Abstract
Amyloid beta fibrillation can lead to major disorder of neurons processes and is associated with several neuronal diseases (e.g., Alzheimer’s disease). We report here an importance of slight temperature changes, in the physiological range (35–42 °C), on the amyloid fibrillation process in the presence and absence of hydrophilic (silica) and hydrophobic (polystyrene) nanoparticles (NPs). The results highlight the fact that slight increases in temperature can induce inhibitory and acceleratory effects of hydrophobic and hydrophilic NPs on the fibrillation process, respectively. Using further in vivo considerations, the outcomes of this study can be used for considerable modifications on the current diagnosis and treatment approaches in amyloid-involved diseases.
Keywords: Amyloid beta, fibrillation, physiological temperature, hydrophilic NPs, hydrophobic NPs
The misfolding of amyloid proteins (e.g., amyloid beta (Aβ) peptide,1−4 prion protein,5 α-synuclein,6 polyglutamine,7 glucagon,8 and β2-microglobulin9,10) followed by their fibrillation is the hallmark of over 40 human diseases, ranging from neurodegenerative disorders (e.g., Alzheimer’s disease, Parkinson's disease, Creutzfeld–Jakob disease, and Gerstmann–Sträussler–Scheinker syndrome) to non-neuropathic disorders (e.g., amyloid heart disease, rheumatoid arthritis, and type II diabetes).11,12 Among various amyloidogenic proteins, Aβ peptides are widely used as model proteins to investigate the effect of NPs on fibrillogenesis.13 Monomeric Aβ is actually soluble in a physiological condition and has shown to be unstructured;14 however, the fibrillar form has a characteristic cross-β structure with stacking of β strands perpendicular to the long axis of the fiber.15−17
It is well recognized that nanoparticles (NPs) have significant effect on the fibrillation process.18−20 Interestingly, it was very recently found that graphene oxide sheets (GO) with a protein corona (protein/biomolecular coated GO in biological medium21) can slow amyloid the fibrillation process.22 Although there are few reports on the effect of temperature on the kinetics of amyloid fibrillation process,23−26 a crucial effect of slight temperature changes (i.e., in the physiological range) on the amyloid fibrillation process in the presence of NPs has not been investigated. This point is very important for the in vivo NP application to humans, specifically for treatment of amyoloid proteins (e.g., Alzheimer’s and Parkinson’s disease).27 The local temperature in different brain diseases/tumors for different individuals is in the range from 33.4 to 42.0 °C.28−32 In normal body, the body temperature, during sleep, decreases and manual work leads to an increase of up to 2 °C. This means that the body temperature for healthy humans varies in the range from 35 to 39 °C and can find a maximum of 42 °C in the case of fever.33 Although there are significant reports on the effects of various NPs on the amyloid fibrillation process,34−38 as far as we know there is no report on the effects of slight temperature changes, in the physiological range, on the interactions between NPs and Aβ; thus, we focused our attention on the effects slight temperature changes have on amyloid fibrillation in the absence and presence of two commercially available and compositionally different NPs (i.e., “hydrophobic” carboxylated polystyrene NPs and hydrophilic silica). It is notable that we employed these particular NPs because of their importance as the first group of materials which were evaluated for safety at the nanoscale.39−41
Results and Discussion
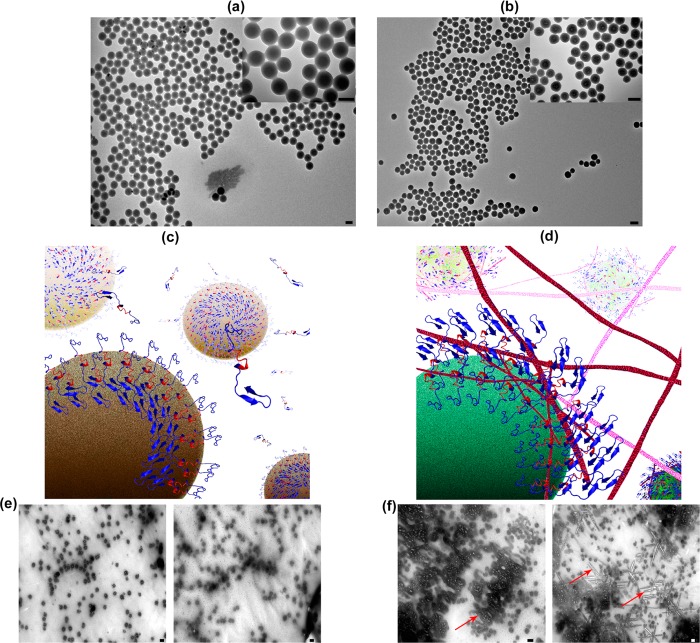
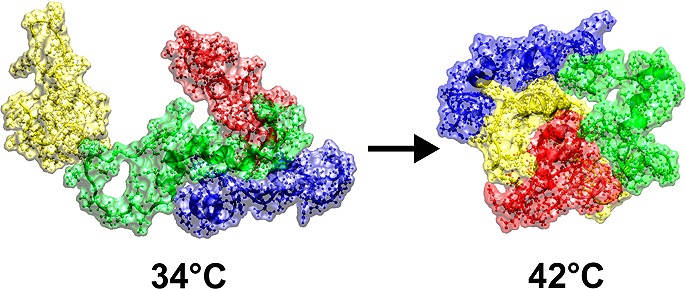
The amino acid sequence of 17–24 (i.e., KLVFFAED) is known to form amyloids on its own, and most likely has a crucial role in the fibrillation process;38 thus, we used both molecular dynamic (MD) simulation methods and experimental methods, against monoclonal antibody, to detect the availability/exposure of this sequence at various physiological temperatures (see Figure 1). According to the results, one can observe that, by increasing the temperature from 37 to 42 °C, the availability/exposure of the KLVFFAED sequence in the amyloid backbone is improved. In the next step, using Thioflavin T (ThT) assay, we probed the exposure of the exposed hydrophobic sequence of Aβ with both hydrophilic and hydrophobic NPs, at various temperatures (see Figure 2 for detail); as seen, the lag time for the pure Aβ (i.e., in the absence of NPs) is decreased gradually, by increasing the temperature from 37 to 42 °C; this happened due to the fact that the core part of the fibrillation process (i.e., KLVFFAED sequence) is exposed to each other resulting in faster formation of amyloid oligomers. Both polystyrene and silica NPs had acceleration effects on the fibrillation process at 37 °C; however, the most striking observation is that dual effects were observed at higher temperature (i.e., 42 °C). More specifically, the acceleration effect of silica NPs was significantly enhanced by increasing the temperature; in contrast, the polystyrene NPs demonstrated strong inhibitory effects on the fibrillation process by slight temperature enhancement. The possible mechanism (see Figure 3) for the observed dual effects of various NPs at the same conditions may strongly relate to the surface properties of NPs. For silica NPs (i.e., hydrophilic), the hydrophilic part of Aβ monomers would be attached to the surface of silica NPs, resulting in placement of hydrophobic site in the outer shell of NPs; by increasing temperature, the availability of these hydrophobic sites would be increased, causing the enhancement in acceleration effect on the fibrillation process. In contrast, hydrophobic NPs (i.e., polystyrene NPs) tend to bind with the hydrophobic part of Aβ monomers, which are more available at higher temperature; thus, the concentration of hydrophilic sites in the outer shell of polystyrene NPs would be increased by increasing the temperature. In this case, one can conclude that the increase in the interaction temperature can induce considerable inhibitory effect on the hydrophobic NPs. In order to further confirm the proposed mechanism, the transmission electron microscopy (TEM) method was employed for the interaction temperature of 42 °C. Quiet remarkably, TEM images (see Figure 3) were in good agreement with the schemes and they illustrated the formation of fibrils at the surface of silica NPs; these fibrils were drastically enhanced by increasing the interaction time. In contrast, there was no trace of fibrillation on the polystyrene NP batch at 42 °C.
Figure 1.
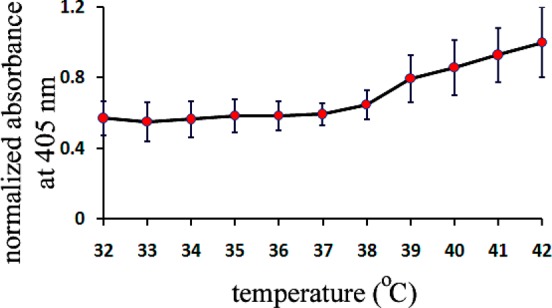
Antibody affinity toward the hydrophobic section of Aβ at various temperatures.
Figure 2.
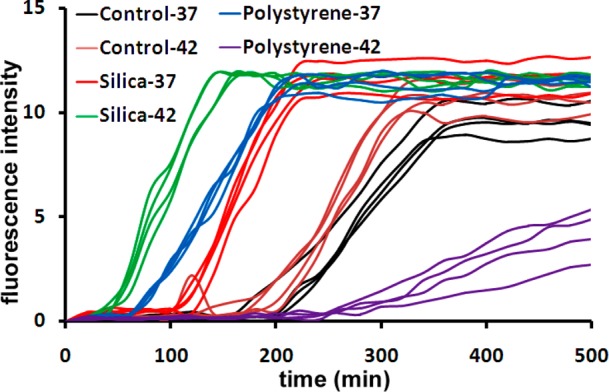
Kinetics of Aβ fibrillation (concentration of 5 μM) with and without NPs at temperatures of 37 and 42 °C.
Figure 3.
TEM images of (a) polystyrene and (b) silica NPs with various magnifications, showing the existence of spherical NPs with narrow size distribution. (c,d) Representative schemes showing the exposure of the amyloids’ hydrophilic and hydrophobic backbone to the free amyloid monomers after interaction with polystyrene and silica particles at 42 °C, respectively. (e,f) TEM images of the amyloid interacted proteins with polystyrene and silica particles at 42 °C. As seen, there is no trace of fibrillation in (e); however, severe fibrillation (see red arrows as example) were observed in (f). In (e) and (f), left and right images correspond to interaction of amyloid with nanoparticles at 20 and 400 min, respectively; scale bar is 100 nm.
Molecular dynamic simulations (see the Supporting Information) were performed to probe the underlying mechanism of the observed results. The results, in various temperatures, clearly showtime dependence of the molecule structure as it is expected. Figure S1 of the Supporting Information shows the atomic root-mean-square displacement (RMSD) of the amyloid structure from the initial state in the simulations. The average radius of gyration of the molecule also confirms structural deformations and also shows that the molecule has the most compact form at 37 °C (see Figure S2 of the Supporting Information). To calculate the radius of gyration, the last 25 ns of any run is considered. Comparing the RMSD from average configuration at 37 °C also shows large deviations at 42 and 27 °C which indicates both hot and cold denaturation (see Figure 4a). In the simulations with more than one molecule (2 and 4), we clearly see that the molecules in higher temperatures bind each other faster and stronger. The range of the investigated temperatures spans about 5% temperature difference. The effect of such temperature change on diffusion of the molecule cannot explain the observed change on the speed of fibrillation process and its dynamics. Looking at the distance between the center of mass of the molecules (Figure S3 of the Supporting Information), one can see that the molecules approach each other almost in the same time, but their equilibrium distance is smaller at higher temperature. The effect of temperature on the dynamics of fibrillation is also investigated by the mean of all atom simulations (see Supporting Information for full detail). Figure 4b shows that an aggregation of four amyloids is formed not only faster in higher temperatures, but also it is more compact there. This is more interesting when we know from single molecule simulations that the molecule in these two temperatures is swollen in comparison to its physiological temperature, almost with the same degree (see Figures 4a and S2 of the Supporting Information).
Figure 4.
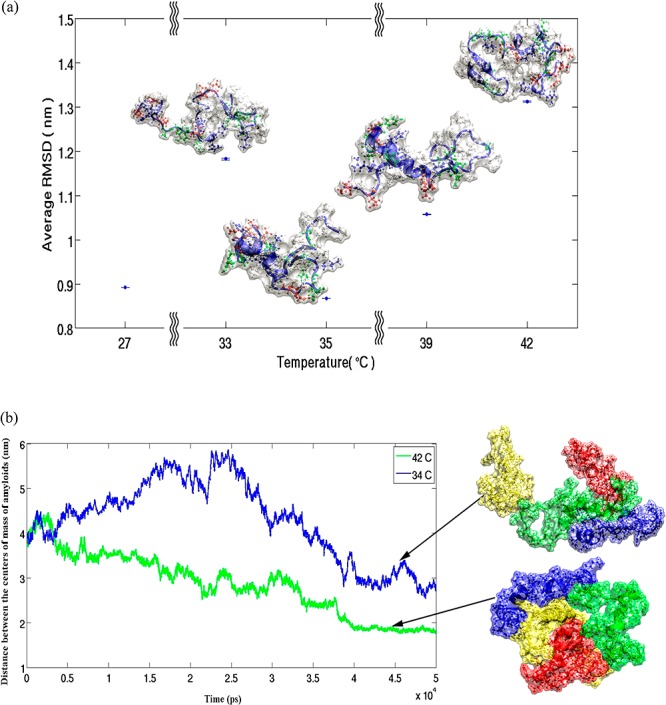
(a) Average RMSD of amyloid structure from mean configuration at 37 °C (protein conformations at defined temperatures are shown). (b) Average distance between the centers of mass of four amyloids at 34 °C (blue line) and 42 °C are varying in temperature in different ways (green line).
Conclusion
We report here a crucial ignored factor (i.e., slight temperature changes in the physiological range) on the protein fibrillation mechanisms in the presence and absence of NPs. From both experimental and simulation methods, it was revealed that the core hydrophobic backbone of Aβ monomers can be more available for interactions by increasing the temperature from 37 to 42 °C. It is also found that hydrophobic NPs (i.e., polystyrene) have the capability to show dual effects (i.e., acceleratory and inhibitory) on the fibrillation process by slight temperature enhancement; however, for hydrophilic NPs (i.e., silica), the acceleratory effects on the fibrillation process can be significantly increased. These findings need in vivo considerations in the future.
Supporting Information Available
Experimental and simulation details. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Author Present Address
# M.M.: School of Chemical Sciences, University of Illinois at Urbana−Champaign, 600 South Mathews Avenue, Urbana, Illinois 61801.
This paper was published on the Web on December 14, 2014, with the second author's last name misspelled. The corrected version was reposted on December 18, 2012.
Supplementary Material
References
- Sipe J. D. (1992) Amyloidosis. Annu. Rev. Biochem. 61, 947–975. [DOI] [PubMed] [Google Scholar]
- Serpell L. C. (2000) Alzheimer’s amyloid fibrils: structure and assembly. Biochim. Biophys. Acta 1502, 16–30. [DOI] [PubMed] [Google Scholar]
- Hardy J.; Selkoe D. J. (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297, 353–356. [DOI] [PubMed] [Google Scholar]
- Williams A. D.; Portelius E.; Kheterpal I.; Guo J.; Cook K. D.; Xu Y.; Wetzel R. (2004) Mapping abeta amyloid fibril secondary structure using scanning proline mutagenesis. J. Mol. Biol. 335, 833–842. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. (1998) Prions. Proc. Natl. Acad. Sci. U.S.A. 95, 13363–13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana R.; Ionescu-Zanetti C.; Pope M.; Li J.; Nielson L.; Ramirez-Alvarado M.; Regan L.; Fink A. L.; Carter S. A. (2003) A general model for amyloid fibril assembly based on morphological studies using atomic force microscopy. Biophys. J. 85, 1135–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.; Berthelier V.; Hamilton J. B. O. N., B.; Wetzel R. (2002) Amyloid-like features of polyglutamine aggregates and their assembly kinetics. Biochemistry 41, 7391–7399. [DOI] [PubMed] [Google Scholar]
- Ferkinghoff-Borg J.; Fonslet J.; Andersen C. B.; Krishna S.; Pigolotti S.; Yagi H.; Goto Y.; Otzen D.; Jensen M. H. (2010) Stop-and-go kinetics in amyloid fibrillation. Phys. Rev. E 82, 010901. [DOI] [PubMed] [Google Scholar]
- Naiki H. (1977) Amyloid. Int. J. Exp. Clin. Invest. 4, 223–232. [Google Scholar]
- McParland V. J.; Kalverda A. P.; Homans S. W.; Radford S. E. (2002) Structural properties of an amyloid precursor ofβ2-microglobulin. Nat. Struct. Biol. 9, 326–331. [DOI] [PubMed] [Google Scholar]
- Chiti F.; Dobson C. M. (2006) Protein Misfolding, Functional Amyloid, and Human Disease. Annu. Rev. Biochem. 75, 333–366. [DOI] [PubMed] [Google Scholar]
- Campioni S.; Mannini B.; Zampagni M.; Pensalfini A.; Parrini C.; Evangelisti E.; Relini A.; Stefani M.; Dobson C. M.; Cecchi C.; Chiti F. (2010) A causative link between the structure of aberrant protein oligomers and their toxicity. Nat. Chem. Biol. 6, 140–147. [DOI] [PubMed] [Google Scholar]
- Fei L.; Perrett S. (2009) Int. J. Mol. Sci. 10, 646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D. M.; Hartley D. M.; Kusumoto Y.; Fezoui Y.; Margaret M.; Condron M. M.; Lomakin A.; Benedek G. B.; Selkoe D. J.; Teplow D. B. (1999) Amyloid β-Protein Fibrillogenesis. J. Biol. Chem. 274, 25945–25952. [DOI] [PubMed] [Google Scholar]
- Nelson R.; Eisenberg D. (2006) Structural models of amyloid-like fibrils. Adv. Protein Chem. 73, 235–282. [DOI] [PubMed] [Google Scholar]
- Sato T.; Kienlen-Campard P.; Ahmed M.; Liu W.; Li H.; Elliott J. I.; Aimoto S.; Constantinescu S. N.; Octave J. N.; Smith S. O. (2006) Inhibitors of amyloid toxicity based on beta-sheet packing of Abeta40 and Abeta42. Biochemistry 45, 5503–5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkova A. T.; Yau W. M.; Tycko R. (2006) Experimental constraints on quaternary structure in Alzheimer’s beta-amyloid fibrils. Biochemistry 45, 498–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linse S.; Cabaleiro-Lago C.; Xue W.-F.; Lynch I.; Lindman S.; Thulin E.; Radford S. E.; Dawson K. A. (2007) Nucleation of protein fibrillation by nanoparticles. Proc. Natl. Acad. Sci. U.S.A. 104, 8691–8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabaleiro-Lago C.; Quinlan-Pluck F.; Lynch I.; Lindman S.; Minogue A. M.; Thulin E.; Walsh D. M.; Dawson K. A.; Linse S. (2008) Inhibition of Amyloid β Protein Fibrillation by Polymeric Nanoparticles. J. Am. Chem. Soc. 130, 15437–15443. [DOI] [PubMed] [Google Scholar]
- Cabaleiro-Lago C.; Lynch I.; Dawson K. A.; Linse S. (2009) Inhibition of IAPP and IAPP(20–29) Fibrillation by Polymeric Nanoparticles. Langmuir 26, 3453–3461. [DOI] [PubMed] [Google Scholar]
- Mahmoudi M.; Lynch I.; Ejtehadi M. R.; Monopoli M. P.; Bombelli F. B.; Laurent S. (2011) Protein–Nanoparticle Interactions: Opportunities and Challenges. Chem. Rev. 111, 5610–5637. [DOI] [PubMed] [Google Scholar]
- Mahmoudi M.; Akhavan O.; Ghavami M.; Rezaee F.; Ghiasi S. M. A. (2012) Graphene oxide strongly inhibits amyloid beta fibrillation. Nanoscale 4, 7322–7325. [DOI] [PubMed] [Google Scholar]
- Sabaté R.; Gallardo M.; Estelrich J. (2005) Temperature dependence of the nucleation constant rate in β amyloid fibrillogenesis. Int. J. Biol. Macromol. 35, 9–13. [DOI] [PubMed] [Google Scholar]
- Gursky O.; Aleshkov S. (2000) Temperature-dependent β-sheet formation in β-amyloid Aβ1–40 peptide in water: uncoupling β-structure folding from aggregation. Biochim. Biophys. Acta 1476, 93–102. [DOI] [PubMed] [Google Scholar]
- Shehi E.; Fusi P.; Secundo F.; Pozzuolo S.; Bairati A.; Tortora P. (2003) Temperature-Dependent, Irreversible Formation of Amyloid Fibrils by a Soluble Human Ataxin-3 Carrying a Moderately Expanded Polyglutamine Stretch (Q36). Biochemistry 42, 14626–14632. [DOI] [PubMed] [Google Scholar]
- Chu H.-L.; Lin S.-Y. (2001) Temperature-induced conformational changes in amyloid β(1–40) peptide investigated by simultaneous FT-IR microspectroscopy with thermal system. Biophys. Chem. 89, 173–180. [DOI] [PubMed] [Google Scholar]
- Krol S., Macrez R., Docagne F., Defer G., Laurent S., Rahman M., Hajipour M. J., Kehoe P. G., and Mahmoudi M. (2012) Therapeutic Benefits from Nanoparticles: The Potential Significance of Nanoscience in Diseases with Compromise to the Blood Brain Barrier. Chem. Rev. published online Nov 19, 2012. DOI: 10.1021/cr200472g. [DOI] [PubMed] [Google Scholar]
- Ishigaki D.; Ogasawara K.; Yoshioka Y.; Chida K.; Sasaki M.; Fujiwara S.; Aso K.; Kobayashi M.; Yoshida K.; Terasaki K.; Inoue T.; Ogawa A. (2009) Brain Temperature Measured Using Proton MR Spectroscopy Detects Cerebral Hemodynamic Impairment in Patients With Unilateral Chronic Major Cerebral Artery Steno-Occlusive Disease. Stroke 40, 3012–3016. [DOI] [PubMed] [Google Scholar]
- Colbourne F.; Nurse S. M.; Corbett D. (1993) Temperature changes associated with forebrain ischemia in the gerbil. Brain Res. 602, 264–267. [DOI] [PubMed] [Google Scholar]
- Sukstanskii A. L.; Yablonskiy D. A. (2006) Theoretical model of temperature regulation in the brain during changes in functional activity. Proc. Natl. Acad. Sci. U.S.A. 103, 12144–12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett R.; Laptook A.; Weatherall P. (1997) Noninvasive Measurements of Human Brain Temperature Using Volume-Localized Proton Magnetic Resonance Spectroscopy. J. Cereb. Blood Flow Metab. 17, 363–369. [DOI] [PubMed] [Google Scholar]
- Collins C. M.; Smith M. B.; Turner R. (2004) Model of local temperature changes in brain upon functional activation. J. Appl. Physiol. 97, 2051–2055. [DOI] [PubMed] [Google Scholar]
- Hasday J. D.; Singh I. S. (2000) Fever and the heat shock response: distinct, partially overlapping processes. Cell Stress Chaperones 5, 471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei L.; Perrett S. (2009) Effect of Nanoparticles on Protein Folding and Fibrillogenesis. Int. J. Mol. Sci. 10, 646–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K.; Okada T.; Sawada S.-i.; Akiyoshi K.; Matsuzaki K. (2006) Inhibition of the formation of amyloid β-protein fibrils using biocompatible nanogels as artificial chaperones. FEBS Lett. 580, 6587–6595. [DOI] [PubMed] [Google Scholar]
- Skaat H.; Shafir G.; Margel S. (2011) Acceleration and inhibition of amyloid-β fibril formation by peptide-conjugated fluorescent-maghemite nanoparticles. J. Nanoparticle Res. 13, 3521–3534. [Google Scholar]
- Kogan M. J.; Bastus N. G.; Amigo R.; Grillo-Bosch D.; Araya E.; Turiel A.; Labarta A.; Giralt E.; Puntes V. F. (2005) Nanoparticle-Mediated Local and Remote Manipulation of Protein Aggregation. Nano Lett. 6, 110–115. [DOI] [PubMed] [Google Scholar]
- Laurent S.; Ejtehadi M. R.; Rezaei M.; Kehoe P. G.; Mahmoudi M. (2012) Interdisciplinary challenges and promising theranostic effects of nanoscience in Alzheimer’s disease. RSC Adv. 2, 5008–5033. [Google Scholar]
- Monopoli M. P.; Walczyk D.; Campbell A.; Elia G.; Lynch I.; Baldelli Bombelli F.; Dawson K. A. (2011) Physical–Chemical Aspects of Protein Corona: Relevance to in Vitro and in Vivo Biological Impacts of Nanoparticles. J. Am. Chem. Soc. 133, 2525–2534. [DOI] [PubMed] [Google Scholar]
- Walczyk D.; Bombelli F. B.; Monopoli M. P.; Lynch I.; Dawson K. A. (2010) What the Cell “Sees” in Bionanoscience. J. Am. Chem. Soc. 132, 5761–5768. [DOI] [PubMed] [Google Scholar]
- Cedervall T.; Lynch I.; Foy M.; Berggård T.; Donnelly S. C.; Cagney G.; Linse S.; Dawson K. A. (2007) Detailed Identification of Plasma Proteins Adsorbed on Copolymer Nanoparticles. Angew. Chem., Int. Ed. 119, 5856–5858. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.