Abstract
Bacillus anthracis, the causative agent of anthrax, elaborates a tripartite toxin composed of two enzymatically active subunits, lethal factor (LF) and edema factor (EF) which, when associated with a cell-binding component, protective antigen (PA), form lethal toxin (LT) and edema toxin (ET), respectively. In this preliminary study we characterised the toxin-specific antibody responses observed in 17 individuals infected with cutaneous anthrax. The majority of the toxin-specific antibody responses observed following infection were directed against LF with IgG detected as early as 4 days after onset of symptoms in contrast to the later and lower EF- and PA-specific IgG responses. Unlike the case with infection, the predominant toxin-specific antibody response of those immunized with the US AVA and UK AVP licensed anthrax vaccines was directed against PA.. We observed that the LF-specific human antibodies were, like anti-PA antibodies, able to neutralize toxin activity, suggesting the possibility that they may contribute to protection. We conclude that an antibody response to LF might be a more sensitive diagnostic marker of anthrax than to PA. The ability of human LF-specific antibodies to neutralize toxin activity supports the possible inclusion of LF in future anthrax vaccines.
Keywords: anthrax, lethal factor, edema factor, protective antigen
Introduction
Anthrax is a zoonotic disease caused by Bacillus anthracis, a gram-positive spore-forming microbe whose manifestations in humans depends on the route of infection. The cutaneous form of the disease accounts for more than 95% of reported cases (Shafazand et al, 1999) and with treatment, does not usually pose a threat to human life (Little & Ivins, 1999). The gastrointestinal and inhalational forms of the disease, though not as common, are much more severe (Little & Ivins, 1999). The ability of the organism to form environmentally resistant spores, be dispersed as aerosols, and cause lethal infection following inhalation has resulted in its development and use as a biological weapon.
Following infection, spores are phagocytosed by macrophages and transported to the draining lymph nodes where they germinate into vegetative bacilli and escape from the macrophage (Lincoln et al, 1965; Dixon et al, 2000; Guidi-Rontini et al, 2001). In cutaneous anthrax, this results in a localized infection; in inhalational anthrax, the bacilli multiply in the lymphatic system and spread to the blood, resulting in massive bacteremia and toxemia (Fish & Lincoln, 1968).
Within three hours of spore germination, expression of the toxin proteins begins (Guidi-Rontini et al, 1999). The extracellular tripartite toxin of anthrax is composed of two enzymatically active subunits, lethal factor (LF) and edema factor (EF), and a cell-binding and translocation component, protective antigen (PA). Both lethal (PA+LF) and edema (PA+EF) toxins are able to suppress key parts of the innate immune response to the developing infection (Erwin et al, 2001; Popov et al, 2002; Duesbury et al, 1998; Pellilzzari et al, 1999; O'Brien et al, 1985; Wright & Mandell, 1986; Kalns et al, 2002; Moayeri et al, 2003). Later in the disease process, high levels of lethal toxin induce the cytokine independent shock-like death associated with anthrax (Moayeri et al, 2003).
Animal studies suggest that as the concentration of toxin increases the likelihood of successfully treating an infected individual decreases until it reaches a level at which antibiotics are no longer effective (Albrecht et al., 2007; Baillie, 2009). Given that early detection of toxin is a key diagnostic marker, it surprising how little is known of the time course of toxin production in humans and thus we sought to characterise the early immune responses to individuals infected with anthrax (Baillie, 2009).
To achieve this aim, clinical serum samples previously obtained at various time points post infection from individuals who had contracted cutaneous anthrax were examined for the presence of toxin-specific IgM and IgG antibodies. In addition we compared these antibody responses to those seen following immunisation with the US Anthrax Vaccine Absorbed (AVA) and UK Anthrax Vaccine Precipitated (AVP) licensed human anthrax vaccines. Finally, the protective function of the toxin specific antibody responses stimulated following infection were assessed using an assay which measures toxin neutralization, a recently demonstrated correlate of protection (Little et al, 2004; Reuveny et al, 2001).
Material and methods
Expression and purification of toxin components
The PA, LF and EF genes were cloned into the E. coli expression vector pQE-30 (QIAGEN) and confirmed by sequencing (Read et al., 2003). Proteins were expressed from either the M15 (PA) or SG13009 (LF and EF) strain of E. coli. Host strains were grown in LB medium to an OD600 of 0.55-0.65 and induced with 1 mM isopropyl β-D-thiogalactoside either 4 hours at 37°C (PA) or 20 hours at 25°C (LF and EF). Cells were pelleted and lysed by French press at 16000 psi; lysates were cleared at 45,000×g for 15 minutes. Recombinant proteins were purified by cobalt affinity chromatography. Cleared lysate was batch-bound to TALON resin (Clontech) then washed with 10 CV 300 mM NaCl, 50 mM Na2HPO4, 20 mM imidazole, pH 7.0. Proteins were eluted in 5 CV 300 mM NaCl, 50 mM Na2HPO4, 150 mM imidazole, pH 7.0. Fractions containing protein (determined by SDS-PAGE) were pooled and dialyzed into 10 mM HEPES, 50 mM NaCl, pH 7.5. Proteins purified by this procedure were approximately 90% pure as assessed by SDS-PAGE with Coomassie staining.
Serum samples
Serum samples were obtained from volunteers who had received at least a priming series of the AVA (six Maryland-based volunteers) or AVP (four UK-based volunteers visiting Maryland) vaccines. Control samples were obtained from six non-immunised, non-infected Maryland-based individuals. All samples were obtained under a protocol approved by the University of Maryland and the Naval Medical Research Center's Institutional Review Boards, as well as by the Ethics Committee at Erciyes University. Informed consent was obtained from all individuals. Clinical samples were obtained from seventeen cutaneous anthrax patients attending the infectious diseases clinic at Erciyes University in Turkey, (Table 1). Serum samples were not collected prospectively from patients under a set protocol but were instead collected when patients presented to the outpatient clinics for up to 21 days after the initial visit. Anthrax was diagnosed by exposure history, clinical presentation consistent with anthrax, Gram stain and positive culture from the lesion.
Table 1.
The details for the patients with cutaneous anthrax
| Patient no | Incubation period (day) | aContact with a contaminated animal materials | Site of the lesion | bGram stain for B.anth. | bPositive culture for B.anth. | Previous antibiotic use | *Antibiotic initiation day of disease | Given antibiotic | Duration of therapy (day) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | + | right eye lids | - | - | yes | 3rd | Pen G | 15 | left deep tissue scar, recovered |
| 2 | 15 | + | right arm | - | - | yes | 4th | Pen G + Cipro | 10 | recovered |
| 3 | 1 | + | right arm | - | - | yes | 2nd | Pan G | 10 | recovered |
| 4 | 6 | + | right hand | – | – | yes | 4th | Pen G | 10 | recovered |
| 5 | 9 | + | left hand | - | - | no | 6th | Pen G | 7 | recovered |
| 6 | 10 | + | left forearm | - | - | yes | 5th | Pen G | 10 | recovered |
| 7 | 5 | + | right hand fingers | + | + | no | 5th | Pen G | 7 | recovered |
| 8 | 4 | + | Left hand finger | + | - | yes | 4th | Pen G | 15 | recovered |
| 9 | 12 | + | both hands | - | - | yes | 3rd | Pen G | 14 | recovered |
| 10c | 2 | + | anterior neck | + | + | no | 2nd | Pen G | 14 | left deep tissue scar recovered |
| 11 | 8 | + | The eye lid | + | + | no | 3rd | Pen G | 10 | recovered |
| 12 | 8 | + | left eye lids | + | - | yes | 3rd | Pen G | 14 | recovered |
| 13 | 5 | + | right wrist | + | - | yes | 5th | Pen G | 10 | recovered |
| 14 | ? | + | left elbow | - | - | yes | 10th | Pen G | 10 | recovered |
| 15 | 5 | + | right face | + | - | yes | 5th | Pen G | 10 | recovered |
| 16 | 1 | + | Anterior neck | + | - | no | 7th | Pen G | 7 | recovered |
| 17 | 7 | + | Right arm and left wrist | - | - | yes | 4th | Pen G | 10 | left deep tissue scar, recovered |
The patient gave a history of contact with ill animal or contaminated animal materials (such as slaughtering of ill animals, skinning, chopping meat, carrying raw skin or splashing blood from dying animal during slaughtering). One case gave a history of fly bite.
The swabs for Gram stain and culture were taken from the vesicles of fluid or under the crust if developed.
In this case, anthrax sepsis was developed from the cutaneous lesion
Time from first symptoms to diagnose and initiation antibiotic treatment
Pen G: Penicillin G; Cipro: Ciprofloxacin
Anti-toxin IgG and IgM ELISA
Antitoxin IgG and IgM levels were measured by enzyme-linked immunosorbent assay (ELISA) as previously described, with minor variations (Hepburn et al, 2007). Data values were compared to a standard curve of purified human IgG or IgM (Sigma). Data in the linear portion of the ELISA graph and within the range of the standard curve were used to calculate the quantitative titer (μg/ml) for the serum sample. For each antigen, four to six naïve serum samples were assayed and their titers were averaged (geometric mean) and the 95% confidence interval of the distribution was calculated. Experimental data were scored as a positive result only if the calculated titer exceeded the upper limit of the confidence interval of the naïve control samples.
Lethal toxin neutralization assay
The toxin neutralization assay was performed on the mouse monocyte cell line J774A.1 (ATCC) as previously described with cell viability determined by addition of DMEM containing XTT (sodium 3’-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis (4-methoxy-6-nitro) benzene sulfonic acid hydrate)) (Roche) for 16 hours . The assay was read at 480 nm. The dilution series data (absorbance at 480nm versus toxin concentration) were modeled with 4-parameter logistic (4PL) curves of the form:
The data were fit by Matlab software via a nonlinear least-squares analysis, yielding the parameters (ß1, ß2, ß3, ß4) of the best fit. The inflection point of the fit (ß4 parameter) corresponds to the serum dilution able to ensure the survival of 50% of the cells in the assay (ED50) (Quinn et al, 2004).
Results
Clinical characteristics
Cutaneous anthrax in the patients was diagnosed by the history of contact with ill animals and animal products, typical cutaneous lesions, and demonstration of Gram positive bacilli (8 cases) and/or positive cultures (3 cases) from the lesion (Table 1). The upper body was the usual site of the cutaneous anthrax lesion. The finding that cultures were positive only in 3 cases was presumed to be due to the fact that many patients had taken an antibiotic before their initial visit. With cultures of both the lesion and blood being positive, one patient was diagnosed with anthrax sepsis originating from the cutaneous lesion., The incubation period varied between 1-15 days. Seventeen patients gave a history of contact with an ill animal or contaminated materials of animals that died. The time from first symptom to the initiation of antibiotics ranged from 2-10 days, with 16 subjects receiving antibiotics, predominantly penicillin, within the first seven days, while ten patients received antibiotics within four days. Typically, the patients were treated for 7-15 days, and all recovered. Three subjects (patients1, 10 and 17), however, were left with deep tissue scars.
Anti-toxin IgM responses
In order to examine the early immune response to infection, the serum IgM levels against PA, LF and EF were measured and compared to the anti-toxin IgM titer of six naïve volunteers. Of the seventeen infected individuals, six (35%) had an IgM response to at least one of the toxin components which was higher than that of the naïve controls (Table 2). There was no statistical difference in the level of toxin-specific IgM between the remaining 11 patients and the naïve controls. An anti-LF specific IgM was detected in 5 individuals within the first and second weeks following symptoms with the earliest response detected at day 4. In contrast, an anti-PA IgM was detected in 4 individuals but not until the second and third weeks following symptoms, with the earliest titers appearing at day 12. Finally, anti-EF titers were detected in only two patients with titers occurring in the second week after symptoms.
Table 2.
Comparison of serum anti-toxin IgM levels in cutaneous anthrax patients
| Patient | Days after Onset of Symptoms | Anti-PA IgM | Anti-LF IgM | Anti-EF IgM |
|---|---|---|---|---|
| Naïve | --- | 1.2 (1.3) | 4.7 (3.3) | 2.2 (2.0) |
| 1 | 5 | <NB | <NB | <NB |
| 12 | 7.1 (1.8) | 11.3 (1.1) | <NB | |
| 2 | 4 | <NB | 8.9 (0.2) | <NB |
| 13 | 2.7 (0.1) | 10.2 (1.0) | 4.7 (2.5) | |
| 5 | 6 | <NB | <NB | <NB |
| 13 | <NB | 9.3 (2.0) | <NB | |
| 7 | 5 | <NB | <NB | <NB |
| 15 | 3.4 (0.3) | 8.6 (0.8) | <NB | |
| 10a | 7 | <NB | 8.3 (0.1) | <NB |
| 16 | 14 | <NB | <NB | 5.1 (2.3) |
| 21 | 2.6 (0.8) | <NB | <NB |
Data are in micrograms/milliliter and represent the mean titer (+/- standard deviation) as determined by ELISA
Patient developed septicemia from cutaneous lesion
<NB, less than the naïve baseline (titer indistinguishable from naïve controls)
Anti-toxin IgG responses
The serum IgG titers against the anthrax toxin proteins in seventeen infected individuals were measured and compared to the geometric mean IgG titer of six naïve volunteers. In contrast to the IgM responses, eleven of the seventeen patients (65%) demonstrated a measurable IgG titer to at least one of the toxin components on the days assayed (Table 3). While each positive patient generated an anti-LF IgG response with titers ranging from 13.6-817 μg/ml, only 4 individuals showed an anti-EF IgG response (patients 5, 6, 8 and 16) and only 3 patients (patients 2, 6 and 7) had IgG specific for PA. The anti-LF IgG response in all infected individuals exceeded that directed against PA and EF. During the second week of infection, the geometric mean IgG titer (GMT) of all responders to LF (69.3 μg/ml) was almost twice the anti-EF titer (37.4 μg/ml) and three times the anti-PA titer (22.6 μg/ml).
Table 3.
Serum anti-toxin IgG levels in cutaneous anthrax patients
| Patient | Days after Onset of Symptoms | Anti-PA IgG | Anti-LF IgG | Anti-EF IgG |
|---|---|---|---|---|
| Naive | --- | 11.6(5.4) | 5.6(4.1) | 6.5(6.0) |
| 1 | 5 | <NB | <NB | <NB |
| 12 | <NB | 147.4(19.0) | <NB | |
| 2 | 4 | <NB | <NB | <NB |
| 13 | 21.6 (6.4) | 117.3(26.8) | <NB | |
| 3 | 4 | <NB | 13.6(3.2) | <NB |
| 9 | <NB | 16.0(3.7) | <NB | |
| 5 | 6 | <NB | 15.7(2.1) | 14.7(1.5) |
| 13 | <NB | 141.0(19.3) | 27.1(2.7) | |
| 6 | 5 | <NB | <NB | <NB |
| 20 | 26.1(2.2) | 430.8(91.0) | 24.3(1.6) | |
| 7 | 5 | <NB | <NB | <NB |
| 15 | 19.3(4.8) | 71.9(18.1) | <NB | |
| 8 | 4 | <NB | <NB | <NB |
| 9 | <NB | 35.6(13.6) | 60.5(7.9) | |
| 13 | <NB | 68.7(18.5) | 31.9(8.2) | |
| 9 | 15 | <NB | 28.9(9.6) | <NB |
| 10a | 7 | <NB | 31.0(8.2) | <NB |
| 16 | 14 | <NB | 214.3(24.4) | <NB |
| 21 | <NB | 817.6(202.8) | 26.2(7.6) | |
| 17 | 7 | <NB | <NB | <NB |
| 15 | <NB | 60.2(10.9) | <NB |
Data are in micrograms/milliliter and represent the mean titer (+/- standard deviation) as determined by ELISA
Patient developed septicemia from cutaneous lesion
<NB, less than the naïve baseline (titer indistinguishable from naïve controls)
LF-specific IgG responses were detected as early as 4 days post onset of symptoms while anti-EF IgG responses were not seen until day 6 and anti-PA IgG responses first being detected at day 13. These preliminary results would appear to indicate that of the three toxins components the antibody response to LF represents the most appropriate early diagnostic indicator.
To further characterise the protective nature of the antibody response induced by infection, we compared the spectrum of the resulting toxin-specific IgG responses to those seen following immunization of healthy volunteers with the US AVA and the UK AVP licensed human anthrax vaccines (Baillie et al, 2003; Baillie et al, 2004; Pittman et al, 2002). As can be seen from Figures 1A and IB all of the vaccinees mounted a PA-specific IgG response, the level of which varied depending on the vaccine used and the immunization schedule of each individual. In contrast to the responses observed following infection, little if any LF-specific IgG was detected and then only in recipients of the UK vaccine. While we were unable to draw any conclusions as to the relative efficacy of each vaccine due to the small sample size, we could conclude that PA comprised the major immunogen within the vaccine, particularly those immunized with the AVA vaccine (Figure 1A).
Figure 1.
Serum anti-toxin titers of immunized individuals. The anti-toxin responses of individuals immunized with the US (AVA) or UK (AVP) licensed vaccine were compared. Responses to protective antigen (PA) and lethal factor (LF) and edema factor (EF) are shown in Figures 1A and 1B respectively. Data represent the mean titer (+/- standard deviation) of four trials as determined by ELISA.
Neutralization of lethal toxin
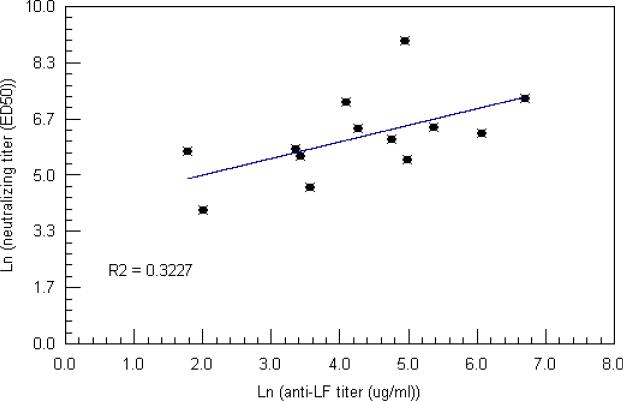
The ability of the AVA and AVP vaccines to stimulate a protective immune response has been demonstrated across a number of animal studies and is thought to be due to the production of PA-specific antibodies capable of neutralizing anthrax toxin activity (Baillie 2009). Indeed, toxin neutralizing PA-specific IgG antibodies have been identified as a correlate of protection (Reuveny et al, 2001 Little et al, 2004). To determine if serum from infected individuals also contained toxin neutralizing antibodies, we assayed the activity of samples collected from 10 infected individuals at the time point which had the highest anti-toxin IgG titer (Figure 2). Neutralizing activity was detected in all samples with ED50 values ranging from 103.8 (patient #8) to 7983 (patient #5). The presence of LF-specific antibodies suggested a possible correlation between LF antibody titer and toxin neutralization. However, linear regression analysis of the ED50 values versus anti-LF IgG produced only a weakly positive correlation (R2 = 0.323) possibly due to the small number of samples included in the analysis (Figure 3). No correlate was observed between the ED50 values and anti-PA IgG, anti-PA IgM or anti-LF IgM titers (data not shown).
Figure 2.
Lethal toxin neutralizing antibody titers of cutaneous anthrax patients. For patients with more than one serum sample, neutralizing titers were measured at the time point with the highest IgG titer. Neutralizing titer ED50 values ranged from 103.8-7983. Data represent the ED50 (β4 parameter) generated by a 4-parameter logistic fit of 4 tests.
Figure 3.
Relationship of the anti-LF ELISA titer with the lethal toxin neutralizing ED50 in cutaneous anthrax patients. There was a weak positive correlation (R2 = 0.3227) between anti-LF IgG levels and neutralizing titer in infected individuals. No correlation was observed between anti-PA levels and neutralizing titer (R2 = 0.005).
Discussion
Anthrax is primarily a disease of animals which occasionally infects humans and as a consequence opportunities to study the pathology of the disease in human are rare (Baillie et al., 2009). The early antibody responses of a small number of individuals suffering from cutaneous anthrax were analyzed for their ability to recognize the individual components of the tripartite toxin produced by B. anthracis. In the context of infection one would expect the initial antibody response to comprise IgM class antibodies and while this was true for a small number of individuals the majority showed a mixed IgM/IgG toxin-specific antibody response at the first sampling point. In general, the IgM responses were considerably lower than the corresponding IgG levels for all patients, a pattern similar to that observed in AVA immunized primates (Ivins et al, 1998). Together, these data imply that class switching from IgM to IgG occurs early in the anthrax infection.
We observed differences in the ability of infected individuals to recognize toxin components. While 11(65 %) patients produced an LF-specific IgG response, only four (24%) mounted an IgG response to EF while only three (18%) demonstrated an IgG specific immune response to PA. In those patients that showed the presence of anti-LF and anti-PA IgG antibodies (patients 2, 6 and 7), the LF titers exceeded the PA response by a factor of a least 3. Since both proteins are produced early in infection (Guidi-Rontini et al, 1999), this disparity in response may reflect fundamental differences in how each protein is processed by the immune system. For example, LF is known to elicit higher IgG antibody titers than PA when administered to experimental animals (Hermanson et al, 2004; Price et al, 2001; Flick-Smith et al, 2005).
Indeed the overall disparity between anti-PA and anti-LF IgG responses observed in this study is likely to be the result of a combination of factors such as the virulence of the infecting strain, the route of infection and the health and genetic background of the individual. The initiation of antimicrobial therapy is also likely to have affected the level of toxin expression and may explain the the absence of detectable response to any anthrax toxin component in six infected individuals (Stepanov et al, 1996; Athamna et al, 2004).
In an anthrax outbreak, it is crucial to identify infected individuals as quickly as possible to enable the initiation of timely treatment. The central role of the toxins in the progression of the infection has lead to the proposal to employ the antibody response to PA as a diagnostic marker of exposure to anthrax (Quinn et al, 2004; Quinn et al, 2002). This recommendation was based on the analysis of the PA-specific antibody response of patients with bioterrorism-related infections (Quinn et al, 2004). While individuals with inhalation anthrax demonstrated an IgG response to PA as early as 11 days following the onset of symptoms, those with the cutaneous infections did not develop anti-PA titers until 21 to 34 days after the onset of symptoms and those titers did not peak until days 30-60 (Quinn et al, 2004), confirming our findings that the anti-PA immune response develops slowly. Unlike our study Quinn and colleagues did not screen their samples for the presence of an anti-LF immune response. If they had, they may have also found that the LF-specific antibody response preceded that of the PA response. We found that the LF-specific IgG was detected as early as day 4 after the onset of symptoms, and that all patients had developed anti-LF titers by day 15. In contrast, PA-specific IgG was first detected 13 days after the onset of symptoms, and had appeared in only three patients by day 21. The early production and prevalence of anti-LF antibodies after the development of symptoms suggest that the overall kinetics of the immune response is a rapid anti-LF response followed by a slower, longer anti-PA response. The speed, strength and prevalence of the anti-LF response in infected patients clearly demonstrate that the presence of an anti-LF response might be a better diagnostic marker of infection than an anti-PA response.
We also sought to compare the response following immunization with the US AVA and UK AVP licensed anthrax vaccines. While both are subunit vaccines, they differ primarily in their means of production. The current U.S. licensed vaccine, AVA, is produced from a cell-free culture filtrate of an anaerobically grown B. anthracis strain V770-NP1-R (a non-encapsulated, non-proteolytic variant of a bovine strain isolated in Florida in 1951) and consists largely of PA adsorbed onto aluminum hydroxide (Baillie, 2001). In contrast, the UK vaccine, AVP is produced from an alum precipitate of the cell-free culture filtrate of a static, aerobic culture of the B. anthracis Sterne strain 34F2. In addition to large amounts of PA, the vaccine also contains trace amounts of LF and other bacterially-derived, immunogenic antigens, which have been shown to stimulate antibody responses in recipients and may contribute to protection (Baillie et al, 2003; Baillie et al, 2004; Whiting et al, 2004). Indeed the presence of these additional proteins may also account for the transient reactogenicity seen in some individuals (Turnbull, 2000). The efficacy of both vaccines has been demonstrated in numerous animal models including primates and has revealed a significant correlation between PA IgG titer and toxin neutralizing activity which is thought to be indicative of protection (Ivins et al, 1998; Little et al, 2004; Reuveny et al, 2001; Quinn et al, 2004, Phipps et al, 2004; Pittman et al, 2005).
In this study we observed that the LF-specific human antibody were able to neutralize toxin activity. Several patients with high anti-LF IgG titers but lacking anti-PA IgG titers had measurable toxin neutralization titers. This neutralization activity was not due to non-specific protection as serum from six naïve individuals failed to protect macrophages (data not shown). Neither could neutralization be attributed to the presence of anti-PA antibodies, as samples obtained from patients 5, 8, 9, 10 and 17 showed no detectable anti-PA IgM or IgG antibodies, but still had quantifiable neutralizing activity. To our knowledge this is the first report of the production of LF-specific neutralizing antibodies by humans following infection. The levels of neutralizing antibody generated by these individuals was roughly equivalent to levels generated by AVP-immunized individuals (Hepburn et al., 2007). The ability of LF to stimulate toxin neutralizing antibodies is not surprising given that LF must first bind to PA before it can be transported into the cytosol of the susceptible cell where it exerts its toxic effects. Indeed, animal studies have demonstrated the ability of toxin neutralizing LF-specific antibodies to protect rabbits against aerosol spore challenge (Hermanson et al, 2004; Zhao et al, 2003; Lim et al, 2005). While we found no such correlation among our patients for PA, we did observe a weak correlation between LF-specific IgG and toxin neutralizing titers. Since little is known about either the human response to anthrax or the ability of LF to generate protective antibodies, further work is required to determine the factors mediating LF-specific toxin neutralization.
Concerns over the ability to circumvent the current licensed vaccines by altering the antigenic structure of PA has spurred researchers to focus on additional vaccine targets (Schneerson et al, 2003; Hoffmaster et al, 2004). The immunogenicity of LF and its ability to stimulate the production of toxin neutralizing antibodies makes a biologically inactive form of LF a strong candidate for inclusion in any future anthrax vaccine. We conclude from this preliminary study that an antibody response to LF might be a more sensitive diagnostic marker of anthrax than one to PA. In addition, the ability of human LF-specific antibodies to neutralize toxin activity supports the possible inclusion of LF in future anthrax vaccines.
Acknowledgments
We thank and acknowledge Dr. Emine Alp for her assistance. For their assistance in providing samples and information, we acknowledge Dr. Basak Dokuzoguz at Ankara Numune Clinical Research and Education Hospital (Ankara), Dr. Mehmet Parlak at Atatürk University (Erzurum), Dr. Ayhan Akbulut at Firat University (Elazig), and Dr. Ilyas Dokmetas at Cumhuriyet University (Sivas). We also acknowledge Stephanie Gray for her administrative efforts. Finally, we thank all the volunteers for their cooperation in donating their time and blood samples to this study.
Financial support . This work was supported by the Defense Threat Reduction Agency under the work unit number 80000.000.000.A0031 and NIH U54 AI057168-01.
Footnotes
The views in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor of the U.S. government.
Copyright statement: I am an employee of the U.S. government. This work was prepared as part of my official duties. Title 17 U.S.C. Section 105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. Section 101 defines a U.S. Government work as a work prepared by the military service member or employee of the U.S. Government as part of that person's official duties.
Potential conflicts of interest. All authors: no conflicts.
References
- Albrecht MT, Li H, Williamson ED, et al. Human monoclonal antibodies against anthrax lethal factor and protective antigen act independently to protect against Bacillus anthracis infection and enhance endogenous immunity to anthrax. Infect Immun. 2007;75:5425–33. doi: 10.1128/IAI.00261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athamna A, Massalha M, Athamna M, et al. In vitro susceptibility of Bacillus anthracis to various antibacterial agents and their time-kill activity. J Antimicrob Chemother. 2004;53:247–251. doi: 10.1093/jac/dkh016. [DOI] [PubMed] [Google Scholar]
- Baillie LW. Is new better than old? The development of human vaccines for anthrax. Human Vaccines. 2009;5:806–816. doi: 10.4161/hv.9777. [DOI] [PubMed] [Google Scholar]
- Baillie L. The development of new vaccines against Bacillus anthracis. J Appl Microbiol. 2001;91:609–613. doi: 10.1046/j.1365-2672.2001.01498.x. [DOI] [PubMed] [Google Scholar]
- Baillie L, Hebdon R, Flick-Smith H, Williamson D. Characterization of the immune response to the UK human anthrax vaccine. FEMS Immunol Med Microbiol. 2003;36:83–86. doi: 10.1016/S0928-8244(03)00085-3. [DOI] [PubMed] [Google Scholar]
- Baillie L, Townend T, Walker N, Erikson U, Williamson D. Characterization of the human immune response to the UK anthrax vaccine. FEMS Immunol Med Microbiol. 2004;42:267–270. doi: 10.1016/j.femsim.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Dixon TC, Fadl AA, Koehler TM, Swanson JA, Hanna PC. Early Bacillus anthracis-macrophage interactions: intracellular survival and escape. Cell Microbiol. 2000;2:453–463. doi: 10.1046/j.1462-5822.2000.00067.x. [DOI] [PubMed] [Google Scholar]
- Duesbery NS, Webb CP, Leppla SH, et al. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science. 1998;280:734–737. doi: 10.1126/science.280.5364.734. [DOI] [PubMed] [Google Scholar]
- Erwin JL, DaSilva LM, Bavari S, Little SF, Friedlander AM, Chanh C. Macrophage-derived cell lines do not express proinflammatory cytokines after exposure to Bacillus anthracis lethal toxin. Infect Immun. 2001;69:1175–1177. doi: 10.1128/IAI.69.2.1175-1177.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish DC, Lincoln RE. In vivo-produced anthrax toxin. J Bacteriol. 1968;95:919–924. doi: 10.1128/jb.95.3.919-924.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flick-Smith HC, Waters EL, Wlaker NJ, et al. Mouse model characterization for anthrax vaccine development: comparison of one inbred and one outbred mouse strain. Microb Pathog. 2005;38:33–40. doi: 10.1016/j.micpath.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Guidi-Rontani C, Levy M, Ohayow H, Mock M. Fate of germinated Bacillus anthracis spores in primary murine macrophages. Mol Microbiol. 2001;42:931–938. doi: 10.1046/j.1365-2958.2001.02695.x. [DOI] [PubMed] [Google Scholar]
- Guidi-Rontani C, Weber-Levy M, Labruyere E, Mock M. Germination of Bacillus anthracis spores within alveolar macrophages. Mol Microbiol. 1999;31:9–17. doi: 10.1046/j.1365-2958.1999.01137.x. [DOI] [PubMed] [Google Scholar]
- Hepburn MJ, Dyson EH, Simpson AJH, et al. Immune response to two different dosing schedules of the anthrax vaccine precipitated (AVP) vaccine. Vaccine. 2007;25:6089–6097. doi: 10.1016/j.vaccine.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Hermanson G, Whitlow V, Parker S, et al. A cationic lipid-formulated plasmid DNA vaccine confers sustained antibody-mediated protection against aerosolized anthrax spores. Proc Natl Acad Sci U S A. 2004;101:13601–13606. doi: 10.1073/pnas.0405557101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmaster AR, Ravel J, Rasko D, et al. Identification of anthrax toxin genes in a Bacillus cereus associated with an illness resembling inhalation anthrax. Proc Natl Sci U S A. 2004;101:8449–8454. doi: 10.1073/pnas.0402414101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivins BE, Pitt ML, Fellows PF, et al. Comparative efficacy of experimental anthrax vaccine candidates against inhalation anthrax in rhesus macaques. Vaccine. 1998;16:1141–1148. doi: 10.1016/s0264-410x(98)80112-6. [DOI] [PubMed] [Google Scholar]
- Kalns J, Scruggs J, Millenbaugh N, Vivkananda J, Shealy D, Eggers J, Kiel J. TNF receptor 1, IL1 receptor, and iNOS genetic knockout mice are not protected from anthrax infection. Biochem Biophys Res Commun. 2002;292:41–44. doi: 10.1006/bbrc.2002.6626. [DOI] [PubMed] [Google Scholar]
- Lim NK, Kim JH, Oh MS, et al. An anthrax lethal factor-neutralizing monoclonal antibody protects rats before and after challenge with anthrax toxin. Infect Immun. 2005;73:6547–6551. doi: 10.1128/IAI.73.10.6547-6551.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln RE, Hodges DR, Klein F, et al. Role of the lymphatics in the pathogenesis of anthrax. J Infect Dis. 1965;115:481–494. doi: 10.1093/infdis/115.5.481. [DOI] [PubMed] [Google Scholar]
- Little SF, Ivins BE. Molecular pathogenesis of Bacillus anthracis infection. Microbes Infect. 1999;1:131–139. doi: 10.1016/s1286-4579(99)80004-5. [DOI] [PubMed] [Google Scholar]
- Little SF, Ivins BE, Fellows PF, Pitt ML, Norris SL, Andrews GP. Defining a serological correlate of protection in rabbits for a recombinant anthrax vaccine. Vaccine. 2004;22:422–430. doi: 10.1016/j.vaccine.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Moayeri M, Haines D, Young HA, Leppla SH. Bacillus anthracis lethal toxin induces TNF-alpha-independent hypoxia-mediated toxicity in mice. J Clin Invest. 2003;112:670–682. doi: 10.1172/JCI17991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J, Friedlander A, Dreier T, Ezzell J, Leppla S. Effects of anthrax toxin components on human neutrophils. Infect Immun. 1985;47:306–310. doi: 10.1128/iai.47.1.306-310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellizzari R, Guidi-Rontani C, Vitale G, Mock M, Montecucco C. Anthrax lethal factor cleaves MKK3 in macrophages and inhibits the LPS/IFNgamma-induced release of NO and TNFalpha. FEBS Lett. 1999;462:199–204. doi: 10.1016/s0014-5793(99)01502-1. [DOI] [PubMed] [Google Scholar]
- Phipps AJ, Premanandan C, Barnewall RE, Lairmore MD. Rabbit and nonhuman primate models of toxin-targeting human anthrax vaccines. Microbiol Mol Biol Rev. 2004;68:617–629. doi: 10.1128/MMBR.68.4.617-629.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman PR, Kim-Ahn G, Pifat DY, et al. Anthrax vaccine: immunogenicity and safety of a dose-reduction, route-change comparison study in humans. Vaccine. 2002;20:1412–1420. doi: 10.1016/s0264-410x(01)00462-5. [DOI] [PubMed] [Google Scholar]
- Pittman PR, Leitman SF, Oro JG, et al. Protective antigen and toxin neutralization antibody patterns in anthrax vaccines undergoing serial plasmapheresis. Clin Diagn Lab Immunol. 2005;12:713–721. doi: 10.1128/CDLI.12.6.713-721.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov SG, Villasmil R, Bernardin J, Grene E, Cardwell J, Wu A, Alibek D, et al. Lethal toxin of Bacillus anthracis causes apoptosis of macrophages. Biochem Biophys Res Commun. 2002;293:349–355. doi: 10.1016/S0006-291X(02)00227-9. [DOI] [PubMed] [Google Scholar]
- Price BM, Liner AL, Park S, Leppla SH, Mateczun A, Galloway DR. Protection against anthrax lethal toxin challenge by genetic immunization with a plasmid encoding the lethal factor protein. Infect Immun. 2001;69:4509–4515. doi: 10.1128/IAI.69.7.4509-4515.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn CP, Dull PM, Semenova V, et al. Immune responses to Bacillus anthracis protective antigen in patients with bioterrorism-related cutaneous or inhalation anthrax. J Infect Dis. 2004;190:1228–1236. doi: 10.1086/423937. [DOI] [PubMed] [Google Scholar]
- Quinn CP, Semenova VA, Elie CM, et al. Specific, sensitive, and quantitative enzyme-linked immunosorbent assay for human immunoglobulin G antibodies to anthrax toxin protective antigen. Emerg Infect Dis. 2002;8:1103–1110. doi: 10.3201/eid0810.020380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read TD, Peterson SN, Tourasse N, Baillie LW, Paulsen IT, Nelson KE, Tettelin H, et al. The genome sequence of Bacillus anthracis Ames and comparison to closely related bacteria. Nature. 2003;423:81–86. doi: 10.1038/nature01586. [DOI] [PubMed] [Google Scholar]
- Reuveny S, White MD, Adar YY, et al. Search for correlates of protective immunity conferred by anthrax vaccine. Infect Immun. 2001;69:2888–2893. doi: 10.1128/IAI.69.5.2888-2893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneerson R, Kubler-Kleib J, Liu TY, et al. Poly(gamma-D-glutamic acid) protein conjugates induce IgG antibodies in mice to the capsule of Bacillus anthracis: a potential addition to the anthrax vaccine. Proc Natl Sci U S A. 2003;100:8945–8950. doi: 10.1073/pnas.1633512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafazand S, Doyle R, Ruoss S, Weinacker A, Raffin TA. Inhalational anthrax: epidemiology, diagnosis, and management. Chest. 1999;116:1369–1376. doi: 10.1378/chest.116.5.1369. [DOI] [PubMed] [Google Scholar]
- Stepanov AV, Marinin LI, Pomerantsev AP, Staritsin NA. Development of novel vaccines against anthrax in man. J Biotechnol. 1996;44:155–160. doi: 10.1016/0168-1656(95)00092-5. [DOI] [PubMed] [Google Scholar]
- Turnbull PC. Current status of immunization against anthrax: old vaccines may be here to stay for awhile. Curr Opin Infect Dis. 2000;13:113–120. doi: 10.1097/00001432-200004000-00004. [DOI] [PubMed] [Google Scholar]
- Whiting GC, Rijpkema S, Adams T, Corbel MJ. Characterization of adsorbed anthrax vaccine by two-dimensional gel electrophoresis. Vaccine. 2004;22:4245–4251. doi: 10.1016/j.vaccine.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Wright GG, Mandell GL. Anthrax toxin blocks priming of neutrophils by lipopolysacchraide and by muramyl dipeptide. J Exp Med. 1986;164:1700–1709. doi: 10.1084/jem.164.5.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Liang X, Kalbfleisch J, et al. Neutralizing monoclonal antibody against anthrax lethal factor inhibits intoxication in a mouse model. Hum Antibodies. 2003;12:129–135. [PubMed] [Google Scholar]