Summary
Impediments to DNA replication are known to induce gross chromosomal rearrangements (GCR) and copy number variations (CNV). GCRs/CNVs underlie human genomic disorders1 and are a feature of cancer2. During cancer development environmental factors and oncogene-driven proliferation promote replication stress. Resulting GCRs/CNVs are proposed to contribute to cancer development and therapy resistance3. When stress arrests replication, the replisome remains associated with the fork DNA (stalled fork) and is protected by the inter-S phase checkpoint. Stalled forks efficiently resume when the stress is relieved. However, if the polymerases dissociate from the fork (fork collapse) or the fork structure breaks (broken fork), replication restart can proceed either by homologous recombination (HR) or microhomology-primed re-initiation (FoSTeS/MMBIR)4,5. Here we ascertain the consequences of replication with a fork restarted by HR. We identify a new mechanism of chromosomal rearrangement: recombination-restarted forks have an exceptionally high propensity to execute a U-turn at small inverted repeats (up to 1:40 replication events). We propose that the error-prone nature of restarted forks contributes to the generation of GCRs and gene amplification in cancer and to non-recurrent CNVs in genomic disorders.
In eukaryotes, multiple origins are licenced but only a subset fire. If one fork collapses, replication is completed by a converging fork6. Alternatively, if both converging forks collapse, dormant origins can fire to rescue the situation7. However, when converging forks collapse without an intervening dormant origin, i.e. at a fragile site8,9, or if a single fork collapses at a unidirectionally-replicated locus10 one replisome will likely be rebuilt by HR. To study replication fork collapse and restart we use a programmed replication terminator sequence (RTS1) to arrest the replisome at a defined genomic locus in fission yeast11,12. Fork arrest at RTS1 is controlled by regulating rtf1+ transcription11. Rtf1, a Myb-like DNA binding protein, is required for arrest at RTS1. Following induction >90% of forks arrest at RTS1 and require HR proteins to restart13.
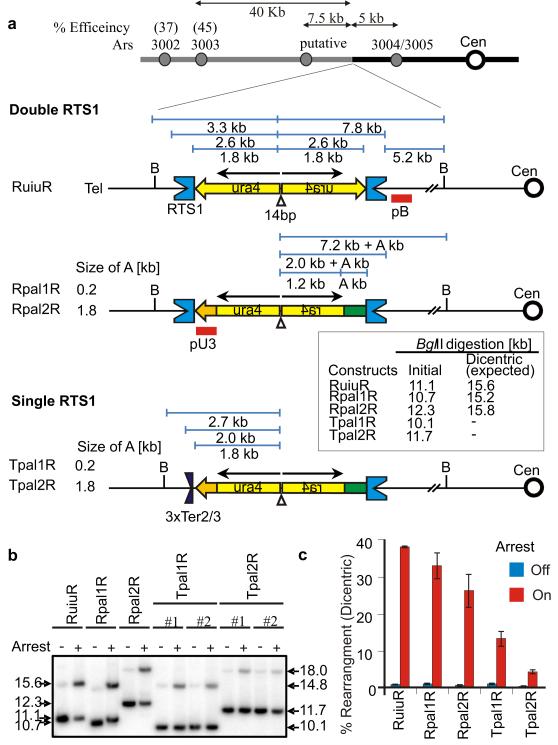
In S. pombe collapsed forks restart by an HR-dependent, but double strand break-independent, mechanism12,13. Our model (Supp. Figure S1) suggests HR proteins associate with the nascent strand behind the collapsed fork and subsequent strand invasion at the collapse site facilitates accurate HR-dependent fork restart with the correct template. However, if a DNA sequence homologous to the collapse site is nearby, an erroneous strand invasion can occur such that replication reinitiates ectopically. This leads to non-allelic homologous recombination (NAHR)11-13. When the homologous sequences are in an inverted repeat (IR) orientation, NAHR associated with inaccurate restart results in acentric and dicentric isochromosomes13. We also observed that, when the fork barrier sequence formed the flanking regions of a small palindrome12, GCR rates increased ~10 fold (contrast RuraR and RuiuR constructs, Suppl. Figure S2). The main distinction between the two constructs is that RuiuR contains RTS1 in context of the 5.3kb palindrome as opposed to an IR separated by 1.8kb (RuraR). We thus speculated that, upon NAHR, branch migration of the invading strand (which is not possible in the IR construct) formed a single HJ at the palindrome centre which drove the increased chromosome rearrangement.
To prevent the predicted half-crossover migrating in RuiuR to the palindrome centre we replaced 550bp of the centromere-proximal ura4+ gene with 0.2 or 1.8kb of his3+ creating Rpal1R and Rpal2R (Figure 1A). To prevent any possibility of rearrangement by NAHR we created two further constructs in which the telomere-proximal RTS1 sequences of Rpal1R and Rpal2R were replaced with three copies of rDNA fork barrier, Ter2/3, to create Tpal1R and Tpal2R. Ter2/3 serves simply to pause the converging fork, allowing more time for the RTS1-collapsed fork to restart. Ter2/3 differs from RTS1 in sequence and arrests forks in an Rtf1-independent fashion14,15 (Suppl. Figure S3A,B). Unlike RTS1, where forks collapse and require HR to rebuild the replisome for restart, forks pause transiently at Ter2/3, do not require HR for resumption and the arrest site does not accumulate Rad52. GCRs are thus not induced (Suppl. Figure S3C-F).
Figure 1.
Alternative mechanism for inverted chromosomal fusion.
A. Cartoon of double and single RTS1 constructs. Replication origins (ars) on Chromosome 3, their distance from ura4 and predicted efficiencies are shown. Open circle indicates centromere 3. Concave blue and dark boxes represent RTS1 or 3×Ter2/3 as indicated. Yellow arrows/boxes represent ura4 sequences and green box represents his3 sequences. Black arrow indicates IRs of the ura4 sequences. Open triangle shows 14 bps interrupting sequence at the palindrome centre. Red bars represent probes. B indicates BglII restriction site. Sizes of initial and predicted dicentric chromosome BglII fragments generated by replication template exchange are shown for each relevant strain. B. A representative southern blot of double and single RTS1 strains with arrest off or arrest on. Genomic DNA was digested with BglII and probed with pB. C. Quantitation of rearranged fragment in B. Mean value and standard deviation of the values are calculated from at least three independent experiments.
To assay for GCRs, fork arrest was induced at RTS1 by inducing Rtf1 (Ter2/3 arrest is constitutive) and genomic DNA analysed by Southern blot. We predicted no GCRs in strains with a single RTS1 (Tpal1R and Tpal2R) and were interested to establish if double RTS1 constructs (Rpal1R and Rpal2R) significantly reduced GCR levels (Figure 1A). Surprisingly, all four constructs generated GCRs in an RTS1 fork arrest-dependent manner (Figure 1B,C). Double RTS1 systems accumulated 25-30% GCRs, similar to that observed in RuiuR, while single RTS1 strains showed ~5-15% rearrangement.
NAHR between RTS1 sequences occurring upon restart should produce dicentric chromosomes with an expected BglII fragment of 15.2kb (Rpal1R) or 15.8kb (Rpal2R). The observed fragment lengths were 14.8kb (Rpal1R) and 18kb (Rpal2R). These correspond to double the size from the cen-proximal BglII site to the palindrome centre. Identical size fragments are observed in the corresponding single RTS1 constructs. These data suggest a novel mechanism of chromosomal rearrangement: the collapsed replication fork resumes accurately with the correct template, but later reverses the orientation of DNA replication (U-turn) as it replicates through the palindrome centre. This leads to isodicentric chromosome formation.
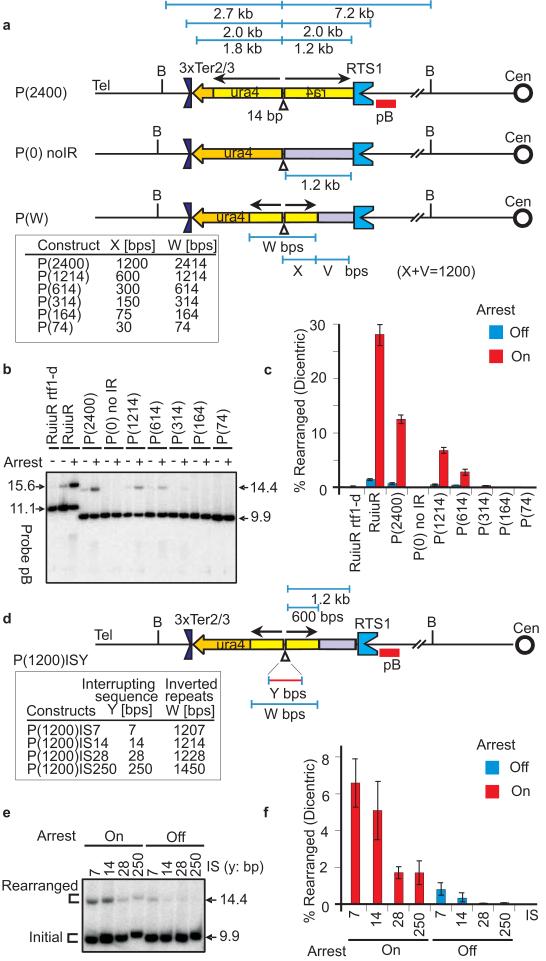
To characterise the effect of palindrome size in promoting restarted fork-dependent GCRs, a series of constructs werte made where the palindrome size [P(bp)] varied between P(74) and P(2400), but its centre of symmetry remained a constant distance from the site of fork restart (Figure 2A). All constructs contained the 14bp interrupting sequence at the palindrome centre. To establish GCR levels, genomic DNA was analysed by Southern blot using two flanking probes, pB or pA.
Figure 2.
Rearrangement frequency is dependent on the repeat size and interrupting sequence size.
A. Cartoon of constructs with varying repeat size. P(2400), P(0) noIR and intermediates P(W),are indicated as in Figure 1A. W represents the size of the whole palindrome in bps. X shows the size of the ura4 fragment creating the IR. Grey box indicates heterologous sequence (V). The sum of X and V is always 1200 bps. Probes pB is indicated as red bar. B. Southern blot analyses of P(W) strains for arrest off or arrest on. Genomic DNA was digested with BglII and probed with pB. C. Quantitation of rearranged fragment in B. Average values and standard deviation are calculated from at least three independent experiments. D. Cartoon of constructs varying interrupting sequence [P(1200)IS(Y)] indicated as in Figure 2A. Y and W represent the size of interrupting sequence and the whole palindrome in bps, respectively. E. Southern blot analysis of P(1200)IS(Y) strains for arrest off or arrest on. Southern blot was performed as described in Figure 1B. F. Quantitation of rearranged fragment in E as described in Figure 1C. Mean value and standard deviation of the values are calculated from at least three independent experiments.
For P(2400), probe pB revealed ~10% of the DNA corresponded to the rearranged product, migrating at 14.4kb (Figure 2B). This is twice 7.2kb, the distance from the palindrome centre to the centromere-proximal BglII site. Probe pA revealed a similar proportion of a 5.4kb fragment (twice 2.7kb, the distance between the palindrome centre and the telomere proximal BglII site, Figure S4).
These rearranged products correspond, respectively, to dicentric and acentric isochromosomes (data not shown). As expected, P(0), which has no IRs, showed no detectable rearrangement products confirming that the 14.4kb and 5.4kb signals do not represent replication intermediates or broken forks. All induced rearrangements were dependent on replication fork arrest at RTS1 and the %GCR was dependent on palindrome size (Figure 2B, C and S4B,D). P(314) was the smallest palindrome allowing GCR detection by Southern analysis.
Palindromes are prone to form secondary structures including cruciforms (dsDNA) and hairpins (ssDNA). Secondary structure formation is influenced by interrupting sequence (IS) size16,17. To establish if GCR formation was related to IS size, we used P(1214) as a base construct and varied the IS (Figure 2D). A 7bp IS showed slightly higher GCR levels than a 14bp IS. A 28bp IS reduced levels ~3 fold to 2%. This did not reduce further when the IS was increased to 250bp (Figure 2E,F). These results indicate that a potential for structured DNA formation promotes restarted fork U-turn but is not essential.
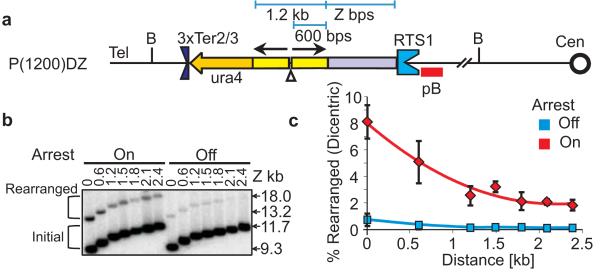
The data from Figure 1B,C suggested that the distance of the palindrome centre from the site of restart influences the U-turn frequency. To clarify this, a further series was constructed where different sizes of heterologous sequence separated a 1.2kb palindrome and RTS1 (Figure 3A). A maximum GCR amount was observed when the palindrome directly abutted RTS1 (~8%). As the distance between the palindrome and RTS1 was increased to ~1.5kb, the GCR level decreased ~4 fold to 2%. Further extension of the distance did not result in further decreases (Figure 3B,C). These data suggest that the restarted fork is initially highly error-prone, but matures as it travels the first few kb. However, the constant rate of GCR observed in constructs separating the palindrome from RTS1 by >1.5kb implies that such “matured” forks are not canonical and remain significantly error-prone.
Figure 3.
Fidelity of HR-restarted fork improves with distance.
A. Cartoon of constructs varying distance from restart site [P(1200)D(Z)]indicated as in Figure 2A. The whole palindrome is 1214 bps. Z indicates distance of the palindrome from RTS1 in kb. B. Southern blot analysis of P(1200)D(Z) strains for arrest off or arrest on. Southern blot was performed as described in Figure 1B. C. Quantitation of rearranged fragment in E as described in Figure 1C. Mean value and standard deviation of the values are calculated from at least three independent experiments.
While it has not been possible to establish if recombination-restarted forks become error-free over greater distances due to the limitations of our system, it is notable that break induced replication (BIR) forks analysed in S. cerevisiae18 remain prone to replication slippage19 and template exchange with homologous chromosomes20 over 10’s and 100’s of kb. BIR initiates from a DSB and occurs outside of S phase in G2 arrested cells. Our recombination-restarted forks restart without a DSB intermediate during S phase. It is thus unclear how closely the two systems equate and if similar replisome configurations underlie restarted replication in both systems.
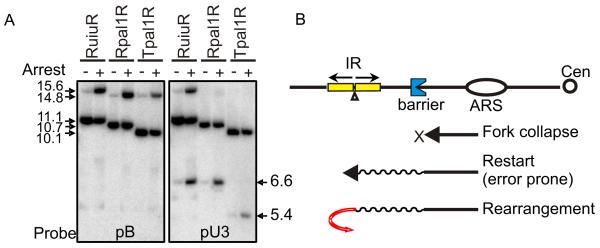
NAHR occurring during the restart event generates acentric and dicentric isochromosomes containing the intervening sequence originally present between the inverted RTS1 repeats. Conversely, the isochromosomes generated by recombination-restarted forks executing a U-turn at the palindrome centre contain either two cen-proximal or two tel-proximal sequences (defined from the centre of the IR/palindrome). Thus, we can establish the relative contribution of these two distinct mechanisms in generating isochromosomes from the double RTS1 palindrome construct, Rpal1R.
Double RTS1 constructs (Figure 1A) potentially cause isochromosome formation by either NAHR or by recombination-restarted fork U-turn. The derivatives with a single RTS1 sequence cannot undergo NAHR and only generate isochromosomes by recombination-restarted fork U-turn. We used Southern blot to distinguish these products (Figure 4A). The major mechanism for the rearrangement for inverted RTS1 constructs is recombination-restarted fork U-turn, with a minor contribution from NAHR. Therefore, the original palindromic RuiuR construct, which showed a much higher level of isochromosome formation compared with the original RuraR construct12, does so because of an additional defect associated with forks restarted by HR can be visualised.
Figure 4.
U-turn at palindrome centre is major mechanism for inverted fusion in double RTS1.
A. Southern blot analyses of RuiuR, Rpal1R and Tpal1R for arrest off or arrest on. Genomic DNA was digested with BglII and probed with pB or pU3 - see Figure 1A. Note that the majority of the rearrangement in Rpal1R detected by probe pU3 is acentric 6.6kb. B. Model for error-prone progression of a recombination-restarted replication fork. Oval, blue concave, and yellow box represent replication origin ARS, obstacle and repeat sequences, respectively. When a replication fork collapses, homologous recombination restarts the collapsed fork. However, the restarted fork is non-canonical and error-prone, causing GCRs at IRs due to executing a U-turn.
The junction consists of two sister chromatids fused at the repeat centre, suggesting that the recombination-restarted replisome performs a U-turn by exchanging template strands between the repeats. This would be consistent with the nascent strand frequently dissociating transiently from, and then re-annealing to, its template. Synthesis continuing on an incorrect inverted template would result in a “closed Y” structure at the repeat centre. Intriguingly, large interrupting sequences reduce, but do not eliminate, the rearrangement, suggesting homology either with or without structured DNA can drive a U-turn.
The genome rearrangement profiles in cancer are complex, including simple CNVs, chromothripsis (multiple linked rearrangements)21, translocations and gene amplifications that often initiate from isochromosomes3. Similarly, rearrangements in genomic disorders include simple recurrent CNV caused by NAHR during meiosis (or occasionally in mitotic cells), inverted duplication deletions22, non-recurrent CNVs typified by microhomology (or no homology) at the junction23, and complex multi-junction events which, in some cases, suggest multiple contiguous replication errors4,24,25.
Our data show recombination-restarted forks are error-prone, with an unexpectedly high propensity (up to 1:40 events) to U-turn between short IRs which can be separated by many kilobases. Likewise, recombination-restarted forks cause increased microhomology-dependent insertions and deletions26. Current models for the generation of replication-associated rearrangements almost invariably assume a double strand break (DSB) as the initiating event, which subsequently undergoes an incorrect choice of restart site based on homology or microhomology. We propose that inaccurate replication from forks correctly restarted without a DSB intermediate also makes a significant contribution to genome rearrangement. Once a fork is restarted at the correct sequence it is particularly prone to U-turn between IRs. While our physical assay can detect these events at IRs of ~150bp, the relationship between repeat size and frequency (Figure 2C) suggests shorter repeats will still generate a significant rate.
While we cannot directly establish if recombination-restarted forks are responsible for genome rearrangements in human cells, our data predicts that isochromosome formation in cancer cells will be elevated at fragile sites, where replication forks are prone to collapse and low origin density necessitates fork restart. Interestingly, isodicentric chromosome formation and subsequent Breakage-Fusion-Bridge (BFB) cycle-dependent rearrangements initiate gene amplification27 and fragile sites have been associated with amplification boundaries as well as other cancer-related GCRs28. Similarly, slippage at microhomology by recombination-restarted forks likely contributes to the frequent CNVs associated with cancer19,26.
Equally, several classes of genomic disorders are compatible with a contribution from recombination-restarted forks: one well characterised rearrangement involves a triplicated segment embedded within a duplication29 mediated by small inverted low copy repeats. DUP-TRP/INV-DUP involves two “breakpoint” junctions, one within the repeats and a second showing microhomology. Such a rearrangement can be explained by two distinct events associated with a single fork restart. Likewise, several other genomic disorders result from inverted duplication deletions22, which are predicted to be the stabilised events of breakage of an isodicentric chromosome during BFB cycles.
Methods
Standard Genetics and Molecular Biology Techniques
Strains were constructed using standard genetic techniques30. The Schizosaccharomyces pombe strains used in this study are listed in Supp. Table S1. Culture conditions, genomic DNA preparation in agarose plugs, Southern blot techniques and the quantitation of rearranged DNA were performed as described12,13. Genomic DNA was digested with 150 units of BglII in the recommended buffer. Probes pA and pB are described in Mizuno et al. 2009 as Cen and Tel respectively. Probe pU3 is a 550 bps fragment of ura4 genomic DNA digested by EcoRV and SpeI. Probe p ura is a 1.8kb ura4 fragment and probe ura45 a HindIII SpeI fragment comprising the ura4 and ura5 genes.
Ter2/3 rDNA Fork Barrier
Primers PRR1-F (5′-p-AATTCTACTACTATTTTGTGCATTACCCTTACCTTTTTTTTC-3′) and PRR1-R (5′-p-AATTGAAAAAAAAGGTAAGGGTAATGCACAAAATAGTAGTAG-3′) were annealed and ligated. Ter2/3 consensus sequence is underlined31. The ligated DNA was digested with EcoRI and MfeI to eliminate inverted repeated configuration, size-fractionated by agarose gel and fragments of ~130 bps (3X tandem repeats) used to replace the telomere-proximal RTS1. The construct was confirmed by sequencing.
Supplementary Material
Acknowledgments
We thank E. Hoffman, J. Baxter, M. Neale and members of the Carr and Murray laboratories for discussions. J.M.M. acknowledges CRUK grant C9601/A9484: A.M.C. acknowledges MRC grant G0600233. K.M.,
Footnotes
Author Information
The authors declare no competing interests.
References
- 1.Lupski JR. Genomic disorders: structural features of the genome can lead to DNA rearrangements and human disease traits. Trends in genetics: TIG. 1998;14:417–422. doi: 10.1016/s0168-9525(98)01555-8. [DOI] [PubMed] [Google Scholar]
- 2.Liu P, Carvalho CM, Hastings P, Lupski JR. Mechanisms for recurrent and complex human genomic rearrangements. Curr Opin Genet Dev. 2012;22:211–220. doi: 10.1016/j.gde.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mondello C, Smirnova A, Giulotto E. Gene amplification, radiation sensitivity and DNA double-strand breaks. Mutat Res. 2010;704:29–37. doi: 10.1016/j.mrrev.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Lee JA, Carvalho CM, Lupski JR. A DNA replication mechanism for generating nonrecurrent rearrangements associated with genomic disorders. Cell. 2007;131:1235–1247. doi: 10.1016/j.cell.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 5.Hastings PJ, Ira G, Lupski JR. A microhomology-mediated break-induced replication model for the origin of human copy number variation. PLoS Genet. 2009;5:e1000327. doi: 10.1371/journal.pgen.1000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petermann E, Helleday T. Pathways of mammalian replication fork restart. Nat Rev Mol Cell Biol. 2010:11. doi: 10.1038/nrm2974. [DOI] [PubMed] [Google Scholar]
- 7.Blow JJ, Ge XQ, Jackson DA. How dormant origins promote complete genome replication. Trends Biochem Sci. 2011;36:405–414. doi: 10.1016/j.tibs.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Letessier A, et al. Cell-type-specific replication initiation programs set fragility of the FRA3B fragile site. Nature. 2011;470:120–123. doi: 10.1038/nature09745. [DOI] [PubMed] [Google Scholar]
- 9.Ozeri-Galai E, et al. Failure of origin activation in response to fork stalling leads to chromosomal instability at fragile sites. Mol Cell. 2011;43:122–131. doi: 10.1016/j.molcel.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 10.Murray JM, Carr AM. Smc5/6: a link between DNA repair and unidirectional replication? Nat Rev Mol Cell Biol. 2008;9:177–182. doi: 10.1038/nrm2309. [DOI] [PubMed] [Google Scholar]
- 11.Lambert S, Watson A, Sheedy DM, Martin B, Carr AM. Gross chromosomal rearrangements and elevated recombination at an inducible site-specific replication fork barrier. Cell. 2005;121:689–702. doi: 10.1016/j.cell.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Mizuno K, Lambert S, Baldacci G, Murray JM, Carr AM. Nearby inverted repeats fuse to generate acentric and dicentric palindromic chromosomes by a replication template exchange mechanism. Genes Dev. 2009;23:2876–2886. doi: 10.1101/gad.1863009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambert S, et al. Homologous recombination restarts blocked replication forks at the expense of genome rearrangements by template exchange. Mol Cell. 2010;39:346–359. doi: 10.1016/j.molcel.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez-Gorostiaga A, Lopez-Estrano C, Krimer DB, Schvartzman JB, Hernandez P. Transcription termination factor reb1p causes two replication fork barriers at its cognate sites in fission yeast ribosomal DNA in vivo. Molecular and cellular biology. 2004;24:398–406. doi: 10.1128/MCB.24.1.398-406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krings G, Bastia D. swi1- and swi3-dependent and independent replication fork arrest at the ribosomal DNA of Schizosaccharomyces pombe. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:14085–14090. doi: 10.1073/pnas.0406037101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams WL, Muller UR. Effects of palindrome size and sequence on genetic stability in the bacteriophage phi X174 genome. J Mol Biol. 1987;196:743–755. doi: 10.1016/0022-2836(87)90401-3. [DOI] [PubMed] [Google Scholar]
- 17.Voineagu I, Narayanan V, Lobachev KS, Mirkin SM. Replication stalling at unstable inverted repeats: interplay between DNA hairpins and fork stabilizing proteins. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9936–9941. doi: 10.1073/pnas.0804510105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lydeard JR, et al. Break-induced replication requires all essential DNA replication factors except those specific for pre-RC assembly. Genes Dev. 2010;24:1133–1144. doi: 10.1101/gad.1922610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deem A, et al. Break-induced replication is highly inaccurate. PLoS Biol. 2011;9:e1000594. doi: 10.1371/journal.pbio.1000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith CE, Llorente B, Symington LS. Template switching during break-induced replication. Nature. 2007;447:102–105. doi: 10.1038/nature05723. [DOI] [PubMed] [Google Scholar]
- 21.Stephens PJ, et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell. 2011;144:27–40. doi: 10.1016/j.cell.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuffardi O, Bonaglia M, Ciccone R, Giorda R. Inverted duplications deletions: underdiagnosed rearrangements?? Clin Genet. 2009;75:505–513. doi: 10.1111/j.1399-0004.2009.01187.x. [DOI] [PubMed] [Google Scholar]
- 23.Arlt MF, Wilson TE, Glover TW. Replication stress and mechanisms of CNV formation. Curr Opin Genet Dev. 2012;22:204–210. doi: 10.1016/j.gde.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu P, et al. Chromosome catastrophes involve replication mechanisms generating complex genomic rearrangements. Cell. 2011;146:889–903. doi: 10.1016/j.cell.2011.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kloosterman WP, et al. Constitutional chromothripsis rearrangements involve clustered double-stranded DNA breaks and nonhomologous repair mechanisms. Cell Rep. 2012;1:648–655. doi: 10.1016/j.celrep.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Iraqui I, et al. Recovery of arrested replication forks by homologous recombination is error-prone. PLoS Genet. 2012 doi: 10.1371/journal.pgen.1002976. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelliccia F, Bosco N, Rocchi A. Breakages at common fragile sites set boundaries of amplified regions in two leukemia cell lines K562 - Molecular characterization of FRA2H and localization of a new CFS FRA2S. Cancer Lett. 2010;299:37–44. doi: 10.1016/j.canlet.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Blumrich A, et al. The FRA2C common fragile site maps to the borders of MYCN amplicons in neuroblastoma and is associated with gross chromosomal rearrangements in different cancers. Hum Mol Genet. 2011;20:1488–1501. doi: 10.1093/hmg/ddr027. [DOI] [PubMed] [Google Scholar]
- 29.Carvalho CM, et al. Inverted genomic segments and complex triplication rearrangements are mediated by inverted repeats in the human genome. Nat Genet. 2011;43:1074–1081. doi: 10.1038/ng.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez-Gorostiaga A, Lopez-Estrano C, Krimer DB, Schvartzman JB, Hernandez P. Transcription termination factor reb1p causes two replication fork barriers at its cognate sites in fission yeast ribosomal DNA in vivo. Molecular and cellular biology. 2004;24:398–406. doi: 10.1128/MCB.24.1.398-406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.