Abstract
Background
L-ascorbic acid is an essential part of the human diet and has been associated with a wide-range of chronic complex diseases including cardiovascular outcomes. To date, there are no confirmed genetic correlates of circulating levels of L-ascorbic acid.
Objectives
We aimed to confirm the existence of association between common variation at the SLC23A1 gene locus and circulating levels of L-ascorbic acid.
Design
We employed a two-stage design which used a discovery cohort (the British Women’s Heart and Health Study) and a series of follow-up cohorts and meta-analysis (totalling 15087 participants) to assess the relationship between variation at SLC23A1 and circulating levels of L-ascorbic acid.
Results
In the discovery cohort, variation at rs33972313 was associated with a reduction in circulating levels of L-ascorbic acid (−4.15μmol/L (95%CI −0.49, −7.81), p=0.03 reduction per minor allele). Pooled analysis of the relationship between rs33972313 and circulating L-ascorbic acid across all studies confirmed this, showing that each additional rare allele was associated with a reduction in circulating levels of L-ascorbic acid of −5.98μmol/L (95%CI −8.23, −3.73), p=2.0×10−7 per minor allele.
Conclusion
Work here has identified a genetic variant (rs33972313) in the SLC23A1 vitamin C active transporter locus that is reliably associated with circulating levels of L-ascorbic acid in the general population. This finding has implications more generally for the epidemiological investigation of relationships between circulating L-ascorbic acid and health outcomes.
Keywords: Vitamin C, genotype, L-ascorbic acid
Introduction
Humans are unable to synthesise L-ascorbic acid (vitamin C) owing to a series of loss-of-function mutations in the gulonolactone oxidase (GULO) locus(1, 2). Consequently, L-ascorbic acid has to be acquired from dietary sources(3). L-ascorbic acid has long been known to be an essential part of the human diet, with its deficiency ultimately resulting in scurvy(4). More recently variation in L-ascorbic acid levels have been associated with a wide-range of chronic complex diseases. These associations are thought to result from a contribution of L-ascorbic acid to antioxidant mechanisms and the synthesis of biological entities such as collagen and hormones(5-11).
Specifically, L-ascorbic acid is an electron donor(12) and through this mechanism contributes to the prevention of oxidative damage, which is thought to contribute to human diseases such as atherosclerosis (through the oxidation of low density lipoprotein cholesterol(13-15)), type 2 diabetes (through oxidative stress on the beta cell(9, 16)) and cancer (through oxidation-related DNA and DNA repair mechanism damage)(7). In addition L-ascorbic acid is essential for the biosynthesis of collagen and L-carnitine (important to membrane integrity during pregnancy(3, 17, 18)) and for the conversion of dopamine to norepinephrine(19).
L-ascorbic acid is obtained from the diet and is transported across the cell membrane, including intestinal cells, through both facilitated and active modes of transport. The active transport is achieved by sodium L-ascorbic acid (“vitamin C”) co-transporters (SVCTs) which transport ascorbate into the cell(20, 21). There are two isoforms of SVCT, hSVCT1 (slc23a1) and hSVCT2 (slc23a2) coded for by the genes SLC23A2 and SLC23A1 respectively(22, 23). The actual roles of SVCT1 and SVCT2 differ, with the two forms having different capacity for L-ascorbic acid transport. SVCT1 exhibits a higher maximum velocity and is recognized as a high capacity/low affinity carrier. The role of SVCT1 in sodium dependent L-ascorbic acid uptake has been recorded and confirmed in functional studies. Not only have these implicated SVCT1 in the absorption of dietary L-ascorbic acid across the intestinal barrier, but this work has highlighted the role of SVCT1 in kidney based reabsorption(24-26). As a result, SVCT1 is primarily involved in whole body homeostasis and transport of L-ascorbic acid and SVCT2 is primarily involved in the regulation of L-ascorbic acid in specific metabolically active tissues(27).
Genetic variation in the sodium channel proteins SLC23A2 and SLC23A1 shows differential patterns of linkage disequilibrium in each locus suggesting the possible action of different selection pressures through human population history(28). Variation in SLC23A2 appears constrained in human populations (being consistent across both European and African populations), however variation is present in SLC23A1(28) and may be associated with changes in physiological function which have escaped immediate selection pressure. In this study, we have measured part of the genetic variation at SLC23A1 in a series of European population based cohorts in order to identify single nucleotide polymorphisms (SNPs) robustly associated with circulating levels of L-ascorbic acid.
Subjects and Methods
The study design for this investigation had three parts. Initially, genetic associations were tested for in a discovery population. From this, further genotyping and replication analyses would be undertaken in first and second stage replication studies to validate initial findings. The studies involved are described below.
Discovery study
British Women’s Heart and Health Study (BWHHS)
Between 1999 and 2001 4,286 women aged 60 to 79 years, were randomly selected from 23 British towns and were interviewed, examined and completed medical questionnaires. Methods used at baseline assessment have been previously described(29, 30). Within the BWHHS, 12 women were described by the examining nurse as non-white and were excluded from further analyses.
SNPs were genotyped using the KASPar chemistry which is a competitive allele-specific PCR SNP genotyping system using FRET quencher cassette oligos. All genotyping was performed by KBioscience (http://www.kbioscience.co.uk). Three stages of internal quality control were employed during genotyping. Known locations of non-DNA test controls were used to assure unique plate identity, a small sample of duplicate DNAs were genotyped for all SNPs and initial assay validations were performed on a sub-sample of 96 chromosomes before genotyping the whole sample set. A total of 3425 women (80% of the 4258 eligible white women who gave consent for genetic studies) had complete genotype and phenotype data and were included in analyses.
Blood samples were taken after a minimum 6-hour fast and samples were used for assessment of circulating L-ascorbic acid (which was assayed in duplicate, with the mean of the two values used in all analyses - see Supplementary Material)(31).
In this discovery study we also examined whether genotype and phenotype associations might be confounded. A priori we thought that due to population selection allele frequency might vary by birth geography and that since mobility in this cohort is generally low that this might also be related to variation in L-ascorbic acid levels and therefore this might confound gene-phenotype associations. In order to account for the geographic origin of participants, we matched the town/city of birth (reported by women on their first assessment) to geographical grid references giving the distance North and East of these locations from the British National Grid reference (located close to the Isle of Scilly, measured in metres). These measurements give an indication of latitude (distance North) and longitude (distance East) of place of birth. For other replication studies information on birth geography were not available and therefore we used the effect of adjusting for latitude and longitude of birth in BWHHS on the main association to consider how likely the unadjusted findings in other studies were to be influenced by this confounding. A number of characteristics affect variation in vitamin C levels including socioeconomic position, physical activity, alcohol consumption and cigarette smoking, but we a priori assumed these would not be associated with genetic variants(32). However, we examined this assumption by assessing genotype associations with these characteristics as well as phenotype associations with these. Full details of how these characteristics were measured are presented in the Supplementary Material. These associations were not further explored in the replication studies.
Replication studies
Full details, including how DNA was extracted, genotype measured and L-ascorbic acid levels measured of all replication studies can be found in the Supplementary Material.
European Prospective Investigation of Cancer Norfolk Study (EPIC-Norfolk)
As first stage replication, we analysed data from a random sub-study of 5000 participants (EPIC5000) from the Norfolk arm of the European Prospective Investigation on Cancer (EPIC-Norfolk) study. Described in detail previously(33), EPIC-Norfolk is an ongoing prospective study of men and women aged between 40 and 79 years, resident in Norfolk, UK. For analyses here between 4500 and 4600 individuals were available with both genotypic and phenotypic data (the number varied depending on the SNP assessed).
MIDSPAN family study
The name MIDSPAN is given to 4 separate occupational and general population cohort studies based in Scotland. The 3 original studies took place between 1964 and 1976. Twenty years later in 1996 the next generation was studied when offspring of couples in the original Renfrew/Paisley Study were recruited into the Family Study(34). This latter group is the subject of the present analysis. For analyses here 1814 samples were available with genetic, phenotype and family data available.
10 Towns
The Ten Towns Study was a longitudinal study of the development of cardiovascular risk among children and adolescents in ten British towns, five with high and five with low adult cardiovascular mortality rates(35, 36). Analyses presented here are restricted to 1359 children of white European origin with genetic and phenotype data available.
British Regional Heart Study (BRHS)
In a study design very similar to that described in the BWHHS 7735 men aged 40-59 were recruited in 1978-80 from a single general practice in each of 24 towns across Great Britain, and have been followed ever since(37). In 1998-2000, when the participants were aged 60-79 years, 4252 were re-examined and most provided a whole blood sample. For analyses here 3740 samples were available with genetic and phenotype data available.
Genetic variation at SLC23A1
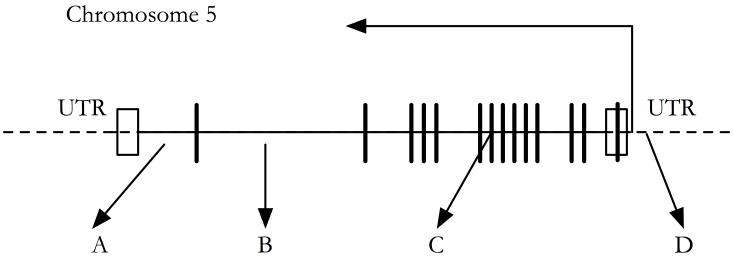
The locus SLC23A1 was chosen as a locus of interest on the basis of previous available evidence suggesting a role for this locus in L-ascorbic acid transport(17, 22). Having identified this locus, SNPs were chosen on the basis of linkage disequilibrium and known genetic variation in this region in four populations in the USA (www.snp500cancer.nci.nih.gov)(28). The four SNPs chosen for analysis within the discovery cohort were distributed equally across the SLC23A1 locus and tagged variation at this locus both in un-translated and genic regions. The location of SNPs across the SLC23A1 locus is summarized in Figure 1.
Figure 1.
Schematic of genetic variation assessed across the SLC23A1 locus.
Variants assessed in discovery cohort, A - rs6596471 (138733487), B - rs6596473 (138738475), C - rs33972313 (138743401), D - rs10063949 (138747425). Open boxes represent 5′ and 3′ untranslated regions, strong bars represent exons. Base positions from Reference Assembly NC_000005.8
Statistical methods
Discovery study analyses
Hardy-Weinberg equilibrium was tested at each SNP locus using an exact test(38). Linkage disequilibrium (LD) estimates were quantified by D′ and r2 values calculated using the Stata 11 (Stata Corp) package “pwld” (www-gene.cimr.cam.ac.uk/clayton).
We used linear regression to assess the association of circulating L-ascorbic acid levels with genetic variation at SLC23A1 assuming an additive genetic model. L-ascorbic acid was not transformed for analysis as its variance (assessed from duplicate assays in BWHHS data), increased roughly linearly but only weakly with its mean, and a square-root transformation (which provided the best approximation to a normal distribution) would considerably hinder interpretability of the results. Although the distribution of L-ascorbic acid was somewhat positively skewed, the large sample sizes ensure robustness of the statistical methods to non-normality(39).
To examine the associations of L-ascorbic acid with potential confounding factors, continuously measured variables (socioeconomic position score, longitude and latitude) were split into tertiles. Mean differences in L-ascorbic acid by tertile of these variables and by category of categorical confounding variables were examined by linear regression. To examine genotype/confounder associations, mean differences of continuous variables and proportion of categorical variables are presented by genotype and were assessed again through the use of linear regression.
Replication study analyses
Associations between genetic variation and circulating L-ascorbic acid level in the discovery study (BWHHS) were first replicated in the EPIC replication study to determine whether primary results for rs10063949, rs6596473 and rs33972313 were robust. The same analyses were performed for the association between genotype and L-ascorbic acid adjusting for age and sex.
Following this, the sole replicating signal (rs33972313) was genotyped in the remaining three independent studies. In the MIDSPAN study linear regression of phenotype on genotype was carried out using a linear mixed model with the Stata 11 (Stata Corp) command “xtmixed” including rs3397213, age, sex, L-ascorbic acid and a variable representing family identity. This ensured that the standard errors were correct for non-independence of participants.
Summary statistics (regression estimates and standard errors) from the regressions of circulating L-ascorbic acid (with sex and age included in the model and assuming an additive genetic contribution) on rs33972313 were pooled using meta-analysis with appropriate metrics for consistency(40). As the BWHHS, BRHS and 10 Towns studies were all assayed for L ascorbic acid in the same way and EPIC and MIDSPAN by different protocols, we anticipated the presence of some heterogeneity in pooled estimates. We accounted for this by using a random effects model and also conducted separate sensitivity meta-analyses in groups determined by assay protocol for purposes of comparison. Meta-analysis was performed in Stata 11 (Stata Corp), using the command “metan”.
Results
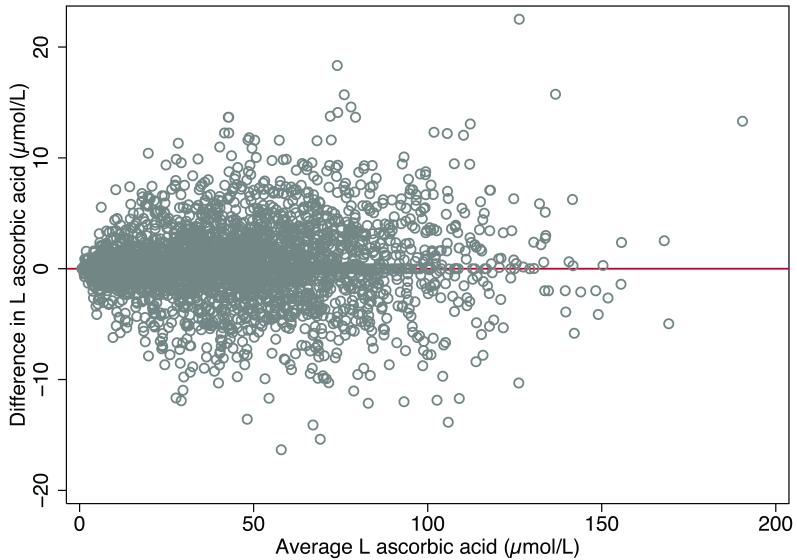
The median value for circulating L-ascorbic acid level in the BWHHS (mean age 68.9) was 39.78μmol/L(inter-quartile range 21.22, 60.14). Comparison of the duplicate measures of vitamin C in the BWHHS showed reasonable repeatability in this measurement (Figure 2) (standard deviation of difference 3.2μmol/L).
Figure 2.
Relationship between mean and difference in two repeat measurements of circulating L ascorbic acid (Bland Altman plot) in the British Women’s Heart and Health Study (n = 3592).
Within the BWHHS minor (m) allele frequencies at SNPs rs10063949(T/Cm), rs6596473(G/Cm), rs6596471(A/Gm) and rs33972313(C/Tm) were 0.32, 0.28, 0.25 and 0.03, respectively. rs10063949, rs6596473 and rs33972313 all adhered to HWE (p>0.3), though rs6596471 showed a nominal departure consistent with a slight over-representation of heterozygotes (p=0.01). Measurements of the degree of linkage disequilibrium (LD) between these variants are shown in Table 1. Variants rs6596473 and rs33972313 were correlated with each other (independent of allele frequency, r2>0.8), but all other pairwise comparisons showed low LD.
Table 1.
Linkage disequilibrium between SNPs across SLC23A1 in the BWHHS.
| Pos | SNP | ||||
|---|---|---|---|---|---|
| 138733487bp | rs6596471 | 0.25* | |||
| 138738475bp | rs6596473 | 0.48 | 0.28* | ||
| 138743401bp | rs33972313 | 0.01 | 0.01 | 0.03* | |
| 138747425bp | rs10063949 | 0.37 | 0.82 | 0.06 | 0.32* |
| rs6596471 | rs6596473 | rs33972313 | rs10063949 |
LD values (r2)
HAPMAP build 36 chromosomal order (chr 5)
Values on diagonal (italics*) represent minor allele frequency of variants
In the BWHHS, three SNPs showed evidence for association with circulating L-ascorbic acid. For each additional minor allele of rs10063949 and rs6596473, there was an associated increase in circulating levels of L-ascorbic acid (1.91μmol/L (95%CI 0.47, 3.34), p=0.009 and 2.86μmol/L (95%CI 1.39, 4.33), p=0.0001 per minor allele respectively). In contrast, for rs33972313 the addition of each rare allele was associated with a reduction on circulating levels of L-ascorbic acid (−4.15μmol/L (95%CI −0.49, −7.81), p=0.03 reduction per minor allele). These findings are summarized in Table 2.
Table 2.
Circulating L ascorbic acid by allelic variation at SCL23A1 in the British Women’s Heart and Health Study.
| Genotype at rs33972313 (n=3252) | |||||
|---|---|---|---|---|---|
| L ascorbic acid μmol/L |
GG | GA | AA | Per allele effect | p |
| 43.77 (42.80, 44.74) |
38.63 (34.90, 42.37) |
52.61 (30.16, 75.06) |
−4.15 (−7.81, −0.49) |
0.03 | |
| Genotype at rs10063949 (n=3365) | |||||
| L ascorbic acid μmol/L |
AA | AG | GG | Per allele effect | p |
| 42.54 (41.15, 43.93) |
43.85 (42.40, 45.30) |
47.01 (44.07, 49.96) |
1.91 (0.47, 3.34) |
0.009 | |
| Genotype at rs6596473 (n=3215) | |||||
| L ascorbic acid μmol/L |
CC | CG | GG | Per allele effect | p |
| 42.04 (40.72, 43.36) |
44.35 (42.86, 45.83) |
48.56 (45.31, 51.81) |
2.86 (1.39, 4.33) |
0.0001 | |
| Genotype at rs6596471 (n=3184) | |||||
| L ascorbic acid μmol/L |
TT | TC | CC | Per allele effect | p |
| 43.01 (41.73, 44.29) |
43.68 (42.17, 45.19) |
45.56 (41.55, 49.58) |
0.95 (−0.63, 2.53) |
0.2 | |
Means (95%CI) by genotype are adjusted for age.
Per allele effects and adjusted means are derived from linear regression.
In the BWHHS, there was evidence for the association of circulating L-ascorbic acid with six of eight potential confounding factors assessed (Table 3). Analysis of the relationship between the confounding features measured in the BWHHS and genetic variation at the SLC23A1 locus showed there to be no strong evidence for any associations (Table 4).
Table 3.
Circulating L ascorbic acid levels by levels of continuous and binary confounding factors in the British Women’s Heart and Health Study
|
|
|||||
|---|---|---|---|---|---|
| Mean (95%CI) L-ascorbic acid | |||||
|
| |||||
| Confounder | n | Tertile 1 | Tertile 2 | Tertile 3 | p |
| SEP | 2968 | 49.33 (47.71, 50.94) |
42.72 (41.15, 44.29) |
36.50 (34.26, 38.74) |
2.8×10−20 |
| Latitude (m) | 3404 | 46.43 (44.82, 48.05) |
39.08 (37.43, 40.74) |
43.16 (41.58, 44.74) |
0.006 |
| Longitude (m) | 3404 | 40.89 (39.32, 42.45) |
44.23 (42.66, 45.80) |
43.95 (42.20, 45.70) |
0.007 |
|
| |||||
| Mean (95%CI) L-ascorbic acid | |||||
|
| |||||
| Confounder | n | No | Yes | p | |
|
| |||||
| <1hr vigorous activity/wk |
2972 | 43.56 (42.45, 44.67) |
41.24 (38.93, .55) |
0.08 | |
| < 2 drinks/day | 3281 | 42.09 (41.04, 43.14) |
49.49 (47.31, 51.67) |
2.2×10−09 | |
| Parental Cardiovascular disease |
3320 | 43.54 (42.19, 44.89) |
43.89 (42.54, 45.25) |
0.7 | |
| Hormone replacement therapy |
3397 | 43.06 (41.98, 44.14) |
45.94 (43.85, 48.04) |
0.02 | |
| Current smoker |
3590 | 44.67 (43.71, 45.64) |
32.47 (29.71, 35.22) |
3.4e-16 | |
Mean (95%CI) L ascorbic acid levels by continuous and binary confounding variables.
SEP denotes socioeconomic position score.
Tests of difference by confounder levels were derived from linear regression.
Table 4.
Potential confounding factors by allelic variation at rs33972313 of SCL23A1 in the British Women’s Heart and Health Study
|
|
|||||
|---|---|---|---|---|---|
| Mean or proportion (95%CI) of each confounder |
|||||
| Genotype at rs33972313 | |||||
|
| |||||
| Confounder | n | GG | GA | AA | p |
| *SEP | 3062 | 4.42 (4.33, 4.50) |
4.54 (4.21, 4.88) |
2.75 (0.50, 5.00) |
0.8 |
| *Latitude (m) | 3512 | 395251.4 (389175.8, 401327) |
397481.8 (374060, 420903.5) |
300689.8 (158551.1, 442828.4) |
0.8 |
| *Longitude (m) | 3512 | 405369.2 (401935.5, 408802.8) |
408850.3 (395613.5, 422087) |
378235.2 (297905.6, 458564.9) |
0.8 |
| <1hr vigorous activity/wk (y/n) |
3125 | 18.39 (17.00, 19.86) |
19.58 (14.81, 25.43) |
20.76 (11.74, 34.04) |
0.7 |
| < 2 drinks/day (y/n) |
3385 | 18.64 (17.32, 20.03) |
19.26 (14.63, 24.93) |
19.92 (11.32, 32.64) |
0.8 |
| Parental CVD (y/n) |
3419 | 50.12 (48.39, 51.86) |
52.12 (45.61, 58.56) |
54.16 (41.13, 66.65) |
0.5 |
| HRT (y/n) |
3507 | 18.56 (17.12, 20.08) |
20.91 (16.23, 26.52) |
23.68 (14.27, 36.66) |
0.3 |
| Current smoker (y/n) |
3703 | 10.87 (9.87, 11.96) |
8.53 (5.67, 12.65) |
6.68 (2.87, 14.77) |
0.3 |
Proportions/means (95%CI) of confounding features by allele derived from linear regression.
Indicates continuous variable.
SEP denotes socioeconomic position score.
First stage replication of genotype-L-ascorbic acid association in the EPIC study showed null results for rs10063949 (Table 5). In contrast to this, rs33972313 and rs6596473 showed association results consistent with those found in the BWHHS (−8.31μmol/L (95%CI −10.51, −6.11), p=1.7×10−13 and 1.01μmol/L (95%CI 0.14, 1.87), p=0.02 per minor allele respectively). Given the nature of the LD between these loci (Table 1) and the nature of their effects, we decided to follow-up the single SNP rs33972313 within three further studies (details of which can be seen in Supplementary Table S1).
Table 5.
Circulating L ascorbic acid by allelic variation at SCL23A1 in the European Prospective Investigation of Cancer study.
|
|
|||||
|---|---|---|---|---|---|
| Mean (95%CI) L-ascorbic acid |
|||||
| Genotype at rs33972313 (n=4501) | |||||
|
| |||||
| GG | GA | AA |
Mean difference
in L-ascorbic acid per minor allele |
p | |
| L ascorbic acid μmol/L |
56.66 (56.08, 57.24) |
48.21 (45.99, 50.43) |
43.38 (28.02, 58.73) |
−8.31 (−10.51, −6.11) |
1.7×10−13 |
|
| |||||
| Genotype at rs6596473 (n=4614) | |||||
|
| |||||
| CC | CG | GG |
Mean difference in
L-ascorbic acid per minor allele |
p | |
|
| |||||
| L ascorbic acid μmol/L |
55.54 (54.73, 56.35) |
56.90 (56.05, 57.75) |
57.09 (55.24, 58.94) |
1.01 (0.14, 1.87) |
0.02 |
|
| |||||
| Genotype at rs10063949 (n=4539) | |||||
|
| |||||
| AA | AG | GG |
Mean difference in
L-ascorbic acid per minor allele |
p | |
|
| |||||
| L ascorbic acid μmol/L |
56.16 (55.31, 57.01) |
56.19 (55.36, 57.03) |
55.98 (54.29, 57.68) |
−0.05 (−0.90, 0.80) |
0.9 |
Means (95% CI) by genotype are adjusted for age and sex.
Per allele effects and adjusted means are derived from linear regression.
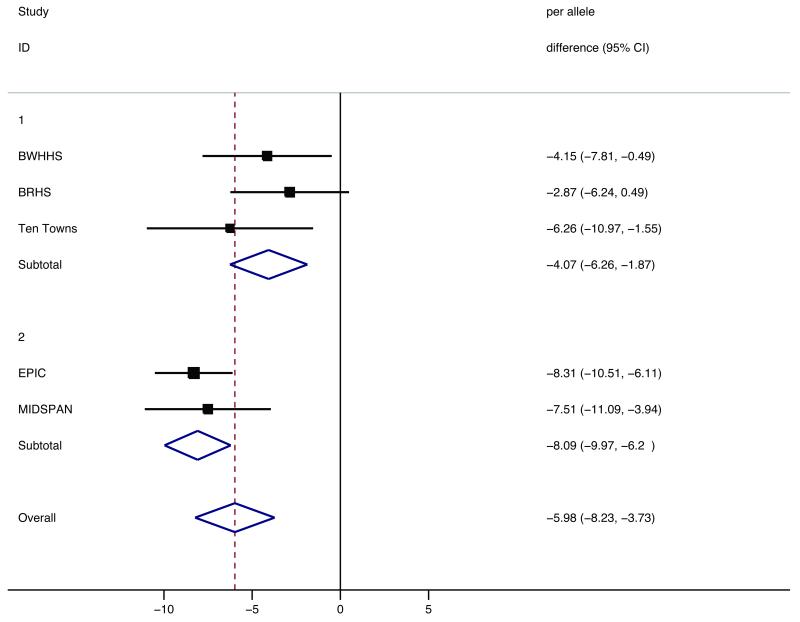
Pooled analysis of the relationship between rs33972313 and circulating L-ascorbic acid across all five studies showed that each additional rare allele was associated with a reduction in circulating levels of vitamin C of −5.98μmol/L (95%CI −8.23, −3.73), p=2.0×10-7 per minor allele (Figure 3). There was evidence of heterogeneity when all five studies were included in the pooled analysis (I2 value of 55.2 (95%CI 0.0, 83.0)%, phet value = 0.06). This appeared to be largely accounted for by the differing assay protocols used to measure L ascorbic acid and in sensitivity analyses (shown in Supplementary Table S2 & Figure 3) pooling the BWHHS, the BRHS and the Ten Towns studies (L-ascorbic acid measured for these in the same laboratory with the same protocol), the per allele effect was −4.07μmol/L (95%CI −6.26, −1.87) with the pooled result for MIDSPAN and EPIC being −8.09μmol/L (95%CI −9.97, −6.22).
Figure 3.
Meta-analysis summary of rs33972313 association with circulating vitamin C from discovery and replication studies.
X axis represents associated difference in L-ascorbic acid per rare allele at rs33972313 (μmol/L). Sections 1 and 2 show sub-analyses by L-ascorbic assay type. BWHHS n=3425, BRHS n=3740, Ten Towns n=1359, EPIC n=4501, MIDSPAN n=1814. Overall - a meta-analysed pooled estimate of per allele association (random effects).
Discussion
We have identified a genetic variant (rs33972313) in the SLC23A1 vitamin C active transporter locus that is reliably associated with circulating levels of L-ascorbic acid in the general population. We have also shown that unlike direct measurements of L-ascorbic acid, genotypes associated with circulating levels are not confounded by a series of factors which often make the interpretation of observational data difficult. This variant provides a reliable, non-confounded proxy for variation in L-ascorbic acid at the population level and has the potential for application to applied epidemiological investigations concerned with the impact of chronic variation in L-ascorbic acid levels in the general population (32, 41-43).
The variant implicated in this study is found to lie within the SLC23A1 gene which encodes the active L-ascorbic acid transporter SVCT1 and confirms a predicted association between variation at this locus and circulating levels of L-ascorbic acid(44). Although functional assays of this variant have not been performed, rs33972313 is known to lie in exon 8 of SLC23A1 and to yield a missense change delivering a methionine (Meth/ATG) form in the presence of the rare A allele as opposed to the common G allele derived valine (Val/GTG) form. It is this rare allelic form which is associated with lower levels of circulating L-ascorbic acid and which is assumed to be the by-product of a conformational change or protein failure which impairs active transport. Knockout experiments have shown that Slc23a1−/− mice exhibit lower plasma L-ascorbic acid, failure to accumulate L-ascorbic acid, very high levels of body store L-ascorbic acid excretion and a surprising level of compensatory L-ascorbic acid synthesis (an ability not found in humans)(44). Furthermore, Slc23a1 null offspring had greater perinatal mortality associated with their lower plasma L-ascorbic acid levels, although this could be avoided by supplementation during pregnancy. Notably, this supplementation not only rescued perinatal mortality (through placental transfer), but also raised maternal L-ascorbic acid levels suggesting that other routes to intestinal absorption do exist.
As well as being involved in the regulation of circulating levels of L-ascorbic acid, SVCT1 has been shown to be active in a series of locations including the intestine(24, 45), the kidney(26) and the respiratory system(27). The involvement of the sister isoform of SVCT1 (SVCT2) in both similar functions (relating to active ascorbic acid transport in the brain, respiratory system, intestine, adrenal glands, bone and the eye (46-48)) and with health outcomes directly(49) goes further to substantiate the likely role for SLC23A1 variation in vitamin C regulation, however the direct relationship between rs33972313 and the function of SVCT2 remain unknown.
There are several limitations to the work presented in this investigation. Firstly, genotypic data available across the SLC23A1 locus does not represent all genetic variation in this region for all populations. Whilst we are able to capture and assess the reliability of association signals at specific SNPs, this is not a comprehensive screen of the SLC23A1 locus. Not only are our results limited in inference to genetic variation specific to populations of European descent, we have only examined variation at or around the SLC23A1 gene. Eck et al(28) examine both variation here and in the related SLC23A2 gene and suggest that whilst variation at the former may be tolerated (and as such provide informative variation in genomic code for the purpose of association studies), it appears that such variation has been selectively removed from the population in the case of SLC23A2. Other than an interesting population genetic observation, this does suggest that variation at this “protected” locus may be more relevant to biological function and indeed may be valuable to the exploration of inherent differences in L-ascorbic acid transport (an issue highlighted by the specific tissue activity of SVCT2(27)). Lastly, we acknowledge that methods used to measure circulating L-ascorbic acid levels were not uniform across the five studies and this may have contributed to some of the heterogeneity that we found between studies. Despite this, the findings from the five studies on associations between circulating levels and the SLC23A1 locus were directionally similar and broadly consistent lending validity to our conclusions.
Despite these limitations, this report brings attention to a confirmed genetic associate of genetic variation in SLC23A1 and circulating measures of L-ascorbic acid with this association being robust across people from five independent studies. This finding is important for understanding the mechanisms involved in variation in this essential vitamin between humans. It also has potential implications for the assessment of causal relationships between circulating L-ascorbic acid and health outcomes through the application of this genetic variant as a proxy measure for variation in circulating L-ascorbic acid. Specifically, this genetic variant could be used as an instrumental variable to determine the causal effect of lifetime variation in vitamin C levels on risk of cardiovascular disease, diabetes, cancer and other chronic disease outcomes that have been found to be associated with variation in vitamin C but for which causality remains debated(32, 41-43). This is because this genetic variant (like most genetic variation) is likely to be unrelated to many of the common characteristics that confound the association of circulating L-ascorbic acid with these chronic diseases and since genotype is allocated at conception its association with disease outcomes could not be affected by reverse causality(32, 43). The use of genetic variation at SLC23A1 will, however, require a single study with very large sample size or pooling of several large studies(43).
Supplementary Material
Acknowledgements
The British Women’s Heart & Health Study is co-directed by Professor Shah Ebrahim, Professor Peter Whincup, Dr Goya Wannamethee and Professor Debbie A Lawlor. We thank Carol Bedford, Alison Emerton, Nicola Frecknall, Karen Jones, Rita Patel, Mark Taylor and Katherine Wornell for collecting and entering data, all of the general practitioners and their staff who have supported data collection, and the women who have participated in the study.
We thank Victor Hawthorne who initiated the original Renfrew/Paisley (MIDSPAN) Study, Dr Mark Upton who co-ordinated and led the first phase of the MIDSPAN Family Study, and Dr Carole Hart and Mrs Pauline Mckinnon who have maintained the original and subsequent family study data set.
The Ten Towns Heart Health Study is co-directed by Professor Peter Whincup and Professor Derek Cook. DNA extraction was supervised by Professor Ian Day. We thank Claire Nightingale for maintaining the database and all the schools and children who participated in the study
The British Regional Heart Study was established by Professor AG Shaper and is co-directed by Professor Peter Whincup, Professor Richard Morris and Dr Goya Wannamethee. We thank Professor Aroon Hingorani (UCL) for his support and input for development of the DNA resource for the study, and thank Devi Kundu and Asmeret Kidane for technical support.
For the EPIC cohort, we thank the general practitioners and volunteers for their participation and the EPIC Norfolk study team for their helpful input.
Funding
NJT is funded through the MRC Centre grant (MRC CAiTE Centre) - G0600705. GDS and DAL works within the MRC Centre for Causal Analyses in Translational Epidemiology which is capacity funded by grant G0600705.
RMH is supported in part by MRC project grant G0601625.
S.P. was supported by a BHF intermediate research fellowship FS/05/095/19937.
British Women’s Heart & Health Study is supported by grants from British Heart Foundation and Department of Health policy research division.
The offspring study in MIDSPAN was supported by grants from the Wellcome Trust and the NHS Research and Development Programme.
The Ten Towns Heart Health Study was supported by a project grant from The Wellcome Trust (051187/Z/97/A) and the genetic studies by a grant from the Medical Research Council (G9900686).
The British Regional Heart Study is a British Heart Foundation Research Group. The measurements and laboratory analyses reported here were supported by British Heart Foundation Project Grants PG97012 and PG97027. DNA extraction was supported in part by British Heart Foundation Senior Research Fellowship FS05/125.
The EPIC Norfolk study is supported by grant funding from the Cancer Research Campaign, the Medical Research Council, the Stroke Association, the British Heart Foundation, the Department of Health, the Europe Against Cancer Programme Commission of the European Union and the Ministry of Agriculture, Fisheries and Food.
Footnotes
Disclosures
None
Contributions
NJT conceived, performed analysis for and wrote the paper. GDS, DAL, RMH and MJB provided analytical and writing support for the paper. All other authors provided data, cohort information and support throughout the drafting process.
References
- 1.Nishikimi M, Koshizaka T, Ozawa T, Yagi K. Occurrence in humans and guinea pigs of the gene related to their missing enzyme L-gulono-gamma-lactone oxidase. Archives of Biochemistry & Biophysics. 1988;267:842–6. doi: 10.1016/0003-9861(88)90093-8. [DOI] [PubMed] [Google Scholar]
- 2.Nishikimi M, Fukuyama R, Minoshima S, Shimizu N, Yagi K. Cloning and chromosomal mapping of the human nonfunctional gene for L-gulono-gamma-lactone oxidase, the enzyme for L-ascorbic acid biosynthesis missing in man. Journal of Biological Chemistry. 1994;269:13685–8. [PubMed] [Google Scholar]
- 3.Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA. 1999:1415–1423. doi: 10.1001/jama.281.15.1415. [DOI] [PubMed] [Google Scholar]
- 4.Lind J. Treatise of the scury. Sands, Murray and Cochran for A Kincaid and A Donaldson; Edinburgh: 1753. [Google Scholar]
- 5.Khaw KT, Bingham S, Welch A, et al. Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: a prospective population study. Lancet. 2001;357:657–663. doi: 10.1016/s0140-6736(00)04128-3. [DOI] [PubMed] [Google Scholar]
- 6.McRae MP. Vitamin C supplementation lowers serum low-density lipoprotein cholesterol and triglycerides: a meta-analysis of 13 randimised controlled trials. Journal of Chiropractic Medicine. 2008;7:48–58. doi: 10.1016/j.jcme.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Padayatty SJ, Katz A, Wang Y, et al. Vitamin C as an antioxidant: evaluation of its role in disease prevention. Journal of the American College of Nutrition. 2003;22:18–35. doi: 10.1080/07315724.2003.10719272. [DOI] [PubMed] [Google Scholar]
- 8.Padayatty SJ, Levine M, Padayatty SJ, Levine M. Fruit and vegetables: think variety, go ahead, eat. American Journal of Clinical Nutrition. 2008;87:5–7. doi: 10.1093/ajcn/87.1.5. [DOI] [PubMed] [Google Scholar]
- 9.Harding AH, Wareham NJ, Bingham SA, et al. Plasma vitamin C level, fruit and vegetable consumption, and the risk of new-onset type 2 diabetes mellitus: the European prospective investigation of cancer--Norfolk prospective study. Archives of Internal Medicine. 2008;168:1493–9. doi: 10.1001/archinte.168.14.1493. [DOI] [PubMed] [Google Scholar]
- 10.Britton JR, Pavord ID, Richards KA, et al. Dietary antioxidant vitamin intake and lung function in the general population. American Journal of Respiratory & Critical Care Medicine. 1995;151:1383–7. doi: 10.1164/ajrccm.151.5.7735589. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz J, Weiss ST. Relationship between dietary vitamin C intake and pulmonary function in the First National Health and Nutrition Examination Survey (NHANES I) American Journal of Clinical Nutrition. 1994;59:110–4. doi: 10.1093/ajcn/59.1.110. [DOI] [PubMed] [Google Scholar]
- 12.Mandl J, Szarka A, Banhegyi G. Vitamin C: update on physiology and pharmacology. British Journal of Pharmacology. 2009;157:1097–1110. doi: 10.1111/j.1476-5381.2009.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polidori MC, Mecocci P, Levine M, et al. Short-term and long-term vitamin C supplementation in humans dose-dependently increases the resistance of plasma to ex vivo lipid peroxidation. Archives of Biochemistry & Biophysics. 2004;423:109–15. doi: 10.1016/j.abb.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Balkan J, Dogru-Abbasoglu S, Aykac-Toker G, Uysal M. Serum pro-oxidant-antioxidant balance and low-density lipoprotein oxidation in healthy subjects with different cholesterol levels. Clinical & Experimental Medicine. 2004;3:237–42. doi: 10.1007/s10238-004-0031-6. [DOI] [PubMed] [Google Scholar]
- 15.Jialal I, Vega GL, Grundy SM. Physiologic levels of ascorbate inhibit the oxidative modification of low density lipoprotein. Atherosclerosis. 1990;82:185–91. doi: 10.1016/0021-9150(90)90039-l. [DOI] [PubMed] [Google Scholar]
- 16.Salmeron J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. Jama. 1997;277:472–7. doi: 10.1001/jama.1997.03540300040031. [DOI] [PubMed] [Google Scholar]
- 17.Erichsen HC, Engel SA, Eck PK, et al. Genetic variation in the sodium-dependent vitamin c transporters, SLC23A1, and SLC23A2 and risk for preterm delivery. American Journal of Epidemiology. 2006;163:245–54. doi: 10.1093/aje/kwj035. [DOI] [PubMed] [Google Scholar]
- 18.Wideman GL, Baird GH, Bolding OT. Ascorbic acid deficiency and premature rupture of fetal membranes. American Journal of Obstetrics & Gynecology. 1964;88:592–5. doi: 10.1016/0002-9378(64)90885-3. [DOI] [PubMed] [Google Scholar]
- 19.Rebouche CJ. Ascorbic acid and carnitine biosynthesis. American Journal of Clinical Nutrition. 1991;54:1147S–1152S. doi: 10.1093/ajcn/54.6.1147s. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Schellhorn HE, Li Y, Schellhorn HE. New developments and novel therapeutic perspectives for vitamin C. Journal of Nutrition. 2007;137:2171–84. doi: 10.1093/jn/137.10.2171. [DOI] [PubMed] [Google Scholar]
- 21.Wilson JX. Regulation of vitamin C transport. Annual Review of Nutrition. 2005;25:6.1–6.21. doi: 10.1146/annurev.nutr.25.050304.092647. [DOI] [PubMed] [Google Scholar]
- 22.Takanaga H, Mackenzie B, Hediger MA, Takanaga H, Mackenzie B, Hediger MA. Sodium-dependent ascorbic acid transporter family SLC23. Pflugers Archiv - European Journal of Physiology. 2004;447:677–82. doi: 10.1007/s00424-003-1104-1. [DOI] [PubMed] [Google Scholar]
- 23.Stratakis CA, Taymans SE, Daruwala R, Song J, Levine M. Mapping of the human genes (SLC23A2 and SLC23A1) coding for vitamin C transporters 1 and 2 (SVCT1 and SVCT2) to 5q23 and 20p12, respectively. Journal of Medical Genetics. 2000;37:E20. doi: 10.1136/jmg.37.9.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maulen NP, Henriquez EA, Kempe S, et al. Up-regulation and polarized expression of the sodium-ascorbic acid transporter SVCT1 in post-confluent differentiated CaCo-2 cells. Journal of Biological Chemistry. 2003;278:9035–41. doi: 10.1074/jbc.M205119200. [DOI] [PubMed] [Google Scholar]
- 25.Bowers-Komro DM, McCormick DB. Characterization of ascorbic acid uptake by isolated rat kidney cells. Journal of Nutrition. 1991;121:57–64. doi: 10.1093/jn/121.1.57. [DOI] [PubMed] [Google Scholar]
- 26.Lee JH, Oh CS, Mun GH, et al. Immunohistochemical localization of sodium-dependent L-ascorbic acid transporter 1 protein in rat kidney. Histochemistry & Cell Biology. 2006;126:491–4. doi: 10.1007/s00418-006-0186-1. [DOI] [PubMed] [Google Scholar]
- 27.Savini I, Rossi A, Pierro C, Avigliano L, Catani MV. SVCT1 and SVCT2: key proteins for vitamin C uptake. Amino Acids. 2008;34:347–55. doi: 10.1007/s00726-007-0555-7. [DOI] [PubMed] [Google Scholar]
- 28.Eck P, Erichsen HC, Taylor JG, et al. Comparison of the genomic structure and variation in the two human sodium-dependent vitamin C transporters, SLC23A1 and SLC23A2. Human Genetics. 2004;115:285–94. doi: 10.1007/s00439-004-1167-x. [DOI] [PubMed] [Google Scholar]
- 29.Lawlor DA, Bedford C, Taylor M, Ebrahim S. Geographical variation in cardiovascular disease, risk factors, and their control in older women: British Women’s Heart and Health Study. Journal of Epidemiology & Community Health. 2003;57:134–40. doi: 10.1136/jech.57.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawlor DA, Ebrahim S, Davey Smith G, British women’s h, health s Socioeconomic position in childhood and adulthood and insulin resistance: cross sectional survey using data from British women’s heart and health study. Bmj. 2002;325:805. doi: 10.1136/bmj.325.7368.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawlor DA, Davey Smith G, Bruckdorfer KR, Kundu D, Ebrahim S. Those confounded vitamins: What can we learn from the difference between observational versus randomised trial evidence. Lancet. 2004;363:1724–1727. doi: 10.1016/S0140-6736(04)16260-0. [DOI] [PubMed] [Google Scholar]
- 32.Davey Smith G, Lawlor DA, Harbord R, Timpson N, Day I, Ebrahim S. Clustered environments and randomized genes: a fundamental distinction between conventional and genetic epidemiology. PLoS Medicine/Public Library of Science. 2007;4:e352. doi: 10.1371/journal.pmed.0040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Day NE, Oakes S, R L, et al. EPIC-Norfolk: study design and characteristics of the cohort: European Prospective Investigation of Cancer. British Journal of Cancer. 1999;80:95–103. [PubMed] [Google Scholar]
- 34.Hart CL, MacKinnon PL, Watt GC, et al. The Midspan studies. International Journal of Epidemiology. 2005;34:28–34. doi: 10.1093/ije/dyh348. [DOI] [PubMed] [Google Scholar]
- 35.Whincup PH, Gilg JA, Owen CG, et al. British South Asians aged 13-16 years have higher fasting glucose and insulin levels than Europeans. Diabetic Medicine. 2005;22:1275–7. doi: 10.1111/j.1464-5491.2005.01587.x. [DOI] [PubMed] [Google Scholar]
- 36.Whincup PH, Owen CG, Sattar N, Cook DG. School dinners and markers of cardiovascular health and type 2 diabetes in 13-16 year olds: cross sectional study. Bmj. 2005;331:1060–1. doi: 10.1136/bmj.38618.540729.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walker M, Whincup PH, Shaper AG. The British Regional Heart Study 1975-2004. International Journal of Epidemiology. 2004;33:1185–92. doi: 10.1093/ije/dyh295. [DOI] [PubMed] [Google Scholar]
- 38.Guo SW, Thompson EA. Performing the exact test of Hardy-Weinberg proportion for multiple alleles. Biometrics. 1992;48:361–72. [PubMed] [Google Scholar]
- 39.Armitage P, Berry G. Statistical methods in medical research. Third ed Blackwell Scientific Publications; Oxford: 1994. [Google Scholar]
- 40.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davey Smith G, Ebrahim S. ‘Mendelian Randomisation’: can genetic epidemiology contribute to understanding environmental determinants of disease? International Jounal of Epidemiology. 2003;32:1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 42.Davey Smith G, Ebrahim S. Mendelian Randomisation: prospects, pitfalls and limitations. International Jounal of Epidemiology. 2003;33:30–42. doi: 10.1093/ije/dyh132. [DOI] [PubMed] [Google Scholar]
- 43.Lawlor DA, Harbord RM, Sterne JAC, Timpson NJ, Davey Smith G. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Statistics in Medicine. 2008;27:1133–1163. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- 44.Corpe CP, Tu H, Eck P, et al. Vitamin C Transporter Slc23a1 links renal reabsorption, vitamin C tissue accumulation, and perinatal survival in mice. The Journal of Clinical Investigation. 2010;120:1069–1083. doi: 10.1172/JCI39191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacDonald L, Thumser AE, Sharp P, MacDonald L, Thumser AE, Sharp P. Decreased expression of the vitamin C transporter SVCT1 by ascorbic acid in a human intestinal epithelial cell line. British Journal of Nutrition. 2002;87:97–100. doi: 10.1079/BJN2001492. [DOI] [PubMed] [Google Scholar]
- 46.Tsukaguchi H, Tokui T, Mackenzie B, et al. A family of mammalian Na+− dependent L-ascorbic acid transporters. Nature. 1999;399:70–5. doi: 10.1038/19986. [DOI] [PubMed] [Google Scholar]
- 47.Jin SN, Mun GH, Lee JH, et al. Immunohistochemical study on the distribution of sodium-dependent vitamin C transporters in the respiratory system of adult rat. Microscopy Research & Technique. 2005;68:360–7. doi: 10.1002/jemt.20255. [DOI] [PubMed] [Google Scholar]
- 48.Boyer JC, Campbell CE, Sigurdson WJ, et al. Polarized localization of vitamin C transporters, SVCT1 and SVCT2, in epithelial cells. Biochemical & Biophysical Research Communications. 2005;334:150–6. doi: 10.1016/j.bbrc.2005.06.069. [DOI] [PubMed] [Google Scholar]
- 49.Wright ME, Andreotti G, Lissowska J, et al. Genetic variation in sodium-dependent ascorbic acid transporters and risk of gastric cancer in Poland. European Journal of Cancer. 2009;45:1824–30. doi: 10.1016/j.ejca.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.