Abstract
Background and Aim
Overexpression of ecotropic viral integration site 1 (EVI1) is associated with aggressive disease in myeloid leukemia. We therefore studied its expression and function in cluster of differentiation 34 positive (CD 34+) primary human hematopoietic progenitor cells.
Materials and Methods
CD34+ cells were differentiated into various myeloid lineages using appropriate cytokines. EVI1 expression was measured by quantitative real time reverse transcriptase polymerase chain reaction (qRT-PCR) and intranuclear fluorescence activated cell sorting (FACS). Experimental manipulation of EVI1 levels was achieved using retroviral infection.
Results
EVI1 mRNA and its variant myelodysplastic syndrome 1 (MDS1)/EVI1, which gives rise to a partially antagonistic protein, were detectable in CD34+ cells, but their levels declined rapidly during differentiation into the granulocytic, monocytic, dendritic, erythroid, and megakaryocytic lineages. Similarly, EVI1 protein levels decreased during myeloid differentiation. Attempts to experimentally express EVI1 in CD34+ and U937 cells indicated that ectopic expression of EVI1 may cause growth arrest, apoptosis and/or senescence of human hematopoietic cells.
Conclusion
EVI1 is expressed in human hematopoietic progenitor cells, but is down-regulated during differentiation. Ectopic expression of EVI1 may activate cellular safeguards against oncogene activation.
Keywords: EVI1, leukemia, primary human hematopoietic cells, myeloid differentiation
Ecotropic viral integration site 1 (EVI1) is an oncogene whose overexpression is associated with aggressive disease in myeloid leukemia, as well as in certain types of solid tumor (1-3). It codes for a nuclear zinc finger protein and functions mainly as transcription factor (4, 5). Use of alternative promoters and alternative splicing lead to the formation of several different mRNA and protein variants (6). The most abundant and best characterized of these are EVI1, a 1053 amino acid protein with a well-established oncogenic function, and myelodysplastic syndrome 1 (MDS1)/EVI1, which, in addition to the entire EVI1 sequence, harbours an N-terminal extension of 188 amino acids and, in some experimental settings, acts as an antagonist of EVI1 (6). Several recent studies have shown that in the murine hematopoietic system, Evi1 is mostly expressed in stem and progenitor cells and is required for their maintenance and proliferation (7-10). Evi1−/− mice had reduced numbers of hematopoietic stem cells (HSCs) as determined by i) fluorescence activated cell sorting (FACS) for HSC-associated cell surface markers, ii) the ability of hematopoietic stem and progenitor cell fractions to form colonies in semisolid media, and iii) engraftment in competitive or non-competitive transplantation assays (7, 8). Even animals heterozygous for Evi1 displayed reduced HSC activities in similar experiments (8, 10). Conversely, experimental overexpression of Evi1 increased colony formation and replating efficiency in semisolid media and delayed the differentiation of murine hematopoietic progenitor cells (9, 10). Nevertheless, mice transplanted with hematopoietic progenitor cells that had been infected with Evi1-encoding retroviral vectors did not develop acute myeloid leukemia (AML), but rather succumbed to MDS, a disease characterized by reduced numbers of mature blood cells (11). While progenitor cells freshly infected with Evi1 retrovirus gave rise to increased numbers of colonies in semisolid media supplemented with granulocyte-macrophage colony-stimulating factor (GM-CSF), the opposite was true for hematopoietic cells isolated from moribund mice (11). Very recently, using a green fluorescent protein (GFP) marker gene knocked into the Evi1 locus, Kataoka et al. reported that the Evi1 regulatory regions were active almost exclusively in hematopoietic stem and progenitor cells (10). GFP-, and by inference Evi1-, positive cells were strongly enriched for cells with multilineage and long-term repopulation potential, suggesting that Evi1 was a highly specific and discriminative marker for murine HSCs. Furthermore, GFP-positive progenitor cells yielded higher numbers of highly proliferative colonies in single-cell culture, and more colonies in semisolid media than their GFP-negative counterparts (10). In summary, a number of reports have shown that expression of Evi1 causes increased proliferation and delayed differentiation of murine hematopoietic stem and progenitor cells. However, human cells have more elaborate mechanisms than mouse cells to protect them from the action of oncogenes. In fact according to some recent studies, EVI1 had growth-inhibitory, rather than -stimulatory, effects on several human hematopoietic cell lines (12-15). We therefore set out to study the expression and function of EVI1 in primary human cluster of differentiation 34 positive (CD34+) hematopoietic progenitor cells.
Materials and Methods
Isolation, culture, and infection of primary human CD34+ cells and of U937 cells
The human hematopoietic cell line U937 was cultured in RPMI-1640 (Life Technologies, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS) (Life Technologies) in a humidified incubator at 37°C and 5% CO2. Work with primary human CD34+ cells was carried out in accordance with the declaration of Helsinki. CD34+ cells were isolated from umbilical cord blood (CB) from consenting donors using immunomagnetic beads (StemCell Technologies, Grenoble, France). They were expanded for three days in serum-free X-vivo 15 medium (Lonza, Basel, Switzerland) containing penicillin, streptomycin, glutamax (Sigma-Aldrich, Seelze, Germany), and 50 ng/ml of each fms-related tyrosine kinase 3 ligand (FLT3-L), stem cell factor (SCF), and thrombopoietin (TPO) (Peprotech, Hamburg, Germany). On day 0, expanded cells were transferred to media containing: 20 ng/ml SCF and 100 ng/ml granulocyte colony stimulating factor (G-CSF) for granulocyte differentiation; 20 ng/ml SCF, 50 ng/ml FLT3-L, 100 ng/ml macrophage colony stimulating factor (M-CSF), and 20 ng/ml interleukin-6 (IL-6) for monocyte differentiation; 10 ng/ml SCF and 1 U/ml erythropoietin (EPO) for erythroid differentiation; and 100 ng/ml GM-CSF, 50 ng/ml FLT3-L, 20 ng/ml SCF, 2.5 ng/ml transforming growth factor ß (TGF-ß), and 0.5 ng/ml tumor necrosis factor α(TNFα) (all cytokines from Peprotech) for dendritic (Langerhans cell, LC) differentiation. For megakaryocyte differentiation, cells were expanded for 6 days in StemPro-34 media (Life Technologies) containing 1 ng/ml IL-3, 1 ng/ml SCF, and 50 ng/ml TPO (all from Miltenyi Biotec, Bergisch Gladbach, Germany), and differentiated in media supplemented with 50 ng/ml TPO. Differentiation was allowed to proceed for 7-13 days depending on lineage, and was monitored by FACS for lineage-specific cell surface markers (CD15 for granulocytes, CD14 for monocytes, CD71 for erythrocytes, CD1a for LCs, and CD41a for megakaryocytes). An EVI1 small hairpin ribonucleic acid (shRNA) (AAGGTATATTGCTGTTGACAGTGAGCGCGCACTACGTCTTCCTTAAATATAGTGAAGCCACAGATGTATATTTAAGGAAGACGTAGTGCTTGCCTACTGCCTCG) was cloned into the XhoI and EcoRI sites of the MSCV-LMP vector (Thermo Scientific Open Biosystems, Lafayette, CO, USA). A nontargeting shRNA in the same vector was used as control. The ability of the EVI1 shRNA to silence its target, determined by transient co-transfection with an EVI1 expression vector into 293T cells followed by immunoblot analysis, was >85%. A codon-optimized version of the human EVI1 cDNA (GeneArt, Regensburg, Germany) was cloned into the retroviral vector pMSCV using the BamHI and EcoRI restriction sites. Infection of CD34+ and U937 cells with empty control vector or pMSCV_EVI1 was carried out using standard procedures (16).
RNA isolation, reverse transcription, and quantitative real time reverse transcriptase polymerase chain reaction (qRT-PCR)
RNA was isolated using Trizol (Life Technologies) according to the manufacturer’s instructions, and reverse transcribed by the MMLV enzyme (Life Technologies) primed by random hexamer oligonucleotides. qRT-PCR was performed on an ABI Prism 7700 Sequence Detection System (Applied Biosystems, Carlsbad, CA, USA) using the MESA Green qPCR Mastermix Plus for SYBR Assay (Eurogentec, Seraing, Belgium) and the primers cEVI1_f (acccactcctttctttatggacc), cEVI1_r (tgatcagccagttggaattgtg), MDS1/EVI1_f (ccagcgaatctaatgtacttgagc), MDS1/EVI1_r (ccagttatggatgggagatcttagac), cyclophilin D_f (atattggaaaatgtggaagtgaaagg), and cyclophilin D_r (tcgccagagccatcttttg), detecting the sum of all EVI1 transcripts (cEVI1), MDS1/EVI1, and the housekeeping gene cyclophilin D, respectively. All measurements were performed in triplicate. A restriction fragment containing both the cEVI1 and the MDS1/EVI1 amplicons was included as calibrator in each experiment, thus allowing the expression levels of cEVI1 and MDS1/EVI1 to be directly compared to each other (17). Expression of cEVI1 and of MDS1/EVI1 relative to cyclophilin D, to the standard, and to the day 0 sample used as a calibrator were calculated according to the method of Livak and Schmittgen (18). All experiments were performed at least three times with comparable results, and means and standard deviations (SDs) of replicate measurements of one representative experiment are shown.
FACS analysis for cell surface and nuclear proteins
CD34+ cells were expanded and subjected to granulocyte or monocyte differentiation conditions as described above. Cells were harvested at the indicated time points, blocked with bovine serum albumin (BSA) and Beriglobin (CSL-Behring, King of Prussia, PA, USA), stained with fluorophor-conjugated antibodies to the cell surface markers CD14 (monocytes) or CD15 (granulocytes), and fixed and permeabilized by subsequent incubations with 2% formaldehyde and methanol. Cells were blocked, incubated with a 1:2400 dilution of EVI1 antibody C50E12 (Cell Signalling Technology, Danvers, MA, USA) or isotype control, followed by incubation with a 1:6000 dilution of Alexa Fluor 647 conjugated F(ab’)2 fragment of goat anti-rabbit IgG(H+L) (Molecular Probes, Life Technologies). FACS analysis was carried out on a LSRII instrument (Becton Dickinson, Franklin Lakes, NJ, USA), using FACSDiva software (Becton Dickinson). Data represent the mean +/− standard error of the mean (SEM) from three independent biological replicates. They are expressed as difference between the geometric means of the EVI1 and isotype control fluorescence, divided by the geometric mean of the isotype control in order to make independent biological replicates comparable to each other.
PKH26 assay to monitor cellular proliferation
CD34+ cells were infected in three consecutive cycles with the MSCV-LMP vector containing either the EVI1- or a negative control-shRNA. Twelve hours after the third cycle of infection (three days after CD34+ cell isolation), cell membranes were stained with the PKH26 Red Fluorescent Cell Linker Kit (Sigma). Three and five days later, GFP-positive cells were assayed for PKH26 staining intensity using a FACS LSRII instrument (Becton Dickinson) and FACSDiva software (Becton Dickinson).
Results
EVI1 and MDS1/EVI1 mRNA levels decline during granulocyte, monocyte, dendritic (LC), erythroid, and megakaryocyte differentiation of primary human hematopoietic progenitor cells
To determine the mRNA expression level of EVI1 and its partial antagonist MDS1/EVI1 in immature human hematopoietic progenitor cells and during differentiation into various myeloid lineages, human CB-derived CD34+ cells were subjected to conditions favouring differentiation into the granulocyte, monocyte, dendritic (Langerhans cell, LC), erythroid, or megakaryocyte lineages. Differentiation was monitored by FACS for lineage-specific cell surface markers (see Methods). Cells were harvested at different time points and qRT-PCR with primers detecting either the sum of all EVI1 transcripts (cEVI1) or the MDS1/EVI1 mRNA only was performed. To allow comparison of cEVI1 and MDS1/EVI1 levels, a restriction fragment containing both amplicons was included as an external reference in each experiment. Both cEVI1 and MDS1/EVI1 were expressed in undifferentiated CD34+ cells, but their levels declined rapidly in conditions promoting differentiation into any one of the aforementioned lineages (Figure 1).
Figure 1.
Ecotropic viral integration site 1 (EVI1) and myelodysplastic syndrome 1 (MDS1)/EVI1 mRNA levels decline rapidly during differentiation of primary human hematopoetic progenitor cells into various myeloid lineages. Cluster of differentiation 34 positive (CD34+) cells enriched from human cord blood (CB) were expanded for 6 (megakaryocytes) or 3 days (all other lineages) and on day 0 transferred to media promoting differentiation into the granulocyte (A), monocyte (B), dendritic (C), erythroid (D), or megakaryocyte (E) lineage. Cells were harvested at the indicated time points, RNA was extracted, and quantitative real time reverse transcriptase polymerase chain reaction (qRT-PCR) performed using primers against a region common to all EVI1 mRNA variants (cEVI1), or specifically recognizing the MDS1/EVI1 transcript. The housekeeping gene cyclophilin D was used for normalization. A restriction fragment containing both the cEVI1 and the MDS1/EVI1 amplicon was included in each qRT-PCR run to allow for direct comparison of the levels of cEVI1 and MDS1/EVI1. Each experiment was performed three times and one representative experiment is shown. Error bars represent standard deviations (SDs) from replicate measurements.
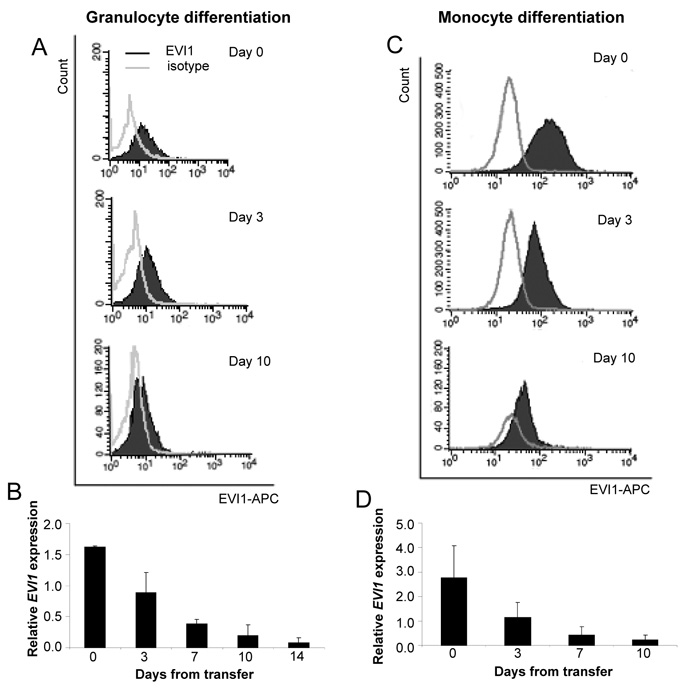
EVI1 protein levels decline during myeloid differentiation of CD34+ cells
To corroborate the mRNA expression data at the protein level, CD34+ cells were subjected to granulocyte or monocyte differentiation conditions, harvested at different time points, and stained for the lineage-specific cell surface markers CD15 (granulocytes) or CD14 (monocytes), as well as for EVI1. In agreement with the mRNA data, EVI1 protein levels declined rapidly during differentiation into both investigated lineages (Figure 2). Interestingly, this decline was not restricted to CD15+ or CD14+ cells, but also occurred in marker-negative cells (data not shown). This suggests that EVI1 is down-regulated rapidly during myeloid differentiation, even before cells acquire lineage-specific markers.
Figure 2.
Ecotropic viral integration site 1 (EVI1) protein levels decline during granulocyte and monocyte differentiation of cluster of differentiation 34 positive (CD34+) cells. CD34+ cells enriched from cord blood (CB) were expanded for 3 days and transferred to media promoting differentiation into the granulocyte (A, B) or monocyte (C, D) lineage on day 0. Cells were harvested at the indicated time points thereafter, stained for nuclear EVI1, and subjected to fluorescence activated cell sorting (FACS) analysis. A, C: Representative FACS plots. B, D: Geometric means of EVI1 expression relative to the isotype control. Data are the mean ± standard error of the mean of three biological replicates.
Functional characterization of EVI1 in primary human hematopoietic progenitor cells and in a human myeloid cell line
Next, we attempted to investigate the function of EVI1 in primary human hematopoietic cells. CD34+ cells were infected with a retroviral vector bearing an EVI1-specific shRNA, which reduced the levels of exogenously expressed EVI1 protein in 293T cells by >85%. Cells were then stained with the membrane dye PKH26, and subjected to FACS analysis. Knockdown of EVI1 altered cellular proliferation only marginally and in a statistically non-significant manner compared to control shRNA-infected cells (Figure 3).
Figure 3.

Knockdown of ecotropic viral integration site 1 (EVI1) does not affect proliferation of cluster of differentiation 34 positive (CD34+) cells. CD34+ cells were infected with lentiviral vectors containing a short hairpin ribonucleic acid (shRNA) against EVI1 (knock-down efficiency >85% as determined in 293T cells) or a scrambled control shRNA, stained with the membrane dye PKH26, and assayed by fluorescence activated cell sorting (FACS) on days 3 and 5 thereafter. Mean PKH26 fluorescence intensities were normalized to those of control shRNA-infected cells, considering only the green fluorescent protein (GFP)-positive populations in both cases. Data are the mean ± standard error of the mean from three biological replicates. Differences between EVI1- and control-shRNA-infected cells were not statistically significant (day 3, p=0.29; day 5, p=0.26; Student’s t-test).
This may indicate that, in contrast to murine cells, endogenous EVI1 does not affect the proliferation of human hematopoietic progenitor cells. Alternatively, due to the fact that EVI1 expression declined rapidly during CD34+ cell culture (which leads to cellular maturation even in the absence of lineage-specific cytokines), its experimental down-regulation may not have an additional effect. To determine whether experimental overexpression of EVI1 would affect the biological behavior of human hematopoietic progenitor cells, a retroviral vector containing a codon-optimized version of the human EVI1 cDNA (pMSCV_co-hEVI1) was constructed. When infected into NIH3T3 cells, pMSCV_co-hEVI1 yielded about half as many GFP-positive cells as the empty pMSCV vector, indicating that its viral titer was lower than but in a comparable range to that of the parental vector. Nevertheless, in CD34+ cells, in repeated experiments and even when parallel infections with empty vector yielded 90% GFP-positive cells, infection rates with pMSCV_co-hEVI1 were <10% and often even <1%. Similar observations were made with a different retroviral vector, pBMN, containing a non-codon-optimized version of the hEVI1 cDNA. These results may indicate that primary human hematopoietic cells ectopically expressing EVI1 proliferate more slowly, senesce, and/or die, and therefore are overgrown by non-infected cells, or lost, between infection and FACS analysis (routinely performed three days later). To further investigate the consequences of EVI1 overexpression in an experimental system that is not limited by the numbers of available cells, the human myeloid cell line U937 was infected with pMSCV_co-mEVI1 or empty vector as a control, and the percentage of GFP-positive cells was monitored by FACS at different time points thereafter. With the EVI1 expression vector, the percentage of GFP positive cells peaked at 23% two days after the last infection cycle (probably reflecting the time point when full expression from the vector had been achieved) and declined continuously to 3% on day 15. In contrast, GFP positivity of empty vector infected cells reached a plateau near 100% on day 3 and remained that high until the end of the experiment (Figure 4).Furthermore, when pMSCV_co-hEVI1 was forcibly introduced into U937 cells via an ectopically expressed murine ecotropic receptor (EcoR), an inverse relation between GFP positivity and cell survival was observed. Infection efficiencies as high as 80% could be achieved, but viability as determined by the FACS forward and side scatters was only ~10% under these conditions (data not shown). At infection efficiencies of ~35%, viability was 79% for MSCV, but only 37% for pMSCV_co-hEVI1 infected cells (Figure 5).
Figure 4.

Ecotropic viral integration site 1 (EVI1)-positive cells are gradually lost after infection of U937 cells with pMSCV_codon-optimized-murine-ecotropic-virus-integration-site 1 (pMSCV_co-mEVI1). U937 cells were infected with pMSCV_co-mEVI1 or pMSCV as control, and the percentage of green fluorescent protein (GFP)-positive cells was determined by fluorescence activated cell sorting (FACS) at the indicated times after the last infection cycle. Diamonds, pMSCV infected cells; squares, pMSCV_co-mEVI1 infected cells.
Figure 5.

Efficient introduction of pMSCV_codon-optimized-human-ecotropic-virus-integration-site 1 (pMSCV_co-hEVI1) into U937 cells leads to increased rates of cell death. U937 cells ectopically expressing murine ecotropic receptor (EcoR) were infected with pMSCV_co-hEVI1 or pMSCV as a control, and subjected to fluorescence activated cell sorting (FACS) analysis. Conditions were chosen so that infection efficiencies were ~35% for both vectors.
Discussion
In this report, we show that both the EVI1 and MDS1/EV1 mRNAs are expressed in immature primary human hematopoietic progenitor cells, and are down-regulated during the differentiation of these cells into various myeloid lineages. Flow cytometry, a novel technique for the quantification of nuclear proteins, confirmed that this was also true at the protein level. These findings are consistent with those reported for primary murine hematopoietic cells (10). They also agree with previous data showing a reduction in EVI1 transcript levels during erythroid differentiation of CD36+ erythroid progenitors from human CB (19). For the megakaryocyte lineage, Shimizu et al. showed that EVI1 was expressed at much lower levels in human bone-marrow derived megakaryocytes than in CD34+ cells from the same source (while it was undetectable in mature monocytes, T-cells, and B-cells from peripheral blood) (12). In contrast, Terui et al. reported induction of EVI1 during in vitro megakaryocyte differentiation of CD34+ cells, and, contradictory to several other reports, no EVI1 expression in undifferentiated CD34+ cells (20). The reason for the discrepancy between their and our data is presently unclear, but could be related to different culture conditions used. In summary, while some previous studies have investigated the expression levels of EVI1 in hematopoietic cells, the present report is as far as we know the first to comprehensively analyze the expression of EVI1 and its partial antagonist MDS1/EVI1 in primary human hematopoietic progenitor cells during differentiation into all major myeloid lineages, and to confirm these data at the protein level. Several groups have reported that Evi1 stimulated the proliferation of primary murine hematopoietic progenitor cells (8-10, 21). Recently, however, growth-inhibitory activities of EVI1 have been demonstrated in human hematopoietic cell lines (12-15), as well as in a human osteosarcoma cell line (22). Similarly, both anti- and proapoptotic activities of EVI1 have been described (13, 23, 24). In the present experiments, a retroviral vector expressing the EVI1 cDNA, despite being capable of efficient infection of NIH3T3 cells, yielded very low percentages of GFP-positive CD34+ cells. This precluded assays directly measuring cellular proliferation, apoptosis, or senescence. We nevertheless hypothesize that the low numbers of GFP-, and by inference EVI1-, positive CD34+ cells indicate that one or several of these processes are affected by EVI1 overexpression in primary human CD34+ cells, leading to a heavy growth disadvantage or loss of successfully infected cells in the three days between the last infection cycle and FACS analysis. This interpretation was supported by experiments with U937 cells: Firstly, introduction of an EVI1-expressing retroviral vector via the very efficient ecotropic virus receptor led to substantial cell death. This was not observed with the control vector which expressed only GFP, indicating that loss of viability was not a nonspecific effect of a high level of expression of an exogenous protein. Secondly, in a time course experiment, cells infected with the EVI1 expression vector (via endogenous virus receptors) reached a peak of GFP positivity soon after the infection and lost marker expression steadily thereafter. In contrast, empty vector-infected cells maintained the percentage of GFP positivity reached after 3 days for at least two weeks. Taken together, our results suggest that primary human hematopoietic progenitor cells, and even transformed human cell lines, may activate cellular defense mechanisms counteracting the oncogenic activity of EVI1, similar to what has been observed for other tumor-promoting genes (25, 26).
Acknowledgements
This work was funded by the Austrian Science Foundation (FWF) grant P19795 to RW.
References
- 1.Nanjundan M, Nakayama Y, Cheng K, Lahad J, Liu J, Lu K, Kuo W, Smith-McCune K, Fishman D, Gray J, Mills G. Amplification of MDS1/EVI1 and EVI1, located in the 3q26.2 amplicon, is associated with favorable patient prognosis in ovarian cancer. Cancer Res. 2007;67:3074–3084. doi: 10.1158/0008-5472.CAN-06-2366. [DOI] [PubMed] [Google Scholar]
- 2.Haas K, Kundi M, Sperr W, Esterbauer H, Ludwig W, Ratei R, Koller E, Gruener H, Sauerland C, Fonatsch C, Valent P, Wieser R. Expression and prognostic significance of different mRNA 5′-end variants of the oncogene EVI1 in 266 patients with de novo AML: EVI1 and MDS1/EVI1 overexpression both predict short remission duration. Genes Chromosomes Cancer. 2008;47:288–298. doi: 10.1002/gcc.20532. [DOI] [PubMed] [Google Scholar]
- 3.Lugthart S, van Drunen E, van Norden Y, van Hoven A, Erpelinck C, Valk P, Beverloo H, Lowenberg B, Delwel R. High EVI1 levels predict adverse outcome in acute myeloid leukemia: Prevalence of EVI1 overexpression and chromosome 3q26 abnormalities underestimated. Blood. 2008;111:4329–4337. doi: 10.1182/blood-2007-10-119230. [DOI] [PubMed] [Google Scholar]
- 4.Yatsula B, Lin S, Read A, Poholek A, Yates K, Yue D, Hui P, Perkins A. Identification of binding sites of EVI1 in mammalian cells. J Biol Chem. 2005;280:30712–30722. doi: 10.1074/jbc.M504293200. [DOI] [PubMed] [Google Scholar]
- 5.Bard-Chapeau E, Jeyakani J, Kok C, Muller J, Chua B, Gunaratne J, Batagov A, Jenjaroenpun P, Kuznetsov V, Wei C, D’Andrea R, Bourque G, Jenkins N, Copeland N. Ecotopic viral integration site 1 (EVI1) regulates multiple cellular processes important for cancer and is a synergistic partner for FOS protein in invasive tumors. Proc Natl Acad Sci USA. 2012;109:2168–2173. doi: 10.1073/pnas.1119229109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wieser R. The oncogene and developmental regulator EVI1: Expression, biochemical properties, and biological functions. Gene. 2007;396:346–357. doi: 10.1016/j.gene.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Yuasa H, Oike Y, Iwama A, Nishikata I, Sugiyama D, Perkins A, Mucenski M, Suda T, Morishita K. Oncogenic transcription factor Evi1 regulates hematopoietic stem cell proliferation through GATA-2 expression. EMBO J. 2005;24:1976–1987. doi: 10.1038/sj.emboj.7600679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goyama S, Yamamoto G, Shimabe M, Sato T, Ichikawa M, Ogawa S, Chiba S, Kurokawa M. Evi-1 is a critical regulator for hematopoietic stem cells and transformed leukemic cells. Cell Stem Cell. 2008;3:207–220. doi: 10.1016/j.stem.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Laricchia-Robbio L, Nucifora G. Significant increase of self-renewal in hematopoietic cells after forced expression of EVI1. Blood Cells Mol Dis. 2008;40:141–147. doi: 10.1016/j.bcmd.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kataoka K, Sato T, Yoshimi A, Goyama S, Tsuruta T, Kobayashi H, Shimabe M, Arai S, Nakagawa M, Imai Y, Kumano K, Kumagai K, Kubota N, Kadowaki T, Kurokawa M. Evi1 is essential for hematopoietic stem cell self-renewal, and its expression marks hematopoietic cells with long-term multilineage repopulating activity. J Exp Med. 2011;208:2403–2416. doi: 10.1084/jem.20110447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buonamici S, Li D, Chi Y, Zhao R, Wang X, Brace L, Ni H, Saunthararajah Y, Nucifora G. Evi1 induces myelodysplastic syndrome in mice. J Clin Invest. 2004;114:713–719. doi: 10.1172/JCI21716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimizu S, Nagasawa T, Katoh O, Komatsu N, Yokota J, Morishita K. EVI1 is expressed in megakaryocyte cell lineage and enforced expression of EVI1 in UT-7/GM cells induces megakaryocyte differentiation. Biochem Biophys Res Commun. 2002;292:609–616. doi: 10.1006/bbrc.2002.6693. [DOI] [PubMed] [Google Scholar]
- 13.Konrad T, Karger A, Hackl H, Schwarzinger I, Herbacek I, Wieser R. Inducible expression of EVI1 in human myeloid cells causes phenotypes consistent with its role in myelodysplastic syndromes. J Leukoc Biol. 2009;86:813–822. doi: 10.1189/jlb.0109042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ichihara E, Kaneda K, Saito Y, Yamakawa N, Morishita K. Angiopoietin1 contributes to the maintenance of cell quiescence in EVI1(high) leukemia cells. Biochem Biophys Res Commun. 2011;416:239–245. doi: 10.1016/j.bbrc.2011.10.061. [DOI] [PubMed] [Google Scholar]
- 15.Yamakawa N, Kaneda K, Saito Y, Ichihara E, Morishita K. The increased expression of integrin alpha 6 (ITGA6) enhances drug resistance in EVI1(high) leukemia. PLoS One. 2012;7:e30706. doi: 10.1371/journal.pone.0030706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taschner S, Koesters C, Platzer B, Jorgl A, Ellmeier W, Benesch T, Strobl H. Down-regulation of RXRalpha expression is essential for neutrophil development from granulocyte/monocyte progenitors. Blood. 2007;109:971–979. doi: 10.1182/blood-2006-04-020552. [DOI] [PubMed] [Google Scholar]
- 17.Vinatzer U, Mannhalter C, Mitterbauer M, Gruener H, Greinix H, Schmidt H, Fonatsch C, Wieser R. Quantitative comparison of the expression of EVI1 and its presumptive antagonist, MDS1/EVI1, in patients with myeloid leukemia. Genes Chromosomes Cancer. 2003;36:80–89. doi: 10.1002/gcc.10144. [DOI] [PubMed] [Google Scholar]
- 18.Livak K, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Scicchitano M, McFarland D, Tierney L, Narayanan P, Schwartz L. In vitro expansion of human cord blood CD36(+) erythroid progenitors: Temporal changes in gene and protein expression. Exp Hematol. 2003;31:760–769. doi: 10.1016/s0301-472x(03)00185-1. [DOI] [PubMed] [Google Scholar]
- 20.Terui K, Takahashi Y, Kitazawa J, Toki T, Yokoyama M, Ito E. Expression of transcription factors during megakaryocytic differentiation of CD34+ cells from human cord blood induced by thrombopoietin. Tohoku J Exp Med. 2000;192:259–273. doi: 10.1620/tjem.192.259. [DOI] [PubMed] [Google Scholar]
- 21.Dickstein J, Senyuk V, Premanand K, Laricchia-Robbio L, Xu P, Cattaneo F, Fazzina R, Nucifora G. Methylation and silencing of miRNA-124 by EVI1 and self-renewal exhaustion of hematopoietic stem cells in murine myelodysplastic syndrome. Proc Natl Acad Sci USA. 2010;107:9783–9788. doi: 10.1073/pnas.1004297107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karakaya K, Herbst F, Ball C, Glimm H, Kramer A, Loffler H. Overexpression of EVI1 interferes with cytokinesis and leads to accumulation of cells with supernumerary centrosomes in G0/1 phase. Cell Cycle. 2012;11:3492–3503. doi: 10.4161/cc.21801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kreider B, Orkin S, Ihle J. Loss of erythropoietin responsiveness in erythroid progenitors due to expression of the Evi-1 myeloid-transforming gene. Proc Natl Acad Sci USA. 1993;90:6454–6458. doi: 10.1073/pnas.90.14.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurokawa M, Mitani K, Yamagata T, Takahashi T, Izutsu K, Ogawa S, Moriguchi T, Nishida E, Yazaki Y, Hirai H. The Evi-1 oncoprotein inhibits c-Jun N-terminal kinase and prevents stress-induced cell death. EMBO J. 2000;19:2958–2968. doi: 10.1093/emboj/19.12.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blagosklonny M. A node between proliferation, apoptosis, and growth arrest. Bioessays. 1999;21:704–709. doi: 10.1002/(SICI)1521-1878(199908)21:8<704::AID-BIES10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 26.Sebastian T, Johnson P. Stop and go: Antiproliferative and mitogenic functions of the transcription factor C/EBPbeta. Cell Cycle. 2006;5:953–957. doi: 10.4161/cc.5.9.2733. [DOI] [PubMed] [Google Scholar]