Abstract
Natriuretic peptides play a fundamental role in cardiovascular homeostasis by modulation of fluid and electrolyte balance and vascular tone. C-type natriuretic peptide (CNP) represents the paracrine element of the natriuretic peptide axis which complements the endocrine actions of atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP). CNP is produced by the endothelium and the heart and appears to play a prominent role in vascular and cardiac function, both physiologically and pathologically. This provides a rationale for the therapeutic potential of pharmacological interventions targeted to CNP signalling. This article provides an overview of the biology and pharmacology of CNP, with emphasis on the cardiovascular system, and discusses pathologies in which drugs designed to manipulate CNP signalling maybe of clinical benefit.
Introduction
The natriuretic peptide (NP) family comprises three principal members which are released in response to hypervolaemic and hypertensive states. Atrial natriuretic peptide (ANP), B-type natriuretic peptide (BNP), and C-type natriuretic peptide (CNP) are all involved in the maintenance of electrolyte-fluid balance and vascular tone; as their name suggests, they promote natriuresis and diuresis resulting in loss of sodium and water thereby lowering blood volume and blood pressure. CNP was the third NP to be identified, first purified in 19901 some 10 years after the discovery of ANP2. CNP is the most widely expressed NP, with hotspots including the brain, chondrocytes and endothelial cells. CNP is thought to act locally, as a paracrine/autocrine regulator, since it is cleared rapidly from the circulation and present at very low concentrations in plasma3. It does not possess the potent diuretic actions that are observed with ANP and BNP3-5, but it is well established that exogenous CNP is a potent arterial- and veno- dilator of isolated mammalian blood vessels6-13 and infusion of CNP lowers blood pressure in several species, including humans14-17. Indeed, CNP has also been characterised as an endothelium-derived hyperpolarizing factor (EDHF)7. CNP also exerts direct cardiac effects where it acts as an inotropic and chronotropic agent18-20 and reduces aldosterone synthesis21. Chronic regulation of blood pressure by CNP also includes a central component through suppression of ACTH21, vasopressin22, and sympathetic outflow23. CNP exerts a number of physiological effects outside the cardiovascular system; perhaps its primary role being regulation of bone growth24-26, but it likely serves other functions such as neuronal development27, neuroprotection28, and reproduction29. CNP gene (Nppc) disruption has revealed the importance of CNP in terms of bone growth (endochondral ossification). Nppc KO mice exhibit dwarfism with the length of femurs, tibiae and vertebrae being 50 – 80 % of their wild-type littermates, in addition to striking narrowing of the growth plate25. Less than 50 % of CNP knockouts (KOs) are able to survive during postnatal development, although targeted expression of CNP in the growth plate chondrocytes improves their survival rate24.
Synthesis, structure and regulation of CNP production
Natriuretic peptides possess a core 17aa ring structure, critical for receptor binding, which is highly conserved between members of the gene family and also amongst species. Of the natriuretic peptides, CNP is the most highly conserved30, 31. The natriuretic peptide genes each encode prepropeptides and in man the CNP gene, Nppc, is located on chromosome 2 and consists of 2 exons32. Following translation, removal of the signal peptide converts the 126aa human prepro-CNP to the propeptide. In turn, pro-CNP is cleaved by furin33, a ubiquitous proprotein convertase resident in the trans-golgi network34, to yield CNP-53. This peptide possesses inherent biological activity3 but is typically processed further, by an as yet unidentified mechanism, to yield the predominant biologically active, 22aa form (CNP).
Unlike its sibling peptides, CNP is not stored and secreted from granules, but it remains unclear whether prepropeptide processing occurs ‘on demand’, particularly in terms of the endothelium-dependent vasorelaxant activity of CNP (see below)7, 35. Nonetheless, there are several important stimuli that promote the secretion of CNP from endothelial cells to alter cardiovascular function including pro-inflammatory cytokines such as tumour necrosis factor and interleukin 1, bacterial lipopolysaccharide, and transforming growth factor-β36, 37; CNP release from the endothelium is also triggered by shear stress akin to nitric oxide (NO)38.
CNP bioactivity is tightly regulated by two principal breakdown pathways, entailing that clearance from the plasma is very rapid (t½~3 min)39. First, CNP has high affinity for the natriuretic peptide ‘clearance’ receptor (NPR-C), which promotes internalisation and lysosomal degradation of each member of the natriuretic peptide family40, 41. Second, CNP is rapidly hydrolysed by neutral endopeptidase, a metalloproteinase present in the plasma and on the endothelial cell surface42.
Natriuretic peptide receptors underlying CNP bioactivity
CNP (and other natriuretic peptides) bring about their biological effects via activation of specific natriuretic peptide receptors (NPR), of which three have been cloned and characterised (NPR-A, NPR-B and NPR-C). The crystal structures of NPR-A and NPR-C bound to natriuretic peptide ligands have been solved, whereas similar structural information for NPR-B has yet to be published43, 44. These proteins exhibit high homology as might be expected, particularly in their N-terminal extracellular ligand binding domains, and short transmembrane regions (NPRs are homodimeric)45. NPR-A and NPR-B also comprise analogous kinase homology and dimerisation domains and, at the C-terminus, a guanylate cyclase functionality that generates the intracellular second messenger cyclic GMP for signalling purposes. In contrast, NPR-C has a short 37 amino acid intracellular tail that lacks cGMP synthesising capability but consists of overlapping Gi/o binding sequences that have been shown to trigger a number of G-protein linked processes including inhibition of adenylate cyclase activity and phospholipase-Cβ3 turnover46. CNP has low affinity and efficacy (i.e. cGMP production) at NPR-A (in comparison to ANP and BNP) and therefore this NPR subtype is not thought to mediate physiological responses to CNP47-49. In contrast, CNP represents the sole endogenous ligand for NPR-B and can also bind and activate NPR-C; it is therefore these NPR subtypes that mediate the bioactivity of CNP and are considered in more detail here.
NPR-B
The human NPR-B gene, Nppb, is located on chromosome 9 and consists of 22 exons producing a 1047 amino acid protein of approximately 120 kDa50. NPR-B is expressed highly in the brain, kidney, heart and vascular tissue50, 51. CNP selectively activates the receptor at physiological concentrations (pM range) and has a binding affinity some 50–500 fold higher than the other natriuretic peptides (CNP>ANP≥BNP)47, 49. In NPR-B KO mice there is dramatic impairment of endochondral ossification and attenuation of longitudinal vertebra or limb-bone growth leading to reduced body size and dwarfism52. Using this model it has also been shown that NPR-B is important in the embryonic development of the female reproductive tract52. In the cardiovascular system, NPR-B is found predominantly on veins, but also in arteries. The vasorelaxant activity of CNP in larger conduit vessels is dependent on NPR-B-triggered cGMP generation, whereas in resistance arteries CNP responses are mediated predominantly via NPR-C (see below)11, 35.
NPR-C
NPR-C is encoded on human chromosome 5 and is a 540 amino acid peptide50. The mature receptor protein exists as a homodimer (each monomer having a size of approximately 60 kDa53) which is stabilised by juxtamembrane intermolecular disulphide bonds50. NPR-C is the most abundantly expressed and widely distributed NPR and is found in major endocrine glands, lungs, kidneys and the vascular wall54 (e.g. vascular endothelial cells). All natriuretic peptides bind to NPR-C with high affinity but it has the following selectivity profile: ANP>CNP>BNP47. The development of NPR-C KO mice has confirmed the clearance function of NPR-C41. The plasma half-life of ANP is significantly extended in these animals, although plasma levels per se are unaffected. Mice lacking NPR-C have increased basal bone turnover and skeletal abnormalities41, which is thought to arise from reduced clearance of CNP that augments bone growth indirectly via NPR-B.
CNP as an EDHF
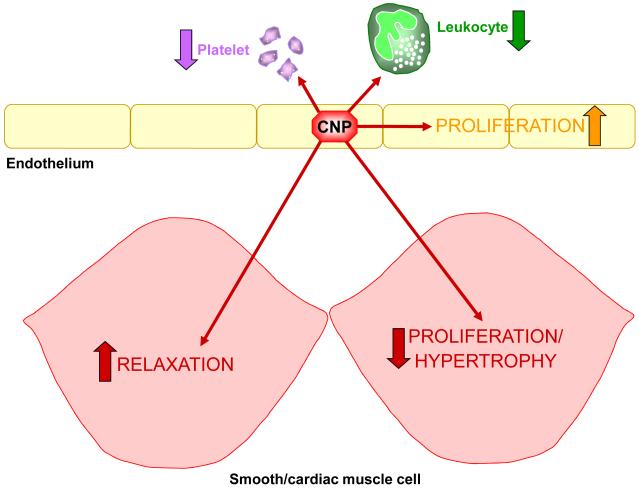
In addition to the clearance function of NPR-C, increasing evidence intimates its importance in regulating blood vessel tone in the resistance vasculature. Endothelium-derived hyperpolarising factor (EDHF) is a term used to describe the mediator(s) responsible for endothelium-dependent relaxation that is non-NO and non-prostanoid in nature, and which brings about hyperpolarisation of vascular smooth muscle via opening of inwardly-rectifying potassium channels (Kir) and/or activation of a Na+/K+-ATPase55. To date, there is no consensus on the identify of EDHF, although several candidates have been proposed including electrical coupling via myoendothelial gap junctions, hydrogen peroxide and cytochrome P450 metabolites55. Evidence from our lab has also added CNP to this list of putative EDHFs7, 35, 56. Furthermore, the EDHF-like actions of CNP have been linked to activation of NPR-C since the selective antagonist for this receptor, M372049, attenuates EDHF responses in rat mesenteric arteries35 and the selective NPR-C agonist cANF4-23 mimics the EDHF activity of CNP. As a result, it has been proposed that NPR-C- and Gi-dependent activation of an inwardly rectifying potassium channel (so called G-protein coupled Kir channels or GIRKs) underlies this activity. Human studies also support an EDHF-like function of CNP; in forearm resistance vessels, CNP-induced vasodilatation is dependent on hyperpolarisation and accentuated by the neutral endopeptidase inhibitor thiorphan16. Moreover, in human penile resistance arteries CNP has been shown to display EDHF-like activity via an NPR-C-dependent mechanism57. Importantly however, the vascular functions of endothelium-derived CNP do not seem to end with regulation of vascular tone. CNP has also been shown to govern platelet and leukocyte reactivity58, endothelial and vascular smooth muscle cell growth59, 60, and cardiac hypertrophy and ischaemia-reperfusion injury56, 61; many of these functions appear to be regulated by NPR-C. These additional biological effects are important when considering the potential for therapeutic modulation of CNP/NPR-C signalling, since this multi-faceted profile of activity brings about a multi-pronged cytoprotective effect in the vasculature (Figure 1); this is discussed further below.
Figure 1.
Schematic depicting the multi-faceted, anti-atherogenic profile of endothelium-derived CNP
Therapeutic potential of interventions targeted to CNP signalling
Hypertension
Hypertension is a significant public health issue in the developed world and its prevalence continues to increase; nearly 100 million adults in the United States are thought to suffer from the disease62-64. Moreover, as many as 30% of hypertensive patients are known to be resistant to medication regardless of compliance, thus highlighting a need for the development of novel therapies65. Hypertension predisposes individuals to myocardial infarction, stroke, renal failure and other cardiovascular diseases66 that account for almost one third of total global deaths (WHO statistics).
Hypertension is associated with aberrant endothelial function, including loss of NO bioactivity, and increased shear stress resulting in vascular remodelling and dysfunction67. Considering that CNP secretion is known to be augmented in response to shear stress38, 68 and that it exerts anti-remodelling effects on vascular smooth muscle and endothelial cells69-72, it is plausible that endothelial CNP is protective against vascular insults associated with hypertension and pharmacological manipulation of the peptide may be of therapeutic benefit. Unfortunately, rigorous functional pharmacological studies investigating the contribution of CNP-NPR-B/NPR-C in regulating blood pressure have been hampered by the fact that genetic deletion of CNP, NPR-B or NPR-C (in mice) results in gross bone deformation and premature death24, 41, 52. Nonetheless, genetic data do highlight a link between CNP (and/or NPR-C) signalling and hypertension in humans. For instance, a polymorphism in the 3′-UTR region of the Nppc gene is associated with hypertension in a Japanese population73. Additionally, a significant increase in circulating CNP correlates positively with hypertension in patients requiring catheterisation for coronary angiography74. Furthermore, allelic variants in the 3′-UTR of NPR-A and NPR-C have been linked to familial hypertension and higher systolic blood pressure75 and the number of tandem repeats in the 5′-flanking region of NPR-C correlates with increased mean arterial blood pressure76. Pharmacological evidence also predicts that targeting (i.e. augmenting) CNP signalling may be clinically useful. Administration of exogenous CNP causes an acute drop in blood pressure in primates14, dogs15 and humans15, 17, and spontaneously hypertensive rats77. CNP also exerts chronic regulation of blood pressure through suppression of ACTH21, vasopressin22, aldosterone synthesis21 and sympathetic outflow23. CNP may also play a protective role in hypertension via NPR-B activation; CNP and NPR-B mRNA levels are increased in the aorta of obese hypertensive rats when compared to lean rats and the NPR-A/B antagonist, HS-142-1, increases systolic blood pressure in obese rats that is accompanied by a decrease in cGMP production78.
Myocardial infarction and cardiac remodelling
Restoration of blood flow, or reperfusion, of ischemic tissues is essential for limiting and salvaging organ function in many cardiovascular disorders, including myocardial infarction (MI). However, reperfusion per se exerts detrimental effects by promoting local inflammation, triggering cell death and facilitating microvascular dysfunction; this phenomenon is known as ischemia/reperfusion (I/R) injury79. The inflammation and vascular dysfunction associated with I/R injury are underpinned by loss of endothelium-derived mediators including nitric oxide (NO); this results in vasoconstriction, decreased perfusion, leukocyte recruitment and platelet activation, which combine to exacerbate damage.
Endogenous and exogenous CNP may offer protection from I/R injury. Ventricular hypertrophy, necrosis, inflammation and functional impairment of cardiac tissue induced by coronary artery ligation is reduced in mice over expressing CNP in cardiomyocytes61. In accord, chronic CNP infusion administered post MI attenuates cardiac fibrosis and left ventricular hypertrophy, and increases survival80. The beneficial effects of CNP (particularly anti-hypertrophic activity) in this scenario may be due to NPR-B activation since transgenic rats over expressing a dominant negative NPR-B exhibit decreased cGMP production and cardiac hypertrophy81. Such a thesis is supported by the observation that CNP down-regulates the expression of many pro-hypertrophic genes/transcription factors, including myocyte enhancer factor 2 (MEF2) and GATA4, and opposes the hypertrophic effects of endothelin-1 and angiotensin II82-84. CNP signalling (perhaps via NPR-C) is also believed to regulate the pacemaker current in the heart85. Furthermore, CNP or drugs mimicking its bioactivity may be beneficial in the treatment of acute MI. Our work has shown that CNP and cANF4-23 reduced infarct size following I/R injury in the rat isolated heart56. Here, NPR-C activation seems to underlie the salutary effects of CNP on the myocardium and in regulating coronary blood flow. Additionally, the activity of CNP is enhanced in the absence of endogenous NO production indicating that CNP may play a compensatory role in protecting the heart and vasculature when NO signalling is impaired.
Angiogenesis & revascularisation
One process thought to play a key role in protecting against I/R injury is re-vascularisation, the growth and development of new blood vessels to re-establish blood flow to the ischaemic tissue. A key facet of this process is angiogenesis, an endothelium-centred response to hypoxia that promotes the migration and proliferation of differentiated endothelial cells to sprout new capillaries from existing blood vessels. This is initiated predominantly by hypoxia inducible factor (HIF)1α that in turn transcriptionally-activates genes including vascular endothelial growth factor (VEGF), VEGF receptors (i.e. Flt-1, Flk-1/KDR & neuropilin-1), angiopoietins, fibroblast growth factor (FGF), platelet-derived growth factor (PDGF) and cytokines such as IL-686-88.
It is now clear that the endothelium plays a pivotal role in integrating re-vascularisation pathways to expedite tissue repair and minimise functional deficit following ischaemia. Of the endothelium-borne signalling molecules that govern re-vascularisation, it is arguably NO that has been best-characterised87, 89. Despite the elucidation of several NO-dependent mechanisms that lead to re-vascularisation, little is known about additional endothelium-derived mediators that may contribute to this process.
Interestingly, several reports in the literature suggest that endothelium-derived CNP can mobilise endothelial cells and promote re-endothelialisation of damaged blood vessels, consistent with a pro-angiogenic activity. For example, adenoviral delivery of CNP slows neointimal hyperplasia and promotes re-endothelialisation in vein grafts69 and following balloon angioplasty60, 70-72, 90, and maintains blood flow and capillary density after hind-limb ischaemia91. This is fitting since many of the primary stimuli for CNP release from endothelial cells, including shear stress38, 68, transforming growth factor (TGF)β92, VEGF and monocyte chemoattractant protein (MCP)-193, 94, are key to the re-vascularisation process and should enlist CNP bioactivity. Further, systemic infusion of CNP enhances endothelial growth and prevents development of intimal thickening in damaged carotid arteries59, and CNP expression is highly up-regulated in human coronary atherosclerotic lesions95 and in the neointima following percutaneous coronary intervention (PCI)96. These observations all intimate that CNP is produced endogenously in response to vessel injury and may represent a further, key endothelium-derived mediator involved in re-vascularisation and a promising therapeutic target.
Heart Failure
Heart failure is defined as an impaired ability of the ventricle to fill with or eject blood, and is characterized by symptoms including dyspnoea and fatigue. It is a serious and growing health concern, with mortality increasing despite advances in treatment. In the USA nearly 5 million people suffer from heart failure, and there are more than 50,000 deaths per annum97.
The development of heart failure involves abnormal signalling in a number of pathways, but particularly those stimulating cGMP production; this encompasses aberrant natriuretic peptide (particulate guanylate cyclase) and NO-mediated (soluble guanylate cyclase) signalling98, 99. NO-donor drugs restore NO levels in heart failure and have been used to treat the disease for over a century, but their long-term use is limited by the development of tolerance and cGMP-independent side effects100. Thus, activation of particulate guanylate cyclases offers a promising alternative. Recently, chimeric natriuretic peptides have been developed that appear to harness many of the desirable biological activities of each natriuretic peptide in heart failure101, 102. This approach is exemplified by the development and clinical evaluation of CD-NP, a chimera of the C-terminus of Dendroaspis natriuretic peptide (DNP) fused to CNP, which reproduces the beneficial renal effects of BNP and vasodilatory actions of CNP without the excessive hypotensive effects that are often observed with treatment of heart failure patients with Carperitide and Nesiritide (synthetic ANP and BNP, respectively). Heart failure is one of the few conditions that results in an increase in plasma CNP levels, so it may be that CNP acts in both a paracrine (endothelial dysfunction is characteristic of heart failure) and endocrine fashion (cardiac function) in this setting. Moreover, cardiac production of CNP and expression of NPR-B (more so than NPR-A) are increased in heart failure, suggesting it is released as a cytoprotective mechanism103-106.
Atherosclerosis
Atherosclerosis is a multi-factorial pathological and inflammatory process of the blood vessel wall leading to distal ischaemic events such as MI and stroke. Development of atherosclerosis begins early in life107 and is a major risk factor for ischaemic cardiovascular diseases which are the primary causes of death in the western world108, 109. Initiation of atherosclerosis is influenced by a number of ‘risk factors’ including smoking, hypertension, hyperlipidaemia, and diabetes mellitus, all of which cause endothelial dysfunction110. Atherosclerotic plaques form in arterial sites where there is decreased shear stress111, 112; in a healthy vascular system shear stress results in a gene expression profile which is crucial for normal vascular function and is atheroprotective by inhibiting proliferation, thrombosis and inflammation of the vessel wall113, 114. Normal laminar flow enhances the expression and phosphorylation (i.e. activation) of eNOS and the production of NO, which promotes vasorelaxation, inhibits platelet activation, and monocyte adhesion115-118. At curvatures, branch ostia and bifurcations, there is disruption to the blood flow and hence reduced shear stress, which is associated with reduced eNOS production, an increase in reactive oxygen species (ROS), leukocyte adhesion, smooth muscle cell proliferation and collagen deposition113, 114. Reduction in shear stress causes up-regulation of endothelial and leukocyte adhesion molecules including selectins, integrins, intercellular adhesion molecule-1, and vascular-cell adhesion molecule-1111. Expression of these molecules on the endothelial surface causes recruitment of monocytes and T-cells and the migration of leukocytes into the artery wall. It is the macrophages in the artery wall that take up oxidised-LDL to become foam cells. The presence of foam cells and T lymphocytes marks the beginning of the fatty streak formation111. The immune cells release a milieu of inflammatory mediators including growth factors and cytokines, which cause migration and proliferation of the underlying smooth muscle cells, which can then release their own mediators including IL-1β, TNF-α and β, IL-6, macrophage colony-stimulating factor, MCP-1, IL-18 and CD-40L resulting in a self-perpetuating inflammatory process within the plaque. As the lesion grows it forms a fibrous cap (often associated with calcification) that isolates the lesion from the lumen and this can become progressively weaker resulting in plaque rupture, thrombosis and distal occlusion.
CNP has been shown to possess anti-atherogenic properties. Immunological studies on human coronary atherosclerotic lesions have revealed that CNP is present in different cell types according to progression of atherosclerosis. Under normal physiological conditions, arterial segments show CNP-positive endothelial cells95 and monocytes/macrophages119. As the development of the atherosclerotic plaques progresses, advanced lesions have CNP-positive macrophages but the majority of the smooth muscle cells within the fibrous cap and the surface endothelial cells are CNP-negative95. Further still, CNP mRNA levels are decreased in human stenosed aortic valves120. In sum, such observations intimate that the severity of atherosclerotic lesions inversely correlates with CNP expression121. It is also interesting to note that oxidized LDL suppresses the secretion of CNP from endothelial cells122. CNP has also been implicated in the regulation of leukocyte adhesion, platelet activation and P-selectin expression58. CNP is able to suppress basal and IL-1β-induced leukocyte recruitment and this is mimicked by the NPR-C selective agonist cANF4-23, implying the involvement of NPR-C. CNP also prevents thrombin-induced platelet aggregation. It is also worth noting that relaxation to CNP in atherosclerotic aortae from cholesterol rabbits is impaired compared to aortae from normal dietary fed rabbits; however, incubation of the atherosclerotic aortae from cholesterol rabbits with a neutral endopeptidase inhibitor attenuates the impairment of the vasorelaxation response to CNP123.
Taken together, the above evidence suggests an anti-atherogenic role for CNP by regulating smooth muscle migration, cellular adhesion and P-selectin expression. Further evidence supporting a cytoprotective role for CNP is provided by studies with vascular endothelial cells which show that the switch from a reductive to an oxidative environment in atherosclerosis (and the production of reactive oxygen species such as superoxide and hydrogen peroxide), and key mediators in inflammation and vascular remodelling augment endothelial secretion of CNP36, 37, 124. Moreover, diabetes associated atherosclerosis might be in part due to impairment of CNP since insulin suppresses CNP, but not endothelin-1, production by cultured endothelial cells125. Interestingly, CNP attenuates the inhibitory effect of Angiotensin-II on Gax (Growth arrest-specific homeobox) mRNA expression in rat aortic vascular smooth muscle cells126. Since Gax over-expression has been reported to reduce neointimal thickening post balloon injury in the rat carotid artery127, Gax may be a pivotal transcription factor involved in the anti-proliferative and anti-remodelling activity of CNP in vascular smooth muscle cells.
Restenosis & vein graft disease
Restenosis describes the recurrence of intimal hyperplasia that is often associated with percutaneous coronary interventions (PCI; balloon angioplasty) and stent insertions in patients with diseased and occluded coronary arteries. It is a significant clinical problem limiting the long term therapeutic success of these procedures128, 129. Drug eluting stents (DES) release agents with anti-proliferative and immunosuppressive actions (e.g. sirolimus/rapamycin) which have been successful in reducing the rate of restenosis to <10%130. However, the complication of stent thrombosis remains129. Stent thrombosis post PCI is categorised as early (0-30 days post PCI), late (>30 days to 1 year post PCI) or very late (>1 year post PCI)131. Stent thrombosis occurs in patients treated with bare metal stents and DES but the rate of late stent thrombosis is almost doubled in patients treated with DES132. Agents released by DES inhibit vascular smooth muscle proliferation and migration, a beneficial action in reducing the incidence of restenosis; however they also inhibit endothelial cell proliferation133, an undesirable effect. PCI and stent deployment cause endothelial denudation, exposing thrombogenic material to the blood, which can lead to thrombosis134, 135. Therefore, an agent that could concomitantly prevent vascular smooth muscle hyperplasia but promote endothelial proliferation should offer a considerable therapeutic advance.
CNP appears an ideal candidate to be released from DES as it promotes endothelial cell proliferation, inhibits VSMC proliferation, and has anti-inflammatory and anti-thrombotic properties. In a porcine model of femoral artery restenosis post balloon angioplasty, liposome-mediated CNP gene and peptide administration reduces neointima formation without compromising re-endothelialisation71. In balloon injured rabbit carotid arteries infection with an adenovirus expressing human CNP leads to increased NO production and suppression of neointimal thickening, ICAM-1 and VCAM-1 expression, and macrophage infiltration136. This profile of activity is also observed following angioplasty in a number of further vessels and species, including rabbit carotid artery, rabbit femoral artery and porcine femoral artery90, 136-138.
A similar beneficial effect of CNP is reported in vein grafts, often used for auto transplantation in patients requiring coronary artery bypass surgery. Akin to (coronary) artery stenosis, following a vein graft procedure the transplanted vessel is affected by thrombosis, intimal hyperplasia and atherosclerosis139. Within the first month, approximately 13% of saphenous vein grafts occlude, principally due to thrombosis140, 141, and the 10 year vein graft patency is ~ 50%141, 142. CNP has been shown to reduce intimal hyperplasia and CD-8 positive cells in a mouse model of vein graft disease, in which the inferior vena cava is interposed into the common carotid artery143. Furthermore, CNP promotes re-endothelialisation and suppresses neointimal hyperplasia and thrombosis in rabbit jugular vein grafts interposed into the carotid artery69. Such studies clearly demonstrate CNP to be effective in suppressing thrombosis and neointimal hyperplasia, two of the main processes involved in vein graft disease.
Pulmonary hypertension
Pulmonary hypertension (PH) is a debilitating disease with a poor prognosis. It is characterised by increased pulmonary artery pressure, re-modelling of the pulmonary resistance vasculature right heart hypertrophy, eventually leading to right heart failure and death144-146. Current therapeutic options include prostacyclin analogues147, 148 endothelin receptor antagonists149, 150, and PDE5 inhibitors151, but despite these advances 2-year mortality remains unacceptably high (~15%)152. Thus, PH represents a clear unmet medical need.
In PH, the bioactivity of NO and other endothelium-dependent vasodilators such as prostacyclin is reduced145, 153, 154, whereas production of endogenous vasoconstrictors such as ET-1 is increased155. This pulmonary endothelial dysfunction precipitates the excessive pulmonary vasoconstriction and remodelling that characterise the disease. The strategy of elevating cGMP by augmenting NO-dependent signalling (e.g. NO inhalation, direct sGC stimulation, PDE5 inhibitors), is clinically effective in PH156. We and others have recently reported that pharmacological interventions targeted to the natriuretic peptide pathways (which also results in augmented cGMP production) may be an alternative and more efficacious approach to the treatment of PH157, 158. Inhibitors of neutral endopeptidase augment natriuretic peptide bioactivity and produce a selective pulmonary dilatation; this mechanism also leads to a reduction in pulmonary vascular remodelling and right ventricular hypertrophy157. Thus, a precedent for a natriuretic peptide-centric approach to PH has been established. As such, altering CNP signalling may prove therapeutically effective. This idea is corroborated by the report that CNP ameliorates the development of PH in an animal model159 and that circulating levels of CNP are increased by hypoxia160. In addition, the anti-remodelling (vascular & cardiac) and anti-inflammatory actions of CNP, coupled with its ability to promote re-endothelialisation (above), intimate it should be protective in PH.
Conclusions
A key role for CNP in regulating cardiac and vascular function has slowly emerged since its identification 20 years ago161, 162. This cardiovascular homeostatic role is perhaps best exemplified by the ability of CNP to control blood flow in the resistance vasculature7, to reduce the reactivity of leukocytes and platelets58, to govern the pacemaker current in the heart85, to protect against myocardial I/R injury56, 80 and cardiac hypertrophy61, 82. Moreover, elevated plasma CNP levels are found in patients with heart failure106, 163, septic shock164 and renal dysfunction165, thereby associating this endothelium-derived peptide with human cardiovascular pathology. The multi-faceted cytoprotective functions assigned to CNP described herein suggest that pharmacological manipulation of this natriuretic peptide, and its receptors (both NPR-B and NPR-C), hold great promise in advancing the treatment of cardiovascular disease. This has already begun in many respects with the clinical evaluation and use of neutral endopeptidase inhibitors166 and CD-NP102, and as our understanding of the cardioprotective roles of CNP expands, so this interest should grow.
References
- (1).Sudoh T, Minamino N, Kangawa K, Matsuo H. C-type natriuretic peptide (CNP): a new member of natriuretic peptide family identified in porcine brain. Biochem Biophys Res Commun. 1990 Apr 30;168(2):863–70. doi: 10.1016/0006-291x(90)92401-k. [DOI] [PubMed] [Google Scholar]
- (2).de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981 Jan 5;28(1):89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- (3).Stingo AJ, Clavell AL, Heublein DM, Wei CM, Pittelkow MR, Burnett JC., Jr Presence of C-type natriuretic peptide in cultured human endothelial cells and plasma. Am J Physiol. 1992 Oct;263(4 Pt 2):H1318–H1321. doi: 10.1152/ajpheart.1992.263.4.H1318. [DOI] [PubMed] [Google Scholar]
- (4).Chen HH, Burnett JC., Jr C-type natriuretic peptide: the endothelial component of the natriuretic peptide system. J Cardiovasc Pharmacol. 1998;32(Suppl 3):S22–S28. [PubMed] [Google Scholar]
- (5).Kalra PR, Anker SD, Struthers AD, Coats AJ. The role of C-type natriuretic peptide in cardiovascular medicine. Eur Heart J. 2001 Jun;22(12):997–1007. doi: 10.1053/euhj.2000.2395. [DOI] [PubMed] [Google Scholar]
- (6).Wiley KE, Davenport AP. Physiological antagonism of endothelin-1 in human conductance and resistance coronary artery. Br J Pharmacol. 2001 Jun;133(4):568–74. doi: 10.1038/sj.bjp.0704119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Chauhan SD, Nilsson H, Ahluwalia A, Hobbs AJ. Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc Natl Acad Sci U S A. 2003 Feb 4;100(3):1426–31. doi: 10.1073/pnas.0336365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Drewett JG, Fendly BM, Garbers DL, Lowe DG. Natriuretic peptide receptor-B (guanylyl cyclase-B) mediates C-type natriuretic peptide relaxation of precontracted rat aorta. J Biol Chem. 1995 Mar 3;270(9):4668–74. doi: 10.1074/jbc.270.9.4668. [DOI] [PubMed] [Google Scholar]
- (9).Brunner F, Wolkart G. Relaxant effect of C-type natriuretic peptide involves endothelium and nitric oxide-cGMP system in rat coronary microvasculature. Cardiovasc Res. 2001 Aug 15;51(3):577–84. doi: 10.1016/s0008-6363(01)00283-8. [DOI] [PubMed] [Google Scholar]
- (10).Wennberg PW, Miller VM, Rabelink T, Burnett JC., Jr Further attenuation of endothelium-dependent relaxation imparted by natriuretic peptide receptor antagonism. Am J Physiol. 1999 Oct;277(4 Pt 2):H1618–H1621. doi: 10.1152/ajpheart.1999.277.4.H1618. [DOI] [PubMed] [Google Scholar]
- (11).Madhani M, Scotland RS, MacAllister RJ, Hobbs AJ. Vascular natriuretic peptide receptor-linked particulate guanylate cyclases are modulated by nitric oxide-cyclic GMP signalling. Br J Pharmacol. 2003 Aug;139(7):1289–96. doi: 10.1038/sj.bjp.0705365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Steinmetz M, Potthast R, Sabrane K, Kuhn M. Diverging vasorelaxing effects of C-type natriuretic peptide in renal resistance arteries and aortas of GC-A-deficient mice. Regul Pept. 2004 Jun 15;119(1-2):31–7. doi: 10.1016/j.regpep.2003.12.005. [DOI] [PubMed] [Google Scholar]
- (13).Barber DA, Burnett JC, Jr, Fitzpatrick LA, Sieck GC, Miller VM. Gender and relaxation to C-type natriuretic peptide in porcine coronary arteries. J Cardiovasc Pharmacol. Jul;199832(1):5–11. doi: 10.1097/00005344-199807000-00002. [DOI] [PubMed] [Google Scholar]
- (14).Seymour AA, Mathers PD, Abboa-Offei BE, Asaad MM, Weber H. Renal and depressor activity of C-natriuretic peptide in conscious monkeys: effects of enzyme inhibitors. J Cardiovasc Pharmacol. 1996 Sep;28(3):397–401. doi: 10.1097/00005344-199609000-00008. [DOI] [PubMed] [Google Scholar]
- (15).Clavell AL, Stingo AJ, Wei CM, Heublein DM, Burnett JC., Jr C-type natriuretic peptide: a selective cardiovascular peptide. Am J Physiol. 1993 Feb;264(2 Pt 2):R290–R295. doi: 10.1152/ajpregu.1993.264.2.R290. [DOI] [PubMed] [Google Scholar]
- (16).Honing ML, Smits P, Morrison PJ, Burnett JC, Jr, Rabelink TJ. C-type natriuretic peptide-induced vasodilation is dependent on hyperpolarization in human forearm resistance vessels. Hypertension. 2001 Apr;37(4):1179–83. doi: 10.1161/01.hyp.37.4.1179. [DOI] [PubMed] [Google Scholar]
- (17).Igaki T, Itoh H, Suga S, et al. C-type natriuretic peptide in chronic renal failure and its action in humans. Kidney Int Suppl. 1996 Jun;55:S144–S147. [PubMed] [Google Scholar]
- (18).Su J, Scholz PM, Weiss HR. Differential effects of cGMP produced by soluble and particulate guanylyl cyclase on mouse ventricular myocytes. Exp Biol Med (Maywood ) 2005 Apr;230(4):242–50. doi: 10.1177/153537020523000403. [DOI] [PubMed] [Google Scholar]
- (19).Pierkes M, Gambaryan S, Boknik P, et al. Increased effects of C-type natriuretic peptide on cardiac ventricular contractility and relaxation in guanylyl cyclase A-deficient mice. Cardiovasc Res. 2002 Mar;53(4) doi: 10.1016/s0008-6363(01)00543-0. [DOI] [PubMed] [Google Scholar]
- (20).Brusq JM, Mayoux E, Guigui L, Kirilovsky J. Effects of C-type natriuretic peptide on rat cardiac contractility. Br J Pharmacol. 1999 Sep;128(1):206–12. doi: 10.1038/sj.bjp.0702766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Guild SB, Cramb G. Characterisation of the effects of natriuretic peptides upon ACTH secretion from the mouse pituitary. Mol Cell Endocrinol. 1999 Jun 25;152(1-2):11–9. doi: 10.1016/s0303-7207(99)00068-4. [DOI] [PubMed] [Google Scholar]
- (22).Shirakami G, Itoh H, Suga S, et al. Central action of C-type natriuretic peptide on vasopressin secretion in conscious rats. Neurosci Lett. 1993 Sep 3;159(1-2):25–8. doi: 10.1016/0304-3940(93)90789-n. [DOI] [PubMed] [Google Scholar]
- (23).Trachte GJ. Depletion of natriuretic peptide C receptors eliminates inhibitory effects of C-type natriuretic peptide on evoked neurotransmitter efflux. J Pharmacol Exp Ther. 2000 Jul;294(1):210–5. [PubMed] [Google Scholar]
- (24).Chusho H, Tamura N, Ogawa Y, et al. Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc Natl Acad Sci U S A. 2001 Mar 27;98(7):4016–21. doi: 10.1073/pnas.071389098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Komatsu Y, Chusho H, Tamura N, et al. Significance of C-type natriuretic peptide (CNP) in endochondral ossification: analysis of CNP knockout mice. J Bone Miner Metab. 2002;20(6):331–6. doi: 10.1007/s007740200048. [DOI] [PubMed] [Google Scholar]
- (26).Kake T, Kitamura H, Adachi Y, et al. Chronically elevated plasma C-type natriuretic peptide level stimulates skeletal growth in transgenic mice. Am J Physiol Endocrinol Metab. 2009 Dec;297(6):E1339–E1348. doi: 10.1152/ajpendo.00272.2009. [DOI] [PubMed] [Google Scholar]
- (27).Schmidt H, Stonkute A, Juttner R, Koesling D, Friebe A, Rathjen FG. C-type natriuretic peptide (CNP) is a bifurcation factor for sensory neurons. Proc Natl Acad Sci U S A. 2009 Sep 29;106(39):16847–52. doi: 10.1073/pnas.0906571106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Ma J, Yu W, Wang Y, et al. Neuroprotective Effects of C-Type Natriuretic Peptide on Rat Retinal Ganglion Cells. Invest Ophthalmol Vis Sci. 2010 Feb 24; doi: 10.1167/iovs.09-5049. [DOI] [PubMed] [Google Scholar]
- (29).McNeill BA, Barrell GK, Wellby M, Prickett TC, Yandle TG, Espiner EA. C-type natriuretic peptide forms in pregnancy: maternal plasma profiles during ovine gestation correlate with placental and fetal maturation. Endocrinology. 2009 Oct;150(10):4777–83. doi: 10.1210/en.2009-0176. [DOI] [PubMed] [Google Scholar]
- (30).Takei Y. Does the natriuretic peptide system exist throughout the animal and plant kingdom? Comp Biochem Physiol B Biochem Mol Biol. 2001 Jun;129(2-3):559–73. doi: 10.1016/s1096-4959(01)00366-9. [DOI] [PubMed] [Google Scholar]
- (31).Inoue K, Naruse K, Yamagami S, Mitani H, Suzuki N, Takei Y. Four functionally distinct C-type natriuretic peptides found in fish reveal evolutionary history of the natriuretic peptide system. Proc Natl Acad Sci U S A. 2003 Aug 19;100(17):10079–84. doi: 10.1073/pnas.1632368100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Ogawa Y, Nakao K, Nakagawa O, et al. Human C-type natriuretic peptide. Characterization of the gene and peptide. Hypertension. 1992 Jun;19(6 Pt 2):809–13. doi: 10.1161/01.hyp.19.6.809. [DOI] [PubMed] [Google Scholar]
- (33).Wu C, Wu F, Pan J, Morser J, Wu Q. Furin-mediated Processing of Pro-C-type Natriuretic Peptide. J Biol Chem. 2003 Jul 11;278(28):25847–52. doi: 10.1074/jbc.M301223200. [DOI] [PubMed] [Google Scholar]
- (34).Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol. 2002 Oct;3(10):753–66. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Villar IC, Panayiotou CM, Sheraz A, et al. Definitive role for natriuretic peptide receptor-C in mediating the vasorelaxant activity of C-type natriuretic peptide and endothelium-derived hyperpolarising factor. Cardiovasc Res. 2007 Jun 1;74(3):515–25. doi: 10.1016/j.cardiores.2007.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Suga S, Nakao K, Itoh H, et al. Endothelial production of C-type natriuretic peptide and its marked augmentation by transforming growth factor-beta. Possible existence of “vascular natriuretic peptide system”. J Clin Invest. 1992 Sep;90(3):1145–9. doi: 10.1172/JCI115933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Suga S, Itoh H, Komatsu Y, et al. Cytokine-induced C-type natriuretic peptide (CNP) secretion from vascular endothelial cells--evidence for CNP as a novel autocrine/paracrine regulator from endothelial cells. Endocrinology. 1993 Dec;133(6):3038–41. doi: 10.1210/endo.133.6.8243333. [DOI] [PubMed] [Google Scholar]
- (38).Chun TH, Itoh H, Ogawa Y, et al. Shear stress augments expression of C-type natriuretic peptide and adrenomedullin. Hypertension. 1997 Jun;29(6):1296–302. doi: 10.1161/01.hyp.29.6.1296. [DOI] [PubMed] [Google Scholar]
- (39).Hunt PJ, Richards AM, Espiner EA, Nicholls MG, Yandle TG. Bioactivity and metabolism of C-type natriuretic peptide in normal man. J Clin Endocrinol Metab. 1994 Jun;78(6):1428–35. doi: 10.1210/jcem.78.6.8200946. [DOI] [PubMed] [Google Scholar]
- (40).Maack T, Suzuki M, Almeida FA, et al. Physiological role of silent receptors of atrial natriuretic factor. Science. 1987 Oct 30;238(4827):675–8. doi: 10.1126/science.2823385. [DOI] [PubMed] [Google Scholar]
- (41).Matsukawa N, Grzesik WJ, Takahashi N, et al. The natriuretic peptide clearance receptor locally modulates the physiological effects of the natriuretic peptide system. Proc Natl Acad Sci U S A. 1999 Jun 22;96(13):7403–8. doi: 10.1073/pnas.96.13.7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Kenny AJ, Bourne A, Ingram J. Hydrolysis of human and pig brain natriuretic peptides, urodilatin, C-type natriuretic peptide and some C-receptor ligands by endopeptidase-24.11. Biochem J. 1993 Apr 1;291(Pt 1):83–8. doi: 10.1042/bj2910083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).He X, Chow D, Martick MM, Garcia KC. Allosteric activation of a spring-loaded natriuretic peptide receptor dimer by hormone. Science. 2001 Aug 31;293(5535):1657–62. doi: 10.1126/science.1062246. [DOI] [PubMed] [Google Scholar]
- (44).Ogawa H, Qiu Y, Ogata CM, Misono KS. Crystal structure of hormone-bound atrial natriuretic peptide receptor extracellular domain: rotation mechanism for transmembrane signal transduction. J Biol Chem. 2004 Jul 2;279(27):28625–31. doi: 10.1074/jbc.M313222200. [DOI] [PubMed] [Google Scholar]
- (45).Potter LR, Hunter T. Guanylyl cyclase-linked natriuretic peptide receptors: structure and regulation. J Biol Chem. 2001 Mar 2;276(9):6057–60. doi: 10.1074/jbc.R000033200. [DOI] [PubMed] [Google Scholar]
- (46).Murthy KS, Teng BQ, Zhou H, Jin JG, Grider JR, Makhlouf GM. G(i-1)/G(i-2)-dependent signaling by single-transmembrane natriuretic peptide clearance receptor. Am J Physiol Gastrointest Liver Physiol. 2000 Jun;278(6):G974–G980. doi: 10.1152/ajpgi.2000.278.6.G974. [DOI] [PubMed] [Google Scholar]
- (47).Suga S, Nakao K, Hosoda K, et al. Receptor selectivity of natriuretic peptide family, atrial natriuretic peptide, brain natriuretic peptide, and C-type natriuretic peptide. Endocrinology. 1992 Jan;130(1):229–39. doi: 10.1210/endo.130.1.1309330. [DOI] [PubMed] [Google Scholar]
- (48).Bennett BD, Bennett GL, Vitangcol RV, et al. Extracellular domain-IgG fusion proteins for three human natriuretic peptide receptors. Hormone pharmacology and application to solid phase screening of synthetic peptide antisera. J Biol Chem. 1991 Dec 5;266(34):23060–7. [PubMed] [Google Scholar]
- (49).Koller KJ, Lowe DG, Bennett GL, et al. Selective activation of the B natriuretic peptide receptor by C-type natriuretic peptide (CNP) Science. 1991 Apr 5;252(5002):120–3. doi: 10.1126/science.1672777. [DOI] [PubMed] [Google Scholar]
- (50).Kone BC. Molecular biology of natriuretic peptides and nitric oxide synthases. Cardiovasc Res. 2001 Aug 15;51(3):429–41. doi: 10.1016/s0008-6363(01)00327-3. [DOI] [PubMed] [Google Scholar]
- (51).Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998 Jul 30;339(5):321–8. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- (52).Tamura N, Doolittle LK, Hammer RE, Shelton JM, Richardson JA, Garbers DL. Critical roles of the guanylyl cyclase B receptor in endochondral ossification and development of female reproductive organs. Proc Natl Acad Sci U S A. 2004 Dec 7;101(49):17300–5. doi: 10.1073/pnas.0407894101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Cohen D, Koh GY, Nikonova LN, Porter JG, Maack T. Molecular determinants of the clearance function of type C receptors of natriuretic peptides. J Biol Chem. 1996 Apr 19;271(16):9863–9. doi: 10.1074/jbc.271.16.9863. [DOI] [PubMed] [Google Scholar]
- (54).Maack T. Receptors of atrial natriuretic factor. Annu Rev Physiol. 1992;54:11–27. doi: 10.1146/annurev.ph.54.030192.000303. [DOI] [PubMed] [Google Scholar]
- (55).Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002 Aug 1;23(8):374–80. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- (56).Hobbs A, Foster P, Prescott C, Scotland R, Ahluwalia A. Natriuretic peptide receptor-C regulates coronary blood flow and prevents myocardial ischemia/reperfusion injury: novel cardioprotective role for endothelium-derived C-type natriuretic peptide. Circulation. 2004 Sep 7;110(10):1231–5. doi: 10.1161/01.CIR.0000141802.29945.34. [DOI] [PubMed] [Google Scholar]
- (57).Kun A, Kiraly I, Pataricza J, et al. C-type natriuretic peptide hyperpolarizes and relaxes human penile resistance arteries. J Sex Med. 2008 May;5(5):1114–25. doi: 10.1111/j.1743-6109.2008.00775.x. [DOI] [PubMed] [Google Scholar]
- (58).Scotland RS, Cohen M, Foster P, et al. C-type natriuretic peptide inhibits leukocyte recruitment and platelet-leukocyte interactions via suppression of P-selectin expression. Proc Natl Acad Sci U S A. 2005 Oct 4;102(40):14452–7. doi: 10.1073/pnas.0504961102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Furuya M, Aisaka K, Miyazaki T, et al. C-type natriuretic peptide inhibits intimal thickening after vascular injury. Biochem Biophys Res Commun. 1993 May 28;193(1):248–53. doi: 10.1006/bbrc.1993.1616. [DOI] [PubMed] [Google Scholar]
- (60).Ueno H, Haruno A, Morisaki N, et al. Local expression of C-type natriuretic peptide markedly suppresses neointimal formation in rat injured arteries through an autocrine/paracrine loop. Circulation. 1997 Oct 7;96(7):2272–9. doi: 10.1161/01.cir.96.7.2272. [DOI] [PubMed] [Google Scholar]
- (61).Wang Y, de Waard MC, Sterner-Kock A, et al. Cardiomyocyte-restricted over-expression of C-type natriuretic peptide prevents cardiac hypertrophy induced by myocardial infarction in mice. Eur J Heart Fail. 2007 Jun;9(6-7):548–57. doi: 10.1016/j.ejheart.2007.02.006. [DOI] [PubMed] [Google Scholar]
- (62).Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004 Oct;44(4):398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- (63).Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988-1994 and 1999-2004. Hypertension. 2008 Nov;52(5):818–27. doi: 10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- (64).Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010 Feb 23;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- (65).Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008 Jun;51(6):1403–19. doi: 10.1161/HYPERTENSIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- (66).Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet. 2007 Aug 18;370(9587):591–603. doi: 10.1016/S0140-6736(07)61299-9. [DOI] [PubMed] [Google Scholar]
- (67).Napoli C, Ignarro LJ. Nitric oxide and pathogenic mechanisms involved in the development of vascular diseases. Arch Pharm Res. 2009 Aug;32(8):1103–8. doi: 10.1007/s12272-009-1801-1. [DOI] [PubMed] [Google Scholar]
- (68).Okahara K, Kambayashi J, Ohnishi T, Fujiwara Y, Kawasaki T, Monden M. Shear stress induces expression of CNP gene in human endothelial cells. FEBS Lett. 1995 Oct 9;373(2):108–10. doi: 10.1016/0014-5793(95)01027-c. [DOI] [PubMed] [Google Scholar]
- (69).Ohno N, Itoh H, Ikeda T, et al. Accelerated reendothelialization with suppressed thrombogenic property and neointimal hyperplasia of rabbit jugular vein grafts by adenovirus-mediated gene transfer of C-type natriuretic peptide. Circulation. 2002 Apr 9;105(14):1623–6. doi: 10.1161/01.cir.0000014985.50017.6e. [DOI] [PubMed] [Google Scholar]
- (70).Kuhnl A, Pelisek J, Tian W, et al. C-type natriuretic peptide inhibits constrictive remodeling without compromising re-endothelialization in balloon-dilated renal arteries. J Endovasc Ther. 2005 Apr;12(2):171–82. doi: 10.1583/1384R.1. [DOI] [PubMed] [Google Scholar]
- (71).Pelisek J, Fuchs AT, Kuehnl A, et al. C-type natriuretic peptide for reduction of restenosis: gene transfer is superior over single peptide administration. J Gene Med. 2006 Jul;8(7):835–44. doi: 10.1002/jgm.905. [DOI] [PubMed] [Google Scholar]
- (72).Morishige K, Shimokawa H, Yamawaki T, et al. Local adenovirus-mediated transfer of C-type natriuretic peptide suppresses vascular remodeling in porcine coronary arteries in vivo. J Am Coll Cardiol. 2000 Mar 15;35(4):1040–7. doi: 10.1016/s0735-1097(99)00625-7. [DOI] [PubMed] [Google Scholar]
- (73).Ono K, Mannami T, Baba S, Tomoike H, Suga S, Iwai N. A single-nucleotide polymorphism in C-type natriuretic peptide gene may be associated with hypertension. Hypertens Res. 2002 Sep;25(5):727–30. doi: 10.1291/hypres.25.727. [DOI] [PubMed] [Google Scholar]
- (74).Palmer SC, Prickett TC, Espiner EA, Yandle TG, Richards AM. Regional release and clearance of C-type natriuretic peptides in the human circulation and relation to cardiac function. Hypertension. 2009 Sep;54(3):612–8. doi: 10.1161/HYPERTENSIONAHA.109.135608. [DOI] [PubMed] [Google Scholar]
- (75).Pitzalis MV, Sarzani R, Dessi-Fulgheri P, et al. Allelic variants of natriuretic peptide receptor genes are associated with family history of hypertension and cardiovascular phenotype. J Hypertens. 2003 Aug;21(8):1491–6. doi: 10.1097/00004872-200308000-00012. [DOI] [PubMed] [Google Scholar]
- (76).Aoi N, Soma M, Nakayama T, et al. Variable number of tandem repeat of the 5′-flanking region of type-C human natriuretic peptide receptor gene influences blood pressure levels in obesity-associated hypertension. Hypertens Res. 2004 Oct;27(10):711–6. doi: 10.1291/hypres.27.711. [DOI] [PubMed] [Google Scholar]
- (77).Caniffi C, Elesgaray R, Gironacci M, Arranz C, Costa MA. C-type natriuretic peptide effects on cardiovascular nitric oxide system in spontaneously hypertensive rats. Peptides. 2010 Apr 2; doi: 10.1016/j.peptides.2010.03.030. [DOI] [PubMed] [Google Scholar]
- (78).Yoshimoto T, Naruse M, Naruse K, et al. Vascular action of circulating and local natriuretic peptide systems is potentiated in obese/hyperglycemic and hypertensive rats. Endocrinology. 1996 Dec;137(12):5552–7. doi: 10.1210/endo.137.12.8940383. [DOI] [PubMed] [Google Scholar]
- (79).Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007 Sep 13;357(11):1121–35. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- (80).Soeki T, Kishimoto I, Okumura H, et al. C-type natriuretic peptide, a novel antifibrotic and antihypertrophic agent, prevents cardiac remodeling after myocardial infarction. J Am Coll Cardiol. 2005 Feb 15;45(4):608–16. doi: 10.1016/j.jacc.2004.10.067. [DOI] [PubMed] [Google Scholar]
- (81).Langenickel TH, Buttgereit J, Pagel-Langenickel I, et al. Cardiac hypertrophy in transgenic rats expressing a dominant-negative mutant of the natriuretic peptide receptor B. Proc Natl Acad Sci U S A. 2006 Mar 21;103(12):4735–40. doi: 10.1073/pnas.0510019103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Tokudome T, Horio T, Soeki T, et al. Inhibitory effect of C-type natriuretic peptide (CNP) on cultured cardiac myocyte hypertrophy: interference between CNP and endothelin-1 signaling pathways. Endocrinology. 2004 May;145(5):2131–40. doi: 10.1210/en.2003-1260. [DOI] [PubMed] [Google Scholar]
- (83).Horio T, Tokudome T, Maki T, et al. Gene expression, secretion, and autocrine action of C-type natriuretic peptide in cultured adult rat cardiac fibroblasts. Endocrinology. 2003 Jun;144(6):2279–84. doi: 10.1210/en.2003-0128. [DOI] [PubMed] [Google Scholar]
- (84).Rosenkranz AC, Woods RL, Dusting GJ, Ritchie RH. Antihypertrophic actions of the natriuretic peptides in adult rat cardiomyocytes: importance of cyclic GMP. Cardiovasc Res. 2003 Feb;57(2):515–22. doi: 10.1016/s0008-6363(02)00667-3. [DOI] [PubMed] [Google Scholar]
- (85).Rose RA, Lomax AE, Kondo CS, Anand-Srivastava MB, Giles WR. Effects of C-type natriuretic peptide on ionic currents in mouse sinoatrial node: a role for the NPR-C receptor. Am J Physiol Heart Circ Physiol. 2004 May;286(5):H1970–H1977. doi: 10.1152/ajpheart.00893.2003. [DOI] [PubMed] [Google Scholar]
- (86).Simons M. Angiogenesis, arteriogenesis, and diabetes: paradigm reassessed? J Am Coll Cardiol. 2005 Sep 6;46(5):835–7. doi: 10.1016/j.jacc.2005.06.008. [DOI] [PubMed] [Google Scholar]
- (87).Simons M. Angiogenesis: where do we stand now? Circulation. 2005 Mar 29;111(12):1556–66. doi: 10.1161/01.CIR.0000159345.00591.8F. [DOI] [PubMed] [Google Scholar]
- (88).Lekas M, Lekas P, Latter DA, Kutryk MB, Stewart DJ. Growth factor-induced therapeutic neovascularization for ischaemic vascular disease: time for a re-evaluation? Curr Opin Cardiol. 2006 Jul;21(4):376–84. doi: 10.1097/01.hco.0000231409.69307.d2. [DOI] [PubMed] [Google Scholar]
- (89).Sessa WC. Molecular control of blood flow and angiogenesis: role of nitric oxide. J Thromb Haemost. 2009 Jul;7(Suppl 1):35–7. doi: 10.1111/j.1538-7836.2009.03424.x. [DOI] [PubMed] [Google Scholar]
- (90).Doi K, Ikeda T, Itoh H, et al. C-type natriuretic peptide induces redifferentiation of vascular smooth muscle cells with accelerated reendothelialization. Arterioscler Thromb Vasc Biol. 2001 Jun;21(6):930–6. doi: 10.1161/01.atv.21.6.930. [DOI] [PubMed] [Google Scholar]
- (91).Yamahara K, Itoh H, Chun TH, et al. Significance and therapeutic potential of the natriuretic peptides/cGMP/cGMP-dependent protein kinase pathway in vascular regeneration. Proc Natl Acad Sci U S A. 2003 Mar 18;100(6):3404–9. doi: 10.1073/pnas.0538059100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).ten DP, Arthur HM. Extracellular control of TGFbeta signalling in vascular development and disease. Nat Rev Mol Cell Biol. 2007 Nov;8(11):857–69. doi: 10.1038/nrm2262. [DOI] [PubMed] [Google Scholar]
- (93).Ma J, Wang Q, Fei T, Han JD, Chen YG. MCP-1 mediates TGF-beta-induced angiogenesis by stimulating vascular smooth muscle cell migration. Blood. 2007 Feb 1;109(3):987–94. doi: 10.1182/blood-2006-07-036400. [DOI] [PubMed] [Google Scholar]
- (94).Deckers MM, van Bezooijen RL, van der HG, et al. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology. 2002 Apr;143(4):1545–53. doi: 10.1210/endo.143.4.8719. [DOI] [PubMed] [Google Scholar]
- (95).Naruko T, Ueda M, van der Wal AC, et al. C-type natriuretic peptide in human coronary atherosclerotic lesions. Circulation. 1996 Dec 15;94(12):3103–8. doi: 10.1161/01.cir.94.12.3103. [DOI] [PubMed] [Google Scholar]
- (96).Naruko T, Itoh A, Haze K, et al. C-Type natriuretic peptide and natriuretic peptide receptors are expressed by smooth muscle cells in the neointima after percutaneous coronary intervention. Atherosclerosis. 2005 Aug;181(2):241–50. doi: 10.1016/j.atherosclerosis.2005.01.023. [DOI] [PubMed] [Google Scholar]
- (97).Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009 Apr 14;53(15):e1–e90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- (98).Boerrigter G, Burnett JC., Jr Recent advances in natriuretic peptides in congestive heart failure. Expert Opin Investig Drugs. 2004 Jun;13(6):643–52. doi: 10.1517/13543784.13.6.643. [DOI] [PubMed] [Google Scholar]
- (99).Hare JM, Stamler JS. NO/redox disequilibrium in the failing heart and cardiovascular system. J Clin Invest. 2005 Mar;115(3):509–17. doi: 10.1172/JCI200524459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Munzel T, Daiber A, Mulsch A. Explaining the phenomenon of nitrate tolerance. Circ Res. 2005 Sep 30;97(7):618–28. doi: 10.1161/01.RES.0000184694.03262.6d. [DOI] [PubMed] [Google Scholar]
- (101).Lisy O, Huntley BK, McCormick DJ, Kurlansky PA, Burnett JC., Jr Design, synthesis, and actions of a novel chimeric natriuretic peptide: CD-NP. J Am Coll Cardiol. 2008 Jul 1;52(1):60–8. doi: 10.1016/j.jacc.2008.02.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Lee CY, Chen HH, Lisy O, et al. Pharmacodynamics of a novel designer natriuretic peptide, CD-NP, in a first-in-human clinical trial in healthy subjects. J Clin Pharmacol. 2009 Jun;49(6):668–73. doi: 10.1177/0091270009336233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Dickey DM, Flora DR, Bryan PM, Xu X, Chen Y, Potter LR. Differential Regulation of Membrane Guanylyl Cyclases in Congestive Heart Failure: NPR-B, Not NPR-A, Is the Predominant Natriuretic Peptide Receptor in the Failing Heart. Endocrinology. 2007 Apr 5; doi: 10.1210/en.2007-0081. [DOI] [PubMed] [Google Scholar]
- (104).Del RS, Cabiati M, Lionetti V, Emdin M, Recchia FA, Giannessi D. Expression of C-type natriuretic peptide and of its receptor NPR-B in normal and failing heart. Peptides. 2008 Dec;29(12):2208–15. doi: 10.1016/j.peptides.2008.09.005. [DOI] [PubMed] [Google Scholar]
- (105).Del RS, Maltinti M, Piacenti M, Passino C, Emdin M, Giannessi D. Cardiac production of C-type natriuretic peptide in heart failure. J Cardiovasc Med (Hagerstown ) 2006 Jun;7(6):397–9. doi: 10.2459/01.JCM.0000228688.94709.5a. [DOI] [PubMed] [Google Scholar]
- (106).Kalra PR, Clague JR, Bolger AP, et al. Myocardial production of C-type natriuretic peptide in chronic heart failure. Circulation. 2003 Feb 4;107(4):571–3. doi: 10.1161/01.cir.0000047280.15244.eb. [DOI] [PubMed] [Google Scholar]
- (107).Malcom GT, McMahan CA, McGill HC, Jr, et al. Associations of arterial tissue lipids with coronary heart disease risk factors in young people. Atherosclerosis. 2009 Apr;203(2):515–21. doi: 10.1016/j.atherosclerosis.2008.07.002. [DOI] [PubMed] [Google Scholar]
- (108).Shoenfeld Y, Sherer Y, Harats D. Atherosclerosis as an infectious, inflammatory and autoimmune disease. Trends in Immunology. 2001 Jun 1;22(6):293–5. doi: 10.1016/s1471-4906(01)01922-6. [DOI] [PubMed] [Google Scholar]
- (109).Sherer Y, Shoenfeld Y. Mechanisms of disease: atherosclerosis in autoimmune diseases. Nat Clin Pract Rheumatol. 2006 Feb;2(2):99–106. doi: 10.1038/ncprheum0092. [DOI] [PubMed] [Google Scholar]
- (110).Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994 Nov 15;24(6):1468–74. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- (111).Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999 Jan 14;340(2):115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- (112).Crowther MA. Pathogenesis of Atherosclerosis. Hematology. 2005 Jan 1;2005(1):436–41. doi: 10.1182/asheducation-2005.1.436. [DOI] [PubMed] [Google Scholar]
- (113).Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest. 2005;85(1):9–23. doi: 10.1038/labinvest.3700215. [DOI] [PubMed] [Google Scholar]
- (114).Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol. 2007 Jun 26;49(25):2379–93. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- (115).Tsao PS, Buitrago R, Chan JR, Cooke JP. Fluid Flow Inhibits Endothelial Adhesiveness: Nitric Oxide and Transcriptional Regulation of VCAM-1. Circulation. 1996 Oct 1;94(7):1682–9. doi: 10.1161/01.cir.94.7.1682. [DOI] [PubMed] [Google Scholar]
- (116).Freedman JE, Sauter R, Battinelli EM, et al. Deficient platelet-derived nitric oxide and enhanced hemostasis in mice lacking the NOSIII gene. Circ Res. 1999 Jun 25;84(12):1416–21. doi: 10.1161/01.res.84.12.1416. [DOI] [PubMed] [Google Scholar]
- (117).Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999 Jun 10;399(6736):601–5. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- (118).Fulton D, Gratton JP, McCabe TJ, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999 Jun 10;399(6736):597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Ishizaka Y, Kangawa K, Minamino N, et al. Isolation and identification of C-type natriuretic peptide in human monocytic cell line, THP-1. Biochem Biophys Res Commun. 1992 Dec 15;189(2):697–704. doi: 10.1016/0006-291x(92)92257-x. [DOI] [PubMed] [Google Scholar]
- (120).Peltonen TO, Taskinen P, Soini Y, et al. Distinct downregulation of C-type natriuretic peptide system in human aortic valve stenosis. Circulation. 2007 Sep 11;116(11):1283–9. doi: 10.1161/CIRCULATIONAHA.106.685743. [DOI] [PubMed] [Google Scholar]
- (121).Casco VH, Veinot JP, Kuroski de, Bold ML, Masters RG, Stevenson MM, de Bold AJ. Natriuretic peptide system gene expression in human coronary arteries. J Histochem Cytochem. 2002 Jun;50(6):799–809. doi: 10.1177/002215540205000606. [DOI] [PubMed] [Google Scholar]
- (122).Sugiyama S, Kugiyama K, Matsumura T, et al. Lipoproteins regulate C-type natriuretic peptide secretion from cultured vascular endothelial cells. Arterioscler Thromb Vasc Biol. 1995 Nov;15(11):1968–74. doi: 10.1161/01.atv.15.11.1968. [DOI] [PubMed] [Google Scholar]
- (123).Kugiyama K, Sugiyama S, Matsumura T, Ohta Y, Doi H, Yasue H. Suppression of Atherosclerotic Changes in Cholesterol-Fed Rabbits Treated With an Oral Inhibitor of Neutral Endopeptidase 24.11 (EC 3.4.24.11) Arterioscler Thromb Vasc Biol. 1996 Aug 1;16(8):1080–7. doi: 10.1161/01.atv.16.8.1080. [DOI] [PubMed] [Google Scholar]
- (124).Chun TH, Itoh H, Saito T, et al. Oxidative stress augments secretion of endothelium-derived relaxing peptides, C-type natriuretic peptide and adrenomedullin. J Hypertens. 2000 May;18(5):575–80. doi: 10.1097/00004872-200018050-00010. [DOI] [PubMed] [Google Scholar]
- (125).Igaki T, Itoh H, Suga S, et al. Insulin suppresses endothelial secretion of C-type natriuretic peptide, a novel endothelium-derived relaxing peptide. Diabetes. 1996 Jul;45(Suppl 3):S62–S64. doi: 10.2337/diab.45.3.s62. [DOI] [PubMed] [Google Scholar]
- (126).Yamashita J, Itoh H, Ogawa Y, et al. Opposite regulation of Gax homeobox expression by angiotensin II and C-type natriuretic peptide. Hypertension. 1997 Jan;29(1 Pt 2):381–7. doi: 10.1161/01.hyp.29.1.381. [DOI] [PubMed] [Google Scholar]
- (127).Weir L, Chen D, Pastore C, Isner JM, Walsh K. Expression of gax, a growth arrest homeobox gene, is rapidly down-regulated in the rat carotid artery during the proliferative response to balloon injury. J Biol Chem. 1995 Mar 10;270(10):5457–61. doi: 10.1074/jbc.270.10.5457. [DOI] [PubMed] [Google Scholar]
- (128).Slottow TL, Waksman R. Drug-eluting stent safety. Am J Cardiol. 2007 Oct 22;100(8B):10M–7M. doi: 10.1016/j.amjcard.2007.08.017. [DOI] [PubMed] [Google Scholar]
- (129).Jaffe R, Strauss BH. Late and very late thrombosis of drug-eluting stents: evolving concepts and perspectives. J Am Coll Cardiol. 2007 Jul 10;50(2):119–27. doi: 10.1016/j.jacc.2007.04.031. [DOI] [PubMed] [Google Scholar]
- (130).Morice MC, Serruys PW, Barragan P, et al. Long-term clinical outcomes with sirolimus-eluting coronary stents: five-year results of the RAVEL trial. J Am Coll Cardiol. 2007 Oct 2;50(14):1299–304. doi: 10.1016/j.jacc.2007.06.029. [DOI] [PubMed] [Google Scholar]
- (131).Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007 May 1;115(17):2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- (132).Pfisterer M, Brunner-La Rocca HP, Buser PT, et al. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol. 2006 Dec 19;48(12):2584–91. doi: 10.1016/j.jacc.2006.10.026. [DOI] [PubMed] [Google Scholar]
- (133).Matter CM, Rozenberg I, Jaschko A, et al. Effects of tacrolimus or sirolimus on proliferation of vascular smooth muscle and endothelial cells. J Cardiovasc Pharmacol. 2006 Dec;48(6):286–92. doi: 10.1097/01.fjc.0000248233.22570.8b. [DOI] [PubMed] [Google Scholar]
- (134).Schwertz DW, Vaitkus P. Drug-eluting stents to prevent reblockage of coronary arteries. J Cardiovasc Nurs. 2003 Jan;18(1):11–6. doi: 10.1097/00005082-200301000-00002. [DOI] [PubMed] [Google Scholar]
- (135).Stahli BE, Camici GG, Tanner FC. Drug-eluting stent thrombosis. Ther Adv Cardiovasc Dis. 2009 Feb;3(1):45–52. doi: 10.1177/1753944708096280. [DOI] [PubMed] [Google Scholar]
- (136).Qian JY, Haruno A, Asada Y, et al. Local expression of C-type natriuretic peptide suppresses inflammation, eliminates shear stress-induced thrombosis, and prevents neointima formation through enhanced nitric oxide production in rabbit injured carotid arteries. Circ Res. 2002 Nov 29;91(11):1063–9. doi: 10.1161/01.res.0000043631.25915.e6. [DOI] [PubMed] [Google Scholar]
- (137).Gaspari TA, Barber MN, Woods RL, Dusting GJ. Type-C natriuretic peptide prevents development of experimental atherosclerosis in rabbits. Clin Exp Pharmacol Physiol. 2000 Aug;27(8):653–5. doi: 10.1046/j.1440-1681.2000.03297.x. [DOI] [PubMed] [Google Scholar]
- (138).Fuchs AT, Kuehnl A, Pelisek J, et al. Inhibition of restenosis formation without compromising reendothelialization as a potential solution to thrombosis following angioplasty? Endothelium. 2008 Jan;15(1):85–92. doi: 10.1080/10623320802092484. [DOI] [PubMed] [Google Scholar]
- (139).Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 1998 Mar 10;97(9):916–31. doi: 10.1161/01.cir.97.9.916. [DOI] [PubMed] [Google Scholar]
- (140).Bourassa MG. Long-term vein graft patency. Curr Opin Cardiol. 1994 Nov;9(6):685–91. doi: 10.1097/00001573-199411000-00008. [DOI] [PubMed] [Google Scholar]
- (141).Fitzgibbon GM, Kafka HP, Leach AJ, Keon WJ, Hooper GD, Burton JR. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol. 1996 Sep;28(3):616–26. doi: 10.1016/0735-1097(96)00206-9. [DOI] [PubMed] [Google Scholar]
- (142).Hassantash SA, Bikdeli B, Kalantarian S, Sadeghian M, Afshar H. Pathophysiology of aortocoronary saphenous vein bypass graft disease. Asian Cardiovasc Thorac Ann. 2008 Aug;16(4):331–6. doi: 10.1177/021849230801600418. [DOI] [PubMed] [Google Scholar]
- (143).Schachner T, Zou Y, Oberhuber A, et al. Perivascular application of C-type natriuretic peptide attenuates neointimal hyperplasia in experimental vein grafts. Eur J Cardiothorac Surg. 2004 Apr;25(4):585–90. doi: 10.1016/j.ejcts.2003.07.013. [DOI] [PubMed] [Google Scholar]
- (144).Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004 Oct 14;351(16):1655–65. doi: 10.1056/NEJMra035488. [DOI] [PubMed] [Google Scholar]
- (145).Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004 Jan 20;109(2):159–65. doi: 10.1161/01.CIR.0000102381.57477.50. [DOI] [PubMed] [Google Scholar]
- (146).Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation. 2010 May 11;121(18):2045–66. doi: 10.1161/CIRCULATIONAHA.108.847707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (147).Rubin LJ, Groves BM, Reeves JT, Frosolono M, Handel F, Cato AE. Prostacyclin-induced acute pulmonary vasodilation in primary pulmonary hypertension. Circulation. 1982 Aug;66(2):334–8. doi: 10.1161/01.cir.66.2.334. [DOI] [PubMed] [Google Scholar]
- (148).Barst RJ, Rubin LJ, Long WA, et al. The Primary Pulmonary Hypertension Study Group A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonaryhypertension. N Engl J Med. 1996 Feb 1;334(5):296–302. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- (149).Channick RN, Simonneau G, Sitbon O, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001 Oct 6;358(9288):1119–23. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- (150).Williamson DJ, Wallman LL, Jones R, et al. Hemodynamic effects of Bosentan, an endothelin receptor antagonist, in patients with pulmonary hypertension. Circulation. 2000 Jul 25;102(4):411–8. doi: 10.1161/01.cir.102.4.411. [DOI] [PubMed] [Google Scholar]
- (151).Galie N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005 Nov 17;353(20):2148–57. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- (152).Provencher S, Sitbon O, Humbert M, Cabrol S, Jais X, Simonneau G. Long-term outcome with first-line bosentan therapy in idiopathic pulmonary arterial hypertension. Eur Heart J. 2006 Mar;27(5):589–95. doi: 10.1093/eurheartj/ehi728. [DOI] [PubMed] [Google Scholar]
- (153).Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995 Jul 27;333(4):214–21. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- (154).Tuder RM, Cool CD, Geraci MW, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med. 1999 Jun;159(6):1925–32. doi: 10.1164/ajrccm.159.6.9804054. [DOI] [PubMed] [Google Scholar]
- (155).Luscher TF, Barton M. Endothelins and endothelin receptor antagonists: therapeutic considerations for a novel class of cardiovascular drugs. Circulation. 2000 Nov 7;102(19):2434–40. doi: 10.1161/01.cir.102.19.2434. [DOI] [PubMed] [Google Scholar]
- (156).Rhodes CJ, Davidson A, Gibbs JS, Wharton J, Wilkins MR. Therapeutic targets in pulmonary arterial hypertension. Pharmacol Ther. 2009 Jan;121(1):69–88. doi: 10.1016/j.pharmthera.2008.10.002. [DOI] [PubMed] [Google Scholar]
- (157).Baliga RS, Zhao L, Madhani M, et al. Synergy between natriuretic peptides and phosphodiesterase 5 inhibitors ameliorates pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008 Oct 15;178(8):861–9. doi: 10.1164/rccm.200801-121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (158).Klinger JR, Thaker S, Houtchens J, Preston IR, Hill NS, Farber HW. Pulmonary hemodynamic responses to brain natriuretic peptide and sildenafil in patients with pulmonary arterial hypertension. Chest. 2006 Feb;129(2):417–25. doi: 10.1378/chest.129.2.417. [DOI] [PubMed] [Google Scholar]
- (159).Itoh T, Nagaya N, Murakami S, et al. C-type natriuretic peptide ameliorates monocrotaline-induced pulmonary hypertension in rats. Am J Respir Crit Care Med. 2004 Dec 1;170(11):1204–11. doi: 10.1164/rccm.200404-455OC. [DOI] [PubMed] [Google Scholar]
- (160).Klinger JR, Siddiq FM, Swift RA, et al. C-type natriuretic peptide expression and pulmonary vasodilation in hypoxia-adapted rats. Am J Physiol. 1998 Oct;275(4 Pt 1):L645–L652. doi: 10.1152/ajplung.1998.275.4.L645. [DOI] [PubMed] [Google Scholar]
- (161).Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb Exp Pharmacol. 2009;(191):341–66. doi: 10.1007/978-3-540-68964-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (162).Ahluwalia A, Hobbs AJ. Endothelium-derived C-type natriuretic peptide: more than just a hyperpolarizing factor. Trends Pharmacol Sci. 2005 Mar;26(3):162–7. doi: 10.1016/j.tips.2005.01.005. [DOI] [PubMed] [Google Scholar]
- (163).Del Ry S, Maltinti M, Piacenti M, Passino C, Emdin M, Giannessi D. Cardiac production of C-type natriuretic peptide in heart failure. J Cardiovasc Med (Hagerstown ) 2006 Jun;7(6):397–9. doi: 10.2459/01.JCM.0000228688.94709.5a. [DOI] [PubMed] [Google Scholar]
- (164).Hama N, Itoh H, Shirakami G, et al. Detection of C-type natriuretic peptide in human circulation and marked increase of plasma CNP level in septic shock patients. Biochem Biophys Res Commun. 1994 Feb 15;198(3):1177–82. doi: 10.1006/bbrc.1994.1166. [DOI] [PubMed] [Google Scholar]
- (165).Totsune K, Takahashi K, Murakami O, Satoh F, Sone M, Mouri T. Elevated plasma C-type natriuretic peptide concentrations in patients with chronic renal failure. Clin Sci (Lond) 1994 Sep;87(3):319–22. doi: 10.1042/cs0870319. [DOI] [PubMed] [Google Scholar]
- (166).Ruilope LM, Dukat A, Bohm M, Lacourciere Y, Gong J, Lefkowitz MP. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet. 2010 Apr 10;375(9722):1255–66. doi: 10.1016/S0140-6736(09)61966-8. [DOI] [PubMed] [Google Scholar]