Abstract
The attachment of dissimilar materials is a major challenge because of the high levels of stress that develop at such interfaces. An effective solution to this problem develops at the attachment of tendon (a compliant “soft tissue”) to bone (a stiff “hard tissue”). This tissue, the “enthesis”, transitions from tendon to bone through gradations in structure, composition, and mechanical properties. These gradations are not regenerated during tendon-to-bone healing, leading to a high incidence of failure after surgical repair. Understanding the development of the enthesis may allow scientists to develop treatments that regenerate the natural tendon-to-bone insertion. Recent work has demonstrated that both biologic and mechanical factors drive the development and morphogenesis of the enthesis. A cascade of biologic signals similar to those seen in the growth plate promotes mineralization of cartilage on the bony end of the enthesis and the formation of fibrocartilage on the tendon end of the enthesis. Mechanical loading is also necessary for the development of the enthesis. Removal of muscle load impairs the formation of bone, fibrocartilage, and tendon at the developing enthesis. This paper reviews recent work on the development of the enthesis, with an emphasis on the roles of biologic and mechanical factors.
Keywords: mechanobiology, enthesis, fibrocartilage
INTRODUCTION
The mechanical challenge of attaching tendon to bone
Tendon and bone display dramatically different mechanical behavior.1, 2 At the hierarchical level of the tissue, tendon has a tensile modulus on the order of 200MPa in the direction of muscle force, but buckles in compression (i.e., it behaves like a rope).2 Bone, on the other hand, has a modulus of 20GPa in both tension and compression, and is rigid and brittle relative to tendon.1 The attachment of a compliant material like tendon to a relatively stiff material like bone is a fundamental engineering challenge.3 As is evident from the study of attachment of engineering materials4, potentially damaging stress concentrations can be expected to arise at the insertion if the insertion is not tuned to this stiffness mismatch. The tendon-to-bone insertion has a complex composition, structure, and mechanical behavior which is effective in transferring stress from tendon to bone. However, this complex attachment results in a particularly difficult challenge for effective response to injury. The unique transitional tissue that exists between uninjured tendon and bone is not recreated during tendon-to-bone healing.5– 9 Surgical reattachment of these two dissimilar biologic materials therefore often fails. For example, failure rates for rotator cuff repair (which requires tendon-to-bone healing) have been reported to range between 20% for repair of small tears to 94% for repair of massive tears.10, 11 Similarly, outcomes after anterior cruciate reconstruction (which also depend on tendon-to-bone healing) have been disappointing.12, 13 Studies in rotator cuff and anterior cruciate ligament animal models indicate that poor healing is due to the lack of regeneration of a specialized tissue to connect tendon and bone.9, 14–18 In order to engineer a replacement tissue for the enthesis or to develop treatments for tendon-to-bone healing, we first need to understand how structure-function relationships develop at the natural interface between tendon and bone.
The graded morphology of the mature tendon-to-bone insertion site
While numerous types of insertions exist between tendon and bone, the most common anatomy is of tendon inserting into bone across a fibrocartilaginous transition.19, 20 This form of attachment has commonly been categorized into four zones.19, 21 The first zone consists of tendon proper, and has properties similar to those found at the tendon mid-substance. This zone consists of well aligned type I collagen fibers with small amounts of the proteoglycan decorin.2, 22 The second zone consists of fibrocartilage and marks the beginning of the transition from tendinous material to bony material. This zone is composed of types II and III collagen, with small amounts of types I, IX, and X collagen, and small amounts of the proteoglycans aggrecan and decorin.22–26 The third zone contains mineralized fibrocartilage, indicating a marked transition towards bony tissue. Here, the predominant collagen is type II, and there are significant amounts of type X collagen as well as aggrecan.23–27 Finally, zone four consists of bone, which is made up predominantly of type I collagen with a relatively high mineral content. As described in subsequent sections, however, this classic four zone description is likely incorrect. Recent studies indicate that there are no sharp boundaries between the different “zones”.28 Rather, a gradation exists in structure and composition between tendon and bone. This continuous change in tissue composition from tendon to bone is presumed to aid in the efficient transfer of load between the two materials.
A number of biologic factors are necessary for the development of tendon, cartilage, and bone
The study of enthesis developmental may allow us to determine which factors are necessary for the formation of an effective tendon-to-bone insertion. While neo- tendon and bone appear at approximately the same time during fetal development, the formation of transitional tissue between them occurs postnatally.29–32 Several critical factors are involved in the formation of the tendon-to-bone insertion site. Scleraxis is a transcription factor associated with tendons and tenogenesis. The scleraxis (Scx) gene is expressed in the progenitors and cells of all tendon tissues 33–36. SOX-9 is necessary for chondrogenesis, is expressed in proliferative chondrocytes, and is responsible for chondrocyte differentiation.37–39 Indian hedgehog (Ihh) and parathyroid hormone related protein (PTHrP) drive chondrocyte proliferation and differentiation and together form a negative feedback loop maintaining a population of proliferative cells available for development at the growth plate 40. These factors are identified in the developing growth plate and may also play a role in the development of the tendon-to-bone insertion site. Gradations in expression of these factors likely result in the graded structure and composition across the tendon-to-bone insertion.
Mechanobiology plays an important role in adult musculoskeletal tissue homeostasis
The hypothesis that mechanobiologic factors play an important role in the development of the enthesis is grounded in observations of how a number of tissues are shaped and maintained by mechanical factors. Tissue response to the mechanical environment was first described in bone, with numerous studies demonstrating that the architecture of trabecular bone and the thickness of cortical bone were dependent on the stress environment. 41–43 A similar responsiveness to the mechanical environment was demonstrated in tendon. Removal of load resulted in rapid deterioration of tendon strength and a change in structure and composition.44–47 A change in the stress mode has also been shown to affect tendon properties. Areas of tendon that were subjected to compression (e.g., the portion of the rotator cuff directly under the acromion and locations where flexor tendons wrap around bony pulleys) produced significant amounts of proteoglycans to resist deformation in the direction of compression.48–51 These studies in bone and tendon imply a role for mechanical factors in the development of the tendon-to-bone insertion.
Mechanobiology influences fetal and postnatal development
Less is known about the role of mechanobiology in development, although studies indicate that mechanical cues influence patterning and growth during fetal joint development.52– 55 In addition to these physiologic examples, a number of pathologies have been associated with abnormal loading during fetal or early postnatal timepoints. For example, traction injury to the brachial plexus during the birth process results in a muscle imbalance across the developing shoulder and to a number of complications.56, 57 The most common deficits resulting from paralysis of the rotator cuff muscles are internal rotation contracture and glenohumeral joint deformities, severely compromising shoulder function.58–61 The responsiveness of mature and developing tendon, cartilage, and bone to mechanical load implies that the mechanobiology plays an important role in the development of the enthesis.
THE STRUCTURE AND FUNCTION OF THE MATURE TENDON-TO-BONE INSERTION
Gradation in biomechanical, compositional, and structural properties along the tendon- to-bone insertion site
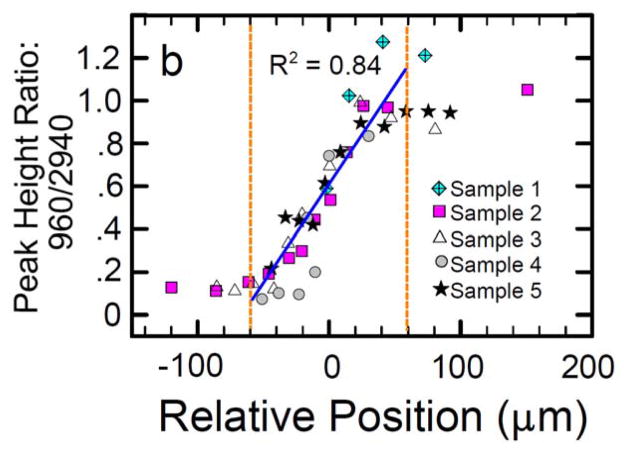
The tendon-to-bone insertion is biomechanically, compositionally, and structurally complex. The four discrete types of tissue recognized under the optical microscope are tendon, fibrocartilage, mineralized fibrocartilage, and bone.19 Between the unmineralized and mineralized fibrocartilage is a narrow zone that darkens markedly during tissue staining. This line was traditionally thought to represent a mineralization front, or, mechanically, a boundary between soft and hard tissue.19 Supraspinatus tendon-to-bone insertions from rats were used to evaluate the gene expression, collagen organization, mineral content, and biomechanical properties of the insertion.23, 28 Assays were performed at various points along the transition zone. For gene expression, in situ hybridization was performed for extracellular matrix genes. The normal insertion site appeared as a fibrocartilaginous transition zone between tendon and bone (Figure 1). There was a variation in gene expression along the length of the insertion; tendon specific matrix genes such as decorin and biglycan were seen only on the tendon end of the insertion while cartilage specific matrix genes such as collagen II and aggrecan were seen only on the bony end of the enthesis. For collagen orientation, sections were viewed under polarized light and angular deviation was calculated. Collagen fibers were less oriented at the insertion compared to the tendon. The mineral along the insertion was examined using individual Raman microprobe analyses. A plot of relative mineral concentration vs. distance across the insertion site showed an approximately linear increase across the interface (Figure 2). Specimens were tested in tension to determine their biomechanical properties. The enthesis was significantly stiffer at the tendon end compared to the bony end. Based on these results, it is apparent that the tendon-to-bone insertion site varies dramatically along its length in collagen structure, extracellular matrix composition, mineral content, geometry, and viscoelastic properties. This gradation in properties likely distributes forces more effectively across the transition from a flexible (i.e., tendon) to a rigid (i.e., bone) material.
Figure 1.
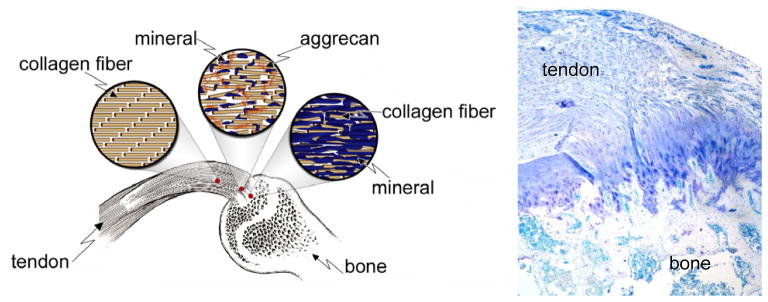
Morphology of the supraspinatus tendon-to-bone insertion site.
Figure 2.
There is a gradual change in the degree of mineralization across the tendon-to-bone insertion of the rotator cuff as evaluated by Raman spectroscopy (right panel, apatite [960 Δcm−1] to collagen [2940 Δcm−1] peak ratios are plotted relative to position. [Adapted with permission from Wopenka B, Kent A, Pasteris JD, Yoon Y, Thomopoulos S. The Tendon-to-Bone Transition of the Rotator Cuff: A Preliminary Raman Spectroscopic Study Documenting the Gradual Mineralization Across the Insertion in Rat Tissue Samples. Appl Spectrosc. 2008 Dec;62(12):1285–94.]
The microstructure of the insertion site is optimized to minimize stress and strain concentrations
A finite element model of the tendon-to-bone insertion was generated to examine structure-function relationships at the insertion.62 It was hypothesized that the microscopic structure of the insertion measured experimentally is optimized to minimize stress and strain concentrations associated with load transfer from the relatively compliant tendon to the relatively rigid bone. To explore this, collagen fiber orientation distributions were used to derive material properties for a two-dimensional mechanical model of an insertion. Comparison between stress concentrations in an idealized model and those in three comparison models showed that the microstructure serves to (1) simultaneously reduce stress concentrations and material mass, and (2) shield the insertion’s outward splay from the highest stresses.
Effective tendon-to-bone attachment is achieved through a functional grading in mineral content and collagen fiber orientation
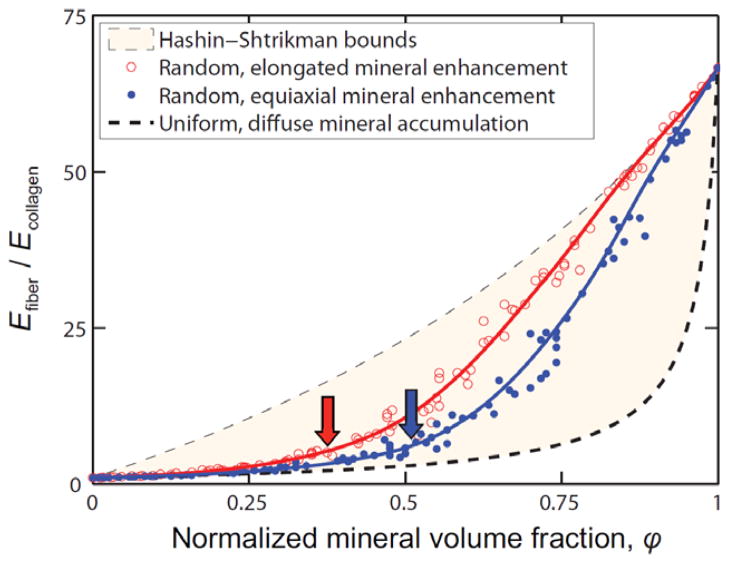
The effect of mineral content on load transfer was modeled at the insertion.63 It was demonstrated that mineral content and collagen fiber orientation combine to give the tendon-to-bone transition a unique grading in mechanical properties. Results supported a new organ-level physiological model of continuous tissue transition from tendon to bone. The linear increase in mineral accumulation on collagen fibers (Figure 2) provides significant stiffening of the partially mineralized fibers, but only for concentrations of mineral above a “percolation threshold” corresponding to formation of a mechanically continuous mineral network (Figure 3). Increasing dispersion in the orientation distribution of collagen fibers from tendon to bone is a second major determinant of tissue stiffness. The combination of these two factors results in the previously reported, non-monotonic variation of stiffness over the length of the tendon-to-bone insertion, and describes how tendon-to-bone attachment is achieved in nature through a functionally graded material composition. These experimental and modeling results provide a nano- and micro-mechanical understanding of how we must combine collagen and mineral to achieve the grading of material properties that is necessary for tissue engineered tendon-to-bone grafts.
Figure 3.
Bounds (lines) and Monte Carlo estimates (circles) for the elastic modulus (E) of collagen fibrils containing mineral deposits up to the level of mineralization found in bone. The stiffening of collagen fibrils by mineral increases dramatically above a critical mineral concentration called the “percolation threshold” (arrows). This concentration is a function of the shape and distribution of mineral (red: aspect ratio of 1:1; blue: aspect ratio of 2:1). [Adapted with permission from Genin GM, Kent A, Birman V, Wopenka B, Pasteris JD, JP M, Thomopoulos S. Functional grading of mineral and collagen in the attachment of tendon to bone. Biophysical Journal. 2009;97(4).]
INSERTION SITE DEVELOPMENT: BIOLOGIC FACTORS
Transitional tissue between tendon and bone does not develop until postnatal timepoints
The complex gradations in structure, composition, and mechanical properties in the mature enthesis develop during fetal and post-natal timepoints due to numerous biologic and mechanical factors. An understating of the natural development of the tendon-to-bone insertion site will allow us to design biomimetic strategies for insertion site tissue engineering and to propose treatments for tendon-to-bone healing. To this end, in situ hybridization experiments were performed to examine the normal development of the supraspinatus tendon-to-bone insertion.64 Mouse shoulders were obtained at specific fetal and postnatal timepoints and in situ hybridization was performed on tissue sections for collagen I (characteristic of fibroblasts and osteoblasts), collagen II (characteristic of chondrocytes), and collagen X (characteristic of hypertrophic chondrocytes).9, 14, 23, 31 Precursors to the rotator cuff tendons were evident adjacent to the humeral head at 15.5 days post conception (dpc). A transition zone between the tendon and bone did not begin to form until 7 days postnatally. The transition did not form into a mature fibrocartilaginous insertion site until 21 days postnatally. The fibroblasts of the supraspinatus tendon expressed type I collagen at all timepoints studied (Figure 4). The chondrocytes in the humeral head expressed type II collagen until 14 days postnatally. The chondrocytes near the insertion became hypertrophic at this point and began expressing type X collagen. Type I collagen and type II collagen were expressed by two adjacent populations of cells; collagen I was expressed by the fibroblasts in zones 1 and 2, while collagen II was expressed by the chondrocytes in zones 3 and 4. It was also shown that type X collagen expression was not turned on until 14 days postnatally, consistent with a study in the rat Achilles tendon insertion32. Notably, many of the critical events in tendon-to-bone insertion development occurred at postnatal timepoints.
Figure 4.
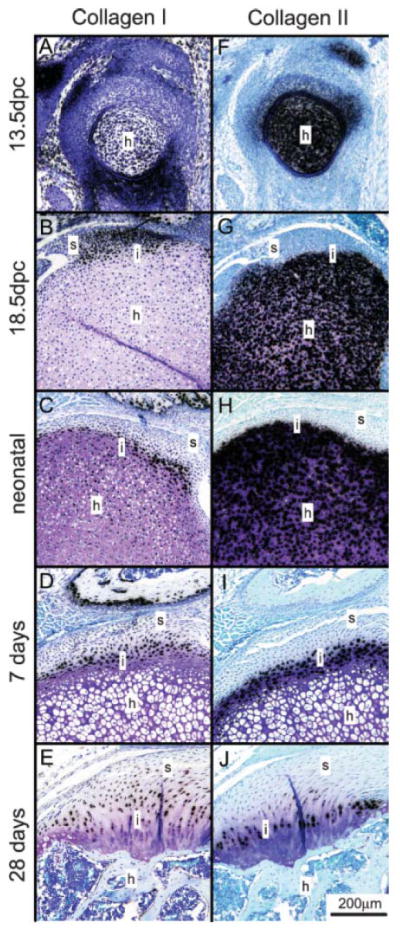
Serial sections for collagen I (A–E) and collagen II (F–J) expression at 13.5 dpc (A, F), 18.5 dpc (B, G), neonatal (C, H), 7 days (D, I), and 28 days (E, J) (Toluidine blue stain). Type I collagen expression mirrored type II collagen expression across the insertion site. Type I collagen was always found on the tendinous side of the insertion, and type II collagen was always found on the bony side of the insertion. Positive staining is indicated by black grains. s, supraspinatus tendon; h, humeral head; i, interface. [Adapted with permission from Galatz L, Rothermich S, VanderPloeg K, Petersen B, Sandell L, Thomopoulos S. Development of the supraspinatus tendon-to-bone insertion: localized expression of extracellular matrix and growth factor genes. J Orthop Res. 2007 Dec;25(12):1621–8.]
Tendon-to-bone insertion development follows pathways similar to those seen in growth plate development
The growth plate is divided into different zones according to the morphology of the cells as well as the state of calcification 65. These zones include the reserve zone, the proliferative zone, and the hypertrophic zone. Cells in the reserve zone are small cells organized singly or in pairs. These chondrocytes then proliferate (in the proliferative zone) and organize into longitudinal columns extending down into the metaphysis. Cells in the upper portion of the proliferative zone become flattened in morphology. Cells hypertrophy (in the hypertrophic zone) and become progressively larger and spherical in morphology. They are eventually loaded with calcium which accumulates in the matrix between the cells and mineralize in what is called the zone of provisional calcification. It is likely that the growth and transcription factors that modulate growth plate maturation also play a role in enthesis maturation. Similar chondrocyte-like cells appear at the developing enthesis and eventually mineralize to create the interface between the tendon and bone.
Parathyroid hormone related protein (PTHrP) has been localized to the fibrous insertion sites of both tendons and ligaments. Histologically, it is localized to the fibroblast like cells that lie in the zone between the dense connective tissue of the tendon and the tissue that blends with the mineralized cortical bone 66. To a lesser extent, PTHrP is localized to the periosteum in general. It is seen in a layer of fibroblast-like cells external to the bone itself. Production of PTHrP is known to be heavily influenced by the mechanical environment 67. When tendon insertion sites were unloaded, either by tail suspension or complete transsection of the more proximal tendon, PTHrP expression decreased markedly, greater in the transsected versus suspended specimens. It is hypothesized that PTHrP plays a role in the migration of the tendon or ligament insertion site during growth as the diaphyseal portion of the bone lengthens.66, 68, 69 Osteoclastic activity is noted at the leading edge of the insertion site while osteoblastic activity is seen along the trailing edge, thus allowing the insertion to accommodate for the growing bone.
PTHrP has been localized to proliferating chondrocytes, preventing proliferating chondrocytes in the proliferative zone from becoming hypertrophic chondrocytes which eventually mineralize 66. Therefore, PTHrP maintains populations of proliferating chondrocytes available for growth and may help prevent mineralization at inappropriate locations in the growth plate. Its role at the tendon-bone insertion site may be analogous in creating a transition between mineralized and unmineralized tissue.
Collagen X is also localized to the mineralizing front in the growth plate as well as in the tendon-to-bone insertion site 31. Collagen X is expressed by hypertrophic chondrocytes in both of these anatomic locations. The fibrocartilaginous zone of the tendon insertion site develops at approximately 14 days postnatally in a murine model 31. The development of this zone is characterized by the presence of type X collagen. While the hypertrophic chondrocytes at the mineralization front of the tendon-to-bone insertion site eventually mature into the fibrocartilaginous transition zone, collagen X persists even after the hypertrophic chondrocytes are no longer present. Therefore, collagen X may also have an important role in maintaining a transition between mineralized and unmineralized tissues.
Ihh and PTHrP influence chondrocyte differentiation 40. Proliferative or prehypertrophic chondrocytes secrete Ihh as they enter the hypertrophic phase of growth. Ihh binds to the cell surface receptor Patched 1 (Ptc1) allowing the transmembrane protein smoothened to accumulate 70. Smoothened leads to induction of PTHrP synthesis which blocks the recruitment of more chondrocytes into this pathway, maintaining a population of proliferative cells 40. Interestingly, Ihh, PTHrP, and Ptc1 are also localized to the developing tendon-bone-insertion sites. Ptc1 expression was high at 14 and 21 days in a murine model (Figure 5). This coincided with the onset of mineralization at the enthesis. Expression of Ptc1 is influenced by the biomechanical environment, as decreased expression was seen in specimens where muscle load was removed using botulinum toxin A. Other studies have also found that expression of Ihh and PTHrP is mechanosensitive 67. Expression was significantly decreased in the unloaded groups of these studies.
Figure 5.
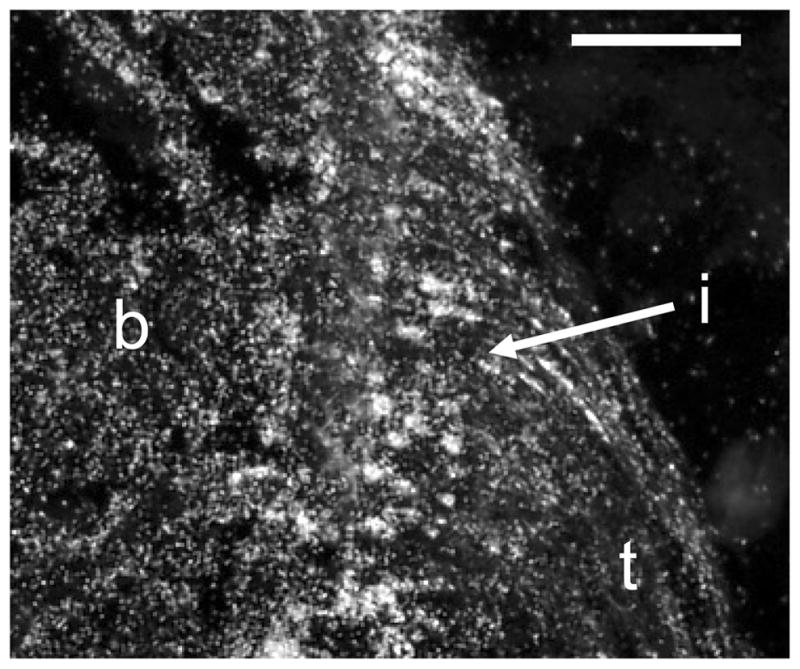
Expression of Patched-1, the receptor for Ihh, was localized to the developing enthesis (21 days postnatally, t: tendon, b: bone, i: insertion, scale bar = 100μm).
Finally, two factors necessary for chondrogenesis and tenogenesis, SOX-9 and scleraxis, respectively, are likely also important for enthesis development.38 SOX-9 is necessary for chondrogenesis37, 39; it is localized to proliferative chondrocytes at the developing growth plate and is also likely expressed at the developing tendon enthesis. SOX-9 is not seen in the hypertrophic chondrocyte region.39 Scleraxis is a transcription factor required for tenogenesis 33– 36. The scleraxis gene serves as a marker for connective tissues including ligaments, tendons, and capsules 19, 35. Expression of scleraxis has been shown to be mechanosensitive. Mesenchymal stem cells responded to cyclic stretching with an upregulation in the expression of scleraxis. Scaffolds seeded with mesenchymal stem cells exposed to dynamic tensile mechanical stimulation also expressed scleraxis. Scleraxis expression has also been localized to the developing tendon enthesis.
Formation of a functional tendon-to-bone attachment: roles for scleraxis, SOX-9, Ihh/PTHrP
These and other tissue specific factors may appear in a graded fashion across the developing insertion, creating the transition from tendon to cartilage to mineralized cartilage to bone. In the immature developing tendon insertion sites, scleraxis is localized to the tendon precursor. Collagen X and Patched 1 (Ptc1) are localized to the developing insertion site. Interestingly, Ptc1 and Collagen X are not seen until 14 days after birth in our mouse model, and they appear simultaneously at the insertion site. Early in development, SOX-9 is expressed in the proliferating chondrocytes, deep to the hypertrophic chondrocytes and this area eventually mineralizes to form bone. Expression of each of these individual factors has been shown to be mechanically sensitive. As described in the next section, it has been shown that the insertion site development is highly sensitive to mechanical factors. The study of the factors listed above may explain the mechanisms for the mechanosensitivity.
INSERTION SITE DEVELOPMENT: MECHANICAL FACTORS
An animal model to study the role of mechanobiology in tendon-to-bone insertion development
A number of experiments were performed to examine the role of mechanical loading on the postnatal development of the supraspinatus tendon-to-bone insertion.64, 71 Supraspinatus intramuscular injections of botulinum toxin A were made in the left shoulders mice within 24 hours of birth (‘Botox’ group). The supraspinatus muscles of right shoulders were injected with saline to serve as contralateral controls (‘Saline’ group). Local paralysis was maintained until sacrifice through repeated botulinum toxin injections. A separate group of neonatal mice were injected with saline in both shoulders and served as fully mobile controls (‘Normal’ group).
The volume of supraspinatus muscles injected with botulinum toxin was decreased by 66% compared to the contralateral saline-injected muscles at 56 days. Decreases in muscle volume corresponded to decreases in muscle force generation (Figure 6). After 28 and 56 days of postnatal paralysis, saline-injected and normal supraspinatus muscles generated dramatically higher forces compared to botulinum-injected muscles. This animal model closely mimics the clinical condition neonatal brachial plexus palsy. In this condition, injury to the brachial plexus during childbirth leads to paralysis of the shoulder and subsequent complications (e.g., shoulder dislocation, posterior shoulder subluxation, humeral head flattening, and glenoid dysplasia.58, 59).
Figure 6.
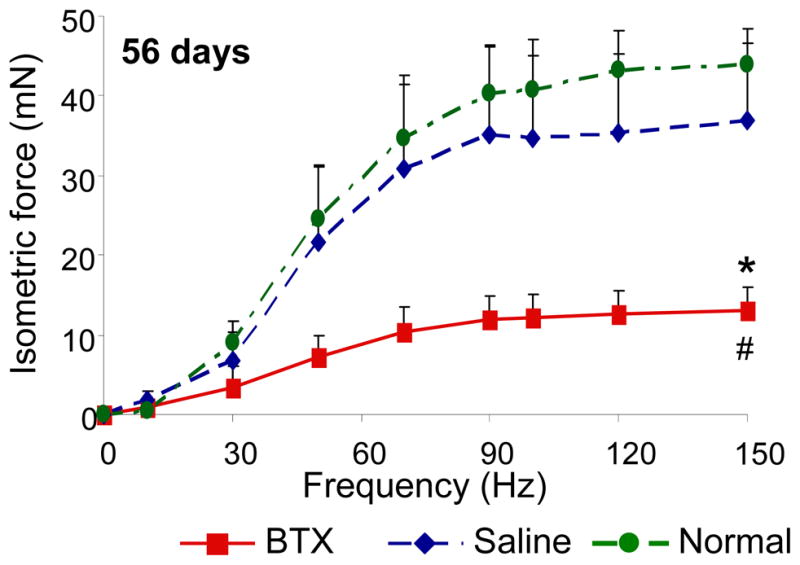
The contractile properties of BTX muscles were significantly lower compared to the Saline and Normal muscles (* p <0.05, paired t-test, BTX vs. Saline; # p <0.05, BTX or Saline vs. Normal).
Reduced loading during development impairs mineral deposition at the insertion
Micro-computed tomography images of the humeral head during postnatal development revealed dramatic differences in the amount of mineralized bone when comparing saline-injected to botulinum toxin-injected shoulders, demonstrating the sensitivity of bone to its mechanical environment (Figure 7).64, 71 There were no differences in any bone measure between Saline and Botox at 14 days. However, most measures were significantly different when comparing Saline to Botox at 21, 28, and 56 days. Similar trends were seen for the percentage of bone that was lined with osteoclasts (Figure 7). Therefore, the lack of mineral accrual in unloaded insertions was at least in part due to increased levels of bone resorption. Our results suggest that genetic cues may drive early postnatal development (as evidenced by the lack of differences at 14 days), but mechanical load is necessary for the bone at the insertion site to mature.
Figure 7.
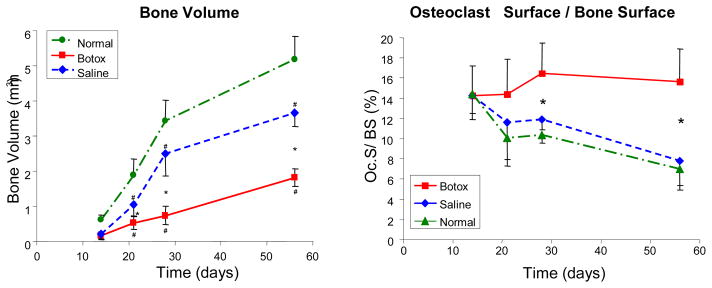
Bone volume was similar when comparing Botox to Saline at 14 days. Bone volume, however, was significantly different when comparing Botox to Saline at 21, 28, and 56 days. The percentage of bone surface covered with osteoclasts (Oc.S/BS) was similar in all groups at 14 days but significantly different when comparing Saline or Normal to the Botox group at 21–56 days. [* Significantly different- Botox vs. Saline, # Significantly different- Botox/Saline vs. Normal, p < 0.05]. [Adapted with permission from Thomopoulos S, Kim HM, Rothermich SY, Biederstadt C, Das R, Galatz LM. Decreased muscle loading delays maturation of the tendon enthesis during postnatal development. J Orthop Res. 2007 Sep;25(9):1154–63.]
Reduced loading during development impairs fibrocartilage formation at the insertion
There was evidence of transitional fibrocartilage forming between the supraspinatus tendon and its humeral head insertion in both the saline and botulinum toxin injected shoulders 14 days postnatally.64 By 21 days, fibrocartilage was well developed in the Saline group between the tendon and the bone. The fibrochondrocytes at the insertion were arranged in a columnar pattern perpendicular to the subchondral bone plate. Contralateral botulinum toxin-injected shoulders showed no fibrocartilage between the tendon and bone. A layer of disorganized primitive mesenchymal like cells and hypertrophic chondrocytes were present at the insertion site. By 56 days, the tendon-to-bone transition in the Saline group consisted of fully developed transitional fibrocartilage with the classically described structure. The insertion of the contralateral botulinum toxin-injected shoulders, on the other hand, showed poor fibrocartilage development. Consistent with the study examining mineral accumulation, removal of mechanical loading cues postnatally dramatically impaired the fibrocartilage formation at the rotator cuff – humeral head insertion.
Reduced loading during development leads to disorganized fiber distribution and inferior mechanical properties in tendon at the insertion
Tendon angular deviation (a measure of collagen disorganization) was significantly higher in the Botox group compared to the Saline group at 28 and 56 days (Figure 8), indicating a less organized collagen matrix. Biomechanically, the Botox group had significantly decreased modulus and ultimate stress compared to Saline at 56 days, indicating inferior material properties (Figure 8). While it is possible for larger quantities of inferior tissue to outperform smaller quantities of healthy tissue, this was not the case: ultimate load was also significantly decreased in the Botox group compared to Saline, indicating inferior structural performance. There were no significant differences between the Saline group and the Normal group. Decreased loading postnatally resulted in a decrease in collagen organization leading to a decrease in the structural (ultimate load) and material (maximum stress and modulus) properties of the tendon-to-bone insertion. Mechanobiology therefore plays a critical role in defining the collagen alignment for optimal load transfer between tendon and bone.
Figure 8.
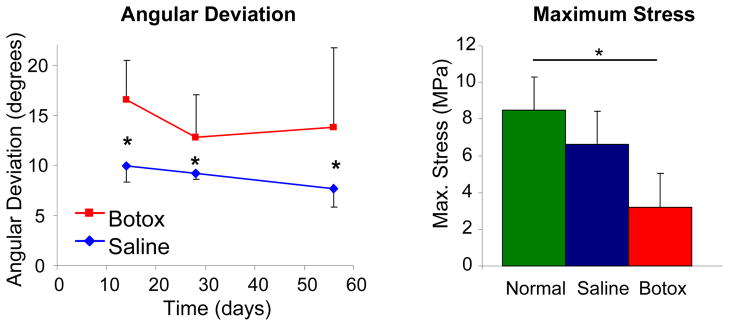
Tendon angular deviation was significantly higher (i.e., the collagen fiber distribution was less organized) in the Botox group compared to the Saline group. Ultimate stress was significantly lower in the Botox group compared to the Saline and Normal groups (* p < 0.05).
CONCLUSIONS
Studies on the mature tendon-to-bone insertion site demonstrate that it is a functionally graded structure where grading occurs at both microscopic and nanoscopic levels.23, 62 Such variation is responsible for a micromechanically optimum stress distribution with limited expenditure of material. Potentially damaging stress concentrations would arise at the insertion if the insertion was not tuned to the stiffness mismatch between tendon and bone. Notably, in most animal studies a gradation in properties does not exist at healing insertions.9, 14, 15, 18, 72–74 The poor outcomes seen in tendon-to-bone healing may therefore be due to the high stress concentrations that arise at the interface between the two dissimilar materials. Understanding the development and morphogenesis of the enthesis may allow us to develop strategies for improving outcomes for tendon-to-bone repair. Recent results show that both biologic factors and mechanobiology play important roles in the development of the tendon-to-bone insertion.31, 64, 71 It is likely that gradations in biologic factors promote gradations in cell differentiation and subsequent tissue formation (Figure 9). The absence of load during enthesis development impairs mineral accumulation, fibrocartilage formation, and collagen fiber organization.64, 71 This results in an attachment between tendon and bone that is mechanically inferior to the naturally occurring attachment. The sensitivity of the tendon-to-bone insertion to mechanical load provides investigators with the opportunity to design specific structure and function in engineered constructs for use in tendon-to-bone repair.
Figure 9.
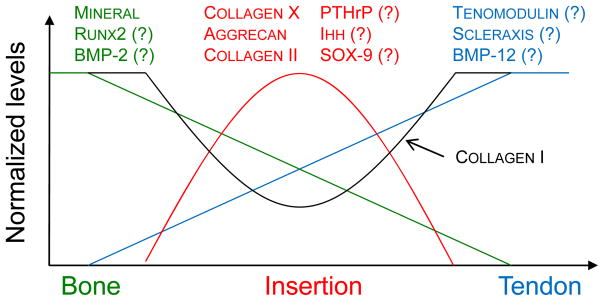
Gradations in biologic factors may promote gradations in cell differentiation and subsequent tissue formation. The measured gradations in mineral at the mature enthesis (shown in green, highest in bone and lowest in tendon) may be due to a graded expression of osteogenic factors such Runx2 and BMP-2. The measured gradations in fibrocartilage (shown in red, low in both tendon and bone and highest at the insertion) may be due to a graded expression of factors such as PTHrP, Ihh, and Sox9. Differences in tenogenesis (shown in blue, highest in tendon and lowest in bone) may be due a graded expression of tendon specific factors such as scleraxis, BMP-12, and tenomodulin.
Acknowledgments
The work presented in this paper supported by grants from the National Institutes of Health (EB004347 and AR055580) and the Orthopaedic Research and Education Foundation.
References
- 1.Bostrom MPG, Boskey A, Kauffman JK, Einhorn TA. Form and function of bone. In: Buckwalter JA, Einhorn TA, Simon SR, editors. Orthopaedic Basic Science. 2. AAOS; 2000. pp. 319–70. [Google Scholar]
- 2.Woo SL, An K, Frank CB, Livesay GA, Ma CB, Zeminski JA, Wayne JS, Myers BS. Orthopaedic Basic Science. 2. Rosemont, IL: AAOS; 2000. Anatomy, biology, and biomechanics of tendon and ligament; pp. 581–616. [Google Scholar]
- 3.Jones RM. Mechanics of composite materials. 2. Philadelphia: Taylor & Francis, Inc; 1999. [Google Scholar]
- 4.Williams ML. Stress singularities resulting from various boundary conditions in angular corners of plates in extension. Journal of Applied Mechanics. 1952;19:526–8. [Google Scholar]
- 5.Aoki M, Oguma H, Fukushima S, Ishii S, Ohtani S, Murakami G. Fibrous connection to bone after immediate repair of the canine infraspinatus: the most effective bony surface for tendon attachment.[see comment] Journal of Shoulder & Elbow Surgery. 2001;10(2):123–8. doi: 10.1067/mse.2001.111963. [DOI] [PubMed] [Google Scholar]
- 6.Fujioka H, Thakur R, Wang GJ, Mizuno K, Balian G, Hurwitz SR. Comparison of surgically attached and non-attached repair of the rat Achilles tendon-bone interface. Cellular organization and type X collagen expression. Connective Tissue Research. 1998;37(3–4):205–18. doi: 10.3109/03008209809002440. [DOI] [PubMed] [Google Scholar]
- 7.Liu SH, Panossian V, al-Shaikh R, Tomin E, Shepherd E, Finerman GA, Lane JM. Morphology and matrix composition during early tendon to bone healing. Clinical Orthopaedics & Related Research. 1997;(339):253–60. doi: 10.1097/00003086-199706000-00034. [DOI] [PubMed] [Google Scholar]
- 8.St Pierre P, Olson EJ, Elliott JJ, O’Hair KC, McKinney LA, Ryan J. Tendon-healing to cortical bone compared with healing to a cancellous trough. A biomechanical and histological evaluation in goats. Journal of Bone & Joint Surgery - American Volume. 1995;77(12):1858–66. doi: 10.2106/00004623-199512000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. Journal of Biomechanical Engineering. 2003;125(1):106–13. doi: 10.1115/1.1536660. [DOI] [PubMed] [Google Scholar]
- 10.Harryman DT, 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA., 3rd Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. Journal of Bone & Joint Surgery. 1991;73(7):982–9. [PubMed] [Google Scholar]
- 11.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004 Feb;86-A(2):219–24. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Fu FH, Bennett CH, Lattermann C, Ma CB. Current trends in anterior cruciate ligament reconstruction. Part I: Biology and biomechanics of reconstruction. Am J Sports Med. 1999 Nov-Dec;27(6):821–30. doi: 10.1177/03635465990270062501. [DOI] [PubMed] [Google Scholar]
- 13.Fu FH, Bennett CH, Ma CB, Menetrey J, Lattermann C. Current trends in anterior cruciate ligament reconstruction. Part II. Operative procedures and clinical correlations. Am J Sports Med. 2000 Jan-Feb;28(1):124–30. doi: 10.1177/03635465000280010801. [DOI] [PubMed] [Google Scholar]
- 14.Thomopoulos S, Hattersley G, Rosen V, Mertens M, Galatz L, Williams GR, Soslowsky LJ. The localized expression of extracellular matrix components in healing tendon insertion sites: an in situ hybridization study. Journal of Orthopaedic Research. 2002;20(3):454–63. doi: 10.1016/S0736-0266(01)00144-9. [DOI] [PubMed] [Google Scholar]
- 15.Galatz LM, Sandell LJ, Rothermich SY, Das R, Mastny A, Havlioglu N, Silva MJ, Thomopoulos S. Characteristics of the rat supraspinatus tendon during tendon-to-bone healing after acute injury. J Orthop Res. 2006 Mar;24(3):541–50. doi: 10.1002/jor.20067. [DOI] [PubMed] [Google Scholar]
- 16.Wong MWN, Qin L, Lee KM, Leung KS. Articular Cartilage Increases Transition Zone Regeneration in Bone-tendon Junction Healing. Clinical Orthopaedics and Related Research. 2009 Apr;467(4):1092–100. doi: 10.1007/s11999-008-0606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong MWN, Tai KO, Lee KM, Qin L, Leung KS. Bone formation and fibrocartilage regeneration at bone tendon junction with allogeneic cultured chondrocyte pellet interposition. Journal of Bone and Mineral Research. 2007 Jul;22(7):1128–9. [Google Scholar]
- 18.Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. Journal of Bone & Joint Surgery -American Volume. 1993;75(12):1795–803. doi: 10.2106/00004623-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Benjamin M, Kumai T, Milz S, Boszczyk BM, Boszczyk AA, Ralphs JR. The skeletal attachment of tendons--tendon “enthuses”. Comp Biochem Physiol A Mol Integr Physiol. 2002 Dec;133(4):931–45. doi: 10.1016/s1095-6433(02)00138-1. [DOI] [PubMed] [Google Scholar]
- 20.Benjamin M, Toumi H, Ralphs JR, Bydder G, Best TM, Milz S. Where tendons and ligaments meet bone: attachment sites (‘entheses’) in relation to exercise and/or mechanical load. Journal of Anatomy. 2006 Apr;208(4):471–90. doi: 10.1111/j.1469-7580.2006.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woo SL, Maynard J, Butler D. Ligament, tendon, and joint capsule insertions to bone. In: Woo SL, editor. Injury and Repair of the Musculoskeletal Soft Tissues. Savannah GA: American Academy of Orthopaedic Surgeons; 1987. pp. 129–66. [Google Scholar]
- 22.Waggett AD, Ralphs JR, Kwan AP, Woodnutt D, Benjamin M. Characterization of collagens and proteoglycans at the insertion of the human Achilles tendon. Matrix Biology. 1998;16(8):457–70. doi: 10.1016/s0945-053x(98)90017-8. [DOI] [PubMed] [Google Scholar]
- 23.Thomopoulos S, Williams GR, Gimbel JA, Favata M, Soslowsky LJ. Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. Journal of Orthopaedic Research. 2003;21(3):413–9. doi: 10.1016/S0736-0266(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 24.Kumagai J, Sarkar K, Uhthoff HK, Okawara Y, Ooshima A. Immunohistochemical distribution of type I, II and III collagens in the rabbit supraspinatus tendon insertion. Journal of Anatomy. 1994;185(Pt 2):279–84. [PMC free article] [PubMed] [Google Scholar]
- 25.Fukuta S, Oyama M, Kavalkovich K, Fu FH, Niyibizi C. Identification of types II, IX and X collagens at the insertion site of the bovine achilles tendon. Matrix Biology. 1998;17(1):65–73. doi: 10.1016/s0945-053x(98)90125-1. [DOI] [PubMed] [Google Scholar]
- 26.Visconti C, Kavalkovich K, Wu J, Niyibizi C. Biochemical analysis of collagens at the ligament-bone interface reveals presence of cartilage-specific collagens. Archives of Biochemistry & Biophysics. 1996;328(1):135–42. doi: 10.1006/abbi.1996.0153. [DOI] [PubMed] [Google Scholar]
- 27.Ralphs JR, Benjamin M, Waggett AD, Russell DC, Messner K, Gao J. Regional differences in cell shape and gap junction expression in rat Achilles tendon: relation to fibrocartilage differentiation. J Anat. 1998 Aug;193( Pt 2):215–22. doi: 10.1046/j.1469-7580.1998.19320215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wopenka B, Kent A, Pasteris JD, Yoon Y, Thomopoulos S. The Tendon-to-Bone Transition of the Rotator Cuff: A Preliminary Raman Spectroscopic Study Documenting the Gradual Mineralization Across the Insertion in Rat Tissue Samples. Appl Spectrosc. 2008 Dec;62(12):1285–94. doi: 10.1366/000370208786822179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bland YS, Ashhurst DE. Fetal and postnatal development of the patella, patellar tendon and suprapatella in the rabbit; changes in the distribution of the fibrillar collagens. Journal of Anatomy. 1997;190(Pt 3):327–42. doi: 10.1046/j.1469-7580.1997.19030327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bland YS, Ashhurst DE. The hip joint: the fibrillar collagens associated with development and ageing in the rabbit. Journal of Anatomy. 2001;198(Pt 1):17–27. doi: 10.1046/j.1469-7580.2001.19810017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galatz L, Rothermich S, VanderPloeg K, Petersen B, Sandell L, Thomopoulos S. Development of the supraspinatus tendon-to-bone insertion: localized expression of extracellular matrix and growth factor genes. J Orthop Res. 2007 Dec;25(12):1621–8. doi: 10.1002/jor.20441. [DOI] [PubMed] [Google Scholar]
- 32.Fujioka H, Wang GJ, Mizuno K, Balian G, Hurwitz SR. Changes in the expression of type-X collagen in the fibrocartilage of rat Achilles tendon attachment during development. Journal of Orthopaedic Research. 1997;15(5):675–81. doi: 10.1002/jor.1100150508. [DOI] [PubMed] [Google Scholar]
- 33.Cserjesi P, Brown D, Ligon KL, Lyons GE, Copeland NG, Gilbert DJ, Jenkins NA, Olson EN. Scleraxis: a basic helix-loop-helix protein that prefigures skeletal formation during mouse embryogenesis. Development - Supplement. 1995;121(4):1099–110. doi: 10.1242/dev.121.4.1099. [DOI] [PubMed] [Google Scholar]
- 34.Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001 Oct;128(19):3855–66. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- 35.Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors.[comment] Cell. 2003;113(2):235–48. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- 36.Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, Schweitzer R. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007 Jul;134(14):2697–708. doi: 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- 37.Akiyama H. Control of chondrogenesis by the transcription factor Sox9. Mod Rheumatol. 2008;18(3):213–9. doi: 10.1007/s10165-008-0048-x. [DOI] [PubMed] [Google Scholar]
- 38.Asou Y, Nifuji A, Tsuji K, Shinomiya K, Olson EN, Koopman P, Noda M. Coordinated expression of scleraxis and Sox9 genes during embryonic development of tendons and cartilage. Journal of Orthopaedic Research. 2002;20(4):827–33. doi: 10.1016/S0736-0266(01)00169-3. [DOI] [PubMed] [Google Scholar]
- 39.Huang W, Chung UI, Kronenberg HM, de Crombrugghe B. The chondrogenic transcription factor Sox9 is a target of signaling by the parathyroid hormone-related peptide in the growth plate of endochondral bones. Proc Natl Acad Sci U S A. 2001 Jan 2;98(1):160–5. doi: 10.1073/pnas.011393998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilbert SF. Cell-cell communication in development. In: Gilbert SF, editor. Developmental Biology. 2. Sinauer Associates; 2006. [Google Scholar]
- 41.Wolff J. Das Gesetz der Transformation der Knochen (Berlin A. Hirchwild) In: Maquet P, Furlong R, editors. The Law of Bone Remodeling. Berlin: Springer-Verlag; 1892. [Google Scholar]
- 42.Goldstein SA. The mechanical properties of trabecular bone: dependence on anatomic location and function. Journal of Biomechanics. 1987;20(11–12):1055–61. doi: 10.1016/0021-9290(87)90023-6. [DOI] [PubMed] [Google Scholar]
- 43.Mullender MG, Huiskes R. Proposal for the regulatory mechanism of Wolff’s law. Journal of Orthopaedic Research. 1995;13(4):503–12. doi: 10.1002/jor.1100130405. [DOI] [PubMed] [Google Scholar]
- 44.Amiel D, Woo SL, Harwood FL, Akeson WH. The effect of immobilization on collagen turnover in connective tissue: a biochemical-biomechanical correlation. Acta Orthopaedica Scandinavica. 1982;53(3):325–32. doi: 10.3109/17453678208992224. [DOI] [PubMed] [Google Scholar]
- 45.Woo SL, Gomez MA, Woo YK, Akeson WH. Mechanical properties of tendons and ligaments. II. The relationships of immobilization and exercise on tissue remodeling. Biorheology. 1982;19(3):397–408. doi: 10.3233/bir-1982-19302. [DOI] [PubMed] [Google Scholar]
- 46.Woo SL, Gomez MA, Sites TJ, Newton PO, Orlando CA, Akeson WH. The biomechanical and morphological changes in the medial collateral ligament of the rabbit after immobilization and remobilization. Journal of Bone & Joint Surgery - American Volume. 1987;69(8):1200–11. [PubMed] [Google Scholar]
- 47.Walsh S, Frank C, Hart D. Immobilization alters cell metabolism in an immature ligament. Clinical Orthopaedics & Related Research. 1992;(277):277–88. [PubMed] [Google Scholar]
- 48.Vogel KG, Koob TJ. Structural specialization in tendons under compression. International Review of Cytology. 1989;115:267–93. doi: 10.1016/s0074-7696(08)60632-4. [DOI] [PubMed] [Google Scholar]
- 49.Vogel KG, Ordog A, Pogany G, Olah J. Proteoglycans in the compressed region of human tibialis posterior tendon and in ligaments. Journal of Orthopaedic Research. 1993;11(1):68–77. doi: 10.1002/jor.1100110109. [DOI] [PubMed] [Google Scholar]
- 50.Vogel KG, Sandy JD, Pogany G, Robbins JR. Aggrecan in bovine tendon. Matrix Biology. 1994;14(2):171–9. doi: 10.1016/0945-053x(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 51.Berenson MC, Blevins FT, Plaas AH, Vogel KG. Proteoglycans of human rotator cuff tendons. Journal of Orthopaedic Research. 1996;14(4):518–25. doi: 10.1002/jor.1100140404. [DOI] [PubMed] [Google Scholar]
- 52.Carter DR, van der Meulen MC, Beaupre GS. Mechanobiologic regulation of osteogenesis and arthrogenesis. In: Buckwalter J, Ehrlich HP, Sandell LJ, Trippel SB, editors. Skeletal Growth and Development: Clinical Issues and Basic Science Advances. Rosemont, IL: AAOS; 1998. pp. 99–130. [Google Scholar]
- 53.Mikic B, Isenstein AL, Chhabra A. Mechanical modulation of cartilage structure and function during embryogenesis in the chick. Ann Biomed Eng. 2004 Jan;32(1):18–25. doi: 10.1023/b:abme.0000007787.39262.a7. [DOI] [PubMed] [Google Scholar]
- 54.Mikic B, Johnson TL, Chhabra AB, Schalet BJ, Wong M, Hunziker EB. Differential effects of embryonic immobilization on the development of fibrocartilaginous skeletal elements. J Rehabil Res Dev. 2000 Mar-Apr;37(2):127–33. [PubMed] [Google Scholar]
- 55.Slack C, Flint MH, Thompson BM. The effect of tensional load on isolated embryonic chick tendons in organ culture. Connect Tissue Res. 1984;12(3–4):229–47. doi: 10.3109/03008208409013685. [DOI] [PubMed] [Google Scholar]
- 56.Birch R. Obstetric brachial plexus palsy. J Hand Surg [Br] 2002 Feb;27(1):3–8. doi: 10.1054/jhsb.2001.0722. [DOI] [PubMed] [Google Scholar]
- 57.Mehta SH, Blackwell SC, Bujold E, Sokol RJ. What factors are associated with neonatal injury following shoulder dystocia? J Perinatol. 2006 Feb;26(2):85–8. doi: 10.1038/sj.jp.7211441. [DOI] [PubMed] [Google Scholar]
- 58.Waters PM, Smith GR, Jaramillo D. Glenohumeral deformity secondary to brachial plexus birth palsy. J Bone Joint Surg Am. 1998 May;80(5):668–77. doi: 10.2106/00004623-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 59.Moukoko D, Ezaki M, Wilkes D, Carter P. Posterior shoulder dislocation in infants with neonatal brachial plexus palsy. J Bone Joint Surg Am. 2004 Apr;86-A(4):787–93. doi: 10.2106/00004623-200404000-00018. [DOI] [PubMed] [Google Scholar]
- 60.Smith NC, Rowan P, Benson LJ, Ezaki M, Carter PR. Neonatal brachial plexus palsy. Outcome of absent biceps function at three months of age. J Bone Joint Surg Am. 2004 Oct;86-A(10):2163–70. [PubMed] [Google Scholar]
- 61.Kirkos JM, Kyrkos MJ, Kapetanos GA, Haritidis JH. Brachial plexus palsy secondary to birth injuries. J Bone Joint Surg Br. 2005 Feb;87(2):231–5. doi: 10.1302/0301-620x.87b2.14739. [DOI] [PubMed] [Google Scholar]
- 62.Thomopoulos S, Marquez JP, Weinberger B, Birman V, Genin GM. Collagen fiber orientation at the tendon to bone insertion and its influence on stress concentrations. J Biomech. 2006;39(10):1842–51. doi: 10.1016/j.jbiomech.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 63.Kostrominova TY, Dow DE, Dennis RG, Miller RA, Faulkner JA. Comparison of gene expression of 2-mo denervated, 2-mo stimulated-denervated, and control rat skeletal muscles. Physiol Genomics. 2005 Jul 14;22(2):227–43. doi: 10.1152/physiolgenomics.00210.2004. [DOI] [PubMed] [Google Scholar]
- 64.Thomopoulos S, Kim HM, Rothermich SY, Biederstadt C, Das R, Galatz LM. Decreased muscle loading delays maturation of the tendon enthesis during postnatal development. J Orthop Res. 2007 Sep;25(9):1154–63. doi: 10.1002/jor.20418. [DOI] [PubMed] [Google Scholar]
- 65.Provot S, Schipani E. Molecular mechanisms of endochondral bone development. Biochem Biophys Res Commun. 2005 Mar 18;328(3):658–65. doi: 10.1016/j.bbrc.2004.11.068. [DOI] [PubMed] [Google Scholar]
- 66.Chen X, Macica CM, Dreyer BE, Hammond VE, Hens JR, Philbrick WM, Broadus AE. Initial characterization of PTH-related protein gene-driven lacZ expression in the mouse. J Bone Miner Res. 2006 Jan;21(1):113–23. doi: 10.1359/JBMR.051005. [DOI] [PubMed] [Google Scholar]
- 67.Chen JM, Willers C, Xu J, Wang A, Zheng MH. Autologous tenocyte therapy using porcine-derived bioscaffolds for massive rotator cuff defect in rabbits. Tissue Eng. 2007 Jul;13(7):1479–91. doi: 10.1089/ten.2006.0266. [DOI] [PubMed] [Google Scholar]
- 68.Chen X, Nasiri A, Dreyer B, Judex S, Broadus AE. The principal domain of PTHrP is the circumference of long bones, in articular cartilage, periosteum, and entheses. Journal of Bone and Mineral Research. 2006 Sep;21:S445-S. [Google Scholar]
- 69.Chen XS, Macica C, Nasiri A, Judex S, Broadus AE. Mechanical regulation of PTHrP expression in entheses. Bone. 2007 Nov;41(5):752–9. doi: 10.1016/j.bone.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koyama E, Ochiai T, Rountree RB, Kingsley DM, Enomoto-Iwamoto M, Iwamoto M, Pacifici M. Synovial joint formation during mouse limb skeletogenesis: roles of Indian hedgehog signaling. Ann N Y Acad Sci. 2007 Nov;1116:100–12. doi: 10.1196/annals.1402.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim HM, Galatz LM, Patel N, Das R, Thomopoulos S. Recovery potential after postnatal shoulder paralysis. An animal model of neonatal brachial plexus palsy. J Bone Joint Surg Am. 2009 Apr;91(4):879–91. doi: 10.2106/JBJS.H.00088. [DOI] [PubMed] [Google Scholar]
- 72.Silva MJ, Thomopoulos S, Kusano N, Zaegel MA, Harwood FL, Matsuzaki H, Havlioglu N, Dovan TT, Amiel D, Gelberman RH. Early healing of flexor tendon insertion site injuries: Tunnel repair is mechanically and histologically inferior to surface repair in a canine model. J Orthop Res. 2006 May;24(5):990–1000. doi: 10.1002/jor.20084. [DOI] [PubMed] [Google Scholar]
- 73.Thomopoulos S, Zampiakis E, Das R, Silva MJ, Gelberman RH. The effect of muscle loading on flexor tendon-to-bone healing in a canine model. J Orthop Res. 2008 Dec;26(12):1611–7. doi: 10.1002/jor.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rodeo SA, Suzuki K, Deng XH, Wozney J, Warren RF. Use of recombinant human bone morphogenetic protein-2 to enhance tendon healing in a bone tunnel. American Journal of Sports Medicine. 1999;27(4):476–88. doi: 10.1177/03635465990270041201. [DOI] [PubMed] [Google Scholar]