Abstract
Background and Purpose
Cigarette smoke is a major cause for chronic obstructive pulmonary disease (COPD). Andrographolide is an active biomolecule isolated from the plant Andrographis paniculata. Andrographolide has been shown to activate nuclear factor erythroid-2-related factor 2 (Nrf2), a redox-sensitive antioxidant transcription factor. As Nrf2 activity is reduced in COPD, we hypothesize that andrographolide may have therapeutic value for COPD.
Experimental Approach
Andrographolide was given i.p. to BALB/c mice daily 2 h before 4% cigarette smoke exposure for 1 h over five consecutive days. Bronchoalveolar lavage fluid and lungs were collected for analyses of cytokines, oxidative damage markers and antioxidant activities. BEAS-2B bronchial epithelial cells were exposed to cigarette smoke extract (CSE) and used to study the antioxidant mechanism of action of andrographolide.
Key Results
Andrographolide suppressed cigarette smoke-induced increases in lavage fluid cell counts; levels of IL-1β, MCP-1, IP-10 and KC; and levels of oxidative biomarkers 8-isoprostane, 8-OHdG and 3-nitrotyrosine in a dose-dependent manner. Andrographolide promoted inductions of glutathione peroxidase (GPx) and glutathione reductase (GR) activities in lungs from cigarette smoke-exposed mice. In BEAS-2B cells, andrographolide markedly increased nuclear Nrf2 accumulation, promoted binding to antioxidant response element (ARE) and total cellular glutathione level in response to CSE. Andrographolide up-regulated ARE-regulated gene targets including glutamate-cysteine ligase catalytic (GCLC) subunit, GCL modifier (GCLM) subunit, GPx, GR and heme oxygenase-1 in BEAS-2B cells in response to CSE.
Conclusions
Andrographolide possesses antioxidative properties against cigarette smoke-induced lung injury probably via augmentation of Nrf2 activity and may have therapeutic potential for treating COPD.
Keywords: chronic obstructive pulmonary disease, glutathione, glutathione peroxidase, glutathione reductase, antioxidant, heme oxygenase-1
Introduction
Chronic obstructive pulmonary disease (COPD) is a global health problem and is predicted to become the third leading cause of death by 2020 (Rabe et al., 2007). COPD is a chronic airway disease characterized by progressive airflow limitation associated with dysregulated inflammatory responses of the lungs and increased macrophage and neutrophil infiltration, leading to obstruction of small airways and destruction of lung parenchyma as a consequence of skewed activation of metalloproteases (Cosio et al., 2009; Brusselle et al., 2011; Mocchegiani et al., 2011). In sharp contrast to asthma, COPD is largely resistant to the anti-inflammatory effects of corticosteroids (Adcock and Barnes, 2008). At present, there are no specific treatments in halting the disease progression and suppressing the lung inflammation effectively in COPD (Barnes, 2010; Morjaria et al., 2010). Although the PDE4 inhibitor roflumilast has been newly approved for the treatment of COPD, it suffers from dose-limiting major side effects such as diarrhoea, headache, nausea and weight loss (Rabe, 2011; Page and Spina, 2012). There is an urgent need to discover novel compounds for COPD.
Cigarette smoking is a major risk factor for the development of COPD. Cigarette smoke is a rich source of potent oxidants and free radicals causing oxidative damage to airway epithelium and alveolar wall, infiltration of macrophages and neutrophils, and imbalance of oxidants and antioxidants (Cantin, 2010; Yao and Rahman, 2011). Pulmonary levels of oxidant biomarkers such as 3-nitrotyrosine (3-NT), 8-isoprostane and 8-hydroxydeoxyguanosine (8-OHdG) have been shown to correlate positively with COPD severity (Louhelainen et al., 2008; Yao and Rahman, 2011). Nuclear factor erythroid-2-related factor 2 (Nrf2) is a redox-sensitive transcription factor and is critical in protecting the lung against oxidative stress, as impaired Nrf2 has been implicated in several pulmonary diseases including acute respiratory distress syndrome, pulmonary fibrosis, asthma and COPD (Cho et al., 2006; Boutten et al., 2011). Nrf2 gene disruption resulted in enhanced susceptibility to emphysema after cigarette smoke exposure (Rangasamy et al., 2004). Nrf2 has been shown to regulate expression of antioxidants such as glutathione peroxidase (GPx) and glutathione reductase (GR) (Adair-Kirk et al., 2008; Hubner et al., 2009). Activation of Nrf2 by triterpenoids such as CDDO-imidazolide has demonstrated beneficial effects in a cigarette smoke-induced emphysema animal model (Sussan et al., 2009). Drug discovery of novel compounds with property of strengthening antioxidant defence is a vital strategy for COPD drug development.
Andrographolide is a labdane diterpene lactone isolated from the Andrographis paniculata plant (Lim et al., 2012). A. paniculata has long been used as herbal medicine for the prevention and treatment of upper respiratory tract infection in Asian countries and in Scandinavia (Coon and Ernst, 2004; Chang et al., 2008). Andrographolide has also been shown to possess hepatoprotective (Negi et al., 2008), antiviral (Lin et al., 2008), anti-cancer (Lim et al., 2012) and anti-inflammatory (Abu-Ghefreh et al., 2009; Bao et al., 2009) activities. Recently, it has been reported that andrographolide promoted Nrf2 nuclear translocation in a human endothelial cell line EA.hy926 by probing Nrf2 protein in the cell nuclear extracts and by measuring Nrf2-induced antioxidant response element (ARE) activation in cells transfected with ARE-luciferase construct (Yu et al., 2010).
We hypothesized that andrographolide may have protective effects against oxidative damage induced by cigarette smoke in COPD. In this study, we aimed to investigate potential antioxidative effects and mechanism of action of andrographolide in a cigarette smoke-induced lung injury mouse model. Our findings reveal for the first time that andrographolide can augment antioxidant gene expressions and activities probably via up regulation of Nrf2 activity in experimental models of cigarette smoke exposure. The reduction in cigarette smoke-induced lung oxidative damage conferred by andrographolide is probably associated with the significant decline in bronchoalveolar lavage (BAL) fluid inflammatory cell counts, chemokines, cytokines and proteases, and up-regulation of glutathione (GSH) redox defence system and heme oxygenase-1 (HO-1) expression. These findings support a novel therapeutic value for andrographolide in the treatment of COPD.
Methods
Animals
Female BALB/c mice of 6 to 8 weeks old (Animal Resources Centre, Canning Vale, Western Australia, Australia) were maintained in the animal housing unit on a 12 h light–dark cycle with food and water available ad libitum. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). Animal experiments were performed according to the Institutional guidelines for Animal Care and Use Committee of the National University of Singapore.
Cigarette smoke-induced lung injury and andrographolide treatment protocol
Mice were placed in a ventilated chamber filled with 4% cigarette smoke delivered by a peristaltic pump (Masterflex, Cole-Parmer Instrument Co., Niles, IL, USA) at a constant rate of 1 L·min−1 as described (Chan et al., 2009). Total suspended particulate of 4% cigarette smoke was 493.5 ± 49.6 mg·m−3 (n = 4) recorded using the MicroDust Pro-aerosol monitor (Casella CEL, Bedford, UK). The level of total suspended particulates observed was comparable to that reported by other laboratories (Morris et al., 2008; Thatcher et al., 2008; Vlahos et al., 2010). To develop cigarette smoke-induced lung injury, mice were exposed to 10 sticks of 3R4F research cigarettes (Tobacco and Health Research Institute, University of Kentucky, Lexington, KY, USA) over a period of 60 min a day for five consecutive days (Braber et al., 2011). Mice in the sham air group were simultaneously placed in another ventilated chamber but exposed to fresh air. Andrographolide (0.1, 0.5, and 1 mg·kg−1; Sigma, St. Louis, MO, USA) (Figure 1A) or vehicle (1% DMSO) in 0.1 mL saline was given by i.p. injection 2 h before each cigarette smoke exposure as described by Bao et al. (2009). Mice were killed 24 h after the last cigarette smoke or sham air exposure, and lung samples were collected for various analyses.
Figure 1.
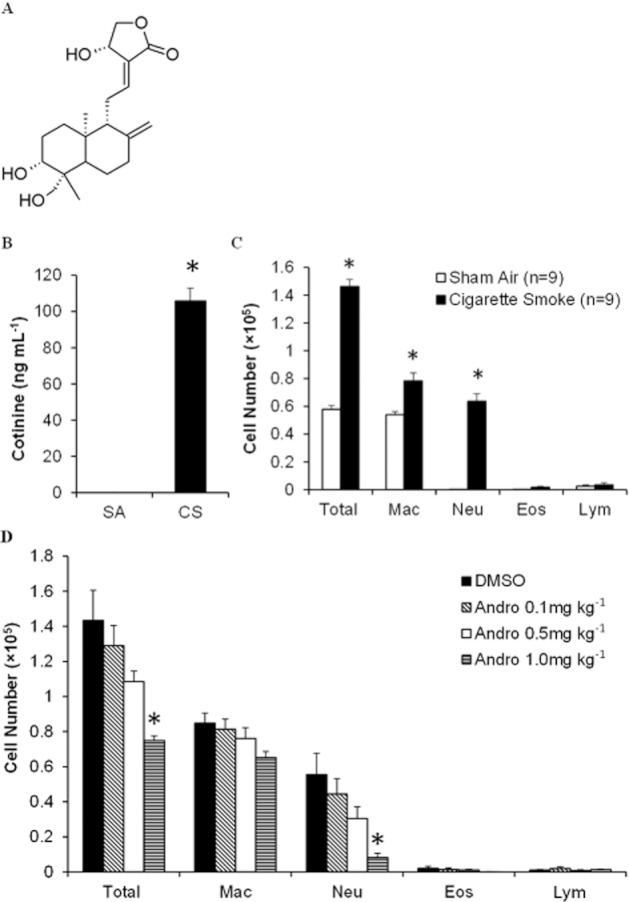
Effects of andrographolide on cigarette smoke-induced inflammatory cell recruitment. (A) The chemical structure of andrographolide. (B) Blood was collected immediately after 1 h 4% cigarette smoke exposure, and plasma cotinine levels were measured using elisa (n = 6). (C) Inflammatory cell counts in BAL fluid obtained from mice 24 h after the last sham air (n = 9 mice per group) or cigarette smoke (n = 9 mice per group) exposure. (D) Andrographolide dose-dependently reduced cigarette smoke-induced inflammatory cell counts in BAL fluid from mice 24 h after the last cigarette smoke challenge (DMSO, n = 6; 0.1 mg·kg−1, n = 6; 0.5 mg·kg−1, n = 6; and 1 mg·kg−1, n = 8 mice per group). Differential cell counts were performed on a minimum of 500 cells to identify eosinophil (Eos), macrophage (Mac), neutrophil (Neu) and lymphocyte (Lym). Values shown are the mean ± SEM. *Significant difference from DMSO, P < 0.05.
Cotinine measurement
Cotinine is the major metabolite of nicotine (Benowitz, 2009). Plasma cotinine level was measured as a marker of cigarette smoking. Immediately after sham air or cigarette smoke exposure, blood was collected and plasma cotinine levels were measured using elisa (Bio-Quant, San Diego, CA, USA) according to the manufacturer's instructions. Absorbance was measured at 450 nm in a microplate reader (Tecan Infinite F200; Mannedorf, Switzerland).
BAL fluid analysis and differential cell counts
BAL was performed as described (Bao et al., 2009). BAL fluid total and differential cell counts were determined. BAL fluid levels of keratinocyte chemoattractant (KC or CXCL1), IFN-γ-inducible protein 10 (IP-10 or CXCL10), monocyte chemoattractant protein-1 (MCP-1 or CCL2) and IL-1β were measured using elisa (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions.
Measurements of oxidative lung damage and antioxidant activities
BAL fluid levels of oxidative damage markers were measured using enzyme immunoassays for 3-NT (Upstate/Millipore, Billerica, MA, USA), and for 8-isoprostane (8-iso-PGF2α) and 8-OHdG (Cayman Chemicals, Ann Arbor, MI, USA). Frozen lung tissues were homogenized in PBS, and the supernatants were used to determine the activities of catalase, superoxide dismutase (SOD), GPx and GR using enzymatic assay kits (Cayman Chemicals).
Preparation of cigarette smoke extract
Cigarette smoke extract (CSE) was freshly prepared immediately before each experiment by bubbling smoke from one cigarette to 10 mL of RPMI 1640 medium supplemented with 1% FBS at a rate of one cigarette every 10 min as described (Kode et al., 2008). CSE was adjusted to pH 7.4 and sterile-filtered through a 0.45-μm Acrodisc filter (Pall, Ann Arbor, MI, USA). CSE preparation was standardized by setting the optical density at 0.8 ± 0.05 with absorbance wavelength at 340 mm. Control medium was prepared in the same way as CSE except for bubbling fresh air into 10 mL of RPMI medium.
Bronchial epithelium exposure to CSE and andrographolide treatment
Human transformed bronchial epithelial cells BEAS-2B (American Type Tissue Collection, Rockville, MD, USA) were cultured in RPMI 1640 supplemented with 1% FBS. Cells were pre-incubated with a non-toxic concentration of 30 μM andrographolide (Bao et al., 2009) or vehicle (0.05% DMSO) for 1 h before exposure to 2% CSE for indicated times. Using the MTS assay, 2% CSE had no adverse effect on the viability of BEAS-2B cells (data not shown).
Nrf2 analysis
BEAS-2B cells were stimulated with CSE for 4 and 24 h in the presence and absence of andrographolide (30 μM). Cytoplasmic and nuclear extractions were performed using a nuclear extraction kit (Active Motif, Carlsbad, CA, USA), and the nuclear extracts were used for measuring Nrf2 binding activity to immobilized antioxidant response elements (ARE) using a TransAM™ Nrf2 kit (Active Motif). For Nrf2 nuclear translocation, both cytoplasmic and nuclear proteins (30 μg per lane) were separated by 10% SDS-PAGE, and immunoblots were probed with rabbit anti-Nrf2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse anti-β-actin and mouse anti-TATA box binding protein (TBP) (Abcam, Cambridge, UK) antibodies. Band intensity was quantitated using ImageJ software (NIH) as described previously (Goh et al., 2012).
GSH assay
BEAS-2B cells were stimulated with CSE for 24 h in the presence and absence of andrographolide (30 μM). Cell lysates were obtained and deproteinated, and glutathione levels were determined using a GSH assay kit (Cayman Chemical).
Quantitative real-time PCR analysis
Total mRNA was extracted from mouse lung tissues and BEAS-2B cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and then used for first-strand cDNA synthesis. Primers for inflammatory and antioxidant genes are listed in Table 1. Template cDNA (100 ng) in PCR mixture containing Fast SYBR Green Master Mix (Applied Biosystems, Carlsbad, CA, USA) was amplified and quantitated using a sequence detector (ABI 7500 Cycler; Applied Biosystems). The mRNA expression levels for all samples were normalized to the level of the housekeeping gene 18S.
Table 1.
Primer sets for reverse transcriptase-PCR analysis
| Targets | Sequences | |
|---|---|---|
| Forward | Reverse | |
| mMMP-12 | 5′-TTTCTTCCATATGGCCAAGC-3′ | 5′-GGTCAAAGACAGCTGCATCA-3′ |
| mTIMP-1 | 5′-GTGGGAAATGCCGCAGAT-3′ | 5′-GGGCATATCCACAGAGGCTTT-3′ |
| mGM-CSF | 5′-GGGCGCCTTGAACATGAC-3′ | 5′-TTGTGTTTCACAGTCCGTTTCC-3′ |
| mTNF-α | 5′-TCGAGTGACAAGCCCGTAGC-3′ | 5′- CTCAGCCACTCCAGCTGCTC-3′ |
| mMIP-2α | 5′-AGTGAACTGCGCTGTCAATGC-3′ | 5′-AGGCAAACTTTTTGACCGCC-3′ |
| miNOS | 5′-CGGGCAAACATCACATTCAGATCCCG-3′ | 5′-TATATTGCTGTGGCTCCCATGTT-3′ |
| hGCLM | 5′-AATCAACCCAGATTTGGTCAGG-3′ | 5′-GAGATACAGTGCATTCCAAGACA-3′ |
| hGCLC | 5′-GGAGGAAACCAAGCGCCAT-3′ | 5′-CTTGACGGCGTGGTAGATGT-3′ |
| hGR | 5′-CACTTGCGTGAATGTTGGATG-3′ | 5′-TGGGATCACTCGTGAAGGCT-3′ |
| hGPx-2 | 5′-GGTAGATTTCAATACGTTCCGGG-3′ | 5′-AGCCACATTCTCAATCAGCAC-3′ |
| hHO-1 | 5′-GCAGAGGGTGATAGAAGAGGC-3′ | 5′-GATGTTGAGCAGGAACGCAGT-3′ |
| 18S | 5′-GCCGCTAGAGGTGAAATTCTTG-3′ | 5′-CATTCTTGGCAAATGCTTTCG-3′ |
Statistical analysis
Data are presented as means ± SEM. One-way anova followed by Dunnett's test was used to determine significant differences between treatment groups. Significant level was set at P < 0.05.
Results
Andrographolide attenuates cigarette smoke-induced lung inflammation
Cotinine is a stable metabolite of nicotine, and it is often used as a surrogate maker for nicotine exposure (Benowitz, 2009). Plasma cotinine level in 4% cigarette smoke-exposed mice was sharply elevated above 100 ng·mL−1. In contrast, plasma cotinine level in sham air control mice was undetectable (Figure 1B). BAL fluid was collected 24 h after the last cigarette smoke or sham air exposure. Cigarette smoke inhalation markedly increased total cell and neutrophil counts, with moderate but significant elevation in macrophage count, as compared with sham air control (Figure 1C). Andrographolide (0.1, 0.5 and 1 mg·kg−1) significantly suppressed the total inflammatory cell and neutrophil counts in BAL fluid in a dose-dependent manner as compared with the DMSO control (Figure 1D). Andrographolide (1 mg·kg−1) showed a moderate inhibitory effect on macrophage count but did not reach statistical significance.
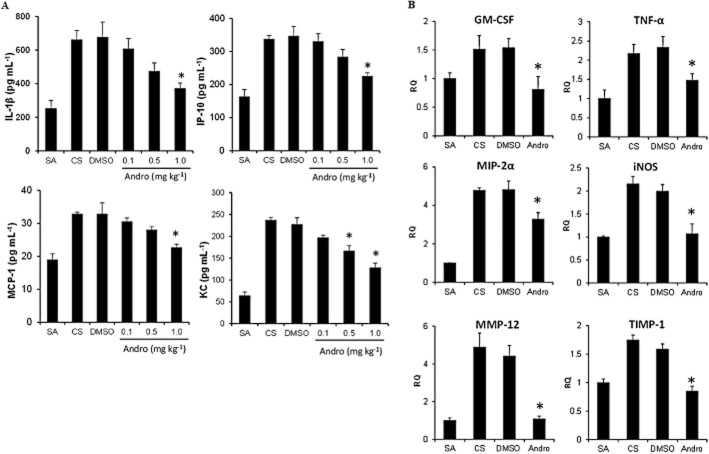
BAL fluid levels of IL-1β, IP-10 (CXCL10), MCP-1 (CCL2) and KC (CXCL1) were significantly raised in cigarette smoke-exposed mice as compared with sham air control mice (Figure 2A). Andrographolide was able to abate the BAL fluid levels of IL-1β, IP-10, MCP-1 and KC in a dose-dependent manner, reaching significant inhibition at the dose of 1 mg·kg−1.
Figure 2.
Effects of andrographolide on cigarette smoke-induced BAL fluid cytokine and chemokine levels, and lung tissue pro-inflammatory gene expression in mice. (A) BAL fluid levels of IL-1β, MCP-1, KC and IP-10 were analysed using elisa (n = 6 mice per group). Lower limits of detection were as follows: IL-1β at 9 pg·mL−1; IP-10 at 15 pg·mL−1; and MCP-1 and KC at 16 pg·mL−1. (B) Real-time PCR analyses of cytokine, chemokine and protease gene expressions in lung tissues (n = 6 mice per group). The mRNA expression levels for all samples were normalized to the level of the housekeeping gene 18S. TIMP-1, tissue inhibitor of metalloproteinases-1; MIP-2α, macrophage inflammatory protein 2-α; iNOS, inducible NOS. Values shown are the mean ± SEM. *Significant difference from DMSO, P < 0.05.
In addition, andrographolide (1 mg·kg−1) significantly suppressed the elevated gene expression of lung tissue GM-CSF, TNF-α and MIP-2α (as known as GRO-β or CXCL2) induced by cigarette smoking (Figure 2B). Cigarette smoke also up-regulated the gene expression of inducible NOS (iNOS), a pro-oxidant enzyme responsible for NO production and subsequent oxidative lung damage. Andrographolide markedly reversed the iNOS gene expression down to basal level (Figure 2B). Furthermore, the increase in gene expression of MMP-12 and tissue inhibitor of metalloproteinase-1 (TIMP-1), a metalloproteinase and an anti-metalloproteinase critical for lung remodelling and repair in COPD, by cigarette smoke was significantly inhibited by 1 mg·kg−1 andrographolide (Figure 2B).
Andrographolide protects against cigarette smoke-induced oxidative lung damage
BAL fluid was used to assay for 3-NT, a product of protein nitration indicative of oxidative protein damage; 8-OHdG, a marker for oxidative DNA damage; and 8-isoprostane, an indicator for lipid peroxidation, using elisa. Cigarette smoking significantly elevated BAL fluid levels of 3-NT, 8-OHdG and 8-isoprostane as compared to the sham air control (Figure 3). Andrographolide markedly suppressed the levels of 3-NT, 8-OHdG and 8-isoprostane at all three doses used, ameliorating oxidative damage to proteins, DNA and lipids induced by cigarette smoking.
Figure 3.
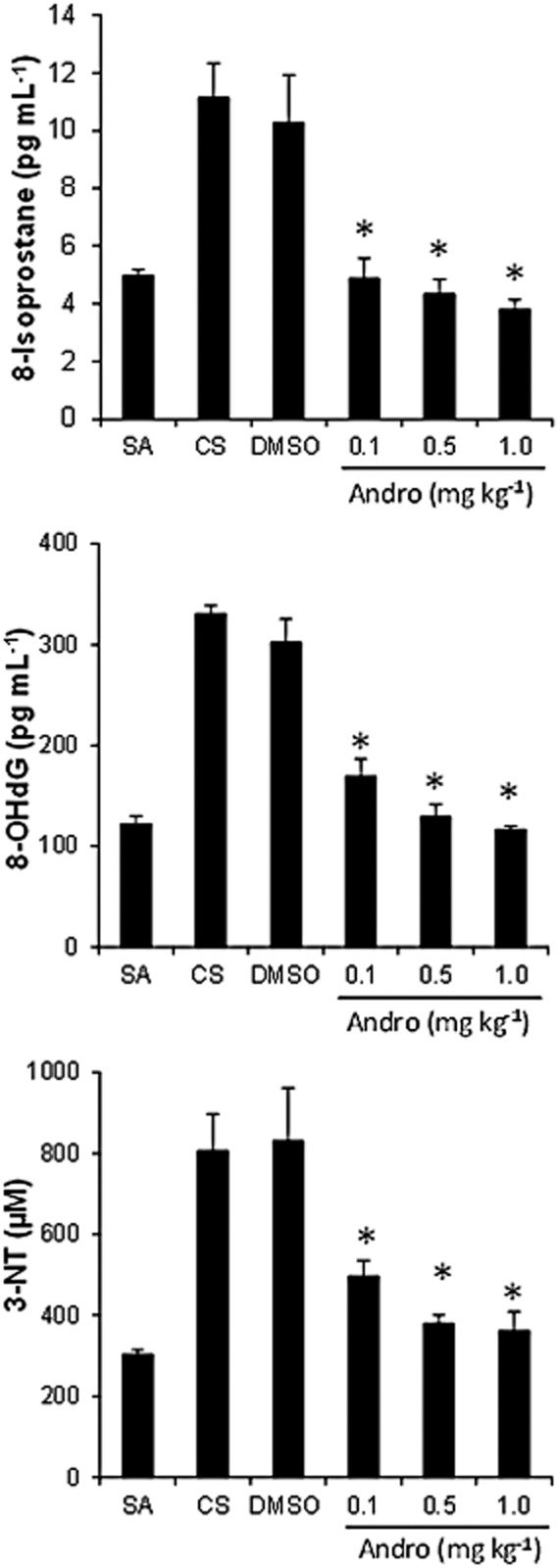
Effects of andrographolide on cigarette smoke-induced BAL fluid oxidative damage marker levels. 8-Isoprostane, 8-OHdG and 3-NT levels were measured using elisa. Lower limits of detection were as follows: 8-isoprostane at 2.7 pg·mL−1; 8-OHdG at 33 pg·mL−1; and 3-NT at 0.1 μM. Values are means ± SEM for six mice per group. *Significant difference from DMSO, P < 0.05.
Andrographolide augments the GPx and GR activities
Cigarette smoke exposure induced a marked adaptive increase in the lung catalase activity as compared with sham air. Andrographolide (1 mg·kg−1) significantly reduced the catalase activity. In contrast, lung SOD activity was not altered by cigarette smoke challenge or by andrographolide treatment (Figure 4). The activity of GPx, an antioxidant enzyme capable of reducing H2O2 to H2O by oxidizing GSH, was not altered by cigarette smoke but was substantially augmented in cigarette smoke-exposed mouse lungs by andrographolide. On the other hand, lung GR activity was markedly abated by cigarette smoke exposure, but andrographolide treatment totally restored GR activity (Figure 4). In brief, andrographolide promoted the GSH-related enzyme activities, which may be attributable to its antioxidant effects.
Figure 4.
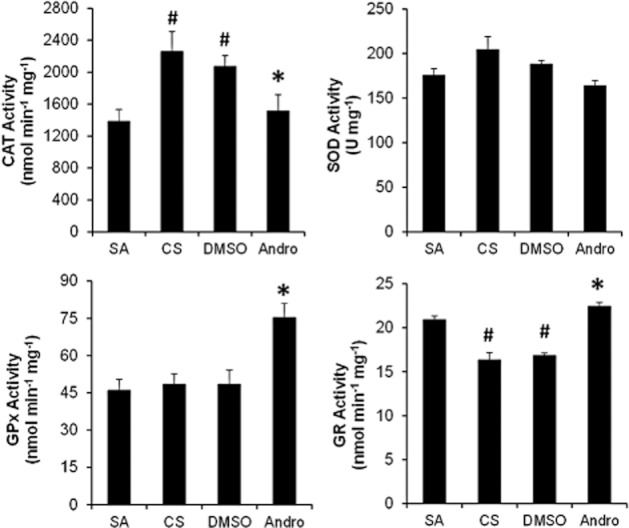
Effects of andrographolide on cigarette smoke-induced lung antioxidant enzymatic activities. Mice were exposed to 4% cigarette smoke for 1 h daily for five consecutive days with or without andrographolide treatment. Enzymatic activities of SOD, catalase, GPx and GR in lung tissues obtained 24 h after the last cigarette smoke challenge were measured using elisa. Values are means ± SEM for six mice per group. #Significant different from sham air control and *significant difference from DMSO, P < 0.05.
Andrographolide promotes nuclear Nrf2 accumulation and GSH level
To investigate if the antioxidative effects of andrographolide were mediated through Nrf2 activation, we studied nuclear Nrf2 translocation and transactivation in BEAS-2B human bronchial epithelial cells. Addition of 2% CSE to BEAS-2B cells resulted in marked nuclear translocation of Nrf2 at 4 h, and andrographolide (30 μM) had no effect on nuclear Nrf2 level. In contrast, at 24 h after CSE exposure, nuclear Nrf2 dropped to the basal level, but andrographolide treatment maintained the nuclear Nrf2 level elevated in BEAS-2B cells (Figure 5A). In contrast, cytoplasmic levels of Nrf2 were very low in control and all treatment groups (Figure 5A). Using Nrf2 transactivation assay, Nrf2 binding to ARE remained significantly higher in andrographolide-treated BEAS-2B cells 24 h after 2% CSE exposure (Figure 5B). Furthermore, cellular GSH level was also strongly elevated by andrographolide in BEAS-2B cells exposed to 2% CSE for 24 h (Figure 5C). Our data suggest that andrographolide is able to sustain nuclear Nrf2 accumulation and activation in cells exposed to cigarette smoke, leading to augmented production of GSH.
Figure 5.
Effects of andrographolide on nuclear Nrf2 and cellular GSH levels. (A) Cytoplasmic and nuclear extracts of BEAS-2B cells treated with 2% CSE in the presence and absence of 30 μM andrographolide for 4 h and 24 h were separated in 10% SDS-PAGE. Immunoblots were probed for Nrf2. β-actin and TBP were used as internal controls for cytosolic and nuclear proteins respectively. The experiments were repeated four times with similar pattern of results. Protein band intensities were analysed using ImageJ software and were normalized to β-actin controls. Values shown are the mean ± SEM. (B) ARE-binding activity of Nrf2 in nuclear extracts of BEAS-2B cells stimulated with 2% CSE in the presence and absence of 30 μM andrographolide for 4 h and 24 h was determined using a TransAM™ Nrf2 elisa kit. (C) Cellular GSH levels at 24 h after exposure to 2% CSE in the presence and absence of 30 μM andrographolide were measured using a GSH assay kit. Experiments were repeated four times, and values are expressed as means ± SEM. #Significant different from control and *significant difference from DMSO, P < 0.05.
Andrographolide augments Nrf2-regulated gene targets
To investigate biological responses to nuclear Nrf2 accumulation and activation, we examined Nrf2-specific antioxidant gene expressions including glutamate-cysteine ligase catalytic (GCLC) subunit, glutamate-cysteine ligase modifier (GCLM) subunit, GR, GPx-2 and HO-1 in BEAS-2B cells exposed to 2% CSE for 12 h and 24 h. Andrographolide significantly enhanced the expression of antioxidants GCLM, GCLC, GR, GPx-2 and HO-1 in CSE-exposed BEAS-2B cells (Figure 6). Notably, andrographolide augmented gene expression of both GPx-2 and HO-1 by about 8- to 12-fold as compared with DMSO controls. Our results clearly indicate that andrographolide is a potent enhancer of antioxidant expression in response to cigarette smoke.
Figure 6.
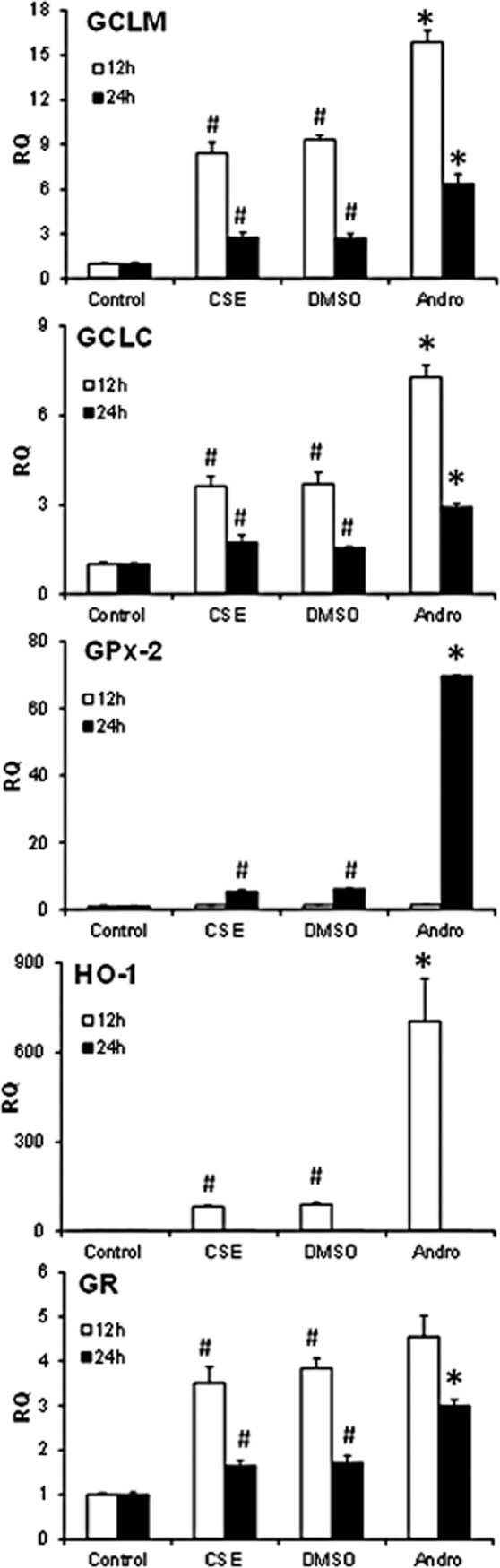
Effects of andrographolide on antioxidant gene expression. Total RNA was extracted from BEAS-2B cells treated with 2% CSE in the presence and absence of 30 μM andrographolide for 12 and 24 h, and gene expression was quantified using real-time PCR and normalized to 18S control gene. Experiments were repeated three times and values are expressed as means ± SEM. #Significant different from control and *significant difference from DMSO, P < 0.05.
Discussion
Cigarette smoke contains high concentrations of potent oxidants and free radicals including reactive aldehyde, quinone, hydroquinone, semiquinone and superoxide (Cantin, 2010; Yao and Rahman, 2011). Exposure to cigarette smoke causes lung epithelial cell damage and triggers production of pro-inflammatory cytokines and chemokines, leading to inflammatory cell infiltration and activation, especially the neutrophils and macrophages. Both neutrophils and macrophages are rich sources of endogenous reactive oxygen species (ROS), reactive nitrogen species (RNS) and tissue proteases such as elastase and metalloproteinases, entailing additional oxidant burden to the lungs and facilitating destruction of alveolar walls. KC, GM-CSF and MIP-2α are pivotal for neutrophil lung infiltration and MCP-1 and IP-10 are essential for recruitment of macrophages and monocytes (Louhelainen et al., 2008; Cosio et al., 2009; Brusselle et al., 2011). Our findings reveal for the first time that andrographolide prevented cigarette smoke-induced lung infiltration of neutrophils and, to a lesser extent, macrophages in a dose-dependent manner and was able to suppress the increase of KC, IP-10, MCP-1, IL-1β, GM-CSF, TNF-α and MIP-2α in lungs from cigarette smoke-exposed mice.
We also observed a sharp rise in lung MMP-12 expression together with an adaptive increase in lung TIMP-1 expression in cigarette smoke-exposed mice. MMP-12 is able to degrade elastin, disrupting lung architecture leading to airspace enlargement (Mocchegiani et al., 2011). Mice deficient in MMP-12 were protected against cigarette smoke-induced lung inflammation and emphysema (Hautamaki et al., 1997). An association study has strongly suggested that MMP12 plays a role in determining lung function and susceptibility to COPD in adult smokers (Hunninghake et al., 2009). Andrographolide reversed the elevation of lung MMP-12 expression and the adaptive increase in TIMP-1 in cigarette smoke-exposed mice. These findings implicate a protective role of andrographolide against airway inflammation, remodelling and emphysema in COPD.
Oxidative stress emitted by the cigarette smoke and generated from the infiltrated macrophages and neutrophils play a pivotal role in the pathogenesis in COPD. Excessive accumulation of ROS and RNS in the lungs results in protein denaturation, lipid peroxidation and DNA damage as determined by the levels of 3-NT (Sugiura and Ichinose, 2011), 8-isoprostane (Louhelainen et al., 2008) and 8-OHdG (Tzortzaki et al., 2011; Aoshiba et al., 2012), respectively, in BAL fluid or in induced sputum. We observe that andrographolide completely blocked the increase of all three oxidant biomarkers in BAL fluid from cigarette smoke-exposed mice in a dose-dependent manner. The suppression of 3-NT level can be partly explained by the inhibitory effect of andrographolide on the iNOS expression, leading to a drop in NO production, peroxynitrite formation and protein nitration of tyrosine residues (Sugiura and Ichinose, 2011). Alternatively, andrographolide has also demonstrated potent antioxidant activity by enhancing both GPx and GR activities in lung tissues from cigarette smoke-exposed mice. GPx and GR play a critical role in generating GSH, an endogenous non-protein thiol with potent free radical scavenging capacity (Biljak et al., 2010; Cantin, 2010; Gould et al., 2011).
To investigate the molecular mechanism of antioxidant action for andrographolide, we studied the effects of andrographolide on nuclear translocation and activation of the redox-sensitive transcription factor Nrf2 and the gene expression of Nrf2-regulated antioxidant gene targets in CSE-treated BEAS-2B human bronchial epithelial cells. Nrf2 plays a predominant role in antioxidant protection against oxidative damage in the lungs incurred by cigarette smoking (Boutten et al., 2011). In line with other studies (Lee and Lee, 2011; Taguchi et al., 2011), cytoplasmic levels of Nrf2 in BEAS-2B cells in control and all treatment groups were almost undetectable. It is mainly due to rapid ubiquitination and proteasomal degradation of Nrf2 targeted by Kelch-like ECH-associated protein 1 (Keap1), a E3 ubiquitin ligase. Andrographolide strongly promoted Nrf2 nuclear stabilization and accumulation, and binding to ARE in BEAS-2B cells exposed to CSE for 24 h. Indeed, andrographolide has been shown to elevate Nrf2 nuclear level in human endothelial cells (Yu et al., 2010). Furthermore, in the process of developing a reporter gene assay for monitoring Nrf2 activation, andrographolide was found to be the most potent Nrf2 activator among 2000 biologically active compounds tested (Smirnova et al., 2011). In addition, andrographolide noticeably enhanced cellular level of GSH in BEAS-2B cells in response to CSE, providing a powerful first-line antioxidant defence against cigarette smoke-induced epithelial damage. Andrographolide has also been shown to promote GSH level in nicotine-stimulated peripheral blood lymphocytes (Das et al., 2009). Our findings strongly support the view that activation of Nrf2 pathway is an attractive therapeutic approach to the treatment of COPD (Boutten et al., 2011).
In patients with advanced COPD, Nrf2 activities are reduced in peripheral lungs, resulting in reduced antioxidant responses and persistent oxidative stress. The reduction of Nrf2 activity may be due to a drop in the Nrf2-positive regulator DJ-1 level, which permits destabilization of Nrf2 protein via rapid proteasomal degradation by Keap1 (Malhotra et al., 2008). In animals, Nrf2 gene disruption resulted in enhanced susceptibility to emphysema after cigarette smoke exposure (Rangasamy et al., 2004). Nrf2 activator such as sulforaphane was able to enhance Nrf2 antioxidant defence in response to cigarette smoke in DJ-1-disrupted human epithelial cells, indicating that Nrf2 is an effective target for COPD drug development (Malhotra et al., 2008). In the present study, we used acute cigarette smoke exposure and, instead of a drop in Nrf2 activity, we observed a slight promotion of nuclear Nrf2 level. Andrographolide further enhanced Nrf2 protein nuclear accumulation and activity, and Nrf2 antioxidant defence. To ensure therapeutic value of andrographolide in COPD, a chronic cigarette smoke-induced lung injury mouse model needs to be developed with clinical features similar to humans such as reductions in Nrf2 protein and activities, and the activator effect of andrographolide on Nrf2 antioxidant defence can then be evaluated in vivo.
In line with the Nrf2 activation by andrographolide in BEAS-2B cells, we have also observed strong inductions of Nrf2-regulated gene targets including GCLC, GCLM, GR, GPx-2 and HO-1 (Adair-Kirk et al., 2008; Hubner et al., 2009) by andrographolide in response to CSE. GCL is the rate-limiting enzyme in the GSH biosynthesis pathway. This enzyme is a heterodimer comprising of the catalytic subunit GCLC and the regulatory subunit GCLM (Biljak et al., 2010; Gould et al., 2011). Overexpression of GCL in human granulosa tumour cells has been shown to increase total GSH levels and protect against H2O2-induced cell death (Cortes-Wanstreet et al., 2009). In addition to being the most abundant cellular free radical scavenger, GSH also functions as a reducing substrate in the redox cycle to facilitate the reduction of H2O2 by GPx to H2O and formation of glutathione disulphide (GSSG), to prevent peroxide-induced DNA damage, lipid peroxidation and protein degradation. GSSG is a substrate for GR to regenerate GSH to sustain the redox cycle (Biljak et al., 2010; Cantin, 2010). GPx and GR are important endogenous antioxidant enzymes responsible for the oxidative balance in the lungs in response to cigarette smoke (Biljak et al., 2010; Gould et al., 2011). It has been reported that GPx activity was markedly decreased in patients with COPD (Biljak et al., 2010). It is noteworthy that andrographolide was able to augment the GPx-2 expression level by at least 12-fold in BEAS-2B cells in response to CSE stimulation. The observed slow rate of GPx-2 gene induction starting at 24 h is consistent with that reported by Singh et al. (2006) that Nrf2 activation-induced GPx-2 expression occurred after 24 h and peaked at 72 h. The slow induction of GPx-2 might be due to the slow degradation rate of this protein (Chu et al., 1999). Our findings clearly indicate that activation of Nrf2 activity leading to up-regulation of GSH level is an antioxidant mechanism of action for andrographolide against cigarette smoking.
Aside from the GSH cycle, andrographolide also induced a rapid eightfold increase in HO-1 gene expression in BEAS-2B cells exposed to CSE within 12 h. Our findings are in line with those reported that HO-1 mRNA induction peaked at 6–8 h and returned to basal level at 24 h in human lung fibroblasts stimulated with CSE (Baglole et al., 2008) and in human bronchial epithelial cells treated with Nrf2 activator (Kumar et al., 2011). Under oxidative stress condition, free heme, a pro-oxidant catalysing production of free radicals, is released from hemoproteins to induce oxidative damages. HO-1 can be rapidly induced under the control of the Nrf2 transcription factor to catabolize heme into labile iron (Fe), carbon monoxide and biliverdin. The latter end product can be further converted by biliverdin reductase into bilirubin. All three reactive products have been shown to possess antioxidant and anti-inflammatory actions (Fredenburgh et al., 2007; Gozzelino et al., 2010). Patients with COPD showed reduced levels of HO-1 in BAL fluid alveolar macrophages (Fredenburgh et al., 2007). Adenovirus-mediated gene transfer of HO-1 in mice ameliorated elastase-induced inflammation and airspace enlargement (Shinohara et al., 2005). Polymorphisms of the HO-1 promoter associated with reduced HO-1 expression have been linked to increased susceptibility to emphysema development (Exner et al., 2004). The antioxidant actions of andrographolide against cigarette smoke-induced lung injury may also be linked to augmented HO-1 expression.
In conclusion, this study reveals for the first time that andrographolide can augment antioxidant gene expression and activities probably via up regulation of Nrf2 activity in experimental models of cigarette smoke exposure. The antioxidant mechanisms of action for andrographolide are summarized in Figure 7. In addition, inhibition of NF-κB is another well-established mechanism of action for andrographolide to bring about anti-inflammatory effects (Bao et al., 2009; Lim et al., 2012), which may contribute to some of the protective effects observed in the present study. The reduction in cigarette smoke-induced lung oxidative damage afforded by andrographolide is likely associated with the significant decline in BAL fluid inflammatory cell counts, in chemokine, cytokine and protease productions, and the up-regulation of GSH redox defence system and HO-1 expression. These findings support a novel therapeutic value for andrographolide in the treatment of COPD.
Figure 7.
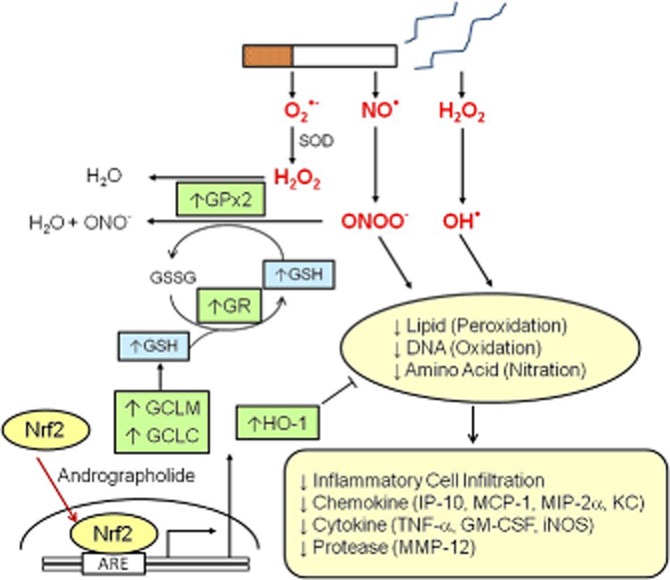
Summary of proposed antioxidant mechanisms of action of andrographolide in cigarette smoke-induced oxidative damage to the lungs. Cigarette smoke contains reactive oxygen and reactive nitrogen species such as NO•, superoxide anion (O2•−) and hydrogen peroxide (H2O2), which can be transformed into more damaging free radicals such as hydroxide radical (OH•) or peroxynitrate (ONOO-), that will lead to lipid peroxidation, DNA oxidation and amino acid nitration. Oxidative damage induces expression of pro-inflammatory cytokines and chemokines, and subsequent pulmonary inflammatory cell infiltration. Andrographolide promotes Nrf2 nuclear translocation and binding to antioxidant response element (ARE) leading to up-regulation of antioxidants GCLM, GCLC, GPx-2, GR and HO-1, and subsequent production of GSH and removal of H2O2 and ONOO-. These actions contribute to the protective effects of andrographolide in cigarette smoke-induced lung injury.
Acknowledgments
This research work was supported in part by a research grant BMRC 09/1/21/19/595 from the BioMedical Research Council of Singapore and by a COT grant HQ/S10-095COT0_21.
Glossary
- 3-NT
3-nitrotyrosine
- 8-OHdG
8-hydroxydeoxyguanosine
- ARE
antioxidant response elements
- BAL
bronchoalveolar lavage
- COPD
chronic obstructive pulmonary disease
- CSE
cigarette smoke extract
- GCLC
glutamate-cysteine ligase catalytic
- GCLM
glutamate-cysteine ligase modifier
- GPx
glutathione peroxidase
- GR
glutathione reductase
- GSH
glutathione
- GSSG
glutathione disulphide
- HO-1
heme oxygenase-1
- iNOS
inducible NOS
- IP-10
IFN-γ-inducible protein 10
- KC
keratinocyte chemoattractant
- MCP-1
monocyte chemoattractant protein-1
- Nrf2
nuclear factor erythroid-2-related factor 2
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TBP
TATA box binding protein
- TIMP-1
tissue inhibitor of metalloproteinase-1
Conflicts of interest
None.
References
- Abu-Ghefreh AA, Canatan H, Ezeamuzie CI. In vitro and in vivo anti-inflammatory effects of andrographolide. Int Immunopharmacol. 2009;9:313–318. doi: 10.1016/j.intimp.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Adair-Kirk TL, Atkinson JJ, Griffin GL, Watson MA, Kelley DG, DeMello D, et al. Distal airways in mice exposed to cigarette smoke. Nrf2-regulated genes are increased in Clara cells. Am J Respir Cell Mol Biol. 2008;39:400–411. doi: 10.1165/rcmb.2007-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adcock IM, Barnes PJ. Molecular mechanisms of corticosteroid resistance. Chest. 2008;134:394–401. doi: 10.1378/chest.08-0440. [DOI] [PubMed] [Google Scholar]
- Aoshiba K, Zhou F, Tsuji T, Nagai A. DNA damage as a molecular link in the pathogenesis of COPD in smokers. Eur Respir J. 2012;39:1368–1376. doi: 10.1183/09031936.00050211. [DOI] [PubMed] [Google Scholar]
- Baglole CJ, Sime PJ, Phipps RP. Cigarette smoke-induced expression of heme oxygenase-1 in human lung fibroblasts is regulated by intracellular glutathione. Am J Physiol Lung Cell Mol Physiol. 2008;295:L624–L636. doi: 10.1152/ajplung.90215.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Z, Guan SP, Cheng C, Wu S, Wong SH, Kemeny DM, et al. A novel antiinflammatory role for andrographolide in asthma via inhibition of the nuclear factor-κB pathway. Am J Respir Crit Care Med. 2009;179:657–665. doi: 10.1164/rccm.200809-1516OC. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. New therapies for chronic obstructive pulmonary disease. Med Princ Pract. 2010;19:330–338. doi: 10.1159/000316368. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009;49:57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biljak VR, Rumora L, Cepelak I, Pancirov D, Popovic-Grle S, Soric J, et al. Glutathione cycle in stable chronic obstructive pulmonary disease. Cell Biochem Funct. 2010;28:448–453. doi: 10.1002/cbf.1675. [DOI] [PubMed] [Google Scholar]
- Boutten A, Goven D, Artaud-Macari E, Boczkowski J, Bonay M. NRF2 targeting: a promising therapeutic strategy in chronic obstructive pulmonary disease. Trends Mol Med. 2011;17:363–371. doi: 10.1016/j.molmed.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Braber S, Koelink PJ, Henricks PAJ, Jackson PL, Nijkamp FP, Garssen J, et al. Cigarette smoke-induced lung emphysema in mice is associated with prolyl endopeptidase, an enzyme involved in collagen breakdown. Am J Physiol Lung Cell Mol Physiol. 2011;300:L255–L265. doi: 10.1152/ajplung.00304.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusselle GG, Joos GF, Bracke K. New insights into the immunology of chronic obstructive pulmonary disease. Lancet. 2011;378:1015–1026. doi: 10.1016/S0140-6736(11)60988-4. [DOI] [PubMed] [Google Scholar]
- Cantin AM. Cellular response to cigarette smoke and oxidants. Adapting to survive. Proc Am Thorac Soc. 2010;7:368–375. doi: 10.1513/pats.201001-014AW. [DOI] [PubMed] [Google Scholar]
- Chan KH, Ho SP, Yeung SC, So WH, Cho CH, Koo MW, et al. Chinese green tea ameliorates lung injury in cigarette smoke-exposed rats. Respir Med. 2009;103:1746–1754. doi: 10.1016/j.rmed.2009.04.027. [DOI] [PubMed] [Google Scholar]
- Chang J, Zhang RM, Zhang Y, Chen ZB, Zhang ZM, Xu Q, et al. Andrographolide drop-pill in the treatment of acute upper respiratory tract infection with external wind-heat syndrome: a multicenter and randomized controlled trial. J Chin Integr Med. 2008;6:1238–1245. doi: 10.3736/jcim20081206. [DOI] [PubMed] [Google Scholar]
- Cho HY, Reddy SP, Kleeberger SR. Nrf2 defends the lung from oxidative stress. Antioxid Redox Signal. 2006;8:76–87. doi: 10.1089/ars.2006.8.76. [DOI] [PubMed] [Google Scholar]
- Chu FF, Esworthy RS, Lee L, Wilczynski S. Retinoic acid induces Gpx2 gene expression in MCF-7 human breast cancer cells. J Nutr. 1999;129:1846–1854. doi: 10.1093/jn/129.10.1846. [DOI] [PubMed] [Google Scholar]
- Coon JT, Ernst E. Androgaphis paniculata in the treatment of upper respiratory tract infection: a systematic review of safety and efficacy. Planta Med. 2004;70:93–298. doi: 10.1055/s-2004-818938. [DOI] [PubMed] [Google Scholar]
- Cortes-Wanstreet MM, Giedzinski E, Limoli CL, Luderer U. Overexpression of glutamate-cysteine ligase protects human COV434 granulosa tumour cells against oxidative and γ-radiation-induced cell death. Mutagenesis. 2009;24:211–224. doi: 10.1093/mutage/gen073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med. 2009;360:2445–2454. doi: 10.1056/NEJMra0804752. [DOI] [PubMed] [Google Scholar]
- Das S, Neogy S, Gautam N, Roy S. In vitro nicotine induced superxoide mediated DNA fragmentation in lymphocytes: protective role of Andrographis paniculata Nees. Toxicol In Vitro. 2009;23:90–98. doi: 10.1016/j.tiv.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Exner M, Minar E, Wagner O, Schillinger M. The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic Biol Med. 2004;37:1097–1104. doi: 10.1016/j.freeradbiomed.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Fredenburgh LE, Perrella MA, Mitsialis SA. The role of heme oxygenase-1 in pulmonary disease. Am J Respir Cell Mol Biol. 2007;36:158–165. doi: 10.1165/rcmb.2006-0331TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh FY, Upton N, Guan SP, Cheng C, Shanmugam MK, Sethi G, et al. Fisetin, a bioactive flavonol, attenuates allergic airway inflammation through negative regulation of NF-κB. Eur J Pharmacol. 2012;679:109–116. doi: 10.1016/j.ejphar.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Gould NS, Min E, Gauthier S, Martin RJ, Day BJ. Lung glutathione adaptive responses to cigarette smoke exposure. Respir Res. 2011;12:133. doi: 10.1186/1465-9921-12-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–354. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277:2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- Hubner RH, Schwartz JD, De BP, Ferris B, Omberg L, Mezey JG, et al. Coordinate control of expression of Nrf2-modulated genes in the human small airway epithelium is highly responsive to cigarette smoking. Mol Med. 2009;15:203–219. doi: 10.2119/molmed.2008.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake GM, Cho MH, Tesfaigzi Y, Soto-Qiros ME, Avila L, Lasky-Su J, et al. MMP12, lung function, and COPD in high-risk populations. N Engl J Med. 2009;361:2599–2608. doi: 10.1056/NEJMoa0904006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kode A, Rajendrasozhan S, Caito S, Yang SR, Megson IL, Rahman I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L478–L488. doi: 10.1152/ajplung.00361.2007. [DOI] [PubMed] [Google Scholar]
- Kumar V, Kumar S, Hassan M, Wu H, Thimmulappa RK, Kumar A, et al. Novel chalcone derivatives as potent Nrf2 activators in mice and human lung epithelial cells. J Med Chem. 2011;54:4147–4159. doi: 10.1021/jm2002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Lee SH. Sulforaphane induces antioxidative and antiproliferative responses by generating reactive oxygen species in human bronchial epithelial BEAS-2B cells. J Korean Med Sci. 2011;26:1474–1482. doi: 10.3346/jkms.2011.26.11.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JCW, Chan TK, Ng DSW, Sagineedu SR, Stanslas J, Wong WSF. Andrographolide and its analogues: verstaile bioactive molecules for combating inflammation and cancer. Clin Exp Pharmacol Physiol. 2012;39:300–310. doi: 10.1111/j.1440-1681.2011.05633.x. [DOI] [PubMed] [Google Scholar]
- Lin TP, Chen SY, Duh PD, Chang LK, Liu YN. Inhibition of the Epstein-Barr virus lytic cycle by andrographolide. Biol Pharm Bull. 2008;31:2018–2023. doi: 10.1248/bpb.31.2018. [DOI] [PubMed] [Google Scholar]
- Louhelainen N, Myllarniemi M, Rahman I, Kinnula VL. Airway biomarkers of the oxidant burden in asthma and chronic obstructive pulmonary disease: current and future perspectives. Int J Chron Obstruct Pulmon Dis. 2008;3:585–603. doi: 10.2147/copd.s3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra D, Thimmulappa R, Navas-Acien A, Sandford A, Elliott M, Singh A, et al. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J Respir Crit Care Med. 2008;178:592–604. doi: 10.1164/rccm.200803-380OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- McGrath J, Drummond G, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocchegiani E, Giacconi R, Costarelli L. Metalloproteases/anti-metalloproteases imbalance in chronic obstructive pulmonary disease: genetic factors and treatment implicartions. Curr Opin Pulm Med. 2011;17(Suppl. 1):S11–S19. doi: 10.1097/01.mcp.0000410743.98087.12. [DOI] [PubMed] [Google Scholar]
- Morjaria JB, Malerba M, Polosa R. Biologic and pharmacologic therapies in clinical development for the inflammatory response in COPD. Drug Discov Today. 2010;15:396–405. doi: 10.1016/j.drudis.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Morris A, Kinnear G, Wan WY, Wyss D, Bahra P, Stevenson CS. Comparison of cigarette smoke-induced acute inflammation in multiple strains of mice and the effect of a matrix metalloproteinase inhibitor on these responses. J Pharmacol Exp Ther. 2008;327:851–862. doi: 10.1124/jpet.108.140848. [DOI] [PubMed] [Google Scholar]
- Negi AS, Kumar JK, Luqman S, Shanker K, Gupta MM, Khanuja SP. Recent advances in plant hepatoprotectives: a chemical and biological profile of some important leads. Med Res Rev. 2008;28:746–772. doi: 10.1002/med.20115. [DOI] [PubMed] [Google Scholar]
- Page CP, Spina D. Selective PDE inhibitors as novel treatment for respiratory diseases. Curr Opin Pharmacol. 2012;12:1–12. doi: 10.1016/j.coph.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Rabe KF. Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br J Pharmacol. 2011;163:53–67. doi: 10.1111/j.1476-5381.2011.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, et al. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara T, Kaneko T, Nagashima Y, Ueda A, Tagawa A, Ishigatsubo Y. Adenovirus-mediated transfer and overexpression of heme oxygenase 1 cDNA in lungs atenuates elastase-induced pulmonary emphysema in mice. Hum Gene Ther. 2005;16:318–327. doi: 10.1089/hum.2005.16.318. [DOI] [PubMed] [Google Scholar]
- Singh A, Rangasamy T, Thimmulappa RK, Lee H, Osburn WO, Brigelius-Flohe R, et al. Glutathione peroxidase 2, the major cigarette smoke-inducible isoform of GPX in lungs, is regulated by Nrf2. Am J Respir Cell Mol Biol. 2006;35:639–650. doi: 10.1165/rcmb.2005-0325OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova NA, Haskew-Layton RE, Basso M, Hushpulian DM, Payappilly JB, Speer RE, et al. Development of Neh2-luciferase reporter and its application for high throughput screening and real-time monitoring of Nrf2 activators. Chem Biol. 2011;18:752–765. doi: 10.1016/j.chembiol.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura H, Ichinose M. Nitrative stress in inflammatory lung diseases. Nitric Oxide. 2011;25:138–144. doi: 10.1016/j.niox.2011.03.079. [DOI] [PubMed] [Google Scholar]
- Sussan TE, Rangasamy T, Blake DJ, Malhotra D, El-Haddad H, Bedja D, et al. Targeting Nrf2 with the triterpenoid CDDO-imidazolide attenuates cigarette smoke-induced emphysema and cardiac dysfunction in mice. Proc Natl Acad Sci U S A. 2009;106:250–255. doi: 10.1073/pnas.0804333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi K, Motohashi H, Yamamoto M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells. 2011;16:123–140. doi: 10.1111/j.1365-2443.2010.01473.x. [DOI] [PubMed] [Google Scholar]
- Thatcher TH, Benson RP, Phipps RP, Sime PJ. High-dose but not low-dose mainstream cigarette smoke suppresses allergic airway inflammation by inhibiting T cell function. Am J Physiol Lung Cell Mol Physiol. 2008;295:L412–L421. doi: 10.1152/ajplung.00392.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzortzaki EG, Kimakou K, Neofytou E, Tsikritsaki K, Samara K, Avgousti M, et al. Oxidative DNA damage and somatic mutations: a link to the molecular pathogenesis of chronic inflammatory airway diseases. Chest. 2011;141:1243–1250. doi: 10.1378/chest.11-1653. [DOI] [PubMed] [Google Scholar]
- Vlahos R, Bozinovski S, Chan SP, Ivanov S, Linden A, Hamilton JA, et al. Neutralizing granulocyte/macrophage colony-stimulating factor inhibits cigarette smoke-induced lung inflammation. Am J Respir Crit Care Med. 2010;182:34–40. doi: 10.1164/rccm.200912-1794OC. [DOI] [PubMed] [Google Scholar]
- Yao H, Rahman I. Current concepts on oxidative/carbonyl stress, inflammation and epigenetics in pathogenesis of chronic obstructive pulmonary disease. Toxciol Appl Pharmacol. 2011;254:72–85. doi: 10.1016/j.taap.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AL, Lu CY, Wang TS, Tsai CW, Liu KL, Cheng YP, et al. Induction of heme oxygenase 1 and inhibition of tumor necrosis factor α-induced intercellular adhesion molecule expression by andrographolide in EA.hy926 cells. J Agric Food Chem. 2010;58:7641–7648. doi: 10.1021/jf101353c. [DOI] [PubMed] [Google Scholar]