Abstract
Vaccination with purified capsular polysaccharide Vi antigen from Salmonella Typhi can protect against typhoid fever, although the mechanism for its efficacy is not clearly established. Here, we have characterised the B cell response to this vaccine in wild-type and T cell-deficient mice. We show that immunization with Typhim Vi rapidly induces proliferation in B1b peritoneal cells, but not in B1a cells or marginal zone (MZ) B cells. This induction of B1b proliferation is concomitant with the detection of splenic Vi-specific antibody secreting cells and protective antibody and Rag1-deficient B1b cell chimeras generated by adoptive transfer induced specific antibody after Vi immunization. Furthermore, antibody derived from peritoneal B cells is sufficient to confer protection against Salmonella that express Vi antigen. Expression of Vi by Salmonella during infection did not inhibit the development of early antibody responses to non-Vi antigens. Despite this, the protection conferred by immunization of mice with porin proteins from Salmonella, which induce antibody-mediated protection, was reduced after infection with Vi-expressing Salmonella, although protection was not totally abrogated. This work therefore suggests that in mice, B1b cells contribute to the protection induced by Vi antigen and targeting non-Vi antigens as sub-unit vaccines may offer an attractive strategy to augment current Vi-based vaccine strategies.
Keywords: B1b cells, Vi antigen, capsular polysaccharide, antibody, Salmonella Typhi
Introduction
Typhoid infections, caused by Salmonella enterica serovar Typhi (ST), are major killers responsible for over 200 000 deaths yearly (1). This organism can infect humans and other higher primates but no other animal reservoir has been recognized. This suggests that it may be possible to eliminate typhoid through the use of vaccination and other public health measures. The death toll from typhoid would be higher except for the availability of vaccines. The two well-tolerated vaccines in use in humans provide partial and temporally limited protection and are quite distinct (2-4). One is a live attenuated typhoid bacterium - strain Ty21a - and is administered orally. How this attenuated bacterium confers protection is not fully understood but antibody is likely to be important. This live vaccine lacks galE and so has an impaired capacity to synthesize LPS O-chain and also lacks the capsular polysaccharide (CP) Vi antigen, both considered major targets of protective antibody (5). The importance of antibody to Vi is evidenced by the use of purified Vi antigen as a stand-alone vaccine. Vi antigen is made of repeating units (1-4)-2-deoxy-2-N-acetyl galacturonic acid encoded within the viaB locus from ST(6). Immunization provides protection against typhoid at levels comparable to Ty21a in adults and older children in the first two years post-immunization (2, 3). The protection conferred by immunization with Vi antigen is likely to be mediated via systemic antibody as it has not been found to induce pronounced mucosal antibody responses, nor have a requirement for T cell involvement (7, 8). Therefore understanding the nature of antibody responses to Vi antigen and other vaccines based on CP is likely to be important in understanding the basis of immunity to many pathogens and improving vaccines that target them.
Classically, CP are regarded as T-independent type-II antigens with splenic marginal zone (MZ) B cells playing an important role in mediating responses to this class of antigens (9-12). This association became apparent in part because of the poor responses to CP seen in asplenic adults (13, 14), and because B cells with the MZ phenotype and responsiveness are mainly located in the spleen. Also, infants have aberrant responses to TI-2 antigens and appear to lack a mature MZ B cell compartment (15). Whilst it is clear that MZ B cells contribute to these responses (9) recent studies have shown that CP induces a more complex response than previously thought, with increasing evidence suggesting that, at least in mice, B1 cells contribute to TI-2 responses (11). Two subsets of B1 cells are recognized in mice - B1a and B1b cells (16) - and though typically found in areas such as the peritoneal cavity they can be present in other anatomical sites such as the spleen. The role of B1 cell subsets in immune responses can be complex. Thus, the phosphorylcholine moiety CP from Streptococcus pneumoniae is a target of natural antibody produced by B1a cells, but after immunization with pneumococcal CP specific-antibody is produced by B1b cells (17). Furthermore, when B1b cells are recruited into responses against model TI-2 antigens such as NP-Ficoll, then plasmablast differentiation occurs in the spleen, highlighting the importance of this site for both MZ and B1b responses (18).
B1 cells can be identified by their surface marker phenotype. B1a and B1b cells are IgM+IgDloCD21loCD23loB220intCD19+ and are CD11b variable (16, 19). B1a cells are discriminated from B1b cells by their expression of CD5. Their importance has been identified in responses to many pathogens including pneumococcus, Borrelia and Salmonella Typhimurium (STm) (17, 19, 20). Interestingly, B1b responses to antigens from these pathogens can occur in the absence of MZ B cells, indicating that B1b cells can be sufficient for protective immunity (19, 20). In light of this, and in the absence of a clear mechanism for the protection induced by Vi antigen, we studied the mode of action of the vaccine Typhim Vi in mice. We show that peritoneal B1b cells, but not other B cell subsets, are selectively induced to proliferate and differentiate to antibody producing cells in response to immunization with purified Vi antigen in a TI manner. Furthermore, transfer of peritoneal B cells is sufficient to confer protection against infection with a STm strain engineered to express Vi antigen (STmVi+). Lastly, we show that Vi expression by Salmonella during infection impairs, but does not ablate, the capacity of antibody to other antigens to control infection. This work further emphasizes the importance of B1b cells in conferring protective responses to bacterial pathogens.
Materials and Methods
Animals, bacteria, antigens and immunizations
Animal studies were performed with ethical approval and within Home Office guidelines. Age and sex-matched C57BL/6J (WT) were obtained from HO Harlan Laboratories OLAC (Bicester, UK) and TCRβδ−/− mice from Jax. Mouse colonies were maintained in the Biomedical Services Unit, University of Birmingham under specific pathogen free conditions. Mice were immunized with 10μg Typhim Vi (Sanofi Pasteur MSD, Maidenhead UK), 20μg porins (21) or 5×105 STm AroC-deficient bacteria or 5×105 STmVi+ AroC-deficient bacteria. STm strains containing an aroC deletion and that either express Vi (strain RAK109) or an isogenic strain that is Vi negative due to deletion of the tviB gene (strain RAK112) were constructed by modification of previously described strains, C5.507 (strain C5 containing ~300kb of S. Typhi genomic sequence including the SPI-7 island encoding the viaB locus) and SGB1 (C5.507 ΔtviB::cat) (22). A strain in which the aroC gene was replaced by the cat gene was constructed using red recombinase mediated allelic exchange in strain SL1344 containing pSIM18 using a PCR product amplified from pKD3 using primers 5′ gcgctactgacaaaccatgccagcagcgcaatcgcggtttttttcatttcTGTGTAGGCTGGAGCTGCTTCG 3′ and 5′ atttataaagattaaaacacgcaaacgacaacaacgataacggagccgtgCATATGAATATCCTCCTTAG 3′ (23, 24). These primers were designed to precisely delete the aroC gene and replace it with the cat gene. The aroC deletion was transferred by P22 mediated transduction by selecting for the aroC::cat using chloramphenicol in the recipient strain. Transduction into recipient strain C5.507 gave rise to strain RAK109, and transduction into a strain derived from strain SGB1 by removal of the cat gene from the tviB locus (maintaining the deletion of tviB) by expression of FLP recombinase from plasmid pCP20, gave rise to strain RAK112. At the end of the experiment peritoneal exudate cells, spleens and serum were extracted for further analysis. TLR grade STm LPS was purchased from Axxora and FliC was generated as described elsewhere (25). To generate STm OmpA, the transmembrane domain (residues 22-211) plus an N terminal (MHHHHHHSSC-) purification tag was synthesised and cloned into pET8c (Integrated DNA Technologies, Glasgow) as described in (26) to create plasmid pStOmpA. The StOmpA protein was expressed from plasmid pStOmpA as inclusion bodies in E. coli, purified and refolded described previously (27).
For opsonisation experiments a single serum was used per mouse and all sera were heat-inactivated at 56 °C for 0.5 h. Bacteria (2.5 × 106/mL) and sera (1:1-200) were mixed for 0.5 h before infection and bacterial viability and lack of agglutination confirmed.
Flow cytometry
For flow cytometric analysis single cell suspensions of spleen or peritoneal exudate cells were blocked using CD16/32 antibody (eBiosciences, Hatfield, UK) and were subsequently stained using combinations of the following; CD23 PE, B220 PE Texas Red, CD5 PE Cy5, CD11b Pacific Blue, CD21 APC, CD19 APC Cy7 (all BD Biosciences, Oxford, UK) and IgM PE Cy7 (eBiosciences). When Ki67 antibody staining was required cell suspensions were fixed and stained intracellularly using the Fix/perm kit from BD Biosciences according to the manufacturer’s instructions and then stained using the FITC Mouse Anti-Human Ki-67 Set which cross reacts with mouse (BD Biosciences). Samples were acquired using a CyAn Flow Cytometer (Beckman Coulter, High Wycombe, UK) and analyzed using FlowJo software (TreeStar, Oregon, USA).
Immunohistology
6μm spleen sections were stained using anti mouse antibodies: IgD (Abcam, Cambridge, UK), IgG3 and CD138 (BD Biosciences) and IgM (Abd Serotec, Kidlington, UK) for 1 hr in Tris buffer as described elsewhere (28). Secondary reagents were donkey anti sheep HRP (The Binding Site, Birmingham, UK) and Rabbit anti Rat biotin (Dako, Cambridge, UK) for 45 minutes followed by streptavidin conjugated alkaline phosphatase (Dako) for 30 minutes. Colors were developed using 3,3′-Diaminobenzidine tetrahydrochloride and Fast blue substrates. CD138+ cells were evaluated by point-counting technique
ELISA and ELISPOT
Serum antibody to Vi or STm and its antigens was assessed by enzyme-linked immunosorbent assay (ELISA) as described previously (29) and Vi-specific antibody producing cells were detected by enzyme-linked immunosorbent spot (ELISPOT) assay. Briefly, ELISA was performed on Nunc Immunosorb 96 well plates (Nunc) coated with 5 μg/ml of antigen in carbonate buffer. Serum was added at a 1/50 dilution and was titrated in 3-fold steps. Bound antibody was detected using goat anti-IgM, IgG3 or IgG conjugated to alkaline phosphotase (Southern Biotech) followed by p-Nitrophenyl phosphate (Sigma). Plates were read at 405nm to determine absorbance. Relative antibody titres were calculated after plotting the OD of each well against the serum dilution and were derived from linear portion of the resulting curves.
ELISPOT was carried out on pre wetted MultiScreenHTS IP 96 well plates (Millipore, Billerica, USA) coated with 5μg/ml Typhim Vi. Plates were blocked with 1% BSA PBS. 105 splenocytes or peritoneal exudate cells were added to each well and plates were incubated for 6 hours at 37°C at 5% CO2. After incubation, plates were washed and then anti-IgM antibody conjugated to alkaline phosphatase (Southern Biotech) was added in 1% BSA PBS over night at 4°C. Final detection was carried out using 5-Bromo-4-chloro-3-indolyl phosphate/Nitro blue tetrazolium (Sigma). Spots were counted using an AID ELISPOT reader system (Autoimmun Diagnostika GmbH, Strassburg, Germany) with Eli4 software (Autoimmun Diagnostika GmbH).
Cell transfer
Peritoneal B cells were harvested from TCRβδ−/− or WT mice. 106 peritoneal B cells were transferred into recipient C57BL/6 Rag1−/− mice i.v. Peritoneal cells were sorted using a MoFlow cell sorter (Beckman Coulter) after staining with CD23 PE, CD5 PE-Cy5 and CD3 FITC and sort purity assessed using B220 APC. 2×105 cells were transferred i.v. into C57BL/6 Rag1−/− mice as above. Recipient mice were left for 2 weeks and reconstitution confirmed by performing ELISA for IgM antibody from tail bleeds. After reconstitution mice were immunized as stated in the text.
Statistics
All statistics were calculated using the non parametric Mann-Whitney U test with p ≤ 0.05 accepted as significant.
Results
Typhim Vi induces a T cell independent antibody response
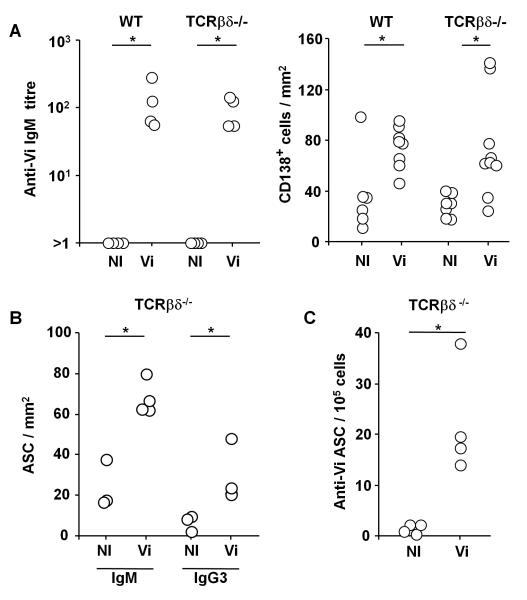
The response to Vi antigen in WT mice was assessed 4 days after i.p. immunization with 10 μg of purified Vi. This antigen induced a rapid induction of Vi-specific serum IgM antibody in parallel with a significant increase in CD138+ splenic antibody secreting cells (ASC) at this time (Fig 1A). This response did not require T cells since T cell-deficient (TCRβδ−/−) mice, that lack all T cells, induced a similar response (Fig. 1A). Thus purified Vi induces a classical T-independent response.
FIGURE 1.
The response to Vi antigen is T-independent. C57BL/6J (WT) and TCRβδ−/− mice were either non-immunized (NI) or immunized i.p. with 10 μg Vi antigen (Vi) for 4d. A, Vi-specific IgM antibody titres determined by serum ELISA (left panel) and the density of of splenic CD138+ cells determined by immunohistology (right panel). B, Quantification of the IgM and IgG3 ASC response in TCRβδ−/− mice with and without immunization with Vi antigen. C, ELISPOT to quantify Vi-specific IgM antibody secreting cells (ASC) in the spleens of NI or Vi antigen immunized TCRβδ−/− mice. Representative of 2 experiments. * = p ≤ 0.05 by two-tailed Mann Whitney U test.
Further assessment of this response in TCRβδ−/− mice at day 4 post-immunization reveals that IgM+ ASC dominated the response, but some IgG3 switched ASC were also observed (Fig 1B). The specificity of this antibody response was confirmed by detecting ASC that secreted Vi-specific antibody by ELISPOT (Fig. 1C). Whilst anti-Vi ASC were readily detectable in the spleen, no anti-Vi ASC were detected in the peritoneal cavity at any stage after immunization (data not shown). Thus, Vi antigen induces T-independent responses that result in Vi-specific ASC development in the spleen.
Vi antigen induces similar responses after immunization through different routes
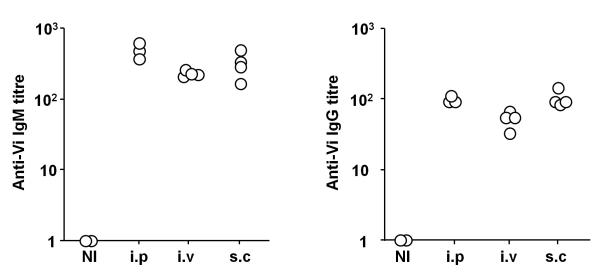
To assess whether the route of immunization affected the T-independent response to Vi antigen, TCRβδ−/− mice were immunized i.p. or i.v. in the tail vein or s.c. over the shoulder and responses assessed 7 days later (Fig. 2). These experiments show that immunization via all routes induced similar IgM and IgG anti-Vi titres, although the response after i.v. immunization was marginally lower than that after i.p. immunization. Thus, route of immunization has little effect on the T-independent antibody response induced to Vi antigen.
FIGURE 2.
The T-independent antibody response to Vi antigen is not dependent upon injection route. TCRβδ−/− mice were immunized with 10 μg Vi antigen i.p., i.v. or s.c. for 7d. IgM (left) and total IgG (right) Vi-specific serum antibody titres were determined by ELISA. Data is representative of 2 independent experiments. NI = non-immunized.
Vi immunization selectively induces B1b cell proliferation
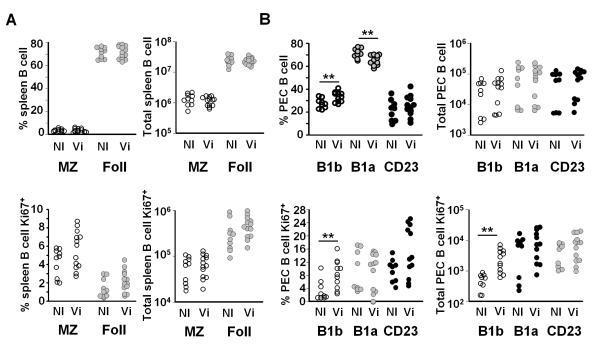
To study which B cell populations respond to Vi, changes in the distribution and proliferative state of splenic and peritoneal B cell subsets was assessed in TCRβδ−/− mice by flow cytometry 4 days after i.p. immunization with 10μg Vi. In the spleen, no significant difference in the proportion or numbers of marginal zone or follicular CD23+ B cells was seen after immunization (Fig. 3A). Nor was there a significant difference in the proportion or absolute numbers of these splenic B cell subsets in cell cycle as determined by Ki67 expression using flow cytometry (Fig. 3A). This was the case independent of whether Vi antigen was given i.p. or i.v. (data not shown). In contrast, examination of the response in peritoneal B1a, B1b and CD23+ cell subsets revealed there was a significant increase in the proportion of B1b cells after i.p. immunization, although not in absolute numbers (Fig. 3B). Furthermore, there was a marked increase in the proportion and numbers of B1b cells expressing Ki67, suggesting they had entered cell cycle in response to immunization with Vi antigen (Fig. 3B). This increase in Ki67 expression was not seen in other peritoneal B cell subsets. Therefore peritoneal B1b cells are induced to proliferate in response to Vi antigen.
FIGURE 3.
Vi antigen selectively induces peritoneal B1b cells to proliferate. Non-immunized (NI) TCRβδ−/− mice or TCRβδ−/− mice were immunized with 10 μg Vi antigen (Vi) i.p. and 4 days later the splenic and peritoneal B cell responses were assessed by flow cytometry. A, The percentage and number of all B cells in splenic B cell subsets (top). Marginal zone B cells, MZ, were identified as IgM+CD19+B220+CD21+CD23low/− and follicular B cells as IgM+CD19+B220+CD23+CD21low B cells, Foll = follicular. The bottom graphs show the numbers and proportion of these subsets that are Ki67+. B, The number and proportion of peritoneal B1 and recirculating B cells, from the same TCRβδ−/− mice as A above, that were in the B1a, B1b and CD23 subset. All B1 cells were identified as IgM+CD19+CD21−CD23− B220+ cells and B1a cells by coexpression of CD5. CD23 cells were IgM+ CD19+ CD23+ CD21−. The bottom graphs show the number and proportion of peritoneal B1 and recirculating B cells that express Ki67. For gating protocols see Supp Fig 1 and methods. NS = not significant, ** = p ≤ 0.01 as assessed by two-tailed Mann Whitney U test. Pooled data from 3 experiments.
Peritoneal B1b cells are sufficient to generate antibody against Vi antigen
As the above data suggest that B1b cells could respond to Vi we assessed whether peritoneal B cells were sufficient to generate protective immunity after Vi immunization. B cell chimeras were generated by transferring 106 peritoneal cells from non-immunized T cell-deficient mice into Rag1-deficient mice that lack both T and B cells. Mice were left to reconstitute for 14 days and success of reconstitution was confirmed by examining tail-vein blood for the presence of total IgM by ELISA. Some chimeras were then immunized with 10 μg Vi and at day 7 antibody titres compared between non-immunized and immunized chimeras. This showed that whilst anti-Vi serum IgM was undetectable in non-immunized controls, anti-Vi IgM was detectable after immunization (Fig. 4A). To dissect this response further, B cell subset Rag1−/− chimeras were generated by transferring 2×105 cell sorted B1a or B1b or CD23+ peritoneal B cells. After 14 days, assessment of tail-bleeds from the chimeras showed that they all had similar levels of serum IgM, indicating successful B cell reconstitution. In the absence of immunization chimeras lacked anti-Vi antibody. Chimeras were then immunized with 10 μg Vi and at day 11 anti-Vi titers were assessed. All B1b chimeras generated anti-Vi antibody in response to Vi, whereas in the other groups responses were either absent or weak (Fig. 4B). Therefore, peritoneal B1b cells are sufficient to respond to Vi antigen.
FIGURE 4.
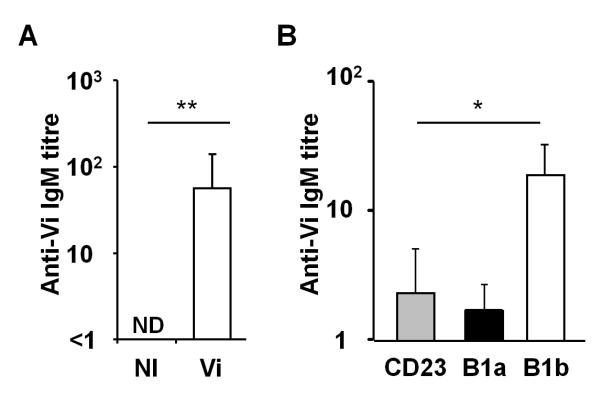
Peritoneal B cells are sufficient to produce anti Vi antibody. A, Peritoneal B cell chimeras were generated by transferring 106 peritoneal cells from TCRβδ−/− mice into B6 Rag−/− mice and allowing these cells to reconstitute for 14 days. After this, chimeras were either non-immunized (NI) or immunized i.p. with 10 μg Vi antigen and 7 days later Vi-specific serum IgM antibody titres were assessed by ELISA. B, B cell subset chimeras were generated by transferring sorted B1a, B1b or CD23+ B cells into Rag1−/− mice and allowing these cells to reconstitute for 14 days. Mice were immunized i.p. with 10 μg Vi antigen and 11 days later Vi-specific serum IgM antibody titres were assessed by ELISA. Graph shows mean and 1 S.D. * = p ≤ 0.05; ** = p ≤ 0.01, two-tailed Mann Whitney U test. ND = not detected.
Antibody from Vi-immunized peritoneal B cell chimeras is sufficient to impair infection
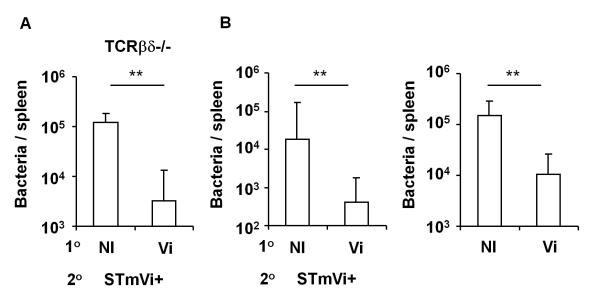
Next, we tested whether T cell independent antibody against Vi could protect against Salmonella infection by challenging mice with an AroC-derivative of STm strain C5.507 engineered to express Vi on its surface (called STmVi+ and detailed in (22, 30)). T cell-deficient mice were immunized i.p. with 10 μg Vi for 14 days before i.p. challenge with STmVi+. Four days after infection, bacterial burdens were assessed in the spleen. This showed that median bacterial numbers were >10-fold lower in immunized mice compared to non-immunized controls (Fig. 5A). These experiments were repeated by challenging non-immunized or Vi immunized peritoneal B cell chimeras. This shows that Vi immunized B cell chimeras had lower bacterial burdens than non-immunized control chimeras (Fig. 5B left panel). To show that antibody was the protective component in these experiments STmVi+ bacteria were opsonised immediately before injection with complement-inactivated sera from Vi-immunized or non-immunized B cell chimeras. Mice infected with bacteria opsonised with anti-Vi antibody had lower levels of bacterial colonization compared to those mice that received bacteria opsonised with sera from non-immunized mice (Fig. 5B right panel). Therefore, antibody to Vi induced in a T-independent manner from peritoneal B cells is sufficient to impair bacterial colonization by STmVi+.
FIGURE 5.
Antibody derived from B1 cells is sufficient to protect against infection with Vi-expressing Salmonella. A, TCRβδ−/− mice were either immunized with 10 μg Vi antigen (Vi) i.p. injection or remained non-immunized (NI). After 14 days mice were challenged with 106 STmVi+ and splenic bacterial burdens assessed 4 days after infection. Data from 2 experiments combined. B, Left graph shows bacterial burdens in NI peritoneal B cell chimeras (generated as in Fig. 4) and peritoneal B cell chimeras immunized for 14 days with Vi antigen before challenge with 106 STmVi+ for 4 days. Right graph shows splenic bacterial burdens from mice that had been infected with STmVi+ opsonised with sera from naive (NI) or from Vi antigen-challenged peritoneal B cell chimeras. Graphs show mean and S.D from two independent experiments combined. ** = p ≤ 0.01, Mann Whitney two-tailed U test.
Bacterial Vi expression does not prevent antibody-mediated protection to other antigens
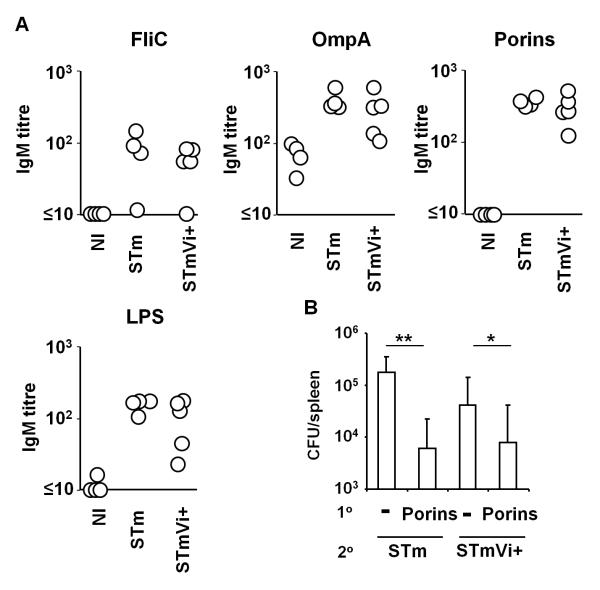
Vi can promote immunoevasion through the inhibition of IL-8-mediated neutrophil recruitment (31, 32). To assess whether Vi expression can also promote immunoevasion through reducing the induction of antibody responses to non-Vi antigens we examined the early IgM antibody response to LPS, porins (OmpF, C and D), flagellin and OmpA 7 days after primary i.p. infection with 5×105 STm or STmVi+. This shows that at day 7 post-infection IgM titres were similar irrespective of Vi expression (Fig. 6A). Therefore, Vi expression does not inhibit the development of the early antibody response to STm. Finally, we assessed whether Vi expression can affect antibody-mediated protection after immunization with non-Vi antigens. To do this mice were immunized with 20 μg purified porins (OmpC, OmpD and OmpF), which can provide protection via an antibody-dependent mechanism (19). Fourteen days later we infected porin-immunized mice alongside non-immunized controls with STm or STmVi+ (Fig. 6B). Three days after infection it was apparent that porin immunization reduced bacterial colonization independently of Vi expression but that this reduction was more pronounced in mice infected with non-Vi expressing STm (median 28 fold reduction for STm compared to 6 fold for STmVi+). This suggests that although Vi expression does not inhibit the development of antibody responses to non-Vi antigens it can provide some limited, protection against non-Vi targeting antibody.
FIGURE 6.
Expression of Vi antigen does not prevent antibody-mediated protection induced by porins. A, TCRβδ−/− mice were infected with 106 STm or STmVi+ by i.p. injection for 4 days and serum antibody responses to FliC, OmpA, porins and LPS in these mice and control non-immunized (NI) mice were assessed by ELISA. Data representative of 4 independent experiments. B, Splenic bacterial burdens in non-immunized mice and mice i.p. immunized with 20 μg porins 14 days before and subsequently challenged with STm or STmVi+ for 3 days. * = p ≤ 0.05, ** = p ≤ 0.01 by the two-tailed Mann Whitney U test. Graph shows mean and 1 S.D.
Discussion
In this study we show that B1b cells respond to Vi antigen and that anti-Vi antibody derived from peritoneal cells is sufficient to impair infection with a Vi-expressing strain of STm. Finally, Vi expression was shown not to ablate antibody-mediated protection to heterologous antigens. Vaccines derived from CP have been important tools in controlling a host of infections including typhoid (2). In recent years numerous studies in mice have implicated a role for B1 cells, and particularly B1b cells, against CP and non-proteinaceous antigens such as the bacterial polysaccharide α-1,3 dextran and Ficoll (17, 18, 33). In addition B1b cells are important in responses to proteins from other pathogens such as Borrelia and STm and the response to these proteins can be T-independent (19, 20). One of the reasons B1 cells have received limited attention has been the lack of a clear counterpart in humans, in part because many non-B1 human B cells express CD5. Indirect evidence that the immunological mechanisms are conserved to TI-2 antigens, such as CP, in mice and humans is the similarity in the nature of these responses (34). These include a hyporesponsiveness in infants and a limited longevity of the response and the recognition by human B cells of known murine B1b antigens (35). However, a purported B1 phenotype (CD20+CD27+CD43+CD70-) in humans has recently been described and a B1-like cell population has been identified in the blood of patients with common variable immunodeficiency (36, 37). Future studies will assess whether further phenotyping into multiple subsets is possible. A second concern about the relationship between B1 cells and responses to TI-2 antigens has been the importance of the role of MZ B cells in these responses (9, 11). Nevertheless, evidence suggests that B1b cells in infant mice respond poorly to antigen despite being present in near normal numbers (38), thus resembling the diminished MZ response seen in infants. There is evidence that this is because in infant mice B1b cells lack sensitivity to IL-7 (38). Therefore, for differing reasons, MZ and B1b responses may both be defective in infant mice. In contrast to B1b cells, B1a cells do not appear to be recruited in response to CP (17). If a similar situation occurs in humans, it may explain the reduced or absent of responsiveness to CP in infants under 5. Lastly, the potential importance of B cell subsets other than MZ B cells, including B1b cells, is highlighted by experiments performed in mice that are MZ cell-deficient (9). These mice can mount responses to pneumococcal CP with only marginally impaired IgM responses. This suggests there is some redundancy in responses to CP, perhaps because of a selective advantage conferred by being able to respond to TI-2 antigens. Therefore it is likely that antibody-producing cells responding to CP can derive from multiple B cell subsets.
It is significant that the genus Salmonella contains multiple B1b antigens - Vi and the porins OmpC, D and F(19) and suggests that naturally occurring B1b antigens are likely to be more common than previously thought. The antigens do not share any obvious structural similarities, but porins do share the property of Vi antigen to form oligomers and thus present the immune system with numerous repeating epitopes (39). Since antibody targeted to the porin OmpD from STm and Vi antigen from ST can protect against infection, it offers the tantalizing possibility that there may be a high frequency of protective antigens that are recognized by B1b cells. Therefore, characterizing B1b responses to bacteria may be a profitable way to identify novel vaccine candidates to a range of pathogens.
We have previously shown that the early, extrafollicular antibody response to STm occurs with unusually rapid kinetics (28), suggesting that there is no limit in antigen availability to drive such an extensive response. In the current study, expression of Vi in STm did not markedly impair the development of the antibody responses to a number of STm antigens such as OmpA, porins, LPS or FliC. This suggests that Vi antigen does not necessarily act to restrict antigen availability from B cells at any significant level. During primary infection antibody does not control bacterial clearance (40, 41). This is consistent with the major effect of Vi antigen being to reduce innate stimulation, cell recruitment and uptake after infection and the production of cytokines rather than to limit B cell responses (31, 42, 43). This is consistent with a role of Vi expression in supporting the dissemination of the organism through the host.
The failure of Vi expression to impair the development of primary antibody responses led us to assess whether antibody to non-Vi antigens can still moderate infection when mice were challenged with Vi-expressing STm. We did this by assessing whether immunizing mice with porins and subsequently infecting with Vi+ and Vi− STm affected the protection afforded. The advantage of using porins in these types of experiments is that porin molecules are integral outer membrane proteins that only have relatively short surface loops exposed from the surface of the organism (39). In this situation anti-porin antibodies were still protective, although the fold reduction in bacterial colonization was not as great as in the absence of Vi. Indeed antibodies to typhoid porins are bactericidal to Vi-expressing ST strains (44). Therefore at best, Vi expression provides partial protection against non-Vi antibody-mediated immunogen. This is significant and encouraging, since it indicates that targeting antigens other than Vi may also offer protective immunity. Further support is evidenced from humans immunized with Ty21a - a vaccine that is protective despite lacking Vi antigen and full competency for LPS O-chain expression (5). This may be significant for the development of future vaccines against typhoid. Although the current purified Vi vaccine is likely to be superseded by more sophisticated conjugated Vi vaccines (45, 46), which may offer greater, longer lasting protection in all age ranges. Nevertheless, since in some instances the use of conjugated vaccines can be problematic (34) it may necessitate the development of other anti-typhoid vaccines that are not Vi-derived. If so then the current study suggests that other non-Vi antigens will be effective protective targets for antibody.
Supplementary Material
Acknowledgments
We are grateful to staff at the Biomedical Services Unit, University of Birmingham for their help and support.
1 This work is supported by a grant from the U.K. Medical Research Council to A.F.C and by National Council for Science and Technology (CONACYT) grants: SEP-2003-CO2-45261, SALUD 2004-01-132, and SALUD-2007-C01-69779 for CL-M.
3 Abbreviations used in this paper
- ASC
antibody secreting cells
- APC
allophycocyanin
- ST
Salmonella enterica serovar Typhi
- STm
Salmonella enterica serovar Typhimurium
- WT
wild-type
Footnotes
Disclosures The authors have no financial conflicts of interest.
References
- 1.Crump JA, Mintz ED. Global trends in typhoid and paratyphoid Fever. Clin Infect Dis. 2010;50:241–246. doi: 10.1086/649541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engels EA, Falagas ME, Lau J, Bennish ML. Typhoid fever vaccines: a meta-analysis of studies on efficacy and toxicity. BMJ. 1998;316:110–116. doi: 10.1136/bmj.316.7125.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fraser A, Paul M, Goldberg E, Acosta CJ, Leibovici L. Typhoid fever vaccines: Systematic review and meta-analysis of randomised controlled trials. Vaccine. 2007;25:7848–7857. doi: 10.1016/j.vaccine.2007.08.027. [DOI] [PubMed] [Google Scholar]
- 4.Guzman CA, Borsutzky S, Griot-Wenk M, Metcalfe IC, Pearman J, Collioud A, Favre D, Dietrich G. Vaccines against typhoid fever. Vaccine. 2006;24:3804–3811. doi: 10.1016/j.vaccine.2005.07.111. [DOI] [PubMed] [Google Scholar]
- 5.Germanier R, Furer E. Isolation and Characterization of Gal-E Mutant Ty 21a of Salmonella-Typhi - Candidate Strain for a Live, Oral Typhoid Vaccine. J. Infect. Dis. 1975;131:553–558. doi: 10.1093/infdis/131.5.553. [DOI] [PubMed] [Google Scholar]
- 6.Raffatellu M, Wilson RP, Winter SE, Baumler AJ. Clinical pathogenesis of typhoid fever. J Infect Dev Ctries. 2008;2:260–266. doi: 10.3855/jidc.219. [DOI] [PubMed] [Google Scholar]
- 7.Breen JF, Apicella MA. Immunogenicity of gonococcal Gc2 polysaccharide: comparative studies with pneumococcal type III polysaccharide and Salmonella typhosa Vi antigen. Infect Immun. 1978;22:195–199. doi: 10.1128/iai.22.1.195-199.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lake JP, Reed ND, Ulrich JT, Varitek VA. Development of a localized hemolysis-in-gel assay for Vi antigen: characterization of the Vi-specific PFC response of nude and normal mice. Immunol Commun. 1977;6:149–165. doi: 10.3109/08820137709055808. [DOI] [PubMed] [Google Scholar]
- 9.Guinamard R, Okigaki M, Schlessinger J, Ravetch JV. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat Immunol. 2000;1:31–36. doi: 10.1038/76882. [DOI] [PubMed] [Google Scholar]
- 10.Martin F, Kearney JF. Marginal-zone B cells. Nature reviews. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 11.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 12.Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Annu Rev Immunol. 2009;27:267–285. doi: 10.1146/annurev.immunol.021908.132607. [DOI] [PubMed] [Google Scholar]
- 13.Amlot PL, Grennan D, Humphrey JH. Splenic dependence of the antibody response to thymus-independent (TI-2) antigens. Eur J Immunol. 1985;15:508–512. doi: 10.1002/eji.1830150516. [DOI] [PubMed] [Google Scholar]
- 14.Amlot PL, Hayes AE. Impaired human antibody response to the thymus-independent antigen, DNP-Ficoll, after splenectomy. Implications for post-splenectomy infections. Lancet. 1985;1:1008–1011. doi: 10.1016/s0140-6736(85)91613-7. [DOI] [PubMed] [Google Scholar]
- 15.Timens W, Boes A, Rozeboom-Uiterwijk T, Poppema S. Immaturity of the human splenic marginal zone in infancy. Possible contribution to the deficient infant immune response. J Immunol. 1989;143:3200–3206. [PubMed] [Google Scholar]
- 16.Stall AM, Adams S, Herzenberg LA, Kantor AB. Characteristics and development of the murine B-1b (Ly-1 B sister) cell population. Ann N Y Acad Sci. 1992;651:33–43. doi: 10.1111/j.1749-6632.1992.tb24591.x. [DOI] [PubMed] [Google Scholar]
- 17.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Hsu MC, Toellner KM, Vinuesa CG, Maclennan IC. B cell clones that sustain long-term plasmablast growth in T-independent extrafollicular antibody responses. Proc Natl Acad Sci U S A. 2006;103:5905–5910. doi: 10.1073/pnas.0601502103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gil-Cruz C, Bobat S, Marshall JL, Kingsley RA, Ross EA, Henderson IR, Leyton DL, Coughlan RE, Khan M, Jensen KT, Buckley CD, Dougan G, MacLennan ICM, LÃ3pez-MacÃ-as C, Cunningham AF. The porin OmpD from nontyphoidal Salmonella is a key target for a protective B1b cell antibody response. Proceedings of the National Academy of Sciences. 2009;106:9803–9808. doi: 10.1073/pnas.0812431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 21.Salazar-Gonzalez RM, Maldonado-Bernal C, Ramirez-Cruz NE, Rios-Sarabia N, Beltran-Nava J, Castanon-Gonzalez J, Castillo-Torres N, Palma-Aguirre JA, Carrera-Camargo M, Lopez-Macias C, Isibasi A. Induction of cellular immune response and anti-Salmonella enterica serovar typhi bactericidal antibodies in healthy volunteers by immunization with a vaccine candidate against typhoid fever. Immunology letters. 2004;93:115–122. doi: 10.1016/j.imlet.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Jansen AM, Hall LJ, Clare S, Goulding D, Holt KE, Grant AJ, Mastroeni P, Dougan G, Kingsley RA. A salmonella typhimurium-typhi genomic chimera: a model to study vi polysaccharide capsule function in vivo. PLoS Pathog. 2011;7:e1002131. doi: 10.1371/journal.ppat.1002131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan W, Costantino N, Li R, Lee SC, Su Q, Melvin D, Court DL, Liu P. A recombineering based approach for high-throughput conditional knockout targeting vector construction. Nucleic Acids Res. 2007;35:e64. doi: 10.1093/nar/gkm163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bobat S, Flores-Langarica A, Hitchcock J, Marshall JL, Kingsley RA, Goodall M, Gil-Cruz C, Serre K, Leyton DL, Letran SE, Gaspal F, Chester R, Chamberlain JL, Dougan G, Lopez-Macias C, Henderson IR, Alexander J, MacLennan IC, Cunningham AF. Soluble flagellin, FliC, induces an Ag-specific Th2 response, yet promotes T-bet-regulated Th1 clearance of Salmonella typhimurium infection. Eur J Immunol. 2011;41:1606–1618. doi: 10.1002/eji.201041089. [DOI] [PubMed] [Google Scholar]
- 26.Politou AS, Gautel M, Pfuhl M, Labeit S, Pastore A. Immunoglobulin-type domains of titin: same fold, different stability? Biochemistry. 1994;33:4730–4737. doi: 10.1021/bi00181a604. [DOI] [PubMed] [Google Scholar]
- 27.Le Brun AP, Holt SA, Shah DS, Majkrzak CF, Lakey JH. The structural orientation of antibody layers bound to engineered biosensor surfaces. Biomaterials. 2011;32:3303–3311. doi: 10.1016/j.biomaterials.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 28.Cunningham AF, Gaspal F, Serre K, Mohr E, Henderson IR, Scott-Tucker A, Kenny SM, Khan M, Toellner KM, Lane PJ, MacLennan IC. Salmonella induces a switched antibody response without germinal centers that impedes the extracellular spread of infection. J Immunol. 2007;178:6200–6207. doi: 10.4049/jimmunol.178.10.6200. [DOI] [PubMed] [Google Scholar]
- 29.Cunningham AF, Serre K, Mohr E, Khan M, Toellner KM. Loss of CD154 impairs the Th2 extrafollicular plasma cell response but not early T cell proliferation and interleukin-4 induction. Immunology. 2004;113:187–193. doi: 10.1111/j.1365-2567.2004.01951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janis C, Grant AJ, McKinley TJ, Morgan FJ, John VF, Houghton J, Kingsley RA, Dougan G, Mastroeni P. In vivo regulation of the Vi antigen in Salmonella and induction of immune responses with an in vivo-inducible promoter. Infect Immun. 2011;79:2481–2488. doi: 10.1128/IAI.01265-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raffatellu M, Chessa D, Wilson RP, Dusold R, Rubino S, Baumler AJ. The Vi capsular antigen of Salmonella enterica serotype Typhi reduces Toll-like receptor-dependent interleukin-8 expression in the intestinal mucosa. Infect Immun. 2005;73:3367–3374. doi: 10.1128/IAI.73.6.3367-3374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raffatellu M, Chessa D, Wilson RP, Tukel C, Akcelik M, Baumler AJ. Capsule-mediated immune evasion: a new hypothesis explaining aspects of typhoid fever pathogenesis. Infect Immun. 2006;74:19–27. doi: 10.1128/IAI.74.1.19-27.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foote JB, Kearney JF. Generation of B cell memory to the bacterial polysaccharide alpha-1,3 dextran. J Immunol. 2009;183:6359–6368. doi: 10.4049/jimmunol.0902473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez-Fernandez A, Faro J, Fernandez C. Immune responses to polysaccharides: lessons from humans and mice. Vaccine. 2008;26:292–300. doi: 10.1016/j.vaccine.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 35.Vuyyuru R, Liu H, Manser T, Alugupalli KR. Characteristics of Borrelia hermsii infection in human hematopoietic stem cell-engrafted mice mirror those of human relapsing fever. Proc Natl Acad Sci U S A. 2011;108:20707–20712. doi: 10.1073/pnas.1108776109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rakhmanov M, Keller B, Gutenberger S, Foerster C, Hoenig M, Driessen G, van der Burg M, van Dongen JJ, Wiech E, Visentini M, Quinti I, Prasse A, Voelxen N, Salzer U, Goldacker S, Fisch P, Eibel H, Schwarz K, Peter HH, Warnatz K. Circulating CD21low B cells in common variable immunodeficiency resemble tissue homing, innate-like B cells. Proc Natl Acad Sci U S A. 2009;106:13451–13456. doi: 10.1073/pnas.0901984106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shriner AK, Liu H, Sun G, Guimond M, Alugupalli KR. IL-7-dependent B lymphocytes are essential for the anti-polysaccharide response and protective immunity to Streptococcus pneumoniae. J Immunol. 2010;185:525–531. doi: 10.4049/jimmunol.0902841. [DOI] [PubMed] [Google Scholar]
- 39.Nikaido H. Molecular Basis of Bacterial Outer Membrane Permeability Revisited. Microbiology and molecular biology reviews. 2003;67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McSorley SJ, Jenkins MK. Antibody is required for protection against virulent but not attenuated Salmonella enterica Serovar Typhimurium. Infect Immun. 2000;68:3344–3348. doi: 10.1128/iai.68.6.3344-3348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mastroeni P, Simmons C, Fowler R, Hormaeche CE, Dougan G. Igh-6−/− (B-cell-deficient) mice fail to mount solid acquired resistance to oral challenge with virulent Salmonella enterica serovar typhimurium and show impaired Th1 T-cell responses to Salmonella antigens. Infect Immun. 2000;68:46–53. doi: 10.1128/iai.68.1.46-53.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson RP, Raffatellu M, Chessa D, Winter SE, Tukel C, Baumler AJ. The Vi-capsule prevents Toll-like receptor 4 recognition of Salmonella. Cell Microbiol. 2008;10:876–890. doi: 10.1111/j.1462-5822.2007.01090.x. [DOI] [PubMed] [Google Scholar]
- 43.Wilson RP, Winter SE, Spees AM, Winter MG, Nishimori JH, Sanchez JF, Nuccio SP, Crawford RW, Tukel C, Baumler AJ. The Vi capsular polysaccharide prevents complement receptor 3-mediated clearance of Salmonella enterica serotype Typhi. Infect Immun. 2011;79:830–837. doi: 10.1128/IAI.00961-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Secundino I, Lopez-Macias C, Cervantes-Barragan L, Gil-Cruz C, Rios-Sarabia N, Pastelin-Palacios R, Villasis-Keever MA, Becker I, Puente JL, Calva E, Isibasi A. Salmonella porins induce a sustained, lifelong specific bactericidal antibody memory response. Immunology. 2006;117:59–70. doi: 10.1111/j.1365-2567.2005.02263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Micoli F, Rondini S, Pisoni I, Proietti D, Berti F, Costantino P, Rappuoli R, Szu S, Saul A, Martin LB. Vi-CRM 197 as a new conjugate vaccine against Salmonella Typhi. Vaccine. 2011;29:712–720. doi: 10.1016/j.vaccine.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin FY, Ho VA, Khiem HB, Trach DD, Bay PV, Thanh TC, Kossaczka Z, Bryla DA, Shiloach J, Robbins JB, Schneerson R, Szu SC. The efficacy of a Salmonella typhi Vi conjugate vaccine in two-to-five-year-old children. N Engl J Med. 2001;344:1263–1269. doi: 10.1056/NEJM200104263441701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.