Abstract
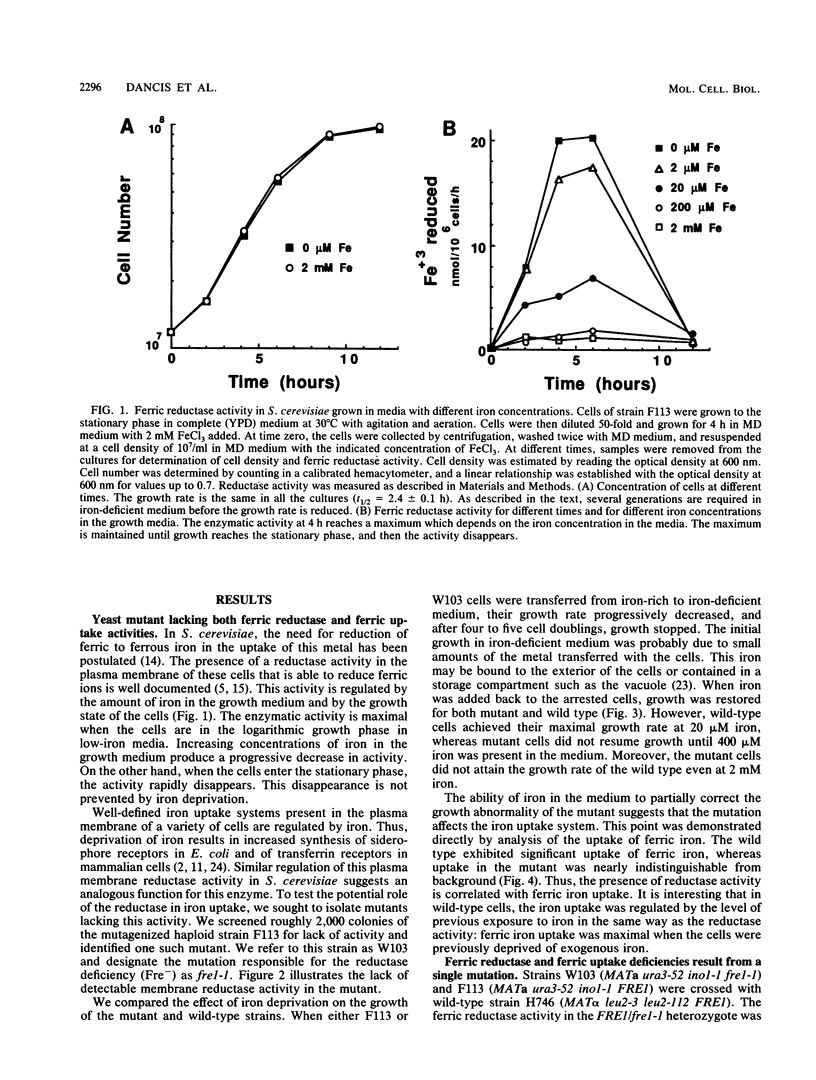
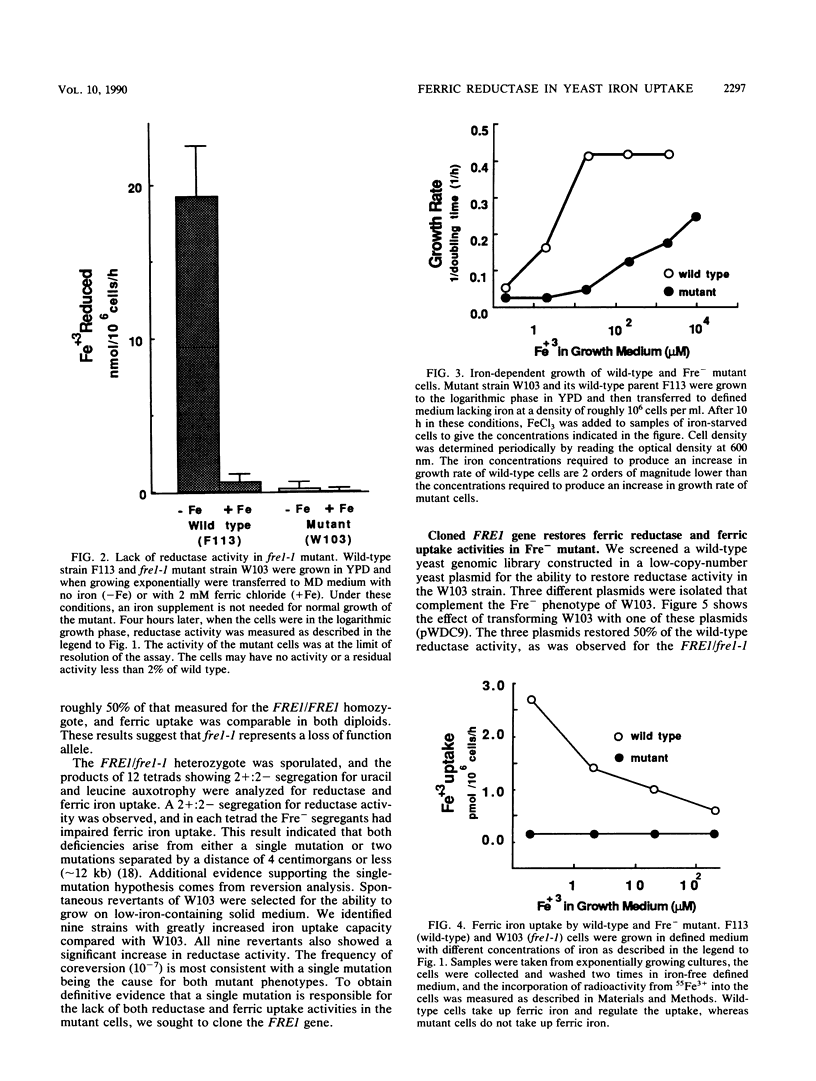
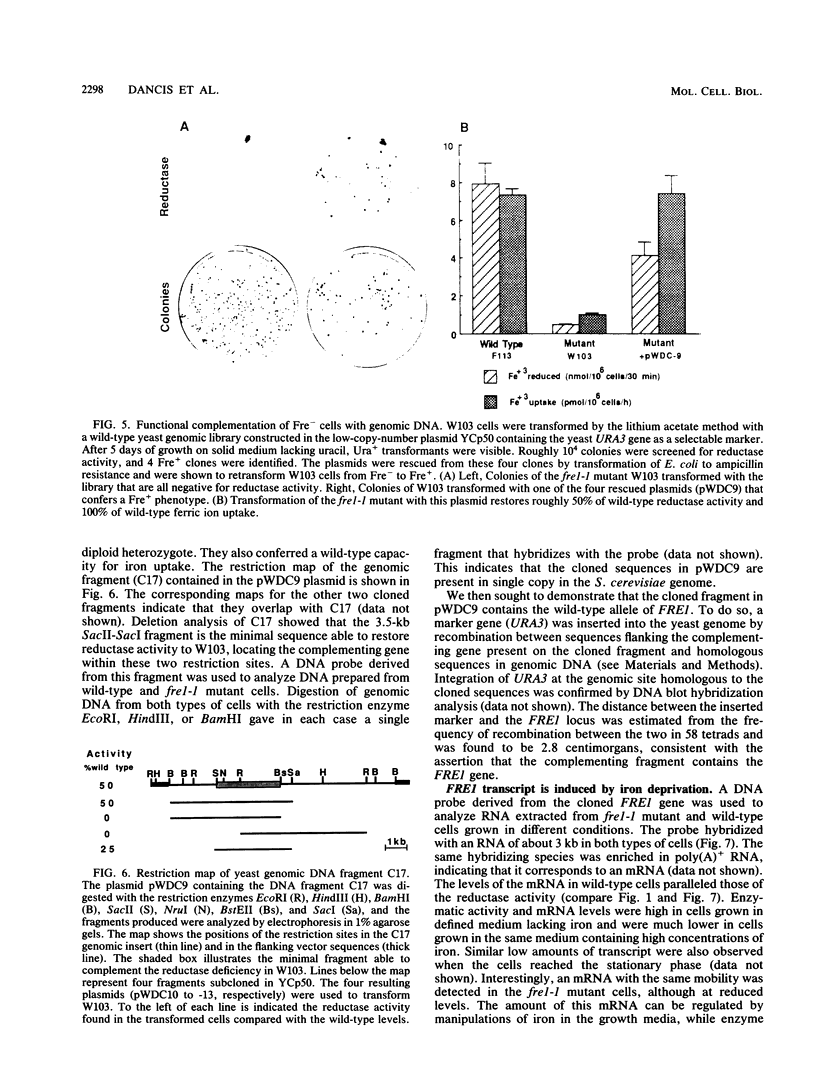
The requirement for a reduction step in cellular iron uptake has been postulated, and the existence of plasma membrane ferric reductase activity has been described in both procaryotic and eucaryotic cells. In the yeast Saccharomyces cerevisiae, there is an externally directed reductase activity that is regulated by the concentration of iron in the growth medium; maximal activity is induced by iron starvation. We report here the isolation of a mutant of S. cerevisiae lacking the reductase activity. This mutant is deficient in the uptake of ferric iron and is extremely sensitive to iron deprivation. Genetic analysis of the mutant demonstrates that the reductase and ferric uptake deficiencies are due to a single mutation that we designate fre1-1. Both phenotypes cosegregate in meiosis, corevert with a frequency of 10(-7), and are complemented by a 3.5-kilobase fragment of genomic DNA from wild-type S. cerevisiae. This fragment contains FRE1, the wild-type allele of the mutant gene. The level of the gene transcript is regulated by iron in the same was as the reductase activity. The ferrous ion product of the reductase must traverse the plasma membrane. A high-affinity (Km = 5 microM) ferrous uptake system is present in both wild-type and mutant cells. Thus, iron uptake in S. cerevisiae is mediated by two plasma membrane components, a reductase and a ferrous transport system.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M., SHAVIT N. A SENSITIVE AND SIMPLE METHOD FOR DETERMINATION OF FERROCYANIDE. Anal Biochem. 1963 Dec;6:549–554. doi: 10.1016/0003-2697(63)90149-0. [DOI] [PubMed] [Google Scholar]
- Bagg A., Neilands J. B. Mapping of a mutation affecting regulation of iron uptake systems in Escherichia coli K-12. J Bacteriol. 1985 Jan;161(1):450–453. doi: 10.1128/jb.161.1.450-453.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienfait H. F. Regulated redox processes at the plasmalemma of plant root cells and their function in iron uptake. J Bioenerg Biomembr. 1985 Apr;17(2):73–83. doi: 10.1007/BF00744199. [DOI] [PubMed] [Google Scholar]
- Bowen B. J., Morgan E. H. Anemia of the Belgrade rat: evidence for defective membrane transport of iron. Blood. 1987 Jul;70(1):38–44. [PubMed] [Google Scholar]
- Crane F. L., Roberts H., Linnane A. W., Löw H. Transmembrane ferricyanide reduction by cells of the yeast Saccharomyces cerevisiae. J Bioenerg Biomembr. 1982 Jun;14(3):191–205. doi: 10.1007/BF00745020. [DOI] [PubMed] [Google Scholar]
- Crichton R. R., Charloteaux-Wauters M. Iron transport and storage. Eur J Biochem. 1987 May 4;164(3):485–506. doi: 10.1111/j.1432-1033.1987.tb11155.x. [DOI] [PubMed] [Google Scholar]
- Eastham E. J., Bell J. I., Douglas A. P. Iron-transport characteristics of vesicles of brush-border and basolateral plasma membrane from the rat enterocyte. Biochem J. 1977 May 15;164(2):289–294. doi: 10.1042/bj1640289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egyed A. Carrier mediated iron transport through erythroid cell membrane. Br J Haematol. 1988 Apr;68(4):483–486. doi: 10.1111/j.1365-2141.1988.tb04241.x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet. 1981;182(2):288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- Iacopetta B. J., Morgan E. H. The kinetics of transferrin endocytosis and iron uptake from transferrin in rabbit reticulocytes. J Biol Chem. 1983 Aug 10;258(15):9108–9115. [PubMed] [Google Scholar]
- Klausner R. D., Van Renswoude J., Ashwell G., Kempf C., Schechter A. N., Dean A., Bridges K. R. Receptor-mediated endocytosis of transferrin in K562 cells. J Biol Chem. 1983 Apr 25;258(8):4715–4724. [PubMed] [Google Scholar]
- Lesuisse E., Raguzzi F., Crichton R. R. Iron uptake by the yeast Saccharomyces cerevisiae: involvement of a reduction step. J Gen Microbiol. 1987 Nov;133(11):3229–3236. doi: 10.1099/00221287-133-11-3229. [DOI] [PubMed] [Google Scholar]
- Myers C. R., Nealson K. H. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science. 1988 Jun 3;240(4857):1319–1321. doi: 10.1126/science.240.4857.1319. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Iron absorption and transport in microorganisms. Annu Rev Nutr. 1981;1:27–46. doi: 10.1146/annurev.nu.01.070181.000331. [DOI] [PubMed] [Google Scholar]
- Nunez M. T., Cole E. S., Glass J. The reticulocyte plasma membrane pathway of iron uptake as determined by the mechanism of alpha, alpha'-dipyridyl inhibition. J Biol Chem. 1983 Jan 25;258(2):1146–1151. [PubMed] [Google Scholar]
- Raguzzi F., Lesuisse E., Crichton R. R. Iron storage in Saccharomyces cerevisiae. FEBS Lett. 1988 Apr 11;231(1):253–258. doi: 10.1016/0014-5793(88)80742-7. [DOI] [PubMed] [Google Scholar]
- Rao K. K., Shapiro D., Mattia E., Bridges K., Klausner R. Effects of alterations in cellular iron on biosynthesis of the transferrin receptor in K562 cells. Mol Cell Biol. 1985 Apr;5(4):595–600. doi: 10.1128/mcb.5.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. D., Novick P., Thomas J. H., Botstein D., Fink G. R. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60(2-3):237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- Sun I. L., Navas P., Crane F. L., Morré D. J., Löw H. NADH diferric transferrin reductase in liver plasma membrane. J Biol Chem. 1987 Nov 25;262(33):15915–15921. [PubMed] [Google Scholar]
- Thorstensen K., Romslo I. Uptake of iron from transferrin by isolated rat hepatocytes. A redox-mediated plasma membrane process? J Biol Chem. 1988 Jun 25;263(18):8844–8850. [PubMed] [Google Scholar]
- VAN STEVENINCK, BOOIJ H. L. THE ROLE OF POLYPHOSPHATES IN THE TRANSPORT MECHANISM OF GLUCOSE IN YEAST CELLS. J Gen Physiol. 1964 Sep;48:43–60. doi: 10.1085/jgp.48.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston F., Chumley F., Fink G. R. Eviction and transplacement of mutant genes in yeast. Methods Enzymol. 1983;101:211–228. doi: 10.1016/0076-6879(83)01016-2. [DOI] [PubMed] [Google Scholar]
- Wright T. L., Brissot P., Ma W. L., Weisiger R. A. Characterization of non-transferrin-bound iron clearance by rat liver. J Biol Chem. 1986 Aug 15;261(23):10909–10914. [PubMed] [Google Scholar]
- van Renswoude J., Bridges K. R., Harford J. B., Klausner R. D. Receptor-mediated endocytosis of transferrin and the uptake of fe in K562 cells: identification of a nonlysosomal acidic compartment. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6186–6190. doi: 10.1073/pnas.79.20.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]