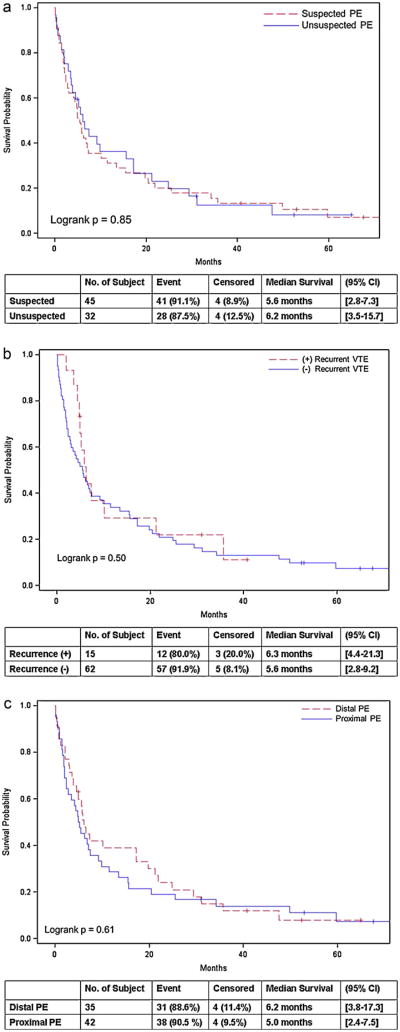
Table 3 summarizes the time between the diagnosis of lung cancer to the diagnosis of PE between suspected vs. unsuspected groups, patients with vs. without recurrent VTE, and those with proximal vs. distal PE. The interval between diagnoses of lung cancer and PE was shorter in patients who later developed recurrent VTE than in those who did not (4.0 months vs. 7.5 months, respectively, p = 0.04). Table 4 summarizes the comparison of the survival after the diagnosis of PE in these groups, which did not demonstrate significant difference (Fig. 1).
Table 3.
Time between the diagnosis of lung cancer to the diagnosis of PE.
| Median time between diagnosis of lung cancer to PE (months) [IQR] | p valuea | |
|---|---|---|
| Suspected PE | 6.0 [1.0–18.0] | 0.20 |
| Unsuspected PE | 7.0 [4.0–17.5] | |
| Recurrent VTE | 4.0 [0.0–8.0] | 0.04 |
| Non-recurrent VTE | 7.5 [2.0–20.0] | |
| Proximal PE | 6.0 [2.0–21.0] | 0.99 |
| Distal PE | 7.0 [2.0–16.0] | |
IQR, interquartile range.
Value in bold denotes statistically significant differences.
Wilcoxon rank-sum test.
Table 4.
The survival after the diagnosis of PE.
| Median survival after PE (months) [95% CI] | p valuea | |
|---|---|---|
| Suspected PE | 5.6 [2.8–7.3] | 0.85 |
| Unsuspected PE | 6.2 [3.5–15.7] | |
| Recurrent VTE | 6.3 [4.4–21.3] | 0.50 |
| Non-recurrent VTE | 5.6 [2.8–9.2] | |
| Proximal PE | 5.0 [2.4–7.5] | 0.61 |
| Distal PE | 6.2 [3.8–17.3] | |
aLog-rank test.
Fig. 1.
The survival after the diagnosis of pulmonary embolism were compared between the suspected and unsuspected groups (A), between patients with recurrent VTE and those without (B), as well as proximal and distal PE (C).
On multivariate analysis with adjustment using the propensity score, the adjusted hazard ratio (HR) of death for patients with unsuspected PE compared to those with suspected PE was 1.09 (95% CI, 0.58–2.05; p = 0.79). The adjusted HR of death for patients with proximal PE compared to those with distal PE was 1.38 (95% CI, 0.83–2.33; p = 0.22). Two patients with unsuspected PE in whom PE was not treated had survival of 0.1 and 1.5 months after the diagnosis of PE. In one patient with suspected PE in whom the treatment-related information was not available, the survival after PE was 0.8 months.
4. Discussion
Management of incidentally detected PE in patients with lung cancer is a frequently encountered clinical challenge, given the high prevalence of lung cancer, its common association with PE, and frequent chest CT scanning in these patients. The increased risk of PE, including that of recurrent PE in these patients needs to be balanced with the risks associated with anticoagulation, mainly bleeding complications. The current the American College of Chest Physicians (ACCP) guidelines recommend treating patients with incidentally detected PE in a similar fashion to those with clinically suspected PE (grade 1C recommendation) [17]. However, there is a lack of data on the differences in the treatment, clinical outcome and frequency of recurrent PE between patients with suspected and unsuspected PE, and therefore there is a need for evidence-based support for this recommendation.
A few small series with limited follow-up duration have been reported on the outcome of unsuspected PE in oncology patient population [18–20], however these studies do not compare clinically suspected and unsuspected PE. A recent article by den Exter et al. reported similar high rates of recurrent VTE, bleeding complications and the mortality in patients with suspected and unsuspected PE in cancer population [14], however, this study included a heterogeneous population with several types of malignancies with only eight lung cancer patients. Focusing on PE in lung cancer, our study investigated a cohort of 77 patients with lung cancer who developed PE, since this population has not been extensively studied despite its high prevalence and frequent association with PE. There was no difference between the patients with suspected and unsuspected PE in terms of treatment for PE, incidence of hemorrhagic complications, the rate of recurrent VTE, and survival after PE. We also found that adenocarcinoma was more commonly noted in the unsuspected PE group while squamous cell carcinoma more prevalent in the suspected PE group.
In our study, 70% of patients had metastatic disease at the time of PE and 79% were receiving systemic anti-cancer therapy at the initial diagnosis of PE; both these factors have been described as risk factors for PE in cancer patients [21–25]. A total of 42% patients had clinically unsuspected PE, indicating that high index of suspicion is needed for diagnosis of PE in lung cancer patients, both from clinicians seeing the patients and radiologists interpreting CT.
A higher proportion of patients with clinically unsuspected PE had adenocarcinoma, and all 11 cases with squamous cell carcinoma had clinically suspected PE, which could conceivably represent characteristics of individual cancer subtypes, which has not been previously described. Among 11 squamous cell lung cancer patients who had suspected PE, 4 patients (36%) had central tumor with endobronchial component at the time of diagnosis of PE. The presence of endobronchial tumor may have predisposed patients developing shortness of breath and other respiratory symptoms that raised a suspicion for PE. Otherwise, there was no remarkable features in these patients that might explain the high association between squamous cell cancer and suspected PE, such as platelet counts, prevalence of brain metastasis, and antitherapeutic regimens. Five patients were receiving no treatment at the time of PE, 4 patients were on chemotherapy with 4 different regimens, one patient was receiving concurrent chemotherapy and chest radiation, and one patient was receiving chest radiotherapy.
Patients with clinically unsuspected PE were more frequently found to be on anti-cancer agents. However, this apparent difference could be explained by the fact that patients on systemic anti-cancer therapy get frequent follow-up CT studies. In addition, several patients presented with suspected PE before any chemotherapy was started, and 4 patients were found to have lung cancer on CT pulmonary angiography performed for PE.
We noticed that clinically suspected PE more frequently involve proximal pulmonary arteries, whereas unsuspected PE more commonly involves peripheral branches. Although this seems intuitive, prior studies have shown no significant difference in the location of suspected and unsuspected PE [9,26]. However, these studies included patients of several different cancer types and did not focus on lung cancer alone. Patients with lung cancer are more prone to pulmonary symptoms, which may lead to a higher index of suspicion for PE by clinicians.
Given the recommendation from ACCP for treatment of unsuspected PE, the treatment of clinically unsuspected PE at our institution was essentially identical to that of clinically suspected PE; vast majority of patients were treated with anticoagulation, most commonly using LMWH. Whether PE was clinically suspected or unsuspected had little impact on the treatment-related decisions because the vast majority of patients were treated for PE. Since the natural history and clinical significance of clinically unsuspected PE is uncertain, this approach seems justifiable, is in accordance with ACCP guidelines, and similar approach has been reported by others [14,17,20,27,28]. The rate of bleeding complications was similar for clinically suspected and unsuspected PE. Recurrent VTE occurred in 20% of patients, without any difference between suspected and unsuspected groups, consistent with a prior report in patients with various cancer types [14]. Also, there was no obvious difference in the survival between these two groups after the diagnosis of PE. Therefore, treatment of each episode of PE seems justifiable. The rate of recurrent VTE and survival could not be compared between patients in whom PE was treated and in those who did not receive any treatment for PE because of small number of untreated patients (n = 3) and short survival of these patients after PE.
Interestingly, the interval between the diagnosis of lung cancer and first episode of PE was shorter in patients who later developed recurrent VTE (4.0 months vs. 7.5 months, p = 0.04). This may represent predisposition of these patients to thromboembolic events. We could not identify any specific risk factors in these patients predisposing them to VTE, however, studies on larger population may identify specific risk factors predisposing certain patients to recurrent VTE.
One of the limitations of this study is its retrospective design which may predispose to information bias. However, in order to avoid this, we followed a predefined protocol for data collection and the medical records were thoroughly evaluated in all the patients. Diagnosis of PE was based on the original radiology report of CT scans, and a second retrospective review of CT images was not performed, which may provide another limitation of the study; however, given the lack of “gold standard” to definitively confirm the presence of PE and the wide acceptance of CT as a diagnostic test for PE in clinical practice, we chose to define the study population based on the official radiology reports that were prospectively provided and were available for clinicians who were making decisions for patient care. Given the heterogeneity of the study population and the length of time from the initial diagnosis of lung cancer to the development of PE (up to 8 years), it was not possible to accurately obtain the initial tumor stage and the performance status in every patient. Therefore, it was not possible to adjust for these factors in the multivariate analysis for survival. The number of untreated patients in this study was very small and therefore we could not compare the rate of recurrent VTE and survival between treated and untreated patients.
In conclusion, lung cancer patients with unsuspected PE had similar rate of recurrent VTE, treatment for PE, incidence of hemorrhagic complications, and survival after PE compared to those with suspected PE. Adenocarcinoma was more common in unsuspected PE while squamous cell carcinoma was more common in suspected PE. Larger studies are needed to understand the natural course of unsuspected PE and investigate the impact of anticoagulation in lung cancer patients with PE.
Footnotes
The investigators were supported by 2009–11 Agfa Health Care/RSNA Research Scholar Grant and 1K23CA157631 (NCI) (M.N.), Grants 1RO1CA114465-01 (B.E.J.) and 5R21 CA11627-02 (H.H.) from the National Institutes of Health, Grant No. 2P50CA090578-06 (B.E.J.) from the National Cancer Institute Specialized Program of Research Excellence in Lung Cancer, and a grant from Genentech Inc., as well as by the Doris and William Krupp Research Fund in Thoracic Oncology and American Society of Clinical Oncology Translational Research Professorship.
Conflict of interest statement: None declared.
References
- 1.Lyman GH, Khorana AA. Cancer, clots and consensus: new understanding of an old problem. J Clin Oncol. 2009 Oct;27(29):4821–6. doi: 10.1200/JCO.2009.22.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gosselin MV, Rubin GD, Leung AN, Huang J, Rizk NW. Unsuspected pulmonary embolism: prospective detection on routine helical CT scans. Radiology. 1998 Jul;208(1):209–15. doi: 10.1148/radiology.208.1.9646815. [DOI] [PubMed] [Google Scholar]
- 3.Levine M, Hirsh J, Gent M, Arnold A, Warr D, Falanga A, et al. Double-blind randomised trial of a very-low-dose warfarin for prevention of thromboembolism in stage IV breast cancer. Lancet. 1994 Apr;343(8902):886–9. doi: 10.1016/s0140-6736(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 4.Agnelli G, Gussoni G, Bianchini C, Verso M, Mandalà M, Cavanna L, et al. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study. Lancet Oncol. 2009 Oct;10(10):943–9. doi: 10.1016/S1470-2045(09)70232-3. [DOI] [PubMed] [Google Scholar]
- 5.Perry JR, Julian JA, Laperriere NJ, Geerts W, Agnelli G, Rogers LR, et al. PRODIGE a randomized placebo-controlled trial of dalteparin low-molecular-weight heparin thromboprophylaxis in patients with newly diagnosed malignant glioma. J Thromb Haemost. 2010 Sep;8(9):1959–65. doi: 10.1111/j.1538-7836.2010.03973.x. [DOI] [PubMed] [Google Scholar]
- 6.Streiff MB. Diagnosis and initial treatment of venous thromboembolism in patients with cancer. J Clin Oncol. 2009 Oct;27(29):4889–94. doi: 10.1200/JCO.2009.23.5788. [DOI] [PubMed] [Google Scholar]
- 7.Coleman R, MacCallum P. Treatment and secondary prevention of venous thromboembolism in cancer. BrJ Cancer. 2010 Apr;102(Suppl. 1):S17–23. doi: 10.1038/sj.bjc.6605601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kakkar AK. Prevention of venous thromboembolism in the cancer surgical patient. J Clin Oncol. 2009 Oct;27(29):4881–4. doi: 10.1200/JCO.2009.23.2009. [DOI] [PubMed] [Google Scholar]
- 9.Shinagare AB, Guo M, Hatabu H, Krajewski KM, Andriole K, Van den Abbeele AD, et al. Incidence of pulmonary embolism in oncologic outpatients at a tertiary cancer center. Cancer. 2011 Aug;117(16):3860–6. doi: 10.1002/cncr.25941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010 Oct;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 11.Tesselaar ME, Osanto S. Risk of venous thromboembolism in lung cancer. Curr Opin Pulm Med. 2007 Sep;13(5):362–7. doi: 10.1097/MCP.0b013e328209413c. [DOI] [PubMed] [Google Scholar]
- 12.Tagalakis V, Levi D, Agulnik JS, Cohen V, Kasymjanova G, Small D. High risk of deep vein thrombosis in patients with non-small cell lung cancer: a cohort study of 493 patients. J Thorac Oncol. 2007 Aug;2(8):729–34. doi: 10.1097/JTO.0b013e31811ea275. [DOI] [PubMed] [Google Scholar]
- 13.Chew HK, Davies AM, Wun T, Harvey D, Zhou H, White RH. The incidence of venous thromboembolism among patients with primary lung cancer. J Thromb Haemost. 2008 Apr;6(4):601–8. doi: 10.1111/j.1538-7836.2008.02908.x. [DOI] [PubMed] [Google Scholar]
- 14.den Exter PL, Hooijer J, Dekkers OM, Huisman MV. Risk of recurrent venous thromboembolism and mortality in patients with cancer incidentally diagnosed with pulmonary embolism: a comparison with symptomatic patients. J Clin Oncol. 2011 Jun;29(17):2405–9. doi: 10.1200/JCO.2010.34.0984. [DOI] [PubMed] [Google Scholar]
- 15.Gomes MPV, Deitcher SR. Diagnosis of venous thromboembolic disease in cancer patients. Oncology (Williston Park, NY) 2003 Jan;17(1):126–35. 139. discussion 139–144. [PubMed] [Google Scholar]
- 16.Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol. 2005 Apr;40(2):90–7. doi: 10.1053/j.ro.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE. Comerota AJ.Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. (8th) 2008 Jun;133(Suppl. 6):454S–545S. doi: 10.1378/chest.08-0658. [DOI] [PubMed] [Google Scholar]
- 18.Gladish GW, Choe DH, Marom EM, Sabloff BS, Broemeling LD, Munden RF. Incidental pulmonary emboli in oncology patients: prevalence CT evaluation, and natural history. Radiology. 2006 Jul;240(1):246–55. doi: 10.1148/radiol.2401051129. [DOI] [PubMed] [Google Scholar]
- 19.Douma RA, Kok MGM, Verberne LM, Kamphuisen PW, Büller HR. Incidental venous thromboembolism in cancer patients: prevalence and consequence. Thromb Res. 2010 Jun;125(6):e306–9. doi: 10.1016/j.thromres.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Browne AM, Cronin CG, English C, NiMhuircheartaigh J, Murphy JM, Bruzzi JF. Unsuspected pulmonary emboli in oncology patients undergoing routine computed tomography imaging. J Thorac Oncol. 2010 Jun;5(6):798–803. doi: 10.1097/JTO.0b013e3181d6153a. [DOI] [PubMed] [Google Scholar]
- 21.Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006 Feb;166(4):458–64. doi: 10.1001/archinte.166.4.458. [DOI] [PubMed] [Google Scholar]
- 22.Sallah S, Wan JY, Nguyen NP. Venous thrombosis in patients with solid tumors: determination of frequency and characteristics. Thromb Haemost. 2002 Apr;87(4):575–9. [PubMed] [Google Scholar]
- 23.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007 Nov;110(10):2339–46. doi: 10.1002/cncr.23062. [DOI] [PubMed] [Google Scholar]
- 24.Blom JW, Vanderschoot JPM, Oostindiër MJ, Osanto S, van der Meer FJM, Rosendaal FR. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J Thromb Haemost. 2006 Mar;4(3):529–35. doi: 10.1111/j.1538-7836.2006.01804.x. [DOI] [PubMed] [Google Scholar]
- 25.Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009 Oct;27(29):4839–47. doi: 10.1200/JCO.2009.22.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palla A, Rossi G, Falaschi F, Marconi L, Pistolesi M, Prandoni P. Is incidentally detected pulmonary embolism in cancer patients less severe? A case–control study. Cancer Investigation. 2012 Feb;30(2):131–4. doi: 10.3109/07357907.2011.633295. [DOI] [PubMed] [Google Scholar]
- 27.Franklin JM, Rahman N, Gleeson FV. The clinician's response to a report of an incidental pulmonary embolism detected on multidetector CT. Postgrad Med J. 2011 Nov;87(1033):746–9. doi: 10.1136/pgmj.2011.118497. [DOI] [PubMed] [Google Scholar]
- 28.O'Connell C, Razavi P, Ghalichi M, Boyle S, Vasan S, Mark L, et al. Unsuspected pulmonary emboli adversely impact survival in patients with cancer undergoing routine staging multi-row detector computed tomography scanning. J Thromb Haemost. 2011 Feb;9(2):305–11. doi: 10.1111/j.1538-7836.2010.04114.x. [DOI] [PubMed] [Google Scholar]