Abstract
Rationale
Calcium entry through Orai1 channels drives vascular smooth muscle cell migration and neointimal hyperplasia. The channels are activated by the important growth factor platelet-derived growth factor (PDGF). Channel activation is suggested to depend on store-depletion which redistributes and clusters stromal interaction molecule 1 (STIM1) which then co-clusters and activates Orai1.
Objective
To determine the relevance of STIM1 and Orai1 redistribution in PDGF responses.
Methods and Results
Vascular smooth muscle cells were cultured from human saphenous vein. STIM1 and Orai1 were tagged with green and red fluorescent proteins to track them in live cells. Under basal conditions the proteins were mobile but mostly independent of each other. Inhibition of sarco-endoplasmic reticulum calcium ATPase led to store-depletion and dramatic redistribution of STIM1 and Orai1 into co-clusters. PDGF did not evoke redistribution even though it caused calcium-release and Orai1-mediated calcium entry in the same time period. After chemical blockade of Orai1-mediated calcium entry, however, PDGF caused redistribution. Similarly, mutagenic disruption of calcium flux through Orai1 caused PDGF to evoke redistribution, showing that calcium flux through the wild-type channels had been filling the stores. Acidification of the extracellular medium to pH 6.4 caused inhibition of Orai1-mediated calcium entry and conferred capability for PDGF to evoke complete redistribution and co-clustering.
Conclusions
The data suggest that PDGF has a non-clustering mechanism by which to activate Orai1 channels and maintain calcium stores replete. Redistribution and clustering become important, however, when the ER stress signal of store-depletion arises, for example when acidosis inhibits Orai1 channels.
Keywords: calcium channel, calcium stores, vascular smooth muscle cells, growth factor, acidosis
Introduction
In its contractile phenotype the vascular smooth muscle cell (VSMC) in the medial layer of arteries and veins is important for vascular integrity and tone. During development, however, the VSMCs can also exist in a non-contracting phenotype that has proliferating and migrating properties, which are important for the generation of new blood vessels1. Features of these modulated VSMCs are altered Ca2+ entry and Ca2+ uptake mechanisms2, 3. In the adult, VSMCs may also switch to this state to enable vascular remodeling. One of the drivers of the proliferating phenotype is platelet-derived growth factor BB (PDGF), a pro-migratory signaling peptide4. In addition to its roles in physiology, PDGF contributes in cardiovascular diseases that include pulmonary arterial hypertension and restenosis5, 6.
The signaling pathways downstream of PDGF were only recently recognized to include activation of a calcium entry channel generated by Orai1 proteins and involving the calcium-sensing regulatory protein STIM13, 7, 8. Orai1 was found to be a positive modulator of VSMC migration8, with knock-down of its expression suppressing neointimal formation after vascular injury9. The mechanism by which PDGF activates Orai1 channels has not been extensively studied but the effect is suggested to occur via PDGFRβ and the down-stream pathways of phospholipase C and Ca2+-release7. Orai1 channels are activated by store-depletion via a mechanism that involves sensing of luminal Ca2+ in the stores by STIM1 and then physical interaction of STIM1 with Orai1 channels in the plasma membrane. It has, therefore, been assumed that PDGF-evoked Ca2+-release leads to store-depletion, which is sensed by STIM1 which then activates Orai1.
Orai1 was originally identified in the immune system10, 11. It is suggested that Orai1 provides the molecular basis for the ion pore-forming subunits of Ca2+-selective channels that are activated by store-depletion and have commonly been referred to as CRAC channels10. The mechanism proposed for activation of the channels by store-depletion is striking10, 12. Activation ultimately involves physical interaction between STIM1 (of the stores) and Orai1 (of the plasma membrane) but, first, there are major cellular redistributions of STIM1 and Orai1. Prior to store-depletion, STIM1 is dynamically active as a partner of the microtubule binding protein EB113. The STIM1 has an N-terminus that is localized to the store lumen and contains an EF-hand with affinity for Ca2+. When the stores are full, STIM1 is bound to luminal Ca2+. However, depletion of Ca2+ in the stores leads to Ca2+ dissociation from STIM1 and a chain of events that ends with major redistribution of STIM1 from microtubules to static oligomerized focal clusters (puncta) under the plasma membrane. Simultaneous studies of clustering and Ca2+ influx have suggested that STIM1 translocation precedes Orai1 channel opening.
Each Orai1 protein is thought to contain four transmembrane segments with intracellular N- and C-termini10. Channels are considered to arise from four Orai1 proteins assembled around a central Ca2+-selective pore. Prior to store-depletion, the Orai1 channels are reasonably uniformly distributed across the cell surface. However, store-depletion leads to Orai1 recruitment to sites of STIM1 clusters, such that the Orai1 and STIM1 become aggregated10, 14. An interaction domain in the cytosolic STIM1 C-terminus (the CAD or SOAR domain) then directly binds Orai1 to activate the channels and enable Ca2+ influx12. Therefore, Orai1 activation is considered to require not only Ca2+-sensing by STIM1 but extensive redistribution and co-clustering.
The purpose of this investigation was to determine the relevance of the redistribution and clustering of Orai1 and STIM1 in the PDGF responsiveness of proliferating VSMCs. In order to achieve this objective we used fluorescently tagged Orai1 and STIM1 so that sub cellular dynamics of these proteins could be specifically tracked in real-time in living VSMCs.
Methods
Cell preparation and culture
Freshly discarded human saphenous vein segments were obtained anonymously and with informed consent from patients undergoing open heart surgery. Approval was granted by the Leeds Teaching Hospitals Local Research Ethics Committee. Proliferating VSMCs were prepared using an explant technique15 and grown in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10 % fetal calf serum, penicillin/streptomycin and L-glutamine at 37 oC in a 5 % CO2 incubator. Experiments were performed on VSMCs passaged 3-5 times; all of the cells stained positively for smooth muscle α-actin and smooth muscle myosin heavy chain and were non-contractile16.
Molecular biology
cDNA encoding wild type mCherry-tagged Orai1 was from S. Muallem and cDNA encoding eYFP-STIM1 was from T. Meyer. mCherry-Orai1 R91W mutagenesis was performed using mutagenic primers and Phusion high fidelity polymerase (NEB) according to the manufacturer’s instructions. Full-length cDNA for Orai1 (BC013386) was purchased from Geneservice (Source Bioscience LifeSciences, UK) and sub cloned into pcDNA6/V5-His (Invitrogen, Paisley, UK) at EcoRI/XhoI sites. The hemagglutinin (HA) tag (YPYDVPDYA) was inserted into the S3-S4 linker between Lys214 and Ala220 using a PCR fusion protocol. The first PCR fragment was used to linearize Orai1 in pcDNA6 from Ala220 to Lys214 and insert a 3′ Gly, Ser linker (forward primer: 5′ gccagtggcgcagcagcc; reverse primer 5′ gcttccgcttcccttgctggtgggcct). The second PCR product amplified full length HA with sequences overlapping the first PCR fragment (forward primer: 5′ caagggaagcggaagctatccatatgat; reverse primer: 5′ tgcgccactggcagcataatctggaac). The fragments were recombined using the BD In-fusion™ PCR cloning kit (BD Biosciences) as per the manufacturer’s instructions. A construct encoding eGFP fused to the C-terminus of Orai1-HA was prepared through PCR cloning as described above. Clones were sequenced to confirm accuracy and identity.
Cell transfection, live cell imaging, and image analysis
0.5-2 ×106 VSMCs were centrifuged (100×g) for 5 min, resuspended in Basic Nucleofector solution (Amaxa GmbH, Germany), mixed with 1.5 μg eYFP-STIM1 and 0.5 μg mCherry-Orai1 and transferred into a cuvette for electroporation (Amaxa). Cells were transferred from cuvettes to pre-warmed culture medium and incubated in a 5 % CO2 incubator at 37 °C. Excess STIM1 relative to Orai1 was previously suggested to be important for channel function. In pilot studies we observed that a STIM1:Orai1 ratio of 3:1 was optimum for observing thapsigargin-evoked clustering in VSMCs.
VSMCs, 24-48 hr post-transfection, were detached and transferred to 35 mm glass-bottomed dishes with fresh culture medium and allowed to spread for 24 hr. Cells were serum-starved for 2-5 hr prior to imaging. During imaging, the extracellular solution contained (mmole/L): 130 NaCl, 5 KCl, 8 D-glucose, 10 HEPES, 1.5 CaCl2 and 1.2 MgCl2, titrated to pH 7.4 or 6.4 with NaOH. In some cells, eYFP-STIM1 fluorescence appeared as intense and large static patches; in these cases the ER was judged to be perturbed and the cells were not used for investigation. We imaged VSMCs expressing low to moderate levels of STIM1 and Orai1 in an effort to mimic the physiological expression. After baseline image acquisition in 120 μL of extracellular solution, 120 μL of the same solution containing twice the final concentration of agent (e.g. PDGF) was applied, minimizing mechanical disturbance. VSMCs were visualized on an Olympus IX-70 inverted microscope using a ×100 UPLAN objective (NA 1.35) supported by a DeltaVision deconvolution system (Applied Precision LLC) with SoftWoRx image acquisition and analysis software. Images were captured on a Roper CoolSNAP HQ CCD camera at 0.5 s exposure every 10 s and epifluorescence was recorded using filter sets for FITC/TRITC. ImageJ was used after acquisition to process and prepare micrographs. To compensate fluorophore bleaching, images were enhanced by a normalization feature so the pixel range, at 0.4 % maximum saturation, was equal to the maximum range for each frame. Recordings were made at room temperature (21±2 °C) unless otherwise stated.
Only VSMCs containing eYFP-STIM1 and mCherry-Orai1 that clustered in response to TG or PDGF were included. Quantification of clustering used Image J particle analysis point picker in a region of interest of each cell at the optimal focal point. Positive identification was a spot of intense mCherry fluorescence with 0.4-3.0 μm lateral diameter. Such spots included all focal intensities of this size and so clusters were recognized as new spots arising in response to PDGF or TG. In the figures, this can be seen as the difference between the basal number of focal intensities (Pre) and the number after exposure to PDGF or TG. Clustering as determined by mCherry analysis was confirmed qualitatively as co-clustering by visual inspection of the eYFP fluorescence over-lay on mCherry clusters (see Figures and Supplemental Material). The data were multilevel, with repeated exposures per cell, which required statistical methods that account for this. Modeling the combined dataset indicated that data followed a Poisson distribution and so a multilevel Poisson model was used. Plots compare freely-derived and hypothesis-driven estimated mean counts with associated 95 % confidence intervals. The hypothesis tested was that PDGF does not elevate wild type Orai1 spots at pH 7.4 at 21 °C, whereas PDGF elevates spots for Orai1 mutants at pH 7.4 at 21 °C and wild type Orai1 at pH 6.4 at 21 °C, though not for the wild type Orai1 at pH 7.4 at 37 °C. Further details and discussion of the statistical methods are provided in the Supplemental Material.
Intracellular Ca2+ measurement
VSMCs were incubated with 2 μmole/L fura-2AM for 1 hr at 37 °C followed by a 0.5 hr wash at room temperature. Measurements were made at room temperature on a 96-well plate reader (FlexStation, Molecular Devices). The change (Δ) in intracellular calcium (Ca2+i) concentration was indicated as the ratio of fura-2 emission intensities for 340 nm and 380 nm excitation (F ratio). The recording solution contained (mmole/L): 130 NaCl, 5 KCl, 8 D-glucose, 10 HEPES, and 1.2 MgCl2, titrated to pH 7.4 with NaOH, with 0.3 mmole/L CaCl2 or without (Ca2+-free). Recordings were made at room temperature. Data are presented as mean±s.e.mean where n is the number of independent experiments and N the number of individual wells used in the 96-well plates.
Immunostaining
VSMCs, 48 hr post-transfection, were detached and transferred to glass cover slips with fresh culture medium and allowed to spread for 24 hr. After treatments, the cells were fixed in 2 % paraformaldehyde for 5 min and, if stated, permeabilised in 0.1% TritonX-100 for 10 min at room temperature. Transfected cells were either incubated with anti-HA 1:500 (Covance) or anti-GFP (Abcam) as stated and for 1 hr at room temperature, then washed and incubated with the appropriate secondary Dylight 594 IgG (Stratech Scientific, Jacksons Immuno Research). All cells were mounted onto glass slides using ProLong Gold anti-fade (Molecular Probes, Invitrogen, Paisley, UK).
Reagents
Synta 668 was synthesized by Dr R Foster (Leeds). Other reagents were from Sigma unless indicated otherwise.
Results
Constitutive subcellular localisation and dynamics of Orai1 and STIM1
The VSMCs adhered to the substrate as thin structures such that there was difficulty distinguishing plasma membrane from intracellular membranes and other structures. To investigate Orai1 that spanned the plasma membrane we generated human Orai1 containing extracellular HA epitope tag and intracellular GFP ([HA]-Orai1-GFP). Non-permeabilised cells were exposed to anti-HA antibody to label only surface-exposed Orai1. The [HA]-Orai1-GFP was evident throughout VSMCs but there was thin, more intense, staining along the cell perimeter (Figure 1A). Anti-GFP antibody failed to label these cells because they were non-permeabilised (Figure 1B). After cell permeabilisation, anti-HA staining was similar but vesicular structures were now also evident (Figure 1C). Anti-GFP antibody, which was also detected by red secondary antibody, gave a similar staining pattern to anti-HA antibody (Figure 1D). The data suggest that Orai1 localised to the plasma membrane. This Orai1 was evident at the cell perimeter and across the surface of these flat cells. Visualization of plasma membrane Orai1 dynamics was therefore possible across the entire VSMC. The data suggest that the tags did not prevent surface localisation of Orai1 and that Orai1 existed in intracellular vesicles as well as the plasma membrane.
Figure 1. Constitutive localization and dynamics of Orai1 and STIM1.
(A-D) [HA]-Orai1-GFP in fixed VSMCs stained with anti-HA (A, C) or anti-GFP antibody (B, D). VSMCs were non-permeabilised (A, B) or permeabilised (C, D). Arrows point to the cell perimeter (A) or intracellular vesicles (C, D). The scale bar in B is 20 μm and applies to (A-D). (E, F) Live VSMC imaging. (E) mCherry-Orai1. (i) Intracellular vesicles. (ii) Ruffles with expanded views depicting the backwards ebb at 0, 60 and 120 s. (iii) Example spiny protrusion. (F) mCherry-Orai1 (red) and eYFP-STIM1 (green). Arrows point to a ruffle and spiny protrusion that contained mCherry-Orai1 but not eYFP-STIM1. The expanded views for areas outlined by white boxes show: (left) a large protrusion where mCherry-Orai1 and eYFP-STIM1 were co-localized (yellow); and (right) eYFP-STIM1 in comet-like structures and ER network. The scale bar in E is 10 μm and applies to (E, F).
For simultaneous live-cell imaging of Orai1 and STIM1 dynamics we used human Orai1 tagged with mCherry to visualise it as a red protein and human STIM1 tagged with eYFP to visual it as a green protein (Figure 1E-F). Care was taken to use VSMCs expressing the minimum levels of STIM1 and Orai1 required for detection, avoiding over-expressing cells. The appearance of mCherry-Orai1 was similar to that of [HA]-Orai1-GFP labeled by anti-HA antibody in permeabilised cells (Figure 1E cf Figure 1C). There was Orai1 throughout the cells but areas of greater intensity were observed in dynamic structures: intracellular vesicles of 0.4 to 1 μm diameter; plasma membrane ruffles; and plasma membrane spiny protrusions (Figure 1E). Membrane ruffles, which are actin-rich waves, rippled away from the cell edges (Online Video I). Spiny protrusions emanated from the ruffles, appearing and disappearing (Figure 1E arrow head, Online Video I).
eYFP on STIM1 restricts STIM1 from the plasma membrane17. Therefore, eYFP tagged STIM1 does not address plasma membrane STIM118, only STIM1 localized to the ER or other intracellular structures. In VSMCs, the eYFP-STIM1 mostly had localization that was distinct from mCherry-Orai1 (Figure 1F). Particularly obvious was separation of the proteins in peripheral regions, which contained mCherry-Orai1 but not eYFP-STIM1 (Figure 1F). There were, nevertheless, instances when mCherry-Orai1 and eYFP-STIM1 were together at newly generated protrusions (Figure 1F) but they did not co-localize in ruffles or spiny protrusions (Figure 1F). eYFP-STIM1 was localized predominantly to the lacy network of the ER (Figure 1F). As expected because of EB1 binding, eYFP-STIM1 displayed comet-like movements (Figure 1F; Online Video II).
The data suggest that under non-stimulated conditions Orai1 and STIM1 were: localized as expected; constitutively dynamic; and operating largely independently.
Redistribution and co-clustering of Orai1 and STIM1 in response to thapsigargin
It is expected that Orai1 and STIM1 redistribute and co-cluster in response to passive store depletion evoked by thapsigargin, an inhibitor of SERCA. Consistent with this expectation, VSMCs exposed to thapsigargin showed redistribution of eYFP-STIM1 into clusters (Figure 2A, B). Similarly mCherry-Orai1 redistributed into clusters that were distinct from the Orai1-containing intracellular vesicles (Figure 2C, D). eYFP-STIM1 and mCherry-Orai1 co-localised once the clusters formed (Figure 2E, F; Online Video III). The co-clusters were static after they had formed and were evident except in regions where eYFP-STIM1 had not been initially expressed (Figure 2E, F; Online Video III). The data suggest that Orai1 and STIM1 dynamically redistributed and co-clustered in VSMCs, as reported for other cell types exposed to thapsigargin.
Figure 2. Thapsigargin-evoked clustering of Orai1 and STIM1.
Live VSMC imaging. (A-F) Example eYFP-STIM1 (A, B), mCherry-Orai1 (C, D) and merged (E, F) images captured 60 s before (Pre) and 300 s after application of 2.5 μmole/L thapsigargin (TG). Arrows point to typical TG-induced clusters (single-lined; B, D) and co-clusters (double-lined; F). A typical pre-existing, non-clustering, intracellular Orai1 vesicle is indicated by a thick arrow replicated in C and E. The scale bar in B is 10 μm and applies to (A-F).
Ca2+-release but not redistribution or clustering in response to PDGF
To investigate the relevance of STIM1 and Orai1 dynamics to the action of PDGF we exposed VSMCs to PDGF, instead of thapsigargin. PDGF evokes Ca2+-release and then Orai1-dependent Ca2+ entry in human saphenous vein VSMCs8. As expected8, STIM1 also contributed substantially to PDGF-evoked Ca2+ entry in these cells (Online Figure I). This Orai1- and STIM1-dependent Ca2+ entry is blocked specifically by the Synta 66 compound (S66) without effect on Ca2+-release8. S66-sensitive Ca2+ entry occurred within 180 s after PDGF exposure8 and it was the dominant Ca2+ signal after 300 s (Online Figure II).
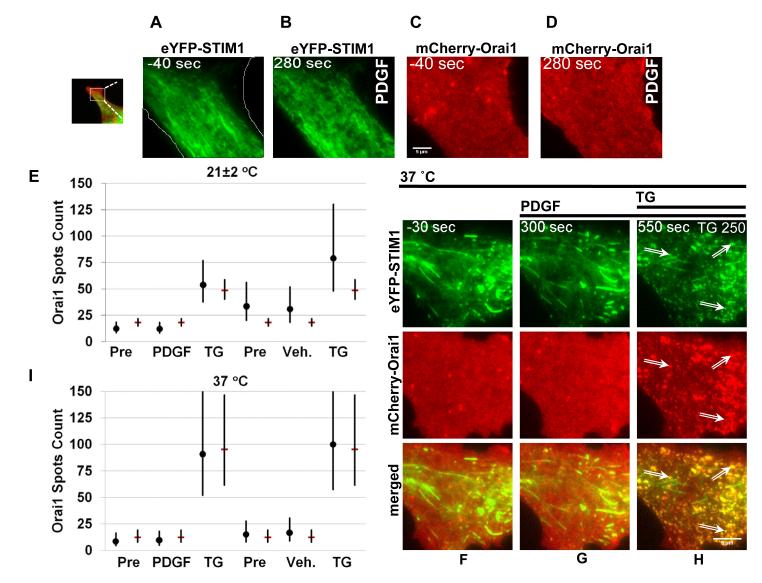
PDGF failed to induce redistribution or co-clustering of mCherry-Orai1 and eYFP-STIM1 (Figure 3A-D; Online Video IV). mCherry-Orai1 continued in its constitutive dynamic pattern in vesicular structures, ruffles, and spiny protrusions (Figure 3C-D; Online Video IV). Even at a concentration of 500 ng/mL PDGF failed to evoke changes in the localisation or dynamics of mCherry-Orai1 (Online Figure III). To be sure that the PDGF-resistant eYFP-STIM1 and mCherry-Orai1 were capable of clustering, the same VSMCs were first exposed to PDGF and then thapsigargin (Figure 3E). There was no change in response to PDGF but marked reorganisation and co-clustering when thapsigargin was applied (Figure 3E). For quantification, intense spots of mCherry-Orai1 fluorescence were counted before PDGF, after PDGF, and then after thapsigargin (Figure 3E). PDGF had no effect on the number of Orai1 spots but the number of spots was significantly increased by thapsigargin (Figure 3E; for the statistical testing of hypotheses, see Supplemental Material). We interpret the spots prior to PDGF (Figure 3E) as intracellular vesicles, or possibly small ruffles. Evaluation of 52 VSMCs showed that PDGF never caused redistribution of mCherry-Orai1 or co-clustering of eYFP-STIM1 and mCherry-Orai1.
Figure 3. Lack of clustering in response to PDGF.
Live VSMC imaging. (A-D) show images for one VSMC, (F-H) for another VSMC, both of which were co-transfected with eYFP-STIM1 (green) and mCherry-Orai1 (red). The scale bars are 5 μm. (A-D) eYFP-STIM1 (A, B) and mCherry-Orai1 (C, D) 40 s before and 280 s after application of 100 ng/mL PDGF. The small image to the left of A shows a wider view of the cell, where the white box indicates the region selected for the main panels. The white lines drawn on image A indicate the outer perimeter of the cell, which was not reached by eYFP-STIM1. (E) Quantification for results of the type illustrated in (A-D) and in which thapsigargin was also applied to the VSMCs (n/N= 6/10). Spots of intense mCherry-Orai1 fluorescence were counted before (Pre) and 240-300 s after PDGF exposure (or vehicle, Veh.), and then 110-290 s after TG. Shown are the freely-derived (black circles) and hypothesis-driven (red bars) estimated mean counts with 95 % confidence intervals. (F-I) As for (A-E) but at 37 °C (n/N= 3/4 PDGF, n/N= 2/4 Veh.).
The above recordings were made at room temperature, but STIM1 is heat-sensitive19. Therefore we repeated the experiments at 37 °C (Figure 3F-I; Online Video V). At this higher temperature, there were also no effects of PDGF on mCherry-Orai1 dynamics and there was no co-clustering with eYFP-STIM1 (Figure 3G, I) until thapsigargin was applied (Figure 3H, I; Online Video V). The effect of thapsigargin was more marked at 37 °C (Figure 3I). We interpret the thapsigargin-induced spots as clusters because they co-localised with redistributed STIM1 (Figure 3H).
The data suggest that PDGF activates Orai1 channels in VSMCs without causing redistribution or co-clustering of Orai1 and STIM1, contrasting with the effects of thapsigargin.
PDGF-evoked redistribution and clustering when Ca2+ flux through Orai1 channels is inhibited
We hypothesized that Ca2+ influx through the activated Orai1 channels was sufficient to keep the stores full, restoring the Ca2+ that had been released by PDGF so that there was no store-depletion and thus no trigger for redistribution or clustering. To test the hypothesis we first inhibited Orai1 channels using S66 (Figure 4A-E). PDGF now promptly evoked mCherry-Orai1 redistribution and co-clustering with eYFP-STIM1 (Figure 4C-E; Online Video VI).
Figure 4. PDGF-evoked clustering in Synta 66.
Live cell imaging for a VSMC co-transfected with eYFP-STIM1 (green) and mCherry-Orai1 (red). The VSMC was pre-treated for 15 min with 5 μmole/L Synta 66 and then exposed to 100 ng/mL PDGF in the presence of Synta 66. Example images are shown for 40 s before PDGF (A) and then 0, 100, 200 and 280 s after PDGF (B-E). The small image in the top left corner shows a wider view of the cell, where the white box indicates the region selected for the main panels. In the main panels of C and D, aspects of the VSMC are highlighted by double-lined arrows to indicate examples of STIM1-Orai1 co-clusters. The experiment is representative of 8 out of 9 independent experiments which showed STIM1-Orai1 co-clusters induced by PDGF. The scale bar is 5 μm.
To investigate the specific role of Orai1 with more certainty we made the R91W mutation in mCherry-Orai1 (Figure 5A-E) because this mutation prevents Orai1 function without affecting its localisation20. In these experiments it was striking that PDGF promptly evoked mCherry-Orai1-[R91W] redistribution and co-clustering with eYFP-STIM1 (Figure 5D-E; Online Video VII). Statistical analysis is provided in Figure 5F and the Supplemental Material. The E106A mutation was also made in mCherry-Orai1 because it is an alternative approach for inhibiting ion permeation21. Significant clustering of mCherry-Orai1-[E106A] was evoked by PDGF (Figure 5G cf Figure 3E).
Figure 5. PDGF-evoked clustering of non-conducting Orai1 mutants.
Live cell imaging for a VSMC co-transfected with eYFP-STIM1 (green) and mCherry-Orai1-[R91W] (red). The VSMC was exposed to 100 ng/mL PDGF. Example images are shown for 40 s before PDGF (A) and then 0, 100, 200 and 280 s after PDGF (B-E). The small image in the top left corner shows a wider view of the cell, where the white box indicates the region selected for the main panels. In the main panels of C and D, aspects of the VSMC are highlighted by double-lined arrows to indicate examples of STIM1-Orai1-[R91W] co-clusters induced by PDGF. The scale bar is 5 μm. (F) Quantification of results of the type illustrated (A-E). Shown are the freely-derived (black circles) and hypothesis-driven (red bars) estimated mean counts with 95 % confidence intervals. (G) For the same type of experiment but in which the mutation in Orai1 was E106A. Spots of intense mCherry-Orai1-[mutant] fluorescence were counted before (Pre) and 240-300 s after PDGF or vehicle (Veh.) exposure, and then 100-240 s after TG. The numbers of experiments were: (F) n/N= 3/5 (PDGF) and 3/10 (Veh.) for R91W; and (G) n/N= 3/7 (PDGF) and 3/6 (Veh.) for E106A.
The data suggest that PDGF was capable of redistributing and co-clustering mCherry-Orai1 and eYFP-STIM1 but that these events were normally prevented by Ca2+ entry through mCherry-Orai1 channels. That is, wild-type mCherry-Orai1 channels must have been activated by PDGF and generated Ca2+ entry that maintained the stores replete, preventing store-depletion that would have resulted in clustering of the ER Ca2+ sensor STIM1. The wild-type mCherry-Orai1 channels were, therefore, behaving physiologically and yet they were activated by PDGF without clustering.
PDGF-evoked redistribution and clustering conferred by acidosis
The above data suggest that redistributions and co-clustering of Orai1 and STIM1 were not involved in responses to PDGF. Nevertheless, the data also show that the redistribution and co-clustering processes exist in VSMCs. Therefore, we hypothesized that there may be conditions in which these processes are required by VSMCs. Previous studies on other cell types have suggested that Orai1 channels are strongly inhibited by acidification of the extracellular medium to pH 6.0-6.5, potentially through the glutamate residue E106 in Orai122. Such acidification occurs in ischemia and conditions of stress or pathology23, 24. Therefore we investigated VSMCs and their PDGF responses in acidic conditions.
We first investigated if acidic extracellular pH inhibited Ca2+ influx through Orai1 channels in VSMCs (Figure 6A). VSMCs were pretreated with thapsigargin and then extracellular Ca2+ was added back to observe Ca2+ entry (Figure 6A). Previous studies showed that this Ca2+ entry is strongly inhibited by S66 and suppressed by Orai1 siRNA in VSMCs8. This Ca2+ entry was inhibited by 65.5 % at pH 6.4 and the fitted Hill equation suggested 50 % inhibition at pH 6.6 (Figure 6A). Therefore, moderate acidity significantly reduced S66-sensitive Ca2+ entry in VSMCs.
Figure 6. PDGF-evoked clustering of wild-type Orai1 during acidosis.
(A) Summary data for intracellular Ca2+ measurement from VSMCs (n/N=3/26). Cells were pre-treated with 2.5 μmole/L TG in Ca2+ free solution, washed in Ca2+-free buffer (pH 6.4, 6.7, 7.1 or 7.4), and then extracellular Ca2+ (0.3 mmol/L) was added at pH 6.4, 6.7, 7.1 or 7.4. The maximum amplitude of the Ca2+ add-back signal was measured and fitted curve is a Hill equation. (B-E) Live cell imaging for a VSMC co-transfected with eYFP-STIM1 (green) and mCherry-Orai1 (red). The VSMC was imaged at pH 6.4 and exposed to 100 ng/mL PDGF and then 2.5 μmole/L thapsigargin (TG). Example images are shown for 20 s before PDGF (B), 0 and 230 s after PDGF (C-D), and then 140 s after TG (E). The panels of D contain double-lined arrows that indicate examples of STIM1-Orai1 co-clusters induced by PDGF at pH 6.4. The scale bar is 5 μm. (F) Quantification for results of the type illustrated in (B-E). Shown are the freely-derived (black circles) and hypothesis-driven (red bars) estimated mean counts with 95 % confidence intervals. Spots of intense mCherry-Orai1 fluorescence were counted before (Pre) and 240-300 s after PDGF or vehicle (Veh.) exposure, and then 80-260 s after TG (n/N=3/6).
The eYFP-STIM1 and mCherry-Orai1 dynamics were observed in VSMCs at pH 6.4 and in response to PDGF at pH 6.4 (Figure 6B-E, F). Application of PDGF caused highly significant redistribution and co-clustering (Figure 6D-E, F; Supplemental Material; Online Video VIII).
The data suggest that redistribution and co-clustering of Orai1 and STIM1 are important in VSMCs when Ca2+ flux through Orai1 channels is compromised by acidosis.
Discussion
Primary findings of this study are that: (i) In non-stimulated conditions, Orai1 and STIM1 were dynamic and mostly independent of each other; (ii) In non-stimulated conditions, Orai1 was localized to intracellular vesicles and fairly uniformly distributed in the plasma membrane, except for intensities in ruffles and spiny protrusions; (iii) In non-stimulated conditions, eYFP-STIM1 was localized to the ER and associated with dynamic comet-like structures; (iv) PDGF activated physiologically-functional (i.e. store-filling) Orai1 channels without causing redistribution or clustering of Orai1 and STIM1; (v) Ca2+-influx through PDGF-activated Orai1 channels inhibited redistribution or co-clustering of Orai1 and STIM1; and (vi) Inhibition of Orai1 channels by extracellular acidosis changed the PDGF response so that it became associated with redistribution and clustering of Orai1 and STIM1. The findings suggest that redistribution and co-clustering of Orai1 and STIM1 are not normally required for PDGF-evoked activation of Orai1 channels. These non-clustered channels nevertheless serve to maintain Ca2+ in the stores, much as clustered Orai1 channels serve to refill depleted stores. Despite this finding of activated non-clustered Orai1 channels the study does not suggest lack of importance of the redistribution and clustering phenomena. Instead it suggests that they become important when there is risk of store-depletion, for example in situations in which Orai1 channels are compromised such as in acidosis. Online Figure IV provides a diagrammatic summary of the findings and possible interpretations.
Thapsigargin depletes stores by blocking SERCA and allowing domination of constitutive Ca2+ leak. The depletion is detected by STIM1, which is an ER Ca2+ sensor whose response to store-depletion is redistribution and clustering (i.e. clustering is an indicator of store-depletion). We know, therefore, that PDGF did not cause store-depletion in VSMCs because there was no clustering of eYFP-STIM1. That is, PDGF was causing Ca2+-release but not store-depletion. We know that there was PDGF-evoked Ca2+-release because we could routinely measure it in Ca2+ measurement experiments. Furthermore, use of a mCherry-Orai1 mutant that failed to conduct Ca2+ led to PDGF-evoked clustering. Therefore, wild-type mCherry-Orai1 channels must have been activated by PDGF and then conferred Ca2+ entry that prevented store-depletion. That is, mCherry-Orai1 channels were not only activated by PDGF but also behaving physiologically to keep Ca2+ stores replete, yet clustering was not involved. It follows that the stores should have depleted when Ca2+-influx through the PDGF-activated Orai1 channels became compromised (e.g. by chemical blockade, mutation, acidosis), which is what we observed. Another inhibitor of the channels may be hypoxia, as suggested by studies on airway smooth muscle25. Similarly, from an experimental perspective, it is important to recognize that Ca2+ influx is also lost when cells are studied in the absence of extracellular Ca2+, as has been common in other studies of physiological agonists. Although use of Ca2+-free solution facilitates the distinction of Ca2+-release and Ca2+-entry, it leads the physiological agonist to cause store-depletion because the stores cannot refill after Ca2+-release. Addition of Ca2+ back to the extracellular medium then leads to observation of Ca2+ entry through clustered Orai1 channels, as in thapsigargin experiments. In support of the suggestion that physiological agonists (in the presence of extracellular Ca2+) normally cause Ca2+-release without causing significant store-depletion, simultaneous measurements of cytosolic and ER Ca2+ in a human umbilical vein endothelial cell line showed that histamine caused substantial Ca2+-release but only modest loss of total ER Ca2+ content26.
If there is Orai1 channel activation without redistribution and co-clustering, how does the activation occur? One possibility is that a small fraction of the STIM1 and Orai1 proteins is constitutively assembled in non-clustered units. Such units would be initially inactive until PDGF generated a signal for STIM1’s CAD domain to bind Orai1 and open the channel. Given the complexity of the superficial ER27 it is hard to rule out a local depletion event as the activation signal, although it would need to be tightly contained in order to avoid evoking clustering. Alternatively the signal could be a second messenger or phosphorylation step arising from the activated PDGF receptor. A recent study suggested pre-formed STIM1-Orai1 units that enable rapid responsiveness in skeletal muscle28 but such complexes required a long form of STIM1 that is not expressed in human saphenous vein VSMCs18.
The finding that non-clustered Orai1 channels are sufficient to maintain stores replete raises a question about why the cells would then need to trigger the more dramatic process of redistribution and co-clustering when Orai1 channels are inhibited or ER stress is threatened by some other means (e.g. SERCA inhibition). We suggest that it is to maximize the chance for interaction between all of the Orai1 and STIM1 proteins in a way that increases the possibility for efficient store-refilling without raising cytosolic Ca2+, thus minimizing the risk of store-depletion, ER stress and elevated cytosolic Ca2+, which are triggers for cell death29. Privileged focal loading of stores was suggested in early studies30 and by more recent studies on STIM1-depleted HELA cells31. Redistribution may also serve to recruit additional Ca2+-handling proteins to the clusters, such as TRPC channels and plasma membrane Ca2+ ATPase3.
The insight provided by this study on the dynamics of a subset of Orai1 channels has depended on the real-time tracking of fluorescently-tagged proteins in living cells. One possible concern is that the expression of exogenous mCherry-Orai1 increased the number of store-refilling channels, preventing depletion of stores that would otherwise have occurred in response to PDGF. Nevertheless, it is clear that PDGF activated the exogenous Orai1 channels without clustering and that these channels were functional in maintaining the stores replete. Furthermore, it is clear that the exogenous proteins were correctly localized and quite capable of redistributing and clustering. Alternative methods for obtaining this type of insight are not currently available. Immunofluorescence studies of endogenous proteins do not, for example, enable reliable identification of dynamic clusters of protein relative to the same protein in other compartments (e.g. in static intracellular vesicles) and co-immunoprecipitation cannot distinguish between increased aggregation (clustering) and binding (STIM1-Orai1 coupling) (see Supplemental Material for further discussion).
In summary, the study suggests that a central concept of the previously described Orai1-STIM1 channel mechanism (i.e. redistribution and co-clustering) is not required for PDGF activation of physiologically functional Orai1 Ca2+ entry channels that serve to maintain a high Ca2+ concentration in the stores. Moreover, Ca2+ entry through these non-clustered channels actively prevents redistribution and co-clustering (because it prevents store-depletion). These findings suggest that the cells have an energy-efficient (non-clustering) process to maintain stores replete in normal physiological situations when the cells respond to a sustained signal such as an elevated concentration of a growth factor. These findings do not, however, argue against the existence or importance of the redistribution and co-clustering phenomena but suggest that they are reserved for promoting Ca2+ entry in conditions such as ischemia that may lead to adverse store-depletion, ER stress, and cell death.
Supplementary Material
Novelty and Significance.
What Is Known?
Orai1 forms calcium channels that are activated by PDGF and facilitate vascular smooth muscle cell remodeling.
Activation of Orai1 channels by store-depletion follows sub cellular redistribution of STIM1 and Orai1 into clusters.
Extracellular acidosis inhibits Orai1 channels.
What New Information Does This Article Contribute?
PDGF can activate Orai1 channels without redistribution and clustering STIM1 and Orai1 in vascular smooth muscle cells
Non-clustered Orai1 channels allow Ca2+ entry that prevents store-depletion and clustering of STIM1 and Orai1.
Acidosis inhibits Orai1 channels in vascular smooth muscle cells and, in so doing, confers PDGF-evoked clustering of STIM1 and Orai1.
Seminal discoveries in 2005 and 2006 were the identifications of STIM1 and Orai1 proteins as components of calcium-selective channels that are activated following depletion of intracellular calcium stores. The findings arose primarily from the immunology field but more recently the significance was appreciated in vascular smooth muscle and endothelial cells. Fascinating features of the mechanism are the existence of STIM1 and Orai1 in separate sub cellular compartments prior to cellular stimulation and major redistribution and co-clustering in response to store-depletion. It has been suggested that only after this co-clustering do the proteins interact and calcium entry occurs through channels formed by Orai1. Our studies of human vascular smooth muscle cells reveal that activation of the channels by a critical growth factor, PDGF, does not require redistribution and co-clustering, suggesting an alternative mechanism that may involve a fraction of the Orai1 and STIM1 proteins being constitutively co-assembled. Furthermore, we show that the non-clustered channels enable calcium entry that actively maintains stores replete and prevents redistribution of Orai1 and STIM1. We show that this situation leads to a striking phenomenon in acidosis, which suppresses the PDGF-activated Orai1 channels, removing their inhibitory effect on redistribution. Consequently, in acidosis, PDGF evokes marked redistribution and co-clustering of STIM1 and Orai1. The results suggest that physiological activation of Orai1 channels does not require a central dogma previously proposed for activation of these channels but that redistribution is important as a safety mechanism to mitigate cell death in ischemia and pathology states.
Acknowledgments
Sources of Funding The work was supported by Wellcome Trust and Medical Research Council grants to DJB and a British Heart Foundation PhD Studentship to NKM.
Non-standard abbreviations and acronyms
- vascular smooth muscle cell
(VSMC)
- platelet-derived growth factor
(PDGF)
- thapsigargin
(TG)
- stromal interaction molecule 1
(STIM1)
- sarco-endoplasmic reticulum Ca2+ ATPase
(SERCA)
- endoplasmic reticulum
(ER)
Footnotes
Disclosures None.
References
- 1.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 2.Beech DJ. Ion channel switching and activation in smooth-muscle cells of occlusive vascular diseases. Biochem Soc Trans. 2007;35:890–894. doi: 10.1042/BST0350890. [DOI] [PubMed] [Google Scholar]
- 3.Beech DJ. Orai1 calcium channels in the vasculature. Pflugers Arch. 2012 doi: 10.1007/s00424-012-1090-2. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 5.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N, Seeger W, Grimminger F. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115:2811–2821. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamasaki Y, Miyoshi K, Oda N, Watanabe M, Miyake H, Chan J, Wang X, Sun L, Tang C, McMahon G, Lipson KE. Weekly dosing with the platelet-derived growth factor receptor tyrosine kinase inhibitor SU9518 significantly inhibits arterial stenosis. Circ Res. 2001;88:630–636. doi: 10.1161/01.res.88.6.630. [DOI] [PubMed] [Google Scholar]
- 7.Bisaillon JM, Motiani RK, Gonzalez-Cobos JC, Potier M, Halligan KE, Alzawahra WF, Barroso M, Singer HA, Jourd’heuil D, Trebak M. Essential role for STIM1/Orai1-mediated calcium influx in PDGF-induced smooth muscle migration. Am J Physiol Cell Physiol. 2010;298:C993–1005. doi: 10.1152/ajpcell.00325.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, McKeown L, Ojelabi O, Stacey M, Foster R, O’Regan D, Porter KE, Beech DJ. Nanomolar potency and selectivity of a Ca2+ release-activated Ca2+ channel inhibitor against store-operated Ca2+ entry and migration of vascular smooth muscle cells. Br J Pharmacol. 2011;164:382–393. doi: 10.1111/j.1476-5381.2011.01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W, Halligan KE, Zhang X, Bisaillon JM, Gonzalez-Cobos JC, Motiani RK, Hu G, Vincent PA, Zhou J, Barroso M, Singer HA, Matrougui K, Trebak M. Orai1-mediated I(CRAC) is essential for neointima formation after vascular injury. Circ Res. 2011;109:534–542. doi: 10.1161/CIRCRESAHA.111.246777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cahalan MD. Stimulating store-operated Ca2+ entry. Nat Cell Biol. 2009;11:669–677. doi: 10.1038/ncb0609-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 12.Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–890. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grigoriev I, Gouveia SM, van der Vaart B, Demmers J, Smyth JT, Honnappa S, Splinter D, Steinmetz MO, Putney JW, Jr., Hoogenraad CC, Akhmanova A. STIM1 is a MT-plus-end-tracking protein involved in remodeling of the ER. Curr Biol. 2008;18:177–182. doi: 10.1016/j.cub.2007.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu P, Lu J, Li Z, Yu X, Chen L, Xu T. Aggregation of STIM1 underneath the plasma membrane induces clustering of Orai1. Biochem Biophys Res Commun. 2006;350:969–976. doi: 10.1016/j.bbrc.2006.09.134. [DOI] [PubMed] [Google Scholar]
- 15.Porter KE, Dickinson T, London NJ. Inhibition of neointima formation in an organ culture of human saphenous vein: A comparison of dual endothelin-converting enzyme/neutral endopeptidase and selective neutral endopeptidase inhibition. J Vasc Surg. 2001;34:548–554. doi: 10.1067/mva.2001.115960. [DOI] [PubMed] [Google Scholar]
- 16.Madi HA, Riches K, Warburton P, O’Regan DJ, Turner NA, Porter KE. Inherent differences in morphology, proliferation, and migration in saphenous vein smooth muscle cells cultured from nondiabetic and type 2 diabetic patients. American journal of physiology. Cell physiology. 2009;297:C1307–1317. doi: 10.1152/ajpcell.00608.2008. [DOI] [PubMed] [Google Scholar]
- 17.Hauser CT, Tsien RY. A hexahistidine-Zn2+-dye label reveals STIM1 surface exposure. Proc Natl Acad Sci U S A. 2007;104:3693–3697. doi: 10.1073/pnas.0611713104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Sukumar P, Milligan CJ, Kumar B, Ma ZY, Munsch CM, Jiang LH, Porter KE, Beech DJ. Interactions, functions, and independence of plasma membrane STIM1 and TRPC1 in vascular smooth muscle cells. Circ Res. 2008;103:e97–104. doi: 10.1161/CIRCRESAHA.108.182931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao B, Coste B, Mathur J, Patapoutian A. Temperature-dependent STIM1 activation induces Ca2+ influx and modulates gene expression. Nat Chem Biol. 2011;7:351–358. doi: 10.1038/nchembio.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derler I, Fahrner M, Carugo O, Muik M, Bergsmann J, Schindl R, Frischauf I, Eshaghi S, Romanin C. Increased hydrophobicity at the N terminus/membrane interface impairs gating of the severe combined immunodeficiency-related Orai1 mutant. J Biol Chem. 2009;284:15903–15915. doi: 10.1074/jbc.M808312200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNally BA, Yamashita M, Engh A, Prakriya M. Structural determinants of ion permeation in CRAC channels. Proc Natl Acad Sci U S A. 2009;106:22516–22521. doi: 10.1073/pnas.0909574106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scrimgeour NR, Wilson DP, Rychkov GY. Glutamate 106 in the Orai1 pore contributes to fast Ca2+-dependent inactivation and ph dependence of Ca2+ release-activated CA2+ (CRAC) current. Biochem J. 2011 doi: 10.1042/BJ20110558. [DOI] [PubMed] [Google Scholar]
- 23.Orchard CH, Cingolani HE. Acidosis and arrhythmias in cardiac muscle. Cardiovasc Res. 1994;28:1312–1319. doi: 10.1093/cvr/28.9.1312. [DOI] [PubMed] [Google Scholar]
- 24.Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: A perfect storm for cancer progression. Nat Rev Cancer. 2011;11:671–677. doi: 10.1038/nrc3110. [DOI] [PubMed] [Google Scholar]
- 25.Mancarella S, Wang Y, Deng X, Landesberg G, Scalia R, Panettieri RA, Mallilankaraman K, Tang XD, Madesh M, Gill DL. Hypoxia-induced acidosis uncouples the STIM-Orai calcium signaling complex. J Biol Chem. 2011 doi: 10.1074/jbc.M111.303081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malli R, Naghdi S, Romanin C, Graier WF. Cytosolic Ca2+ prevents the subplasmalemmal clustering of STIM1: An intrinsic mechanism to avoid Ca2+ overload. J Cell Sci. 2008;121:3133–3139. doi: 10.1242/jcs.034496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franzini-Armstrong C. The sarcoplasmic reticulum and the control of muscle contraction. FASEB J. 1999;13(Suppl 2):S266–270. doi: 10.1096/fasebj.13.9002.s266. [DOI] [PubMed] [Google Scholar]
- 28.Darbellay B, Arnaudeau S, Bader CR, Konig S, Bernheim L. STIM1L is a new actin-binding splice variant involved in fast repetitive Ca2+ release. J Cell Biol. 2011;194:335–346. doi: 10.1083/jcb.201012157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minamino T, Komuro I, Kitakaze M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ Res. 2010;107:1071–1082. doi: 10.1161/CIRCRESAHA.110.227819. [DOI] [PubMed] [Google Scholar]
- 30.Casteels R, Droogmans G. Exchange characteristics of the noradrenaline-sensitive calcium store in vascular smooth muscle cells or rabbit ear artery. J Physiol. 1981;317:263–279. doi: 10.1113/jphysiol.1981.sp013824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jousset H, Frieden M, Demaurex N. STIM1 knockdown reveals that store-operated Ca2+ channels located close to sarco/endoplasmic Ca2+ ATPases (SERCA) pumps silently refill the endoplasmic reticulum. J Biol Chem. 2007;282:11456–11464. doi: 10.1074/jbc.M609551200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.