Abstract
Prevalence of cigarette smoking among opioid-maintained patients is more than threefold that of the general population and associated with increased morbidity and mortality. Relatively few studies have evaluated smoking interventions in this population. The purpose of the present study was to examine the efficacy of contingency management for promoting initial smoking abstinence. Forty methadone- or buprenorphine-maintained cigarette smokers were randomly assigned to a contingent (n = 20) or noncontingent (n = 20) experimental group and visited the clinic for 14 consecutive days. Contingent participants received vouchers based on breath carbon monoxide levels during Study Days 1 to 5 and urinary cotinine levels during Days 6 to 14. Voucher earnings began at $9.00 and increased by $1.50 with each subsequent negative sample for maximum possible of $362.50. Noncontingent participants earned vouchers independent of smoking status. Although not a primary focus, participants who were interested and medically eligible could also receive bupropion (Zyban). Contingent participants achieved significantly more initial smoking abstinence, as evidenced by a greater percentage of smoking-negative samples (55% vs. 17%) and longer duration of continuous abstinence (7.7 vs. 2.4 days) during the 2 week quit attempt than noncontingent participants, respectively. Bupropion did not significantly influence abstinence outcomes. Results from this randomized clinical trial support the efficacy of contingency management interventions in promoting initial smoking abstinence in this challenging population.
Keywords: contingency management, smoking cessation, methadone, buprenorphine, bupropion
Prevalence rates of cigarette smoking among methadone-maintained (MM) patients range from 84 to 94% (Clemmey, Brooner, Chutuape, Kidorf, & Stitzer, 1997; Nahvi, Richter, Li, Modali, & Arnsten, 2006; Richter, Gibson, Ahluwalia, & Schmelzle, 2001) compared to approximately 25% in the general U.S. adult population (Centers for Disease Control, 2005; Substance Abuse and Mental Health Services Administration, 2007). Smoking among MM patients is associated with smoking-related morbidity and mortality (Engstrom, Adamsson, Allebeck, & Rydberg, 1991; Hser, McCarthy, & Anglin, 1994). For example, the mortality rate of opioid-dependent smokers is fourfold greater than that of opioid-dependent nonsmokers (Hser et al., 1994), and individuals who abuse alcohol and other drugs are more likely to die of problems related to tobacco than other drug use (Hurt et al., 1996).
Many MM patients report a desire to quit smoking (Clark, Stein, McGarry, & Gogineni, 2001; Clemmey et al., 1997; Frosch, Shoptaw, Jarvik, Rawson, & Ling, 1998; Kozlowski, Skinner, Kent, & Pope, 1989; Richter et al., 2001; Sees & Clark, 1993). For example, 50% of MM patients report making at least one serious attempt to quit smoking in the past year (Nahvi et al., 2006) and 75% express interest in a smoking-cessation program if it were offered by their clinic (Richter et al., 2001). Further, MM and other opioid-substitution treatment modalities, such as office-based buprenorphine treatment, may be uniquely suited for implementing smoking-cessation interventions (Reid et al., 2007). Many patients become stable in treatment, achieve prolonged periods of abstinence from illicit drug use, and remain engaged in treatment for extended periods of time. This combination of frequent and extended contact and clinical stability could offer an ideal setting in which to implement a smoking-cessation intervention. In addition, MM clinics generally adhere to a relatively uniform set of state and federal regulations. As such, a successful smoking-cessation intervention developed within the context of an MM program could have significant potential for dissemination to other clinics throughout the country.
Of the relatively few scientific efforts to develop smoking-cessation interventions for opioid-maintained patients, most have used pharmacotherapy or behavioral interventions. The four studies that administered pharmacotherapies for smoking abstinence reported generally modest outcomes (Reid et al., 2008; Richter, McCool, Catley, Hall, & Ahuwalia, 2005; Stein et al., 2006; Story & Stark, 1991). Five studies have examined contingency management (CM) to promote smoking abstinence among MM patients (Dunn, Sigmon, Thomas, Heil, & Higgins, 2008; Mooney et al., 2008; Schmitz, Rhoades, & Grabowski, 1995; Shoptaw, Jarvik, Ling, & Rawson, 1996; Shoptaw et al., 2002). CM is a behavioral approach where patients earn tangible incentives contingent on providing evidence of behavior change and has shown promise in reducing smoking among nondrug using populations (see Sigmon, Lamb, & Dallery, 2008, for a recent review). Thus far, results of CM smoking interventions in opioid-maintained patients have been modest. Although some evidence of potential efficacy exists (Shoptaw et al., 1996; Shoptaw et al., 2002), other studies have reported no effects (Mooney et al., 2008; Schmitz et al., 1995). It is possible the modest outcomes were related to the relatively low frequency of testing (e.g., 2 to 5 times per week) or relatively high carbon monoxide (CO) cutoffs (8 to 10 ppm) often employed in these studies, which may have permitted low-level smoking to go undetected. In addition, these studies provided relatively low magnitude of weekly voucher earnings ($10.00 to $37.29). Notably, larger magnitude incentives and more frequent urinalysis monitoring have been associated with more favorable drug abstinence outcomes within CM interventions (Griffith, Rowan-Szal, Roark, & Simpson, 2000; Lussier, Heil, Mongeon, Badger, & Higgins, 2006).
Dunn et al. (2008) recently conducted a pilot study that sought to increase the frequency and sensitivity of abstinence monitoring, and to increase the magnitude of voucher earnings, in an attempt to determine whether initial smoking abstinence could be achieved within a population of opioid-maintained smokers. Participants in that study (n = 20) were randomly assigned to a contingent or noncontingent group and attended the study daily for 14 consecutive days. Breath CO and urinary cotinine (a nicotine metabolite) samples were collected daily. Contingent participants earned voucher-based incentives (maximum possible of $362.50) for meeting abstinence criteria and noncontingent participants could receive a similar amount of vouchers independent of smoking status. Contingent participants achieved significantly more smoking abstinence than noncontingent participants, as evidenced by more smoking-negative samples (55% vs. 5%) and longer durations of continuous smoking abstinence (6.3 vs. 0.6 days), respectively. Similar findings were evident across all measures of smoking, including breath CO levels, urine cotinine levels, and self-reported number of cigarettes smoked. Data from this pilot study provided initial support for the efficacy of a brief yet intensive CM intervention in producing initial smoking abstinence among MM patients.
In the present study, we sought to replicate and extend those initial results. The method were generally similar to those used in the pilot. As in the pilot, our goal was to examine the possibility of promoting initial smoking abstinence in opioid-maintained smokers during a 2-week period, as smoking abstinence during the first 2 weeks of a quit attempt have been shown to robustly predict long-term outcomes (Frosch, Nahom, & Shoptaw, 2002; Gourlay, Forbes, Marriner, Pethica, & McNeil, 1994; Kenford et al., 1994; Yudkin, Jones, Lancaster, & Fowler, 1996). For example, one study that examined predictors of abstinence following a 7-week nicotine patch administration reported smoking status during the first 2 weeks of the intervention was the most robust predictor of abstinence at a 6-month follow-up (Kenford et al., 1994). A second study evaluated predictors of smoking abstinence among MM patients enrolled in a 12-week smoking cessation treatment and found that smoking abstinence during the second and third weeks of treatment was predictive of longer term cessation (Frosch et al., 2002). Therefore, although it was not expected that a 14-day intervention would likely be sufficient to promote extended smoking abstinence, this study represented a necessary first step toward achieving the initial abstinence that could then be sustained with a longer duration intervention.
Several differences between this trial and the pilot study should also be noted. First, the present study involved a larger sample size (i.e., 40 vs. 20 randomized smokers). Second, both methadone- and buprenorphine-maintained patients were recruited to participate. As of yet, there has been only one study that examined smoking abstinence among buprenorphine patients (Mooney et al., 2008). Buprenorphine can be prescribed directly by primary care physicians and represents a new and important modality for treating opioid dependence. Finally, participants who were interested and medically eligible were offered bupropion (Zyban). Although the primary focus of this research was the behavioral intervention, pharmacotherapies such as nicotine replacement therapies and bupropion are considered first-line treatments for smoking (Fiore, Bailey, & Cohen, 2000; U.S. Public Health Service, 2003) and are used by 45.5% of smokers trying to quit annually (Centers for Disease Control, 2006). Considering the elevated rates of smoking and smoking-related illnesses, as well as the history of modest outcomes in this challenging population, we felt it prudent to include an optional pharmacotherapy that could be delivered in conjunction with the behavioral intervention. Bupropion was chosen as the pharmacotherapy for this study as nicotine replacement therapy (NRT) products would confound our use of cotinine for biochemical verification of smoking abstinence. It is important to note that bupropion was offered as an optional, rather than required, component of treatment, as mandating it would likely have excluded as many as half of participants from the study due to lack of interest or medical contraindications (Richter, Choi, & Alford, 2005; Vogt, Hall & Marteau, 2008). The administration of bupropion only to interested and medically eligible participants also reflects real-world conditions and increases the generality of these findings to a clinical setting. Finally, to preserve experimental control, bupropion use was included as a stratification variable to ensure comparable distribution between experimental groups. Overall, these procedures enabled us to offer an empirically supported ancillary pharmacotherapy while not undermining our primary aim of evaluating the efficacy of the behavioral intervention.
Method
Participants
Participants interested in smoking cessation were recruited from a local methadone clinic and office-based buprenorphine providers via mailings and flyers. Eligible participants were required to be 18 to 65 years old and report smoking ≥ 10 cigarettes a day for ≥ 1 year. Because opioid and cocaine administration can increase smoking (Chait & Griffiths, 1984; Mello, Lukas, & Mendelson, 1985; Mello, Mendelson, Sellers, & Kuehnle, 1980; Mutschler, Stephen, Teoh, Mendelson, & Mello, 2002; Roll, Higgins, & Tidey, 1997), participants were required to be on a stable methadone or buprenorphine dose and have a relatively low occurrence of illicit drug use (≤ 30% samples testing positive for illicit drugs) for ≥ 30 days preceding intake. Duration of time at dose and urinalysis results were obtained from the treatment provider after obtaining written permission from the participant. Finally, because marijuana use may confound breath CO readings, participants were required to provide a marijuana-negative urine sample at intake. Participants who were pregnant or presented with active severe mental illness (e.g., evidence of current suicidal thoughts, active hallucinations) that might interfere with study comprehension and participation were excluded. The study was approved by the IRB and all participants provided informed consent to participate. Overall, 95 opioid-maintained patients expressed interest in the study and completed a phone screening form; 33 were excluded due to an inability to confirm opioid-treatment criteria (e.g., failed to sign a release form, n = 29, or physicians could not be contacted, n = 4), 7 declined to participate and 15 were excluded from participating for not meeting eligibility criteria.
Intake
Participants completed a wide range of self-report and experimenter-administered instruments at intake. A semi-structured questionnaire was used to assess basic demographic and smoking characteristics (e.g., age started smoking, average number of cigarettes/day, time to first cigarette in the morning, number of previous quit attempts). Smoking questionnaires included the Fagerström Test of Nicotine Dependence, a measure of nicotine dependence (FTND; Fagerström & Schneider, 1989; Heatherton, Kozlowski, Frecker, & Fagerström, 1991), the Minnesota Nicotine Withdrawal Questionnaire (MNWQ; Hughes & Hatsukami, 1986), the Nicotine Dependence and Syndrome Scale (Shiffman, Waters, & Hickcox, 2004), which yields a global dependence score as well as five subscales of nicotine dependence (e.g., Drive, Priority, Tolerance, Continuity, and Stereotypy), and the Questionnaire on Smoking Urges (QSU; Tiffany & Drobes, 1991), a two-factor measure of craving that examines desire/intention to smoke and relief from negative affect, respectively. General mental health was assessed using the Beck Depression Inventory (BDI-II; Beck & Beck, 1972) and the Brief Symptom Inventory (BSI; Derogatis, 1993), and use of substances other than nicotine was assessed using the Addiction Severity Index (ASI; McLellan et al., 1985) and Michigan Alcoholism Screening Test (MAST; Selzer, 1971). Finally, breath CO and urine samples were collected to determine baseline carbon monoxide and cotinine levels. Participants were compensated $35 for completing the intake. Participants also completed a subset of these questionnaires (BDI, BSI, MNWQ, NDSS, QSU) and received $35 at the end of the intervention (e.g., Day 14) and at three follow-up assessments (e.g., 30, 60, 90 days).
Eligible participants were randomly assigned to a contingent or noncontingent group and received a copy of the National Cancer Society’s booklet “Clearing the Air Today” (National Cancer Institute, 2003), which was reviewed with them by a staff member who emphasized reasons to quit smoking, aspects of nicotine dependence, triggers for smoking and how to manage cravings, tips for not smoking, and ways to manage symptoms of nicotine withdrawal. Immediately following this brief educational session, the staff member helped the participant establish a “quit date,” which was required to be within 7 days of providing study consent. The quit-date represented the first day of the 14-day intervention and was not rescheduled if the participant was positive for smoking on that day.
Study Details
Participants attended the study for 14 consecutive days. The smoking study was not affiliated with any opioid-treatment program and participants’ opioid treatment was not influenced by their status in the smoking cessation study. At each visit, they completed the MNWQ and the Questionnaire of Smoking Urges-Brief Version (QSU-B; Cox, Tiffany, & Christen, 2001), which is an abbreviated version of the QSU that also yields factors for desire/intention to smoke and relief from negative affect, respectively. Also completed were modified versions of the Time Line Follow-Back (Sobell, Sobell, Leo, & Cancilla, 1988) to monitor cigarette use, changes in opioid-maintenance dose, any use of any NRT, and, for those receiving pharmacotherapy as part of the study, ingestion of bupropion. Participants also provided a breath CO sample, assessed using hand-held meters (Bedfont EC50 Smokerlyzer, Bedfont Scientific Ltd., Kent, England) and a urine sample that was tested for the nicotine metabolite cotinine. Cotinine was measured immediately using an onsite enzyme multiplied immunoassay test (EMIT) with a semiquantitative cotinine assay (Microgenics, Fremont CA).
Although both CO and cotinine samples were collected daily, the abstinence criteria (e.g., either CO or cotinine) varied depending on study day. Smoking abstinence was defined as a breath CO ≤ 6 ppm during Study Days 1 to 5 and a urine cotinine ≤ 80 ng/ml on Days 6 to 14. A brief note on this combination of criteria is warranted. Due to the relatively short half-life of CO (2 to 3 hr), smokers can meet the 6 ppm abstinence criterion within 12 to 24 hr of stopping smoking (Society for Research on Nicotine and Tobacco Subcommittee on Biochemical Verification, 2002); however, the relatively long half-life of cotinine requires several continuous days of abstinence in order to meet the abstinence criterion (Society for Research on Nicotine and Tobacco Subcommittee on Biochemical Verification, 2002). Thus, CO was used early in the intervention to allow us to reinforce initial smoking abstinence, and the cotinine measure was used later to provide a more sensitive test likely to detect even low levels of ongoing smoking. This method of transitioning from CO to cotinine for monitoring of smoking status has been shown to be effective for promoting smoking abstinence with CM in prior studies by Dunn et al. (2008), Heil et al. (2008), and Higgins et al. (2004).
Experimental Groups
Contingent participants (n = 20) earned voucher-based incentives contingent on providing biochemical evidence of smoking abstinence. Vouchers were provided on an escalating schedule (cf. Higgins et al., 1991) beginning at $9 for the first negative sample and increasing by $1.50 for each subsequent negative sample. A smoking-positive sample resulted in no vouchers being given and voucher values were reset to the initial starting value ($9). However, to encourage participants to become abstinent again, two consecutive negative samples returned the voucher schedule to the value at which it would be have been at prior to the reset. In addition, to further encourage early continuous smoking abstinence, participants received a $10 bonus for any CO samples ≤ 4 ppm during Days 1 to 5, as well as a $50 bonus on Study Day 6 if they successfully met the 80 ng/ml cotinine criteria. Maximum earnings possible in the contingent group was $362.50. All vouchers were redeemable for goods and services at local stores or bill payments. Study staff purchased all vouchers; no participant received cash directly.
Noncontingent participants (n = 20) earned voucher-based incentives independent of smoking status, and voucher earnings were yoked to the mean earnings of the contingent group during the pilot study ($216.50). Voucher earnings were randomly determined by a computer program developed by us to generate a random distribution of payments for each noncontingent participant based on three parameters (i.e., maximum earnings, monetary range of individual payments, number of payments to be delivered during the study). Each noncontingent participant could earn a total of $216.50 (mean earnings by the contingent group in the previous pilot trial) in vouchers, with daily values ranging from $10 to $20 over the 14-day period. Noncontingent participants were informed at the beginning of the study that voucher values would be determined randomly and that daily amounts to be earned would be unpredictable. All other aspects of the study were identical across the two experimental groups (e.g., frequency of visits, monitoring of smoking, data collection, protocol for spending vouchers).
Pharmacotherapy
Participants were offered an opportunity to receive bupropion as a pharmacotherapy to assist with their cessation attempt. Interested participants completed a medical history at intake and met with the study physician for a brief physical examination. Exclusion criteria included history of seizures, acute alcohol withdrawal, eating disorders, head trauma, or compromised liver function. Only one participant who was interested in bupropion was deemed medically ineligible.
On physician approval, participants began bupropion therapy 1 week prior to the scheduled quit attempt and were provided bupropion for the 2-week study as well as an additional 4 weeks, which is consistent with the 7-week regimen recommended in published guidelines (Zyban Prescribing Information; GlaxoSmithKline, 2009). Adherence and any potential side effects were assessed at each study visit. Fifteen participants (nine contingent, six noncontingent) ingested ≥ 1 bupropion dose during the study. There was a relatively low rate of bupropion-related side effects, with only three participants reporting any potential side effects (e.g., sweating, dry mouth, rash) and discontinuing bupropion treatment. There were no significant differences between experimental groups in percentage of participants taking bupropion, χ2(1, N = 40) = 0.96, p = .33.
It should also be noted that because NRT could confound urinary cotinine testing, participants were asked at intake to abstain from using NRT during the 14-day intervention. A time-line followback of NRT use was conducted at each daily visit and revealed that two participants (one in each experimental group) reported use of NRT. The contingent participant reported taking four to seven puffs from a nicotine inhaler on 4 study days, however never provided a biochemical sample that exceeded the relevant abstinence criterion and therefore did not lose any potential voucher earnings. The noncontingent participant reported 14 mg nicotine patch use on 11 of the 14 study days, however due to the noncontingent nature of the group assignment that participant did not lose any voucher earnings for nicotine patch use. The noncontingent participant also reported smoking on each study day, thereby precluding an evaluation of the contribution nicotine patch use may have had on urinary cotinine.
Follow-Up Assessments
Follow-up assessments were conducted at 30-, 60- and 90-days postintake. At each assessment, breath and urine samples were collected to determine carbon monoxide and cotinine levels, and a subset of the intake questionnaires (e.g., BDI, BSI, MNWQ, NDSS, QSU) was administered. Participants were compensated $35 for completing each follow-up assessment, independent of smoking status.
Statistical Analyses
Experimental groups were compared on baseline demographic and other characteristics using analyses of variance for continuous variables and chi-square tests for categorical variables. Primary analyses included all participants who were randomized to experimental conditions, independent of early dropout, noncompliance, and so forth, consistent with an intent-to-treat approach to clinical trials (Armitage, 1983). Missed samples were considered to be smoking positive. The primary outcome measure was the percentage of biochemical samples meeting the abstinence criteria, compared between the two experimental groups using a chi-square test. Mean days of continuous abstinence were analyzed using an independent groups t test. Abstinence at Day 14, and at the 30-, 60-, and 90-day follow-ups, was defined as a self-report of no smoking in the past 7 days and a negative urine cotinine test (≤ 80 ng/ml) and was compared using a chi-square test. Contribution of bupropion- and opioid-treatment modality on smoking abstinence was examined using an analysis of variance (ANOVA) test, and percentage retention over the 14-day intervention was compared using a chi-square test. Outcomes of self-report measures (e.g., self-reported smoking, nicotine withdrawal) across experimental group and time were analyzed using repeated-measures ANOVA (SAS Version 9, PROC MIXED).
MNWQ data were analyzed in a manner consistent with prior reports, with seven individual items averaged for a single severity score, and desire to smoke analyzed separately as an individual item (Hughes & Hatsukami, 1998). MNWQ analyses were restricted to participants who achieved high levels (> 85%) of smoking abstinence (e.g., responders) to prevent any continued smoking from confounding the examination of withdrawal. Withdrawal within responders was also evaluated as a function of receiving bupropion, which has been shown to reduce symptoms of nicotine withdrawal (Gonzales et al., 2006; Jorenby et al., 2006; Shiffman et al., 2000; West, Baker, Cappelleri, & Bushmakin, 2008).
Results
Participants
Forty participants were enrolled into the study and randomized into contingent (n = 20) and noncontingent (n = 20) groups. There were no significant group differences in demographic, opioid treatment, smoking, drug use, and other characteristics at intake (see Table 1).
Table 1.
Baseline Demographic and Smoking Characteristics
| Total sample (N = 40) |
Contingent (n = 20) |
Noncontingent (n = 20) |
p valuea | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 31.0 ± 1.8 | 31.0 ± 1.8 | 31.0 ± 1.7 | .89 |
| Education (years) | 12.5 ± 0.3 | 12.0 ± 0.3 | 13.0 ± 0.3 | .22 |
| Male (%) | 33 | 40 | 25 | .31 |
| Never married (%) | 58 | 55 | 60 | .75 |
| Opioid-treatment characteristics | ||||
| Methadone maintained (%) | 63 | 60 | 65 | .74 |
| Methadone dose (mgs) | 107.6 ± 8.8 | 114.4 ± 12.6 | 101.4 ± 12.5 | .47 |
| Buprenorphine dose (mgs) | 14.9 ± 1.3 | 15.5 ± 1.9 | 14.3 ± 1.7 | .65 |
| Length of time at maintenance dose (days) | 171.8 ± 48.2 | 144.6 ± 41.5 | 198.9 ± 88.0 | .58 |
| Smoking characteristics | ||||
| Cigarettes smoked in the past 7 days (no. per day) | 18.5 ± 1.8 | 17.0 ± 1.9 | 20.0 ± 1.7 | .21 |
| Age at first cigarette (years) | 12.7 ± 0.6 | 13.3 ± 0.7 | 12.0 ± 0.6 | .14 |
| M no. of years smoked regularly | 10.9 ± 1.3 | 12.4 ± 2.1 | 9.5 ± 1.6 | .29 |
| Fagerström Test for Nicotine Dependence (0–10)b | 5.4 ± 0.4 | 5.2 ± 0.4 | 5.6 ± 0.4 | .73 |
| Baseline CO (ppm) | 15.5 ± 1.6 | 13.0 ± 1.6 | 17.0 ± 1.6 | .06 |
| Baseline cotinine (ng/ml) | 1488.8 ± 136.1 | 1377.0 ± 121.6 | 1601.0 ± 150.6 | .25 |
| Planning to quit next 30 days (%) | 93 | 90 | 95 | .56 |
| Believe maintenance treatment increases smoking (%) | 45 | 45 | 45 | 1.00 |
| Smoking quit history | ||||
| Ever tried to quit (%) | 93 | 100 | 85 | .07 |
| Previous quit attempts (no.) | 7.0 ± 1.5 | 7.0 ± 1.7 | 7.0 ± 1.3 | .76 |
| Longest duration quit (days) | 280.3 ± 120.0 | 138.7 ± 50.0 | 421.8 ± 190.0 | .13 |
| Counselor ever advised delay quitting smoking (%) | 50 | 45 | 55 | .53 |
| Counselor ever advised never quit smoking (%) | 0.5 | 0 | 1 | .31 |
| ASI subscale scores | ||||
| Medical (0–1) | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.1 | .42 |
| Employment (0–1) | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 | .30 |
| Alcohol (0–1) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | .78 |
| Drug (0–1) | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | .14 |
| Opiate (0–1) | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | .10 |
| Cocaine (0–1) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | .65 |
| Legal (0–1) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.0 | .29 |
| Family/Social (0–1) | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.1 | .30 |
| Psychiatric (0–1) | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.2 ± 0.4 | .94 |
Note. Values represent M ± SEM unless otherwise indicated. ASI = Addiction Severity Index.
p values based on independent sample t tests for continuous variables and chi-square measures for categorical variables.
Represent score range.
Retention
Noncontingent participants attended significantly more study visits than contingent participants, t(38) =−2.18, p = .04, with 67.8% and 92.9% visits attended by contingent and noncontingent groups, respectively. There also was a significant difference in the percentage of participants retained at Day 14, χ2(1, N = 40) = 4.44, p < .04, with 65% and 95% of contingent and noncontingent participants retained at the end of the study, respectively.
There were no significant differences between contingent and noncontingent participants in the percentage completing follow-up assessments at the 30-day, χ2 (1, N = 40) = 0.00, p = 1.00, 60-day, χ2(1, N = 40) = 0.00, p = 1.00, and 90-day, χ2(1, N = 40) = 0.11, p = .74 assessments. Specifically, 80% and 70% of both contingent and noncontingent participants completed the 30- and 60-day assessments, respectively, and 65% and 60% of contingent and noncontingent participants, respectively, completed the 90-day assessment.
Smoking Outcomes
Abstinence during intervention
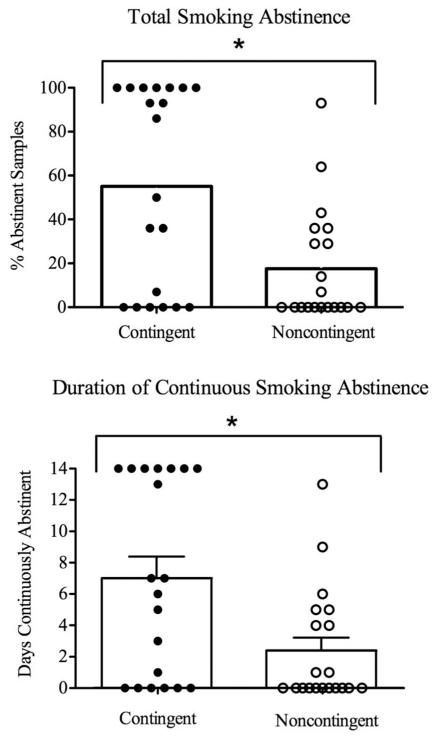
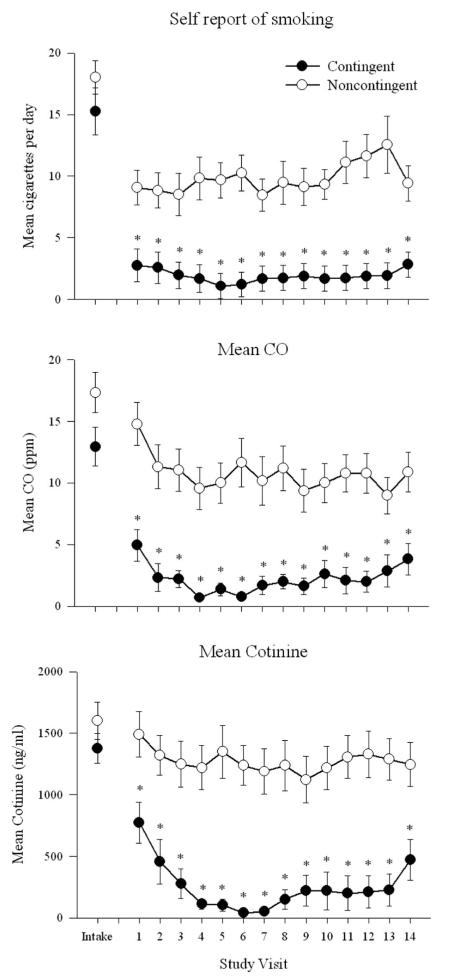
Contingent participants achieved more smoking abstinence than noncontingent participants during the 14-day intervention. Specifically, contingent participants submitted a greater percentage of smoking-abstinent samples than noncontingent participants, with 55% and 17% of samples meeting the abstinence criterion, respectively, t(30.1) = 3.24, p < .01 (Figure 1, top panel). Contingent participants also achieved significantly longer durations of continuous smoking abstinence than noncontingent participants (7.7 vs. 2.4 days, respectively), t(30.1) = 3.24, p < .01 (Figure 1, bottom panel). Analyses of daily smoking data also showed this effect. For example, contingent participants reported smoking significantly fewer cigarettes per day than noncontingent, F(1, 34) = 13.75, p ≤ .01 (Figure 2, top panel). Contingent participants provided significantly more CO samples, F(1, 35) = 18.17, p < .01 (Figure 2, middle panel), and cotinine samples, F(1, 35) = 9.67, p < .01 that met the abstinence criteria than noncontingent participants at each of the 14 study visits (Figure 2, bottom panel).
Figure 1.
Smoking abstinence for contingent (filled symbols) and noncontingent (open symbols) participants. Data are collapsed across 14-day intervention. Bars represent group mean; symbols represent individual data. Top panel: Total percentage smoking-abstinent samples. Bottom panel: Mean longest duration of smoking abstinence. Error bars on bottom panel represent SEM. Asterisks indicate significant group effects between contingent and noncontingent groups.
Figure 2.
Daily smoking abstinence across the 14-day intervention for contingent (filled symbols) and noncontingent (open symbols) participants. Error bars represent SEM. Top panel: Self-report of cigarettes per day at each study visit. Middle panel: Mean breath carbon monoxide (CO) levels. Bottom panel: Mean urine cotinine levels. Asterisks indicate significant group effects between contingent and noncontingent groups.
Abstinence at Day 14
Contingent participants were significantly more likely to be abstinent than noncontingent participants, χ2(1, N = 40) = 7.02, p < .01, with 45% and 10% of contingent and noncontingent participants meeting this abstinence criterion at Day 14, respectively.
Abstinence at follow-up
Smoking abstinence did not differ significantly between the experimental groups at the 30-day, χ2(1, N = 40) = 2.11, p = .15, 60-day, χ2(1, N = 40) = 2.11, p = .15, or 90-day, χ2(1, N = 40) = 2.11, p = .15, follow-up assessments, with 10% and 0% of contingent and noncontingent participants abstinent at the 30- and 60-day follow-up assessments, and 5% and 0% abstinent at the 90-day follow-up assessment, respectively.
Treatment responders
Inspection of individual participant data in the contingent group revealed a bimodal distribution whereas 50% of contingent participants achieved near perfect levels of abstinence, although 50% achieved moderate to low levels of abstinence (see Figure 1). In an effort to determine whether any baseline characteristics might have predicted better treatment response, contingent participants were dichotomized post hoc into responders (n = 10) and nonresponders (n = 10) based on level of smoking abstinence achieved during the 14-day intervention. These two groups were compared on a number of baseline demographic, smoking, and opioid-treatment characteristics. There were no significant differences between the responders and nonresponders on any of the baseline characteristics examined.
Self-Report Measures
Self-report measures during daily visits
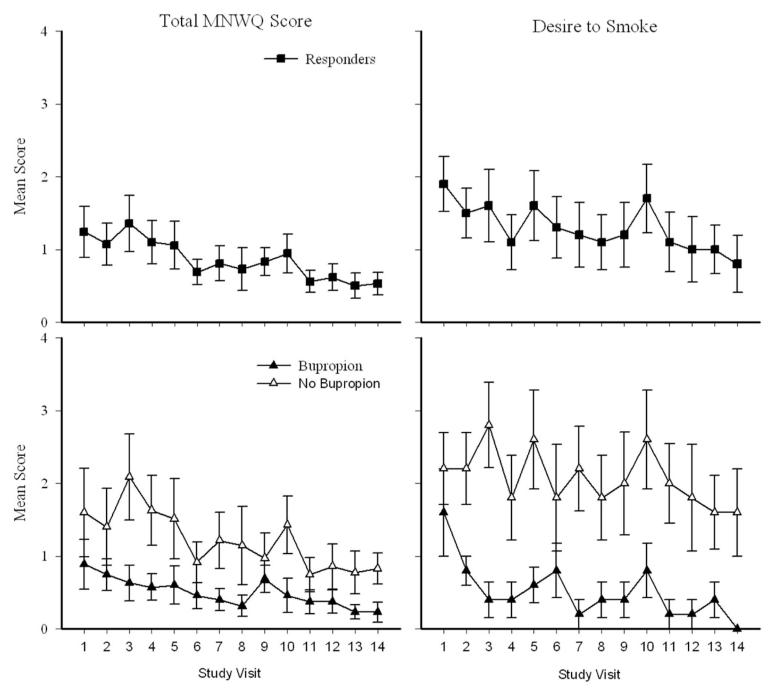
Contingent participants reported significant reductions over time in the QSU-B Factor 1, F(13, 39.6) = 2.14, p = .01, and Factor 2, F(1, 33.9) = 4.62, p = .04, during the 14-day intervention. Mean MNWQ scores, F(1, 13) = 2.23, p < .01 (Figure 3 top-left panel), and desire to smoke, F(1, 13) = 2.21, p < .01 (Figure 3 top-right panel) decreased significantly over the 14-day intervention in contingent responders. In addition, bupropion significantly reduced MNWQ total scores, F(1, 13) = 6.55, p = .02 (Figure 3 bottom-left panel) and desire to smoke, F(1, 13) = 15.5, p < .001 (Figure 3 bottom-right panel) over the 14-day intervention in this group.
Figure 3.
Nicotine withdrawal across the 14-day intervention for responders in the contingent group. Data presented are from the Minnesota Nicotine Withdrawal Questionnaire (MNWQ). Mean total score (left panels) and desire to smoke (right panels) are presented. Top panels: MNWQ data from all contingent responders (collapsed across bupropion groups). Bottom panels: MNWQ data as a function of bupropion (filled symbols) or no bupropion (open symbols). Error bars represent SEM.
Self-report measures at intake and Day 14
Contingent participants reported significant reductions between intake and Day 14 on the NDSS Global Dependence scale, F(1, 40) = 6.18, p < .01, and on the Drive subscale, F(1, 40) = 9.03, p < .01. Contingent participants also reported a significant increase between intake and Day 14 on the NDSS Priority subscale, F(1, 40) = 2.95, p = .02. There were no significant changes on any NDSS scales in the noncontingent group. Contingent participants reported significant reductions between intake and Day 14 on the QSU Factor 1, F(1, 40) = 5.02, p = .03. There were no significant changes on the QSU Factor 2 within the contingent group and no changes on any QSU scales in the noncontingent group. Finally, there were no significant differences observed between intake and Day 14 on the BDI or BSI scales within contingent or noncontingent participants.
Contribution of Bupropion to Study Outcomes
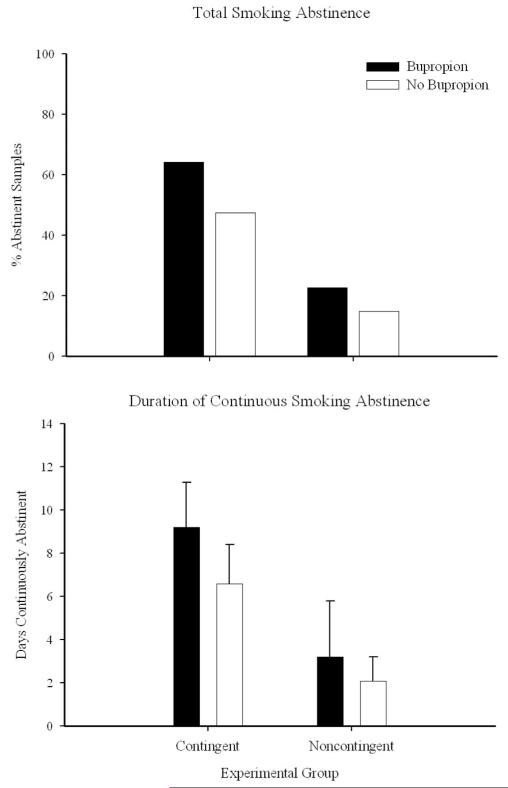
Smoking abstinence, collapsed across the 14-day study, was evaluated as a function of bupropion administration. Although somewhat more participants with bupropion achieved more smoking abstinence, differences were not statistically significant, F(1, 40) = 0.99, p = .33. For example, contingent participants with and without bupropion achieved 64% and 47% abstinence, whereas noncontingent participants with and without bupropion achieved 23% and 15% abstinence, respectively (Figure 4, top panel). Similarly, contingent participants with and without bupropion achieved 9.2 and 6.6 day of continuous abstinence, whereas noncontingent participants with and without bupropion achieved 3.2 and 2.1 day of continuous abstinence, respectively, F(1, 40) = 1.16, p = .28 (Figure 4, bottom panel).
Figure 4.
Smoking abstinence among contingent (left bars) and noncontingent (right bars) participants as a function of bupropion (solid bars) or no bupropion (open bars) administration. Data are collapsed across the 14-day intervention. Top panel: Total percentage smoking-abstinent samples. Bottom panel: Mean longest duration of smoking abstinence. Bars represent group mean; error bars represent SEM.
Contribution of Opioid-Treatment Modality to Study Outcomes
The contribution of opioid-treatment modality (methadone or buprenorphine) on primary study outcomes also was evaluated. Although there were slightly more participants receiving methadone in the noncontingent versus contingent group (65% vs. 60%, respectively), the difference was not statistically significant, χ2(1, N = 40) = 0.11, p = .74. Opioid-treatment modality had no significant effect on total smoking abstinence, F(1, 40) = 0.31, p = .58, longest duration of continuous abstinence, F(1, 40) = 0.37, p = .54, or smoking status at Day 14, χ2(1, N = 40) = 0.89, p = .34. There was also no significant effect of opioid-treatment modality on number of study visits attended, F(1, 40) = 0.26, p = .61, or the percentage of participants retained in treatment at Day 14, F(1, 40) = 0.64, p = .43.
Discussion
In the present study we sought to examine whether a CM intervention would promote initial smoking abstinence in opioid-maintained smokers. Participants randomly assigned to the contingent experimental group achieved more smoking abstinence than noncontingent participants, as evidenced by 55% and 17% smoking-negative samples submitted by contingent and noncontingent participants, respectively. These results are consistent with those of prior studies (55% and 5%, respectively; Dunn et al., 2008). Contingent participants also achieved longer durations of smoking abstinence, with contingent and noncontingent participants achieving 7.7 and 2.4 days of continuous smoking abstinence, respectively. These results also are consistent with the Dunn et al. (2008) study, which found 6.3 versus 0.6 days of continuous smoking-negative samples between contingent and noncontingent groups, respectively. One potential difference between studies is the higher degree of smoking abstinence among the noncontingent group in the present trial compared to the Dunn et al. 2008 study. At first glance this could be related to bupropion, which had not been used previously but was offered in this study. However, although nine noncontingent participants provided one or more smoking-negative samples, only four of those participants were using bupropion. Further, when abstinence rates were compared between noncontingent participants with and without bupropion, those receiving bupropion (n = 4) provided 34% smoking-abstinent samples whereas those not taking bupropion (n = 5) submitted 48% abstinent samples. Overall, the reasons for the higher abstinence levels among noncontingent participants in this study remain unknown, though these data may suggest that a subset of opioid-maintained patients may not need an intensive smoking cessation intervention to successfully abstain from smoking. It would be important for future studies to evaluate the characteristics of this group.
Taken together, these data replicate the findings of the Dunn et al. (2008) study and provide further evidence that smoking among methadone- and buprenorphine-maintained patients is sensitive to CM-based interventions. These results also compare favorably to prior efforts by others to use CM to promote abstinence with an opioid-maintained population (Mooney et al., 2008; Schmitz et al., 1995; Shoptaw et al., 1996; Shoptaw et al., 2002). Abstinence rates in those studies were modest and ranged from 0 to 24%. The more robust abstinence effects observed in this study may be attributable to several methodological differences between the current and prior studies. First, we used a combination of breath CO and urinary cotinine as biochemical markers of smoking status, whereas prior studies had relied solely on CO as an indicator of smoking. The addition of the urinary cotinine measure may have enabled a more rigorous monitoring protocol than in prior studies. It also provides support for the utility of using a CO-to-cotinine transition, and urinary cotinine more generally, in contingency-management interventions for cigarette smoking. Second, participants in the current study were required to attend the clinic daily during the 14-day intervention; this stands in contrast to 2 to 3 times per week visits in prior studies (Schmitz et al., 1995; Shoptaw et al., 1996; Shoptaw et al., 2002). This relatively intensive frequency of biochemical monitoring allowed us to reinforce abstinence frequently and potentially minimize low levels of smoking that might have gone undetected with less frequent monitoring. Third, participants in this study could earn $181.25/week and a total of $362.50 over the 2-week study. Potential earnings in prior CM studies averaged $18.63/week (range: $10.00 to $37.29). Magnitude of voucher earnings has been shown to influence abstinence from licit and illicit drugs (Higgins et al., 2007; Lussier et al., 2006; Silverman, Chutuape, Bigelow, & Stitzer, 1999) and may have contributed to the favorable outcomes observed in the current study. Finally, it is possible that the brief duration of the present study may have contributed to the favorable outcomes. That is, it may be easier to achieve smoking abstinence for a short-term (i.e., 2 week) intervention than to sustain that abstinence throughout a longer duration (i.e., 12 week) smoking-cessation treatment. This is an empirical question and we know of no studies that have experimentally investigated duration of a behavioral smoking-cessation treatment. However, we are currently conducting a longer term, 12-week randomized trial that may help to inform this question. It is also the case that, when smoking abstinence rates are compared over equivalent time periods between this and longer duration studies, the favorable outcomes in the present trial are evident. For example, in the 12-week randomized clinical trial by Shoptaw and colleagues (2002), only 6 to 12% of contingent participants met the abstinence criterion at the end of the initial 2 weeks, compared to 48% of participants in the current study. These data suggest that our favorable outcomes in the contingent group are likely not just a function of comparing abstinence rates between two interventions that vary markedly in duration.
Inspection of abstinence data revealed marked differences across individuals in response to the intervention. For example, as can be seen in Figure 1, 50% (n = 10) of contingent participants achieved nearly complete abstinence whereas 50% (n = 10) achieved low to moderate levels of abstinence. This finding is consistent with our prior study in this population (Dunn et al., 2008). We examined possible individual differences in responders and nonresponders, but no significant differences between the two groups on any of the baseline characteristics were observed. This stands in contrast to some prior studies that have found lower nicotine dependence to be associated with better smoking outcomes (Fagerström & Schneider, 1989; Heatherton, Kozlowski, Frecker, Rickert, & Robinson, 1989; John, Meyer, Rumpf, & Hapke, 2004; Lerman et al., 2004). The lack of significant differences in this study may be due to the relatively small sample size used in these analyses (i.e., 10 per responder group). It would be important for future studies with larger sample sizes to examine the characteristics that predict response to a smoking cessation intervention within an opioid-maintained population. For example, it is possible that participants who do not respond favorably to a single treatment strategy may benefit more from a combination of multiple-smoking cessation treatments, including CM, pharmacotherapies, cognitive-behavioral or relapse-prevention techniques. Once an effective combination strategy is identified and implemented, future research could then aim to dismantle and examine the relative contribution of each treatment component. Overall, future studies should examine, using larger sample sizes, the individual demographic and drug use characteristics that may predict success or failure in smoking cessation, as well as how to enhance outcomes in subsets of particularly challenging smokers.
In the present study, noncontingent participants had greater study retention than contingent participants, which is likely related to the lack of contingencies placed on their smoking abstinence. That is, due to the nature of their group assignment, noncontingent participants were able to earn vouchers simply for attending study visits, whereas contingent participants were required to provide evidence of smoking-abstinence to earn vouchers. We saw similar retention results in a previous study (Dunn et al., 2008). It is important to note, however, that despite the poorer retention, contingent participants still provided significantly more smoking-negative samples than noncontingent participants. This suggests that the greater rate of abstinence among contingent participants is indeed a function of the experimental intervention and not related solely to study attendance. A more scientifically problematic finding would have been for retention differences to lean in the other direction. That is, if contingent participants would have had better retention than noncontingent, it would have been difficult to distinguish whether the increased smoking abstinence in the contingent group was due to the delivery of contingent reinforcement of smoking abstinence or, alternatively, due simply to the enhanced retention.
A high rate of relapse to smoking was observed following completion of this brief intervention, even among participants who achieved smoking abstinence during the study. For example, only 5% of all participants were abstinent at the 30- and 60-day follow-ups, and only 3% remained abstinent at the 90-day follow-up. These results are consistent with the findings in the pilot study, where 15% of participants remained abstinent at the 30- and 60-day follow-ups and 10% were abstinent at the 90-day assessment (Dunn et al., 2008). Our primary aim was to develop an intervention that would promote initial smoking abstinence among opioid-maintained smokers. Although we did not necessarily expect that this brief intervention would be sufficient in-and-of itself to produce extended abstinence, initial abstinence is a necessary step in developing an efficacious intervention to promote longer term smoking abstinence. In the present study we established a proof-of-concept that opioid-maintained patients can quit smoking, which provides a positive control from which to work in terms of developing more sustained interventions. Indeed, these data suggest that this population would benefit from the inclusion of an extended abstinence-monitoring period to sustain the abstinence achieved early in the quit attempt. A 12-week randomized trial is currently being conducted to experimentally investigate this question.
Although there were no significant changes between intake and Day 14 on any of the psychosocial (e.g., BDI, BSI) measures, there were some significant effects within the contingent group on several smoking-related questionnaires. For example, significant decreases were observed in some measures of nicotine dependence (e.g., NDSS), craving (e.g., QSU, QSU-B), and nicotine withdrawal (e.g., MNWQ). Overall, these data suggest that several aspects related to nicotine dependence decreased over the course of the 14-day intervention, and are consistent with prior studies using a 14-day smoking-cessation intervention in non-opioid dependent smokers (Heil, Alessi, Lussier, Badger, & Higgins, 2004; Lussier, Higgins, & Badger, 2005; Yoon, Higgins, Bradstreet, Badger, & Thomas, 2009). Several challenges associated with interpreting the MNWQ data should also be noted. First, although the MNWQ is a widely used measure of nicotine withdrawal, there are no standardized norms available. Second, there is substantial variability in scores across studies that have reported MNWQ scores. Third, prior studies also have often collapsed MNWQ scores across a period of time (e.g., over a 1-week period) rather than providing daily withdrawal data as is presented in the current study. However, because a great deal of relapse to smoking occurs during the initial few days of a quit attempt (Hughes et al., 1992; Hughes, Keely, & Naud, 2004), it may be particularly important to examine withdrawal on a daily basis during that initial period. Finally, we know of no prior smoking cessation studies among opioid-maintained patients that have reported withdrawal outcomes. As a result, it is challenging to identify a specific cutpoint that may distinguish clinically meaningful withdrawal or to directly compare the results of the current trial with previously published studies. However, as this study represents the first examination to our knowledge of nicotine withdrawal in opioid-maintained populations, we hope that including that data here will encourage other researchers to also investigate the relationship between nicotine withdrawal, bupropion, and cessation outcomes among opioid-maintained patients. Overall, it will be important to learn whether opioid-maintained patients report a nicotine withdrawal syndrome similar to other smokers, or whether they may have unique needs and thus may warrant different treatment strategies.
Bupropion did not significantly influence smoking outcomes. The lack of a significant bupropion effect is inconsistent with several prior reports in the literature on this topic. For example, a recent meta-analysis of the effectiveness of bupropion administration to reduce smoking among non-opioid populations reported bupropion can approximately double the odds of quitting smoking (Hughes, Stead, & Lancaster, 2009). However, these data are consistent with several prior studies that have used pharmacotherapies for smoking cessation among opioid-maintained patients. For example, bupropion did not contribute significantly to smoking abstinence in two prior studies with opioid-maintained patients (Mooney et al., 2008; Richter, McCool et al., 2005), and the nicotine patch has failed to substantially impact smoking outcomes among opioid-maintained populations (Shoptaw et al., 2002; Stein et al., 2006). It is also possible that the limited sample size in the present study reduced statistical power and prevented us from seeing a significant effect of bupropion on smoking outcomes. For example, as seen in Figure 4, bupropion produced a nonsignificant increase in percent smoking abstinence and duration of continuous abstinence in the contingent and noncontingent groups. Although not statistically significant, these data suggest that bupropion may influence smoking outcomes. Bupropion administration also was associated with significant reductions in nicotine withdrawal, consistent with other reports suggesting that bupropion influences the symptoms of nicotine withdrawal (Gonzales et al., 2006; Jorenby et al., 2006; Shiffman et al., 2000; West et al., 2008). Overall, future studies that employ larger sample sizes and experimentally control bupropion administration are needed to examine the contribution of bupropion to a CM intervention for opioid-maintained smokers.
The effect of opioid-treatment modality on smoking and retention outcomes also was evaluated. Although prior studies have suggested that opioid agonists can increase the reinforcing effects of cigarettes (Chait & Griffiths, 1984; Mello et al., 1980, 1985; Mutschler et al., 2002; Story & Stark, 1991), it is not clear whether methadone and buprenorphine would differentially impact smoking-cessation outcomes. Further, a potentially greater difference between these two pharmacological treatments may be the clinical setting in which they are administered. For example, although methadone clinics typically involve extensive over-sight of clients, office-based buprenorphine treatment is more variable (Walley et al., 2008). Despite these potential differences between treatment modalities, the present study provided no evidence that smoking and study retention outcomes varied as a function of opioid-treatment setting. This study also provides the first experimental evidence that buprenorphine-maintained patients are sensitive to a CM smoking-cessation intervention. As such, these data extend the prior research with MM patients and suggest that this smoking-cessation intervention holds potential utility for buprenorphine-maintained patients as well. Continued research efforts with buprenorphine-maintained smokers will be important, particularly as this treatment modality becomes increasingly available throughout the United States (Fiellin, 2007).
Several limitations of the current study should be noted. First, participants were required to be relatively stable on their opioid-agonist dose and abstinent from illicit drugs to participate. Although these procedures may limit the generality of our findings to unstable patients, we believe this is not likely to be a significant concern. Many opioid-maintained patients achieve stability and become abstinent from illicit drugs for extended periods, and this subset of patients may be strong candidates for a smoking-cessation intervention. Further, this eligibility criterion prevented only 15% of interested participants from being enrolled into the study, suggesting it was not so restrictive that it prevented a significant number of interested people from participating. Second, the duration of this study was brief and, although this intervention was successful in producing short-term abstinence, the majority of participants resumed smoking following the study. As noted previously, although abstinence during the initial 2 weeks of a quit attempt has been shown to be a robust predictor of long-term abstinence within smoking cessation interventions (Frosch et al., 2002; Gourlay et al., 1994; Kenford et al., 1994; Yudkin et al., 1996), we did not necessarily expect that a 2-week intervention would result in long-term smoking abstinence. Rather, the 2-week timeframe was chosen as an initial first step toward determining whether opioid-maintained participants would be sensitive to a CM smoking cessation intervention. Additional studies are needed to examine how to maintain the abstinence achieved during the initial 2-week period. Finally, although the current study included a larger sample of opioid-maintained participants than a previous pilot study (40 vs. 20 participants, respectively), additional studies with even larger sample sizes will be important for replicating these findings in the larger population of opioid-maintained smokers.
In summary, the present study demonstrates the efficacy of a brief voucher-based CM intervention in promoting initial smoking abstinence among opioid-maintained patients. Although demonstrating the efficacy of CM for smoking cessation per se is not new, demonstration that its efficacy extends to this particularly challenging clinical population represents a unique contribution to the scientific literature on this topic. That is, it had seemed reasonable to wonder whether opioid-dependent patients would even respond to a smoking-cessation intervention, particularly when one considers the modest outcomes in prior studies and the scientific evidence suggesting that opioids themselves can increase the reinforcing effects of cigarettes (Bigelow, Stitzer, Griffiths & Liebson, 1981; Chait & Griffiths, 1984; Mello et al., 1985, 1980; Mutschler et al., 2002; Richter et al., 2007; Schmitz, Grabowski, & Rhoades, 1994). The development of effective smoking cessation programs in the opioid-treatment setting also holds potential for wide dissemination to methadone and buprenorphine treatment programs throughout the country, as well as to clinics treating other forms of drug abuse. Indeed, as one exciting example, the New York State Office of Alcoholism and Substance Abuse Services (Office of Alcoholism and Substance Abuse Services, 2007) recently implemented new regulations to prohibit use of tobacco products in all drug abuse prevention, treatment, or recovery services throughout the state, as well as to establish treatment modalities for patients who use tobacco. Overall, the results from this clinical trial suggest that behavioral treatments, such as contingency management, offer significant promise for efforts to promote smoking cessation among drug abusers.
Acknowledgments
This research was supported by National Institute on Drug Abuse Grant R01 DA019550 (Sigmon) and National Institute of Drug Abuse Grant T32 DA007242 (Higgins). We thank Kathryn Saulsgiver and Allison Necheles for their assistance in conducting this study and Joan Skelly for statistical assistance.
Contributor Information
Kelly E. Dunn, Department of Psychiatry, Johns Hopkins University School of Medicine
Stacey C. Sigmon, Departments of Psychiatry and Psychology, University of Vermont
Edward F. Reimann, Department of Psychiatry, University of Vermont
Gary J. Badger, Department of Medical Biostatistics, University of Vermont
Sarah H. Heil, Departments of Psychiatry and Psychology, University of Vermont
Stephen T. Higgins, Departments of Psychiatry and Psychology, University of Vermont
References
- Armitage P. Exclusions, losses to follow-up, and with-drawals in clinical trials. In: Shapiro SH, Lewis TA, editors. Clinical trials: Issues and approaches. Dekker; New York, NY: 1983. pp. 99–113. [Google Scholar]
- Beck AT, Beck RW. Screening depressed patients in family practice: A rapid technique. Postgraduate Medicine. 1972;52(6):81–85. doi: 10.1080/00325481.1972.11713319. [DOI] [PubMed] [Google Scholar]
- Bigelow GE, Stitzer ML, Griffiths RR, Liebson IA. Contingency management approaches to drug self-administration and drug abuse: Efficacy and limitations. Addictive Behaviors. 1981;6:241–252. doi: 10.1016/0306-4603(81)90022-8. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control Annual smoking-attributable mortality, years of potential life lost, and economic costs— United States, 1997-2001. Morbidity and Mortality Weekly Report. 2005;55:625–628. [PubMed] [Google Scholar]
- Centers for Disease Control Use of cessation methods among smokers aged 16-24 years—United States 2003. Morbidity and Mortality Weekly Report. 2006;55:1351–1354. [PubMed] [Google Scholar]
- Chait LD, Griffiths RR. Effects of methadone on human cigarette smoking and subjective ratings. Journal of Pharmacology and Experimental Therapeutics. 1984;229:636–640. [PubMed] [Google Scholar]
- Clark JG, Stein MD, McGarry KA, Gogineni A. Interest in smoking cessation among injection drug users. American Journal on Addictions. 2001;10:159–166. doi: 10.1080/105504901750227804. [DOI] [PubMed] [Google Scholar]
- Clemmey P, Brooner R, Chutuape MA, Kidorf M, Stitzer M. Smoking habits and attitudes in a methadone maintenance treatment population. Drug and Alcohol Dependence. 1997;4:123–132. doi: 10.1016/s0376-8716(96)01331-2. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the Brief Questionnaire of Smoking Urges (QSU-B) in laboratory and clinical settings. Nicotine and Tobacco Research. 2001;3(1):7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. Brief Symptom Inventory. National Computer Systems; Minneapolis, MN: 1993. [Google Scholar]
- Dunn KE, Sigmon SC, Thomas CS, Heil SH, Higgins ST. Voucher-based contingent reinforcement of smoking abstinence among methadone-maintained patients: A pilot study. Journal of Applied Behavior Analysis. 2008;41:527–538. doi: 10.1901/jaba.2008.41-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrom A, Adamsson C, Allebeck P, Rydberg U. Mortality in patients with substance abuse: A follow-up in Stockholm County, 1973-84. International Journal of Addictions. 1991;26(1):91–106. doi: 10.3109/10826089109056241. [DOI] [PubMed] [Google Scholar]
- Fagerström KO, Schneider NG. Measuring nicotine dependence: A review of the Fagerström Tolerance Questionnaire. Journal of Behavioral Medicine. 1989;12:159–182. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- Fiellin DA. The first three years of buprenorphine in the United States: Experience to date and future directions. Journal of Addiction Medicine. 2007;1(2):62–67. doi: 10.1097/ADM.0b013e3180473c11. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Bailey WC, Cohen SJ. Treating tobacco use and dependence: Clinical practice guideline. U.S. Dept of Health and Human Services, U.S. Public Health Service; Rockville, MD: 2000. [Google Scholar]
- Frosch DL, Nahom D, Shoptaw S. Optimizing smoking cessation outcomes among the methadone maintained. Journal of Substance Abuse Treatment. 2002;23:425–430. doi: 10.1016/s0740-5472(02)00280-5. [DOI] [PubMed] [Google Scholar]
- Frosch DL, Shoptaw S, Jarvik ME, Rawson RA, Ling W. Interest in smoking cessation among methadone maintained outpatients. Journal of Addictive Disorders. 1998;17(2):9–19. doi: 10.1300/J069v17n02_02. [DOI] [PubMed] [Google Scholar]
- GlaxoSmithKline . Zyban Prescribing Information. GlaxoSmithKline; Research Triangle Park, NC: 2009. [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Varenicline Phase 3 Study Group Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs. sustained-release bupropion and placebo for smoking cessation: A randomized controlled trial. Journal of the American Medical Association. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Gourlay SG, Forbes A, Marriner T, Pethica D, McNeil JJ. Prospective study of factors predicting outcome of transdermal nicotine treatment in smoking cessation. British Medical Journal. 1994;309(6958):842–846. doi: 10.1136/bmj.309.6958.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith JD, Rowan-Szal GA, Roark RR, Simpson DD. Contingency management in outpatient methadone treatment: A meta-analysis. Drug and Alcohol Dependence. 2000;58(1-2):55–66. doi: 10.1016/s0376-8716(99)00068-x. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: Using self-reported time to first cigarette of the day and number of cigarettes smoked per day. British Journal of Addiction. 1989;84:791–799. doi: 10.1111/j.1360-0443.1989.tb03059.x. [DOI] [PubMed] [Google Scholar]
- Heil SH, Alessi SM, Lussier JP, Badger GJ, Higgins ST. An experimental test of the influence of prior cigarette smoking abstinence on future abstinence. Nicotine & Tobacco Research. 2004;6:471–479. doi: 10.1080/14622200410001696619. [DOI] [PubMed] [Google Scholar]
- Heil SH, Higgins ST, Bernstein IM, Solomon LJ, Rogers RE, Thomas CS, Lynch ME. Effects of voucher-based incentives on abstinence from cigarette smoking and fetal growth among pregnant women. Addiction. 2008;103:1009–1018. doi: 10.1111/j.1360-0443.2008.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Delaney DD, Budney AJ, Bickel WK, Hughes JR, Foerg F, Fenwick JW. A behavioral approach to achieving initial cocaine abstinence. American Journal of Psychiatry. 1991;148:1218–1224. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Dantona R, Donham R, Matthews M, Badger GJ. Effects of varying monetary value of voucher-based incentives on abstinence achieved during and following treatment among cocaine-dependent outpatients. Addiction. 2007;102:271–281. doi: 10.1111/j.1360-0443.2006.01664.x. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Solomon LJ, Lussier JP, Abel RL, Lynch ME, Badger GJ. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine and Tobacco Research. 2004;6:1015–1020. doi: 10.1080/14622200412331324910. [DOI] [PubMed] [Google Scholar]
- Hser YI, McCarthy WJ, Anglin MD. Tobacco use as a distal predictor of mortality among long-term narcotic addicts. Preventative Medicine. 1994;23:61–69. doi: 10.1006/pmed.1994.1009. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Gulliver SB, Fenwick JW, Valliere WA, Cruser K, Pepper S, Flynn BS. Smoking cessation among self-quitters. Health Psychology. 1992;11:331–334. doi: 10.1037//0278-6133.11.5.331. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Errors in using the tobacco withdrawal scale. Tobacco Control. 1998;7(1):92–93. doi: 10.1136/tc.7.1.92a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99:29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. The Cochrane Database of Systematic Reviews. 2009:1. doi: 10.1002/14651858.CD000031. doi:10.1002/14651858.CD000031.pub3. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, Melton LJ. Mortality following inpatient addictions treatment: Role of tobacco use in a community-based cohort. Journal of the American Medical Association. 1996;275:1097–1103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- John U, Meyer C, Rumpf HJ, Hapke U. Smoking, nicotine dependence and psychiatric comorbidity- a population-based study including smoking cessation after three years. Drug and Alcohol Dependence. 2004;76:287–295. doi: 10.1016/j.drugalcdep.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Varenicline Phase 3 Study Group Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs. placebo or sustained-release bupropion for smoking cessation: A randomized clinical trial. Journal of the American Medical Association. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB. Predicting smoking cessation: Who will quit with and without the nicotine patch. Journal of the American Medical Association. 1994;271:589–594. doi: 10.1001/jama.271.8.589. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT, Skinner W, Kent C, Pope MA. Prospects for smoking treatment in individuals seeking treatment for alcohol and other drug problems. Addictive Behavior. 1989;14:273–278. doi: 10.1016/0306-4603(89)90058-0. [DOI] [PubMed] [Google Scholar]
- Lerman C, Kaufmann V, Rukstalis M, Patterson F, Perkins K, Audrain-McGovern J, Benowitz N. Individualizing nicotine replacement therapy for the treatment of tobacco dependence: A randomized trial. Annals of Internal Medicine. 2004;140:426–433. doi: 10.7326/0003-4819-140-6-200403160-00009. [DOI] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GA, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- Lussier JP, Higgins ST, Badger GJ. Influence of the duration of abstinence on the relative reinforcing effects of cigarette smoking. Psychopharmacology. 2005;181:486–495. doi: 10.1007/s00213-005-0008-5. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, O’Brien CP. New data from the Addiction Severity Index: Reliability and validity in three centers. Journal of Nervous and Mental Disease. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Mello NK, Lukas SE, Mendelson JH. Buprenorphine effects on cigarette smoking. Psychopharmacology. 1985;86:417–425. doi: 10.1007/BF00427902. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Sellers ML, Kuehnle JC. Effects of heroin self-administration on cigarette smoking. Psychopharmacology. 1980;67:45–52. doi: 10.1007/BF00427594. [DOI] [PubMed] [Google Scholar]
- Mooney ME, Poling J, Gonzalez G, Gonsai K, Kosten T, Sofuoglu M. Preliminary study of buprenorphine and bupropion for opioid-dependent smokers. American Journal on Addictions. 2008;17:287–292. doi: 10.1080/10550490802138814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutschler NH, Stephen BJ, Teoh SK, Mendelson JH, Mello NK. An inpatient study of the effects of buprenorphine on cigarette smoking in men concurrently dependence on cocaine and opioids. Nicotine and Tobacco Research. 2002;4:223–228. doi: 10.1080/14622200210124012. [DOI] [PubMed] [Google Scholar]
- Nahvi S, Richter K, Li X, Modali L, Arnsten J. Cigarette smoking and interest in quitting in methadone maintenance patients. Addictive Behaviors. 2006;31:2127–2134. doi: 10.1016/j.addbeh.2006.01.006. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute . Clearing the air: Quit smoking today. National Institutes of Health, U.S. Department of Health and Human Services; Bethesda, MD: 2003. Pub. No. P133. [Google Scholar]
- Office of Alcoholism and Substance Abuse Services OASAS announces New York the first in the nation to go tobacco-free in all prevention and treatment programs. 2007 [Press release]. Retrieved from http://www.oasas.state.ny.us/pio/press/ pr-7-23-07.cfm.
- Reid MS, Fallon B, Sonne S, Flammino F, Nunes EV, Jiang H, Rotrosen J. Smoking cessation treatment in community-based substance-abuse rehabilitation programs. Journal of Substance Abuse Treatment. 2008;35:68–77. doi: 10.1016/j.jsat.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Reid MS, Fallon B, Sonne S, Nunes EV, Lima J, Jiang H, Rotrosen J. Implementation of a smoking cessation treatment study at substance abuse rehabilitation programs: Smoking behavior and treatment feasibility across varied community-based outpatient programs. Journal of Addiction Medicine. 2007;1:154–160. doi: 10.1097/ADM.0b013e31813872e4. [DOI] [PubMed] [Google Scholar]
- Richter KP, Choi WS, Alford DP. Smoking policies in U.S. outpatient drug treatment facilities. Nicotine and Tobacco Research. 2005;7:475–480. doi: 10.1080/14622200500144956. [DOI] [PubMed] [Google Scholar]
- Richter KP, Gibson CA, Ahluwalia JS, Schmelzle KH. Tobacco use and quit attempts among methadone maintenance clients. American Journal of Public Health. 2001;91:296–299. doi: 10.2105/ajph.91.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter KP, Hamilton AK, Hall S, Catley D, Cox LS, Grove J. Patterns of smoking and methadone dose in drug treatment patients. Experimental and Clinical Psychopharmacology. 2007;15:144–153. doi: 10.1037/1064-1297.15.2.144. [DOI] [PubMed] [Google Scholar]
- Richter KP, McCool RM, Catley D, Hall M, Ahuwalia JS. Dual pharmacotherapy and motivational interviewing for tobacco dependence among drug treatment patients. Journal of Addictive Diseases. 2005;24(4):79–90. doi: 10.1300/j069v24n04_06. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Tidey JW. Cocaine use can increase cigarette smoking: Evidence from laboratory and naturalistic settings. Experimental and Clinical Psychopharmacology. 1997;5:263–268. doi: 10.1037//1064-1297.5.3.263. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Grabowski J, Rhoades H. The effects of high and low doses of methadone on cigarette smoking. Drug and Alcohol Dependence. 1994;34:237–242. doi: 10.1016/0376-8716(94)90162-7. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Rhoades H, Grabowski J. Contingent reinforcement for reduced carbon monoxide levels in methadone maintenance patients. Addictive Behaviors. 1995;20:171–179. doi: 10.1016/0306-4603(94)00059-x. [DOI] [PubMed] [Google Scholar]
- Sees KL, Clark HW. When to begin smoking cessation in substance abusers. Journal of Substance Abuse Treatment. 1993;10:189–195. doi: 10.1016/0740-5472(93)90044-3. [DOI] [PubMed] [Google Scholar]
- Selzer ML. The Michigan Alcoholism Screening Test: The quest for a new diagnostic instrument. American Journal of Psychiatry. 1971;127:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Johnston JA, Khayrallah M, Elash CA, Gwaltney CJ, Paty JA, DeVeaugh-Geiss J. The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology. 2000;148:33–40. doi: 10.1007/s002130050022. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters A, Hickcox M. The nicotine dependence syndrome scale: A multidimensional measure of nicotine dependence. Nicotine and Tobacco Research. 2004;6:327–348. doi: 10.1080/1462220042000202481. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Jarvik ME, Ling W, Rawson RA. Contingency management for tobacco smoking in methadone-maintained opiate addicts. Addictive Behavior. 1996;21:409–412. doi: 10.1016/0306-4603(95)00066-6. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Rotheram-Fuller E, Yang X, Frosch D, Nahom D, Jarvik ME, Ling W. Smoking cessation in methadone maintenance. Addiction. 2002;97:1317–1328. doi: 10.1046/j.1360-0443.2002.00221.x. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Lamb RJ, Dallery J. Tobacco. In: Higgins ST, Silverman K, Heil SH, editors. Contingency management in substance abuse treatment. Guilford Press; New York, NY: 2008. pp. 99–119. [Google Scholar]
- Silverman K, Chutuape MA, Bigelow GE, Stitzer ML. Voucher-based reinforcement of cocaine abstinence in treatment-resistant methadone patients: Effects of reinforcement magnitude. Psychopharmacology. 1999;146:128–138. doi: 10.1007/s002130051098. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: Assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. British Journal of Addiction. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Society for Research on Nicotine and Tobacco Subcommittee on Biochemical Verification Biochemical verification of tobacco use and cessation. Nicotine and Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Stein MD, Weinstock MC, Herman DS, Anderson BJ, Anthony JL, Niaura R. A smoking cessation intervention for the methadone-maintained. Addiction. 2006;101:599–607. doi: 10.1111/j.1360-0443.2006.01406.x. [DOI] [PubMed] [Google Scholar]
- Story J, Stark MJ. Treating cigarette smoking in methadone maintenance clients. Psychoactive Drugs. 1991;23:203–215. doi: 10.1080/02791072.1991.10472237. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results from the 2006 National Survey on Drug Use and Health: National findings. Office of Applied Studies; Rockville, MD: 2007. Office of Applied Studies, NSDUH Series H-32, DHHS Pub. No. SMA 07-4293. [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. British Journal of Addiction. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- U.S. Public Health Service Treating tobacco use and dependence-clinician’s packet. A how-to guide for implementing the public health service clinical practice guideline. 2003 Retrieved from http://www.surgeongeneral.gov/tobacco/clinpack.html.
- Vogt F, Hall S, Marteau TM. Understanding why smokers do not want to use nicotine dependence medications to stop smoking: Qualitative and quantitative studies. Nicotine and Tobacco Research. 2008;10:1405–1413. doi: 10.1080/14622200802239280. [DOI] [PubMed] [Google Scholar]
- Walley AY, Alperen JK, Cheng DM, Botticelli M, Castro-Donlan C, Samet JH, Alford DP. Office-based management of opioid dependence with buprenorphine: Clinical practices and barriers. Journal of General Internal Medicine. 2009;23:1393–1398. doi: 10.1007/s11606-008-0686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology. 2008;197:371–377. doi: 10.1007/s00213-007-1041-3. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Higgins ST, Bradstreet MP, Badger GJ, Thomas CS. Changes in the relative reinforcing effects of cigarette smoking as a function of initial abstinence. Psychopharmacology. 2009;205:305–318. doi: 10.1007/s00213-009-1541-4. [DOI] [PubMed] [Google Scholar]
- Yudkin PL, Jones L, Lancaster T, Fowler GH. Which smokers are helped to give up smoking using transdermal nicotine patches? Results from a randomized, double-blind, placebo-controlled trial. British Journal of General Practice. 1996;46(404):145–148. [PMC free article] [PubMed] [Google Scholar]