Abstract
Aims
To examine the characteristics associated with early dyspnoea relief during acute heart failure (HF) hospitalization, and its association with 30-day outcomes.
Methods and results
ASCEND-HF was a randomized trial of nesiritide vs. placebo in 7141 patients hospitalized with acute HF in which dyspnoea relief at 6 h was measured on a 7-point Likert scale. Patients were classified as having early dyspnoea relief if they experienced moderate or marked dyspnoea improvement at 6 h. We analysed the clinical characteristics, geographical variation, and outcomes (mortality, mortality/HF hospitalization, and mortality/hospitalization at 30 days) associated with early dyspnoea relief. Early dyspnoea relief occurred in 2984 patients (43%). In multivariable analyses, predictors of dyspnoea relief included older age and oedema on chest radiograph; higher systolic blood pressure, respiratory rate, and natriuretic peptide level; and lower serum blood urea nitrogen (BUN), sodium, and haemoglobin (model mean C index = 0.590). Dyspnoea relief varied markedly across countries, with patients enrolled from Central Europe having the lowest risk-adjusted likelihood of improvement. Early dyspnoea relief was associated with lower risk-adjusted 30-day mortality/HF hospitalization [hazard ratio (HR) 0.81; 95% confidence interval (CI) 0.68–0.96] and mortality/hospitalization (HR 0.85; 95% CI 0.74–0.99), but similar mortality.
Conclusion
Clinical characteristics such as respiratory rate, pulmonary oedema, renal function, and natriuretic peptide levels are associated with early dyspnoea relief, and moderate or marked improvement in dyspnoea was associated with a lower risk for 30-day outcomes.
Keywords: Acute heart failure, Dyspnoea relief, Prognosis, Outcomes
Introduction
Heart failure (HF) is a major and growing public health problem worldwide.1,2 Despite advances in the care of chronic HF, the prognosis of patients hospitalized for acute HF remains poor.3 Fluid retention and congestion are responsible for the majority of HF hospitalizations,4 and greater severity of congestion is associated with worse outcomes.5 Several analyses have investigated the association between dyspnoea relief and acute HF outcomes.6–8 These studies were relatively modest in size and investigated dyspnoea relief over the first several days of hospitalization. However, studies have suggested that dyspnoea relief with usual care may occur significantly earlier following hospitalization.9–11 The patient characteristics associated with early dyspnoea improvement and the relationship between early dyspnoea relief and outcomes are not well characterized.
Given the uncertainty surrounding dyspnoea in acute HF, we used data from the international ASCEND-HF trial12 (Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure) to examine the characteristics of acute HF patients associated with moderate or marked dyspnoea relief at 6 h following study drug initiation. We also describe the regional variation in dyspnoea relief and the association of early dyspnoea relief with 30-day outcomes.
Methods
Study design
The design and results of the ASCEND-HF trial have been reported previously.12,13 Briefly, the trial evaluated nesiritide vs. placebo in 7141 patients with acute HF enrolled within 24 h of the first i.v. HF-related treatment. Participants were required to have the following at the time of randomization: dyspnoea at rest or with minimal activity, ≥1 accompanying HF sign, and ≥1 objective measure of HF. Exclusion criteria germane to this analysis were severe pulmonary disease and clinically significant anaemia (the full criteria are available elsewhere).12
Study definitions, endpoints, and statistical analysis
Dyspnoea was measured with a self-reported 7-point categorical Likert scale, ranging from ‘markedly better’ to ‘markedly worse’ at 6 h after study drug initiation as compared with the degree of dyspnoea present at the start of study drug administration. For the present analysis, patients were classified as having early dyspnoea relief if they experienced moderate or marked improvement in dyspnoea at 6 h in comparison with other categories.
Post-hoc analyses were performed on the randomized population with complete dyspnoea data (n = 6902), with patients grouped based on early dyspnoea relief status. Demographics, physical and laboratory findings, medical history, and therapies were compared using the Student's t-test or Wilcoxon rank sum test for continuous variables, and χ2 tests for categorical variables, as appropriate. Baseline characteristics of the study population were summarized as frequencies and percentages for categorical variables and by the medians and 25th and 75th percentiles for continuous variables.
Logistic regression modelling was used to assess the predictors of early dyspnoea relief. Pre-specified baseline factors were selected from the case report form (Supplementary Material, Table S1). The impact of these variables on dyspnoea relief was assessed. Stepwise selection was performed on the factors, using a criterion of ≤0.05 to be included in the model and to stay in the model. The linearity of relationships between outcome and continuous variables was evaluated. The restricted cubic spline model's likelihood ratio χ2 statistic was compared with the model including a linear term exclusively. If the relationship between a predictor and outcome did not appear to be linear, then other transformations such as quadratic terms, log transformation, or linear splines were applied. No adjustments for multiple comparisons were made. We assessed the frequency of missing values for all candidate predictors and outcomes. Variables missing ≤20% of values were identified, and Proc MI was used to impute the missing data. Imputation was not performed for BNP and NT-proBNP because they were missing >60% of the values. To assess their importance in predicting dyspnoea at 6 h, these factors were added to the original model. We also used these methods to determine the predictors of moderate–marked dyspnoea relief at 24 h. The discrimination ability of each model was assessed using a C index. The C index and calibration for the models were internally validated using bootstrap resampling to adjust for overfitting optimism.
The downstream clinical endpoints for the present analyses were mortality/HF hospitalization, all-cause mortality, and mortality/hospitalization at 30 days. Multivariate logistic regression models were developed to assess which baseline factors were predictive of these three outcomes. Factors with P-values ≤0.01 were included in the final model. Rehospitalization and fatal events within 30 days after randomization were reviewed and categorized by an independent, blinded clinical events committee.12 Univariate time to event comparisons between those with and without dyspnoea relief were made using log-rank tests. Kaplan–Meier estimates of the event rates were calculated over the entire follow-up period. Hazard ratios (HRs) and corresponding confidence intervals (CIs) were calculated relative to dyspnoea relief status using a Cox proportional hazards model with and without adjustment for baseline covariates. Statistical significance was assessed using two-sided P-values. A P-value <0.05 was considered statistically significant. All statistical computations were generated using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Clinical characteristics
Early dyspnoea relief occurred in 2984 patients (43%). There were 239 patients for whom evaluation was not done. Baseline characteristics based on dyspnoea relief status are shown in Table 1. Patients with early dyspnoea relief tended to be slightly older with lower NYHA class symptoms and less ischaemic aetiology for HF or HF hospitalizations in the previous year compared with those without dyspnoea relief (all P < 0.01). Patients with early dyspnoea relief also had slightly higher systolic blood pressure and serum haemoglobin, but lower serum blood urea nitrogen (BUN) and red cell distribution width compared with those without dyspnoea relief (all P < 0.001). At baseline, patients with early dyspnoea relief were less likely to be receiving aldosterone antagonists, loop diuretics, or nitrates. Time from hospitalization to randomization was shorter in those patients with early dyspnoea relief.
Table 1.
Baseline clinical and demographic characteristics of heart failure patients with and without moderate/marked dyspnoea improvement at 6 h
| Characteristic | No early dyspnoea improvement [n = 3918 (57%)] | Early dyspnoea improvement [n = 2984 (43%)] | P-value |
|---|---|---|---|
| Demographics | |||
| Age, median (25th, 75th), years | 66 (56, 76) | 67 (57, 77) | 0.01 |
| Male, % | 65.8 | 65.7 | 0.86 |
| Race, % | < 0.001 | ||
| White | 55.5 | 55.8 | |
| Black | 14.8 | 15.3 | |
| Asian | 26.4 | 23.1 | |
| Other | 3.2 | 5.8 | |
| Weight, median (25th, 75th), kg | 78 (64, 95) | 78 (65, 94) | 0.72 |
| Height, median (25th, 75th), cm | 168 (160, 175) | 168 (160, 175) | 0.38 |
| Region, % | <0.001 | ||
| Asia-Pacific | 26.3 | 23.1 | |
| Central Europe | 15.7 | 11.6 | |
| Latin America | 6.9 | 12.2 | |
| Western Europe | 6.9 | 6.6 | |
| North America | 44.1 | 46.5 | |
| Medical history | |||
| NYHA class III/IV, % | 79.1 | 74.2 | <0.0001 |
| Ischaemic heart disease, % | 61.5 | 58.3 | 0.01 |
| HF hospitalization in past year, % | 40.5 | 36.7 | 0.001 |
| Ejection fraction, median (25th, 75th), % | 30 (20, 36) | 30 (20, 37) | 0.62 |
| LVEF <40% in past year, % | 20.1 | 21.4 | 0.24 |
| Hypertension, % | 71.3 | 73.3 | 0.07 |
| Diabetes, % | 42.3 | 43.4 | 0.35 |
| Coronary artery disease, % | 55.7 | 53.2 | 0.03 |
| Cerebrovascular disease, % | 11.4 | 12.3 | 0.26 |
| Peripheral arterial disease, % | 10.3 | 10.7 | 0.56 |
| Chronic respiratory disease, % | 16.0 | 17.0 | 0.28 |
| Atrial fibrillation, % | 37.1 | 37.7 | 0.59 |
| CRT, % | 9.3 | 8.4 | 0.19 |
| Baseline physical and laboratory findings, median (25th, 75th) | |||
| Systolic BP, mmHg | 122 (110, 138) | 125 (110, 140) | <0.001 |
| Heart rate, b.p.m. | 82 (72, 95) | 82 (72, 95) | 0.68 |
| Respiratory rate, breaths/min | 24 (21, 26) | 23 (21, 25) | 0.01 |
| Sodium, mg/dL | 139 (136, 141) | 139 (136, 141) | 0.23 |
| Potassium, mmol/L | 4.1 (3.7, 4.5) | 4.1 (3.7, 4.4) | 0.45 |
| BUN, mg/dL | 26 (19, 40) | 24 (17, 36) | <0.001 |
| Creatinine, mg/dL | 1.2 (1.0, 1.6) | 1.2 (1.0, 1.6) | 0.02 |
| Haemoglobin, g/dL | 12.6 (11.3, 13.9) | 12.8 (11.5, 14.1) | <0.001 |
| BNP, pg/mL | 967 (520, 1820) | 1000 (571, 1856) | 0.19 |
| NT-proBNP, pg/mL | 4458 (2040, 9177) | 4536 (2127, 9020) | 0.35 |
| RDW, % | 15.4 (14.2, 17.1) | 15.2 (14.0, 16.8) | <0.001 |
| Medications before randomization, % | |||
| ACE inhibitor/ARB | 61.6 | 59.8 | 0.12 |
| Beta-blockers | 58.3 | 58.0 | 0.82 |
| Aldosterone-blocking agents | 29.1 | 26.2 | 0.01 |
| Chronic loop diuretics | 66.5 | 59.8 | <0.001 |
| Chronic thiazide diuretics | 6.5 | 6.9 | 0.49 |
| Nitrates (oral or topical) | 25.2 | 21.4 | <0.001 |
| Hydralazine | 7.3 | 7.4 | 0.91 |
| Digoxin | 26.3 | 26.6 | 0.80 |
| Calcium channel blockers | 12.3 | 13.6 | 0.09 |
| Oral anticoagulants | 25.0 | 22.8 | 0.03 |
| Aspirin | 49.7 | 48.6 | 0.38 |
| I.v. dobutamine | 3.3 | 3.1 | 0.60 |
| I.v. nitroglycerin | 13.6 | 15.0 | 0.12 |
| I.v. opiates | 3.7 | 4.7 | 0.03 |
| From qualifying episode to randomization (early hospital use) | |||
| Loop diuretic from qualifying episode to randomization, % | 88.8 | 89.8 | 0.18 |
| Thiazide diuretic from qualifying episode to randomization, % | 4.3 | 4.0 | 0.55 |
| I.v. furosemide equivalent dosing from 0–6 h post-randomization, median (25th, 75th), mg | 40.0 (40.0, 80.0) | 40.0 (30.0, 60.0) | <0.001 |
| I.v. furosemide equivalent dosing from 0–24h post-randomization, median (25th, 75th), mg | 80.0 (40.0, 160.0) | 80.0 (56.3, 124.8) | 0.17 |
| Time from hospitalization to randomization, median (25th, 75th), h | 16.7 (5.7, 22.3) | 13.7 (5.1, 21.2) | <0.0001 |
BP, blood pressure; BUN, blood urea nitrogen; HF, heart failure; RDW, red cell distribution width.
Multivariable predictors of dyspnoea relief
The multivariable model for the predictors of early dyspnoea relief is presented in Table 2. Lower BUN was a predictor of early dyspnoea relief. Additional independent predictors of dyspnoea relief included older age and oedema on chest radiograph; higher systolic blood pressure, respiratory rate, and natriuretic peptide level; and lower serum sodium and haemoglobin. Chronic respiratory disease and smoking status were not found to be independent predictors of early dyspnoea relief. C indexes were generated for the 25 imputed databases. The mean C index was 0.590, with a minimum value of 0.587 and a maximum of 0.594.
Table 2.
Multivariate clinical predictors of moderate–marked dyspnoea relief at 6 h
| Variable | OR represents | OR | 95% CI | t-statistic | P-value |
|---|---|---|---|---|---|
| Serum BUN, mg/dL | Doubling of BUN | 0.84 | 0.79–0.90 | –5.21 | <0.0001 |
| Systolic BP, mm Hg | 10 units increase | 1.07 | 1.04–1.11 | 4.25 | <0.0001 |
| Serum haemoglobin, g/dL | 1 units increase | 0.94 | 0.90–0.97 | –3.45 | <0.001 |
| Age, years | 10 year increase | 1.06 | 1.02–1.10 | 3.16 | <0.01 |
| Respiratory rate, breaths/min | 2 units increase | 1.05 | 1.02–1.09 | 2.86 | <0.01 |
| Serum sodium, mg/dL | 1 units increase | 0.96 | 0.94–0.99 | –2.51 | 0.01 |
| Oedema on chest radiograph | Yes vs. no | 1.17 | 1.02–1.34 | 2.26 | 0.02 |
| NYHA classa | |||||
| II | 0.73 | 0.55–0.96 | –2.25 | 0.02 | |
| III | 0.61 | 0.46–0.79 | –3.69 | <0.001 | |
| IV | 0.73 | 0.56–0.96 | –2.23 | 0.03 | |
| Raceb | |||||
| Black | 1.09 | 0.93–1.26 | 1.04 | 0.30 | |
| Asian | 0.92 | 0.81–1.04 | –1.38 | 0.17 | |
| Other | 1.66 | 1.30–2.12 | 4.07 | <0.0001 | |
| BNP, pg/mL | Doubling | 1.06 | 1.02–1.09 | 8.3648 | <0.01 |
| NT-proBNP, pg/mL | Doubling | 1.05 | 1.01–1.08 | 7.9264 | <0.01 |
BP, blood pressure; BUN, blood urea nitrogen; CI, confidence interval; OR, odds ratio.
aReference group is NYHA class I.
bReference group is white.
Table 3 presents the association between additional factors and early dyspnoea relief adjusted for the variables in Table 2. Chronic use of aldosterone antagonists, loop diuretics, and nitrates was associated with less dyspnoea relief (P < 0.01).
Table 3.
Association between additional factors and moderate–marked dyspnoea relief at 6ha
| Variable | OR | 95% CI | χ2 | P-value |
|---|---|---|---|---|
| ACE inhibitor/ARB | 0.92 | 0.84–1.02 | 2.37 | 0.12 |
| Beta-blocker | 0.99 | 0.90–1.09 | 0.1 | 0.82 |
| Aldosterone antagonist | 0.87 | 0.78–0.96 | 7.0 | <0.01 |
| Chronic loop diuretic | 0.75 | 0.68–0.83 | 32.6 | <0.0001 |
| I.v furosemide equivalent doseb | 0.92 | 0.87–0.98 | 6.52 | 0.01 |
| Chronic thiazide diuretic | 1.01 | 0.88–1.29 | 0.4691 | 0.49 |
| Pre-randomization oral/topical nitrates | 0.81 | 0.72–0.91 | 13.60 | <0.001 |
| Pre-randomization i.v. nitroglycerin | 1.11 | 0.97–1.28 | 2.43 | 0.12 |
| Pre-randomization nitroprusside | 1.35 | 0.87–2.11 | 1.78 | 0.18 |
| Pre-randomization hydralazine | 1.01 | 0.84–1.21 | 0.0133 | 0.91 |
| Pre-randomization dobutamine | 0.93 | 0.71–1.22 | 0.28 | 0.60 |
| Baseline RDWc | 0.94 | 0.88–1.00 | 3.65 | 0.06 |
| Prior CRT | 0.61 | 0.39–0.96 | 4.73 | 0.03 |
| World regiond | ||||
| Central Europe | 0.71 | 0.55–0.92 | 6.71 | 0.01 |
| Western Europe | 0.75 | 0.56–1.00 | 3.79 | 0.051 |
| Asia | 0.91 | 0.53–1.57 | 0.11 | 0.74 |
| Latin America | 1.19 | 0.90–1.58 | 1.46 | 0.23 |
| Planned nesiritide | 1.10 | 0.97–1.24 | 2.22 | 0.14 |
| Time to treatmente | 0.99 | 0.98–0.99 | 17.50 | <0.0001 |
Overall test Wald χ2 14.4420; P = 0.0060.
Variables in bold text are statistically significant (P-value <0.05).
CI, confidence interval; OR, odds ratio; RDW, red cell distribution width.
aRelationships are adjusted for all factors reported in Table 2.
bI.v. furosemide equivalent dose from 0 to 6 h post-randomization, per 40 mg increase.
cRDW, per 1 standard deviation increase (4.11).
dReference is North America.
eTime from hospital presentation to study drug administration.
Table 4 presents the baseline predictors of dyspnoea relief at 24 h. Lower BUN, older age, and increased natriuretic peptide levels were associated with both 6 and 24 h dyspnoea relief. Several predictors were similar between the two time points, specifically pulmonary oedema on exam or chest radiograph, and higher blood pressure. Additional baseline predictors of dyspnoea relief at 24 h (but not 6 h) included atrial fibrillation or flutter, chronic respiratory disease, and HF hospitalization within the previous year. The mean C index for the 24 h dyspnoea model was 0.601, with a minimum value of 0.599 and a maximum of 0.603.
Table 4.
Multivariate clinical predictors of moderate–marked dyspnoea relief at 24h
| Variable | OR represents | OR | 95% CI | t-statistic | P-value |
|---|---|---|---|---|---|
| Serum BUN, mg/dL | Doubling of BUN | 0.87 | 0.80–0.93 | –4.18 | <0.001 |
| Diastolic BP, mm Hg | 10 units increase | 1.08 | 1.04–1.12 | 3.68 | <0.01 |
| Age, years | 10 year increase | ||||
| Age <60 years | 0.97 | 0.90–1.04 | –0.89 | 0.38 | |
| Age >60 years | 1.11 | 1.03–1.20 | 2.54 | 0.01 | |
| Jugular venous distension | Yes vs. no | 1.12 | 1.02–1.23 | 2.12 | 0.03 |
| Baseline AF/AFL | Yes vs. no | 0.87 | 0.76–0.99 | –2.32 | 0.02 |
| HF hospitalization within 1 year | Yes vs. no | 0.79 | 0.68–0.90 | –4.30 | <0.001 |
| Chronic respiratory disease | Yes vs. no | 0.75 | 0.62–0.89 | –4.02 | <0.001 |
| Respiratory rate, breaths/min | 2 units increase | ||||
| <26 breaths/min | 1.01 | 0.97–1.05 | 0.52 | 0.60 | |
| >26 breaths/min | 0.94 | 0.88–1.00 | –2.11 | 0.04 | |
| Pulmonary oedema | Yes vs. no | ||||
| <1/3 lung field | 1.40 | 1.24–1.57 | 4.13 | <0.001 | |
| >1/3 lung field | 1.50 | 1.35–1.66 | 5.12 | <0.001 | |
| Racea | |||||
| Black | 1.03 | 0.87–1.21 | 0.31 | 0.76 | |
| Asian | 1.02 | 0.89–1.16 | 0.22 | 0.82 | |
| Other | 2.57 | 1.86–3.57 | 5.68 | <0.0001 | |
| BNP, pg/mL | Doubling | 1.03 | 0.99–1.07 | 2.71 | <0.01 |
| NT-proBNP, pg/mL | Doubling | 1.05 | 1.01–1.08 | 2.70 | <0.01 |
AF/AFL, atrial fibrillation/flutter; BP, blood pressure; BUN, blood urea nitrogen; CI, confidence interval; HF, heart failure; OR, odds ratio.
aReference group is white.
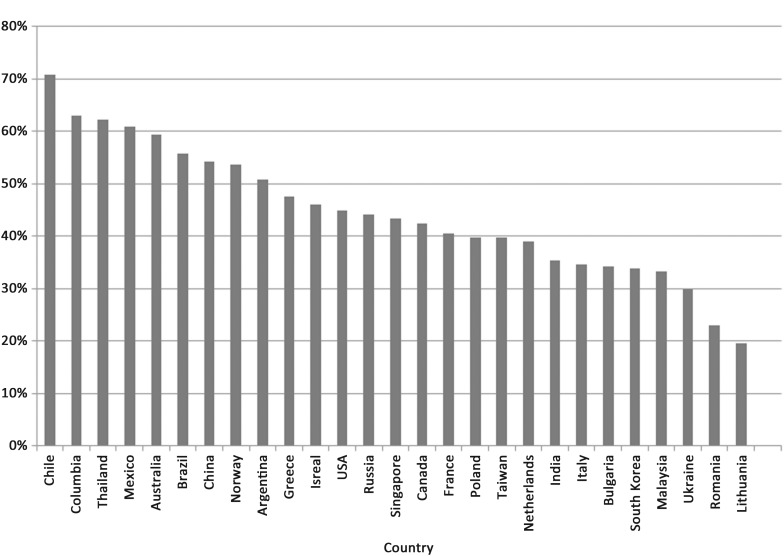
Early dyspnoea relief was the greatest in Latin America (57% of patients); intermediate in North American (45%), Western Europe (42%), and Asia-Pacific (40%); and lowest in Central Europe (36%). The geographical variation in early dyspnoea relief is presented in Figure 1.
Figure 1.
Observed moderate to marked improvement in dyspnoea at 6 h by country of enrolment.
Outcomes
Table 5 presents the association between early dyspnoea relief and outcomes. On univariate analysis, early dyspnoea relief was associated with reduced risk for 30-day mortality, mortality/HF hospitalization, and mortality/hospitalization. After risk adjustment, patients with early dyspnoea relief were at decreased risk for 30-day mortality/HF hospitalization (HR 0.81; 95% CI 0.68–0.96) and mortality/hospitalization (HR 0.85; 95% CI 0.74–0.99), but similar 30-day mortality.
Table 5.
Association between moderate–marked dyspnoea improvement at 6 hand outcomes
| Outcomes | Early dyspnoea improvement | No early dyspnoea improvement | Unadjusted |
Adjusteda |
||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |||
| 30-day mortality | 93/2980 (3.1) | 167/3911 (4.3) | 0.72 (0.56–0.94) | 0.014 | 0.81 (0.62–1.07) | 0.14 |
| 30-day mortality or HF hospitalization | 240/2907 (8.3) | 414/3834 (10.8) | 0.74 (0.63–0.88) | 0.0005 | 0.81 (0.68–0.96) | 0.018 |
| 30-day mortality or hospitalization | 397/2908 (13.7) | 629/3837 (16.4) | 0.81 (0.70–0.92) | 0.002 | 0.85 (0.74–0.99) | 0.031 |
BUN, blood urea nitrogen; CI, confidence interval; HF, heart failure; HR, hazard ratio.
aAdjustment variables were as follows.
Predictors of mortality by day 30 (at the P ≤0.01 level): age, BUN, sodium, systolic blood pressure, baseline dyspnoea.
Predictors of mortality by day 30 and HF hospitalization (at the P ≤ 0.01 level): age, BUN, baseline cerebrovascular disease, creatinine, depression, systolic blood pressure, baseline dyspnoea, HF hospitalization in the year prior to admission, serum sodium, elevated jugular venous pressure, and history of chronic respiratory disease.
Predictors of mortality by day 30 and all-cause hospitalization (at the P ≤ 0.01 level): age, BUN, cerebrovascular disease systolic blood pressure, creatinine, depression, HF hospitalization in the year prior to admission, serum sodium, elevated jugular venous pressure, history of chronic respiratory disease, and baseline weight.
Discussion
ASCEND-HF represents the largest experience in examining dyspnoea relief in patients with acute HF. Approximately 45% of randomized patients experienced moderate to marked dyspnoea relief 6 h after study drug initiation; however, there was significant regional variation. While there are several clinical characteristics such as age, renal function, and natriuretic peptide levels associated with early dyspnoea relief, the degree to which these factors account for dyspnoea relief is modest. The discrimination ability of the model was poor (C ndex <0.60), suggesting significant unexplained factors associated with dyspnoea improvement. Nevertheless, early improvement in dyspnoea may be a good prognostic indicator as it is associated with lower risk-adjusted 30-day outcomes of mortality or rehospitalization compared with patients with only minimal or no improvement in dyspnoea at 6 h. However, the lack of association between early dyspnoea relief and 30-day mortality on adjusted analysis suggests that the patient-reported outcome of dyspnoea relief is not strongly linked with the clinical outcome of mortality.
While nearly half of patients randomized had moderate to marked improvement in dyspnoea in 6 h, there was significant regional variation. There may be multiple explanations for such variation, including differences in patient profiles, cultural interpretation of dyspnoea, treatment strategies before randomization, and other factors. The impact of regional variation in the interpretation of dyspnoea and use of specific dyspnoea measurements warrants investigation especially considering that instruments used for dyspnoea have not been well validated.14 For instance, variability around the timing of dyspnoea assessment and the persistence of dyspnoea despite ongoing therapy may influence the evaluation of dyspnoea in clinical trials.15 Improved standardization of dyspnoea measurements has been proposed15 and has recently been incorporated in clinical trials.11,16 A recent ASCEND-HF investigation of the change in peak expiratory flow rate over the first 24 h during acute HF management and its association with dyspnoea improvement by Likert scale17 supports the use of objective metrics for future dyspnoea evaluations. The RED-ROSE study (Reliable Evaluation of Dyspnoea in the Heart Failure Network ROSE Study) is investigating whether a provocative dyspnoea evaluation is a more sensitive index of variability in dyspnoea status than the dyspnoea visual analogue scale assessment (Clinicaltrials.gov: NCT01132846). In sum, future acute HF studies should consider a standardized provocative dyspnoea evaluation that incorporates baseline dyspnoea information as well as relative change in dyspnoea in combination with objective metrics (e.g. peak expiratory flow rate) and quality control oversight to minimize geographical variation.
Previous studies have identified few predictors of dyspnoea relief in acute HF,6,7 and limited data exist with respect to dyspnoea relief in the early period following hospitalization. We found that BUN, natriuretic peptide levels, respiratory rate, and oedema on chest radiograph were associated with early dyspnoea relief. These characteristics may represent the degree of congestion. The association between neurohormonal activation, renal function, and BUN levels may explain, in part, the underlying pathophysiology.18 More modest degrees of neurohormonal activation and preserved renal function, as captured in a lower BUN, may afford more rapid return to cardiac compensation with brisk diuresis upon initiation of acute HF therapies. Similarly, the association between lower haemoglobin and early dyspnoea relief may be due to relative haemodilution that responds to aggressive decongestion. This hypothesis is supported by data demonstrating the positive association between haemoconcentration, haemodynamic changes, and outcomes during the management of acute HF.19 The association between the use of aldosterone antagonists, loop diuretics, and nitrates and a decreased likelihood of early dyspnoea relief may be related to greater severity of illness or chronicity of HF. Alternatively, aldosterone antagonists, loop diuretics, and nitrate use, which have known benefits on haemodynamics and pulmonary decongestion,20,21 may not exert the same benefit in patients receiving these therapies chronically compared with treatment-naïve patients.22 Therefore, the association between medication use, particularly post-randomization, and dyspnoea relief requires further prospective investigation.
Previous studies of characteristics associated with dyspnoea relief in acute HF are limited. In the PROTECT pilot study of 303 acute HF patients, only a history of pulmonary disease was associated with dyspnoea relief at 24–48 h.6 A dyspnoea analysis in the Pre-RELAX-AHF study of 232 patients found that respiratory rate, systolic blood pressure, and baseline haemoglobin were associated with dyspnoea relief over the first 24 h.7 Our study found similar relationships for respiratory rate and systolic blood pressure, but, in contrast, patients with lower haemoglobin had greater dyspnoea relief in ASCEND-HF. The modest predictive capacity of the ASCEND-HF model suggests that significant uncertainty remains with respect to the factors associated with dyspnoea relief. Focused studies to understand the underlying pathophysiology of this subjective patient-reported outcome are needed.
In a large international acute HF population, we demonstrated that early dyspnoea relief was associated with a 19% decreased risk for 30-day mortality/HF hospitalization and a 15% decreased risk for 30-day mortality/hospitalization, but similar mortality. Previous studies investigating the association between dyspnoea relief over the first several days following hospitalization and 30- to 60-day outcomes have been mixed.6–8 In the PROTECT study8 and the PROTECT pilot study,6 dyspnoea relief at 2–3 days was associated with reduced mortality at 30 and 60 days, respectively. However, in Pre-RELAX-AHF,7 dyspnoea relief within 24 h was not associated with a significant difference in mortality at 30 or 60 days. Moreover, in both the PROTECT pilot study and Pre-RELAX-AHF, dyspnoea relief was not associated with reduced 60-day composite endpoints including mortality or hospitalization. Importantly, a comparison of patients with and without dyspnoea relief at 6 h is different from a comparison between patients with and without dyspnoea relief within 24 h or at 2–3 days. In general, dyspnoea relief is not a surrogate for clinical outcomes, but is a patient-reported outcome or potentially a prognostic factor. Thus, evaluating a patient's symptoms of dyspnoea early in a hospitalization, in addition to other risk factors, may be used to identify patients at high risk of poor outcomes.
These results have applications to both the clinical and research enterprise. First, while there are clinical characteristics associated with early dyspnoea relief, much more research is needed to understand a patient's experience of dyspnoea as well as its association with downstream clinical outcomes. Certainly, those patients without dyspnoea relief are higher risk for poor outcomes and should be monitored more carefully. The implications of this analysis on dyspnoea improvement as a key endpoint for clinical trials are several fold. The geographic variation in dyspnoea relief may indicate differences in patient experiences or perceptions which warrant further refinement and validation of instruments evaluating dypnoea as a patient-reported outcome. The Food and Drug Administration (FDA)-established guidelines in 2009 for patient-reported outcomes and future studies that use dyspnoea as a primary endpoint may require instruments that have content validation.23 Those without early dyspnoea relief may also be used for selection of high-risk patients for acute HF trials.
Limitations
Our study should be interpreted in the context of several limitations. First, this was a retrospective analysis. Despite covariate adjustment, other measured and unmeasured factors may have influenced these findings. The study was from a clinical trial with inclusion and exclusion criteria, and may not be fully representative of the overall acute HF population. While the entry criteria may select a patient population that varies from other cohorts such as those entered into a registry, ASCEND-HF is the largest study to examine dyspnoea in acute HF, and previous registries have had limited evaluation of dyspnoea. Moreover, the presence of continental and regional differences in the aetiology, severity, and management of acute HF24 highlights the importance of additional studies across geographical and cultural boundaries. Also, dyspnoea relief status was defined by a 7-point Likert scale at 6 h after study drug initiation. Since the median time from presentation to randomization was 15 h, our dyspnoea evaluation reflects an ‘early’ evaluation in a clinical trial but still represents a relatively later time point in a patient's hospital course.
Conclusion
In ASCEND-HF, nearly 45% of acute HF patients experienced early dyspnoea relief. Age, systolic blood pressure, respiratory rate, and serum BUN, sodium, and haemoglobin levels are modest predictors of early dyspnoea relief, which is associated with improved 30-day HF outcomes. Given the modest predictive capacity of baseline clinical variables for early dyspnoea relief, future investigations will need to clarify further the characteristics associated with early HF symptom improvement. The significant geographical variation in the percentage of patients with early dyspnoea improvement highlights the importance of standardization and validation of dyspnoea measurements.
Supplementary material
Supplementary material is available at European Journal of Heart Failure online.
Funding
Johnson and Johnson provided funding for the overall ASCEND-HF trial.
Conflict of interest: J.R.T. has received research grants for his role as a Steering committee member in ASCEND-HF from Scios Inc., the sponsor of this trial. He received research support and/or consultation fees from Amgen, Bayer, Corthera, Cytokinetics, Novartis, and Trevena. R.C. has received research grants and consulting fees from Johnson & Johnson. All other industry relations are publicly displayed at www.dcri.org/about-us. J.E. is currently conducting research sponsored by Johnson & Johnson. B.M. has received consulting fees/honoraria from DCRI. G.M.F. has received consulting fees from Amgen, Trevena, Otsuka, Roche Diagnostics, Novartis, Merck, and BG Medicine, and research funding from NIH, Amgen, Otsuka, and Roche Diagnostics. P.P. has received fees in respect of consultancy and speaker's bureau from Scios Inc., Corthera, Novartis, Johnson & Johnson, and Bayer. All other authors declare no conflict of interest.
References
- 1.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P ESC guidelines for the diagnosis treatment of acute chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol. 2009;53:557–573. doi: 10.1016/j.jacc.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 4.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JE, Cleland JG, Dickstein K, Drazner MH, Fonarow GC, Jaarsma T, Jondeau G, Sendon JL, Mebazaa A, Metra M, Nieminen M, Pang PS, Seferovic P, Stevenson LW, van Veldhuisen DJ, Zannad F, Anker SD, Rhodes A, McMurray JJ, Filippatos G. Assessing and grading congestion in acute heart failure: a scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail. 2010;12:423–433. doi: 10.1093/eurjhf/hfq045. [DOI] [PubMed] [Google Scholar]
- 6.Metra M, Cleland JG, Weatherley BD, Dittrich HC, Givertz MM, Massie BM, O'Connor CM, Ponikowski P, Teerlink JR, Voors AA, Cotter G. Dyspnoea in patients with acute heart failure: an analysis of its clinical course, determinants, relationship to 60-day outcomes in the PROTECT pilot study. Eur J Heart Fail. 2010;12:499–507. doi: 10.1093/eurjhf/hfq021. [DOI] [PubMed] [Google Scholar]
- 7.Metra M, Teerlink JR, Felker GM, Greenberg BH, Filippatos G, Ponikowski P, Teichman SL, Unemori E, Voors AA, Weatherley BD, Cotter G. Dyspnoea and worsening heart failure in patients with acute heart failure: results from the Pre-RELAX-AHF study. Eur J Heart Fail. 2010;12:1130–1139. doi: 10.1093/eurjhf/hfq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metra M, O'Connor CM, Davison BA, Cleland JG, Ponikowski P, Teerlink JR, Voors AA, Givertz MM, Mansoor GA, Bloomfield DM, Jia G, DeLucca P, Massie B, Dittrich H, Cotter G. Early dyspnoea relief in acute heart failure: prevalence, association with mortality effect of rolofylline in the PROTECT Study. Eur Heart J. 2011;32:1519–1534. doi: 10.1093/eurheartj/ehr042. [DOI] [PubMed] [Google Scholar]
- 9.Publication Committee for the VMAC Investigators (Vasodilatation in the Management of Acute CHF) Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;287:1531–1540. doi: 10.1001/jama.287.12.1531. [DOI] [PubMed] [Google Scholar]
- 10.Cleland JG, Freemantle N, Coletta AP, Clark AL. Clinical trials update from the American Heart Association: REPAIR-AMI, ASTAMI, JELIS, MEGA, REVIVE-II, SURVIVE, and PROACTIVE. Eur J Heart Fail. 2006;8:105–110. doi: 10.1016/j.ejheart.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Mebazaa A, Pang PS, Tavares M, Collins SP, Storrow AB, Laribi S, Andre S, Mark Courtney D, Hasa J, Spinar J, Masip J, Frank Peacock W, Sliwa K, Gayat E, Filippatos G, Cleland JG, Gheorghiade M. The impact of early standard therapy on dyspnoea in patients with acute heart failure: the URGENT-dyspnoea study. Eur Heart J. 2010;31:832–841. doi: 10.1093/eurheartj/ehp458. [DOI] [PubMed] [Google Scholar]
- 12.O'Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF, Jr, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalan R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Mendez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 13.Hernandez AF, O'Connor CM, Starling RC, Reist CJ, Armstrong PW, Dickstein K, Lorenz TJ, Gibler WB, Hasselblad V, Komajda M, Massie B, McMurray JJ, Nieminen M, Rouleau JL, Swedberg K, Califf RM. Rationale, design of the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure Trial (ASCEND-HF) Am Heart J. 2009;157:271–277. doi: 10.1016/j.ahj.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 14.West RL, Hernandez AF, O'Connor CM, Starling RC, Califf RM. A review of dyspnea in acute heart failure syndromes. Am Heart J. 2010;160:209–214. doi: 10.1016/j.ahj.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 15.Gheorghiade M, Adams KF, Cleland JG, Cotter G, Felker GM, Filippatos GS, Fonarow GC, Greenberg BH, Hernandez AF, Khan S, Komajda M, Konstam MA, Liu PP, Maggioni AP, Massie BM, McMurray JJ, Mehra M, Metra M, O'Connell J, O'Connor CM, Pang PS, Pina IL, Sabbah HN, Teerlink JR, Udelson JE, Yancy CW, Zannad F, Stockbridge N. Phase III clinical trial end points in acute heart failure syndromes: a virtual roundtable with the Acute Heart Failure Syndromes International Working Group. Am Heart J. 2009;157:957–970. doi: 10.1016/j.ahj.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Teerlink JR, Metra M, Felker GM, Ponikowski P, Voors AA, Weatherley BD, Marmor A, Katz A, Grzybowski J, Unemori E, Teichman SL, Cotter G. Relaxin for the treatment of patients with acute heart failure (Pre-RELAX-AHF): a multicentre, randomised, placebo-controlled, parallel-group, dose-finding phase IIb study. Lancet. 2009;373:1429–1439. doi: 10.1016/S0140-6736(09)60622-X. [DOI] [PubMed] [Google Scholar]
- 17.Ezekowitz JA, Hernandez AF, O'Connor CM, Starling RC, Proulx G, Weiss MH, Bakal JA, Califf RM, McMurray JJV, Armstrong PW. Assessment of dyspnea in acute decompensated heart failure: insights from ASCEND-HF (Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure) on the contributions of peak expiratory flow. J Am Coll Cardiol. 2012;59:1441–1448. doi: 10.1016/j.jacc.2011.11.061. [DOI] [PubMed] [Google Scholar]
- 18.Kazory A. Emergence of blood urea nitrogen as a biomarker of neurohormonal activation in heart failure. Am J Cardiol. 2010;106:694–700. doi: 10.1016/j.amjcard.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 19.Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122:265–272. doi: 10.1161/CIRCULATIONAHA.109.933275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.den Uil CA, Lagrand WK, Spronk PE, van der Ent M, Jewbali LS, Brugts JJ, Ince C, Simoons ML. Low-dose nitroglycerin improves microcirculation in hospitalized patients with acute heart failure. Eur J Heart Fail. 2009;11:386–390. doi: 10.1093/eurjhf/hfp021. [DOI] [PubMed] [Google Scholar]
- 21.Felker GM, Mentz RJ. Diuretics and ultrafiltration in acute decompensated heart failure. J Am Coll Cardiol. 2012;59:2145–2153. doi: 10.1016/j.jacc.2011.10.910. [DOI] [PubMed] [Google Scholar]
- 22.Packer M. The clinical significance of nitrate tolerance in patients with chronic heart failure. Eur Heart J. 1989;10(Suppl A):20–25. doi: 10.1093/eurheartj/10.suppl_a.20. [DOI] [PubMed] [Google Scholar]
- 23.Speight J, Barendse SM. FDA guidance on patient reported outcomes. BMJ. 2010;340:c2921. doi: 10.1136/bmj.c2921. [DOI] [PubMed] [Google Scholar]
- 24.Chioncel O, Vinereanu D, Datcu M, Ionescu DD, Capalneanu R, Brukner I, Dorobantu M, Ambrosy A, Macarie C, Gheorghiade M. The Romanian Acute Heart Failure Syndromes (RO-AHFS) registry. Am Heart J. 2011;162 doi: 10.1016/j.ahj.2011.03.033. 142–53 e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.