Abstract
Our understanding of the interaction of leukocytes and the vessel wall during leukocyte capture is limited by an incomplete understanding of the mechanical properties of the endothelial surface layer. It is known that adhesion molecules on leukocytes are distributed non-uniformly relative to surface topography 3, that topography limits adhesive bond formation with other surfaces 9, and that physiological contact forces (≈ 5.0 − 10.0 pN per microvillus) can compress the microvilli to as little as a third of their resting length, increasing the accessibility of molecules to the opposing surface 3, 7. We consider the endothelium as a two-layered structure, the relatively rigid cell body, plus the glycocalyx, a soft protective sugar coating on the luminal surface 6. It has been shown that the glycocalyx can act as a barrier to reduce adhesion of leukocytes to the endothelial surface 4. In this report we begin to address the deformability of endothelial surfaces to understand how the endothelial mechanical stiffness might affect bond formation. Endothelial cells grown in static culture do not express a robust glycocalyx, but cells grown under physiological flow conditions begin to approximate the glycocalyx observed in vivo 2. The modulus of the endothelial cell body has been measured using atomic force microscopy (AFM) to be approximately 5 to 20 kPa 5. The thickness and structure of the glycocalyx have been studied using electron microscopy 8, and the modulus of the glycocalyx has been approximated using indirect methods, but to our knowledge, there have been no published reports of a direct measurement of the glycocalyx modulus in living cells. In this study, we present indentation experiments made with a novel AFM probe on cells that have been cultured in conditions to maximize their glycocalyx expression to make direct measurements of the modulus and thickness of the endothelial glycocalyx.
Keywords: Biomedical Engineering, Issue 72, Bioengineering, Cellular Biology, Biophysics, Molecular Biology, Endothelium, Vascular, Membrane Glycoproteins, Receptors, Leukocyte-Adhesion, bioengineering (general), glycocalyx, mechanical properties, atomic force microscopy, ATM, Endothelial cells, leukocytes, cell wall, cell culture, microscopy, imaging
Protocol
1. Methods
1.1 Cell Flow Chamber
A flow chamber, shown in Figure 1, was constructed so that cells could be grown under a shear of 1.0 Pa (10 dyn/cm2) and then transferred directly to an Asylum MFP3D AFM (Santa Barbara, CA).
The flow chamber was prepared for the experiment by first cleaning the glass slides in piranha solution (3:1 H2SO4:H2O2) for 15 min and then washing them with distilled water. They were then baked to dry and coated with aminopropyl triethoxy silane (APTES) in a vacuum deposition chamber.
A silicone gasket was cut using a Silhouette SD cutting tool. This allowed us to finely control the flow chamber dimensions for control of the flow rate and shear stress during cell growth. Typically, a channel was cut 6.4 mm wide by 19 mm long from a sheet of 0.4 mm silicone. The flow rate necessary to generate a shear stress of 1.0 Pa (10 dyn/cm2) is calculated assuming laminar flow in a rectangular channel with the equation:
![]() where Q is the flow rate, τ is the shear stress, μ is the viscosity of the medium, assumed here to be 1.0 mPa (0.01 dyn*sec/cm2), h is the height and w is the width of the flow chamber.
where Q is the flow rate, τ is the shear stress, μ is the viscosity of the medium, assumed here to be 1.0 mPa (0.01 dyn*sec/cm2), h is the height and w is the width of the flow chamber.
The top piece of the flow chamber was aligned with the gasket in the cell culture dish and secured with a magnetic ring. The assembly was filled with isopropyl alcohol (IPA) for sterilization.
The full flow system was assembled. The flow ports in the cell culture dish were connected to three-way valves. The valves were connected to open 30 ml syringes. IPA was flushed through the system, which was then washed with 30 ml of McCoy's medium with 4% Fetal Calf Serum (FCS). The system was then filled with 20 ml of the Vec Technologies cell growth medium. Covers were placed on the tops of the syringes. The catch reservoir syringe cap had a needle in place to move medium back to the feed reservoir. Sterile 0.2 μm filters were attached to the air inlets in the covers to prevent contamination of the system. The flow chamber was then ready for cell seeding.
1.2 Cell Culture
Human umbilical vein endothelial cells (HUVEC's) and growth medium were purchased from Vec Technologies (Rensselaer, NY) and grown to confluence in a T25 Flask.
The growth medium was removed from the flask and the monolayer of cells released with 2 ml of 2.5% trypsin. Once the cells were in solution, the trypsinization was quenched with the addition of 10 ml of cell culture medium to the flask.
The cell suspension was centrifuged for 5 min and the supernatant was removed. The cells were resuspended in 1 ml of cell culture medium (containing serum) for injection into the flow chamber.
The cell suspension (0.5 ml, ~50,000 cells) was loaded into a syringe and injected into the flow chamber through a three-way valve.
The cells were allowed to settle and adhere to the glass substrate for 2 hr before flow was started. The cells were grown under flow in an incubator at 37 °C for 1 to 5 days until confluent.
1.3 Cantilever Preparation and Cell Indentation
Tipless AFM cantilevers (NanoWorld, Switzerland) were cleaned in nitric acid for 5 min and functionalized with aminopropyl triethoxy silane (APTES) in a vapor deposition chamber.
A solution of 5 mg/ml by weight NHS-sulfo-LC-biotin in Hank's Buffered Salt Solution (HBSS) was prepared. The cantilevers were submerged in the solution for 15 min to conjugate the silane with N-Hydroxysuccinimide (NHS) chemistry.
A solution of biotin free medium was made by incubating 20 ml of Vec Technologies cell culture medium (including serum) with 200 μl of streptavadin beads for 12 hr. The beads were removed from the medium with a magnet and the medium was filtered through a 0.22 μm sterile filter.
The flow chamber was removed from the cell culture dish and the cells were washed in 37 °C biotin-free medium.
A stock solution of 1 μl of 2.4 μm beads coated with streptavidin in 1 ml of biotin free medium was prepared, and 100 μl of the stock was added to the cell culture dish.
Streptavidin beads were picked up with the cantilever by landing the tip on the glass surface next to a bead, retracting, positioning the apex of the cantilever over the bead, and then pressing the cantilever down onto the bead and resting for several seconds.
The sensitivity of the cantilever was measured by indenting onto a region of bare glass and using the slope of the curve to set the tip deflection as a function of voltage.
The spring constant of the cantilever was then calculated from a thermal calibration in the MFP3D software.
The calibrated cantilever was then used to indent the samples, as shown in Figure 2. These 2.4 μm beads offer a larger contact area with the cell surface so that the mechanical properties of the soft glycocalyx layer can be detected. The cantilever was positioned over a cell near the cell nucleus and a soft approach of the tip onto the cell was used to set the cantilever height approximately 3 μm above the cell surface. The software was set for 20 repeated indentations at a rate of 1 μm/sec to a maximum force of 7 nN. Approximately 6 sec elapsed between successive contacts. It is possible to use different rates of indentation to test for time-dependent properties of the glycocalyx, although in these initial experiments, only a single indentation rate (1 μm/sec) was used.
2. Indentation Theory
Indentation into an elastic half-space with a sphere of radius R can be described using Hertz theory where the force of indentation, F, is given by the equation:
![]() Where δ is the indentation depth and E* is the reduced modulus of the material under test (Figure 3). In the case of an infinitely stiff indenter impinging a uniform elastic half-space, E* is given by the equation:
Where δ is the indentation depth and E* is the reduced modulus of the material under test (Figure 3). In the case of an infinitely stiff indenter impinging a uniform elastic half-space, E* is given by the equation:
![]() where E is the elastic modulus and ν is the Poisson ratio of the material. Recent work with polymer films has inspired the development of a two layer model for determining the modulus and thickness of thin films 1. We are applying this model to cell biology by treating the glycocalyx as a uniform thin soft film on the surface of the cell body. Using this model, the reduced modulus of the system becomes:
where E is the elastic modulus and ν is the Poisson ratio of the material. Recent work with polymer films has inspired the development of a two layer model for determining the modulus and thickness of thin films 1. We are applying this model to cell biology by treating the glycocalyx as a uniform thin soft film on the surface of the cell body. Using this model, the reduced modulus of the system becomes:
![]() Where EGC is the modulus of the glycocalyx, Ecell is the modulus of the cell body, P, Q and n are constants which have been empirically determined from the polymer fits, and z is given by the equation:
Where EGC is the modulus of the glycocalyx, Ecell is the modulus of the cell body, P, Q and n are constants which have been empirically determined from the polymer fits, and z is given by the equation:
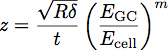 Where t is the thickness of the glycocalyx layer. A schematic of these parameters is shown in Figure 3. The model has been shown to be an accurate way of determining the modulus and thickness of a thin film on stiffer substrate 1. This equation can be used to fit the curves obtained from indentation into cells to determine the modulus and thickness of the endothelial glycocalyx, as shown in Figure 4.
Where t is the thickness of the glycocalyx layer. A schematic of these parameters is shown in Figure 3. The model has been shown to be an accurate way of determining the modulus and thickness of a thin film on stiffer substrate 1. This equation can be used to fit the curves obtained from indentation into cells to determine the modulus and thickness of the endothelial glycocalyx, as shown in Figure 4.
Representative Results
In a typical experiment, 20 force-vs-distance curves were obtained from a given region of the cell, typically in the perinuclear region, near, but not on, the nucleus (within ~2 μm). The curves were aligned to account for any sample drift over the duration of the measurement and then averaged to remove cantilever noise, as shown in Figure 4. The curves were analyzed and fit with the two layer model that was developed for determining the modulus and thickness of thin polymer films 1. From fits of the curves of 25 cells, we have determined that the modulus of the luminal layer is 0.7 ± 0.5 kPa and the thickness is 380 ± 50 nm, as shown in Figure 5. The modulus of the cell body is 16 ± 6 kPa. These values are in good agreement with previous measurements of the modulus of the cell body and thickness of the glycocalyx 5, 8.
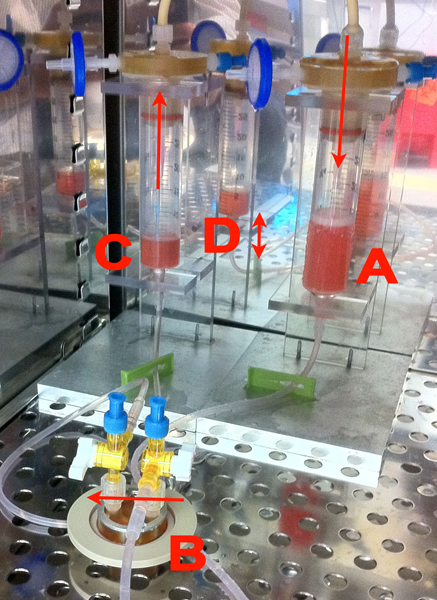 Figure 1. Our cell culture device. Flow across the chamber was driven by gravity from reservoir A to C. Cells were plated in the closed chamber B which was held with magnets to the surface of a closed cell dish for an Asylum AFM (Asylum, Santa Barbara CA). Three-way valves on B enabled us to stop the flow. A silicone gasket created a flow channel 6.4 mm wide by 19 mm long by 0.4 mm high. The closed chamber B was easily removed and the closed cell dish moved directly into the AFM for experimentation. The height difference D was maintained by pumping fluid from reservoir C to A with a peristaltic pump (not shown). The whole assembly was placed in an incubator for the duration of cell culture.
Figure 1. Our cell culture device. Flow across the chamber was driven by gravity from reservoir A to C. Cells were plated in the closed chamber B which was held with magnets to the surface of a closed cell dish for an Asylum AFM (Asylum, Santa Barbara CA). Three-way valves on B enabled us to stop the flow. A silicone gasket created a flow channel 6.4 mm wide by 19 mm long by 0.4 mm high. The closed chamber B was easily removed and the closed cell dish moved directly into the AFM for experimentation. The height difference D was maintained by pumping fluid from reservoir C to A with a peristaltic pump (not shown). The whole assembly was placed in an incubator for the duration of cell culture.
 Figure 2. Left: Image of a cantilever with a 2.4 μm bead (arrow) attached by a biotin-streptavidin linkage. Right: Monolayer of HUVECs grown under flow.
Figure 2. Left: Image of a cantilever with a 2.4 μm bead (arrow) attached by a biotin-streptavidin linkage. Right: Monolayer of HUVECs grown under flow.
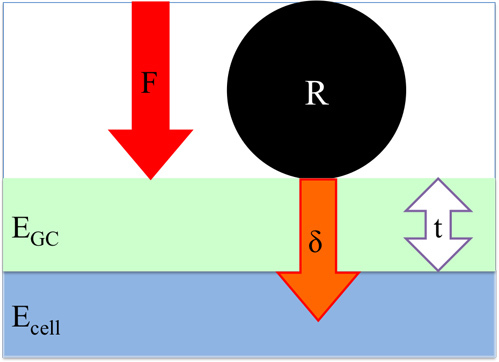 Figure 3. Geometry of the interaction of the bead of radius R indenting a distance δ into the cell. The glycocalyx, shown in green, has a modulus of EGC and a thickness t. The cell body has a modulus Ecell. The force applied by the bead to the cell, F, is measured and the force versus distance curve, shown in Figure 4, is obtained.
Figure 3. Geometry of the interaction of the bead of radius R indenting a distance δ into the cell. The glycocalyx, shown in green, has a modulus of EGC and a thickness t. The cell body has a modulus Ecell. The force applied by the bead to the cell, F, is measured and the force versus distance curve, shown in Figure 4, is obtained.
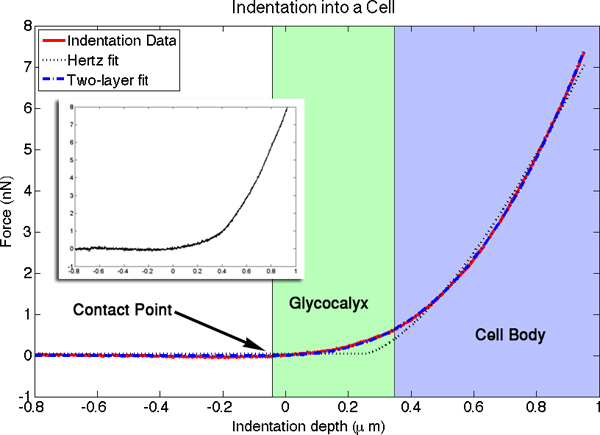 Figure 4. The averaged force versus distance curve for an indentation into a cell is plotted in red. An individual indentation is shown in the inset. Highlighted in the image are the contact point, where the cantilever first touches the luminal surface, the luminal layer, where the slope of the curve is dominated by the stiffness of the glycocalyx, and the cell body, where the slope is primarily a function of cell body modulus. The fitted curve from the two-layer indentation theory is shown in blue, and the fit for the simpler Hertz model for an elastic half space is shown by the dotted black line. There were four free parameters in the two-layer fits. Values determined for this particular fit were: cell modulus = 15.9 kPa, luminal layer modulus = 0.33 kPa, and luminal layer thickness = 420 nm. The fourth fitted parameter is the contact point with the glycocalyx. Positioning of the x-axis origin relative to the cell surface is arbitrary. Click here to view larger figure.
Figure 4. The averaged force versus distance curve for an indentation into a cell is plotted in red. An individual indentation is shown in the inset. Highlighted in the image are the contact point, where the cantilever first touches the luminal surface, the luminal layer, where the slope of the curve is dominated by the stiffness of the glycocalyx, and the cell body, where the slope is primarily a function of cell body modulus. The fitted curve from the two-layer indentation theory is shown in blue, and the fit for the simpler Hertz model for an elastic half space is shown by the dotted black line. There were four free parameters in the two-layer fits. Values determined for this particular fit were: cell modulus = 15.9 kPa, luminal layer modulus = 0.33 kPa, and luminal layer thickness = 420 nm. The fourth fitted parameter is the contact point with the glycocalyx. Positioning of the x-axis origin relative to the cell surface is arbitrary. Click here to view larger figure.
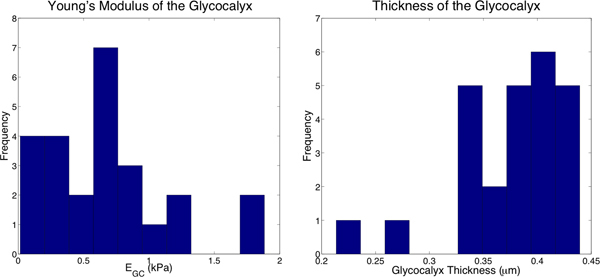 Figure 5. Histograms of the properties of 25 cells from the curve fits. Left: EGC was determined to be 0.7 ± 0.5 kPa. Right: The thickness of the glycocalyx was determined to be 380 ± 50 nm.
Figure 5. Histograms of the properties of 25 cells from the curve fits. Left: EGC was determined to be 0.7 ± 0.5 kPa. Right: The thickness of the glycocalyx was determined to be 380 ± 50 nm.
Discussion
We used values calculated from the two-layer model and Hertz theory to model the interaction of a leukocyte circulating in the blood with the endothelial wall. We have calculated that a microvillus on the leukocyte with a diameter of 50 nm under a 10 pN load would indent approximately 150 nm into the glycocalyx, only a fraction of the total thickness. This indicates that the glycocalyx, with properties as measured in this experiment, is a significant barrier to cell-cell interaction and can be a large steric hindrance which the cells must overcome during the adhesion cascade during leukocyte adhesion.
In the model used here, we approximate the glycocalyx as an isotropic elastic structure. While we are unaware of any mechanical measurements to indicate this is not the case, what is known about the molecular structure of the glycocalyx suggests that this is likely an over-simplification. In fact, the glycocalyx is a complex and varied structure on the surface of cells. It consists of oriented molecular structures, lacks a well-defined outer boundary, and likely becomes denser close to the cell surface. Thus, while the two-layered elastic model used here provides insight into the relative stiffness of the glycocalyx observed by circulating cells, future studies could explore alternative mechanical descriptions that may account for its possible anisotropy and varying density in the thickness direction. It is also possible that the glycocalyx is not uniform across different regions of the cell surface. This would not have been evident from the present data set because all data presented here were taken in the central region of the cell around the nucleus.
The glycocalyx may also exhibit viscoelastic properties that were not investigated in this study. It has been observed that, under static conditions, red blood cells in capillaries can completely compress the glycocalyx, wheres circulating red blood cells do not 10. Forces generated by static red cells are likely to be extremely small (~3-10 Pa). This indicates the glycocalyx may be very soft in response to slow compression, but significantly stiffer during faster compression. Our measurements were performed at indentation rates of 1 μm/sec to simulate the stiffness a circulating cell may encounter, but further work to investigate the time-dependent properties is in progress.
AFM indentation has been used to directly measure the modulus and thickness of the endothelial glycocalyx in living cells. The measurements indicate that the glycocalyx can be a significant barrier to cell-cell contact and adhesion and likely serves as a key factor in regulating the adhesion cascade during inflammation.
Disclosures
No conflicts of interest declared.
Acknowledgments
The authors would like to thank Elena Lomakina, Richard Bauserman, Margaret Youngman, Shay Vaknin, Jessica Snyder, Chris Striemer, Nakul Nataraj, Hung Li Chung, Tejas Khire, and Eric Lam for their assistance with this project. This project was funded by NIH #PO1 HL 018208.
References
- Clifford C, Seah M. Nanoindentation measurement of young's modulus for compliant layers on stiffer substrates including the effect of poisson's ratios. Nanotechnology. 2009. [DOI] [PubMed]
- Gouverneur M, Spaan JAE, Pannekoek H, Fontijn RD, Vink H. Fluid shear stress stimulates incorporation of hyaluronan into endothelial cell glycocalyx. Am. J. Physiol. Heart. Circ. Physiol. 2006;290(1):458–452. doi: 10.1152/ajpheart.00592.2005. [DOI] [PubMed] [Google Scholar]
- Hocde SA, Hyrien O, Waugh RE. Cell adhesion molecule distribution relative to neutrophil surface topography assessed by tirfm. Biophysical Journal. 2009;97(1):379–387. doi: 10.1016/j.bpj.2009.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipowski HH. The endothelial glycocalyx as a barrier to leukocyte adhesion and its mediation by extracellular proteases. Annals of biomedical engineering. 2012;40(4):840–848. doi: 10.1007/s10439-011-0427-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Oswald SJ, Ngu H, Yin FCP. Mechanical properties of actin stress fibers in living cells. Biophysical Journal. 2008;95(12):6060–6071. doi: 10.1529/biophysj.108.133462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Archiv. European Journal of Physiology. 2000;440(5):653–666. doi: 10.1007/s004240000307. [DOI] [PubMed] [Google Scholar]
- Spillmann CM, Lomakina E, Waugh RE. Neutrophil adhesive contact dependence on impingement force. Biophysical Journal. 2004;87(6):4237–4245. doi: 10.1529/biophysj.103.031773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vanden Berg BM, Vink H, Spaan JAE. The endothelial glycocalyx protects against myocardial edema. Circulation Research. 2003;92(6):592–594. doi: 10.1161/01.RES.0000065917.53950.75. [DOI] [PubMed] [Google Scholar]
- Williams TE, Nagarajan S, Selvaraj P, Zhu C. Quantifying the impact of membrane microtopology on effective two-dimensional affinity. J. Biol. Chem. 2001;276(16):13283–138. doi: 10.1074/jbc.M010427200. [DOI] [PubMed] [Google Scholar]
- Vink H, Duling B. Identification of Distinct Luminal Domains for Macromolecules, Erythrocytes, and Leukocytes Within Mammalian Capillaries. Circulation Research. 1996;79:581–589. doi: 10.1161/01.res.79.3.581. [DOI] [PubMed] [Google Scholar]


