Abstract
T-cell tolerance in the thymus is a key step in shaping the developing T-cell repertoire. Thymic medullary epithelial cells play multiple roles in this process including negative selection of autoreactive thymocytes, influencing thymic dendritic cell positioning, and the generation of FoxP3+ Regulatory T-cells. Previous studies show that mTEC development involves haemopoietic crosstalk, and numerous Tumour Necrosis Factor Receptor Superfamily members have been implicated in this process. While CD40 and RANK represent key examples, interplay between these receptors, and the individual cell types providing their ligands at both fetal and adult stages of thymus development, remain unclear. Here, by analysis of the cellular sources of RANKL and CD40L during fetal and adult crosstalk in the mouse, we show that innate immune cells system drive initial fetal mTEC development via expression of RANKL but not CD40L. In contrast, crosstalk involving the adaptive immune system involves both RANKL and CD40L, with analysis of distinct subsets of intrathymic CD4+ T-cells revealing a differential contribution of CD40L by conventional, but not FoxP3+ regulatory, T-cells. We also provide evidence for a stepwise involvement of TNF-Receptors in mTEC development, with CD40 up-regulation induced by initial RANK signalling subsequently controlling proliferation within the mTEC compartment. Collectively, our findings show how multiple haemopoietic cell types regulate mTEC development through differential provision of RANKL/CD40L during ontogeny, revealing molecular differences in fetal and adult haemopoietic crosstalk. They also suggest a stepwise process of mTEC development, in which RANK is a master player in controlling the availability of other TNF-Receptor family members.
Keywords: Thymus, Cell Differentiation, Stromal Cells
Introduction
The thymus provides a specialised microenvironment for the generation and selection of self-tolerant T-cells. During their intrathymic development, T-cell progenitors migrate through distinct cortical and medullary microenvironments that contain phenotypically and functionally distinct epithelial cell types (1). Initial stages in T-cell development occur in the cortex, and include the transition of immature CD4−8− thymocytes to the CD4+8+ stage, a process that involves the generation of, and signalling through, the pre-T-cell receptor complex (2). T-cell progenitor development is also dependent upon interactions between Notch and DL4 (3, 4), the latter being expressed by cortical thymic epithelial cells (cTEC) that can be further defined by their expression of MHC class II and CD205 (5, 6). Positive selection of CD4+8+ thymocytes also occurs within the cortex (7), with cTEC specific expression of the thymoprotesomal subunit β5t (8) and the protease prss16 (9) enabling expression of peptide/MHC complexes to drive a process of thymocyte differentiation that includes the generation and migration of newly generated CD4+ and CD8+ cells into the thymic medulla (1).
Within thymic medullary regions, medullary thymic epithelial cells (mTEC), including those expressing the Autoimmune Regulator (Aire) gene, play a key role in imposing tolerance on positively selected CD4+ and CD8+ thymocytes (7, 10, 11). For example, mTEC expression of self-antigens can trigger negative selection of thymocytes bearing TCRs with potentially autoreactive specificities (12-14), a process that can also include transfer of mTEC-derived antigens to dendritic cells (15-17). In addition, mTEC are involved in the intrathymic emergence of CD4+FoxP3+ Regulatory T-cells (T-Reg), that play an important role in peripheral tolerance mechanisms (18, 19). Most recently, the Aire+ subset of mTEC has been shown to play a role in the intrathymic positioning of dendritic cells, which themselves are potent mediators of tolerance induction in the thymus (19).
Given the importance of Aire+ mTEC in multiple aspects of intrathymic tolerance induction, several studies have examined the cellular and molecular requirements for their maturation. Importantly, early stages in mTEC development were shown to involve the formation of clonal islets from individual mTEC progenitors within medullary areas (20). While further studies have shown that progenitors for Aire+CD80+ mTEC reside within the CD80−MHCIIlow (mTEClow) fraction (21, 22) that are also defined by claudin-3/4 expression (23), earlier experiments demonstrated a role for signals from haemopoietic cells for thymus medulla development (24, 25), a process termed thymus ‘crosstalk’. While our own studies have revealed a role for innate immune cells, namely Lymphoid Tissue inducer (LTi) and invariant Vγ5+ Dendritic Epidermal T-cell (DETC) progenitors, in the generation of the first cohorts of Aire+ mTEC in the fetal thymus (22, 26, 27), other studies demonstrated the importance of αβTCR+ CD4+ thymocytes during postnatal and adult stages (28, 29). Importantly, these studies also collectively helped to identify a role for multiple members of the TNF-R superfamily in the crosstalk mechanisms that control the development of mTEC. Thus, while deficiency in LTβR signalling resulted in an overall disruption of medullary organisation (30), the TNF-Receptors RANK and CD40 were shown to be particularly important for the development of the Aire+ mTEC subset (22, 28, 29, 31). Of importance, studies have also demonstrated a breakdown in T-cell tolerance in mice lacking RANK and CD40, either individually or in combination (22, 31), further highlighting the relevance of these TNF-Receptors in thymus medulla development and αβTCR repertoire selection. Importantly, Aire+ mTEC are absent from the embryonic thymus of RANK deficient mice, and reduced in the adult (22, 28, 31), consistent with the finding that RANK signalling is a potent trigger of Aire+ mTEC differentiation (22, 28, 31). In contrast, deficiency in CD40-CD40L interactions has a lesser impact on Aire+ mTEC development (26, 31), and in vitro ligation of CD40 appears less effective than ligation of RANK in inducing Aire+ mTEC development (31). Thus, while CD40L and CD40 deficient mice have a reduction in overall mTEC numbers (26, 29, 31, 32) the precise role of CD40-CD40L interactions during mTEC development are unclear.
While these studies explain some aspects of the mechanisms controlling mTEC development, others remain poorly understood, including the timing of availability of the cellular sources of RANKL and CD40L during mTEC/haemopoietic crosstalk. For example, much of the data on thymic RANKL and CD40L expression comes from either PCR analysis of cell populations to measure mRNA expression, or flow cytometric analysis where CD40L/RANKL have been analysed individually but never simultaneously, and sometimes following pharmacological stimulation (28, 29, 31, 33, 34). As a result, potential heterogeneity in physiological levels of RANKL and CD40L expression within LTi, DETC progenitors and mature αβTCR+ thymocytes has not been fully addressed, meaning that their ability to provide these signals either singularly or in combination is not known. Moreover, the requirement for both RANK and CD40 signalling, and the possible interplay between these molecules in mTEC development, has not been fully explored.
Here we define, at a per cell level, the provision of RANKL and CD40L during fetal and adult stages of mTEC development. We find that haemopoeitic crosstalk from innate LTi and invariant DETC progenitors that control Aire+ mTEC development in fetal stages involves RANKL but not CD40L, while postnatal control of mTEC development by αβTCRhi CD4+ thymocytes involves both RANKL and CD40L. Separation of thymic resident CD4+ T-cells into distinct subsets based on their activation status (CD69), lineage (CD25) and maturational stage (Rag-GFP) shows that positive selection of conventional CD4+ T-cells involves the sequential acquisition of first RANKL and then CD40L, while CD25+ T-Reg provide RANKL but not CD40L regardless of their maturational stage. Finally, we show that initial RANK signalling is required to upregulate CD40 expression on developing mTEC at a stage that occurs prior to the induction of functionally relevant mTEC molecules including Aire and CD80, and provide evidence that CD40-CD40L signalling controls proliferation, but not the rate of apoptosis, within the mTEC compartment. From our data, we propose that mTEC development in the adult is controlled by a sequence of events initially involving RANK and then CD40, with both stages being influenced by distinct CD4 thymocyte subsets.
Materials and Methods
Mice
Balb/c, C57BL/6, BoyJ, RANK deficient (22) (Tnfrsf11a−/−), CD40L deficient (35) (Cd40lg−/−) and FVB/N RAG2-GFP transgenic mice (36) mice were housed at the Biomedical Services Unit, University of Birmingham under UK Home Office guidelines. For the generation of timed Balb/c embryos, the day of detection of the vaginal plug was designated as day zero.
Cell Isolation
Thymocyte suspensions were obtained from adult thymus or fetal thymus organ cultures (FTOC) by mechanical dissociation. Stromal cells from 2-deoxyguanosine treated FTOC were obtained by disaggregation in 0.25% trypsin/0.02% EDTA (Sigma) solution at 37°C for 5-10min, with a single-cell suspension made by gentle repetitive pipetting (37). Trypsin was removed by washing prior to FACS staining. Stromal cells from postnatal thymuses were isolated as previously described (38). In brief, thymus lobes were cut into 1 mm3 pieces, washed and digested with R-5 medium (Hepes and L-glutamine supplemented RPMI [Sigma], 1mM sodium pyruvate, 100U.mL−1 penicillin, 100mg.mL−1 streptomycin, 10mM Hepes [all from Gibco-Invitrogen], 5% FCS) containing 0.32 Wunsch U.mL−1 of liberase/thermolysin (Roche) and 50 Kunitz U.ml−1 of DnaseI (Sigma) at 37°C for 35 min using an orbital shaker (180rpm, New Brunswick Scientific). Enzymatic treatment was repeated for an additional 20 min followed by incubation with 5mM EDTA on ice for 5-10 min. Remaining tissue fragments were mechanically dispersed by careful pipetting. Cell suspensions from each digestion were pooled and washed in ice-cold PBS containing 2% FCS and 2mM EDTA to prevent aggregate formation. Stromal cells were enriched by MACS immunomagnetic depletion of CD45+ cells (Miltenyi) according to the manufacturers protocol. Cell surface FACS staining was performed in 2% FCS and 2 mM EDTA to prevent aggregate formation.
Cell Suspension Culture And RANKL/CD40L Flow Cytometry Staining
Haematopoietic cells from adult thymus and FTOC were isolated by mechanical dissociation. Cells were counted, and 2ml volumes of DMEM + 10% FCS containing 4×106 cells were cultured in each well of a polystyrene 6 well-plate (Falcon) at 37°C, 5% CO2 for 4 hours 30m. After culture, cell suspensions were harvested and washed before being stained with the antibodies below. DAPI (Invitrogen) was used to exclude dead cells.
Antibodies and Flow Cytometry
The following antibodies were used for flow cytometric analysis (all obtained from eBioscience unless stated otherwise): anti-B220 FITC (RA3-6B2), anti-CD3ε Alexa700 (17A2), anti-CD4 Alexa700 or PEcy7 (GK1.5), anti-CD4 V500 (RM4-5, BD Biosciences), anti-CD8α FITC, APC or V500 (53-6.7, BD Biosciences), anti-CD11c FITC (N418), anti-CD25 APC (PC61, Biolegend), anti-CD40 PE (3/23, BD biosciences), anti-CD40L PE (MR1, BD biosciences), anti-CD45 APC780 (30-F11), anti-CD69 PerCPcy5.5 (H1.2F3), anti-CD80 V421 (16-10A1, Biolegend), anti-CD127 Alexa647 (A7R34), anti-Aire Alexa488 (clone H512, kind gift of Dr H. Scott, Adelaide University), anti-EpCAM1 Alexa647 (G8.8, kind gift from Andy Farr, University of Washington), anti-FoxP3PE (FJK-16s), anti-IAb FITC or biotin (AF6-120.1, BD biosciences), anti-Ki67 PEcy7 (B56, BD biosciences), anti-Ly51 biotin or PE (6C3). Anti-RANKL biotin (IK22.5), anti-TCRb APC780 (H57-597), anti-TCRδ FITC (GL3), anti-Vγ5 FITC (536, BD biosciences), isotype mouse IgG1 PEcy7 (BD biosciences), isotype Rat IgG1 PE (eBRG1), isotype Rat IgG2a biotin (eBR2a), AnnexinV-FITC (BD biosciences). Biotinylated antibodies were revealed with streptavidin PEcy7 or PerCPcy5.5. Surface staining of cell suspensions was performed in PBS 3% FCS solution at 4°C. AnnexinV staining was performed in binding buffer (BD biosciences) for 20 min at room temperature, and staining was stopped by adding an excess of binding buffer containing DAPI (Invitrogen). Intracellular staining for FoxP3 was performed using Fixation buffer (eBioscience) and Permeabilisation buffer (eBioscience) according to the manufacturers protocol. Intracellular staining for Aire was performed as described (27). Intracellular staining for Ki67 was performed following the same protocol. Flow cytometric acquisition was performed on a BD-LSR Fortessa machine using FACSDiva 6.2 software (BD biosciences), and data analysed with FlowJo 8.7 software (Treestar).
RANK Stimulation of 2dGuo-Treated FTOC
2-deoxyguanosine treated FTOC were established from freshly isolated E15 thymus lobes, as previously described (39). After 7 days organ culture in 1.35mM 2-deoxyguanosine, thymic lobes were transferred in center-well organ culture dishes (Falcon) containing fresh DMEM+10% FCS. 5mg/mL of agonist anti-RANK antibody (R&D Systems) was added where indicated in order to induce mTEC maturation (22), and lobes were harvested over a further 4 days at the indicated time points.
Real-Time Quantitative PCR
Expression of the tissue restricted antigens Casein-alpha (Csna) and Salivary protein 1 (Spt1) were analysed before and after RANK stimulation of 2dGuo-treated FTOC. The generation of cDNA, and quantitative PCR was performed exactly as described (27). Expression values for each sample were normalized to β-actin, and fold levels of the indicated genes represent the mean (±SEM) of replicate reactions. Primer sequences are as follows: b-actin (Actb): QuantiTect Mm Actb 1 SG Primer Assay (Qiagen QT00095242); Casein alpha (Csna) Forward CATCATCCAAGACTGAGCCAG, Reverse CCTGTGGAAAGTAAGCCCAAAG; Salivary Protein 1 (Spt1) Forward GGCTCTGAAACTCAGGCAGA, Reverse TGCAAACTCATCCACGTTGT.
Results
Differential Expression of RANKL and CD40L in Cells Regulating Fetal And Adult Haemopoietic Crosstalk During Aire+ mTEC Development
To compare the way in which fetal innate and adult adaptive immune cells influence mTEC development, we first investigated expression of RANKL and CD40L protein simultaneously by flow cytometry on defined cell types, to provide a detailed analysis of the provision of these molecules at the single cell level. Note that isotype control antibodies were used to set flow cytometric gates for levels of RANKL expression, while for CD40L staining, both isotype controls and cells from CD40lg−/− mice were used.
Flow cytometric analysis of Vγ5+TCR DETC progenitors and CD4+3−IL7Rα+ LTi isolated from E15 thymus, explanted for 7 days in organ culture, showed that both populations expressed readily detectable cell surface levels of RANKL (Figure 1A), with higher levels detectable on LTi cells, a finding consistent with qPCR analysis (27). In contrast to RANKL, both LTi and DETC progenitors were found to lack detectable expression of CD40L (Figure 1A), while both RANKL and CD40L were expressed at the cell surface of adult thymocytes (Figure 1B), most notably CD4+TCRβhi mature thymocytes. In addition, clear heterogeneity with regard to RANKL/CD40L expression was observed within this subset (Figure 1B), with some cells expressing either RANKL or CD40L, or both (Figure 1C). Collectively, these data suggest that while RANKL expression is common to both fetal and adult haemopoietic cells that are involved in Aire+ mTEC development, the capacity to regulate mTEC development through CD40-CD40L interactions is limited to a subset of mature CD4+TCRβhi thymocytes.
Figure 1. RANKL and CD40L Are Differentially Expressed In Innate Cells and αβTCR+ Thymocytes.
Lymphoid Tissue inducer (LTi) cells (CD4+IL-7Rα+CD8α−B220−CD11c−TcRγδ−CD3ε−TCRβ−) and Vγ5+TCR (Vγ5+CD3ε+CD4−CD8α−) thymocytes from 7d FTOC (A), together with adult thymocyte subsets defined by expression of CD4, CD8 and TCRβ (B) were analysed for expression of RANKL and CD40L by flow cytometry. Note that gates are set using isotype control antibodies for RANKL, and cells obtained from Cd40lg−/− mice for CD40L expression. Panel C shows frequencies of CD40L+ and RANKL+ cells in adult thymocytes. Error bars represent S.E.M. Open bars, RANKL+CD40L− cells; hatched bars, RANKL+CD40L+ cells, grey bars RANKL−CD40L+ cells. Analysis of Vγ5+TCR and LTi cells was performed on pooled batches of approximately 60 and 100 FTOC, respectively. The dot plots shown are representative of two independent experiments. Dot plots and histograms representing RANKL and CD40L expressions on adult RAG2-GFP thymocytes are representative of two independent experiments with 4 mice analysed in each of these experiments.
RANKL and CD40L Are Expressed At Defined Stages of αβTCR Thymocyte Maturation
CD4+8−TCRβhi cells within the adult thymus are known to consist of a heterogeneous mixture of cell types, including mature medullary-resident thymocytes giving rise to both FoxP3− conventional T-cells and FoxP3+ T-Reg as result of intrathymic positive selection, as well as recirculating CD4+ T-cells that have re-entered the thymus from the periphery (40). To explore further the heterogeneity in RANKL/CD40L expression seen in total CD4+8−TCRβhi cells, we took advantage of Rag2GFP mice in which decreasing levels of GFP expression are directly linked to increasing thymocyte maturity (41, 42). We also analysed RANKL/CD40L expression in Rag2GFP mice in conjunction with the activation marker CD69, and CD25 as a marker of T-Reg. As in our hands, nuclear staining of FoxP3 using antibodies impacts on detection of GFP (not shown), we used CD25 expression as a surrogate T-Reg marker (43), as it largely overlaps with FoxP3 in thymic CD4+TCRβhi cells (Figure 2A), thereby enabling identification of CD25− conventional, and CD25+ regulatory, CD4+8− thymocyte subsets (Figure 2A).
Figure 2. RANKL and CD40L Define Early And Late Stages of αβT-cell Maturation.
Panel A shows CD25 expression and intracellular FoxP3 expression in total CD4+8−TCRbhi adult thymocytes. In panel B, CD4+8−TCRβhi adult thymocytes from adult Rag2GFP mice were divided into CD25− and CD25+ subsets shown in the right dotplot of Panel A, and subsequently analysed for Rag2GFP in conjunction with CD69 expression. Panels C-D show frequencies of RANKL and CD40L expressing cells in the indicated CD25/RagGFP/CD69 subsets of CD4+8−TCRβhi thymocytes. Error bars represent S.E.M. Open bars, RANKL+CD40L− cells; hatched bars, RANKL+CD40L+ cells, grey bars RANKL−CD40L+ cells. The dot plot showing FoxP3 expression is representative of four independent experiments with one mouse analysed in each of these experiments. Dot plots and histograms showing RANKL and CD40L expression alongside CD25, GFP and CD69 on adult RAG2-GFP thymocytes are representative of two independent experiments with 4 mice analysed in each of these experiments.
Figure 2B shows that within the CD25− subset of CD4+TCRβhi cells, a dominant Rag2GFP+CD69+ population is present, representing newly positively selected thymocytes, and a smaller subset of Rag2GFP+CD69− cells, representing later stage mature CD4+ thymocytes (41, 42, 44). Analysis of RANKL/CD40L expression within the Rag2-GFP+ subset of CD25−CD4+TCRβhi cells shows that newly selected Rag2GFP+CD69+ cells are enriched for RANKL+ but not CD40L+ cells (Figure 2C). In contrast, Rag2GFP+CD69− cells contain few RANKL+ cells while CD40L+ cells are readily detectable (Figure 2C), suggesting that RANKL and CD40L expression differentially map to early and late stages of CD4 thymocyte selection. Interestingly, while analysis of the CD25+ subset of Rag2GFP+CD4+TCRβhi thymocytes also revealed RANKL expression within the CD69+ subset (Figure 2E), Rag2GFP+CD69− cells within the CD25+ subset were CD40L− (Figure 2D). Thus, these data suggest that while RANKL expression is a shared feature of both recently produced CD25+ regulatory T-cells and CD25− conventional T-cells, CD40L expression is limited to the latter.
In agreement with an earlier study (42), within CD4+TCRβhi cells, a Rag2GFP− population is detectable within both CD25− and CD25+ subsets, and most predominantly in the latter (Figure 2B). As previously discussed (42), we cannot currently determine what proportions of these Rag2GFP− cells represent either peripheral T-cells that have re-entered the thymus, or thymocytes that have lost GFP expression intrathymically. However, it is interesting to note that CD25−Rag2GFP− cells within the CD4+TCRβhi subset are enriched for CD40L+ cells (Figure 2D). In marked contrast, CD25+Rag2GFP−cells within the CD4+TCRβhi subset (Figure 2F) are predominantly CD40L−RANKL+, irrespective of their CD69 expression. Thus, regardless of whether Rag2GFP−CD4+TCRβhi cells represent re-circulating or longer-term thymus resident cells, expression of RANKL but not CD40L within the CD25+ subset suggests that these cells are able to stimulate RANK-mediated but not CD40-mediated mTEC crosstalk.
Evidence For A Sequential Involvement of RANK and CD40 In mTEC Differentiation And Proliferation
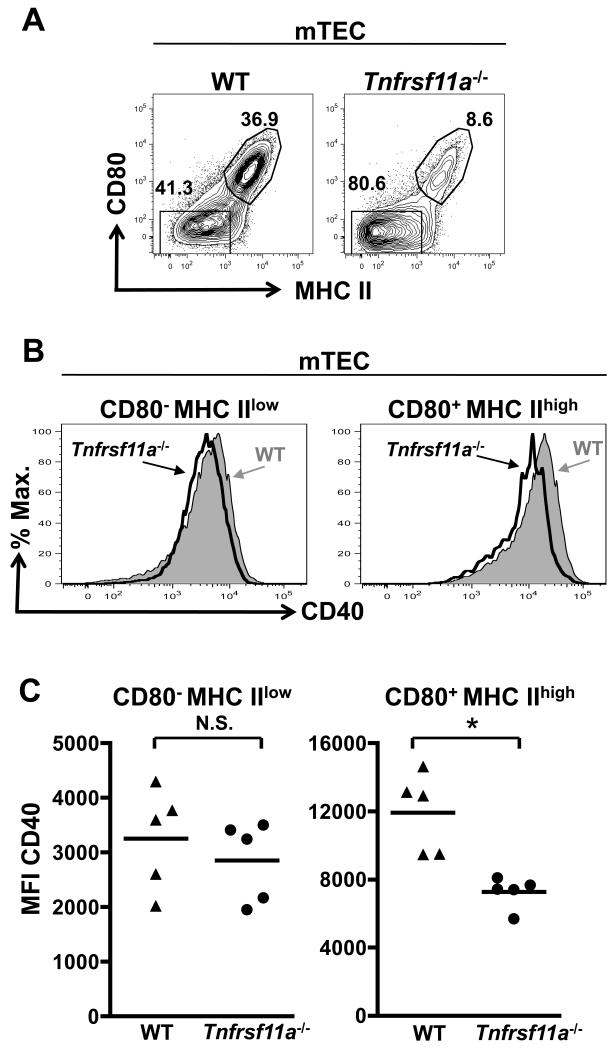
As the data above suggests that in conventional CD4 thymocyte positive selection, RANKL expression appears to map to earlier CD69+ stages while CD40L appears at later CD69− stages, we investigated the possibility that RANK, and then CD40 signals, are sequentially involved in mTEC maturation. Thus, thymuses from 3-6 week old WT and Tnfrsf11a−/− (RANK−/−) littermate controls were enzymatically digested and the CD45−EpCAM+Ly51− mTEC compartment analysed. Consistent with previous studies (22, 28, 31), a reduced proportion of mature CD80+MHChigh mTEC were detected in adult Tnfrsf11a−/− mice (Figure 3A). Interestingly, comparison of the levels of CD40 expression in both CD80−MHCIIlow ‘mTEClow’ and CD80+MHCIIhigh ‘mTEChigh’ cells revealed a slightly lower level of expression of CD40 in mTEChigh cells from Tnfrsf11a−/− mice compared to WT controls (Figure 3B and 3C), suggesting that in vivo levels of CD40 expression within mTEC may be RANK dependent.
Figure 3. CD40 Expression By mTEC Is Reduced In The Absence of The TNF-R Superfamily Member RANK.
Panel A shows expression of MHC class II and CD80, used to define mTEClow and mTEChigh compartments, in total CD45−EpCAM+Ly51low mTEC within freshly digested thymuses from 3-4 week old WT and Tnfrsf11a−/− mice. mTEClow and mTEChigh fractions in WT and Tnfrsf11a−/− mice were compared for expression of CD40 (panel B), and Panel C shows Mean Fluorescence Intensity analysis of CD40 expression on the indicated mTEC subsets. Statistical analysis performed used a Mann-Whitney test (unpaired, two-tailed, 95% of confidence) with * indicating P<0.05 and N.S.: non-significant. Dot plots are representative of two independent experiments on 3 to 6 weeks old mice. In total, five mice of each group were studied.
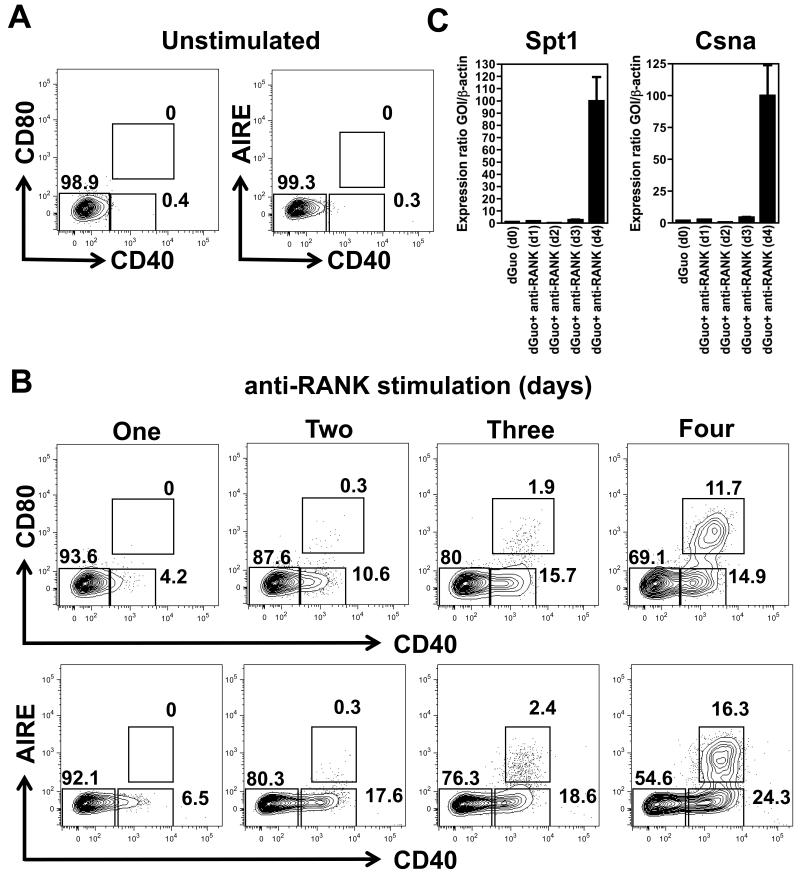
To explore this possibility further, we stimulated 2-deoxyguanosine treated FTOC, deprived of haemopoeitic crosstalk and devoid of mature mTEC(22) with agonistic RANK antibodies, and analysed expression of a panel of mTEC markers, namely CD40, Aire, and the tissue restricted antigens Casein-alpha (Csna) and Salivary Protein 1 (Spt1), over a timecourse of 1-4 days. At the indicated timepoints, lobes were disaggregated and analysed by flow cytometry in the case of Aire and CD40, and qPCR for Csna and Spt1. Interestingly, analysis of mTEC expression of CD40 prior to RANK stimulation shows that these cells are CD40− (Figure 4A), suggesting that CD40 expression is crosstalk dependent. Moreover, in line with the in vivo analysis shown in Figure 3, upregulation of CD40 by mTEC occurred as a consequence of RANK signalling (Figure 4B), with RANK mediated CD40 upregulation occurring prior to the expression of Aire and CD80 (Figure 4B) and the tissue restricted antigens Csna and Spt1 (Figure 4C). Thus anti-RANK stimulation of mTEC progenitors rapidly promotes the upregulation of CD40, consistent with the idea that RANK and CD40 are sequentially involved in mTEC development.
Figure 4. RANK Stimulation of mTEC Induces CD40 Expression Prior to Aire.
2-dGuo treated FTOC were cultured in either the absence (A) or presence (B and C) of 5mg/mL of agonistic anti-RANK antibody for the indicated time. Panel B shows FACS analysis of disaggregated lobes for CD40/CD80 (upper panels) and CD40/Aire expression (lower panels), after gating on CD45−EpCAM+Ly51− mTEC. Note the early induction of CD40 expression. Panel C shows qPCR analysis of Csna and Spt1 mRNA expression during the timecourse of RANK stimulation. Dot plots and qPCR are representative of two independent experiments, with PCR reactions performed in replicate. Three to four pooled 2-dGuo treated FTOC were required for each condition.
While several studies have examined the mTEC compartment in CD40 and/or CD40L deficient mice, the role played by CD40-CD40L mediated crosstalk on mTEC development and/or homeostasis, and the timing of its involvement at particular stages of mTEC development, remain unclear (29, 31, 32). To investigate further the role of CD40-CD40L interactions in mTEC development, we investigated whether this signalling axis may play a role in regulating cellular proliferation within the mTEC compartment. To this end, thymuses from adult WT and Cd40lg−/− mice were disaggregated and the proliferative status of mTEC subsets analysed using anti-Ki67 antibodies. Figure 5A shows that the proportion of cells expressing Ki67 within the total mTEC population is similar in WT and Cd40lg−/− mice. Interestingly however, by subdividing total mTEC into mTEClow and mTEChigh compartments, we saw a significant disruption of the proliferative status of mTEC from Cd40lg−/− mice, with a reduction in the frequency of Ki67+ cells in the mTEClow subset (Figure 5B, C), and an increase in the frequency of Ki67+ cells within the mTEChigh population (Figure 5B, C), suggesting that CD40-CD40L mediated crosstalk may play a key role in controlling mTEC proliferation. Finally, given the observed perturbations in mTEC proliferation in Cd40lg−/− mice reported here, together with the relatively rapid turnover time of mature mTEC (21, 45), and the possible link between Aire expression by mTEC and the induction of apoptosis (45, 46), we next investigated the frequency of apoptotic cells within distinct subsets of mTEC. Freshly digested thymic stromal preparations were analysed by flow cytometry for the presence of apoptotic cells within subsets of mTEC distinguished on the basis of their levels of MHC class II expression, using a combination of Annexin V and DAPI. Interestingly however, despite an increase in the frequency of Ki67+ mTEChigh cells in CD40lg−/− mice, we did not see a significant difference in the levels of apoptosis in WT and CD40lg−/− mTEClow and mTEChigh compartments (Figure 6), suggesting that different mechanisms regulate proliferation and apoptosis within the mTEC compartment.
Figure 5. CD40-CD40L Interactions Control The Balance of Proliferation Within The mTEC Compartment.
Panel A shows quantatitive analysis of Ki67+ cells within the total mTEC population, obtained by flow cytometry analysis, in WT and Cd40lg−/− mice. Panel B shows flow cyometric analysis of digested thymuses from 7-8 week old WT (upper panels) and Cd40lg−/− (lower panels) mice, gated on CD80− MHCIIlow and CD80+ MHCIIhigh subsets of CD45−EpCAM1+Ly51low mTEC. Staining is shown for levels for MHC class II and the proliferation marker Ki67. Panel C shows quantitative analysis of Ki67+ cells within the mTEClow and mTEChigh subsets in WT and Cd40lg−/− mice. Statistical analysis performed used a Mann-Whitney test (unpaired, two-tailed, 95% of confidence) with ** indicating P<0.005 and N.S.: non-significant. The histogram in panel A is a summary of two independent experiments. In total, five mice of each group were studied. The dot plots are representative of three independent experiments on 6-9 week old male mice. In total, seven WT and six Cd40lg−/− mice were studied.
Figure 6. CD40-CD40L Interactions Do Not Control The Rate Of Apoptosis Within The mTEC Compartment.
Panel A shows flow cytometric analysis of DAPI/AnnexinV staining in digested thymuses from 11-15 week old WT (upper panels) and Cd40lg−/− (lower panels) mice, gated on MHC-IIlow and MHC-IIhigh subsets of CD45−EpCAM1+Ly51low mTEC. Panel B shows quantitative analysis of AnnexinV+ DAPI−/Int. cells within mTEClow and mTEChigh subsets in WT and Cd40lg−/− mice. Panel C shows quantitative analysis of AnnexinV+ DAPIhigh cells within mTEClow and mTEChigh subsets in WT and Cd40lg−/− mice. Statistical analysis performed used a Mann-Whitney test (unpaired, two-tailed, 95% of confidence) with N.S.: non-significant. The dot plots are representative of two independent experiments. The histograms are the summary of these two independent experiments. Three WT and three Cd40lg−/− mice were studied in each experiment.
Discussion
The thymic medulla represents a key site for T-cell tolerance induction, a process that is mediated by several cell types including Aire+ medullary epithelial cells (7). Exploring the mechanisms by which medullary thymic epithelial cell (mTEC) microenvironments are induced to develop represents an important aspect in determining how central tolerance mechanisms are controlled. Here, we have investigated a panel of cell types linked to Aire+ mTEC development, with regard to their ability to provide RANKL and CD40L during crosstalk for thymus medulla development at distinct developmental stages. Importantly, our experiments involved simultaneous analysis of cell surface RANKL and CD40L expression in cells without the need for exogenous stimulation.
Detailed flow cytometric analysis revealed that LTi cells and Vγ5 dendritic epidermal T-cell progenitors, both of which are involved in development of the first cohorts of Aire+ mTEC at stages prior to αβT-cell selection (27), express RANKL but not CD40L. Thus, despite expression of CD40 and RANK by fetal mTEC (6, 22), the cells of the innate immune system that drive initial medulla formation do so through provision of RANKL but not CD40L, a finding that fits well with the absence of Aire+ mTEC in the fetal period of RANK deficient mice (22), as well as PCR (31) and histological analysis(33) demonstrating the absence of CD40L expression in total embryonic thymus. Interestingly, a more complex RANKL and CD40L expression pattern was observed when subsets of cells within the αβT-cell lineage of the adult thymus were analysed (Figure 7). In agreement with earlier findings, CD4+8−TCRβhi, but not CD4−8+TCRβhi thymocytes, were found to be the major source of RANKL and CD40L (28, 29). Importantly however, in the experiments described here, use of Rag2GFP mice together with expression of the activation marker CD69 as well as CD25 expression allowed us to reveal marked heterogeneity within CD4+8−TCRβhi thymocytes with regard to cell surface expression of RANKL and CD40L. Thus, RANKL+ cells were abundant within recently positively selected CD69+Rag2GFP+ cells, while CD40L+ cells mapped to later CD69− stages. Interestingly, analysis of the CD25+ subset of CD4+ thymocytes, the majority of which are FoxP3+ T-Reg, showed this population to express RANKL but not CD40L, regardless of their Rag2GFP and CD69 status. Thus, conventional and regulatory subsets of CD4+ thymocytes appear differentially equipped with regard to RANKL/CD40L expression to stimulate mTEC development.
Figure 7. The Involvement of RANK and CD40 During Fetal and Adult mTEC Development.
A model of mTEC development involving the TNF-Receptor superfamily members RANK and CD40 is presented. During the fetal program of thymus development, the generation of the first cohorts of Aire+ mTEC involves RANK but not CD40 signalling, via interactions with RANKL+CD40L− innate like cells. In contrast, in an adult program of mTEC development, RANK signalling is followed by CD40 upregulation and signalling, a two-step process involving interactions with distinct CD4 thymocyte subsets.
That positive selection and maturation of CD4+ thymocytes appears to first enable RANKL- and then CD40L-mediated mTEC crosstalk led us to explore the idea that mTEC development involves RANK and CD40 act in sequence during mTEC development. In support of this idea, mTEC from Tnfrsf11a−/− deficient mice expressed lower levels of CD40 in vivo, and in vitro RANK stimulation of mTEC induced the rapid upregulation of CD40, prior to expression of Aire, tissues restricted antigens Csna and Spt1, and CD80. While our data shows that RANK signalling in mTEC precursors involves the rapid upregulation of CD40 expression, a recent study showed that RANK expression by mTEC is controlled by LTβR signalling (47). Collectively, these observations further support the idea that signalling through individual TNF-Receptors controls the availability of other family members during mTEC development. Moreover, while proliferation within the total mTEC population was not perturbed in Cd40lg−/− mice, analysis of mTEClow and mTEChigh compartments revealed significant alterations. Indeed, reduced proliferation within the mTEClow compartment was observed, together with enhanced proliferation within mTEChigh cells. Given that proliferation within total mTEC was unaltered, these findings suggest that, rather than triggering proliferation directly, CD40 may play a role in controlling its timing during mTEC development, with a delay in proliferation occurring in the absence of CD40 signalling. Interestingly however, no significant difference in the frequency of apoptotic mTEC was found in CD40lg−/− mice, suggesting that while CD40-CD40L interactions impact upon proliferative control of the mTEC compartment, a different mechanism regulates the rate of mTEC apoptosis. Of note, our findings that CD40 influences proliferation within mTEC are of interest in relation to studies linking it to the regulation of epithelial cells of other tissues such as the skin, where it plays a role in the homeostatic control of keratinocyte proliferation (48, 49). Given other studies have shown that epithelial cells within skin and thymus can share certain molecular characteristics (50, 51), including FoxN1 (52) and RANK expression(53), these findings suggest that CD40 represents an additional example of molecules that are involved in the regulation of epithelial compartments within both these tissues.
Taken together, our data reveal complexity in the intrathymic availability of key TNF-Receptor ligands that control mTEC development, and show that while embryonic and adult stages of mTEC development share a common requirement for RANK signalling, provision of CD40L signalling occurs only in the postnatal thymus from interactions with discrete subsets of CD4 thymocytes (Figure 7). Finally, the control of CD40 expression, shown here to be a regulator of mTEC proliferation, by RANK stimulation supports a stepwise involvement for these molecules in mTEC development, in which upregulation of CD40 is an early event, occurring prior to the induction of CD80, Aire and genes encoding tissue restricted antigens.
Acknowledgements
We thank Biomedical Services Unit staff at The University of Birmingham for animal husbandry.
Footnotes
This study was supported by an MRC Programme Grant to GA, PJLL and EJJ and core facilities of the MRC Centre for Immune Regulation. JC is supported by a PhD Studentship from the MRC Centre for Immune Regulation; SB is supported by an EU PhD Studentship from the Integrated Training Network NINA.
References
- 1.Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol. 2006;6:127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- 2.Ciofani M, Zuniga-Pflucker JC. The thymus as an inductive site for T lymphopoiesis. Annu Rev Cell Dev Biol. 2007;23:463–493. doi: 10.1146/annurev.cellbio.23.090506.123547. [DOI] [PubMed] [Google Scholar]
- 3.Hozumi K, Mailhos C, Negishi N, Hirano K, Yahata T, Ando K, Zuklys S, Hollander GA, Shima DT, Habu S. Delta-like 4 is indispensable in thymic environment specific for T cell development. J Exp Med. 2008;205:2507–2513. doi: 10.1084/jem.20080134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koch U, Fiorini E, Benedito R, Besseyrias V, Schuster-Gossler K, Pierres M, Manley NR, Duarte A, Macdonald HR, Radtke F. Delta-like 4 is the essential, nonredundant ligand for Notch1 during thymic T cell lineage commitment. J Exp Med. 2008;205:2515–2523. doi: 10.1084/jem.20080829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiorini E, Ferrero I, Merck E, Favre S, Pierres M, Luther SA, MacDonald HR. Cutting edge: thymic crosstalk regulates delta-like 4 expression on cortical epithelial cells. J Immunol. 2008;181:8199–8203. doi: 10.4049/jimmunol.181.12.8199. [DOI] [PubMed] [Google Scholar]
- 6.Shakib S, Desanti GE, Jenkinson WE, Parnell SM, Jenkinson EJ, Anderson G. Checkpoints in the development of thymic cortical epithelial cells. J Immunol. 2009;182:130–137. doi: 10.4049/jimmunol.182.1.130. [DOI] [PubMed] [Google Scholar]
- 7.Anderson G, Takahama Y. Thymic epithelial cells: working class heroes for T cell development and repertoire selection. Trends Immunol. 2012;33:256–263. doi: 10.1016/j.it.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Murata S, Sasaki K, Kishimoto T, Niwa S, Hayashi H, Takahama Y, Tanaka K. Regulation of CD8+ T cell development by thymus-specific proteasomes. Science. 2007;316:1349–1353. doi: 10.1126/science.1141915. [DOI] [PubMed] [Google Scholar]
- 9.Gommeaux J, Gregoire C, Nguessan P, Richelme M, Malissen M, Guerder S, Malissen B, Carrier A. Thymus-specific serine protease regulates positive selection of a subset of CD4+ thymocytes. Eur J Immunol. 2009;39:956–964. doi: 10.1002/eji.200839175. [DOI] [PubMed] [Google Scholar]
- 10.Irla M, Hollander G, Reith W. Control of central self-tolerance induction by autoreactive CD4+ thymocytes. Trends Immunol. 2010;31:71–79. doi: 10.1016/j.it.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 12.Liston A, Gray DH, Lesage S, Fletcher AL, Wilson J, Webster KE, Scott HS, Boyd RL, Peltonen L, Goodnow CC. Gene dosage--limiting role of Aire in thymic expression, clonal deletion, and organ-specific autoimmunity. J Exp Med. 2004;200:1015–1026. doi: 10.1084/jem.20040581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinterberger M, Aichinger M, Prazeres da Costa O, Voehringer D, Hoffmann R, Klein L. Autonomous role of medullary thymic epithelial cells in central CD4+ T cell tolerance. Nat Immunol. 2010;11:512–519. doi: 10.1038/ni.1874. [DOI] [PubMed] [Google Scholar]
- 14.DeVoss J, Hou Y, Johannes K, Lu W, Liou GI, Rinn J, Chang H, Caspi RR, Fong L, Anderson MS. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. J Exp Med. 2006;203:2727–2735. doi: 10.1084/jem.20061864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallegos AM, Bevan MJ. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J Exp Med. 2004;200:1039–1049. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koble C, Kyewski B. The thymic medulla: a unique microenvironment for intercellular self-antigen transfer. J Exp Med. 2009;206:1505–1513. doi: 10.1084/jem.20082449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hubert FX, Kinkel SA, Davey GM, Phipson B, Mueller SN, Liston A, Proietto AI, Cannon PZ, Forehan S, Smyth GK, Wu L, Goodnow CC, Carbone FR, Scott HS, Heath WR. Aire regulates the transfer of antigen from mTECs to dendritic cells for induction of thymic tolerance. Blood. 2011;118:2462–2472. doi: 10.1182/blood-2010-06-286393. [DOI] [PubMed] [Google Scholar]
- 18.Aschenbrenner K, D’Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, Rolink A, Klein L. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8:351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 19.Lei Y, Ripen AM, Ishimaru N, Ohigashi I, Nagasawa T, Jeker LT, Bosl MR, Hollander GA, Hayashi Y, Malefyt Rde W, Nitta T, Takahama Y. Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. J Exp Med. 2011;208:383–394. doi: 10.1084/jem.20102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodewald HR, Paul S, Haller C, Bluethmann H, Blum C. Thymus medulla consisting of epithelial islets each derived from a single progenitor. Nature. 2001;414:763–768. doi: 10.1038/414763a. [DOI] [PubMed] [Google Scholar]
- 21.Gabler J, Arnold J, Kyewski B. Promiscuous gene expression and the developmental dynamics of medullary thymic epithelial cells. Eur J Immunol. 2007;37:3363–3372. doi: 10.1002/eji.200737131. [DOI] [PubMed] [Google Scholar]
- 22.Rossi SW, Kim MY, Leibbrandt A, Parnell SM, Jenkinson WE, Glanville SH, McConnell FM, Scott HS, Penninger JM, Jenkinson EJ, Lane PJ, Anderson G. RANK signals from CD4+3− inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J Exp Med. 2007;204:1267–1272. doi: 10.1084/jem.20062497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamazaki Y, Fujita H, Kobayashi T, Choi Y, Scott HS, Matsumoto M, Minato N. Medullary thymic epithelial cells expressing Aire represent a unique lineage derived from cells expressing claudin. Nat Immunol. 2007;8:304–311. doi: 10.1038/ni1438. [DOI] [PubMed] [Google Scholar]
- 24.Shores EW, Van Ewijk W, Singer A. Disorganization and restoration of thymic medullary epithelial cells in T cell receptor-negative scid mice: evidence that receptor-bearing lymphocytes influence maturation of the thymic microenvironment. Eur J Immunol. 1991;21:1657–1661. doi: 10.1002/eji.1830210711. [DOI] [PubMed] [Google Scholar]
- 25.Surh CD, Ernst B, Sprent J. Growth of epithelial cells in the thymic medulla is under the control of mature T cells. J Exp Med. 1992;176:611–616. doi: 10.1084/jem.176.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White AJ, Withers DR, Parnell SM, Scott HS, Finke D, Lane PJ, Jenkinson EJ, Anderson G. Sequential phases in the development of Aire-expressing medullary thymic epithelial cells involve distinct cellular input. Eur J Immunol. 2008;38:942–947. doi: 10.1002/eji.200738052. [DOI] [PubMed] [Google Scholar]
- 27.Roberts NA, White AJ, Jenkinson WE, Turchinovich G, Nakamura K, Withers DR, McConnell FM, Desanti GE, Benezech C, Parnell SM, Cunningham AF, Paolino M, Penninger JM, Simon AK, Nitta T, Ohigashi I, Takahama Y, Caamano JH, Hayday AC, Lane PJ, Jenkinson EJ, Anderson G. Rank signaling links the development of invariant gammadelta T cell progenitors and Aire+ medullary epithelium. Immunity. 2012;36:427–437. doi: 10.1016/j.immuni.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hikosaka Y, Nitta T, Ohigashi I, Yano K, Ishimaru N, Hayashi Y, Matsumoto M, Matsuo K, Penninger JM, Takayanagi H, Yokota Y, Yamada H, Yoshikai Y, Inoue J, Akiyama T, Takahama Y. The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity. 2008;29:438–450. doi: 10.1016/j.immuni.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 29.Irla M, Hugues S, Gill J, Nitta T, Hikosaka Y, Williams IR, Hubert FX, Scott HS, Takahama Y, Hollander GA, Reith W. Autoantigen-specific interactions with CD4+ thymocytes control mature medullary thymic epithelial cell cellularity. Immunity. 2008;29:451–463. doi: 10.1016/j.immuni.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Boehm T, Scheu S, Pfeffer K, Bleul CC. Thymic medullary epithelial cell differentiation, thymocyte emigration, and the control of autoimmunity require lympho-epithelial cross talk via LTbetaR. J Exp Med. 2003;198:757–769. doi: 10.1084/jem.20030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akiyama T, Shimo Y, Yanai H, Qin J, Ohshima D, Maruyama Y, Asaumi Y, Kitazawa J, Takayanagi H, Penninger JM, Matsumoto M, Nitta T, Takahama Y, Inoue J. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity. 2008;29:423–437. doi: 10.1016/j.immuni.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 32.Gray DH, Seach N, Ueno T, Milton MK, Liston A, Lew AM, Goodnow CC, Boyd RL. Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood. 2006;108:3777–3785. doi: 10.1182/blood-2006-02-004531. [DOI] [PubMed] [Google Scholar]
- 33.Dunn RJ, Luedecker CJ, Haugen HS, Clegg CH, Farr AG. Thymic overexpression of CD40 ligand disrupts normal thymic epithelial organization. J Histochem Cytochem. 1997;45:129–141. doi: 10.1177/002215549704500116. [DOI] [PubMed] [Google Scholar]
- 34.Spence PJ, Green EA. Foxp3+ regulatory T cells promiscuously accept thymic signals critical for their development. Proc Natl Acad Sci U S A. 2008;105:973–978. doi: 10.1073/pnas.0709071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Renshaw BR, Fanslow WC, 3rd, Armitage RJ, Campbell KA, Liggitt D, Wright B, Davison BL, Maliszewski CR. Humoral immune responses in CD40 ligand-deficient mice. J Exp Med. 1994;180:1889–1900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu W, Nagaoka H, Jankovic M, Misulovin Z, Suh H, Rolink A, Melchers F, Meffre E, Nussenzweig MC. Continued RAG expression in late stages of B cell development and no apparent re-induction after immunization. Nature. 1999;400:682–687. doi: 10.1038/23287. [DOI] [PubMed] [Google Scholar]
- 37.White A, Jenkinson E, Anderson G. Reaggregate thymus cultures. J Vis Exp. 2008;18:905. doi: 10.3791/905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sitnik KM, Kotarsky K, White AJ, Jenkinson WE, Anderson G, Agace WW. Mesenchymal cells regulate retinoic acid receptor-dependent cortical thymic epithelial cell homeostasis. J Immunol. 2012;188:4801–4809. doi: 10.4049/jimmunol.1200358. [DOI] [PubMed] [Google Scholar]
- 39.Jenkinson W, Jenkinson E, Anderson G. Preparation of 2-dGuo-treated thymus organ cultures. J Vis Exp. 2008;18:906. doi: 10.3791/906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ceredig R. The impact of cell re-entry into the primary lymphoid organs on lymphocyte repertoire and functionality. Immunol Cell Biol. 2009;87:13–15. doi: 10.1038/icb.2008.91. [DOI] [PubMed] [Google Scholar]
- 41.Boursalian TE, Golob J, Soper DM, Cooper CJ, Fink PJ. Continued maturation of thymic emigrants in the periphery. Nat Immunol. 2004;5:418–425. doi: 10.1038/ni1049. [DOI] [PubMed] [Google Scholar]
- 42.McCaughtry TM, Wilken MS, Hogquist KA. Thymic emigration revisited. J Exp Med. 2007;204:2513–2520. doi: 10.1084/jem.20070601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 44.Brandle D, Muller S, Muller C, Hengartner H, Pircher H. Regulation of RAG-1 and CD69 expression in the thymus during positive and negative selection. Eur J Immunol. 1994;24:145–151. doi: 10.1002/eji.1830240122. [DOI] [PubMed] [Google Scholar]
- 45.Gray D, Abramson J, Benoist C, Mathis D. Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J Exp Med. 2007;204:2521–2528. doi: 10.1084/jem.20070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liiv I, Haljasorg U, Kisand K, Maslovskaja J, Laan M, Peterson P. AIRE-induced apoptosis is associated with nuclear translocation of stress sensor protein GAPDH. Biochem Biophys Res Commun. 2012;423:32–37. doi: 10.1016/j.bbrc.2012.05.057. [DOI] [PubMed] [Google Scholar]
- 47.Mouri Y, Yano M, Shinzawa M, Shimo Y, Hirota F, Nishikawa Y, Nii T, Kiyonari H, Abe T, Uehara H, Izumi K, Tamada K, Chen L, Penninger JM, Inoue J, Akiyama T, Matsumoto M. Lymphotoxin signal promotes thymic organogenesis by eliciting RANK expression in the embryonic thymic stroma. J Immunol. 2011;186:5047–5057. doi: 10.4049/jimmunol.1003533. [DOI] [PubMed] [Google Scholar]
- 48.Grousson J, Ffrench M, Concha M, Schmitt D, Peguet-Navarro J. CD40 ligation alters the cell cycle of differentiating keratinocytes. J Invest Dermatol. 2000;114:581–586. doi: 10.1046/j.1523-1747.2000.00905.x. [DOI] [PubMed] [Google Scholar]
- 49.Villarroel Dorrego M, Whawell SA, Speight PM, Barrett AW. Transfection and ligation of CD40 in human oral keratinocytes affect proliferation, adhesion and migration but not apoptosis in vitro. Clin Exp Dermatol. 2006;31:266–271. doi: 10.1111/j.1365-2230.2005.02018.x. [DOI] [PubMed] [Google Scholar]
- 50.Yano M, Kuroda N, Han H, Meguro-Horike M, Nishikawa Y, Kiyonari H, Maemura K, Yanagawa Y, Obata K, Takahashi S, Ikawa T, Satoh R, Kawamoto H, Mouri Y, Matsumoto M. Aire controls the differentiation program of thymic epithelial cells in the medulla for the establishment of self-tolerance. J Exp Med. 2008;205:2827–2838. doi: 10.1084/jem.20080046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White AJ, Nakamura K, Jenkinson WE, Saini M, Sinclair C, Seddon B, Narendran P, Pfeffer K, Nitta T, Takahama Y, Caamano JH, Lane PJ, Jenkinson EJ, Anderson G. Lymphotoxin signals from positively selected thymocytes regulate the terminal differentiation of medullary thymic epithelial cells. J Immunol. 2010;185:4769–4776. doi: 10.4049/jimmunol.1002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nehls M, Pfeifer D, Schorpp M, Hedrich H, Boehm T. New member of the winged-helix protein family disrupted in mouse and rat nude mutations. Nature. 1994;372:103–107. doi: 10.1038/372103a0. [DOI] [PubMed] [Google Scholar]
- 53.Duheron V, Hess E, Duval M, Decossas M, Castaneda B, Klopper JE, Amoasii L, Barbaroux JB, Williams IR, Yagita H, Penninger J, Choi Y, Lezot F, Groves R, Paus R, Mueller CG. Receptor activator of NF-kappaB (RANK) stimulates the proliferation of epithelial cells of the epidermo-pilosebaceous unit. Proc Natl Acad Sci U S A. 2011;108:5342–5347. doi: 10.1073/pnas.1013054108. [DOI] [PMC free article] [PubMed] [Google Scholar]