Abstract
Lithium is the most effective mood stabilizer for the treatment of bipolar disorder, but it is toxic at only twice the therapeutic dosage and has many undesirable side effects. It is likely that a small molecule could be found with lithium-like efficacy but without toxicity through target-based drug discovery; however, lithium’s therapeutic target remains equivocal. Inositol monophosphatase is a possible target but no bioavailable inhibitors exist. Here we report that the antioxidant ebselen inhibits inositol monophosphatase and induces lithium-like effects on mouse behaviour, which are reversed with inositol, consistent with a mechanism involving inhibition of inositol recycling. Ebselen is part of the National Institutes of Health Clinical Collection, a chemical library of bioavailable drugs considered clinically safe but without proven use. Therefore, ebselen represents a lithium mimetic with the potential both to validate inositol monophosphatase inhibition as a treatment for bipolar disorder and to serve as a treatment itself.
Bipolar disorder affects 1-3% of the population and the most effective treatment for stabilizing mood is lithium 1. Lithium is also the only agent that reduces suicidal thoughts and actions 2. Unfortunately, lithium is toxic at only twice the therapeutic dosage and has many undesirable side effects including weight gain, thirst, tremor and kidney damage 3. To develop a lithium mimetic—ideally a drug with its therapeutic action but without its disadvantages—would require an understanding of lithium’s mechanism of action, which, even after six decades of use 4, remains controversial 5. Lithium displaces magnesium ions and inhibits at least 10 cellular targets, all of which are components of intracellular signalling pathways5. However, targets inhibited by lithium at therapeutically relevant concentrations (0.6-1 mM) narrows the targets to two: glycogen synthase kinase 3ß6 and inositol monophosphatase 7-9. Both putative targets have experimental evidence for and against them based on genetics and pharmacology6,9-12. Additionally, several chemically distinct bipolar medications (lithium, valproic acid and carbamazapine) all have a common mechanism of action affecting the inositol cycle13. Inhibition of inositol monophosphatase by lithium led to Berridge’s ‘inositol depletion hypothesis’ that suggests that Ins1P accumulates and inositol is depleted7. Given that in neurons regeneration of phosphatidylinositol 4,5-bisphosphate requires recycling of inositol from Ins1P, lithium dampens signalling in cells with overactive signalling through pathways using a G-protein-coupled receptor linked to phospholipase C7.
IMPase remains a potential therapeutic target for bipolar disorder, but its validation requires small molecule inhibitors. However, little progress has been made in regard to inhibitors since a large effort by Merck yielded a potent (IC50 300 nM) antagonist (L-690,330) but neither it nor its esterified prodrug (L-690,488) was bioavailable 14,15. We now report that ebselen inhibits IMPase and acts as a lithium mimetic in mouse models of bipolar disorder.
Results
Repurposing reveals ebselen as an inhibitor of IMPase
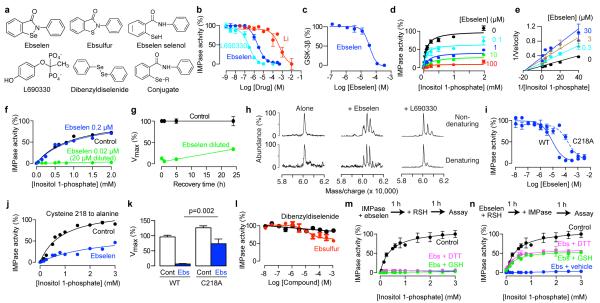
To identify an inhibitor of IMPase, we expressed human IMPase in bacteria and used it in an assay to screen the NIH Clinical Collection provided through the National Institutes of Health Molecular Libraries Roadmap Initiative 16. Compounds in this collection have a history of use in human clinical trials, are drug-like with known safety profiles and may even be appropriate for direct human use in new disease areas (www.nihclinicalcollection.com). A primary screen at 100 μM of each drug in the collection identified ebselen (Fig. 1a) as an inhibitor of IMPase, and we characterized it further with a full concentration-response curve (Fig. 1b). The potency of ebselen against IMPase (IC50 1.5 μM) compared favourably to that of the known but poorly bioavailable inhibitor L-690,33014 (IC50 0.3 μM) and was greater than that of lithium (IC50 0.8 mM; Fig. 1b). Importantly, the greater potency of ebselen for IMPase (Fig. 1b) compared to glycogen synthase kinase 3ß (Fig. 1c) demonstrates selectivity, making ebselen of diagnostic use in determining the therapeutic potential of IMPase inhibition.
Fig. 1. Ebselen inhibits inositol monophosphatase in vitro.
(a) Chemical structures. For the ebselen conjugate, R is glutathione or another ebselen molecule. (b) Concentration–inhibition relationships for ebselen and known inhibitors of IMPase. Assay used expressed human IMPase. (c) Concentration–inhibition relationships for ebselen on glycogen synthase kinase 3ß. (d,e) Effect of ebselen on the enzyme kinetics of IMPase shown as a Michaelis-Menten plot (d) or a Lineweaver-Burk plot (e). (f) Effect of dilution on inhibition of IMPase by ebselen (20 μM before and 0.2 μM after dilution). (g) Effect of recovery time after dilution on inhibition of IMPase by ebselen. (h) Mass spectroscopy under mild denaturing and non-denaturing conditions of IMPase incubated with ebselen or L-690,330 (100 μM each). (i-k) Effect of mutation cysteine-218 to alanine on inhibition of IMPase by ebselen assessed by concentration-inhibition curves (i), Michaelis-Menton kinetics (ebselen 50 μM) (j) and Vmax (k). Analyzed by a pre-planned t-test, n=6. (l) Concentration-response relationships for the sulfur analogue of ebselen and dibenzyldiselenide on IMPase. (m,n) Effect of disulfide reducing agents on inhibition of IMPase by ebselen (50 μM) with either post-incubation with glutathione (GSH, 1 mM) or dithiothreitol (50 mM) (m) or pre-incubation with 5 mM and 250 mM, respectively (n). All error bars represent standard error of the means.
Ebselen inhibition is irreversible and covalent
As increasing concentrations of ebselen decreased Vmax with little effect on Km (Fig. 1d,e) the inhibition is not competitive17. Inhibition of IMPase by ebselen was not fully relieved by dilution (20 μM to 0.2 μM ebselen; Fig. 1f) even after a time course for recovery extending to 25 h (Fig. 1g), thus indicating that inhibition is, for practical purposes, irreversible. (A reversible inhibitor would lose potency upon dilution due to mass action promoting dissociation17.) As irreversible inhibition often arises from covalent binding, we looked for direct evidence of ebselen binding to IMPase. Mass spectrometry revealed that a mixture of IMPase and ebselen formed complexes heavier than pure IMPase dimer by the mass of one or two ebselen molecules under both denaturing and non-denaturing conditions (Fig. 1h), supporting covalent binding and 1:1 stoichiometry per monomer. In contrast, a mixture of IMPase and the reversible inhibitor L-690,33014 formed heavier complexes under non-denaturing conditions, but not under denaturing conditions (Fig. 1h).
Ebselen contains selenium (Fig. 1a), which can form a selenylsulfide (–Se–S–) bond18,19. For bovine IMPase, alkylation of cysteine 218 with the non-selective agent N-ethylmaleimide inhibited activity20. In bovine IMPase, cysteine 218 is near the active site residue aspartate 220, which is required for magnesium ion coordination and catalysis20. The position of this cysteine is conserved in both the mouse and human isoforms based on its crystal structure21. To test the importance of this cysteine in mediating ebselen inhibition, we constructed a human IMPase with a cysteine to alanine mutation (C218A). The C128A mutant was indeed less sensitive to ebselen inhibition, based on the increase in IC50 for ebselen (Fig. 1i) and a smaller decrease in Vmax (Fig. 1j,k). Furthermore, an analogue ebselen in which the selenium is substituted with sulphur (ebsulfur; Fig. 1a) weakly inhibited IMPase (Fig. 1l), whereas a selenium-containing compound with similar electrophilic reactivity (dibenzyldiselenide; Fig. 1a) had no inhibitory effect (Fig. 1l). These data demonstrate that inhibition of IMPase by ebselen requires not just the presence of an electrophilic selenium atom but also an appropriate chemical scaffold. Unlike the case for most other proteins when covalently linked to ebselen18,19,22, inactivation of IMPase was not reversed by post-incubation with the sulfhydryl reducing agents glutathione and dithiothreitol (Fig. 1m). Pre-incubation of ebselen with the reducing agents did, however, reduce inhibition (Fig. 1n) as described in detail below.
Ebselen is pharmacologically active in the brain
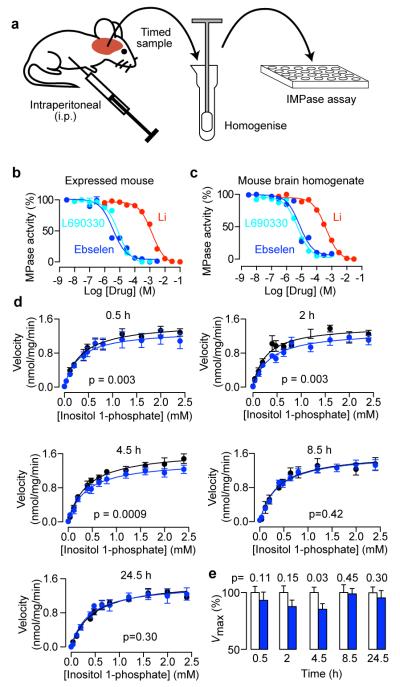
To determine whether ebselen can cross the blood–brain barrier and thus be pharmacologically active in mouse brain, as reported for rat23, we exploited the irreversible inhibition property of ebselen in an ex vivo method based on IMPase activity in brain homogenate (Fig. 2a). As the initial experiments that identified ebselen as an inhibitor used recombinant human IMPase (Fig. 1b), we first needed to ensure that recombinant mouse IMPase was enzymatically active. Recombinant mouse IMPase was inhibited by lithium and L-690, 330 and ebselen (Fig. 2b). Having validated that ebselen inhibited the mouse form of IMPase, we demonstrated that in homogenates of mouse brain, IMPase activity was detectable and inhibited by lithium, L-690,330 and ebselen (Fig. 2c). In an ex vivo experiment, IMPase activity was measured in brain homogenates prepared at various times after intraperitoneal injection of ebselen (Fig. 2a)24. Over time, IMPase inhibition developed and then returned to control levels (Fig. 2d,e). Therefore, systemic administration of ebselen inhibits IMPase in mouse brain in whole animals.
Fig. 2. Ebselen permeates the blood-brain barrier and inhibits endogenous inositol monophosphatase in mouse brain.
(a) Schematic illustrating the experimental protocol for assessing IMPase in ex vivo and brain homogenate experiments. Ebselen was injected at 10 mg/kg. (b,c) Concentration-inhibition relationships for novel and known inhibitors of mouse IMPase expressed in bacteria (b) or present in homogenates from mouse brain (c). (d) Michaelis-Menten plots showing the effect of injected ebselen on IMPase in ex vivo brain homogenate. Statistical significance was determined by a global fit of the Michaelis-Menten equation to the entire data set. (e) Effect of ebselen on the Vmax of IMPase over time after injection, analysed by pre-planned paired t-test between the treatment and control, n=5-6. All error bars represent standard error of the means.
That IMPase inhibition by ebselen was detected in the ex vivo experiments (Fig. 2d,e) is revealing in regard to the likely chemical form of ebselen in intact cells in vivo, as its selenium atom can exist in higher or lower oxidation states, and these have different reactivities18,19. Incubation of ebselen with reduced glutathione forms ebselen–glutathione selenenylsulphide, whereas incubation with dithiothreitol reduces ebselen to its selenol and diselenide (Fig. 1a,l)18,19. When we pre-incubated ebselen with these reducing agents the reduced forms of ebselen (confirmed by mass spectrometry) were weaker inhibitors of IMPase (Fig. 1n), likely because they are less reactive with cysteines18. Therefore, to obtain inhibition of IMPase with ebselen in vivo a fraction of ebselen must exist in a non-conjugated free form in the oxidation state shown in Fig. 1a, despite an intracellular environment with millimolar concentrations of reduced glutathione25.
Ebselen alters the function of the central nervous system
To determine whether ebselen was affecting the function of the central nervous system, we investigated the effect of ebselen on the responses mediated by the serotonergic 5-HT2 receptor26. In humans, lithium reduces phosphoinositide cycle-coupled 5-HT2 receptor function27, and this may be linked to lithium’s antidepressant action. Lithium also reduces 5-HT2 receptor function in mouse as modelled by a 5-HT2 agonist-evoked head-twitch response28. This is mediated by the prefrontal cortex29, which is believed to be the target of lithium in the treatment of bipolar disorder30. Ebselen decreased 5-HT2 agonist-induced head twitches in a dose-dependent manner (Fig. 3a), and this was associated with decreased expression of Arc mRNA (a molecular marker of neural activity26) in the prefrontal cortex (Fig. 3b) and cingulate cortex (Fig. 3c). Thus, ebselen attenuates a cortically mediated 5-HT2 receptor response that is linked to phosphoinositide turnover, as would be predicted for an inhibitor of IMPase.
Fig. 3. Ebselen induces lithium-like behaviour.
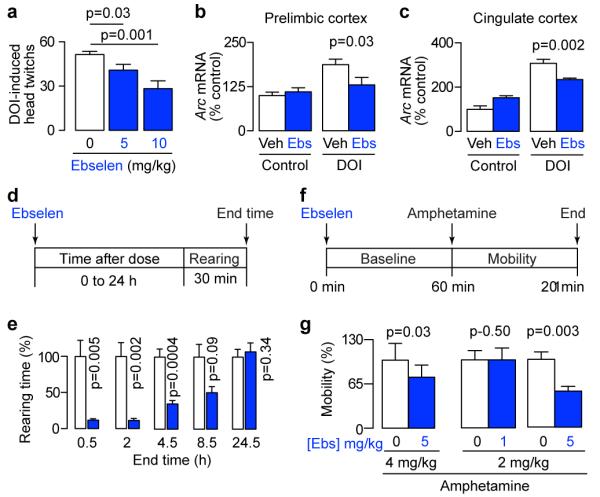
(a) Ebselen attenuates the head-twitch response induced by the 5-HT2A agonist DOI (2 mg/kg), analyzed by pre-planned, one-tailed t-tests, n=6. (b, c) Ebselen (10 mg/kg) attenuates the increase in the immediate-early gene Arc mRNA induced by DOI (2 mg/kg) in two cortical regions. Analyzed by pre-planned, one-tailed t-tests, n=5-6. (d, f) Experimental protocols for assessing the effect of ebselen on behaviour in the open field test during exploratory activity (rearing, d) and amphetamine-induced hyperactivity (mobility, f). (e) Effect of ebselen (10 mg/kg) on rearing over time, analyzed by pre-planned paired t-tests, n=5-6. (g) Effect of ebselen on mobility during amphetamine-induced hyperactivity, analyzed by pre-planned paired t-test, n=6-8. All error bars represent standard error of the means.
Ebselen exhibits lithium-like effects on behaviour
Rodent behaviours are used to model bipolar disorder, and typically focus on either the manic or the depressive pole31. The ‘learned helplessness’ aspect of depression is often modelled with the forced swim test. In this model, ebselen has recently been shown to exhibit anti-depressant action32. Given these findings, we investigated the effect of ebselen in lithium-sensitive mouse models of mania33. In the open field test (Fig. 3d), rearing was decreased by ebselen over time and then returned to baseline (Fig. 3e), a time course that paralleled that for IMPase inhibition in the ex vivo assay (Fig. 2e), as well as plasma ebselen concentrations in humans after oral administration34. Rearing is an exploratory behaviour that correlates with impulsivity33, which in turn correlates with suicidal thoughts and actions35. Mania has also been modelled by amphetamine-induced hyperactivity (Fig. 3f)33,36. Similarly to lithium37, ebselen reduced amphetamine-induced hyperactivity in a manner that depended on both the dose of amphetamine and the dose of ebselen (Fig. 3g), as is the case for lithium37. Baseline mobility was not significantly reduced (one-tailed, paired t-tests: amphetamine 2 mg/kg and ebselen 5 mg/kg, p=0.24; amphetamine 4 mg/kg and ebselen 5 mg/kg, p=0.08).
Ebselen acts through inositol depletion
Finally, if ebselen were mimicking lithium by inhibition of IMPase (and therefore inositol depletion), then one would expect ebselen’s effects to be circumvented by administration of exogenous inositol. This reversal is diagnostic of the ‘inositol depletion hypothesis’ if the addition of inositol re-establishes normal phosphatidylinositol 4,5-bisphosphate signalling7,8,38. We injected inositol intracerebroventricularly (Fig. 4a), and this reversed the effects of ebselen in models of both rearing (Fig. 4b) and amphetamine-induced mobility (Fig. 4c). Furthermore, mice injected intraperitoneally with ebselen, showed a decrease in brain levels of inositol 1 h after administration (Fig. 4d,e) providing direct evidence for inositol depletion. Combined, these results are consistent with the known ability of inositol to reverse the behavioural effects of lithium8,9,38 and support the inositol depletion hypothesis7.
Fig. 4. The pharmacological effects of ebselen are mediated by inositol depletion.
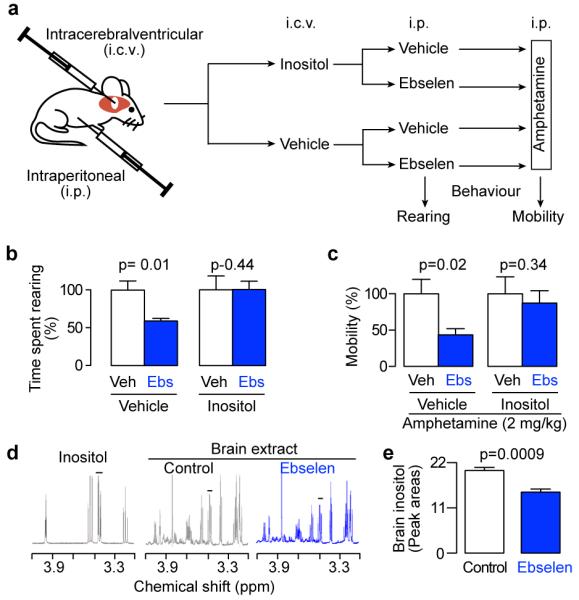
(a) Schematic outlining the experimental protocol used to investigate inositol reversal of behaviours induced by ebselen. Drugs were injected as follows: 1 μL of 0.5 M inositol, 5 mg/kg ebselen and 2 mg/kg amphetamine. (b) Effect of inositol on the ability of ebselen to attenuate rearing, analysed by pre-planned paired t-tests between ebselen and control, n=4-6. (c) Effect of inositol on the ability of ebselen to attenuate amphetamine-induced hyperactivity (mobility) analyzed by pre-planned paired t-tests between ebselen and control, n=5-6. (d) Proton NMR spectra of authentic inositol and brain extracts from mice injected intraperitoneally with either ebselen (10 mg/kg) or hydroxypropyl ß-cyclodextrin(4% w/v, Control). (e), Effect of ebselen (10 mg/kg) on inositol levels in mouse brain. Inositol was quantified by integration of the C1 and C3 peaks (indicated by the bar in d), and analyzed by a pre-planned one-way t-test between ebselen and control, n=4. All error bars represent standard error of the means.
Discussion
Despite 60 years of use since its discovery as a mood stabilizer4, lithium remains the gold standard for the treatment of bipolar disorder1. Although combination therapy with an antidepressant is used to treat bipolar disorder, there is a risk of precipitating mania39. Uniquely, lithium is the only drug reported to reduce suicidal thoughts and behaviour2. Lithium is less than ideal, however, due to its undesirable side effects and toxicity. Therefore, there is a crucial need for drugs that are safe and efficacious for the treatment of bipolar disorder. Currently, drugs fail clinical trials for primarily safety or efficacy40. Ebselen, is known to be clinically safe34,41 and hence its efficacy should be tested.
Ebselen exhibits lithium-like actions at many levels including enzymatic, inositol recycling and animal behaviour making it the best lithium mimetic reported to date5. Additionally, ebelen is bioavailable, blood-brain barrier permeant and safer than lithium based on cellular toxicity42 and Phase 1-3 clinical trials34,41. Ebselen exhibits a polypharmacology profile (http://mli.nih.gov/mli/mlp-probes/) that would be predicted to be beneficial in its role as a lithium mimetic because it directly inhibits the putative therapeutic targets of lithium including IMPase and protein kinase C43 as well as being an antioxidant and inhibitor of cyclooxygenases that promotes neuronal survival44. Polypharmacology is much more common than previously appreciated45. Moreover, polypharmacology is now a desirable property46,47, for example, antipsychotics hitting multiple targets were more efficacious than drugs that were selective48.
Inhibition of IMPase by ebselen is covalent and irreversible. Traditionally, covalent drugs have been disfavoured due to risks relating to immunogenicity49. However, covalent binding alone is not sufficient to cause problems50 and many marketed drugs act covalently49, demonstrating that such risks are compound specific. Importantly, ebselen has a good safety profile in all animal and human experiments reported to date34,41. Moreover, the irreversible action of ebelen on IMPase offers several advantages, as is that case for all irreversible drugs49,51,52. One is that an irreversible inhibition cannot be overcome by accumulation of substrate. Additionally, irreversible inhibition can interact with pharmacokinetic effects to prolong ebselen’s duration of action and increase its selectivity for IMPase. After dosing, the decreasing concentration of ebselen will decrease its inhibition of all its reversible secondary targets. In contrast, IMPase will remain inhibited until new enzyme is synthesized. Such a scenario is known to be the case for many marketed drugs that are covalent and irreversible inhibitors including the well known drugs aspirin, clopidogrel and omeprazole49.
There is an increasingly urgent need for new drugs for the treatment of mental illness, especially given that many large pharmaceutical companies have pulled out of these areas due to high costs and failure rates39,53 prompting speculation as to where new drugs will come from for treating disorders of the central nervous system54,55. Although there is no single solution, repurposing of drugs from their original use to a new use is being strongly promoted by government initiatives such as that announced by the NIH16,56,57. Given that ebselen has been in clinical trials34,41, ebselen offers all the promise of drug repurposing58.
Methods
Recombinant Inositol Monophosphatase
Murine MmImpa1 and human HsImpa1 were amplified from cDNA clones (IMAGE clones 6413389 and 3682657, respectively; Source Bioscience, Cambridge, UK). Cloning and protein expression were carried out as reported21,59. Semi-purified recombinant protein was obtained by heating lysed-cell supernatant (68°C, 1 h) and centrifugation (30,000 g, 30 min, 4°C).
Cysteine 218 to Alanine Mutation in IMPase
Site directed mutagenesis of cysteine 218 was carried out using the Stratagene QuickChange kit. Protein was expressed as before, but without sorbitol and betaine.
IMPase Activity
Phosphate hydrolyzed from Ins1P was detected using the malachite green assay. For the in vitro assays, recombinant HsIMPase (10 ng/well) or MmIMPase (30 ng/well) was incubated (1 h, 37°C) with Ins1P (1mM) in 20 μL Tris buffer (50 mM Tris-HCl, 1 mM EGTA, 3 mM MgCl2, 150 mM KCl, 0.5 mg/mL BSA and 0.01% v/v Triton X pH 7.4). Absorbance was measured at 595 nm for samples and phosphate standards. For the ex vivo assays, brain homogenate (0.5 mg/mL) was incubated (37°C, 1 h) with Ins1P (0.1-2.4 mM) in the presence or absence of LiCl (30 mM) to determine IMPase-specific activity.
Chemical Library and Screening
The NIH Clinical Collection of 450 compounds was provided by the National Institute of Health16 and purchased from BioFocus DPI. Compounds (100 μM) were screened at three concentrations of Ins1P. Initial hits were confirmed with concentration–inhibition curves spanning six orders of magnitude. Subsequent experiments used ebselen from Fisher Scientific. For compound screening, compound at 100 μM (in 0.2% v/v DMSO) was incubated with IMPase (10 min, room temperature) in buffer, before addition of Ins1P (1 mM) to a final volume of 20 μL and further incubated (37°C, 1 h). Phosphatase concentration was determined by the malachite green assay. LiCl and L-690,330 (Tocris) were used as positive controls.
Reduced Ebselen
Ebselen (250 μM) was treated with 0.25 M dithiothreitol (DTT) or 5 mM GSH; reduced ebselen (final concentration 50 μM) was incubated (1 h, room temperature) with HsIMPase before addition of Ins1P (0.1-3 mM) and further incubation (1 h, 37°C). Enzyme activity was determined by the malachite green assay.
Testing for Reversibility of Drug Inhibition
HsIMPase (1 μg/well) was incubated with 20 μM drug for varying times before dilution to 10 ng/well, addition of Ins1P and further incubation (1 h, 37°C). Enzyme activity was determined by the malachite green assay.
Selenyl-Sulfur Reversibility
Conditions were as described above, except that 2 μL of reductant (50 mM DTT or 1 mM GSH) was added to each well, after incubation of IMPase with ebselen.
Mass Spectrometry
IMPase (100 μM) was desalted using a Bio-Spin 6 Column (Bio-Rad) in 15 mM ammonium acetate (pH 7.5) and incubated (room temperature, 15 min) with 10 mM MgCl2 prior to non-denaturing electrospray ionization mass spectrometry analysis (Q-TOF micro, Micromass). Data were processed with MASSLYNX 4.0 (Waters). To investigate IMPase ligand binding, mass spectrometry was used as described60, but with an additional mild denaturing step. HsIMPase (100 μM) was incubated with 100 μM drug (10 min) then diluted (1:10) in 15 mM ammonium acetate buffer (pH 7.4) with 0.1% v/v formic acid. This solution was then subjected to mass spectroscopy.
Animals
All studies used 20-25 g 10-12 week old male C57Bl6 mice (Harlan Olac, UK). Mice were housed with 12 h light-dark cycles with free access to standard lab chow and water. Experiments were carried out in accordance with UK Home Office Animals (Scientific Procedures) Act (1986) and associated code of practice guidelines. Animals were dosed intraperitoneally (i.p.) at 10 μL/g, unless otherwise specified. Lithium was dosed i.p. at 67 μL/g.
Ex Vivo Mouse Brain Homogenate
Mice were injected with ebselen (10 mg/kg) or vehicle (4% w/v hydroxypropyl ß-cyclodextrin) and left for varying amounts of time before euthanization by cervical dislocation, or by CO2 followed by cervical dislocation. Brains were removed and frozen on dry ice immediately. One hemisphere was homogenized using a Precellys 24 bead mill homogenizer (Stretton) and diluted in Tris buffer (50 mM Tris HCl, 3 mM MgCl2, 150 mM KCl, 1 mM EGTA, 0.01% v/v Triton X pH 7.4) to a final concentration of 0.5 mg/mL.
Ex vivo Inositol Measurement by Nuclear Magnetic Resonance
Mice were euthanized by cervical dislocation 1 h after administration of ebselen (10 mg/kg) or vehicle (4% w/v hydroxypropyl ß-cyclodextrin), then brains were extracted and frozen immediately on dry ice. Brains were weighed then homogenized using a Precellys 24 bead mill homogenizer (Stretton). Acetonitrile was added to homogenate (1:1 v/v) to precipitate protein, the sample was centrifuged (13,000×g, 10 min), and the supernatant was prepared for NMR by lyophilization and reconstitution in D2O with 0.008% w/v 3- (trimethylsilyl)propionic 2233d acid sodium salt (600 mg/mL).
Amphetamine-induced Hyperactivity
Mice were treated with ebselen or vehicle and immediately placed in Linton AM1053 X, Y, Z IR Activity Monitor (San Diego Instruments) for 1 h to habituate. Mice were then injected with d-amphetamine/saline and returned to the cage, and activity was monitored for an additional 1 h.
Rearing behavior
Mice were injected with ebselen (10 mg/kg) or vehicle (4% w/v hydroxypropyl ß-cyclodextrin) and left for varying amounts of time before being placed in the Linton AM1053 X, Y, Z IR Activity Monitor (San Diego Instruments) for 30 mins while their activity was monitored. Rearing was measured by counting the number of beam breaks in upper grid.
Intracerebroventricular Injection of Inositol
Inositol reversal experiments were performed as described38. With mice under isoflurane-induced general anaesthesia, a guide cannula was stereotaxically implanted to 1 mm above the injection site in the lateral ventricle, and held in place with dental cement (Aqualox). Mice were left to recover for 7 days before behavioural studies were carried out. Inositol (5 or 1 μL of a supersaturated solution ≥ 278 mM) or control (0.9% w/v NaCl) was injected intracerebroventicularly, then 24 h later amphetamine-induced hyperactivity was assessed as described above. Group means were compared with pre-planned t-tests, one-tailed or paired as appropriate.
DOI-induced Head Twitches
Mice were placed in an arena and left to acclimatize to the novel environment. After 1 h, they were injected with vehicle or ebselen (5 or 10 mg/kg) followed 1 h later by the non-selective 5HT2A agonist 1- (2,5-dimethoxy-4 iodophenyl)-2-aminopropane (DOI, 2 mg/kg). Head twitches were recorded 5 min after agonist injection for 15 min. Mice were constantly monitored by a video camera, and behavioural recordings were analysed offline independently by two observers who were blind to the treatment.
In Situ Hybridization
For quantification of Arc mRNA, brains were removed 1 h after the last injection of drug or vehicle and snap frozen in isopentane cooled by dry ice. Brain tissue sections (12 μm) were cut in a cryostat (-21°C), thaw-mounted onto gelatine-subbed slides and stored (-80°C), then pretreated using standard methods. For in situ hybridisation, oligonucleotides complimentary to Arc mRNA were 3′-tail labelled with [35S]dATP and applied to each section in hybridization buffer (1×10−6 cpm/section). After overnight incubation at 37°C, sections were washed in buffer (3M NaCl and 300 mM citrate, pH 7) first at 55°C (3×20 min) then at room temperature (2×60 min). Sections were then allowed to dry overnight and exposed to Kodak Biomax MR film (Sigma–Aldrich) for 7 days at room temperature. Films were developed and analysed with a computerized image analysis system using densitometric software (MCID, Linton, UK).
Statistical Analyses
Means were compared with pre-planned t-tests (one-tailed or paired as appropriate) using GraphPad Prism software. All data are presented as mean ± standard error of the mean.
ACKNOWLEDGEMENTS
Our research was supported by the Biotechnology and Biological Sciences Research Council through a Project Grant (BB/D012694/1) and a Follow-on Fund grant (BB/J021547/1). Nisha Singh was supported by a Departmental Scholarship, The Vice Chancellor’s and the Radhakrishnan Memorial Bequest Fund. Ivi Antoniadou was supported by a scholarship from the Onassis Foundation. We thank Daniel Rosen, Emma Wallace, Alex Lazare and Simon Hackett for help setting up the IMPase assay, Nathan Lack for advice on protein expression, Bob Sim for advice on protein purification, Edith Sim for use of protein purification equipment, Dave Smith and Fran Platt for advice on and use of the open field apparatus, Anna Nadali, Helen Storr and Tim Claridge for help with NMR and mass spectrometry and Michael Field for proofreading and editing.
Footnotes
Competing Financial Interests Based on the therapeutic effects of Ebselen reported in this paper, all authors have filed the patent entitled Treatment of Bipolar disorder’: WO/2012/107735 A2.
References
- 1.Geddes JR, et al. Lithium plus valproate combination therapy versus monotherapy for relapse prevention in bipolar I disorder (BALANCE): a randomised open-label trial. Lancet. 2010;375:385–395. doi: 10.1016/S0140-6736(09)61828-6. [DOI] [PubMed] [Google Scholar]
- 2.Dolgin E. The ultimate endpoint. Nat. Med. 2012;18:190–193. doi: 10.1038/nm0212-190. [DOI] [PubMed] [Google Scholar]
- 3.McKnight RF, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379:721–728. doi: 10.1016/S0140-6736(11)61516-X. [DOI] [PubMed] [Google Scholar]
- 4.Cade JFJ. Lithium salts in the treatment of psychotic excitement. Med. J. Aust. 1949;2:349–352. doi: 10.1080/j.1440-1614.1999.06241.x. [DOI] [PubMed] [Google Scholar]
- 5.Quiroz JA, Gould TD, Manji HK. Molecular effects of lithium. Mol. Interv. 2004;4:259–272. doi: 10.1124/mi.4.5.6. [DOI] [PubMed] [Google Scholar]
- 6.O’Brien WT, Klein PS. Validating GSK3 as an in vivo target of lithium action. Biochem. Soc. Trans. 2009;37:1133–1138. doi: 10.1042/BST0371133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berridge MJ, Downes CP, Hanley MR. Neural and developmental actions of lithium: a unifying hypothesis. Cell. 1989;59:411–419. doi: 10.1016/0092-8674(89)90026-3. [DOI] [PubMed] [Google Scholar]
- 8.Belmaker RH, Bersudsky Y, Agam G, Levine J, Kofman O. How does lithium work on manic depression? Clinical and psychological correlates of the inositol theory. Annu. Rev. Med. 1996;47:47–56. doi: 10.1146/annurev.med.47.1.47. [DOI] [PubMed] [Google Scholar]
- 9.Agam G, et al. Knockout mice in understanding the mechanism of action of lithium. Biochem. Soc. Trans. 2009;37:1121–1125. doi: 10.1042/BST0371121. [DOI] [PubMed] [Google Scholar]
- 10.Cryns K, et al. IMPA1 is Essential for Embryonic Development and Lithium-Like Pilocarpine Sensitivity. Neuropsychopharmacology. 2007;33:674–684. doi: 10.1038/sj.npp.1301431. [DOI] [PubMed] [Google Scholar]
- 11.Baum AE, et al. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol. Psychiatry. 2008;13:197–207. doi: 10.1038/sj.mp.4002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Purcell SM, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams RSB, Cheng L, Mudge AW, Harwood AJ. A common mechanism of action for three mood-stabilizing drugs. Nature. 2002;417:292–295. doi: 10.1038/417292a. [DOI] [PubMed] [Google Scholar]
- 14.Atack JR. Inositol monophosphatase inhibitors--lithium mimetics? Med Res Rev. 1997;17:215–224. doi: 10.1002/(sici)1098-1128(199703)17:2<215::aid-med3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 15.Atack JR, Broughton HB, Pollack SJ. Inositol monophosphatase--a putative target for Li+ in the treatment of bipolar disorder. Trends Neurosci. 1995;18:343–349. doi: 10.1016/0166-2236(95)93926-o. [DOI] [PubMed] [Google Scholar]
- 16.Austin CP, Brady LS, Insel TR, Collins FS. NIH Molecular Libraries Initiative. Science. 2004;306:1138–1139. doi: 10.1126/science.1105511. [DOI] [PubMed] [Google Scholar]
- 17.Copeland RA. Evaluation Of Enzyme Inhibitors In Drug Discovery: A Guide For Medicinal Chemists And Pharmacologists. John Wiley and Sons; 2005. [PubMed] [Google Scholar]
- 18.Sarma BK, Mugesh G. Antioxidant activity of the anti-inflammatory compound ebselen: a reversible cyclization pathway via selenenic and seleninic acid intermediates. Chemistry. 2008;14:10603–10614. doi: 10.1002/chem.200801258. [DOI] [PubMed] [Google Scholar]
- 19.Haenen GR, De Rooij BM, Vermeulen NP, Bast A. Mechanism of the reaction of ebselen with endogenous thiols: dihydrolipoate is a better cofactor than glutathione in the peroxidase activity of ebselen. Mol. Pharmacol. 1990;37:412–422. [PubMed] [Google Scholar]
- 20.Knowles MR, et al. Bovine inositol monophosphatase. Modification, identification and mutagenesis of reactive cysteine residues. Biochem. J. 1992;285(Pt 2):461–468. doi: 10.1042/bj2850461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh N, et al. Cloning, expression, purification, crystallization and X-ray analysis of inositol monophosphatase from Mus musculus and Homo sapiens. Acta Crystallographica Section F Structural Biology and Crystallization Communications. 2012:68. doi: 10.1107/S1744309112035191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagner G, Schuch G, Akerboom TP, Sies H. Transport of ebselen in plasma and its transfer to binding sites in the hepatocyte. Biochem. Pharmacol. 1994;48:1137–1144. doi: 10.1016/0006-2952(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 23.Imai H, Masayasu H, Dewar D, Graham DI, Macrae IM. Ebselen protects both gray and white matter in a rodent model of focal cerebral ischemia. Stroke. 2001;32:2149–2154. doi: 10.1161/hs0901.095725. [DOI] [PubMed] [Google Scholar]
- 24.Agam G, et al. Lithium inhibitable enzymes in postmortem brain of bipolar patients. J Psychiatr Res. 2003;37:433–442. doi: 10.1016/s0022-3956(03)00044-x. [DOI] [PubMed] [Google Scholar]
- 25.Hansen RE, Roth D, Winther JR. Quantifying the global cellular thiol-disulfide status. Proc. Natl. Acad. Sci. U.S.A. 2009;106:422–427. doi: 10.1073/pnas.0812149106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnes NM, Sharp TA. review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 27.Friston KJ, Sharpley AL, Solomon RA, Cowen PJ. Lithium increases slow wave sleep: possible mediation by brain 5-HT2 receptors? Psychopharmacology (Berl.) 1989;98:139–140. doi: 10.1007/BF00442020. [DOI] [PubMed] [Google Scholar]
- 28.Goodwin GM, DeSouza RJ, Wood AJ, Green AR. Lithium decreases 5-HT1A and 5-HT2 receptor and α2-adrenoreceptor mediated function in mice. Psychopharmacology. 1986;90:482–487. doi: 10.1007/BF00174065. [DOI] [PubMed] [Google Scholar]
- 29.González-Maeso J, et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Schloesser RJ, Martinowich K, Manji HK. Mood-stabilizing drugs: mechanisms of action. Trends Neurosci. 2012;35:36–46. doi: 10.1016/j.tins.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat. Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Posser T, et al. Antidepressant-like effect of the organoselenium compound ebselen in mice: evidence for the involvement of the monoaminergic system. Eur. J. Pharmacol. 2009;602:85–91. doi: 10.1016/j.ejphar.2008.10.055. [DOI] [PubMed] [Google Scholar]
- 33.O’Donnell KC, Gould TD. The behavioral actions of lithium in rodent models: leads to develop novel therapeutics. Neurosci Biobehav Rev. 2007;31:932–962. doi: 10.1016/j.neubiorev.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lynch E, Kil J. Development of Ebselen, a Glutathione Peroxidase Mimic, for the Prevention and Treatment of Noise-Induced Hearing Loss. Seminars in Hearing. 2009;30:047–055. [Google Scholar]
- 35.Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- 36.Jacobs D, Silverstone T. Dextroamphetamine-Induced Arousal in Human Subjects as a Model for Mania. Psychological Medicine. 1986;16:323–329. doi: 10.1017/s0033291700009132. [DOI] [PubMed] [Google Scholar]
- 37.Lerer B, Globus M, Brik E, Hamburger R, Belmaker RH. Effect of treatment and withdrawal from chronic lithium in rats on stimulant-induced responses. Neuropsychobiology. 1984;11:28–32. doi: 10.1159/000118046. [DOI] [PubMed] [Google Scholar]
- 38.Kofman O, Belmaker RH. Intracerebroventricularmyo-inositol antagonizes lithium-induced suppression of rearing behaviour in rats. Brain Research. 1990;534:345–347. doi: 10.1016/0006-8993(90)90155-5. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Frye MA, Shelton RC. Review of pharmacological treatment in mood disorders and future directions for drug development. Neuropsychopharmacology. 2012;37:77–101. doi: 10.1038/npp.2011.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scannell JW, Blanckley A, Boldon H, Warrington B. Diagnosing the decline in pharmaceutical R&D efficiency. Nature Reviews Drug Discovery. 2012;11:191–200. doi: 10.1038/nrd3681. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi T, et al. Ebselen Study Group Ebselen in acute ischemic stroke: a placebo-controlled, double-blind clinical trial. Stroke. 1998;29:12–17. doi: 10.1161/01.str.29.1.12. [DOI] [PubMed] [Google Scholar]
- 42.Nogueira CW, Zeni G, Rocha JBT. Organoselenium and organotellurium compounds: toxicology and pharmacology. Chem. Rev. 2004;104:6255–6285. doi: 10.1021/cr0406559. [DOI] [PubMed] [Google Scholar]
- 43.Zarate CA, Jr, et al. Efficacy of a protein kinase C inhibitor (tamoxifen) in the treatment of acute mania: a pilot study. Bipolar Disord. 2007;9:561–570. doi: 10.1111/j.1399-5618.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- 44.Schewe T. Molecular actions of ebselen--an antiinflammatory antioxidant. Gen. Pharmacol. 1995;26:1153–1169. doi: 10.1016/0306-3623(95)00003-j. [DOI] [PubMed] [Google Scholar]
- 45.Lounkine E, et al. Large-scale prediction and testing of drug activity on side-effect targets. Nature. 2012;486:361–367. doi: 10.1038/nature11159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frantz S. Drug discovery: playing dirty. Nature. 2005;437:942–943. doi: 10.1038/437942a. [DOI] [PubMed] [Google Scholar]
- 47.Roth BL, Sheffler DJ, Kroeze WK. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nature Reviews Drug Discovery. 2004;3:353–359. doi: 10.1038/nrd1346. [DOI] [PubMed] [Google Scholar]
- 48.Conn PJ, Roth BL. Opportunities and challenges of psychiatric drug discovery: roles for scientists in academic, industry, and government settings. Neuropsychopharmacology. 2008;33:2048–2060. doi: 10.1038/sj.npp.1301638. [DOI] [PubMed] [Google Scholar]
- 49.Singh J, Petter RC, Baillie TA, Whitty A. The resurgence of covalent drugs. Nat Rev Drug Discov. 2011;10:307–317. doi: 10.1038/nrd3410. [DOI] [PubMed] [Google Scholar]
- 50.Uetrecht J. Screening for the potential of a drug candidate to cause idiosyncratic drug reactions. Drug Discov. Today. 2003;8:832–837. doi: 10.1016/s1359-6446(03)02816-2. [DOI] [PubMed] [Google Scholar]
- 51.Barf T, Kaptein A. Irreversible Protein Kinase Inhibitors: Balancing the Benefits and Risks. J. Med. Chem. 2012;55:6243–6262. doi: 10.1021/jm3003203. [DOI] [PubMed] [Google Scholar]
- 52.Copeland RA, Pompliano DL, Meek TD. Drug-target residence time and its implications for lead optimization. Nat Rev Drug Discov. 2006;5:730–739. doi: 10.1038/nrd2082. [DOI] [PubMed] [Google Scholar]
- 53.Insel TR, Sahakian BJ. Drug research: a plan for mental illness. Nature. 2012;483:269. doi: 10.1038/483269a. [DOI] [PubMed] [Google Scholar]
- 54.Schwab ME, Buchli AD. Drug research: plug the real brain drain. Nature. 2012;483:267–268. doi: 10.1038/483267a. [DOI] [PubMed] [Google Scholar]
- 55.Schoepp DD. Where will new neuroscience therapies come from? Nat Rev Drug Discov. 2011;10:715–716. doi: 10.1038/nrd3559. [DOI] [PubMed] [Google Scholar]
- 56.Collins FS. Reengineering Translational Science: The Time Is Right. Sci Transl Med. 2011;3:90cm17–90cm17. doi: 10.1126/scitranslmed.3002747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang R, et al. The NCGC Pharmaceutical Collection: A Comprehensive Resource of Clinically Approved Drugs Enabling Repurposing and Chemical Genomics. Sci Transl Med. 2011;3:80ps16–80ps16. doi: 10.1126/scitranslmed.3001862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cavalla D. APT drug R&D: the right active ingredient in the right presentation for the right therapeutic use. Nat Rev Drug Discov. 2009;8:849–853. doi: 10.1038/nrd2981. [DOI] [PubMed] [Google Scholar]
- 59.McAllister G, et al. cDNA cloning of human and rat brain myo-inositol monophosphatase. Expression and characterization of the human recombinant enzyme. Biochem. J. 1992;284(Pt 3):749–754. doi: 10.1042/bj2840749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loo JA. Studying noncovalent protein complexes by electrospray ionization mass spectrometry. Mass Spectrom Rev. 1997;16:1–23. doi: 10.1002/(SICI)1098-2787(1997)16:1<1::AID-MAS1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]