Abstract
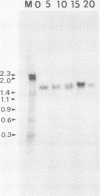
We have identified two novel, very closely related genes, SAS1 and SAS2, from Dictyostelium discoideum. These encode small, approximately 20-kilodaton proteins with amino acid sequences thought to be involved in interaction with guanine nucleotides. The protein sizes, spacings of GTP-binding domains, and carboxyl-terminal sequences suggest their relationship to the ubiquitous ras-type proteins. Their sequences, however, are sufficiently different to indicate that they are not true ras proteins. More extensive sequence identity (approximately 55%) is shared with the YPT1 and SEC4 proteins from Saccharomyces cerevisiae. These yeast proteins are essential for growth and are believed to be involved in intracellular signaling associated with membrane function. SAS1 and SAS2 exhibit distinct patterns of genomic organization and developmentally regulated gene expression. SAS1 contains introns and is associated with a developmentally regulated repetitive element. SAS2 is colinear with its mRNA and does not appear to be closely linked with this repetitive element. Both genes are expressed during growth and throughout development. SAS1 is maximally expressed during cytodifferentiation, when two sizes of SAS1 mRNA are detectable. SAS2 mRNA levels are maximal during culmination. On the basis of the expression patterns of the SAS genes and their relationship to the YPT1 and SEC4 genes, we discuss possible functions of the SAS proteins.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Capon D. J., Chen E. Y., Levinson A. D., Seeburg P. H., Goeddel D. V. Complete nucleotide sequences of the T24 human bladder carcinoma oncogene and its normal homologue. Nature. 1983 Mar 3;302(5903):33–37. doi: 10.1038/302033a0. [DOI] [PubMed] [Google Scholar]
- Chardin P., Tavitian A. The ral gene: a new ras related gene isolated by the use of a synthetic probe. EMBO J. 1986 Sep;5(9):2203–2208. doi: 10.1002/j.1460-2075.1986.tb04485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFeo-Jones D., Scolnick E. M., Koller R., Dhar R. ras-Related gene sequences identified and isolated from Saccharomyces cerevisiae. Nature. 1983 Dec 15;306(5944):707–709. doi: 10.1038/306707a0. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Firtel R. A., van Haastert P. J., Kimmel A. R., Devreotes P. N. G protein linked signal transduction pathways in development: dictyostelium as an experimental system. Cell. 1989 Jul 28;58(2):235–239. doi: 10.1016/0092-8674(89)90837-4. [DOI] [PubMed] [Google Scholar]
- Gallwitz D., Donath C., Sander C. A yeast gene encoding a protein homologous to the human c-has/bas proto-oncogene product. Nature. 1983 Dec 15;306(5944):704–707. doi: 10.1038/306704a0. [DOI] [PubMed] [Google Scholar]
- Gibbs J. B., Marshall M. S. The ras oncogene--an important regulatory element in lower eucaryotic organisms. Microbiol Rev. 1989 Jun;53(2):171–185. doi: 10.1128/mr.53.2.171-185.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Goud B., Salminen A., Walworth N. C., Novick P. J. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell. 1988 Jun 3;53(5):753–768. doi: 10.1016/0092-8674(88)90093-1. [DOI] [PubMed] [Google Scholar]
- Halliday K. R. Regional homology in GTP-binding proto-oncogene products and elongation factors. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(6):435–448. [PubMed] [Google Scholar]
- Hancock J. F., Magee A. I., Childs J. E., Marshall C. J. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989 Jun 30;57(7):1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- Haubruck H., Disela C., Wagner P., Gallwitz D. The ras-related ypt protein is an ubiquitous eukaryotic protein: isolation and sequence analysis of mouse cDNA clones highly homologous to the yeast YPT1 gene. EMBO J. 1987 Dec 20;6(13):4049–4053. doi: 10.1002/j.1460-2075.1987.tb02750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubruck H., Prange R., Vorgias C., Gallwitz D. The ras-related mouse ypt1 protein can functionally replace the YPT1 gene product in yeast. EMBO J. 1989 May;8(5):1427–1432. doi: 10.1002/j.1460-2075.1989.tb03524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes S. R., Raychaudhuri G., Beyer A. L. The Drosophila Hrb98DE locus encodes four protein isoforms homologous to the A1 protein of mammalian heterogeneous nuclear ribonucleoprotein complexes. Mol Cell Biol. 1990 Jan;10(1):316–323. doi: 10.1128/mcb.10.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurnak F. Structure of the GDP domain of EF-Tu and location of the amino acids homologous to ras oncogene proteins. Science. 1985 Oct 4;230(4721):32–36. doi: 10.1126/science.3898365. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Powers S., Cameron S., Fasano O., Goldfarb M., Broach J., Wigler M. Functional homology of mammalian and yeast RAS genes. Cell. 1985 Jan;40(1):19–26. doi: 10.1016/0092-8674(85)90304-6. [DOI] [PubMed] [Google Scholar]
- Kimmel A. R. Different molecular mechanisms for cAMP regulation of gene expression during Dictyostelium development. Dev Biol. 1987 Jul;122(1):163–171. doi: 10.1016/0012-1606(87)90342-3. [DOI] [PubMed] [Google Scholar]
- Kimmel A. R., Firtel R. A. Sequence organization and developmental expression of an interspersed, repetitive element and associated single-copy DNA sequences in Dictyostelium discoideum. Mol Cell Biol. 1985 Aug;5(8):2123–2130. doi: 10.1128/mcb.5.8.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel A. R., Firtel R. A. Sequence organization in Dictyostelium: unique structure at the 5'-ends of protein coding genes. Nucleic Acids Res. 1983 Jan 25;11(2):541–552. doi: 10.1093/nar/11.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P. S., Sun T. J., Saxe C. L., 3rd, Kimmel A. R., Johnson R. L., Devreotes P. N. A chemoattractant receptor controls development in Dictyostelium discoideum. Science. 1988 Sep 16;241(4872):1467–1472. doi: 10.1126/science.3047871. [DOI] [PubMed] [Google Scholar]
- Madaule P., Axel R. A novel ras-related gene family. Cell. 1985 May;41(1):31–40. doi: 10.1016/0092-8674(85)90058-3. [DOI] [PubMed] [Google Scholar]
- Madaule P., Axel R., Myers A. M. Characterization of two members of the rho gene family from the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1987 Feb;84(3):779–783. doi: 10.1073/pnas.84.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui Y., Kikuchi A., Kondo J., Hishida T., Teranishi Y., Takai Y. Nucleotide and deduced amino acid sequences of a GTP-binding protein family with molecular weights of 25,000 from bovine brain. J Biol Chem. 1988 Aug 15;263(23):11071–11074. [PubMed] [Google Scholar]
- McCormick F., Clark B. F., la Cour T. F., Kjeldgaard M., Norskov-Lauritsen L., Nyborg J. A model for the tertiary structure of p21, the product of the ras oncogene. Science. 1985 Oct 4;230(4721):78–82. doi: 10.1126/science.3898366. [DOI] [PubMed] [Google Scholar]
- Mehdy M. C., Ratner D., Firtel R. A. Induction and modulation of cell-type-specific gene expression in Dictyostelium. Cell. 1983 Mar;32(3):763–771. doi: 10.1016/0092-8674(83)90062-4. [DOI] [PubMed] [Google Scholar]
- Mierendorf R. C., Pfeffer D. Direct sequencing of denatured plasmid DNA. Methods Enzymol. 1987;152:556–562. doi: 10.1016/0076-6879(87)52061-4. [DOI] [PubMed] [Google Scholar]
- Molenaar C. M., Prange R., Gallwitz D. A carboxyl-terminal cysteine residue is required for palmitic acid binding and biological activity of the ras-related yeast YPT1 protein. EMBO J. 1988 Apr;7(4):971–976. doi: 10.1002/j.1460-2075.1988.tb02903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pupillo M., Kumagai A., Pitt G. S., Firtel R. A., Devreotes P. N. Multiple alpha subunits of guanine nucleotide-binding proteins in Dictyostelium. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4892–4896. doi: 10.1073/pnas.86.13.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond C. D., Gomer R. H., Mehdy M. C., Firtel R. A. Developmental regulation of a Dictyostelium gene encoding a protein homologous to mammalian ras protein. Cell. 1984 Nov;39(1):141–148. doi: 10.1016/0092-8674(84)90199-5. [DOI] [PubMed] [Google Scholar]
- Reymond C. D., Gomer R. H., Nellen W., Theibert A., Devreotes P., Firtel R. A. Phenotypic changes induced by a mutated ras gene during the development of Dictyostelium transformants. 1986 Sep 25-Oct 1Nature. 323(6086):340–343. doi: 10.1038/323340a0. [DOI] [PubMed] [Google Scholar]
- Robbins S. M., Williams J. G., Jermyn K. A., Spiegelman G. B., Weeks G. Growing and developing Dictyostelium cells express different ras genes. Proc Natl Acad Sci U S A. 1989 Feb;86(3):938–942. doi: 10.1073/pnas.86.3.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A., Novick P. J. A ras-like protein is required for a post-Golgi event in yeast secretion. Cell. 1987 May 22;49(4):527–538. doi: 10.1016/0092-8674(87)90455-7. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt H. D., Puzicha M., Gallwitz D. Study of a temperature-sensitive mutant of the ras-related YPT1 gene product in yeast suggests a role in the regulation of intracellular calcium. Cell. 1988 May 20;53(4):635–647. doi: 10.1016/0092-8674(88)90579-x. [DOI] [PubMed] [Google Scholar]
- Schmitt H. D., Wagner P., Pfaff E., Gallwitz D. The ras-related YPT1 gene product in yeast: a GTP-binding protein that might be involved in microtubule organization. Cell. 1986 Nov 7;47(3):401–412. doi: 10.1016/0092-8674(86)90597-0. [DOI] [PubMed] [Google Scholar]
- Segev N., Botstein D. The ras-like yeast YPT1 gene is itself essential for growth, sporulation, and starvation response. Mol Cell Biol. 1987 Jul;7(7):2367–2377. doi: 10.1128/mcb.7.7.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev N., Mulholland J., Botstein D. The yeast GTP-binding YPT1 protein and a mammalian counterpart are associated with the secretion machinery. Cell. 1988 Mar 25;52(6):915–924. doi: 10.1016/0092-8674(88)90433-3. [DOI] [PubMed] [Google Scholar]
- Sigal I. S., Gibbs J. B., D'Alonzo J. S., Scolnick E. M. Identification of effector residues and a neutralizing epitope of Ha-ras-encoded p21. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4725–4729. doi: 10.1073/pnas.83.13.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touchot N., Chardin P., Tavitian A. Four additional members of the ras gene superfamily isolated by an oligonucleotide strategy: molecular cloning of YPT-related cDNAs from a rat brain library. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8210–8214. doi: 10.1073/pnas.84.23.8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haastert P. J., Kesbeke F., Reymond C. D., Firtel R. A., Luderus E., Van Driel R. Aberrant transmembrane signal transduction in Dictyostelium cells expressing a mutated ras gene. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4905–4909. doi: 10.1073/pnas.84.14.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahraoui A., Touchot N., Chardin P., Tavitian A. Complete coding sequences of the ras related rab 3 and 4 cDNAs. Nucleic Acids Res. 1988 Feb 11;16(3):1204–1204. doi: 10.1093/nar/16.3.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- la Cour T. F., Nyborg J., Thirup S., Clark B. F. Structural details of the binding of guanosine diphosphate to elongation factor Tu from E. coli as studied by X-ray crystallography. EMBO J. 1985 Sep;4(9):2385–2388. doi: 10.1002/j.1460-2075.1985.tb03943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]