Abstract
The SLC4 family consists of ten genes (SLC4A1-5; SLC4A7-11). All encode integral membrane proteins with very similar hydropathy plots—consistent with 10 – 14 transmembrane segments. Nine SLC4 members encode proteins that transport (or a related species, such as ) across the plasma membrane. Functionally, eight of these proteins fall into two major groups: three Cl-HCO3 exchangers (AE1 – 3) and five Na+-coupled transporters (NBCe1, NBCe2, NBCn1, NBCn2, NDCBE). Two of the Na+ - coupled transporters (NBCe1, NBCe2) are electrogenic; the other three Na+-coupled transporters and all three AEs are electroneutral. In addition, two other SLC4 members (AE4, SLC4A9 and BTR1, SLC4A11) do not yet have a firmly established function. Most, though not all, SLC4 members are functionally inhibited by 4,4′-diisothiocyanatostilbene-2,2′-disulfonate (DIDS). SLC4 proteins play important roles many modes of acid-base homeostasis: the carriage of CO2 by erythrocytes, the transport of H+ or by several epithelia, as well as the regulation of cell volume and intracellular pH.
Keywords: SLC4, Bicarbonate, carbonate, chloride, sodium, boron, exchanger, cotransporter
1. Introduction
The SLC4 transporter family1, also known as the bicarbonate-transporter family, includes the products of ten human genes (SLC4A1-5; A7-11). Figure 1 summarizes the interrelatedness of the ten proteins, based on a computerized phylogenetic analysis (http://www.ebi.ac.uk/clustalw/). Table 1 summarizes the HUGO gene nomenclature, preferred protein names, functional information, tissue distribution of the proteins, associated diseases, gene localization, and representative, human sequence accession numbers. Note that human genes are capitalized (e.g., SLC4A1), whereas rodent genes are in lower case (e.g., Slc4a1). The preferred protein names reflect known transport functions, of which there are three: (1) Cl-HCO3 (or “anion”) exchanger (the AEs); (2) Na/HCO3 cotransport (the NBCs), which may be either electrogenic (‘e’ suffix) or neutral (‘n’ suffix); and (3) Na+-driven Cl-HCO3 exchange (NDCBE). Because the functions of the other gene products are not conclusively established (AE4 and BTR1), their protein names should be regarded as provisional until they are eventually assigned permanent, preferred names.
Figure 1. SLC4 family tree.
The computerized phylogenetic analysis was performed using the deduced amino-acid sequence of one representative human splice variant for each of the ten SLC4 genes (available at http://www.ebi.ac.uk/clustalw/). Using sequences from only one species (i.e., human) simplifies the conceptualization and quantitation of sequence identity.
Table 1. Characteristics of the SLC4 family members.
| Human Gene Symbol (HUGO) |
Protein name |
Aliases | Predominant Substrates |
Transport type* |
Tissue distribution and cellular/subcellular expression |
Link to. disease† |
Human gene locus |
Sequence Accession ID |
Splice Variants and their specific features |
|---|---|---|---|---|---|---|---|---|---|
| SLC4A1 | AE1 | Band 3 | Chloride Bicarbonate |
E | Erythrocytes, intercalated cells of renal collecting duct, heart and colon |
Hemolytic anemia, distal renal tubular acidosis |
17q21—q22 | NM_000342 | 2 |
| SLC4A2 | AE2 | Chloride Bicarbonate |
E | Widely distributed; basolateral in most epithelial cells |
7q35—36 | NM_003040 | 5 | ||
| SLC4A3 | AE3 | Chloride Bicarbonate |
E | Brain, retina, heart and smooth muscle. Also epithelial cells of kidney and GI tract |
2q36 | NM_005070 | ≥ 2 | ||
| SLC4A4 | NBCel | NBC, NBC1 |
Sodium Bicarbonate (and/or Carbonate) |
C | NBCe1-A: renal proximal tubule, eye NBCe1-B: widely distributed, pancreas, heart, eye. NBCe1-C: brain |
Severe proximal renal tubular acidosis, ocular abnormalities, short stature |
4q21 | NM_003759 | 5 |
| SLC4A5 | NBCe2 | NBC4 | Sodium Bicarbonate (and/or Carbonate) |
C | Liver, testes, spleen | 2p13 | NM_133478 | ≥1 | |
| SLC4A6 [not used] |
- | - | - | - | - | - | - | - | - |
| SLC4A7 | NBCnl | NBC2, NBC3 |
Sodium Bicarbonate |
C | Widely distributed, spleen, testes, brain, heart, lung, liver and kidney; not skeletal muscle. |
Blindness, auditory impairment |
3p22 | NM_003615 | ≥3 |
| SLC4A8 | NDCBE | kNBC3 | Sodium Bicarbonate Chloride |
C and E | Brain, testes, kidney and ovary |
12q13 | NM_004858 | 4 | |
| SLC4A9 | - | AE4 | Inconclusive | ? | At least kidney | 5q31 | NM 031467 | 1 | |
| SLC4A10 | NBCn2 | NCBE | Sodium Bicarbonate Chloride |
C and E | Brain | 2q23—q24 | NM_022058 | 4 | |
| SLC4A11 | BTR1 | NaBCl | (sodium, borate) | C* | Kidney, salivary gland testis, thyroid, trachea |
20p12 | NM_032034 | 1 |
C: Cotransporter, E: Exchanger, O: Orphan transporter, Disputed
All examples are genetic defects
1.1. Similarities among the SLC4 family members
Nine Slc4-family members with more-or-less well-established functions, all are integral membrane proteins that carry bicarbonate () and/or carbonate (), in addition to at least one monoatomic ion (typically Na+ and/or Cl−), across the plasma membrane. The transporters have at least three other structural or functional similarities.
Table 2 summarizes the % identity of the deduced amino-acid sequences among the family members. The eight family members whose physiological function is more-or-less well defined fall into three major phylogenetic subfamilies that correlate reasonably well with function:
Table 2. Percent identity among SLC4 family members*.
| SLC4A1 | SLC4A2 | SLC4A3 | SLC4A4 | SLC4A5 | SLC4A7 | SLC4A8 | SLC4A9 | SLC4A10 | SLC4A11 | |
|---|---|---|---|---|---|---|---|---|---|---|
| AE1 | AE2 | AE3 | NBCe1 | NBCe2 | NBCn1 | NDCBE | AE4 | NBCn2 | BTR1 | |
| AE1 | 55% | 53% | 34% | 29% | 33% | 30% | 29% | 32% | 19% | |
| AE2 | 56% | 34% | 28% | 30% | 34% | 34% | 33% | 19% | ||
| AE3 | 34% | 30% | 31% | 33% | 33% | 33% | 20% | |||
| NBCe1 | 53% | 50% | 50% | 50% | 50% | 20% | ||||
| NBCe2 | 39% | 39% | 39% | 41% | 14% | |||||
| NBCn1 | 72% | 40% | 71% | 19% | ||||||
| NDCBE | 42% | 76% | 20% | |||||||
| AE4 | 42% | 19% | ||||||||
| NBCn2 | 20% | |||||||||
| BTR1 |
Numbers represent “% identity” of the deduced amino-acid sequences of representative, human splice variants. The comparisons were made using LaserGene’s DNAStar software and Clustal W with gap inclusions.
The Cl-HCO3 exchangers AE1 – 3, which are about 53 – 56% identical to one another at the amino-acid level.
The electrogenic Na/HCO3 cotransporters NBCe1 and NBCe2, which are about 53% identical to each other, and about 28 – 34% identical to the AEs.
The electroneutral Na+-coupled transporters NBCn1, NDCBE, and NBCn2, which are about 71 – 76% identical to each other, about 30 – 34% identical to the AEs, and about 39 – 50% identical to the electrogenic NBCs.
The other two Slc4-family members, whose function is inconclusive (AE4 and BTR1/SLC4A11), are less closely related phylogenetically to any of the others, and themselves.
Most striking is similarity of the hydropathy analysis and, presumably, membrane topology. Figure 2A is a general topological model SLC4 proteins based on hydropathy of NBCe1 and a AE1 model proposed by Zhu and Casey (Zhu et al., 2003). Like all members of the SLC4 family, NBCe1 has a long N-terminal hydrophilic domain and a much shorter C-terminal hydrophilic domain, both of which are intracellular. The N-terminal domain of AE1 is a dimer by X-ray crystallographic analysis (Zhang et al., 2000), consistent with the conventional view that AE1 itself, and perhaps each SLC4 family member, exists in a dimeric state. 10 – 14 transmembrane (TM) segments separate the hydrophilic N and C termini. Most authors agree on the first six TM assignments. Differing experimental approaches, however, have yielded conflicting results for the last 4 – 8 TMs, perhaps because these TMs are more flexible and/or more easily shifted into unnatural conformations. In addition to 13 -helical TMs, the model shown in Figure 2A includes two re-entrant loops, one between TMs #9 and #10, and one just after TM #11. This segment also includes an extended structure that crosses from the extra- to the intracellular fluid just before TM #13. A cautionary note: One should regard the topology model in Figure 2A, as well as any other such model, as one of several educated guesses. In the case of the SLC4 family, other authors have proposed simpler models that include 10 to 14 TMs (Abuladze et al., 2005; Tatishchev et al., 2003; Zhu et al., 2009; Zhu et al., 2010). The value of these models is that they help us to envision relationships among parts of the molecule, and challenge us to design experiments to test the models.
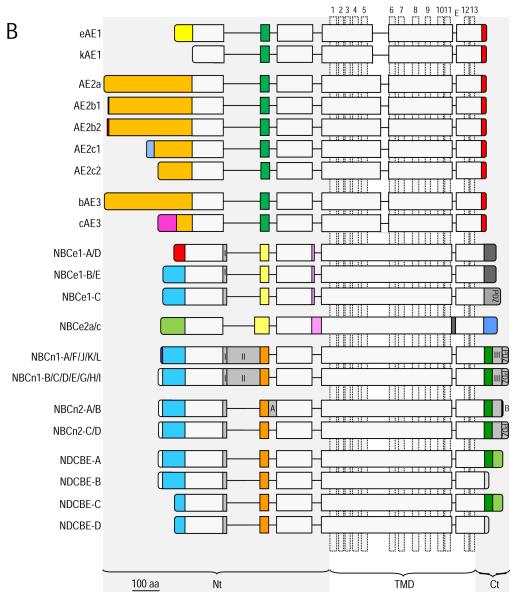
Figure 2. Structural and molecular cassette analysis.
(A) Topology model of SLC4 proteins based on NBC hydropathies and the model of AE1 proposed by Zhu et al (Zhu et al., 2003). (B) Diagrammatic representation of Slc4 splice variants. Each protein sequence is represented as a horizontal bar. Vertically, identical colors denotes protein sequence that is well conserved among Slc4s. Lettered/numbered dark blue regions are splice cassettes that are present or absent in certain variants. NBCe1-A is identical to NBCe1-D, except that NBCe1-D lacks cassette I. NBCe1-B is identical to NBCe1-E, except that NBCe1-E lacks cassette I. The splicing of NBCn1 is complex. NBCn1-A,F,J,K, and L begin with an exon that encodes proteins sequence ‘MERF’ (purple box in NBCn1 rows), NBCn1-B,C,D,E,G, and I begin with an exon that encodes protein sequence that begins ‘MEAD” (white box in NBCn1 rows). NBCn1-A includes cassettes I and II only. NBCn1-F includes cassette I only. NBCn1-J includes cassettes I and III only. NBCn1-K includes cassette II only. NBCn1-L includes cassette III only. NBCn1-B includes cassettes I, and II only. NBCn1-C includes cassettes II, and III only. NBCn1-D includes all three cassettes. NBCn1-E includes cassette I only. NBCn1-G includes cassettes I and III only. NBCn1-H includes only cassette II. NBCn1-I includes cassettes II and III only. Presumably NBCn1 variants yet to be described include the remaining combinations of these three cassettes. NBCn2-A is identical to NBCn2-B, except that NBCn2-A lacks cassette I. NBCn2-C is identical to NBCn2-D, except that NBCn2-C lacks cassette I
A second similarity among some, though not all, members of the SLC4 family is inhibition by disulfonic stilbene derivatives such as DIDS. At least for AE1 and NBCe1, this drug interacts with residues near the extracellular end of TM #5 and perhaps elsewhere (see Figure 2A).
A third similarity among SLC4 family members is glycosylation. Four of the SLC4 family members are known to be N-glycosylated: AE1 is N-glycosylated on only its fourth extracellular loop, whereas AE2, AE3, and NBCe1 are all N-glycosylated on only their third extracellular loops. The rest of the SLC4 family members have at least two consensus N-glycosylation sites on the third, but none on the fourth extracellular loop.
1.2. Differences among the SLC4 family members
While there are clear SLC4 similarities, SLC4 subgroup members differ from one another in important ways. That is, functional (physiological) characteristics distinguish the transporters. The most striking distinguishing property among the SLC4 members is the very nature of transport activity. For example, AE1, AE2 and AE3 exchange monovalent anions from opposite sides of the membrane. The Na/HCO3 cotransporters move Na+ and a -related species from one side of the membrane to the other. Finally, the Na+-driven Cl-HCO3 exchanger NDCBE appears to be a hybrid cotransporter/exchanger that cotransports Na+ and two (or one Na+ plus one , or one ion pair) into the cell in exchange for a single Cl−. Understanding how such closely related proteins can mediate such fundamentally different functions is one of the major challenges, and one of the major opportunities, in transporter research.
A second major distinguishing factor among SLC4 family members is whether, in addition to and/or , the transporter carries an anion or a cation. For some family members, the additional ion is Cl− (i.e., AE1, AE2, and AE3), for some it is Na+ (e.g., NBCe1, NBCe2, NBCn1), and for two, it is both Cl− and Na+ (NBCn2 and NDCBE). However, in the case of human NBCn2, Cl− transport is not net but rather futile Cl-Cl exchange (Parker et al., 2008b).
A third major distinguishing factor is whether or not the transporter is electrogenic. At least two of the members of the SLC4 family are electrogenic (i.e., NBCe1 and NBCe2); that is, one complete cycle of transport activity results in the movement of one or two net negative charges across the membrane, carrying electrical current and causing a shift in membrane potential (Vm). The other six SLC4 family members that have been more-or-less well characterized are electroneutral; that is, a complete transport cycle results in no net movement of electrical charge (i.e., current) across the membrane, and thus no change in Vm.
A fourth distinguishing characteristic among SLC4 family is the third extracellular loop. A characteristic of the Na+-coupled transporters is that, compared to AE1 – 3, their third extracellular loops (i.e., between putative TM #5 and TM #6) are unusually long, consistently the longest TM-to-TM loop in the protein. Moreover, these long third extracellular loops have four highly conserved cysteine residues (see Figure 2A). Among AE1 – 3, only AE2 has even a single cysteine residue in its third extracellular loop. It is notable that AE4, whose function is still controversial, has the four conserved cysteine residues in its third extracellular loop. BTR1, apparently transports borate rather than bicarbonate, has no cysteines in the third extracellular loop.
1.3. Physiological roles of the SLC4 family members
In mammals, the SLC4 membrane proteins are critical for several physiological processes, including the carriage of carbon dioxide (CO2) from the systemic capillaries to the pulmonary capillaries, the secretion or resorption of acid-base equivalents by numerous epithelia (e.g., NaHCO3 reabsorption by the kidney, HCl secretion by the stomach, NaHCO3 secretion by the pancreas and duodenum), NaCl reabsorption by certain epithelia (e.g., ileum, proximal colon), the regulation of cell volume in multiple cell types, and the regulation of intracellular pH (pHi) in nearly every cell of the body. The physiological manifestations are illustrated in both knockout mice as well as the reported human diseases. Table 3 summarizes the human disorders associated with SLC4-sequence variations and mouse Slc4-knockout phenotypes reported in the past decade. While there are clear commonalities, this Table makes it clear that the human and mouse diseases, associated with mutations or defects in these various Slc4 genes, are not identical.
Table 3. Human and Mouse Slc4 phenotypes.
(−) Primary or secondary to gene dysfunction (+) Exacerbated by gene hyperfunction
(SNP) Genetically associated with polymorphism, but direction of effect upon gene function unknown
(GD) Genetically associated with deletions and/or translocations at the gene locus, but direction of effect upon gene function unknown
1.4. Focus of this review
In this review, we focus almost exclusively on vertebrate, especially human, members of the bicarbonate-transporter family. However, it is possible to trace SLC4-related genes to invertebrates (Romero et al., 2000; Virkki et al., 2003), yeast (Zhao and Reithmeier, 2001) and plants (Frommer and von Wiren, 2002; Takano et al., 2002). Given the rich variety of SLC4 transport activity in vertebrates (i.e., exchange vs cotransport vs exchange/cotransport hybrids), we should not be surprised if the SLC4-related gene products from these other life forms are unique either in terms of mechanism or ions transported. Indeed, a plant SLC4 homologue that has been dubbed BOR1 (Takano et al., 2002) mediates the transport of boron (the element), perhaps as borate. As detailed for each SLC4 transporter, many groups have localized mRNA and individual proteins. A few years ago, Damkier and coworkers performed a survey of Na+ coupled Slc4 proteins (Slc4a4, a5, a7, a8, a10 and a11) in mice (Damkier et al., 2007), yet more detailed localizations of many tissues are needed and some are underway.
2. The Cl-HCO3 (anion) exchangers
The three identified anion exchangers (AE1, AE2 and AE3) mediate the electroneutral exchange of one monovalent anion for another across the plasma membrane. The preferred substrates are and Cl− (as well as all other halides), although the AEs can also transport OH−, and AE1 can cotransport plus H+ in exchange for Cl−, but at a very low rate compared to monovalent anion exchange (Jennings, 1976). In living cells, the physiologically relevant transport activity is the exchange of Cl− for , and the transmembrane chemical gradients for these two ions determine the direction of net transport. For most cell types, the inward Cl− chemical gradient dominates, driving the exchange of extracellular Cl− for intracellular . As we shall see, the direction of net Cl-HCO3 exchange in erythrocytes depends on whether the cells are in the systemic or pulmonary capillaries. The disulfonic stilbenes SITS and DIDS block the transport activity of all three AEs.
For a recent summary of the AE literature, refer to the excellent review by Alper et al (Alper, 2009, 2010). Additionally, molecular cassettes of Na+ coupled SLC4 proteins were outlined by Boron and Parker (Boron et al., 2009)
2.1. AE1 (SLC4A1)
AE1, the Cl-HCO3 exchanger of erythrocytes, was one of the first transporters of any sort to be physiologically identified. Among the members of the SLC4 family, AE1 was also the first to be cloned. It is also the family member most intensively studied, and about which the most is known. AE1, “Band 3” due to its position in SDS-polyacrylamide gel electrophoresis of erythrocyte membrane proteins, is the most abundant membrane protein in red blood cells, accounting for about a quarter of all membrane protein. Kopito and Lodish in 1985 reported the cloning of murine AE1(Kopito and Lodish, 1985), paving the way to the cloning other species AE1 as well as AE2 and AE3. Human AE1 consists of 911 amino acids. According to the model of Zhang & Low (Zhang et al., 2000), the cytoplasmic N-terminal domain of human AE1 is 404 amino acids long and serves as an anchorage site for several proteins, including components of the cytoskeleton, some glycolytic enzymes, and hemoglobin.
The membrane-spanning domain of human AE1 consists of ~467amino acids (Zhu et al., 2003), and is responsible for the anion-exchange function of the protein. The final 40 aa of human AE1, the cytoplasmic C-terminal portion contains an anchorage site for GAPDH (PMID: 20980406) and a proposed binding site for cytoplasmic carbonic anhydrase II (CA II) (Vince and Reithmeier, 1998). However, a subsequent study concluded that the binding requires GST and is unlikely to be physiological (Piermarini et al., 2007).
In erythrocytes, AE1 plays a key role in CO2 carriage from the systemic tissues to the lungs. CO2 that is generated by metabolism moves from the mitochondria to the blood plasma (Figure 3), this CO2 enters the erythrocyte. There, CAII binds CO2 plus H2O converting them into and H+. AE1 disposes of the newly generated in exchange for Cl− (the so-called “chloride” or “Hamburger” shift), while de-oxygenated hemoglobin (which has a higher H+ affinity than fully oxygenated hemoglobin) buffers the H+. These two concurrent processes allow additional CO2 uptake by the erythrocyte. This entire process is called the Jacobs-Stewart cycle. In the lungs, this cycle is reversed, releasing CO2 into the blood plasma for migration into the alveoli and release from the body by ventilation. Erythrocytes also express high levels of the aquaporin 1 water channel (Preston and Agre, 1991), which is also permeable to CO2(Cooper and Boron, 1998; Endeward et al., 2006; Musa-Aziz et al., 2009; Nakhoul et al., 1998).
Figure 3. Role of AE1 in the uptake of CO2 erythrocytes in systemic capillaries.
CA II is carbonic anhydrase II, a soluble enzyme that also can bind to the C terminus of AE1. Hb is hemoglobin, Hb-O2 is hemoglobin fully saturated with four O2 molecules, and Hb–H+ is protonated hemoglobin that binds three O2 molecules. CO2 may, in part, enter the cell via the water channel AQP1. In the pulmonary capillaries, all of the reactions reverse, releasing CO2 into the blood plasma.
The cells with the next, highest AE1 expression are the renal -intercalated (or A) cells of the collecting duct. These cells have AE1 in the basolateral membrane (van Adelsberg et al., 1993), playing a key role in the reabsorption from the tubule lumen to the blood. An alternate promoter site produces a unique renal transcript results in an N-terminal variant (kAE1) that lacks the first 65 amino acids in humans (Brosius et al., 1989; Sahr et al., 1994) compared to the erythroid transcript (eAE1). AE1 mRNA is also expressed at lower levels in heart (Sabolic et al., 1997) and colon (Papageorgiou et al., 2001).
Naturally occurring mutations of AE1 can cause human disease affecting the function of erythrocytes and/or intercalated cells of the distal nephron (Alper, 2002, 2009, 2010; Shayakul and Alper, 2000). The deletion 400–408 (encompassing the end of the cytoplasmic N terminus and beginning of TM #1) produces a transporter that, by itself, is nonfunctional. However, the heterodimer consisting of the mutant and wild-type transporters is functional. Moreover, the presence of this mutant AE1 polypeptide increases the rigidity of the erythrocyte membrane and may confer protection against cerebral malaria. The erythrocytes have an altered shape (Southeast Asian ovalocytosis, SAO), but exhibit no clinical pathology.
Mutations throughout the AE1 protein can lead to a form of autosomal-dominant hereditary spherocytosis (HS) that is characterized by increased erythrocyte fragility and thus a hemolytic anemia. However, HS is not typically associated with a renal defect. Curiously, several mutations in the membrane-spanning domain of AE1, several mutations at R589 in TM #6 (Bruce et al., 1997; Jarolim et al., 1998; Karet et al., 1998), S613F in TM #7(Bruce et al., 1997), V850(Bruce et al., 2000), and an 11–amino-acid deletion in the cytoplasmic C terminus (Karet et al., 1998)— produce an autosomal-dominant distal renal tubular acidosis (dRTA) with erythrocyte pathology. Heterologously AE1 expression in Xenopus oocytes reveals that only the dominant mutants have transport consequences, and then only moderate. Expression of recessive AE1-dRTA- mutants in Xenopus oocytes indicate that the renal pathology is due to inappropriate trafficking to the intercalated cell plasma membrane. Interestingly, heterologous co-expression of glycophorin A (an erythroid-restricted, AE1-binding, single span, membrane protein), rescues the surface delivery of recessive mutant AE1 polypeptides in oocytes (Tanphaichitr et al., 1998; Young et al., 2000). Presumably native glycophorin A replicates this in erythrocytes, resulting in a lack of erythroid pathology in recessive dRTA.
All AEs are functionally blocked by the stilbene derivatives SITS and DIDS (Barzilay et al., 1979; Cabantchik and Greger, 1992; Cabantchik and Rothstein, 1972), both of which have two negatively charged sulfonate groups. In addition, SITS has one isothiocyano group capable of covalent interaction with free amines; DIDS has two such groups. SITS interacts with AE1 in two steps: (1) a rapid, reversible electrostatic ionic interaction, and (2) a slower, yet irreversible covalent reaction with an AE1 lysine. In human AE1, Lys 539 (K539, extracellular end of TM #5 in Figure 2A) (Bartel et al., 1989; Landolt-Marticorena et al., 1995; Okubo et al., 1994; Schopfer and Salhany, 1995; Wood et al., 1992) is the covalent reaction site. Based on AE homologies, Kopito (Kopito et al., 1989) proposed a potential consensus DIDS-reaction motif at the putative extracellular end of TM #5: KLXK (where the first K is K539 in human AE1, and X is I or Y).
2.2. AE2 (SLC4A2)
An AE2 fragment was initially cloned by Demuth et al from human kidney and lymphoma cells, and described as a non-erythroid band 3 (Demuth et al., 1986). Alper et al later cloned a full-length mouse ortholog by low-stringency probe strategy with a mouse-kidney library (Alper et al., 1988).
The three AEs are most similar in their membrane-spanning domains; they diverge most markedly in the cytoplasmic N termini. Human and mouse AE2 have three alternative promoters (a, b, and c) that yield a total of five splice variants in mouse: a, b1, b2, c1, and c2 (it is not clear whether humans have distinct c1 and c2 variants) (see Figure 2B). The physiological significance of these variants remains under study. All AE2 variants have cytoplasmic N termini that are longer than that of AE1 as well as other SLC4 proteins. For example, the longer cytoplasmic N terminus of human AE2a is almost entirely responsible for the substantially greater overall length of AE2a (1240 residues) compared to human erythrocyte AE1 (911 residues).
AE2 is the most widely distributed AE, being expressed at the basolateral membranes of most epithelial cells. Expression levels are especially high in gastric parietal cells (Stuart-Tilley et al., 1994), choroid-plexus epithelial cells (Alper et al., 1994), surface enterocytes in colon (Alper et al., 1999), and renal collecting duct (Alper et al., 1997; Stuart-Tilley et al., 1998). In gastric parietal cells, AE2 presumably plays a key role in H+ secretion, exporting into the blood at least some of the that balances the H+ pumped into the lumen of the gastric gland. AE2 may contribute to H+ secretion by the TAL. When arranged in parallel with a Na-H exchanger, AE2 contributes to net NaCl uptake and, in an epithelial cell such as in the choroid plexus, may contribute to the transepithelial movement of Na+ and Cl−. It is reasonable to postulate that, in most cells in which it is expressed, AE2 contributes to the regulation of pHi (by exporting in response to intracellular alkali loads) and/or the regulation of cell volume (by contributing to volume-regulatory increases in cell volume by taking up Cl−). Indeed, the activity of AE2— heterologously expressed in Xenopus oocytes and assessed as 36Cl efflux—is stimulated by (1) increases in either intracellular or extracellular pH (Stewart et al., 2002); (2) hypertonicity, a response that requires the pHi produced by the shrinkage-induced activation of the native Xenopus Na-H exchanger (Humphreys et al., 1995); and (3) (Humphreys et al., 1997), the application of which paradoxically causes oocyte pHi to fall. All of these responses can be modified or inactivated by engineered mutations in the highly conserved “WRETARWIKFEE” motif (Romero et al., 1997) in the cytoplasmic N terminus (Chernova et al., 2003; Stewart et al., 2002).
2.3. AE3 (SLC4A3)
AE3 was first cloned by Kopito et al in 1989 (Kopito et al., 1989). Human AE3 has two alternative promoters that yield two splice variants, the cardiac form (cAE3) and the brain form (bAE3) (Figure 2B). These naming conventions are of historical significance only, both splice variants may be present in heart, brain and elsewhere. We already noted that the three AEs are most similar in their membrane-spanning domains. The cytoplasmic N terminus of human cAE3, like that of AE2c, is somewhat longer than that of AE1 (1034 total residues in human cAE3 vs 911 in eAE1). The cytoplasmic N terminus of human bAE3, like that of AE2a and AE2b, is substantially longer (1232 total residues for human bAE3).
AE3 is found mostly in excitable tissues: brain (Kopito et al., 1989; Kudrycki et al., 1990), retina (Kobayashi et al., 1994), heart (Linn et al., 1992; Yannoukakos et al., 1994) and smooth muscle (Brosius et al., 1997). However, AE3 variants are also expressed in epithelial cells, including those of the kidney and GI tract. The tissue-distribution data is mainly the result of northern blots and RT-PCR, and the relative distribution of AE3 splice variants as determined by these techniques varies with developmental stage and among species.
Like AE2, AE3 is stimulated by increases in pHi. Thus, it is reasonable to postulate that AE3 contributes to the regulation of pHi by exporting in response to intracellular alkali loads. The substitution polymorphism Ala867Asp, which occurs in the extracellular loop between TM #5 and TM #6, has been suggested to confer a small degree of susceptibility to idiopathic generalized epilepsy (Sander et al., 2002) (Table 3).
3. The Electrogenic Na/HCO3 cotransporters
The two identified electrogenic Na/HCO3 cotransporters (NBCe1 and NBCe2) mediate the movement in the same direction of one Na+ and what appears to be either two or three . As a result, one or two negative charges cross the plasma membrane with each Na+, i.e., Na+ movement appears as an anion. In most cases, the electrogenic NBCs appear to move the equivalent of one Na+ and two ions in one complete cycle of transport activity. Given such a 1:2 stoichiometry, as well as typical values of Vm and ion gradients for Na+ and , thermodynamics predicts that NBCe1 and NBCe2 should move Na+ and into the cell, thereby raising [Na+]i and pHi as well as shifting Vm to more negative values. A notable exception is the basolateral membrane of the renal proximal tubule, where NBCe1 appears to have a 1:3 stoichiometry. As predicted by thermodynamics, renal NBCe1 mediates the net movement of Na+, , and net negative charge out of the cell.
For the Na+-coupled transporters, the two electrogenic NBCs and the three electroneutral transporters that we will discuss below, a fundamental uncertainty exists concerning the nature of the -related ion that the protein carries. For example, the electrogenic NBCs with a 1:2 stoichiometry might carry one Na+ and two ions, one Na+ and one , or a single ion pair . Preliminary data suggest that, for both NBCe1 and NBCe2, the -related substrate is either or (Grichtchenko and Boron, 2002; Lee et al., 2011a; Lee et al., 2012).
The disulfonic stilbenes SITS and/or DIDS block the transport activities of both NBCe1 (operating in both 1:2 and 1:3 stoichiometries) and NBCe2. Nevertheless, these compounds are not specific for blockade of NBCe1, NBCe2 or any Na+-coupled transporter. Thus, care must be taken when using these stilbenes in any system other than cellular overexpression of a transporter.
3.1. NBCe1 (SLC4A4)
In 1983 while working on the salamander renal proximal tubule, Boron and Boulpaep described the first Na/HCO3 cotransporter (Boron and Boulpaep, 1983), the electrogenic Na/HCO3 cotransporter. In 1997, Romero et al used an expression-cloning approach to obtain the first cDNA that encodes a Na+-coupled transporter, the renal electrogenic Na/HCO3 cotransporter (Romero et al., 1997). They named this clone NBC for Na/Bicarbonate Cotransporter. We shall refer to this clone as NBCe1-A— ‘e’ for electrogenic, ‘1’ because it represents the first of two genes encoding electrogenic NBCs, and ‘A’ for the first known splice variant of that gene. Subsequent work led to the cloning of NBCe1-A homologues in human (Burnham et al., 1997) and rat (Romero et al., 1998). The renal isoform arises from an alternate promoter in intron 3 (Abuladze et al., 2000). Two other major NBCe1 splice variants are known (Figure 2B). In NBCe1-B, which was first cloned from pancreas (Abuladze et al., 1998) and heart (Choi et al., 1999) and is the most widespread splice variant, arising from the dominant promoter in exon 1 (Abuladze et al., 2000). For NBCe1-B, the N-terminal 85 amino acids of NBCe1-B replace the N-terminal 41 amino acids of NBCe1-A. In NBCe1-C, which is found almost exclusively in the brain (Bevensee et al., 2000), a 97-bp deletion near the C terminus causes a unique 61 C-terminal amino acids to replace the 46 C-terminal amino acids in NBCe1-B. All three NBCe1 splice variants have two motifs similar to the one in AE1 that binds CA II, and data suggest that the common C terminus of NBCe1-A/B can bind CA II in vitro(Davis et al., 2002; Gross et al., 2002; Lu et al., 2006).Two minor isoforms (NBCe1-D and -E) have been found to delete a 27bp segment within the cytosolic Nt (Liu et al., 2011) (see Figure 2B for gene cassettes).
Protein-localization studies have shown that NBCe1 is present in the basolateral membranes of renal proximal tubule (Schmitt et al., 1999), pancreatic ducts (Marino et al., 1999), and epididymis (Jensen et al., 1999), as well as in astrocytes and neurons in several regions of the brain (Schmitt et al., 2000), several tissues within the eye (Bok et al., 2001), and blood vessels and intercalated disks within the heart (Williams et al., 2003).
In the kidney, NBCe1-A mediates the movement of equivalents from the proximal-tubule cell to the blood, thereby completing the reabsorption of from lumen to blood (Figure 4). In the pancreas, NBCe1-B plays a major role in the accumulation of intracellular (Ishiguro et al., 1996); the basolateral Na-H exchanger assists in this task by extruding H+ across the basolateral membrane and driving the cytoplasmic CO2/ equilibrium toward the formation of . The accumulation of cytoplasmic is the first step in the secretion of from the blood to the lumen of the exocrine ducts (Figure 5). Curiously, immuno-studies of NBCe1-B reveals both basolateral and apical localization in pancreatic epithelia (Marino et al., 1999; Satoh et al., 2003; Thévenod et al., 1999). In non-epithelial cells, NBCe1-B and NBCe1-C contribute to pHi regulation by moving alkali into cells in response to intracellular acid loads.
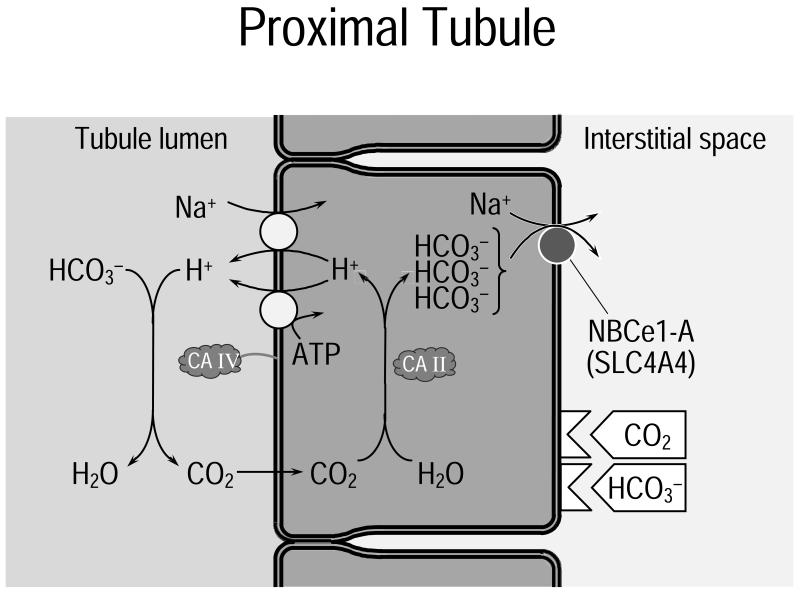
Figure 4. Role of NBCe1-A in reabsorption by the renal proximal tubule.
Two acid extruders in the apical membrane, the Na-H exchanger NHE3 and a vacuolar-type proton pump, transfer H+ to the tubule lumen. There, the GPI-linked enzyme carbonic anhydrase IV (CA IV) uses the H+ to titrate the filtered to CO2 and H2O. These two products enter the cell across the apical membrane; the water channel AQP1 assists in the uptake of H2O and may also play a role in CO2 uptake. Inside the cell, CA II converts CO2 and H2O to H+ (which is extruded into the lumen) and . NBCe1-A, operating with a Na+: stoichiometry of 1:3, exports into the interstitial space. The eventually diffuses into the blood. A CO2-sensing mechanism responds to high levels of [CO2] by increasing the rate of reabsorption.
Figure 5. Role of NBCe1-B in secretion by pancreatic duct.
NBCe1-B at the basolateral membrane appears to operate with a stoichiometry of 1:2, leading to the net uptake of . A basolateral Na-H exchanger (NHE1) contributes to the accumulation of intracellular , in conjunction with carbonic anhydrase II (CA II). At the apical membrane, members of the SLC26a family, which electrogenically exchange Cl− for with different stoichiometries (indicated by ‘n’ and ‘m’ in the figure), export into the lumen (Ko et al., 2002b). Apical CFTR recycles the Cl−. During baseline periods, apical NBCn1 may keep luminal [] relatively low.
An intriguing and still unsettled question is how the Na+: stoichiometry of NBCe1 apparently shifts from 1:2 to 1:3. Measuring transporter currents, intracellular pH and Na+, NBCe1A expression in Xenopus oocytes revealed a 1:2 rather than the 1:3 expected stoichiometry (Sciortino and Romero, 1999). Muller-Berger et al computed the Na+: stoichiometry from reversal potentials and slope conductances measured using inside-out giant patches from Xenopus oocytes heterologously expressing NBCe1-A (Muller-Berger et al., 2001). They found that the stoichiometry was 1:2 when the cytosol-side [Ca2+] was 100 nM or less, but was usually 1:3 when the cytosol-side [Ca2+] was 500 nM. Gross and colleagues have expressed NBCe1-A and NBCe1-B in various epithelial cell lines, mounted confluent monolayers in an Ussing chamber, permeabilized the apical membranes with Amphotericin B, obtained current-voltage (I-V) plots in the presence and absence of the reversible DIDS analog 4,4′-dinitro-2,2′-disulfonate (DNDS), and then computed the Na+: stoichiometry from the reversal potential of the difference current. They found that, when expressed in a mouse renal-proximal-tubule cell line, both NBCe1-A or NBCe1-B had stoichiometries of 1:3, whereas when expressed in a renal-collecting-duct cell line, both had stoichiometries of 1:2 (Gross et al., 2001a). Moreover, it appears that, when NBCe1-A is expressed in a proximal-tubule cell line, phosphorylating Ser-982 (in the second of two putative CA-II binding sites in the cytoplasmic C terminus; see Figure 2B) shifts the stoichiometry from 1:3 to 1:2 (Gross et al., 2001b). Finally, two of the aspartate residues near Ser-982 appear to be necessary for the phosphorylation-induced shift in stoichiometry (Gross et al., 2002). The simplest explanation for the results of Gross et al is that the proximal tubule contains unique components required for a 1:3 stoichiometry. A concern with these experiments is that DNDS produces relatively small changes against rather large background currents (Gross et al., 2001a).
3.1.1. Regulation
A further complication in the stoichiometry models, is that NBCe1-A vs. NBCe1-B/-C activities and regulation are distinct. NBCe1-A is high activity whereas, the B/C isoforms display ~20-30% NBCe1-A activity (McAlear et al., 2006), apparently due to the N-terminal variations resulting in an autoinhibitory domain in the different 85 aa N-terminus. IRBIT (inositol 1,4,5-trisphosphate receptor-binding protein) was shown to increase the NBCe1-B and –C activity but not that of NBCe1-A (Lee et al., 2012; Shirakabe et al., 2006; Yang et al., 2011; Yang et al., 2009). Subsequently, it was hypothesized that IRBIT regulation of NBCe1-C function is mediated by relieving inhibition of an auto inhibitory domain in the B-isoform N-terminus (Lee et al., 2012; Parker et al., 2007; Seki et al., 2008). Muallem’s group working in the pancreas system have data indicating that IRBIT increases membrane surface expression of NBCe1-B by antagonizing WNK/SPAK signaling which otherwise reduces NBCe1-B surface expression (Yang et al., 2011). Thus, increasing IRBIT in pancreatic ductal cells would lead to stimulation of transductal secretion (Yang et al., 2009). Thus, IRBIT interaction with NBCe1-B seems to have the dual effect of stimulating intrinsic NBCe1-B activity as well as antagonizing WNK/SPAK (Lee et al., 2012). Conversely, NBCe1-A is high in activity, not regulated by IRBIT, but can be potentiated 50-80% by PIP2 (Wu et al., 2009). This PIP2 effect is immediate and reversible by spermine suggesting a direct activation rather than surface protein changes. These isoforms and functional N-terminal differences seem to have occurred during teleost development because neither zebrafish (Lee et al., 2011b; Sussman et al., 2009) nor mefugu (Chang et al., 2012) seem to encode a true NBCe1-A (intronic promoter). Nevertheless, activation of NBCe1-B function by IRBIT can be demonstrated in these more simple organisms (Wang et al., 2012). Combined, these data imply that ability to control NBCe1 activity (as opposed to stoichiometry) arose with growing mammalian, systemic complexity, perhaps arising due to chronic systemic buffering needs in contrast to momemt to moment needs of the gut and other tissues.
Like AE1-3 (see discussion of AE1, above), NBCe1 is blocked by the stilbene derivative DIDS (Romero et al., 1997). Moreover, at the putative end of TM #5, NBCe1 has a sequence (KMIK) that is very similar to the consensus DIDS-reaction motif for the AEs (KLXK, X = I or Y). Thus, the actual DIDS-reaction motif might be KXXK, where X is any of several hydrophobic residues. Work on human NBCe1-A suggests that KXXK plays a critical role in reversible DIDS blockade (Lu and Boron, 2007). A lysine-to-asparagine mutation at either position increases both the apparent DIDS binding constant and DIDS off-rate; mutating both lysines produces the greatest effects. Used in the Xenopus oocyte system, tenidap is able to completely block NBCe1-mediate Na+ and dependent currents (Ducoudret et al., 2001; Lu and Boron, 2007; Sussman et al., 2009).
Investigators have reported twelve naturally occurring mutations of NBCe1, all of which cause a severe and persistent recessive proximal renal tubular acidosis—with the pH of arterial blood being as low as 7.10—and ocular abnormalities such as bilateral glaucoma, bilateral cataracts, band keratopathy, and blindness (Demirci et al., 2006; Dinour et al., 2000; Horita et al., 2005; Igarashi et al., 1999; Igarashi et al., 2003; Igarashi et al., 2000; Inatomi et al., 2004; Lo et al., 2011b; Suzuki et al., 2008; Suzuki et al., 2010a). Two missense mutations target Arg residues that are highly conserved among SLC4 family members (Igarashi et al., 1999), one in middle of the cytoplasmic C terminus (R298S in NBCe1-A—see Figure 2A), and one near the extracellular end of proposed TM #3 (R510H in NBCe1-A, see Figure 2A). Another missense mutation (Dinour et al., 2000) targets a Ser residue near the proposed intracellular end of TM #1 (S427L in NBCe1-A, see Figure 2A). At the residue homologous to S427 in NBCe1-A, NBCe2 also has a Ser; the three AEs and the three electroneutral Na+-coupled transporters (NBCn1, NCBE and NDCBE) all have an Ala. One mutation (Igarashi et al., 2000) is a nonsense mutation (Q29X) in the portion of the cytoplasmic N terminus that is unique to NBCe1-A, and produces the same renal phenotype as the other mutations. Probing of these human mutations has revealed which mutations are involved in the transport cycle vs protein trafficking. R298S (Chang et al., 2008), S427L (Dinour et al., 2004) and A799V (Parker et al., 2012) mutations affect the transport cycle. Other mutations effect protein localization by a variety of mechanisms: Q29X (Igarashi et al., 2001), T485S (Horita et al., 2005; Sciortino, 2001), G486R (Suzuki et al., 2008), R510H (Horita et al., 2005), W516X (Lo et al., 2011b), L522P (Demirci et al., 2006), A799V (Parker et al., 2012), R881C (Toye et al., 2006) and nucleotide-2311X (Inatomi et al., 2004). Interestingly, work with R298S illustrated that a structurally common, N-terminal domain exists for the AE’s and NBC’s (Chang et al., 2008). Finally, human single nucleotide polymorphisms (SNPs) in NBCe1 (E122G, S356Y, K558R, and N640I) have also been evaluated for activity changes (Yamazaki et al., 2011), but only K558R changes (decreases) activity.
3.1.2. Recent Advances
Human NBCe1 mutations are physiologically informative; however, phenotypes observed in nbce1(−/−) mice (Gawenis et al., 2007) reveal additional organ and systemic implications of NBCe1 physiology (Gawenis et al., 2007; Ruminot et al., 2011; Yu et al., 2009). These more severe phenotypes include runting, intestinal blockage, decrease colonic anion secretion, altered dentition, splenomegaly, and altered astrocytic glycolytic response to K+ (Table 3). Interestingly, Lacruz and coworkers found that nbce1(+/−) have a moderate enamel phenotype (Lacruz et al., 2010), implying that allelic variations of SLC4A4 may be partially penetrant.
3.2. NBCe2 (SLC4A5)
The nomenclature of the NBCe2 protein (NBC4; see Table 1) is confusing because four of the reported six variants (named a – f) are likely to be amplification products of incompletely spliced pre-mRNA. We shall refer to the questionable variants by their originally published names: NBC4, followed by a letter (e.g., NBC4a). We shall restrict use of the term NBCe2 for clones known to function, of which there is currently only one, NBCe2-C (equivalent to NBC4c). Eventually the field will have to adopt a standard nomenclature that eliminates reference to the questionable clones.
Pushkin et al reported two variants of an NBC-related clone (Pushkin et al., 2000). Both NBC4a and NBC4b contain a unique putative exon just after the beginning of proposed TM #11. In addition, NBC4b contains a unique 16-bp insert in proposed TM #13 that causes a frameshift and premature stop codon. Pushkin et al deposited two other variants in GenBank, one of which (NBC4c) yields a functional protein. The other is missing two exons, resulting in the elimination of all amino acids between the proposed end of TM #10 and near the proposed beginning of TM #13. Subsequent attempts by Virkki & Boron to obtain the four variants by PCR yielded only NBC4c, consistent with the hypothesis that the others are cloning artifacts (Virkki et al., 2002). Both Virkki et al (Virkki et al., 2002) and Sassani et al (Sassani et al., 2002) have now characterized NBC4c (i.e., NBCe2-C, which consists of 1121 amino acids) as an electrogenic NBC with an apparent stoichiometry of 1:2.
Based on northern analysis of mouse, NBCe2 is expressed most abundantly in liver, testes, and spleen; lower levels are present in heart, kidney, stomach, lung, and brain (Pushkin et al., 2000).
3.2.1. Recent Advances
Hypertension
Barkley et al identified SLC4A5 as a positional candidate for hypertension (Barkley et al., 2004). Subsequently, Hunt et al verified that SLC4A5 polymorphisms are associated with change in baseline blood pressures in a 10-year follow-up (Hunt et al., 2006). Both genetic variations in SLC4A5 and salt intake were found to be risk factors for hypertension (Taylor et al., 2009). In mice, disruption of slc4a5 causes both arterial hypertension as well as metabolic acidosis (distal renal tubular acidosis);(Groger et al., 2012).
Neurologic Impairment
Kao et al also disrupted Slc4a5 but these mice had reduced choroid plexus secretion and reduced ventricle size (Kao et al., 2011). These combined issues changed intracranial pressure, CSF electrolytes. In the eye, Kao also reported severe eye pathologies: photoreceptor loss, ganglion cell loss, and retinal detachment.
4. The Electroneutral Na+-couple HCO–3 transporters
At least three genes encode membrane proteins that mediate the electroneutral movement of Na+, (or a related species) across the plasma membrane. One, NBCn1, normally mediates the uptake of one Na+ and one , but no movement of Cl−. A second, NDCBE, appears to mediate the uptake of one Na+ and two , as well as the coupled exit of one Cl− (i.e., it is a Na-driven Cl-HCO3 exchanger). The third, NBCn2, also known as NCBE, was originally described as a Na-driven Cl-HCO3 exchanger, although for human NBCn2 the Cl efflux that accompanies the influx of Na+ and represents futile cycles of Cl-Cl exchange.
4.1. Nomenclature
As discussed earlier, column 1 in Table 1 summarizes the nomenclature of the SLC4 proteins, which is based on homology and the chronological order of discovery. Column 2 summarizes protein nomenclature, which is based on transport function. The alternate protein names of the electroneutral Na/HCO3 cotransporter proteins, listed in column 3 of Table 1, are extremely confusing because various authors assigned names (sometimes conflicting names) before determining with confidence the key functional attributes of three of the transport proteins. The protein name of an SLC4 family member should be regarded as provisional until the following minimal functional characteristics (or lack thereof) have been determined experimentally: (1) transport (at fixed pHi and pHo), (2) Na+ dependence (i.e., dependent Na+ flux), (3) Cl− dependence (i.e., dependent Cl− flux), and (4) electrogenicity. Regarding the last point, it should be noted that electrogenicity cannot be determined by examining the effect of a presumed or even a known effect of a Vm change on transport activity—thermodynamics cannot predict kinetics! Only measurements of Vm and/or current can provide the necessary evidence for distinguishing electrogenicity from electroneutrality. In summary, before assigning protein name, one must be certain that the transporter has precisely the function associated with one of the following candidate names: (1) a Cl-HCO3 exchanger (AE), (2) an electrogenic Na/HCO3 cotransporter (NBCe), (3) an electroneutral Na+/HCO3 cotransporter (NBCn), or a Na-driven Cl-HCO3 exchanger (NDCBE).
The names “SBC2” and NBC2 (Ishibashi et al., 1998) refer to a cloning artifact that consists mainly of the authentic sequence of the human electroneutral NBC (NBCn1). However, the N-terminal portion is missing the amino acids encoded by the first three exons, and instead begins with the amino acids that would be encoded by an inverted exon 4. Additionally, the C-terminus of NBC2 lacks the 26 amino acids encoded by the final exon. Thus, the name NBC2 is no longer in use.
The authentic cDNA sequence for the human electroneutral NBC (NBCn1) was named NBC3 (Pushkin et al., 1999) before experiments were performed to test its electrogenicity. The name NBC3 (Amlal et al., 1999) was also used simultaneously for a fragment of the authentic sequence for the Na+-driven Cl-HCO3 exchanger (NDCBE). Thus, the name NBC3 is degenerate— referring both to SLC4A7 and SLC4A8.
When Romero et al originally cloned the electrogenic Na/HCO3 cotransporter from salamander (Romero et al., 1997), they proposed that the nomenclature of that clone and future clones should follow function. Mindful of that suggestion, and of the confusion that has surrounded numerical names such as NBC2 and NBC3, we recommend that the electrogenic Na/HCO3 cotransporters be referred to as NBCe1 and NBCe2, that electroneutral Na/HCO3 cotransporter be referred to as NBCn1 and the Na+-driven Cl-HCO3 exchanger should be referred to as NDCBE. Under no circumstances should a Na+-driven Cl-HCO3 exchanger be referred to as an “NBC,” a name that implies lack of exchange activity.
4.2. NBCn1 (SLC4A7)
In 1999, Pushkin et al reported a new NBC-related cDNA, cloned from human skeletal muscle. They concluded that the cDNA encoded a Na/HCO3 cotransporter, which they named NBC3, but did not study the electrophysiology of the transporter (Pushkin et al., 1999). Later, Choi et al cloned from rat aorta three cDNAs (variants B, C and D) that are homologous to NBC3 (Choi et al., 2000) and pointed out that the C terminus has a putative PDZ-binding domain. Using microelectrodes to monitor pHi and Vm in oocytes injected with cRNA, they demonstrated that the B variant encodes a Na/HCO3 cotransporter that is electroneutral. They named the clone NBCn1-B. The rat clone is poorly sensitive to DIDS and completely insensitive to EIPA, as recently confirmed by Park et al, who expressed human NBCn1 in HEK cells (Park et al., 2002).
Using a two-electrode voltage clamp to study current-voltage relationships in oocytes expressing NBCn1, Choi et al demonstrated two NBCn1-dependent currents, one of which is a -independent Na+ current that is paradoxically stimulated by DIDS. It is not yet clear whether one or both currents represent novel oocyte channels or “slippage” of the electroneutral transporter. The NBCn1 gene has at least three cassettes that may (or may not) be present at two different positions in the mRNA: (1) a 13-residue ‘A’ or ‘I’ cassette in the cytoplasmic N-terminal region, (2) a 124-residue cassette ‘II’ in the cytoplasmic N-terminal region, and (3) a 36-residue ‘B’ or ‘III’ cassette in the cytoplasmic C-terminal region. Figure 2B summarizes which cassettes are present in which splice variants.
The molecular basis for the poor sensitivity of NBCn1 to DIDS is apparently the lack of an intact DIDS motif (KXXK) at the extracellular end of putative TM #5 (see discussion of NBCe1). In NBCn1, the sequence is KLFH. Preliminary work indicates that mutating the histidine to lysine (KLFK) renders the transporter DIDS sensitive (Choi et al., 2001). The NBCn1 C-terminus concludes with a PDZ binding motif.
In human northern blots probed with a 95-bp oligonucleotide directed only to sequence that encodes cassette II (Figure 2B), NBCn1 expression is apparent only in heart and skeletal muscle. In rat northern blots probed with a 836-bp oligonucleotide (which corresponds to part of the extracellular loop between TM #3 and TM #4, extending to about halfway through TM #9), high levels of NBCn1 mRNA are apparent in spleen and testis, and lower levels in heart, brain, lung, liver and kidney but not in skeletal muscle (Choi et al., 2000).
In the kidney, NBCn1 is in the basolateral membrane of the thick ascending limb of Henle’s loop (Kwon et al., 2002), and inner medullary collecting ducts (Praetorius et al., 2004). The protein abundance is stimulated by acidosis (Kwon et al., 2002), apparently to facilitate net epithelial ammonium transport (Lee et al., 2010).
An NBCn1 knockout mouse was recently created by Bok et al (Bok et al., 2003). The mice develop blindness and auditory impairment as the result of the degeneration of sensory receptors.
4.2.1. Recent Advances
Blood pressure traits
A genome-wide association study links an SNP in SLC4A7 with elevated systolic and diastolic blood pressure in individuals of European and African ancestry (Ehret et al., 2011). Furthermore, NBCn1-null mice are mildly hypertensive at rest (Boedtkjer et al., 2011). NBCn1 is a major pH regulator in vascular smooth muscle cells and is therefore in a position to contribute to the enhanced myogenic tone and the ability to maintain contractile ability during agonist exposure that is observed in mesenteric arteries in the presence vs the absence of CO2/ (Boedtkjer et al., 2006).
Osteoclast survival and function
NBCn1 in the ruffled membrane of osteoclasts is suggested to reabsorb the liberated from the hydroxyapatite matrix during bone remodeling (Riihonen et al., 2010) and also counters the pro-apoptotic effects of acidosis in osteoclasts by mediating influx (Bouyer et al., 2007).
Duodenal secretion
NBCn1 is a major pH regulator in duodenal villus cells, supporting both the basal and forskolin-stimulated rates of secretion by the duodena of mice (Chen et al., 2012), see Figure 5.
Breast cancer
Multiple studies have suggested a link between susceptibility to breast cancer and an SNP in SLC4A7 (Ahmed et al., 2009; Antoniou et al., 2010; Campa et al., 2011; Chen et al., 2007; Han et al., 2011; Izumi et al., 2003; Long et al., 2010; Milne et al., 2010; Mulligan et al., 2011; Peng et al., 2011; Sueta et al., 2011). The breast-cancer cell line MCT-7—when overexpressing a truncated ErbB2 receptor—exhibits enhanced acid-extruding capability in part by increasing the plasma-membrane abundance of NBCn1 protein (Lauritzen et al., 2010).
4.3. NDCBE (SLC4A8)
Historically, Na-driven Cl-HCO3 exchanger was the first acid-base transporter shown to play a role in pHi regulation—based on microelectrode recordings of pHi on squid giant axons (Boron and De Weer, 1976; Boron and Russell, 1983; Russell and Boron, 1976) and snail neurons (Thomas, 1976a, b, 1977). This transporter normally mediates the uptake of one Na+ and the equivalent of two , and the egress of one Cl−. In the squid axon, this transporter has an absolute requirement for intracellular ATP, although the ATP is not stoichiometrically hydrolyzed as fuel (Boron et al., 1988).
The first cDNA encoding activity related to Na+-driven Cl-HCO3 exchange was cloned from Drosophila, and encodes a Na+-driven anion exchanger (NDAE1) that can apparently exchange extracellular Na+ and two (or two OH−) for intracellular Cl− (Romero et al., 2000). The Na+-driven Cl-HCO3 exchanger (NDCBE) cloned from human brain has an absolute dependence on as well as for Na+ and Cl− (Grichtchenko et al., 2001). Northern analysis indicates robust expression of a ~12 kb transcript in brain and testis, with weaker expression in kidney and ovary. A splice variant of NDCBE has an alternatively spliced 3′ end, leading to a protein in which the C-terminal 17 amino acids of human NDCBE are replaced by 66 amino acids (Parker et al., 2008a; Wang et al., 2001). Recently, a squid NDCBE has been cloned and characterized (Virkki et al., 2003), encoded by a gene that presumably shares common ancestry with mammalian SLC4A7, A8, and A10.
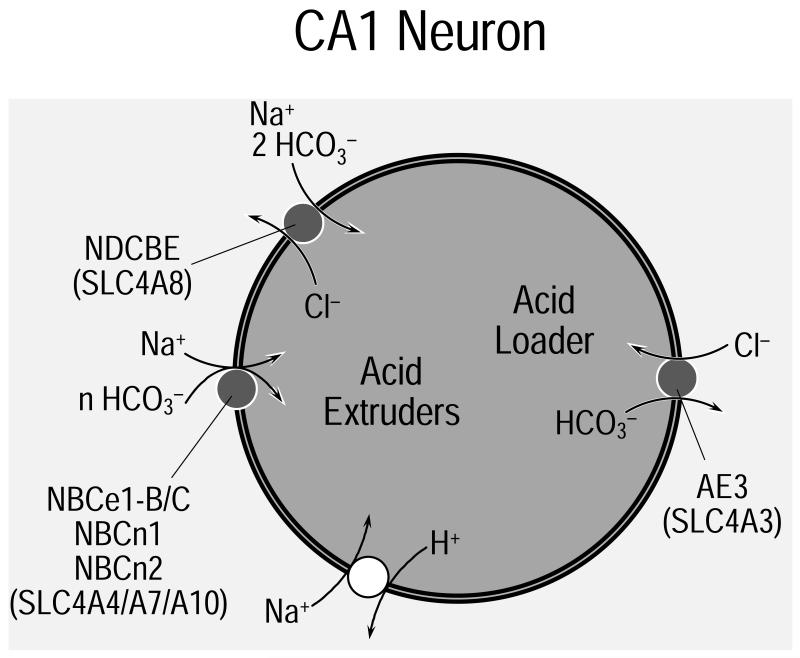
Human NDCBE, as well as the related transporters from Drosophila and squid, are very sensitive to inhibition by DIDS even though none of them have a consensus DIDS-interaction motif (KXXK) at the presumed extracellular end of TM #5. However, human NDCBE (as well as NBCn2) has such a motif at the presumed extracellular end of TM #3, and squid NDCBE has such a motif at the presumed end of TM #12—as discussed in (Virkki et al., 2003). Curiously, the DIDS-sensitive Drosophila NDAE1 does not have the consensus motif at either of these locations. Experiments on freshly dissociated hippocampal CA1 neurons have demonstrated Na+-driven Cl-HCO3 exchange activity as a major mode of pHi regulation (see Figure 6). In response to acid loads (which produce a fall in pHi), a Na-H exchanger and a Na+-driven Cl-HCO3 exchanger extrude acid (Schwiening and Boron, 1994) and thereby return pHi toward normal. The term “acid extrusion” refers to an energy-requiring process used to export acid and/or to take up alkali. In response to alkali loads (which produce a rise in pHi), many neurons use a Cl-HCO3 exchanger (perhaps AE3) to export . This acid-loading process also tends to return pHi toward normal. In the steady state, the actions of the acid extruders and acid loaders oppose each other and thus stabilize pHi within a narrow range of values. In Figure 6, we depict NDCBE and AE3 as playing keys roles in a hypothetical “neuron.” The extent to which specific acid-base transporters contribute to pHi regulation in specific neurons and astrocytes is not yet known. NBCe1 has been identified in neurons (Bevensee et al., 2000; Schmitt et al., 2000), NBCn2 (discussed below) is expressed at high levels in the brain (Wang et al., 2000), and both NBCn1 (Choi et al., 2000) and NBCe2 (Pushkin et al., 2000) also appear to be expressed in the brain. Thus, we should not be surprised if it turns out that different parts of different neurons and glial cells have different complements of acid-base transporters. Nevertheless, the principles underlying Figure 6 are likely to hold for pHi regulation in most cells throughout the brain and the rest of the body.
Figure 6. Proposed role of NDCBE and AE3 in pHi regulation by neurons.
Along with a Na-H exchanger (e.g., NHE5), the Na+-driven Cl-HCO3 exchanger NDCBE responds to intracellular acid loads by extruding acid and thereby returning intracellular pH (pHi) toward normal. A Cl-HCO3 exchanger (e.g., AE3) responds to intracellular alkali loads by exporting and thus returning pHi to normal.
4.3.1. Recent Advances
CSF secretion and neuronal excitability
The basolateral presence of NDCBE protein in the choroid plexus epithelium of fetal rats and adult humans suggests that NDCBE contributes to CSF secretion (Chen et al., 2008); (Damkier et al., 2007). Loss of NDCBE protein in neurons results in the exhibition, in slices of the CA1 hippocampal region from NDCBE-null mice, of reduced network excitability and increased presynaptic plasticity (Sinning et al., 2011). While the exact connection to neuronal excitability is not clear, activity of the NDCBE functional ortholog (ATBS-1 in C. elegans) controls whether GABAA-signaling results in an excitatory rather than an inhibitory input by lowering intracellular [Cl−] presumably in a and pH-dependent manner (Bellemer et al., 2011).
NaCl reabsorption in the CCD
NDCBE-null mice fed a Na+-deficient diet do not upregulate a thiazide-sensitive NaCl reabsorption pathway in the apical membranes of CCD intercalated cells that would tend to enhance Na+ retention (Leviel et al., 2010). The localization of NDCBE itself in the apical membranes of these cells has not been directly demonstrated.
4.4. NBCn2/NCBE (SLC4A10)
In 2000, Wang et al cloned a novel NBC-related cDNA from a mouse insulinoma cell line. Because this clone appeared to encode a Na+-driven Cl-HCO3 exchanger, based on a functional characterization carried out partially in HEK cells and partially in Xenopus oocytes, they named it NCBE (Wang et al., 2000) for Na-driven Cl bicarbonate exchanger. A study of mouse and rat Slc4a10 products in 2011 by Damkier et al, also concludes that rodent Slc4a10 is an NCBE. As expressed in Xenopus oocytes, a human SLC4A10 product has absolute requirements for Na+ and , is blocked by DIDS, but appears not to require Cl−. Instead the efflux of radiolabeled Cl− that is mediated by the transporter represents futile cycles of -dependent Cl-Cl exchange (Parker et al., 2008b). These data are consistent with the hypothesis that human SLC4A10 is a second electroneutral Na/HCO3 cotransporter: NBCn2.
In humans and mice, NBCn2/NCBE is expressed heavily in brain (Wang et al., 2000). In humans, preliminary work indicates the presence of high levels of a ~5.5-kb transcript, and lower levels of a ~9.5-kb transcript, in several regions of human brain (Choi et al., 2002).
4.4.1. Recent Advances
CSF secretion and neuronal excitability (epilepsy and autism)
NBCn2/NCBE protein in the basolateral membrane of choroid plexus epithelium contributes towards CSF secretion: accordingly, NBCn2/NCBE-null mice exhibit a 78% decrease in brain ventricular volume (Jacobs et al., 2008). Loss of NBCn2/NCBE protein from neurons results in the exhibition, in slices of the CA3 hippocampal region from NBCn2/NCBE-null mice, of increased tolerance to seizure-induction, both in terms of latency until onset and in survival rate (Jacobs et al., 2008), indicative of reduced neuronal excitability. Consistent with a role in regulation of neuronal excitability, three disruptions at the SLC4A10 gene locus have been described in epileptic individuals (Gurnett et al., 2008; Krepischi et al., 2010); and one disruption has been described in a pair of autistic twins (Sebat et al., 2007). However, the disruption of genes other than SLC4A10 in these individuals makes it difficult to assess the contribution of NBCn2 loss to these pathologies.
5. Other SLC4 family members
Genome sequencing and analysis of EST databases have revealed two additional members of the SLC4 family, AE4 and BTR1. Because the sequencing of the human genome is virtually complete, it is unlikely that additional SLC4 members will be found.
5.1. AE4 (SLC4A9)
In 2001, Tsuganezawa et al cloned two splice variants (a and b) of a novel NBC-like cDNA from rabbit kidney (Tsuganezawa et al., 2001). Compared to AE4-a, AE4-b lacks 16 amino acids in the cytoplasmic N terminus. As summarized in Figure 1, the cDNA is more closely related to the Na+-coupled transporters than to the Cl-HCO3 exchangers. Nevertheless, when expressed in COS cells, AE4-a appeared to behave as a Na+-independent Cl-HCO3 exchanger, leading to the name AE4. Moreover, Xenopus oocytes expressing AE4-a were reported to mediate a modest level of DIDS-insensitive 36Cl uptake. However, the function of AE4 is still unsettled. Following the cloning of human AE4 by Parker et al (Parker et al., 2001), Parker et al (Parker et al., 2002) attempted to characterize AE4 in Xenopus oocytes. In preliminary electrophysiological experiments, they failed to detect Cl-HCO3 exchange activity in oocytes injected with AE4 cRNA. Recently, Ko et al expressed in HEK and LLC-PK1 cells an AE4 cDNA that they cloned from rat (Ko et al., 2002a). These authors found evidence for Cl-HCO3 exchange that was DIDS sensitive. This last finding is surprising, inasmuch as AE4 lacks a classical DIDS motif at the presumed extracellular ends of TMs #3, #5 and #12. Thus, additional work will be required to establish the function of AE4.
Immunocytochemical data suggest that AE4 protein is predominantly expressed in the intercalated cells of the cortical collecting duct, although the cell type (α vs β) and the polarity of expression (apical vs basolateral) remain unresolved and may even differ between model organisms (e.g., rabbit vs mouse) (Blomqvist et al., 2004; Hentschke et al., 2009; Kurth et al., 2006; Shin et al., 2007; Tsuganezawa et al., 2001).
5.2. BTR1 (SLC4A11)
In 2001, Parker et al identified a novel sequence in GenBank and cloned BTR1 (bicarbonate-transporter-related protein 1) from human kidney(Parker et al., 2001). As indicated in Figure 1, BTR1 is the most unique of the SLC4 members. Northern analysis indicates that BTR1 is present most abundantly in kidney, salivary gland, testis, thyroid and trachea (Parker et al., 2001).
5.2.1. Recent Advances
Slc4a11 has been characterized as a Na+-coupled borate cotransporter, NaBC1 (Park et al., 2004), apparently cotransporting 2 Na+ with B(OH) −4. Knockdown of Slc4a11retards mammalian cell growth and proliferation, and is rescued by boric acid addition (Park et al., 2004). SLC4A11 mutations cause recessive congenital hereditary endothelial dystrophy (CHED2), Fuchs dystrophy and Harboyan syndrome (Desir et al., 2007; Kumar et al., 2007; Ramprasad et al., 2007; Sultana et al., 2007; Vithana et al., 2006). Slc4a11−/− mice have abnormal auditory brain responses and vestibular-evoked potential waveforms (Lopez et al., 2009). The connection between the animal/human phenotypes and Na+/borate cotransport is unclear. That said, one group found that Slc4a11 is needed for renal urinary concentration (Groger et al., 2010b). To date, Slc4a11 is the only SLC4 member which has not been found to transport .
6. Potential Therapeutic/Pharmaceutical Applications
6.1. Myocardial reperfusion injury
Following a period of myocardial ischemia, the very process of reestablishing blood flow, a prerequisite for salvaging the jeopardized myocardium, may paradoxically cause damage in and of itself. At least one contributing factor to this reperfusion injury may be the following. During the period of ischemia, pHi in affected myocardial cells probably falls appreciably. Nevertheless, during the ischemia, the ability of the cells to respond to the intracellular acidosis may be diminished markedly by relatively low levels of ATP, which may indirectly lead to inhibition of the Na-H exchangers and Na+-coupled transporters that would normally be responsible for acid extrusion. During reperfusion, however, rising levels of ATP now allow acid extruders to respond in a delayed fashion to the intracellular acidosis. The result of massive acid-extrusion effort can be not only recovery of pHi from the acid load, but also Na+ overload. A rise in [Na+]ican bias the Na-CA-II+ exchanger in favor of net Ca2+ uptake, leading to a rise in Ca2+ and cell injury. Blocking Na+-H++ exchange can reduce reperfusion injury, at least in clinical models (Allen and Xiao, 2003; Avkiran, 2003; Avkiran and Marber, 2002).
Khandoudi et al reported that an antibody targeted to the third extracellular loop of NBCe1 provides substantial protection to rat hearts subjected to ischemia and reperfusion (Khandoudi et al., 2001). Thus, it is possible that agents targeting NBCe1 and NBCe2 (or other Na+-coupled transporters in the heart) may prove clinically useful for treating reperfusion injury. A similar argument could be made for protecting against reperfusion injury to the brain in stroke. Here, however, the most likely targets would be the major Na+-coupled transporters in the brain: NDCBE and NBCn2/NCBE, as well as NBCe1 and NBCn1.
6.2. Hypertension
An agent that reduces Na+ reabsorption by the kidney will also reduce extracellular fluid volume and thus blood pressure. NBCe1-A is responsible for perhaps ~80% of reabsorption by the kidney, and a not insignificant fraction of Na+ reabsorption. Moderately inhibiting NBCe1-A in the renal proximal tubule might reduce Na+ reabsorption enough to lower blood pressure, and yet not lower reabsorption beyond the point where the H+ pumps in the distal nephron could compensate and thereby maintain a stable arterial pH. Thus, NBCe1-A might be a target for treating high blood pressure. That said, SLC4A5 (NBCe2) sequence variations are associated with hypertension.
6.3. Glaucoma/Cataracts
Human mutations of NBCe1 (SLC4A4) are causative of early onset bilateral glaucoma and bilateral cataracts. This ocular neuropathy is associated with elevated intraocular pressure (IOP), while cataracts are related to an undetermined lens issue. All human, recessive SLC4A4 mutations are associated with decreased function or low/no protein expression. Thus, therapeutics activating NBCe1 in the eye should reduce IOP and if early enough, may prevent lens opacity.
Acknowledgements
We are grateful to Dr. Seth L. Alper for helpful discussions regarding the AE anion exchangers over the years. Finally, we thank the members of and students working with the Romero and Boron laboratories, past and present; the experimental observations of these individuals are the basis for many of the issues discussed in this review. This work was supported by NIH grants DK56218 (MFR), EY017732 (MFR), DK83007 (Mayo O’Brien Urology Research Center), DK90728 (Mayo Translational Polycystic Kidney (PKD) Center), HD32573 (WFB), NS18400 (WFB), DK30344 (WFB), and EY021646 (MDP).
Footnotes
SLC4 has been designated as “2.A.31” by a “transporter classification” database (http://www.tcdb.org/)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
10. References
- Abuladze N, Azimov R, Newman D, Liu W, Tatishchev S, Pushkin A, Kurtz I. Critical amino acid residues involved in the electrogenic sodium bicarbonate cotransporter kNBC1-mediated transport. J Physiol. 2005;565(Pt 3):717–730. doi: 10.1113/jphysiol.2005.084988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuladze N, Lee I, Newman D, Hwang J, Boorer K, Pushkin A, Kurtz I. Molecular Cloning, Chromosomal Localization, Tissue Distribution, and Functional Expression of the Human Pancreatic Sodium Bicarbonate Cotransporter. J Biol Chem. 1998;273(28):17689–17695. doi: 10.1074/jbc.273.28.17689. [DOI] [PubMed] [Google Scholar]
- Abuladze N, Song M, Pushkin A, Newman D, Lee I, Nicholas S, Kurtz I. Structural organization of the human NBC1 gene: kNBC1 is transcribed from an alternative promoter in intron 3. Gene. 2000;251(2):109–122. doi: 10.1016/s0378-1119(00)00204-3. [DOI] [PubMed] [Google Scholar]
- Ahmed S, Thomas G, Ghoussaini M, Healey CS, Humphreys MK, Platte R, Morrison J, Maranian M, Pooley KA, Luben R, et al. Newly discovered breast cancer susceptibility loci on 3p24 and 17q23.2. Nat Genet. 2009;41(5):585–590. doi: 10.1038/ng.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiba Y, Nakamura M, Joshita S, Inamine T, Komori A, Yoshizawa K, Umemura T, Horie H, Migita K, Yatsuhashi H, et al. Genetic polymorphisms in CTLA4 and SLC4A2 are differentially associated with the pathogenesis of primary biliary cirrhosis in Japanese patients. J Gastroenterol. 2011;46(10):1203–1212. doi: 10.1007/s00535-011-0417-7. [DOI] [PubMed] [Google Scholar]
- Aldahmesh MA, Khan AO, Meyer BF, Alkuraya FS. Mutational spectrum of SLC4A11 in autosomal recessive CHED in Saudi Arabia. Invest Ophthalmol Vis Sci. 2009;50(9):4142–4145. doi: 10.1167/iovs.08-3006. [DOI] [PubMed] [Google Scholar]
- Aldave AJ, Yellore VS, Bourla N, Momi RS, Khan MA, Salem AK, Rayner SA, Glasgow BJ, Kurtz I. Autosomal recessive CHED associated with novel compound heterozygous mutations in SLC4A11. Cornea. 2007;26(7):896–900. doi: 10.1097/ICO.0b013e318074bb01. [DOI] [PubMed] [Google Scholar]
- Allen DG, Xiao XH. Role of the cardiac Na+/H+ exchanger during ischemia and reperfusion. Cardiovasc Res. 2003;57(4):934–941. doi: 10.1016/s0008-6363(02)00836-2. [DOI] [PubMed] [Google Scholar]
- Alper SL. Genetic diseases of acid-base transporters. Annu Rev Physiol. 2002;64:899–923. doi: 10.1146/annurev.physiol.64.092801.141759. [DOI] [PubMed] [Google Scholar]
- Alper SL. Molecular physiology and genetics of Na+-independent SLC4 anion exchangers. The Journal of experimental biology. 2009;212(Pt 11):1672–1683. doi: 10.1242/jeb.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper SL. Familial renal tubular acidosis. Journal of nephrology. 2010;23(Suppl 16):S57–76. [PubMed] [Google Scholar]
- Alper SL, Kopito RR, Libresco SM, Lodish HF. Cloning and characterization of a murine band 3-related cDNA from kidney and from a lymphoid cell line. J Biol Chem. 1988;263(32):17092–17099. [PubMed] [Google Scholar]
- Alper SL, Rossmann H, Wilhelm S, Stuart-Tilley AK, Shmukler BE, Seidler U. Expression of AE2 anion exchanger in mouse intestine. Am J Physiol. 1999;277(2 Pt 1):G321–332. doi: 10.1152/ajpgi.1999.277.2.G321. [DOI] [PubMed] [Google Scholar]
- Alper SL, Stuart-Tilley A, Simmons CF, Brown D, Drenckhahn D. The fodrin-ankyrin cytoskeleton of choroid plexus preferentially colocalizes with apical Na+K(+)-ATPase rather than with basolateral anion exchanger AE2. J Clin Invest. 1994;93(4):1430–1438. doi: 10.1172/JCI117120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper SL, Stuart-Tilley AK, Biemesderfer D, Shmukler BE, Brown D. Immunolocalization of AE2 anion exchanger in rat kidney. Am J Physiol. 1997;273(4 Pt 2):F601–614. doi: 10.1152/ajprenal.1997.273.4.F601. [DOI] [PubMed] [Google Scholar]
- Alvarez BV, Gilmour GS, Mema SC, Martin BT, Shull GE, Casey JR, Sauve Y. Blindness caused by deficiency in AE3 chloride/bicarbonate exchanger. PloS one. 2007;2(9):e839. doi: 10.1371/journal.pone.0000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amlal H, Burnham CE, Soleimani M. Characterization of Na+/ cotransporter isoform NBC-3. Am J Physiol. 1999;276(6 Pt 2):F903–913. doi: 10.1152/ajprenal.1999.276.6.F903. [DOI] [PubMed] [Google Scholar]
- Antoniou AC, Beesley J, McGuffog L, Sinilnikova OM, Healey S, Neuhausen SL, Ding YC, Rebbeck TR, Weitzel JN, Lynch HT, et al. Common breast cancer susceptibility alleles and the risk of breast cancer for BRCA1 and BRCA2 mutation carriers: implications for risk prediction. Cancer Res. 2010;70(23):9742–9754. doi: 10.1158/0008-5472.CAN-10-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avkiran M. Basic biology and pharmacology of the cardiac sarcolemmal sodium/hydrogen exchanger. J Card Surg. 2003;18(Suppl 1):3–12. doi: 10.1046/j.1540-8191.18.s1.2.x. [DOI] [PubMed] [Google Scholar]
- Avkiran M, Marber MS. Na(+)/H(+) exchange inhibitors for cardioprotective therapy: progress, problems and prospects. J Am Coll Cardiol. 2002;39(5):747–753. doi: 10.1016/s0735-1097(02)01693-5. [DOI] [PubMed] [Google Scholar]
- Baggio B, Bordin L, Gambaro G, Piccoli A, Marzaro G, Clari G. Evidence of a link between erythrocyte band 3 phosphorylation and anion transport in patients with ‘idiopathic’ calcium oxalate nephrolithiasis. Miner Electrolyte Metab. 1993;19(1):17–20. [PubMed] [Google Scholar]
- Banales JM, Saez E, Uriz M, Sarvide S, Urribarri AD, Splinter P, Tietz Bogert PS, Bujanda L, Prieto J, Medina JF, Larusso NF. Upregulation of mir-506 leads to decreased AE2 expression in biliary epithelium of patients with primary biliary cirrhosis. Hepatology. 2012 doi: 10.1002/hep.25691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA, Chakravarti A, Cooper RS, Ellison RC, Hunt SC, Province MA, Turner ST, Weder AB, Boerwinkle E. Positional identification of hypertension susceptibility genes on chromosome 2. Hypertension. 2004;43(2):477–482. doi: 10.1161/01.HYP.0000111585.76299.f7. [DOI] [PubMed] [Google Scholar]
- Bartel D, Lepke S, Layh-Schmitt G, Legrum B, Passow H. Anion transport in oocytes of Xenopus laevis induced by expression of mouse erythroid band 3 protein--encoding cRNA and of a cRNA derivative obtained by site-directed mutagenesis at the stilbene disulfonate binding site. Embo J. 1989;8(12):3601–3609. doi: 10.1002/j.1460-2075.1989.tb08533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilay M, Ship S, Cabantchik ZI. Anion transport in red blood cells. I. Chemical properties of anion recognition sites as revealed by structure-activity relationships of aromatic sulfonic acids. Membr Biochem. 1979;2(2):227–254. doi: 10.3109/09687687909063866. [DOI] [PubMed] [Google Scholar]
- Bellemer A, Hirata T, Romero MF, Koelle MR. Two types of chloride-extruding transporters are required for GABAA receptor-mediated inhibition in C. elegans. The EMBO journal. 2011;30(9):1852–1863. doi: 10.1038/emboj.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevensee MO, Schmitt BM, Choi I, Romero MF, Boron WF. An electrogenic Na/HCO3 cotransporter (NBC) with a novel C terminus, cloned from rat brain. Am J Physiol Cell Physiol. 2000;278(6):C1200–C1211. doi: 10.1152/ajpcell.2000.278.6.C1200. [DOI] [PubMed] [Google Scholar]
- Blomqvist SR, Vidarsson H, Fitzgerald S, Johansson BR, Ollerstam A, Brown R, Persson AE, Bergstrom GG, Enerback S. Distal renal tubular acidosis in mice that lack the forkhead transcription factor Foxi1. The Journal of clinical investigation. 2004;113(11):1560–1570. doi: 10.1172/JCI20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boedtkjer E, Praetorius J, Matchkov VV, Stankevicius E, Mogensen S, Fuchtbauer AC, Simonsen U, Fuchtbauer EM, Aalkjaer C. Disruption of Na+,HCO cotransporter NBCn1 (slc4a7) inhibits NO-mediated vasorelaxation, smooth muscle Ca(2) sensitivity, and hypertension development in mice. Circulation. 2011;124(17):1819–1829. doi: 10.1161/CIRCULATIONAHA.110.015974. [DOI] [PubMed] [Google Scholar]
- Bok D, Galbraith G, Lopez I, Woodruff M, Nusinowitz S, BeltrandelRio H, Huang W, Zhao S, Geske R, Montgomery C, et al. Blindness and auditory impairment caused by loss of the sodium bicarbonate cotransporter NBC3. Nature Genetics. 2003;34(3):313–319. doi: 10.1038/ng1176. [DOI] [PubMed] [Google Scholar]
- Bok D, Schibler MJ, Pushkin A, Sassani P, Abuladze N, Naser Z, Kurtz I. Immunolocalization of electrogenic sodium-bicarbonate cotransporters pNBC1 and kNBC1 in the rat eye. Am J Physiol Renal Physiol. 2001;281(5):F920–935. doi: 10.1152/ajprenal.2001.281.5.F920. [DOI] [PubMed] [Google Scholar]
- Boron WF, Boulpaep EL. Intracellular pH regulation in the renal proximal tubule of the salamander. Basolateral transport. J Gen Physiol. 1983;81(1):53–94. doi: 10.1085/jgp.81.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron WF, Chen L, Parker MD. Modular structure of sodium-coupled bicarbonate transporters. J Exp Biol. 2009;212(Pt 11):1697–1706. doi: 10.1242/jeb.028563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron WF, De Weer P. Active proton transport stimulated by CO2/HCO3-, blocked by cyanide. Nature. 1976;259(5540):240–241. doi: 10.1038/259240a0. [DOI] [PubMed] [Google Scholar]
- Boron WF, Hogan E, Russell JM. pH-sensitive activation of the intracellular-pH regulation system in squid axons by ATP-gamma-S. Nature. 1988;332(6161):262–265. doi: 10.1038/332262a0. [DOI] [PubMed] [Google Scholar]
- Boron WF, Russell JM. Stoichiometry and ion dependencies of the intracellular-pH-regulating mechanism in squid giant axons. J Gen Physiol. 1983;81(3):373–399. doi: 10.1085/jgp.81.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius FC, 3rd, Pisoni RL, Cao X, Deshmukh G, Yannoukakos D, Stuart-Tilley AK, Haller C, Alper SL. AE anion exchanger mRNA and protein expression in vascular smooth muscle cells, aorta, and renal microvessels. Am J Physiol. 1997;273(6 Pt 2):F1039–1047. doi: 10.1152/ajprenal.1997.273.6.F1039. [DOI] [PubMed] [Google Scholar]
- Brosius F.C.d., Alper SL, Garcia AM, Lodish HF. The major kidney band 3 gene transcript predicts an amino-terminal truncated band 3 polypeptide. J Biol Chem. 1989;264(14):7784–7787. [PubMed] [Google Scholar]
- Bruce LJ, Cope DL, Jones GK, Schofield AE, Burley M, Povey S, Unwin RJ, Wrong O, Tanner MJ. Familial distal renal tubular acidosis is associated with mutations in the red cell anion exchanger (Band 3, AE1) gene. J Clin Invest. 1997;100(7):1693–1707. doi: 10.1172/JCI119694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce LJ, Wrong O, Toye AM, Young MT, Ogle G, Ismail Z, Sinha AK, McMaster P, Hwaihwanje I, Nash GB, Hart S, Lavu E, Palmer R, Othman A, Unwin RJ, Tanner MJ. Band 3 mutations, renal tubular acidosis and South-East Asian ovalocytosis in Malaysia and Papua New Guinea: loss of up to 95% band 3 transport in red cells. Biochem J. 2000;350(Pt 1):41–51. [PMC free article] [PubMed] [Google Scholar]
- Burnham CE, Amlal H, Wang Z, Shull GE, Soleimani M. Cloning and functional expression of a human kidney Na+: cotransporter. J Biol Chem. 1997;272(31):19111–19114. doi: 10.1074/jbc.272.31.19111. [DOI] [PubMed] [Google Scholar]
- Cabantchik ZI, Greger R. Chemical probes for anion transporters of mammalian cell membranes. Am J Physiol. 1992;262(4 Pt 1):C803–827. doi: 10.1152/ajpcell.1992.262.4.C803. [DOI] [PubMed] [Google Scholar]
- Cabantchik ZI, Rothstein A. The nature of the membrane sites controlling anion permeability of human red blood cells as determined by studies with disulfonic stilbene derivatives. J Membr Biol. 1972;10(3):311–330. doi: 10.1007/BF01867863. [DOI] [PubMed] [Google Scholar]
- Campa D, Kaaks R, Le Marchand L, Haiman CA, Travis RC, Berg CD, Buring JE, Chanock SJ, Diver WR, Dostal L, et al. Interactions between genetic variants and breast cancer risk factors in the breast and prostate cancer cohort consortium. J Natl Cancer Inst. 2011;103(16):1252–1263. doi: 10.1093/jnci/djr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai SM, Vithana EN, Venkataraman D, Saleh H, Chekkalichintavida NP, al-Sayyed F, Aung T. Novel human pathological mutations. Gene symbol: SLC4A11. Disease: Corneal endothelial dystrophy 2. Hum Genet. 2010;127(1):110. [PubMed] [Google Scholar]
- Chang M-H, DiPiero JM, Sönnichsen FD, Romero MF. Entry to “HCO3 tunnel” revealed by human mutation and structural model. J Biol Chem. 2008;283(26):18402–18410. doi: 10.1074/jbc.M709819200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M-H, Plata C, Kurita Y, Kato A, Hirose S, Romero MF. Euryhaline Pufferfish NBCe1 differs from non-marine species NBCe1 physiology. Am J Physiol Cell Physiol. 2012;302(8):C1083–1095. doi: 10.1152/ajpcell.00233.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Choong LY, Lin Q, Philp R, Wong CH, Ang BK, Tan YL, Loh MC, Hew CL, Shah N, Druker BJ, Chong PK, Lim YP. Differential expression of novel tyrosine kinase substrates during breast cancer development. Mol Cell Proteomics. 2007;6(12):2072–2087. doi: 10.1074/mcp.M700395-MCP200. [DOI] [PubMed] [Google Scholar]
- Chernova MN, Stewart AK, Jiang L, Friedman DJ, Kunes YZ, Alper SL. Structure-Function Relationships of AE2 Regulation by the Ca2+-sensitive Stimulators, and Hypertonicity. Am J Physiol Cell Physiol. 2003;284(5):C1235–1246. doi: 10.1152/ajpcell.00522.2002. [DOI] [PubMed] [Google Scholar]
- Choi I, Aalkjaer C, Boulpaep EL, Boron WF. An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature. 2000;405(6786):571–575. doi: 10.1038/35014615. [DOI] [PubMed] [Google Scholar]
- Choi I, Kobayashi C, M J, Boron WF. Structure/function analysis of an electroneutral Na/HCO3 cotransporter (NBCn1) FASEB J. 2001;15:A446. [Google Scholar]
- Choi I, Rojas JD, Kobayashi C, Boron WF. Functional characterization of “NCBE”, a Na/HCO3 cotransporter. FASEB J. 2002;16(5):A796. [Google Scholar]
- Choi I, Romero MF, Khandoudi N, Bril A, Boron WF. Cloning and characterization of an electrogenic Na/HCO3 cotransporter from human heart. Am. J. Physiol. 1999;274(3 Pt 1):C576–584. doi: 10.1152/ajpcell.1999.276.3.C576. [DOI] [PubMed] [Google Scholar]
- Cooper GJ, Boron WF. Effect of PCMBS on CO2 permeability of xenopus oocytes expressing aquaporin 1 or its C189S mutant. Am J Physiol. 1998;275(6 Pt 1):C1481–1486. doi: 10.1152/ajpcell.1998.275.6.C1481. [DOI] [PubMed] [Google Scholar]
- Damkier HH, Nielsen S, Praetorius J. Molecular expression of SLC4 derived Na+ dependent anion transporters in selected human tissues. Am J Physiol Regul Integr Comp Physiol. 2007 doi: 10.1152/ajpregu.00356.2007. [DOI] [PubMed] [Google Scholar]
- Damkier HH, Praetorius J. Genetic ablation of Slc4a10 alters the expression pattern of transporters involved in solute movement in the mouse choroid plexus. Am J Physiol Cell Physiol. 2012;302(10):C1452–1459. doi: 10.1152/ajpcell.00285.2011. [DOI] [PubMed] [Google Scholar]
- Davis BA, Choi I, Rojas JD, Virkki LV, Boron WF. Binding of carbonic anhydrase II (CAII) to C termini of the Na-coupled HCO3 transporters NBCe1, NBCn1 and NDCBE1. FASEB J. 2002;16:A796. [Google Scholar]
- Deda G, Ekim M, Guven A, Karagol U, Tumer N. Hypopotassemic paralysis: a rare presentation of proximal renal tubular acidosis. J Child Neurol. 2001;16(10):770–771. doi: 10.1177/088307380101601013. [DOI] [PubMed] [Google Scholar]
- Demirci FY, Chang M-H, Mah TS, Romero MF, Gorin MB. Proximal renal tubular acidosis and ocular pathology: L522P, a novel missense mutation in the gene (SLC4A4) for Sodium Bicarbonate Cotransporter protein (NBCe1) Molecular Vision. 2006;12:324–330. [PubMed] [Google Scholar]
- Demuth DR, Showe LC, Ballantine M, Palumbo A, Fraser PJ, Cioe L, Rovera G, Curtis PJ. Cloning and structural characterization of a human non-erythroid band 3-like protein. Embo J. 1986;5(6):1205–1214. doi: 10.1002/j.1460-2075.1986.tb04348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desir J, Moya G, Reish O, Van Regemorter N, Deconinck H, David KL, Meire FM, Abramowicz MJ. Borate transporter SLC4A11 mutations cause both Harboyan syndrome and non-syndromic corneal endothelial dystrophy. J Med Genet. 2007;44(5):322–326. doi: 10.1136/jmg.2006.046904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinour D, Chang M-H, Satoh J-I, Smith BL, Angle N, Knecht A, Serban I, Holtzman EJ, Romero MF. A novel missense mutation in the sodium bicarbonate cotransporter (NBCe1/SLC4A4) causes proximal tubular acidosis and glaucoma through ion transport defects. J Biol Chem. 2004;279(50):52238–52246. doi: 10.1074/jbc.M406591200. [DOI] [PubMed] [Google Scholar]
- Dinour D, Knecht A, Serban I, Holtzman EJ. A Novel Missense Mutation in the Sodium BicarbonateCotransporter (NBC-1) Causes Congenital Proximal Renal Tubular Acidosis with Ocular Defects. J. Am. Soc. Nephrol. 2000;11:A0012. [Google Scholar]
- Dorfman R, Li W, Sun L, Lin F, Wang Y, Sandford A, Pare PD, McKay K, Kayserova H, Piskackova T, Macek M, Czerska K, Sands D, Tiddens H, Margarit S, Repetto G, Sontag MK, Accurso FJ, Blackman S, Cutting GR, Tsui LC, Corey M, Durie P, Zielenski J, Strug LJ. Modifier gene study of meconium ileus in cystic fibrosis: statistical considerations and gene mapping results. Hum Genet. 2009;126(6):763–778. doi: 10.1007/s00439-009-0724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducoudret O, Diakov A, Mueller-Berger S, Romero MF, Boron WF, Frömter E. The renal Na-HCO3-cotransporter expressed in Xenopus laevis oocytes: Inhibition by tenidap and benzamil and effect of temperature on transport rate and stoichiometry. Pflügers Arch. 2001;442(5):709–717. doi: 10.1007/s004240100594. [DOI] [PubMed] [Google Scholar]
- Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, Smith AV, Tobin MD, Verwoert GC, Hwang SJ, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478(7367):103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endeward V, Musa-Aziz R, Cooper GJ, Chen LM, Pelletier MF, Virkki LV, Supuran CT, King LS, Boron WF, Gros G. Evidence that aquaporin 1 is a major pathway for CO2 transport across the human erythrocyte membrane. Faseb J. 2006;20(12):1974–1981. doi: 10.1096/fj.04-3300com. [DOI] [PubMed] [Google Scholar]
- Frommer WB, von Wiren N. Plant biology: Ping-pong with boron. Nature. 2002;420(6913):282–283. doi: 10.1038/420282a. [DOI] [PubMed] [Google Scholar]
- Gawenis LR, Bradford EM, Prasad V, Lorenz JN, Simpson JE, Clarke LL, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE. Colonic anion secretory defects and metabolic acidosis in mice lacking the NBC1 Na+/ cotransporter. J Biol Chem. 2007;282(12):9042–9052. doi: 10.1074/jbc.M607041200. [DOI] [PubMed] [Google Scholar]
- Gawenis LR, Ledoussal C, Judd LM, Prasad V, Alper SL, Stuart-Tilley A, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE. Mice with a targeted disruption of the AE2 Cl-/HCO3- exchanger are achlorhydric. J Biol Chem. 2004;279(29):30531–30539. doi: 10.1074/jbc.M403779200. [DOI] [PubMed] [Google Scholar]
- Grichtchenko II, Boron WF. Surface-pH gradient measurements in Xenopus oocytes co-expressing the Na+driven Cl-HCO3 exchanger (NDCBE1) and CAIV: evidence for transport. FASEB J. 2002;16(5):A797–A797. [Google Scholar]
- Grichtchenko II, II, Choi II, Zhong X, Bray-Ward P, Russell JM, Boron WF. Cloning, characterization and chromosomal mapping of a human electroneutral Na+-driven Cl-HCO3 exchanger. J Biol Chem. 2001;276:8358–8363. doi: 10.1074/jbc.C000716200. [DOI] [PubMed] [Google Scholar]
- Groger N, Frohlich H, Maier H, Olbrich A, Kostin S, Braun T, Boettger T. SLC4A11 prevents osmotic imbalance leading to corneal endothelial dystrophy, deafness, and polyuria. The Journal of biological chemistry. 2010a;285(19):14467–14474. doi: 10.1074/jbc.M109.094680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groger N, Frohlich H, Maier H, Olbrich A, Kostin S, Braun T, Boettger T. SLC4A11 prevents osmotic imbalance leading to corneal endothelial dystrophy, deafness, and polyuria. The Journal of biological chemistry. 2010b;285(19):14467–14474. doi: 10.1074/jbc.M109.094680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groger N, Vitzthum H, Frohlich H, Kruger M, Ehmke H, Braun T, Boettger T. Targeted mutation of SLC4A5 induces arterial hypertension and renal metabolic acidosis. Hum Mol Genet. 2012;21(5):1025–1036. doi: 10.1093/hmg/ddr533. [DOI] [PubMed] [Google Scholar]
- Gross E, Hawkins K, Abuladze N, Pushkin A, Cotton CU, Hopfer U, Kurtz I. The stiochiometry of the electrogenic sodium bicarbonate cotransporter NBC1 is cell-type dependent. J. Physiol. 2001a;531(3):597–603. doi: 10.1111/j.1469-7793.2001.0597h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross E, Hawkins K, Pushkin A, Sassani P, Dukkipati R, Abuladze N, Hopfer U, Kurtz I. Phosphorylation of Ser(982) in the sodium bicarbonate cotransporter kNBC1 shifts the HCO(3)(−): Na(+) stoichiometry from 3: 1 to 2: 1 in murine proximal tubule cells. J Physiol. 2001b;537(Pt 3):659–665. doi: 10.1111/j.1469-7793.2001.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross E, Pushkin A, Abuladze N, Fedotoff O, Kurtz I. Regulation of the sodium bicarbonate cotransporter kNBC1 function: role of Asp(986), Asp(988) and kNBC1-carbonic anhydrase II binding. J Physiol. 2002;544(Pt 3):679–685. doi: 10.1113/jphysiol.2002.029777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Woo JH, Yu JH, Lee MJ, Moon HG, Kang D, Noh DY. Common genetic variants associated with breast cancer in Korean women and differential susceptibility according to intrinsic subtype. Cancer Epidemiol Biomarkers Prev. 2011 doi: 10.1158/1055-9965.EPI-10-1282. [DOI] [PubMed] [Google Scholar]
- Hemadevi B, Veitia RA, Srinivasan M, Arunkumar J, Prajna NV, Lesaffre C, Sundaresan P. Identification of mutations in the SLC4A11 gene in patients with recessive congenital hereditary endothelial dystrophy. Arch Ophthalmol. 2008;126(5):700–708. doi: 10.1001/archopht.126.5.700. [DOI] [PubMed] [Google Scholar]
- Hentschke M, Hentschke S, Borgmeyer U, Hubner CA, Kurth I. The murine AE4 promoter predominantly drives type B intercalated cell specific transcription. Histochemistry and cell biology. 2009;132(4):405–412. doi: 10.1007/s00418-009-0614-0. [DOI] [PubMed] [Google Scholar]
- Hentschke M, Wiemann M, Hentschke S, Kurth I, Hermans-Borgmeyer I, Seidenbecher T, Jentsch TJ, Gal A, Hubner CA. Mice with a targeted disruption of the Cl-/HCO3-exchanger AE3 display a reduced seizure threshold. Mol Cell Biol. 2006;26(1):182–191. doi: 10.1128/MCB.26.1.182-191.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horita S, Yamada H, Inatomi J, Moriyama N, Sekine T, Igarashi T, Endo Y, Dasouki M, Ekim M, Al-Gazali L, Shimadzu M, Seki G, Fujita T. Functional Analysis of NBC1 Mutants Associated with Proximal Renal Tubular Acidosis and Ocular Abnormalities. J Am Soc Nephrol. 2005;16(8):2270–2278. doi: 10.1681/ASN.2004080667. [DOI] [PubMed] [Google Scholar]
- Humphreys BD, Chernova MN, Jiang L, Zhang Y, Alper SL. NH4Cl activates AE2 anion exchanger in Xenopus oocytes at acidic pHi. Am J Physiol. 1997;272(4 Pt 1):C1232–1240. doi: 10.1152/ajpcell.1997.272.4.C1232. [DOI] [PubMed] [Google Scholar]
- Humphreys BD, Jiang L, Chernova MN, Alper SL. Hypertonic activation of AE2 anion exchanger in Xenopus oocytes via NHE-mediated intracellular alkalinization. Am J Physiol. 1995;268(1 Pt 1):C201–209. doi: 10.1152/ajpcell.1995.268.1.C201. [DOI] [PubMed] [Google Scholar]
- Hunt SC, Xin Y, Wu LL, Cawthon RM, Coon H, Hasstedt SJ, Hopkins PN. Sodium bicarbonate cotransporter polymorphisms are associated with baseline and 10-year follow-up blood pressures. Hypertension. 2006;47(3):532–536. doi: 10.1161/01.HYP.0000196949.26088.3c. [DOI] [PubMed] [Google Scholar]
- Igarashi T, Inatomi J, Sekine T, Cha SH, Kanai Y, Kunimi M, Tsukamoto K, Satoh H, Shimadzu M, Tozawa F, Mori T, Shiobara M, Seki G, Endou H. Mutations in SLC4A4 cause permanent isolated proximal renal tubular acidosis with ocular abnormalities. Nat Genet. 1999;23(3):264–266. doi: 10.1038/15440. [DOI] [PubMed] [Google Scholar]
- Igarashi T, Inatomi J, Sekine T, Seki G, Shimadzu M, Tozawa F, Takeshima Y, Takumi T, Takahashi T, Yoshikawa N, Nakamura H, Endou H. Novel nonsense mutation in the Na+/HCO3- cotransporter gene (SLC4A4) in a patient with permanent isolated proximal renal tubular acidosis and bilateral glaucoma. J Am Soc Nephrol. 2001;12(4):713–718. doi: 10.1681/ASN.V124713. [DOI] [PubMed] [Google Scholar]
- Igarashi T, Inatomi J, Sekine T, Seki G, Yamada H, Horita S, Fujita T. Mutational and Functional Analysis of the Na+/ Cotransporter Gene (SLC4A4) in Patients with Permanent Isolated Proximal Renal Tubular Acidosis and Ocular Abnormalities. J. Am. Soc. Nephrol. 2003;14(Suppl. S, NOV 2003):302A–302A. [Google Scholar]
- Igarashi T, Inatomi J, Sekine T, Takeshima Y, Yoshikawa N, Endou H. A Nonsense Mutation in the Na+/ Cotransporter Gene (SLC4A4) in a Patient with Permanent Isolated Proximal Renal Tubular Acidosis and Bilateral Glaucoma. J. Am. Soc. Nephrol. 2000;11:A0573. doi: 10.1681/ASN.V124713. [DOI] [PubMed] [Google Scholar]
- Igarashi T, Sekine T, Inatomi J, Seki G. Unraveling the molecular pathogenesis of isolated proximal renal tubular acidosis. J Am Soc Nephrol. 2002;13(8):2171–2177. doi: 10.1097/01.asn.0000025281.70901.30. [DOI] [PubMed] [Google Scholar]
- Imamura T, Matsuo T, Yanase T, Kagiyama S. Hereditary spherocytosis associated with a variant of band 3 protein in the erythrocyte membrane. Jpn J Med. 1984;23(3):216–219. doi: 10.2169/internalmedicine1962.23.216. [DOI] [PubMed] [Google Scholar]
- Inaba M, Yawata A, Koshino I, Sato K, Takeuchi M, Takakuwa Y, Manno S, Yawata Y, Kanzaki A, Sakai J, Ban A, Ono K, Maede Y. Defective anion transport and marked spherocytosis with membrane instability caused by hereditary total deficiency of red cell band 3 in cattle due to a nonsense mutation. J Clin Invest. 1996;97(8):1804–1817. doi: 10.1172/JCI118610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inatomi J, Horita S, Braverman N, Sekine T, Yamada H, Suzuki Y, Kawahara K, Moriyama N, Kudo A, Kawakami H, et al. Mutational and functional analysis of SLC4A4 in a patient with proximal renal tubular acidosis. Pflugers Arch. 2004;448(4):438–444. doi: 10.1007/s00424-004-1278-1. [DOI] [PubMed] [Google Scholar]
- Ishibashi K, Sasaki S, Marumo F. Molecular cloning of a new sodium bicarbonate cotransporter cDNA from human retina. Biochem Biophys Res Commun. 1998;246(2):535–538. doi: 10.1006/bbrc.1998.8658. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Namkung W, Yamamoto A, Wang Z, Worrell RT, Xu J, Lee MG, Soleimani M. Effect of Slc26a6 deletion on apical Cl−/ exchanger activity and cAMP-stimulated bicarbonate secretion in pancreatic duct. Am J Physiol Gastrointest Liver Physiol. 2007;292(1):G447–455. doi: 10.1152/ajpgi.00286.2006. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Steward MC, Lindsay AR, Case RM. Accumulation of intracellular by Na+- cotransport in interlobular ducts from guinea-pig pancreas. J Physiol (Lond) 1996;495(Pt 1):169–178. doi: 10.1113/jphysiol.1996.sp021582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S, Ruusuvuori E, Sipila ST, Haapanen A, Damkier HH, Kurth I, Hentschke M, Schweizer M, Rudhard Y, Laatikainen LM, Tyynela J, Praetorius J, Voipio J, Hubner CA. Mice with targeted Sl c4a10 gene disruption have small brain ventricles and show reduced neuronal excitability. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(1):311–316. doi: 10.1073/pnas.0705487105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarolim P, Palek J, Amato D, Hassan K, Sapak P, Nurse GT, Rubin HL, Zhai S, Sahr KE, Liu SC. Deletion in erythrocyte band 3 gene in malaria-resistant Southeast Asian ovalocytosis. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(24):11022–11026. doi: 10.1073/pnas.88.24.11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarolim P, Shayakul C, Prabakaran D, Jiang L, Stuart-Tilley A, Rubin HL, Simova S, Zavadil J, Herrin JT, Brouillette J, Somers MJ, Seemanova E, Brugnara C, Guay-Woodford LM, Alper SL. Autosomal dominant distal renal tubular acidosis is associated in three families with heterozygosity for the R589H mutation in the AE1 (band 3) Cl-/HCO3-exchanger. J Biol Chem. 1998;273(11):6380–6388. doi: 10.1074/jbc.273.11.6380. [DOI] [PubMed] [Google Scholar]
- Jennings ML. Proton fluxes associated with erythrocyte membrane anion exchange. J Membr Biol. 1976;28(2-3):187–205. doi: 10.1007/BF01869697. [DOI] [PubMed] [Google Scholar]
- Jensen LJ, Schmitt BM, Berger UV, Nsumu NN, Boron WF, Hediger MA, Brown D, Breton S. Localization of sodium bicarbonate cotransporter (NBC) protein and messenger ribonucleic acid in rat epididymis. Biol Reprod. 1999;60(3):573–579. doi: 10.1095/biolreprod60.3.573. [DOI] [PubMed] [Google Scholar]
- Jiao X, Sultana A, Garg P, Ramamurthy B, Vemuganti GK, Gangopadhyay N, Hejtmancik JF, Kannabiran C. Autosomal recessive corneal endothelial dystrophy (CHED2) is associated with mutations in SLC4A11. J Med Genet. 2007;44(1):64–68. doi: 10.1136/jmg.2006.044644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juran BD, Atkinson EJ, Larson JJ, Schlicht EM, Lazaridis KN. Common genetic variation and haplotypes of the anion exchanger SLC4A2 in primary biliary cirrhosis. Am J Gastroenterol. 2009;104(6):1406–1411. doi: 10.1038/ajg.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao L, Kurtz LM, Shao X, Papadopoulos MC, Liu L, Bok D, Nusinowitz S, Chen B, Stella SL, Andre M, et al. Severe Neurologic Impairment in Mice with Targeted Disruption of the Electrogenic Sodium Bicarbonate Cotransporter NBCe2 (Slc4a5 Gene) The Journal of biological chemistry. 2011;286(37):32563–32574. doi: 10.1074/jbc.M111.249961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardia SL, Greene MT, Boerwinkle E, Turner ST, Kullo IJ. Investigating the complex genetic architecture of ankle-brachial index, a measure of peripheral arterial disease, in non-Hispanic whites. BMC Med Genomics. 2008;1:16. doi: 10.1186/1755-8794-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karet FE, Gainza FJ, Gyory AZ, Unwin RJ, Wrong O, Tanner MJ, Nayir A, Alpay H, Santos F, Hulton SA, et al. Mutations in the chloride-bicarbonate exchanger gene AE1 cause autosomal dominant but not autosomal recessive distal renal tubular acidosis. Proc Natl Acad Sci U S A. 1998;95(11):6337–6342. doi: 10.1073/pnas.95.11.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandoudi N, Albadine J, Robert P, Krief S, Berrebi-Bertrand I, Martin X, Bevensee MO, Boron WF, Bril A. Inhibition of the cardiac electrogenic sodium bicarbonate cotransporter reduces ischemic injury. Cardiovasc Res. 2001;52(3):387–396. doi: 10.1016/s0008-6363(01)00430-8. [DOI] [PubMed] [Google Scholar]
- Ko SB, Luo X, Hager H, Rojek A, Choi JY, Licht C, Suzuki M, Muallem S, Nielsen S, Ishibashi K. AE4 is a DIDS-sensitive Cl−/ exchanger in the basolateral membrane of the renal CCD and the SMG duct. Am J Physiol Cell Physiol. 2002a;283(4):C1206–1218. doi: 10.1152/ajpcell.00512.2001. [DOI] [PubMed] [Google Scholar]
- Ko SB, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S, Muallem S. A molecular mechanism for aberrant CFTR-dependent transport in cystic fibrosis. Embo J. 2002b;21(21):5662–5672. doi: 10.1093/emboj/cdf580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Morgans CW, Casey JR, Kopito RR. AE3 anion exchanger isoforms in the vertebrate retina: developmental regulation and differential expression in neurons and glia. J Neurosci. 1994;14(10):6266–6279. doi: 10.1523/JNEUROSCI.14-10-06266.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopito RR, Lee BS, Simmons DM, Lindsey AE, Morgans CW, Schneider K. Regulation of intracellular pH by a neuronal homolog of the erythrocyte anion exchanger. Cell. 1989;59(5):927–937. doi: 10.1016/0092-8674(89)90615-6. [DOI] [PubMed] [Google Scholar]
- Kopito RR, Lodish HF. Primary structure and transmembrane orientation of the murine anion exchange protein. Nature. 1985;316(6025):234–238. doi: 10.1038/316234a0. [DOI] [PubMed] [Google Scholar]
- Krepischi AC, Knijnenburg J, Bertola DR, Kim CA, Pearson PL, Bijlsma E, Szuhai K, Kok F, Vianna-Morgante AM, Rosenberg C. Two distinct regions in 2q24.2-q24.3 associated with idiopathic epilepsy. Epilepsia. 2010;51(12):2457–2460. doi: 10.1111/j.1528-1167.2010.02742.x. [DOI] [PubMed] [Google Scholar]
- Kudrycki KE, Newman PR, Shull GE. cDNA cloning and tissue distribution of mRNAs for two proteins that are related to the band 3 Cl-/HCO3- exchanger. J Biol Chem. 1990;265(1):462–471. [PubMed] [Google Scholar]
- Kumar A, Bhattacharjee S, Prakash DR, Sadanand CS. Genetic analysis of two Indian families affected with congenital hereditary endothelial dystrophy: two novel mutations in SLC4A11. Mol Vis. 2007;13:39–46. [PMC free article] [PubMed] [Google Scholar]
- Kurth I, Hentschke M, Hentschke S, Borgmeyer U, Gal A, Hubner CA. The forkhead transcription factor Foxi1 directly activates the AE4 promoter. The Biochemical journal. 2006;393(Pt 1):277–283. doi: 10.1042/BJ20051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon TH, Fulton C, Wang W, Kurtz I, Frokiaer J, Aalkjaer C, Nielsen S. Chronic metabolic acidosis upregulates rat kidney Na-HCO3 cotransporters NBCn1 and NBC3 but not NBC1. Am J Physiol Renal Physiol. 2002;282(2):F341–351. doi: 10.1152/ajprenal.00104.2001. [DOI] [PubMed] [Google Scholar]
- Lacruz RS, Nanci A, White SN, Wen X, Wang H, Zalzal SF, Luong VQ, Schuetter VL, Conti PS, Kurtz I, Paine ML. The sodium bicarbonate cotransporter (NBCe1) is essential for normal development of mouse dentition. J Biol Chem. 2010;285(32):24432–24438. doi: 10.1074/jbc.M110.115188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolt-Marticorena C, Casey JR, Reithmeier RA. Transmembrane helix-helix interactions and accessibility of H2DIDS on labelled band 3, the erythrocyte anion exchange protein. Mol Membr Biol. 1995;12(2):173–182. doi: 10.3109/09687689509027505. [DOI] [PubMed] [Google Scholar]
- Lee S-K, Grichtchenko II, Boron WF. Distinguishing HCO3(−) from CO3(2−) transport by NBCe1-A (Abstract) FASEB J. 2011a;25:656–659. [Google Scholar]
- Lee S, Lee HJ, Yang HS, Thornell IM, Bevensee MO, Choi I. Sodium-bicarbonate cotransporter NBCn1 in the kidney medullary thick ascending limb cell line is upregulated under acidic conditions and enhances ammonium transport. Experimental physiology. 2010;95(9):926–937. doi: 10.1113/expphysiol.2010.053967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Boron WF, Parker MD. Relief of autoinhibition of the electrogenic Na-HCO(3) [corrected] cotransporter NBCe1-B: role of IRBIT vs.amino-terminal truncation. American journal of physiology. Cell physiology. 2012;302(3):C518–526. doi: 10.1152/ajpcell.00352.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YC, Yan JJ, Cruz SA, Horng JL, Hwang PP. Anion exchanger 1b, but not sodium-bicarbonate cotransporter 1b, plays a role in transport functions of zebrafish H+- ATPase-rich cells. American journal of physiology. Cell physiology. 2011b;300(2):C295–307. doi: 10.1152/ajpcell.00263.2010. [DOI] [PubMed] [Google Scholar]
- Leviel F, Hubner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, Hassan H, Parker MD, Kurth I, Kougioumtzes A, et al. The Na+- dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest. 2010;120(5):1627–1635. doi: 10.1172/JCI40145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn SC, Kudrycki KE, Shull GE. The predicted translation product of a cardiac AE3 mRNA contains an N terminus distinct from that of the brain AE3 Cl−/ exchanger. Cloning of a cardiac AE3 cDNA, organization of the AE3 gene, and identification of an alternative transcription initiation site. J Biol Chem. 1992;267(11):7927–7935. [PubMed] [Google Scholar]
- Liu Y, Xu JY, Wang DK, Wang L, Chen LM. Cloning and identification of two novel NBCe1 splice variants from mouse reproductive tract tissues: a comparative study of NCBT genes. Genomics. 2011;98(2):112–119. doi: 10.1016/j.ygeno.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Lo YF, Yang SS, Seki G, Yamada H, Horita S, Yamazaki O, Fujita T, Usui T, Tsai JD, Yu IS, Lin SW, Lin SH. Severe metabolic acidosis causes early lethality in NBC1 W516X knock-in mice as a model of human isolated proximal renal tubular acidosis. Kidney Int. 2011a;79(7):730–741. doi: 10.1038/ki.2010.523. [DOI] [PubMed] [Google Scholar]
- Lo YF, Yang SS, Seki G, Yamada H, Horita S, Yamazaki O, Fujita T, Usui T, Tsai JD, Yu IS, Lin SW, Lin SH. Severe metabolic acidosis causes early lethality in NBC1 W516X knock-in mice as a model of human isolated proximal renal tubular acidosis. Kidney international. 2011b;79(7):730–741. doi: 10.1038/ki.2010.523. [DOI] [PubMed] [Google Scholar]
- Long J, Shu XO, Cai Q, Gao YT, Zheng Y, Li G, Li C, Gu K, Wen W, Xiang YB, Lu W, Zheng W. Evaluation of breast cancer susceptibility loci in Chinese women. Cancer Epidemiol Biomarkers Prev. 2010;19(9):2357–2365. doi: 10.1158/1055-9965.EPI-10-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez IA, Rosenblatt MI, Kim C, Galbraith GC, Jones SM, Kao L, Newman D, Liu W, Yeh S, Pushkin A, Abuladze N, Kurtz I. Slc4a11 gene disruption in mice: cellular targets of sensorineuronal abnormalities. J Biol Chem. 2009;284(39):26882–26896. doi: 10.1074/jbc.M109.008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Boron WF. Reversible and irreversible interactions of DIDS with the human electrogenic Na/HCO3 cotransporter NBCe1-A: role of lysines in the KKMIK motif of TM5. Am J Physiol Cell Physiol. 2007;292(5):C1787–1798. doi: 10.1152/ajpcell.00267.2006. [DOI] [PubMed] [Google Scholar]
- Lu J, Daly CM, Parker MD, Gill HS, Piermarini PM, Pelletier MF, Boron WF. Effect of human carbonic anhydrase II on the activity of the human electrogenic Na/HCO3 cotransporter NBCe1-A in Xenopus oocytes. J Biol Chem. 2006;281(28):19241–19250. doi: 10.1074/jbc.M602181200. [DOI] [PubMed] [Google Scholar]
- Lynn KS, Li LL, Lin YJ, Wang CH, Sheng SH, Lin JH, Liao W, Hsu WL, Pan WH. A neural network model for constructing endophenotypes of common complex diseases: an application to male young-onset hypertension microarray data. Bioinformatics. 2009;25(8):981–988. doi: 10.1093/bioinformatics/btp106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino CR, Jeanes V, Boron WF, Schmitt BM. Expression and distribution of the Na(+)-HCO(−)(3) cotransporter in human pancreas. Am. J. Physiol. 1999;277(2 Pt 1):G487–494. doi: 10.1152/ajpgi.1999.277.2.G487. [DOI] [PubMed] [Google Scholar]
- McAlear SD, Liu X, Williams JB, McNicholas-Bevensee CM, Bevensee MO. Electrogenic Na/HCO3 cotransporter (NBCe1) variants expressed in xenopus oocytes: functional comparison and roles of the amino and carboxy termini. J Gen Physiol. 2006;127(6):639–658. doi: 10.1085/jgp.200609520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF. Role of the anion exchanger 2 in the pathogenesis and treatment of primary biliary cirrhosis. Dig Dis. 2011;29(1):103–112. doi: 10.1159/000324144. [DOI] [PubMed] [Google Scholar]
- Mehta JS, Hemadevi B, Vithana EN, Arunkumar J, Srinivasan M, Prajna V, Tan DT, Aung T, Sundaresan P. Absence of phenotype-genotype correlation of patients expressing mutations in the SLC4A11 gene. Cornea. 2010;29(3):302–306. doi: 10.1097/ICO.0b013e3181ae9038. [DOI] [PubMed] [Google Scholar]
- Milne RL, Gaudet MM, Spurdle AB, Fasching PA, Couch FJ, Benitez J, Arias Perez JI, Zamora MP, Malats N, Dos Santos Silva I, et al. Assessing interactions between the associations of common genetic susceptibility variants, reproductive history and body mass index with breast cancer risk in the breast cancer association consortium: a combined case-control study. Breast Cancer Res. 2010;12(6):R110. doi: 10.1186/bcr2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison AC, Srinivas SK, Elovitz MA, Puschett JB. Genetic variation in solute carrier genes is associated with preeclampsia. Am J Obstet Gynecol. 2010;203(5):491, e413. doi: 10.1016/j.ajog.2010.06.004. e491-491. [DOI] [PubMed] [Google Scholar]
- Muller-Berger S, Ducoudret O, Diakov A, Fromter E. The renal Na-HCO3-cotransporter expressed in Xenopus laevis oocytes: change in stoichiometry in response to elevation of cytosolic Ca2+ concentration. Pflugers Arch. 2001;442(5):718–728. doi: 10.1007/s004240100592. [DOI] [PubMed] [Google Scholar]
- Mulligan AM, Couch FJ, Barrowdale D, Domchek SM, Eccles D, Nevanlinna H, Ramus SJ, Robson M, Sherman M, Spurdle AB, et al. Common breast cancer susceptibility alleles are associated with tumour subtypes in BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2. Breast Cancer Res. 2011;13(6):R110. doi: 10.1186/bcr3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa-Aziz R, Chen LM, Pelletier MF, Boron WF. Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG. Proc Natl Acad Sci U S A. 2009;106(13):5406–5411. doi: 10.1073/pnas.0813231106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhoul NL, Davis BA, Romero MF, Boron WF. Effect of expressing the water channel aquaporin-1 on the CO2 permeability of Xenopus oocytes [see comments] Am J Physiol. 1998;274(2 Pt 1):C543–548. doi: 10.1152/ajpcell.1998.274.2.C543. [DOI] [PubMed] [Google Scholar]
- Oehlschlager S, Fuessel S, Meye A, Herrmann J, Lotzkat U, Froehner M, Albrecht S, Wirth MP. Importance of erythrocyte band III anion transporter (SLC4A1) on oxalate clearance of calcium oxalate monohydrate stone-formering patients vs. normal controls. Urology. 2011;77(1):250, e251–255. doi: 10.1016/j.urology.2010.06.043. [DOI] [PubMed] [Google Scholar]
- Okubo K, Kang D, Hamasaki N, Jennings ML. Red blood cell band 3. Lysine 539 and lysine 851 react with the same H2DIDS (4,4′-diisothiocyanodihydrostilbene-2,2′-disulfonic acid) molecule. J Biol Chem. 1994;269(3):1918–1926. [PubMed] [Google Scholar]
- Papageorgiou P, Shmukler BE, Stuart-Tilley AK, Jiang L, Alper SL. AE anion exchangers in atrial tumor cells. Am J Physiol Heart Circ Physiol. 2001;280(3):H937–945. doi: 10.1152/ajpheart.2001.280.3.H937. [DOI] [PubMed] [Google Scholar]
- Park M, Ko SB, Choi JY, Muallem G, Thomas PJ, Pushkin A, Lee MS, Kim JY, Lee MG, Muallem S, Kurtz I. The cystic fibrosis transmembrane conductance regulator interacts with and regulates the activity of the HCO3- salvage transporter human Na+-HCO3- cotransport isoform 3. J Biol Chem. 2002;277(52):50503–50509. doi: 10.1074/jbc.M201862200. [DOI] [PubMed] [Google Scholar]
- Park M, Li Q, Shcheynikov N, Zeng W, Muallem S. NaBC1 Is a Ubiquitous Electrogenic Na(+)- Coupled Borate Transporter Essential for Cellular Boron Homeostasis and Cell Growth and Proliferation. Mol Cell. 2004;16(3):331–341. doi: 10.1016/j.molcel.2004.09.030. [DOI] [PubMed] [Google Scholar]
- Parker MD, Boron WF, Tanner MJA. Characterization of human ‘AE4’ as an electroneutral, sodium-dependent bicarbonate transporter. FASEB J. 2002;16(5):A796. [Google Scholar]
- Parker MD, Bouyer P, Daly CM, Boron WF. Cloning and characterization of novel human SLC4A8 gene products encoding Na+-driven Cl-/HCO3(−) exchanger variants NDCBE-A, - C, and -D. Physiol Genomics. 2008a;34(3):265–276. doi: 10.1152/physiolgenomics.90259.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MD, Daly C, Skelton LA, Boron WF. IRBIT functionally enhances the electroneutral Na+-coupled bicarbonate transporter NCBE by sequestering and N-terminal autoinhibitory domain (Abstract) FASEB Journal. 2007;21:A1285. [Google Scholar]
- Parker MD, Musa-Aziz R, Rojas JD, Choi I, Daly CM, Boron WF. Characterization of human SLC4A10 as an electroneutral Na/HCO3 cotransporter (NBCn2) with Cl- self-exchange activity. J Biol Chem. 2008b;283(19):12777–12788. doi: 10.1074/jbc.M707829200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MD, Ourmozdi EP, Tanner MJ. Human BTR1, a new bicarbonate transporter superfamily member and human AE4 from kidney. Biochem Biophys Res Commun. 2001;282(5):1103–1109. doi: 10.1006/bbrc.2001.4692. [DOI] [PubMed] [Google Scholar]
- Parker MD, Qin X, Williamson RC, Toye AM, Boron WF. HCO(3)(−)-independent conductance with a mutant Na(+)/HCO(3)(−) cotransporter (SLC4A4) in a case of proximal renal tubular acidosis with hypokalaemic paralysis. The Journal of physiology. 2012;590(Pt 8):2009–2034. doi: 10.1113/jphysiol.2011.224733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Lu B, Ruan W, Zhu Y, Sheng H, Lai M. Genetic polymorphisms and breast cancer risk: evidence from meta-analyses, pooled analyses, and genome-wide association studies. Breast Cancer Res Treat. 2011;127(2):309–324. doi: 10.1007/s10549-011-1459-5. [DOI] [PubMed] [Google Scholar]
- Peters LL, Shivdasani RA, Liu SC, Hanspal M, John KM, Gonzalez JM, Brugnara C, Gwynn B, Mohandas N, Alper SL, Orkin SH, Lux SE. Anion exchanger 1 (band 3) is required to prevent erythrocyte membrane surface loss but not to form the membrane skeleton. Cell. 1996;86(6):917–927. doi: 10.1016/s0092-8674(00)80167-1. [DOI] [PubMed] [Google Scholar]
- Piermarini PM, Kim EY, Boron WF. Evidence against a direct interaction between intracellular carbonic anhydrase II and pure C-terminal domains of SLC4 bicarbonate transporters. J Biol Chem. 2007;282(2):1409–1421. doi: 10.1074/jbc.M608261200. [DOI] [PubMed] [Google Scholar]
- Poupon R, Ping C, Chretien Y, Corpechot C, Chazouilleres O, Simon T, Heath SC, Matsuda F, Poupon RE, Housset C, Barbu V. Genetic factors of susceptibility and of severity in primary biliary cirrhosis. J Hepatol. 2008;49(6):1038–1045. doi: 10.1016/j.jhep.2008.07.027. [DOI] [PubMed] [Google Scholar]
- Praetorius J, Kim YH, Bouzinova EV, Frische S, Rojek A, Aalkjaer C, Nielsen S. NBCn1 is a basolateral Na+ - cotransporter in rat kidney inner medullary collecting ducts. Am J Physiol Renal Physiol. 2004;286(5):F903–912. doi: 10.1152/ajprenal.00437.2002. [DOI] [PubMed] [Google Scholar]
- Preston GM, Agre P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci U S A. 1991;88(24):11110–11114. doi: 10.1073/pnas.88.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushkin A, Abuladze N, Lee I, Newman D, Hwang J, Kurtz I. Cloning, Tissue Distribution, Genomic Organization, and Functional Characterization of NBC3, a New Member of the Sodium Bicarbonate Cotransporter Family. J Biol Chem. 1999;274(23):16569–16575. doi: 10.1074/jbc.274.23.16569. [DOI] [PubMed] [Google Scholar]
- Pushkin A, Abuladze N, Newman D, Lee I, Xu G, Kurtz I. Cloning, characterization and chromosomal assignment of NBC4, a new member of the sodium bicarbonate cotransporter family. Biochim Biophys Acta. 2000;1493(1-2):215–218. doi: 10.1016/s0167-4781(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Ramprasad VL, Ebenezer ND, Aung T, Rajagopal R, Yong VH, Tuft SJ, Viswanathan D, El-Ashry MF, Liskova P, Tan DT, et al. Novel SLC4A11 mutations in patients with recessive congenital hereditary endothelial dystrophy (CHED2). Mutation in brief #958. Online. Hum Mutat. 2007;28(5):522–523. doi: 10.1002/humu.9487. [DOI] [PubMed] [Google Scholar]
- Riazuddin SA, Vithana EN, Seet LF, Liu Y, Al-Saif A, Koh LW, Heng YM, Aung T, Meadows DN, Eghrari AO, et al. Missense mutations in the sodium borate cotransporter SLC4A11 cause late-onset Fuchs corneal dystrophy. Hum Mutat. 2010;31(11):1261–1268. doi: 10.1002/humu.21356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero MF, Fong P, Berger UV, Hediger MA, Boron WF. Cloning and functional expression of rNBC, an electrogenic Na+- cotransporter from rat kidney. Am J Physiol. 1998;274(2 Pt 2):F425–432. doi: 10.1152/ajprenal.1998.274.2.F425. [DOI] [PubMed] [Google Scholar]
- Romero MF, Hediger MA, Boulpaep EL, Boron WF. Expression cloning and characterization of a renal electrogenic Na+/ cotransporter. Nature. 1997;387(6631):409–413. doi: 10.1038/387409a0. [DOI] [PubMed] [Google Scholar]
- Romero MF, Henry D, Nelson S, Harte PJ, Dillon AK, Sciortino CM. Cloning and characterization of a Na+ driven anion exchanger (NDAE1): a new bicarbonate transporter. J Biol. Chem. 2000;275:24552–24559. doi: 10.1074/jbc.M003476200. [DOI] [PubMed] [Google Scholar]
- Ruminot I, Gutierrez R, Pena-Munzenmayer G, Anazco C, Sotelo-Hitschfeld T, Lerchundi R, Niemeyer MI, Shull GE, Barros LF. NBCe1 mediates the acute stimulation of astrocytic glycolysis by extracellular K+ J Neurosci. 2011;31(40):14264–14271. doi: 10.1523/JNEUROSCI.2310-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JM, Boron WF. Role of choloride transport in regulation of intracellular pH. Nature. 1976;264(5581):73–74. doi: 10.1038/264073a0. [DOI] [PubMed] [Google Scholar]
- Sabolic I, Brown D, Gluck SL, Alper SL. Regulation of AE1 anion exchanger and H(+)- ATPase in rat cortex by acute metabolic acidosis and alkalosis. Kidney Int. 1997;51(1):125–137. doi: 10.1038/ki.1997.16. [DOI] [PubMed] [Google Scholar]
- Sahr KE, Taylor WM, Daniels BP, Rubin HL, Jarolim P. The structure and organization of the human erythroid anion exchanger (AE1) gene. Genomics. 1994;24(3):491–501. doi: 10.1006/geno.1994.1658. [DOI] [PubMed] [Google Scholar]
- Salas JT, Banales JM, Sarvide S, Recalde S, Ferrer A, Uriarte I, Oude Elferink RP, Prieto J, Medina JF. Ae2a,b-deficient mice develop antimitochondrial antibodies and other features resembling primary biliary cirrhosis. Gastroenterology. 2008;134(5):1482–1493. doi: 10.1053/j.gastro.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Sander T, Toliat MR, Heils A, Leschik G, Becker C, Ruschendorf F, Rohde K, Mundlos S, Nurnberg P. Association of the 867Asp variant of the human anion exchanger 3 gene with common subtypes of idiopathic generalized epilepsy. Epilepsy Res. 2002;51(3):249–255. doi: 10.1016/s0920-1211(02)00152-3. [DOI] [PubMed] [Google Scholar]
- Sassani P, Pushkin A, Gross E, Gomer A, Abuladze N, Dukkipati R, Carpenito G, Kurtz I. Functional characterization of NBC4: a new electrogenic sodium-bicarbonate cotransporter. Am J Physiol Cell Physiol. 2002;282(2):C408–416. doi: 10.1152/ajpcell.00409.2001. [DOI] [PubMed] [Google Scholar]
- Satoh H, Moriyama N, Hara C, Yamada H, Horita S, Kunimi M, Tsukamoto K, Iso ON, Inatomi J, Kawakami H, Kudo A, Endou H, Igarashi T, Goto A, Fujita T, Seki G. Localization of Na+-HCO-3 cotransporter (NBC-1) variants in rat and human pancreas. Am J Physiol Cell Physiol. 2003;284(3):C729–737. doi: 10.1152/ajpcell.00166.2002. [DOI] [PubMed] [Google Scholar]
- Schmitt BM, Berger UV, Douglas RM, Bevensee MO, Hediger MA, Haddad GG, Boron WF. Na/HCO3 cotransporters in rat brain: expression in glia, neurons, and choroid plexus. J Neurosci. 2000;20(18):6839–6848. doi: 10.1523/JNEUROSCI.20-18-06839.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt BM, Biemesderfer D, Boulpaep EL, Romero MF, Boron WF. Immunolocalization of the electrogenic Na+/ cotransporter in mammalian and amphibian kidney. Am. J. Physiol. 1999;276(1 Pt 2):F27–36. doi: 10.1152/ajprenal.1999.276.1.F27. [DOI] [PubMed] [Google Scholar]
- Schopfer LM, Salhany JM. Characterization of the stilbenedisulfonate binding site on band 3. Biochemistry. 1995;34(26):8320–8329. doi: 10.1021/bi00026a013. [DOI] [PubMed] [Google Scholar]
- Schwiening CJ, Boron WF. Regulation of intracellular pH in pyramidal neurones from the rat hippocampus by Na(+)-dependent Cl(−)-HCO3-exchange. J Physiol (Lond) 1994;475(1):59–67. doi: 10.1113/jphysiol.1994.sp020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciortino CM. Characterization and localization of the sodium mediated bicarbonate transporters NBC and NDAE1, Physiology & Biophysics. Case Western Reserve University; Cleveland, OH: 2001. p. 252. [Google Scholar]
- Sciortino CM, Romero MF. Cation and voltage dependence of rat kidney, electrogenic Na+/ cotransporter, rkNBC, expressed in oocytes. Am. J. Physiol. 1999;277(4 Pt 2):F611–623. doi: 10.1152/ajprenal.1999.277.4.F611. [DOI] [PubMed] [Google Scholar]
- Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, Yamrom B, Yoon S, Krasnitz A, Kendall J, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316(5823):445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki G, Yamada H, Horita S, Suzuki M, Sekine T, Igarashi T, Fujita T. Activation and inactivation mechanisms of Na-HCO3 cotransporter NBC1. J Epithel Biol Pharmacol. 2008;1:35–39. [Google Scholar]
- Shah SS, Al-Rajhi A, Brandt JD, Mannis MJ, Roos B, Sheffield VC, Syed NA, Stone EM, Fingert JH. Mutation in the SLC4A11 gene associated with autosomal recessive congenital hereditary endothelial dystrophy in a large Saudi family. Ophthalmic Genet. 2008;29(1):41–45. doi: 10.1080/13816810701850033. [DOI] [PubMed] [Google Scholar]
- Shayakul C, Alper SL. Inherited renal tubular acidosis. Curr Opin Nephrol Hypertens. 2000;9(5):541–546. doi: 10.1097/00041552-200009000-00014. [DOI] [PubMed] [Google Scholar]
- Shayakul C, Alper SL. Defects in processing and trafficking of the AE1 Cl-/HCO3-exchanger associated with inherited distal renal tubular acidosis. Clin Exp Nephrol. 2004;8(1):1–11. doi: 10.1007/s10157-003-0271-x. [DOI] [PubMed] [Google Scholar]
- Shin JH, Son EJ, Lee HS, Kim SJ, Kim K, Choi JY, Lee MG, Yoon JH. Molecular and functional expression of anion exchangers in cultured normal human nasal epithelial cells. Acta physiologica. 2007;191(2):99–110. doi: 10.1111/j.1748-1716.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakabe K, Priori G, Yamada H, Ando H, Horita S, Fujita T, Fujimoto I, Mizutani A, Seki G, Mikoshiba K. IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein, specifically binds to and activates pancreas-type Na+/ cotransporter 1 (pNBC1) Proc Natl Acad Sci U S A. 2006;103(25):9542–9547. doi: 10.1073/pnas.0602250103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southgate CD, Chishti AH, Mitchell B, Yi SJ, Palek J. Targeted disruption of the murine erythroid band 3 gene results in spherocytosis and severe haemolytic anaemia despite a normal membrane skeleton. Nat Genet. 1996;14(2):227–230. doi: 10.1038/ng1096-227. [DOI] [PubMed] [Google Scholar]
- Stehberger PA, Shmukler BE, Stuart-Tilley AK, Peters LL, Alper SL, Wagner CA. Distal renal tubular acidosis in mice lacking the AE1 (band3) Cl-/HCO3-exchanger (slc4a1) J Am Soc Nephrol. 2007;18(5):1408–1418. doi: 10.1681/ASN.2006101072. [DOI] [PubMed] [Google Scholar]
- Stewart AK, Chernova MN, Shmukler BE, Wilhelm S, Alper SL. Regulation of AE2-mediated Cl(-) Transport by Intracellular or by Extracellular pH Requires Highly Conserved Amino Acid Residues of the AE2 NH(2)-terminal Cytoplasmic Domain. J Gen Physiol. 2002;120(5):707–722. doi: 10.1085/jgp.20028641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart-Tilley A, Sardet C, Pouyssegur J, Schwartz MA, Brown D, Alper SL. Immunolocalization of anion exchanger AE2 and cation exchanger NHE-1 in distinct adjacent cells of gastric mucosa. Am J Physiol. 1994;266(2 Pt 1):C559–568. doi: 10.1152/ajpcell.1994.266.2.C559. [DOI] [PubMed] [Google Scholar]
- Stuart-Tilley AK, Shmukler BE, Brown D, Alper SL. Immunolocalization and tissue-specific splicing of AE2 anion exchanger in mouse kidney. J Am Soc Nephrol. 1998;9(6):946–959. doi: 10.1681/ASN.V96946. [DOI] [PubMed] [Google Scholar]
- Stutz AM, Teran-Garcia M, Rao DC, Rice T, Bouchard C, Rankinen T. Functional identification of the promoter of SLC4A5, a gene associated with cardiovascular and metabolic phenotypes in the HERITAGE Family Study. Eur J Hum Genet. 2009;17(11):1481–1489. doi: 10.1038/ejhg.2009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueta A, Ito H, Kawase T, Hirose K, Hosono S, Yatabe Y, Tajima K, Tanaka H, Iwata H, Iwase H, Matsuo K. A genetic risk predictor for breast cancer using a combination of low-penetrance polymorphisms in a Japanese population. Breast Cancer Res Treat. 2011 doi: 10.1007/s10549-011-1904-5. [DOI] [PubMed] [Google Scholar]
- Sultana A, Garg P, Ramamurthy B, Vemuganti GK, Kannabiran C. Mutational spectrum of the SLC4A11 gene in autosomal recessive congenital hereditary endothelial dystrophy. Mol Vis. 2007;13:1327–1332. [PubMed] [Google Scholar]
- Sussman CR, Zhao J, Plata C, Daly JLC, Angle N, DiPiero J, Drummond IA, Liang JO, Boron WF, Romero MF, Chang M-H. Cloning, localization and functional expression of the electrogenic Na+ bicarbonate cotransporter (NBCe1) from zebrafish. Am J Physiol - Cell Physiol. 2009;297(Oct 2009):C865–C875. doi: 10.1152/ajpcell.00679.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Vaisbich MH, Yamada H, Horita S, Li Y, Sekine T, Moriyama N, Igarashi T, Endo Y, Cardoso TP, de Sa LC, Koch VH, Seki G, Fujita T. Functional analysis of a novel missense NBC1 mutation and of other mutations causing proximal renal tubular acidosis. Pflugers Arch. 2008;455(4):583–593. doi: 10.1007/s00424-007-0319-y. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Van Paesschen W, Stalmans I, Horita S, Yamada H, Bergmans BA, Legius E, Riant F, De Jonghe P, Li Y, et al. Defective membrane expression of the Na(+)-HCO(3)(−) cotransporter NBCe1 is associated with familial migraine. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(36):15963–15968. doi: 10.1073/pnas.1008705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano J, Noguchi K, Yasumori M, Kobayashi M, Gajdos Z, Miwa K, Hayashi H, Yoneyama T, Fujiwara T. Arabidopsis boron transporter for xylem loading. Nature. 2002;420(6913):337–340. doi: 10.1038/nature01139. [DOI] [PubMed] [Google Scholar]
- Tanphaichitr VS, Sumboonnanonda A, Ideguchi H, Shayakul C, Brugnara C, Takao M, Veerakul G, Alper SL. Novel AE1 mutations in recessive distal renal tubular acidosis. Loss-of-function is rescued by glycophorin A. J Clin Invest. 1998;102(12):2173–2179. doi: 10.1172/JCI4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatishchev S, Abuladze N, Pushkin A, Newman D, Liu W, Weeks D, Sachs G, Kurtz I. Identification of Membrane Topography of the Electrogenic Sodium Bicarbonate Cotransporter pNBC1 by in Vitro Transcription/Translation. Biochemistry. 2003;42(3):755–765. doi: 10.1021/bi026826q. [DOI] [PubMed] [Google Scholar]
- Taylor JY, Maddox R, Wu CY. Genetic and environmental risks for high blood pressure among African American mothers and daughters. Biol Res Nurs. 2009;11(1):53–65. doi: 10.1177/1099800409334817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JY, Sampson D, Taylor AD, Caldwell D, Sun YV. Genetic and BMI Risks for Predicting Blood Pressure in Three Generations of West African Dogon Women. Biol Res Nurs. 2011 doi: 10.1177/1099800411419026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thévenod F, Roussa E, Schmitt BM, Romero MF. Cloning and Immunolocalization of a Rat Pancreatic Na+ Bicarbonate Cotransporter. Biochemical and Biophysical Research Communications. 1999;264(1):291–298. doi: 10.1006/bbrc.1999.1484. [DOI] [PubMed] [Google Scholar]
- Thomas RC. The effect of carbon dioxide on the intracellular pH and buffering power of snail neurones. J Physiol (Lond) 1976a;255(3):715–735. doi: 10.1113/jphysiol.1976.sp011305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RC. Ionic mechanism of the H+ pump in a snail neurone. Nature. 1976b;262(5563):54–55. doi: 10.1038/262054a0. [DOI] [PubMed] [Google Scholar]
- Thomas RC. The role of bicarbonate, chloride and sodium ions in the regulation of intracellular pH in snail neurones. J Physiol (Lond) 1977;273(1):317–338. doi: 10.1113/jphysiol.1977.sp012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toye AM, Parker MD, Daly CM, Lu J, Virkki LV, Pelletier MF, Boron WF. The Human NBCe1-A Mutant R881C, Associated With Proximal Renal Tubular Acidosis, Retains Function But Is Mistargeted In Polarized Renal Epithelia. Am J Physiol Cell Physiol. 2006;291(4):C788–801. doi: 10.1152/ajpcell.00094.2006. [DOI] [PubMed] [Google Scholar]
- Tsuganezawa H, Kobayashi K, Iyori M, Araki T, Koizumi A, Watanabe S-I, Kaneko A, Fukao T, Monkawa T, Yoshida T, Kim DK, Kanai Y, Endou H, Hayashi M, Saruta T. A new member of the transporter superfamily is an apical anion exchanger of b -intercalated cells in the kidney. Journal of Biological Chemistry. 2001;276(11):8180–8189. doi: 10.1074/jbc.M004513200. [DOI] [PubMed] [Google Scholar]
- van Adelsberg JS, Edwards JC, al-Awqati Q. The apical Cl/HCO3 exchanger of beta intercalated cells. J Biol Chem. 1993;268(15):11283–11289. [PubMed] [Google Scholar]
- Vince JW, Reithmeier RAF. Carbonic anhydrase II binds to the carboxyl terminus of human band 3, the erythrocyte Cl−/ exchanger. J Biol Chem. 1998;273(43):28430–28437. doi: 10.1074/jbc.273.43.28430. [DOI] [PubMed] [Google Scholar]
- Virkki LV, Choi I, Davis BA, Boron WF. Cloning of a Na+-driven Cl/HCO3 exchanger from squid giant fiber lobe. Am J Physiol Cell Physiol. 2003;285(4):C771–780. doi: 10.1152/ajpcell.00439.2002. [DOI] [PubMed] [Google Scholar]
- Virkki LV, Wilson DA, Vaughan-Jones RD, Boron WF. Functional characterization of human NBC4 as an electrogenic Na/HCO3 cotransporter (NBCe2) Am. J. Physiol. Cell Physiol. 2002;282(6):C1278–1289. doi: 10.1152/ajpcell.00589.2001. [DOI] [PubMed] [Google Scholar]
- Vithana EN, Morgan P, Sundaresan P, Ebenezer ND, Tan DT, Mohamed MD, Anand S, Khine KO, Venkataraman D, Yong VH, et al. Mutations in sodium-borate cotransporter SLC4A11 cause recessive congenital hereditary endothelial dystrophy (CHED2) Nat Genet. 2006;38(7):755–757. doi: 10.1038/ng1824. [DOI] [PubMed] [Google Scholar]
- Vithana EN, Morgan PE, Ramprasad V, Tan DT, Yong VH, Venkataraman D, Venkatraman A, Yam GH, Nagasamy S, Law RW, Rajagopal R, Pang CP, Kumaramanickevel G, Casey JR, Aung T. SLC4A11 mutations in Fuchs endothelial corneal dystrophy. Hum Mol Genet. 2008;17(5):656–666. doi: 10.1093/hmg/ddm337. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Yano H, Nagashima K, Seino S. The Na+-driven Cl-/HCO3-Exchanger. Cloning, tissue distribution, and functional characterization. J Biol Chem. 2000;275(45):35486–35490. doi: 10.1074/jbc.C000456200. [DOI] [PubMed] [Google Scholar]
- Wang Y-F, Holmes HL, Romero MF, Chang M-H. Zebrafish Na+ bicarbonate cotransporter NBCe1 is functionally augmented by IRBIT interaction with the zNBCe1-N-terminus; 10th International Congress on the Biology of Fish; 2012. [Google Scholar]
- Wang Z, Conforti L, Petrovic S, Amlal H, Burnham CE, Soleimani M. Mouse Na+: cotransporter isoform NBC-3 (kNBC-3): Cloning, expression, and renal distribution. Kidney Int. 2001;59(4):1405–1414. doi: 10.1046/j.1523-1755.2001.0590041405.x. [DOI] [PubMed] [Google Scholar]
- Williams JB, Powell PC, Bevensee MO. Electrogenic Na/Bicarbonate Cotransporter (NBCe1) variants in rat heart. FASEB J. 2003;17:A462. [Google Scholar]
- Williamson RC, Toye AM. Glycophorin A: Band 3 aid. Blood Cells Mol Dis. 2008;41(1):35–43. doi: 10.1016/j.bcmd.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Wood PG, Muller H, Sovak M, Passow H. Role of Lys 558 and Lys 869 in substrate and inhibitor binding to the murine band 3 protein: a study of the effects of site-directed mutagenesis of the band 3 protein expressed in the oocytes of Xenopus laevis. J Membr Biol. 1992;127(2):139–148. doi: 10.1007/BF00233286. [DOI] [PubMed] [Google Scholar]
- Wu J, McNicholas CM, Bevensee MO. Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates the electrogenic Na/HCO3 cotransporter NBCe1-A expressed in Xenopus oocytes. Proc Natl Acad Sci U S A. 2009;106(33):14150–14155. doi: 10.1073/pnas.0906303106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki O, Yamada H, Suzuki M, Horita S, Shirai A, Nakamura M, Seki G, Fujita T. Functional characterization of nonsynonymous single nucleotide polymorphisms in the electrogenic Na+- cotransporter NBCe1A. Pflugers Archiv : European journal of physiology. 2011;461(2):249–259. doi: 10.1007/s00424-010-0918-x. [DOI] [PubMed] [Google Scholar]
- Yang D, Li Q, So I, Huang CL, Ando H, Mizutani A, Seki G, Mikoshiba K, Thomas PJ, Muallem S. IRBIT governs epithelial secretion in mice by antagonizing the WNK/SPAK kinase pathway. J Clin Invest. 2011;121(3):956–965. doi: 10.1172/JCI43475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Shcheynikov N, Zeng W, Ohana E, So I, Ando H, Mizutani A, Mikoshiba K, Muallem S. IRBIT coordinates epithelial fluid and secretion by stimulating the transporters pNBC1 and CFTR in the murine pancreatic duct. J Clin Invest. 2009;119(1):193–202. doi: 10.1172/JCI36983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannoukakos D, Stuart-Tilley A, Fernandez HA, Fey P, Duyk G, Alper SL. Molecular cloning, expression, and chromosomal localization of two isoforms of the AE3 anion exchanger from human heart. Circ Res. 1994;75(4):603–614. doi: 10.1161/01.res.75.4.603. [DOI] [PubMed] [Google Scholar]
- Young MT, Beckmann R, Toye AM, Tanner MJ. Red-cell glycophorin A-band 3 interactions associated with the movement of band 3 to the cell surface. Biochem J. 2000;350(Pt 1):53–60. [PMC free article] [PubMed] [Google Scholar]
- Yu H, Riederer B, Stieger N, Boron WF, Shull GE, Manns MP, Seidler UE, Bachmann O. Secretagogue stimulation enhances NBCe1 (electrogenic Na(+)/HCO(3)(−) cotransporter) surface expression in murine colonic crypts. American journal of physiology. Gastrointestinal and liver physiology. 2009;297(6):G1223–1231. doi: 10.1152/ajpgi.00157.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Kiyatkin A, Bolin JT, Low PS. Crystallographic structure and functional interpretation of the cytoplasmic domain of erythrocyte membrane band 3. Blood. 2000;96(9):2925–2933. [PubMed] [Google Scholar]
- Zhao R, Reithmeier RA. Expression and characterization of the anion transporter homologue YNL275w in Saccharomyces cerevisiae. Am J Physiol Cell Physiol. 2001;281(1):C33–45. doi: 10.1152/ajpcell.2001.281.1.C33. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Azimov R, Kao L, Newman D, Liu W, Abuladze N, Pushkin A, Kurtz I. NBCe1-A transmembrane segment 1 lines the ion translocation pathway. J Biol Chem. 2009;284(13):8918–8929. doi: 10.1074/jbc.M806674200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Kao L, Azimov R, Newman D, Liu W, Pushkin A, Abuladze N, Kurtz I. Topological location and structural importance of the NBCe1-A residues mutated in proximal renal tubular acidosis. J Biol Chem. 2010;285(18):13416–13426. doi: 10.1074/jbc.M109.093286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Lee DW, Casey JR. Novel topology in C-terminal region of the human plasma membrane anion exchanger, AE1. J Biol Chem. 2003;278(5):3112–3120. doi: 10.1074/jbc.M207797200. [DOI] [PubMed] [Google Scholar]