Abstract
Background and Purpose
Asthma is an inflammatory disease that involves airway hyperresponsiveness and remodelling. Flavonoids have been associated to anti-inflammatory and antioxidant activities and may represent a potential therapeutic treatment of asthma. Our aim was to evaluate the effects of the sakuranetin treatment in several aspects of experimental asthma model in mice.
Experimental Approach
Male BALB/c mice received ovalbumin (i.p.) on days 0 and 14, and were challenged with aerolized ovalbumin 1% on days 24, 26 and 28. Ovalbumin-sensitized animals received vehicle (saline and dimethyl sulfoxide, DMSO), sakuranetin (20 mg kg–1 per mice) or dexamethasone (5 mg kg–1 per mice) daily beginning from 24th to 29th day. Control group received saline inhalation and nasal drop vehicle. On day 29, we determined the airway hyperresponsiveness, inflammation and remodelling as well as specific IgE antibody. RANTES, IL-5, IL-4, Eotaxin, IL-10, TNF-α, IFN-γ and GMC-SF content in lung homogenate was performed by Bioplex assay, and 8-isoprostane and NF-kB activations were visualized in inflammatory cells by immunohistochemistry.
Key Results
We have demonstrated that sakuranetin treatment attenuated airway hyperresponsiveness, inflammation and remodelling; and these effects could be attributed to Th2 pro-inflammatory cytokines and oxidative stress reduction as well as control of NF-kB activation.
Conclusions and Implications
These results highlighted the importance of counteracting oxidative stress by flavonoids in this asthma model and suggest sakuranetin as a potential candidate for studies of treatment of asthma.
Keywords: sakuranetin, experimental asthma model, airway remodelling, cytokines, oxidative stress
Introduction
Asthma is a chronic allergic disease mainly characterized by airway inflammation and hyperresponsiveness (AHR), which can usually be reversed by bronchodilators (GINA,2011). The chronicity of airway inflammation, associated with pro-inflammatory mediator releases, can induce several structural changes in the lungs, such as extracellular matrix (ECM) component deposition, airway smooth muscle hyperplasia and hypertrophy, and goblet cell hyperplasia, alterations called by airway remodelling (Fahy,2001). Eosinophils and lymphocytes infiltration, particularly Th2 type, and elevated levels of IL-13, IL-4, IL-5, eotaxin, RANTES and other pro-inflammatory mediators are observed in lung from asthmatic patients (Murphy and O'Byrne,2010). An imbalance between Th2 and Th1 cytokines can be also involved in asthmatic pathophysiology (Shi et al.,2011). Among various transcription factors, NF-κB is the master switch for pro-inflammatory genes and has been involved in asthma both in experimental models and in humans (Hart et al.,2000; Poynter et al.,2002). Recently, some studies have addressed the importance of oxidative stress in asthma physiopathology and clearly reported that isoprostanes are an important oxidative marker in asthma (Brussino et al.,2010; Voynow and Kummarapurugu,2011). Brussino et al. (2010) showed that there is an increase in 8-isoprostane in exhaled air after allergen challenge in mild asthmatics. In this regard, using an animal model of asthma, Park et al. (2009) showed that immediately after the first antigen injection, there is an increase in oxidative stress in the lung, and the use of an antioxidant attenuated not only the reactive oxidative stress regeneration but also the inflammation in this animal model.
Although the gold standard treatment for asthma is the glucocorticoids that markedly inhibit the lung inflammation, the persistent AHR even after prolonged anti-inflammatory steroid therapy (Baraket et al.,2012) is observed in some asthmatic patients, particularly in severe asthma. This irreversible obstruction present in some asthmatics is usually associated to remodelled airway wall structure and composition leading to a change in mechanical properties (Kariyawasam et al.,2007; Southam et al.,2007). Some studies have shown that glucocorticoids treatment, even though associated to bronchodilators, do not always revert all these asthmatic features (Baraket et al.,2012). In addition, the significant incidence of adverse effects related to corticosteroids and bronchodilators, particularly β2-agonists, have been described (Miller et al.,2011; Patel et al.,2011; Ernst and Suissa,2012). For this reason, the search for a potent anti-inflammatory and nontoxic compound is inevitable in the asthma treatment.
Flavonoids are commonly found in fruits and vegetables and are consumed frequently. Animal studies revealed that phenolics compounds attenuated chronic inflammation (Rogerio et al.,2010; Taur and Patil,2011; Wu et al.,2011). There were multiples pharmacological effects attributed to flavonoids including anti-inflammatory and antioxidant potential (Havsteen,2002; Hirano et al.,2004). The structure of flavonoid sakuranetin (SK) (5,4′-dihydroxy-7-methoxyflavanone) was previously reported (Grecco et al.,2010), and it is the main component found in Baccharis retusa, a plant. Its antioxidant potential was previously reported by Soares et al. (2005), indicating that this compound displayed high antioxidant potential. In addition, sakuranetin has potential in vivo anti-allergic effects (Ogawa et al.,2007) and in vitro anti-leishmanial and anti-trypanosomal activity (Grecco et al.,2012).
Based on these observations, the present study aimed to evaluate the effects of sakuranetin treatment, by means of a chronic allergic pulmonary inflammation model, in AHR, airway inflammation and remodelling. Considering the anti-inflammatory potential of sakuranetin, we hypothesized that this treatment could attenuate the airway inflammation induced by antigen in mice. In order to understand some possible mechanisms involved in mediating the effects of sakuranetin, we measured lung content of some cytokines, oxidative stress and NF-κB expression in lung. We concluded that sakuranetin treatment attenuates several aspects of allergic airway inflammation by Th2 pro-inflammatory cytokine modulation and oxidative stress control. One possible mechanism involved in the anti-inflammatory effects of sakuranetin is the inhibition of NF-κB activation.
Methods
Ethics statement
All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al.,2010; McGrath et al.,2010). All animals received humane care in compliance which follows the rules of British Pharmacological Society's Ethics Committee. The animals used in this work were kept on a 12 h light/dark cycle in a temperature-controlled room at 21–23°C, with free access to water and food. All procedures described in this study were approved by the internal ethical committee of Faculty of Medicine of the University of São Paulo (Brazil) (Number 338/10).
Plant material
Leaves of B. retusa DC were collected in Campos do Jordão/SP, Brazil, on July 2009. The plant material was identified by Dr Oriana A Fávero, and a voucher specimen has been deposited at Herbarium of Instituto de Botânica-SEMA, São Paulo/SP, Brazil.
Extraction and isolation of sakuranetin
Dried leaves of B. retusa (460 g) were ground and extracted with hexane (4 × 500 mL) at room temperature. Sequentially, the plant material was extracted with MeOH (6 × 700 mL) at room temperature and concentrated under vacuum yielding a crude extract (32 g). This extract was partitioned between MeOH : H2O (1:2) and CH2Cl2. After evaporation under reduced pressure, the CH2Cl2 phase (15 g) was chromatographed by silica gel column chromatography (230–400 mesh; Merck, Darmstadt, Germany) eluted with CH2Cl2 containing increasing amounts of EtOAc (up to 100%) and with EtOAc containing increasing amounts of MeOH (up to 100%), to give eight fractions (F1–F8). Fraction F2 (3.5 g) was purified by silica gel column chromatography (230–400 mesh; Merck) eluted with increasing amounts of EtOAc in CH2Cl2 (up to 100%) to afford 736 mg of sakuranetin, which was characterized by 1H and 13C NMR spectra [spectrometer Bruker DPX-300, using CDCl3 (Aldrich) as solvent and TMS (Aldrich) as internal standard] as well as by low resolution electronic impact mass spectrum (spectrometer HP 5990/5988A).
Sakuranetin
1H NMR (300 MHz, CDCl3) δH: 7.26 (d, J = 8.5 Hz, H-2′/H-6′), 6.83 (d, J = 8.5 Hz, H-3′/H-5′), 6.01 (s, H-6/H-8), 5.32 (dd, J = 13.0 and 3.0 Hz, H-2), 3.77 (s, OCH3-7), 3.08 (dd, J = 17.2 and 13.0 Hz, H-3a), 2.73 (dd, J = 17.2 and 3.0 Hz, H-3b). 13C NMR (75 MHz, CDCl3) δC: 196.5 (C-4), 168.0 (C-4′), 163.6 (C-7), 163.0 (C-5), 157.4 (C-9), 129.0 (C-1′), 127.7 (C-2′/C-6′), 127.6 (C-6), 115.3 (C-3′/C-5′), 102.8 (C-10), 93.9 (C-8), 79.1 (C-2), 55.3 (OCH3), 42.8 (C-3). LREIMS (70 eV) m/z (int. rel.): 286 (67), 193 (33), 180 (39), 167 (100), 138 (24), 120 (44), 95 (38), 69 (25).
Animals
Male BALB/c mice aged 6–8 weeks (25–30 g) and adult male Wister Furth rats (120–200 g) were acquired from University of São Paulo and maintained in our own animal facilities. Male Wister Furth rats were used in the passive cutaneous anaphylaxis (PCA) test for IgE evaluation, and BALB/c was used as an experimental asthma model.
Immunization and challenge protocols
Mice were divided at random into four groups (eight animals in each group): (a) control (submitted to saline protocol and treated with vehicle); (b) OVA (ovalbumin, submitted to the OVA sensitization and treated with vehicle); (c) OVA + SK (ovalbumin–sakuranetin, submitted to the OVA sensitization and treated with sakuranetin); (d) OVA + DX (ovalbumin–dexamethasone, submitted to the OVA sensitization and treated with dexamethasone).
Animals were immunized using OVA (50 μg i.p.) (grade IV, Sigma Aldrich, St. Louis, MO) in the presence of 6 mg of Al(OH)3 adjuvant (Pepsamar, Sandei-Synthelabo SA, Rio de Janeiro, Brazil) diluted in a 0.2 mL saline solution on days 1 and 14, as previously described (Toledo et al.,2011). Aerosolized OVA (1% challenges) were started 1 week after the second immunization, on days 22, 24, 26 and 28. For that, mice were placed in a Plexiglas box (30 cm × 15 cm × 20 cm) coupled to an ultrasonic nebulizer (US-1000; ICEL, São Paulo, Brazil) and exposed to 1% OVA for 30 min. Control mice received the adjuvant (i.p.) and were exposed to a nebulized aerosol of saline (0.9% NaCl) at the same time points.
Study design
Animals subjected to OVA sensitization received daily 20 mg kg–1 (10 μL, intranasal), since day 22 until the end of protocol. Sakuranetin was diluted in DMSO (Sigma Aldrich) and saline 0.9% (1:4) (Vehicle). The dose of sakuranetin used in the present study was based on a pilot study and in some articles that use a compound with similar structure (Jung et al.,2009). In this pilot study, we tested the dose of 10 mg kg–1 and 20 mg kg–1, and we found difference only in the dose of 20 mg kg–1, which was chosen for the whole study. The animals treated with 10 mg kg–1 presented the results of lung mechanic and total cellular in BALF similar to those obtained in non-treated OVA group, suggesting that this dose did not have a therapeutically effect. To compare the sakuranetin efficacy, we also performed a group sensitized with OVA and treated with dexamethasone (DX), as previously described (Roh et al.,2004). The dexamethasone treatment was performed by i.p. injection (5 mg kg–1) in the same time point of sakuranetin treatment. Control animals were subjected to vehicle treatment. All animals were anaesthetized and submitted to mechanical ventilation at day 29, after 30 min of the last dose of sakuranetin, dexamethasone or vehicle treatment.
IgE anti-OVA antibody titration by PCA
Heterologous PCA was performed in Wistar Furth rats for anti-OVA IgE, as previously described (Mota and Wong,1969). The animals' back was shaved and injected intradermally with different serum dilutions. The animals were challenged i.v. with 0.5 mg of OVA in 0.25% Evans Blue solution, after a sensitization period of 18–24 h in rats for IgE titration. The PCA titre was expressed as the reciprocal of the highest dilution that gave an intradermic allergic reaction >5 mm in diameter in duplicate of tests. The detection of threshold of the technique was established at a 1:5 dilution (Mota and Perini,1970).
Evaluation of respiratory mechanics
Mice were anaesthetized (50 mg kg–1 i.p. thiopental), tracheostomized and connected to a rodent ventilator (FlexiVent; SCIREQ, Montreal, Canada) with the tidal volume and frequency set at 10 mL kg−1 and 2 Hz respectively. Oscillatory lung mechanics was performed by producing flow oscillations at different prime frequencies (from 0.25 to 19.625 Hz) for 16 s. Pressure and flow data were obtained and airway impedance was calculated at each frequency (Hantos et al.,2003). Airway resistance (Raw), tissue damping (Gtis) and tissue elastance (Htis) parameters were obtained by applying the constant phase model.
After baseline measurements, we performed a dose–response curve to methacholine, i.v. (10, 30, 100, 300 and 1000 μg mL−1); and values of respiratory system resistance (Rrs) and elastance (Ers) as well as Raw, Gtis and Htis were collected 30 s after each dose. Still under anaesthesia, animals were exsanguinated by vena cava dissection, and bronchoalveolar lavage fluid (BALF) was collected.
BALF
The BALF was performed by introducing 0.5 mL of sterile saline into the lungs via a tracheal cannula and withdrawing the fluid into a test tube on ice. The recovery volume was over 95% of the instilled fluid. This procedure was repeated three times. The fluid collected was centrifuged at 800× g, for 8 min, at 5°C, and the cell pellet was re-suspended in 1 mL of physiological saline. Total cells were counted using a Neubauer hemocytometer chamber and an optical microscope with a magnification of 1000×. BAL differential cell counts were performed using a cytocentrifuge. Slides were prepared by centrifugation of each sample at 900× g for 6 min (Cytospin 2, Shandon Scientific, Pittsburgh, PA). These slides were stained by Diff Quick stain, and differential counts of at least 300 cells were made according to standard morphologic criteria.
Lung histology
Lungs were then removed in block to perform histological analysis, and 3–5 μm thick sections of the lungs were stained with LUNA to detect peribronchial eosinophils (Angeli et al.,2008). Picrosirius (Direct Red 80, C.I. 35780, Sigma Aldrich) was used for bronchial collagen fibre quantification, and Weigert's Resorcin Fuchsin with oxidation was used for elastic fibres (Prado et al.,2006). The slides were coded and the researcher who performed the measurements was unaware of the study groups. Using an Leica DM4000B microscope (Leica Microsystems, Wetzlar, Germany), a digital camera (Leica DFC420 Leica Microsystems) and the image analyses software Image Proplus 4.5 (Media Cybernetics, Bethesda, MD), we evaluated collagen and elastic fibre deposition on the airway wall. Five airways at 400× magnification were evaluated for each animal. Airway collagen and elastic fibre deposition were evaluated in the area compressed between epithelial basal membranes until airway adventitia. The positive area of collagen and elastic fibres was expressed as a percentage of the total airway wall area. The eosinophils were evaluated by point-counting technique (Weibel,1963). To determine the eosinophils/unit area (104 μm2) (Prado et al.,2006; Angeli et al.,2008), we counted the number of points of the integrating eyepiece falling on areas of peribronchiolar inflammation in three areas of each airway wall and the number of eosinophils in this same area. All analyses were performed in randomly selected transversely sectioned non-cartilaginous airways.
Evaluation of 8-PGF-2α and NF-κB expression
Immunohistochemical staining was performed using antibody to anti-8-epi-PGF2α (Oxford Biomedical Research, Rochester Hills, MI) and anti-NF-κB (Oxford Biomedical Research, Rochester Hills, MI) at a 1:500 and 1:300 dilution respectively (Angeli et al.,2008). The ABCKit Vectastain (Vector Elite PK-6105, Burlingame, CA) was used as secondary antibody, and DAB (Sigma Aldrich) was used as chromogen. The sections were counterstained with Harris haematoxylin (Merck). We determined the 8-iso-PGF2α-stained area by image analysis as described above in peribronchiolar wall and the total peribronchiolar wall area. Measurements were carried out at ×400 in each slide, and the results were expressed in percentage. Positive cells immunostaining to NF-κB was evaluated by point-counting technique, as described above in LUNA evaluation. To determine the positive cells/unit area (104 μm2) (Prado et al.,2006; Angeli et al.,2008), we counted the number of points of the integrating eyepiece falling on areas of peribronchiolar inflammation in three areas of each airway wall and the number of positive cells in this same area. All analyses were performed in randomly selected transversely sectioned of five non-cartilaginous airways.
Bioplex
In the other six animals of each group, lungs were removed and quickly frozen to perform cytokine measurements in lung homogenate. A Bio-Plex mouse Plex cytokine assay kit (Bio-Rad Laboratories, Inc., Hercules, CA) was used to test samples for the presence of eight cytokines in lung homogenate. The assay was read on the Bio-Plex suspension array system, and the data were analysed using Bio-Plex Manager software version 4.0. Standard curves ranged from 32 000 to 1.95 pg mL−1, as previously described (Correa-Costa et al.,2010). The results of RANTES, IL-5, IL-4, eotaxin, TNF-α), IFN-γ, and IL-10 in lung homogenate were expressed in pg of cytokines/ pg of total protein.
Statistical analysis
Normality was evaluated by using the Kolmogorov–Smirnov test, and data were expressed as means ± SE. The parametric data were analysed by two-way anova followed by the Student–Newman–Keul's post hoc test, using the Sigma Stat software version 10 (CA). The significance level was adjusted to 5%.
Results
Chemical characterization of sakuranetin
The 1H NMR spectrum showed signals at δ 5.27 (dd, J = 13.0 and 3.0 Hz), 3.04 (dd, J = 17.2 and 13.0 Hz), and at δ 2.70 (dd, J = 17.2 and 3.0 Hz), assigned, respectively, to H-2, H-3a, and H-3b. This spectrum showed also signals attributed to H-3′/H-5′ at δ 6.81 (d, J = 8.7 Hz, 2H) and to H-2′/H-6′ at δ 7.23 (d, J = 8.7 Hz, 2H) as well as one singlet assigned to H-6/H-8 at δ 5.99 (2H). Additionally, was observed one singlet at δ 3.77 (3H), assigned to one methoxyl group linked to C-7. The 13C and DEPT 135° NMR spectra indicated the presence of carbonyl carbons at δ 196.5 (C-4), oxybenzylic carbons at δ 79.1 (C-2) and aliphatic carbons at δ 42.8 (C-3) as well as an additional peak at δ 55.3, assigned to methoxyl group at C-7. Remaining peaks at range δ 94–168 were attributed to aromatic carbons C-5 to C-10 and C-1′ to C-6′. Finally, the LREIMS spectrum analysis indicated the molecular formula C16H14O5, due the ion peak at m/z 286, as well as confirmed the position of methoxyl group at C-7, since the base peak was observed at m/z 167, characteristic of a fragment [C7H5O4]+. Comparison of spectroscopic data with those reported in the literature (Agrawal,1989), allowed the identification of sakuranetin (5,4′-dihydroxy-7-methoxy-flavanone). This compound has 99.9% of purity as indicated by HPLC analysis.
Treatment with vehicle (DMSO plus saline) did not interfere in any lung responses
Considering that the effects of DMSO in inflammation in a matter of controversy, we initially compared whether DMSO treatment plus saline (vehicle) interferes in lung inflammation and function, by comparing control and OVA groups that received vehicle, with control (saline) and OVA that did not received any kind of treatment, respectively. There were no differences between vehicle treated and non-treated groups. In addition, since DMSO has a known antioxidant effect, we also evaluated the 8-isoprostane expression in airways (%) from OVA-sensitized animals treated with vehicle (49.78 ± 3.70%) or non-treated (49.14 ± 0.89%). Both groups presented similar values related to 8-isoprostane expression, and for this reason, we used as a control of the sakuranetin treatment the animals treated with vehicle only.
Treatment with sakuranetin reduces specific OVA antibodies
First, we aimed to see whether the treatment with sakuranetin (SK) and dexametasone in OVA-sensitized animals interferes with specific IgE antibody production measured by PCA technique. Both treatments reduced the amount of IgE (Figure 1) in sensitized animals treated with dexamethasone or sakuranetin compared with sensitized animals only (P < 0.05).
Figure 1.
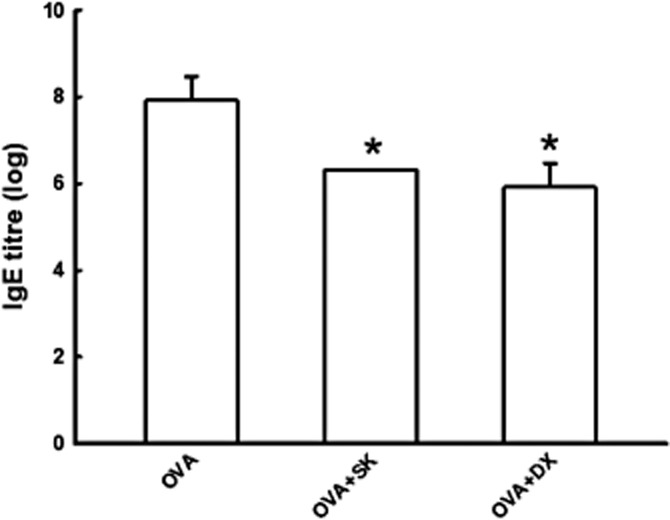
OVA-specific IgE antibodies titre measured by PCA technique. Data are presented as mean ± SE. OVA group: sensitized and treated with vehicle; OVA + SK group: sensitized and treated with sakuranetin; OVA + DX: sensitized and treated with dexamethasone. Note that both sakuranetin and dexamethasone treatments reduced the OVA-specific IgE antibodies in sensitized animals. *P < 0.001 compared with OVA.
Treatment with sakuranetin protects against AHR
In order to evaluate if sakuranetin protects lung against AHR, we performed a dose–response curve to methacholine. As we observed in Figure 2, both sakuranetin and dexametasone treatments markedly reduced the Rrs (A) and Ers (B) in sensitized animals in the final dose (1000 μg mL−1 of methacholine) (P < 0.05 for all comparisons).
Figure 2.
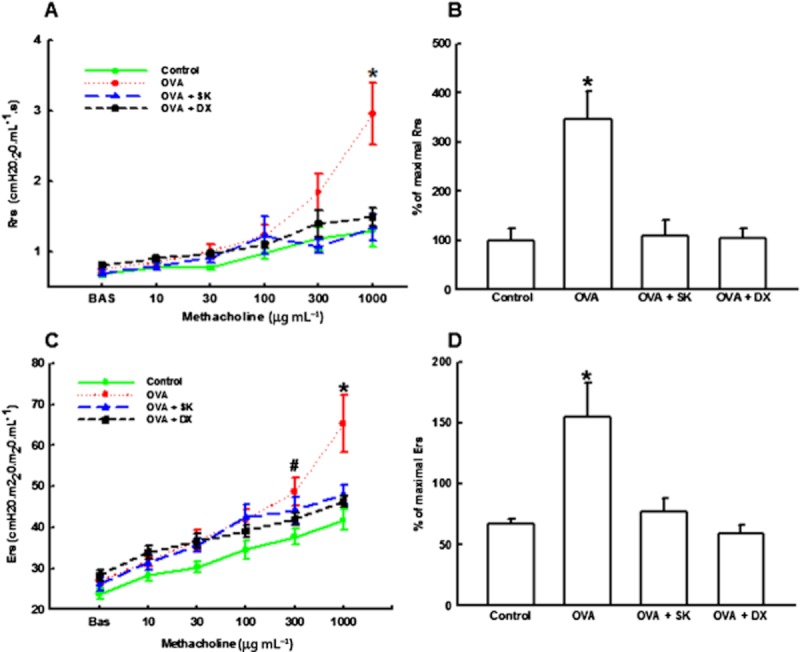
Lung mechanics. (A) Respiratory system resistance (Rrs). (B) Percentage of maximal responses related to baseline of Rrs. (C) Respiratory system elastance (Ers). (D) Percentage of maximal responses related to baseline of Ers. Control group: non-sensitized and non-treated; OVA group: sensitized and vehicle treated; OVA + SK: sensitized and sakuranetin treated; OVA + DX group: sensitized and dexamethasone treated. All results are presented as mean ± SE. *P < 0.05 compared with other groups (Control, OVA + SK and OVA + DX); #P < 0.05 compared with Control only.
We also analysed the percentage (%) of maximal responses related to baseline of Rrs and Ers (C and D respectively). The OVA group showed higher % of increase in Rrs and Ers compared with control group (P < 0.05). OVA + SK and OVA + DX groups presented all these responses reduced when compared with OVA group (P < 0.05).
We know that the responses obtained in respiratory system may be from alterations in airways and in peripheral regions. In order to indirect isolate these responses, we performed the oscillatory force mechanic that analysed airways (Raw, Figure 3A) and tissue responses (Gtis and Htis; Figure 3B and C, respectively). The Raw and Gtis were also increased in OVA group compared with those values obtained in control group (P < 0.05). We noted that both treatments attenuated these responses (P < 0.05).
Figure 3.
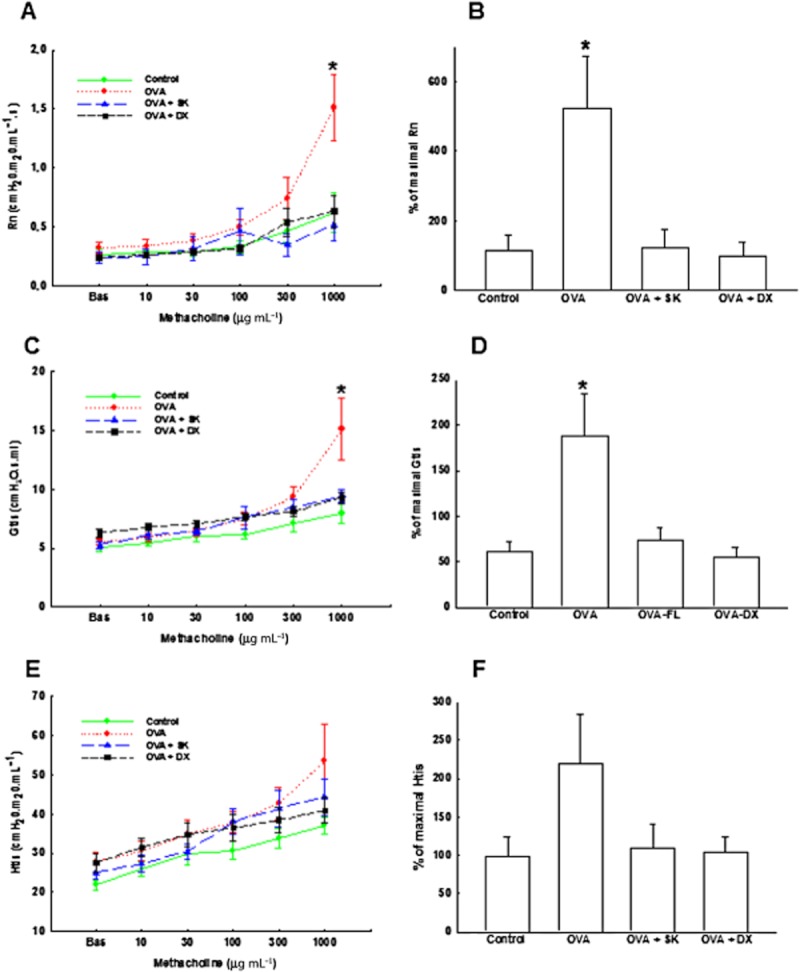
Lung mechanics. (A) Airway resistance (Raw). (B) Percentage of maximal responses related to baseline of Raw. (C) Tissue resistance (Gtis). (D) Percentage of maximal responses related to baseline of Gtis. (E) Tissue elastance (Htis). (F) Percentage of maximal responses related to baseline of Htis. Control group: non-sensitized and non-treated; OVA group: sensitized and vehicle treated; OVA + SK: sensitized and sakuranetin treated; OVA + DX group: sensitized and dexamethasone treated. All results are presented as mean ± SE. *P < 0.05 compared with other groups (Control, OVA + FL and OVA + DX).
We also analysed the percentage of maximal responses related to baseline of Raw, Gtis and Htis presented in Figure 3D–F respectively. The OVA group showed higher % of increase in, Raw and Gtis when compared with control group (P < 0.05). Both treatments reduced all these responses when compared to sensitized animals that received vehicle (P < 0.05). We did not observe any significant change in Htis responses.
Treatment with sakuranetin attenuates lung inflammation in BALF and peribronchial areas and reduced pro-inflammatory cytokines
The total and differential inflammatory cells count was shown in Table 1. We noted that OVA group presented an increase in total inflammatory cells and in eosinophils compared with Control (P < 0.01). The total inflammatory cells and the eosinophils were significantly reduced by both sakuranetin and dexamethasone treatments in ovalbumin-sensitized animals (P < 0.01). Surprisingly, the OVA + SK group presented high values of neutrophils in BALF compared with other groups (P < 0.05).
Table 1.
Effects of flavonone on inflammatory cells in BALF
| Control (cells × 104 mL−1) | OVA (cells × 104 mL−1) | OVA+SK (cells × 104 mL−1) | OVA+DX (cells × 104 mL−1) | |
|---|---|---|---|---|
| Total cells | 2.50 ± 0.31 | 9.74 ± 2.17* | 4.22 ± 0.72 | 2.63 ± 0.58 |
| Macrophages | 2.45 ± 0.31 | 5.27 ± 1.33 | 2.76 ± 0.52 | 2.88 ± 0.57 |
| Neutrophils | 0.03 ± 0.02 | 0.36 ± 0.27 | 1.05 ± 0.26** | 0.12 ± 0.06 |
| Eosinophils | 0.01 ± 0.01 | 3.80 ± 0.82* | 0.04 ± 0.01 | 0.12 ± 0.04 |
| Lymphocytes | 0.01 ± 0.01 | 0.30 ± 0.18 | 0.20 ± 0.09 | 0.01 ± 0.01 |
Data are presented as mean ± SE.
P < 0.01 compared with Control, OVA + SK and OVA + DX groups;
P < 0.05 compared with other groups.
In addition, considering that eosinophil is one of the major cell involved in allergic asthma, we stained lung slices with LUNA, and by morphometric analysis, we counted the numbers of eosinophils in peribronchiolar area in lung tissue. The OVA group presented more airway eosinophilic infiltrate compared with control (P < 0.05) (Figure 4). We noticed that flavonone treatment (OVA + SK group) reduced the number of eosinophils in sensitized animals (OVA group) in similar values obtained by corticosteroid treatment (OVA + DX group), (P < 0.05). There were no significantly difference between OVA + DX and OVA + SK groups, and also comparing these groups to control.
Figure 4.
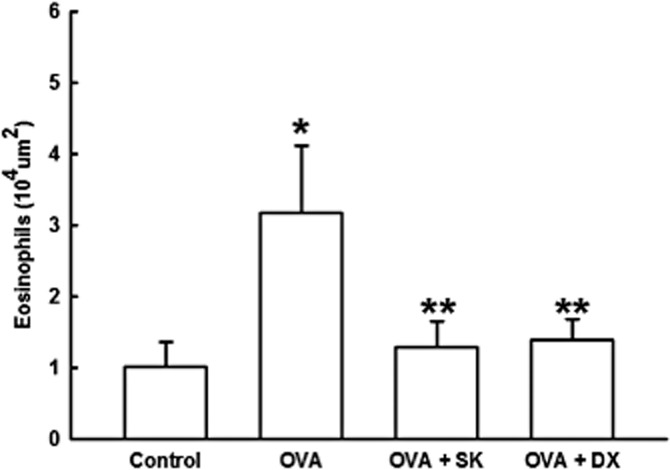
Eosinophilic inflammation. Control group: non-sensitized and non-treated; OVA group: sensitized and vehicle treated; OVA + SK: sensitized and sakuranetin treated; OVA + DX group: sensitized and dexamethasone treated. All results are presented as mean ± SE. *P < 0.05 compared with control; **P < 0.05 compared with OVA.
The values of cytokines measured in lung homogenate (pg of cytokine pg−1 of total protein) by Bioplex are presented in Table 2. We noticed that OVA group presented high levels of RANTES, IL-5, IL-4 and eotaxin compared with control (P < 0.05). In addition, sakuranetin and dexamethasone treatments reduced the levels of all Th2-cytokines (P < 0.05). except IL-4 which was attenuated only in dexamethasone-treated animals (P < 0.05). In order to observed if sakuranetin treatment affect Th1 cytokines we also quantified levels of TNF-α, IFN-γ and GM-CSF in lung homogenate. There was no effect of both sakuranetin and dexamethasone treatment in the levels of TNF-α, IFN-γ and GM-CSF. We also measured the levels of IL-10 in lung, a T-regulatory cytokine involved in anti-inflammatory responses, and we did not find any differences among the experimental groups.
Table 2.
Effects of sakuranetin treatment on cytokine release in lung homogenate quantified by elisa (Bioplex)
| Control (pg mL−1) | OVA (pg mL−1) | OVA + SK (pg mL−1) | OVA + DX (pg mL−1) | |
|---|---|---|---|---|
| RANTES | 10.82 ± 1.74 | 24.70 ± 5.55* | 9.38 ± 1.39 | 3.81 ± 0.45 |
| IL-5 | 1.61 ± 0.34 | 22.60 ± 8.28* | 5.61 ± 1.45 | 1.70 ± 0.37 |
| IL-4 | 3.51 ± 0.52 | 7.57 ± 0.12** | 6.64 ± 0.82 | 2.62 ± 0.73 |
| Eotaxin | 248.87 ± 88.35 | 759.39 ± 107.10* | 436.74 ± 112.78 | 220.56 ± 42.79 |
| IL-10 | 1.73 ± 0.20 | 3.87 ± 2.05 | 2.22 ± 0.70 | 2.50 ± 0.33 |
| TNF-α | 1.97 ± 0.40 | 1.85 ± 0.19 | 1.55 ± 0.46 | 1.25 ± 0.15 |
| IFN-γ | 4.24 ± 0.87 | 5.37 ± 1.98 | 4.76 ± 0.90 | 2.48 ± 0.26 |
| GM-CSF | 5.27 ± 0.61 | 9.70 ± 1.58 | 6.70 ± 0.83 | 5.77 ± 0.60 |
Data are presented as mean ± SE.
P < 0.05 compared with others experimental groups;
P < 0.05 compared only with control and OVA + DX groups.
Treatment with sakuranetin attenuate lung remodelling
Airway remodelling is characterized by structural alteration in airways. We evaluated the collagen and elastic fibre content around airways (Figure 5A and B, respectively). We noted in Figure 5A and F that OVA group presented an increase in collagen and elastic fibre content, respectively, compared with control (P < 0.05). Both sakuranetin and dexamethasone treatments reduced collagen airway content when compared to OVA group (P < 0.05). However, only sakuranetin was able to reduce elastic fibre content in sensitized animals (OVA + SK, P < 0.05) compared with OVA. There was no significantly difference between OVA and OVA + DX group in elastic fibre content.
Figure 5.
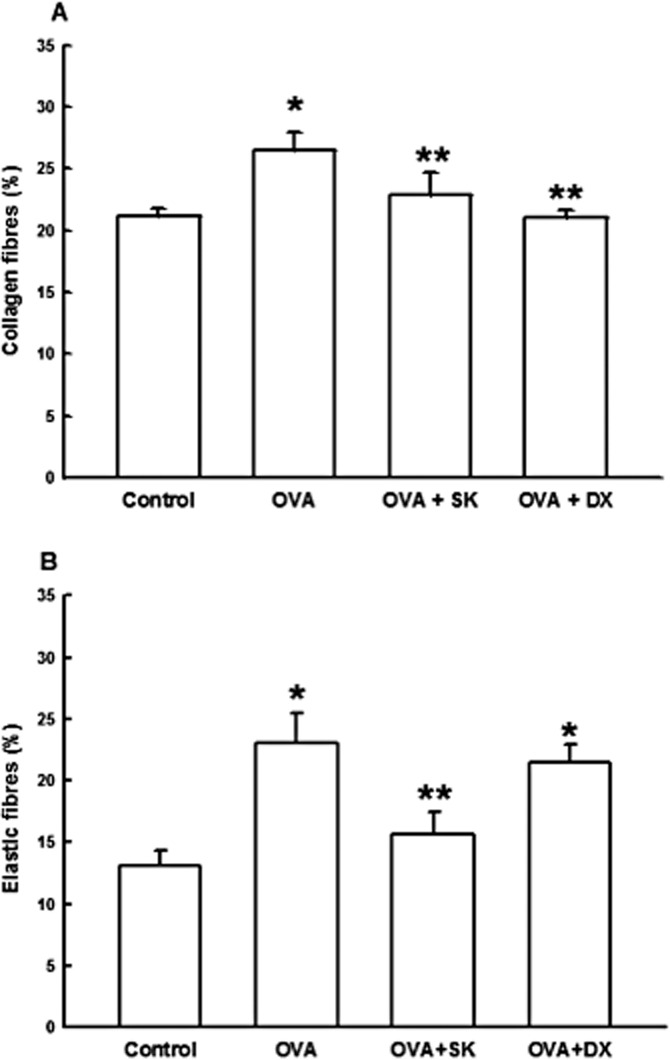
Airway remodelling. Control group: non-sensitized and non-treated; OVA group: sensitized and vehicle treated; OVA + SK: sensitized and sakuranetin treated; OVA + DX group: sensitized and dexamethasone treated. All results are presented as mean ± SE. *P < 0.05 compared with control; **P < 0.05 compared with OVA. (B–E) Representative photomicrographs of airways stained with Picro Sirius for collagen fibres detection from animal of Control, OVA, OVA + SK and OVA + DX groups respectively.
Treatment with sakuranetin reduces pulmonary oxidative stress
The OVA group presented an intense stain for 8-iso-PGF2α in lung compared with control group. The quantification of the stained area around airways (Figure 6A ) revealed that OVA group presented high stained area for 8-iso-PGF2α compared with control (P < 0.01). In addition, the treatments with sakuranetin and corticosteroid reduced the positive airway wall area for 8-iso-PGF2α in ovalbumin-exposed animals (P < 0.05 for both comparisons). There were no significantly differences among 8-iso-PGF2α positive area in OVA + DX, OVA + SK and control groups.
Figure 6.
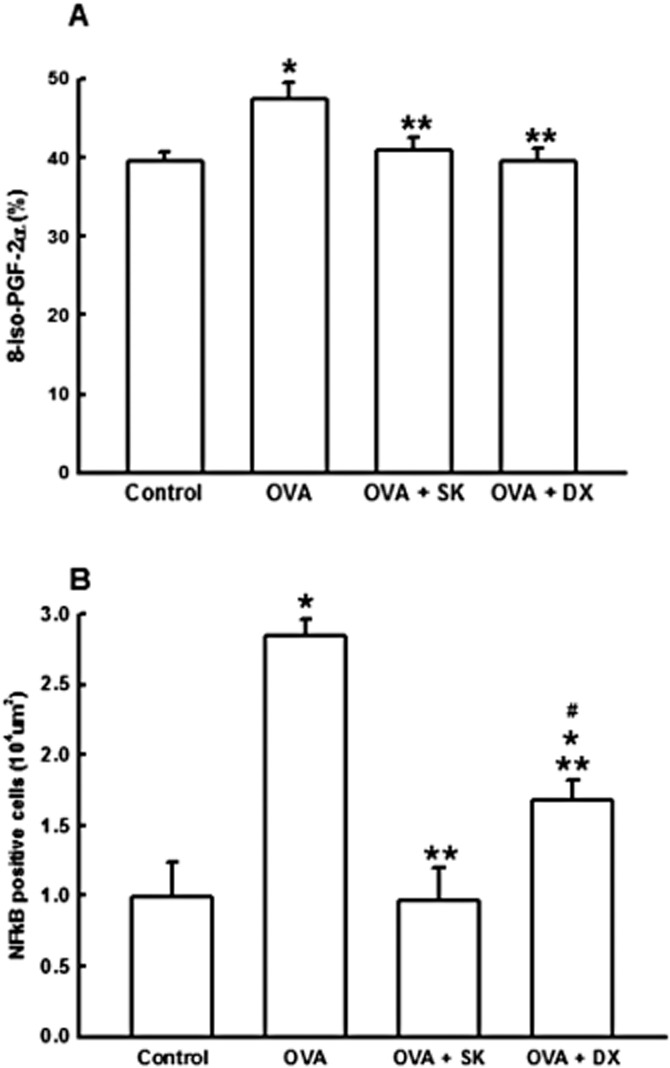
Oxidative stress and NF-kB activation. Control group: non-sensitized and non-treated; OVA group: sensitized and vehicle treated; OVA + SK: sensitized and sakuranetin treated; OVA + DX group: sensitized and dexamethasone treated. All results are presented as mean ± SE. *P < 0.05 compared with control; **P < 0.05 compared with OVA; #P < 0.05 compared with OVA-SK.
Treatment with sakuranetin reduces NF-κB expression in inflammatory cells
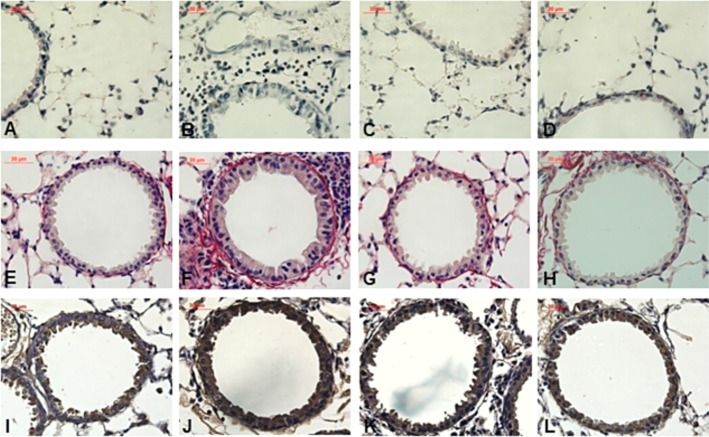
In order to assess the involvement of NF-κB, a transcription factor with a critical role in triggering and coordinating both innate and adaptive immune response, we evaluated the NF-κB-positive cells in peribronchiolar area. The OVA group presented an intense stain for NF-κB compared with the other three groups (P < 0.001; Figure 6B). Sakuranetin and dexamethasone reduced the NF-κB-positive cells in ovalbumin-exposed animals (P < 0.05), with larger effects for sakuranetin, which decreased NF-κB to similar levels of the control group. Figures 7A to D shows representative photomicrographs of peribronchiolar area of the four experimental groups. The airway wall from OVA group presented an intense stain for collagen and elastic fibers, suggesting a remodeling process (Figure 7F). Both treatments reduced these changes in ECM fibers, as observed in representative photomicrographs (Figure 7E to H). In addition, the OVA group presented an intense stain for 8-iso-PGF2 in lung compared to control group, as can be observed in Figures 7I-L.
Figure 7.
Representative photomicrographs of airways stained with LUNA to eosinophil detection (A–D), with Picro Sirius (E–H) and with antibody against 8-iso-PGF-2α (I–L) from animal of Control, OVA, OVA + SK and OVA + DX groups (1st to 4st column respectively). It is clearly that OVA group presented more eosinophils, an intense collagen deposition and increase of 8-iso-PGF-2α positive area around airways. Both sakuranetin and dexamethasone treatments attenuated these responses in airways as shown in panels C, D, G, H, K and L. Control group: non-sensitized and non-treated; OVA group: sensitized and vehicle treated; OVA + SK: sensitized and sakuranetin treated; OVA + DX group: sensitized and dexamethasone treated.
Discussion and conclusion
In the present study, we tested the hypothesis that sakuranetin, the main flavanone obtained from B. retusa, ameliorates the asthma features in this experimental model in mice. Flavanones are phenolic substances derived from different plants that have been attracting much attention due to their diverse biological activities, including anti-leishmanial and anti-trypanosomal (Grecco et al.,2012), anti-inflammatory and antioxidant properties (Havsteen,2002; Hirano et al.,2004; Rogerio et al.,2010; Taur and Patil,2011; Wu et al.,2011). Because of these properties, these species have been used in the folk medicine to treat inflammatory diseases (Zalewski et al.,2011; Goh et al.,2012).
We showed that sakuranetin decreases IgE specific antibodies, eosinophil inflammation, AHR and airway remodelling by reducing oxidative stress, Th2 pro-inflammatory cytokines and chemokines and NF-κB activation in inflammatory cells in an experimental asthma model. Its effects were similar to those observed in animals treated with corticosteroids in the majority of the parameters evaluated. It is important to point that DMSO per se, the vehicle used to dilute sakuranetin, did not affect the parameters evaluated in ovalbumin-sensitized animals, since we first compared it with non-treated animals, and no differences were observed.
Allergic asthma is a chronic inflammatory disease that involves some antigen interaction and complex immune responses, and it is well characterized by allergen-induced airway bronchoconstriction, inflammation markedly involving eosinophils, mucus hypersecretion and airway remodelling. The inflammatory responses are attributed to Th2 cells and other inflammatory factors including mast cells, B cells, eosinophils, cytokines and chemokines produced by these cells (Lee et al.,2010). In chronic asthma, it is well described that Th2 cells infiltrate the lungs and release high quantities of pro-inflammatory cytokines, including IL-4, IL-5 and IL-13. In addition, some chemokines, such as eotaxin and RANTES, were found in high levels in lung of asthmatic patients, acting as an eosinophil trafficking regulator (Rankin et al.,2000; Zimmermann et al.,2003). An imbalance between Th1 and Th2 cytokines is also involved in asthma (Shi et al.,2011).
The experimental model used in this study reproduces some of the features observed in asthmatic patients. This model consists in two different phases: one where the sensitization is induced by i.p. injection and the other made by inhalations challenge with antigen. We showed that ovalbumin-sensitized animals presented AHR to methacholine, eosinophilic inflammation, airway remodelling and elevated levels of IL-4, IL-5, eotaxin and RANTES in lung homogenate. The positive cells to NF-κB were also increased in ovalbumin-exposed animals. In addition, these animals also present an increase in 8-iso-PGF-2a in airways, suggesting an oxidative stress state in the lung.
Since sakuranetin treatment was started after the sensitization period (i.p. injections), this model allows us to design a therapeutic strategy. In order to compare our results with a positive control treatment, we performed a group sensitized and treated with corticosteroid during the same period of flavanone treatment, since the corticosteroid is the gold standard drug used for treatment of asthmatic patients (GINA,2011).
Airway eosinophil recruitment is regulated by a variety of Th2 cytokines (IL-4, IL-5, IL-13) and chemokines (RANTES and Eotaxin) and also by adhesion molecules (Hogan,2007). Eosinophilic inflammation is observed in asthmatic patients (Hogan,2007; Van Rensen et al.,2009), and it is also present in the majority of asthma models (Prado et al.,2006; Hogan,2007; Angeli et al.,2008). Considering the inflammation in airways, sakuranetin also reduced the number of eosinophils around airways and the number of eosinophils presented in BALF, as well as the number of total inflammatory cells. The dexamethasone treatment also attenuated all these cytokines and the number of inflammation cells in sensitized animals (OVA + DX group). These results are in agreement with previous studies suggesting that some medicinal plant treatment is able to inhibit eosinophilic inflammation (Havsteen,2002; Hirano et al.,2004; Rogerio et al.,2010; Taur and Patil,2011; Wu et al.,2011).
Eosinophil transmigration into the airways is a multistep process that is orchestrated by Th2 cytokines such as IL-5, IL-4, IL-13 and coordinated by specific chemokine such as eotaxin, RANTES in combination with adhesion molecules (Bisset and Schmid-Grendelmeier,2005; Hogan,2007). Our results showed that OVA induced an increase in eotaxin, IL- 4, IL-5 and RANTES content in lung homogenate. In fact, sakuranetin treatment in sensitized animals suppressed IL-5, RANTES and eotaxin. Other authors also demonstrated that other flavonoid derivatives, such as fisetin, are able to suppress the pro-inflammatory cytokines and chemokines (Wu et al.,2011; Goh et al.,2012). Bao et al. (2011), using a model of airway inflammation, showed that a dietary intake of the soy isoflavone is able to reduced IL-4 and increase the IFN-α levels. Goh et al., (2012) showed that fisetin reduced IL-4, IL-5 and IL-13 levels in BALF of sensitized animals, and the authors attributed these effects to a negative regulation of NF-κB pathway. The studies above cited did not compare the results obtained by flavanone treatment with corticosteroid. In this context, although dexamethasone treatment seems to have a more pronounced effect in the reduction of these molecules, except for IL-4 (P < 0.01 comparing OVA + DX and OVA + SK), there were no statistically significantly difference between OVA + SK and OVA + DX in IL-5, eotaxin and RANTES lung content (P = 0.36, P = 0.22, P = 0.11 respectively).
Notwithstanding the reduction in inflammation induced by sakuranetin treatment, there is an increase the number of neutrophils in BALF. We first hypothesized that it could be related to some increase in Th1 cytokines. An increase in the Th2 cell response and an imbalance inTh1/Th2 cells were found in patients with allergic asthma across a range of disease severities (Shi et al.,2011). Since IFN-γ and others are effective for directing a primary immunity through Th1 pathways we measured IFN-γ, TNF-α and GM-CSF in lung homogenate. We did not find any significantly difference among the groups. IFN-γ drives Th1 cell production, while IL-4 inhibits Th1 cell production; conversely, IL-4 drives Th2 cell production, and IFN-γ is believed to antagonize some of the effects of IL-4 (Lama et al.,2011). In our study, IL-4 was reduced in OVA group only by dexamethasone and IFN-γ was not altered by any group.
In fact, these effects of sakuranetin on neutrophils could be attributed to increase in IL-8 and MCP-2, neutrophil chemotatic factors, which are also non-inhibited by oral corticosteroid (Fukakusa et al.,2005). Shi et al. (2011) found that IL-17 and IL-22 production define the Th17 cells and play important roles in autoimmune and inflammatory diseases. The increase of Th17 cells and IL-17 can be correlated with eosinophil and neutrophil recruitment into airways in asthma models. In the present study, the increase of neutrophils observed only in sensitized animals treated with sakuranetin could be also explained by Th17 or IL-17 as a regulatory mechanism. However, we did not evaluate this pathway and future studies need to be performed in other to evaluate the exactly mechanisms involved in the increase of neutrophils induced by sakuranetin treatment. It can be some adverse effects of sakuranetin treatment.
T regulatory cells can exert their roles via anti-inflammatory cytokines such as IL-10 and TGF-β, and deficiencies in Treg cells are correlated with the severity of asthma (Shi et al.,2011). In order to evaluate if the effects of sakuranetin could also be attributed to increase in T regulatory cytokine, we also measured IL-10 content in lung that was similar among the experimental groups. Collectively, our findings suggest that sakuranetin attenuated lung inflammation in this asthma model by reducing pro-inflammatory mediators more related to Th2 cytokines, with little interference in Th1 cytokines and also with T regulatory cytokine.
NF-κB has been implicated in the pathogenesis of asthma in experimental models as well as in patients with asthma (Hart et al.,2000; Poynter et al.,2002). Among various transcription factors, NF-κB is the master switch for pro-inflammatory genes, and it is associated to airway hyperresponsiveness in a model of allergic airway disease (Donovan et al.,1999; Das et al.,2001). In other to evaluate some possible anti-inflammatory mechanism of sakuranetin, we detected the activation of NF-κB in inflammatory cells by immunohistochemistry. Sakuranetin treatment reduced NF-κB in inflammatory cells around airways more than dexamethasone.
Several studies reporting the structure/activity relationships of flavonoids have been conducted to find pharmacophoric groups of these compounds. As described by Kim et al. (2004), the presence of hydroxyl/methoxyl groups at C-5 and C-7 (ring A) associated to occurrence of carbonyl group at C-4 are crucial to anti-inflammatory activity. Thus, as sakuranetin presents hydroxyl group at C-7 and methoxyl group at C-5, these groups could be associated to airway anti-inflammatory activity of this compound. Other flavonoids, such as kaempherol, also displayed anti-inflammatory activity and presented an additional hydroxyl group at C-4′ (ring B), similar of sakuranetin. Finally, is important to mention that different flavonoids found in Capparis spinosa, including sakuranetin, are involved in the in vitro inhibition of NF-κB activation, suggesting one possible mechanism of action of this compound (Zhou et al.,2011) and corroborating our data.
Airway remodelling is a determinant of AHR, and it is related to the decrement in lung function observed in asthmatic patients (Kariyawasam et al.,2007; Southam et al.,2007; Kermode et al.,2011). It is well determined that persistence of the inflammatory milieu induces a repair process in the lung, which is characterized by airways structural alterations such smooth muscle cells hyperplasia and hypertrophy, mucus glands hypertrophy and ECM components alterations. The sakuranetin treatment attenuated airway remodelling and the pulmonary responses to antigen challenge, mainly concerning the airway responses. The evaluation of pulmonary mechanics using forced oscillation allowed us to identify the airways and the lung tissue responses (Hantos et al.,2003). The effects of sakuranetin were more intense in Rrs, Raw and Ers than in Htis and Gtis, suggesting an action of flavanone more in airways than in lung tissue response. Previous study also showed the inhibitory effect of sakuranetin in the smooth muscle cells (ileum and uterus) contraction induced by ACh, histamine and Ca2+ in K+-depolarizing solution, suggesting an interference of sakuranetin with calcium metabolism in that cells (Rojas et al.,1996). In this way, the effects of sakuranetin observed in airways could be due to dilating effects in smooth muscle, although other studies are necessary to confirm this hypothesis.
In this model, in order to test if sakuranetin is able to counteract the airway remodelling, we quantified collagen and elastic fibre deposition near the airway (between basement membrane and airway adventitia) by image analysis. We observed that both sakuranetin and dexamethasone attenuated the ECM collagen fibre deposition in airways of ovalbumin-sensitized animals, suggesting that sakuranetin is able to attenuate not only the inflammation but also the remodelling presented in this asthma model. The reduction in fibroblast activation or controlling the imbalance between proteases and ant-ipreoteases could be possible explanations for these finding, previously reported by others (Neaud and Rosenbaum,2008; Chen et al.,2010). The present study corroborates previous ones that found increased elastic fibre density in the airways of guinea pigs and mice sensitized with ovalbumin (Angeli et al.,2008; Vieira et al.,2008; Antunes et al.,2009). This increase could be due to a turnover of the ECM components as part of the remodelling process, as previously described (Araujo et al.,2008). The sakuranetin treatment but not dexamethasone reduced airway elastic fibre deposition. In a recent study performed by our group, we have shown similar response related to corticosteroids which did not affect the elastic fibres in a model of asthma in guinea pigs (Souza et al.,2012). The reasons for these differences are unknown since few studies evaluated the mechanisms and drugs involved in elastic fibre deposition in models of asthma. Some recent findings have shown that an increasing severity of AHR is linked with increased elastin (Slats et al.,2008). However, others showed that airway remodelling did not contribute to AHR (Kermode et al.,2011) and suggest that airway remodelling can be a response in order to maintain the homeostasis and avoid a severe bronchoconstriction (Brown et al.,2010).
Some important issues need to be pointed out. We cannot conclude in the present study that the effects of sakuranetin in airway bronchodilation were a direct effect of this drug relaxing the smooth muscle cells. It is important to consider that the attenuation of inflammatory and remodelling responses can significantly corroborate to attenuation observed in Rrs values. In addition, the isoprostanes are important constrictors of airways (Montuschi et al.,1999). In this way, sakuranetin also reduced the 8-iso-PGF-2 a content in the lung. However, is important to note that sakuranetin treatment attenuated the AHR in the same degree of dexamethasone, suggesting an important therapeutic efficacy of this new compound in order to counteract the airway constriction, by direct or indirectly mechanisms.
Although the role of oxidative stress in asthma physiopathology was not fully determined, an imbalance in reducing and/or oxidizing (redox) systems favouring a more oxidative milieu is linked to asthma pathophysiology. Some authors have described a relationship between oxidative stress and some of the asthmatic symptoms such as airflow limitation, hyperreactivity and airway remodelling (Brussino et al.,2010; Voynow and Kummarapurugu,2011). In this regard, high levels of reactive oxygen (ROS) and nitrogen (RNS) species in lung of asthmatic patients (Shichijo et al.,2003; Shum et al.,2008) and also in experimental models (Prado et al.,2006; Angeli et al.,2008) have been demonstrated. Considering poliphenolic compounds, it was reported some antioxidant effects (Boots et al.,2011). In the present study, we evaluated the oxidative stress by measurement of 8-iso-PGF-2α in airway tissue (Angeli et al.,2008). The isoprostanes is generating by lipid peroxidation due to peroxynitrite formation. The 8-iso-PGF-2α is considered the predominant form generated during free radical attack of cell membranes (Montuschi et al.,1999). Our data showed a reduction in oxidative stress induced by sakuranetin treatment since the airway content of 8-iso-PGF-2α was reduced in the OVA + SK group. The same effect was observed in OVA + DX group, suggesting that both sakuranetin and dexamesthasone reduced the oxidative stress.
Various studies reported the importance of IgE in asthma physiopathology (Holgate,2008). The IgE release by mast cell activation seems to depend of IL-4 and TNF-α and contribute to Th2 maintenance (Galli et al.,2008). Interestingly, in the present study, sakuranetin reduced the specific IgE of ovalbumin-sensitized animals as well as dexamethasone treatment, independent of IL-4 content in lung homogenate. Several other flavonones, such as apigenin, luteolin, diosmetin, fisetin and quercetin, inhibited the antigen-IgE-mediated TNF-α and IL-4 production (Mastuda et al.,2002). One possible explanation is that IgE release depends on other cytokines such as IL-35 and IL-13 (Kaur et al.,2006; Huang et al.,2011).
To the best of our knowledge, no other study has evaluated the effects of sakuranetin in all these features of asthma model. The results obtained in the present study show the potential beneficial effects of sakuranetin in a fully reproducible and a widely used model of experimental asthma (Toledo et al.,2011). However, the data provided here should be careful interpreted when to transpose to the field of humans. It is noteworthy to emphasize that mice model has its known limitations. The major limitation of the present study is related to toxic effects of some flavonoid derivatives. Although no clinical effects of sakuranetin in any animals were observed, and there were no deaths in OVA + SK group, we did not evaluate some possible toxic effects of the isolated flavanone in this dose. At least to our knowledge, there were no studies showing toxic effects related to Baccharis retusa and also to sakuranetin. Oliveira-Filho et al. (2012) described that from 120 different species of Baccharis found in Brazil, only the Baccharis coridifolia and Baccharis megapotamica showed toxic effects. Nogueira et al. (2011) studied Baccharis trimera, a popular plant in Brazil, and they did not observe mutagenicity, inhibition of hepatic enzymes or induction of toxic effects in kidney cells both in vivo and in vitro. However, De Alda et al. (2009) described a case related to spontaneous intoxication in horses, which presented an increase in heart frequency, respiratory frequency, intestinal hypermobility and diarrhoea, induced by B. coridifolia. Although the effects of sakuranetin were similar to those observed in OVA + DX group, it is also interesting test if the association of these drugs could also have a beneficial therapeutically effect.
In conclusion, we reported that treatment with sakuranetin, a flavanone obtained from B. retusa, ameliorates airway hyperresponsiveness, inflammation and remodelling in an experimental model of asthma in mice. The mechanisms involved are related to the effects of sakuranetin reducing pro-inflammatory cytokines and chemokines as well as oxidative stress. Our findings support a therapeutic value of sakuranetin in the treatment of asthma since its effects were similar those obtained with corticosteroid treatment in this model.
Acknowledgments
This work was supported by FAPESP and CNPq grants awarded to CMP, MAM and JHGL. In addition, CMP, MAM, IFLCT, JHGL and NOSC were supported by a fellowship from CNPq, Brazil. The authors thank Meire Ioshie Hiyane for technical support.
Glossary
- AHR
hyperresponsiveness
- BALF
bronchoalveolar lavage fluid
- DAB
3,3′-diaminobenzidine
- DMSO
dimethyl sulfoxide
- DX
dexamethasone
- ECM
extracellular matrix
- Ers
respiratory system elastance
- Gtis
tissue damping
- Htis
tissue elastance
- MCP-2
monocyte chemotactic protein
- OVA
ovalbumin
- OVA + DX
ovalbumin–dexamethasone
- OVA + SK
ovalbumin–sakuranetin
- PCA
passive cutaneous anaphylaxis
- RANTES
Regulated on Activation, normal T-cell expressed and secreted
- Raw
airway resistance
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- Rrs
respiratory system resistance
- SK
sakuranetin (5,4′-dihydroxy-7-methoxyflavanone)
- Th1
type 1 helper T cells
- Th17
type 17 helper T cells
- Th2
type 2 helper T cells
- Treg cells
regulatory T cells
Conflict of interest
All authors have no conflict of interest in this study.
References
- Agrawal PK. Carbon-13 NMR of Flavonones. Amsterdan, The Netherlands: Elsevier Science Publishers; 1989. [Google Scholar]
- Angeli P, Prado CM, Xisto DG, Silva PL, Pássaro CP, Nakazato HD, et al. Effects of chronic L-NAME treatment lung tissue mechanics, eosinophilic and extracellular matrix responses induced by chronic pulmonary inflammation. Am J Physiol Lung Cell Mol Physiol. 2008;294:1197–1205. doi: 10.1152/ajplung.00199.2007. [DOI] [PubMed] [Google Scholar]
- Antunes MA, Abreu SC, Damaceno-Rodrigues NR, Parra ER, Capelozzi VL, Pinart M, et al. Different strains of mice present distinct lung tissue mechanics and extracellular matrix composition in a model of chronic allergic asthma. Respir Physiol Neurobiol. 2009;165:202–207. doi: 10.1016/j.resp.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Araujo BB, Dolhnikoff M, Silva LF, Elliot J, Lindeman JH, Ferreira DS, et al. Extracellular matrix components and regulators in the airway smooth muscle in asthma. Eur Respir J. 2008;32:61–69. doi: 10.1183/09031936.00147807. [DOI] [PubMed] [Google Scholar]
- Bao ZS, Hong L, Guan Y, Dong XW, Zheng HS, Tan GL, et al. Inhibition of airway inflammation, hyperresponsiveness and remodeling by soy isoflavone in a murine model of allergic asthma. Int Immunopharmacol. 2011;11:899–906. doi: 10.1016/j.intimp.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Baraket M, Oliver BG, Burgess JK, Lim S, King GG, Black JL. Is low dose inhaled corticosteroid therapy as effective for inflammation and remodeling in asthma? A randomized, parallel group study. Respir Res. 2012;13:1–11. doi: 10.1186/1465-9921-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisset LR, Schmid-Grendelmeier P. Chemokines and their receptors in the pathogenesis of allergic asthma: progress and perspective. Curr Opin Pulm Med. 2005;11:35–42. doi: 10.1097/01.mcp.0000144502.50149.e0. [DOI] [PubMed] [Google Scholar]
- Boots AW, Drent M, de Boer VC, Bast A, Haenen GR. Quercetin reduces markers of oxidative stress and inflammation in sarcoidosis. Clin Nutr. 2011;30:506–512. doi: 10.1016/j.clnu.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Brown RH, Kaczka DW, Mitzner W. Effect of parenchymal stiffness on canine airway size with lung inflation. PLoS ONE. 2010;5:e10332. doi: 10.1371/journal.pone.0010332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brussino L, Badiu I, Sciascia S, Bugiani M, Heffler E, Guida G, et al. Oxidative stress and airway inflammation after allergen challenge evaluated by exhaled breath condensate analysis. Clin Exp Allergy. 2010;40:1642–1647. doi: 10.1111/j.1365-2222.2010.03604.x. [DOI] [PubMed] [Google Scholar]
- Chen CY, Peng WH, Wu LC, Wu CC, Hsu SL. Luteolin ameliorates experimental lung fibrosis both in vivo and in vitro: implications for therapy of lung fibrosis. J Agric Food Chem. 2010;58:11653–11661. doi: 10.1021/jf1031668. [DOI] [PubMed] [Google Scholar]
- Correa-Costa M, Semedo P, Monteiro AP, Silva RC, Pereira RL, Gonçalves GM, et al. Induction of heme oxygenase-1 can halt and even reverse renal tubule-interstitial fibrosis. PLoS ONE. 2010;5:e14298. doi: 10.1371/journal.pone.0014298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das J, Chen CH, Yang L, Cohn L, Ray P, Ray A. A critical role for NFkB in gata3 expression and Th2 differentiation in allergic airway inflammation. Nat Immunol. 2001;2:45–50. doi: 10.1038/83158. [DOI] [PubMed] [Google Scholar]
- De Alda JL, Sallis ESV, Nogueira CEW, Soares MP, Amaral L, Marcolongo-Pereira C, et al. Intoxicação espontânea por Baccharis cordifolia (Compositae) em equinos no rio Grande do Sul. Pesq Vet Bras. 2009;29:409–414. [Google Scholar]
- Donovan CE, Mark DA, He HZ, Liou HC, Kobzik L, Wang Y, et al. Nf-kB/Rel transcription factors: C-Rel promotes airway hyperresponsiveness and allergic pulmonary inflammation. J Immunol. 1999;163:6827–6833. [PubMed] [Google Scholar]
- Ernst P, Suissa S. Systemic effects of inhaled corticosteroids. Curr Opin Pulm Med. 2012;18:85–89. doi: 10.1097/MCP.0b013e32834dc51a. [DOI] [PubMed] [Google Scholar]
- Fahy JV. Remodeling of the airway epithelium in asthma. Am J Respir Crit Care Med. 2001;164(10 Pt 2):S46–S51. doi: 10.1164/ajrccm.164.supplement_2.2106066. [DOI] [PubMed] [Google Scholar]
- Fukakusa M, Bergeron C, Tulic MK, Fiset PO, Al Dewachi O, Laviolette M, et al. Oral corticosteroids decrease eosinophil and CC chemokine expression but increase neutrophil, IL-8, and IFN-gamma-inducible protein 10 expression in asthmatic airway mucosa. J Allergy Clin Immunol. 2005;115:280–286. doi: 10.1016/j.jaci.2004.10.036. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Tsai M, Piliponsky AM. Nature. Vol. 24. Montreal, Quebec, Canada: Meakins-Christie Laboratories, McGill University; 2008. The development of allergic inflammation; pp. 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Initative for Asthma (GINA) 2011. GINA report, global strategy for asthma management and prevention.
- Goh FY, Upton N, Guan S, Cheng C, Shanmugam MK, Sethi G, et al. Fisetin, a bioactive flavonol, attenuates allergic airway inflammation through negative regulation of NF-κB. Eur J Pharmacol. 2012;679:109–116. doi: 10.1016/j.ejphar.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Grecco SS, Reimão JQ, Tempone AG, Sartorelli P, Romoff P, Ferreira MJ, et al. Isolation of an antileishmanial and antitrypanosomal flavanone from the leaves of Baccharis retusa DC. (Asteraceae) Parasitol Res. 2010;106:1245–1248. doi: 10.1007/s00436-010-1771-8. [DOI] [PubMed] [Google Scholar]
- Grecco SS, Reimão JQ, Tempone AG, Sartorelli P, Cunha RL, Romoff P, et al. In vitro antileishmanial and antitrypanosomal activities of flavanones from Baccharis retusa DC. (Asteraceae) Exp Parasitol. 2012;130:141–145. doi: 10.1016/j.exppara.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Hantos Z, Collins RA, Turner DJ, Jánosi TZ, Sly PD. Tracking of airway and tissue mechanics during TLC maneuvers in mice. J Appl Physiol. 2003;95:1695–1705. doi: 10.1152/japplphysiol.00104.2003. [DOI] [PubMed] [Google Scholar]
- Hart L, Lim S, Adcock I, Barnes PJ, Chung KF. Effects of inhaled corticosteroid therapy on expression and DNA-binding activity of nuclear factor kB in asthma. Am J Respir Crit Care Med. 2000;161:224–231. doi: 10.1164/ajrccm.161.1.9809019. [DOI] [PubMed] [Google Scholar]
- Havsteen BH. The biochemistry and medical significance of the flavonones. Pharmacol Ther. 2002;96:167–202. doi: 10.1016/s0163-7258(02)00298-x. [DOI] [PubMed] [Google Scholar]
- Hirano T, Higa S, Arimitsu J, Naka T, Shima Y, Ohshima S, et al. Flavonones such as luteolin, fisetin and apigenin are inhibitors of interleukin-4 and interleukin-13 production by activated human basophils. Int Arch Allergy Immunol. 2004;134:135–140. doi: 10.1159/000078498. [DOI] [PubMed] [Google Scholar]
- Hogan SP. Recent advances in eosinophil biology. Int Arch Allergy Immunol. 2007;143(Suppl. 1):3–14. doi: 10.1159/000101398. [DOI] [PubMed] [Google Scholar]
- Holgate S. Pathogenesis of asthma. Clin Exp Allergy. 2008;38:872–897. doi: 10.1111/j.1365-2222.2008.02971.x. [DOI] [PubMed] [Google Scholar]
- Huang CH, Loo EX, Kuo IC, Soh GH, Goh DL, Lee BW, et al. Airway inflammation and IgE production induced by dust mite allergen-specific memory/effector Th2 cell line can be effectively attenuated by IL-35. Source J Immunol. 2011;187:462–471. doi: 10.4049/jimmunol.1100259. [DOI] [PubMed] [Google Scholar]
- Jung WK, Choi I, Oh S, Park SG, Seo SK, Lee SW, et al. Anti-asthmatic effect of marine red alga (Laurencia undulata) polyphenolic extrats in a murine model of asthma. Food Chem Toxicol. 2009;47:293–297. doi: 10.1016/j.fct.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Kariyawasam HH, Aizen M, Barkans J, Robinson DS, Kay AB. Remodeling and airway hyperresponsiveness but not cellular inflammation persist after allergen challenge in asthma. Am J Respir Crit Care Med. 2007;175:896–904. doi: 10.1164/rccm.200609-1260OC. [DOI] [PubMed] [Google Scholar]
- Kaur D, Hollins F, Woodman L, Yang W, Monk P, May R, et al. Mast cells express IL-13R alpha 1: IL-13 promotes human lung mast cell proliferation and Fc epsilon RI expression. Allergy. 2006;61:1047–1053. doi: 10.1111/j.1398-9995.2006.01139.x. [DOI] [PubMed] [Google Scholar]
- Kermode JA, Brown NJ, Hardaker KM, Farah CS, Berend N, King GG, et al. The effect of airway remodelling on airway hyper-responsiveness in asthma. Respir Med. 2011;105:1798–1804. doi: 10.1016/j.rmed.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HP, Son KH, Chang HW, Kang SS. Anti-inflammatory plant flavonoids and cellular action mechanisms. J Pharmacol Sci. 2004;96:229–245. doi: 10.1254/jphs.crj04003x. [DOI] [PubMed] [Google Scholar]
- Lama M, Chatterjee M, Nayak CR, Chaudhuri TK. Increased interleukin-4 and decreased interferon-γ levels in serum of children with asthma. Cytokine. 2011;55:335–338. doi: 10.1016/j.cyto.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Lee MY, Seo CS, Ha HK, Jung DY, Lee HY, Lee NH, et al. Protective effects of Ulmus davidiana var. japonica against OVA-induced murine asthma model via upregulation of heme oxygenase-1. J Ethnopharmacol. 2010;130:61–69. doi: 10.1016/j.jep.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Mastuda H, Morikawa T, Ueda K, Managi H, Yoshikawa M. Structural Requirements of Flavonones for Inhibition of Antigen-Induced Degranulation, TNF-α and IL-4 Production from RBL-2H3 Cells. Kyoto: Kyoto Pharmaceutical University; 2002. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DP, Watkins SE, Sampson T, Davis KJ. Long-term use of fluticasone propionate/salmeterol fixed-dose combination and incidence of cataracts and glaucoma among chronic obstructive pulmonary disease patients in the UK General Practice Research Database. Int J Chron Obstruct Pulmon Dis. 2011;6:467–476. doi: 10.2147/COPD.S14247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montuschi P, Curro D, Ragazzoni E, Preziosi P, Ciabattoni G. Anaphylasis increases 8-iso-prostaglandin F2alpha release from guinea-pig lung in vitro. Eur J Pharmacol. 1999;365:59–64. doi: 10.1016/s0014-2999(98)00859-0. [DOI] [PubMed] [Google Scholar]
- Mota I, Perini A. A heat labile mercaptoethanol susceptible homocytotropic antibody in the guinea pig. Life Sci II. 1970;9:923–930. doi: 10.1016/0024-3205(70)90063-9. [DOI] [PubMed] [Google Scholar]
- Mota I, Wong D. Homologous and heterologous passive cutaneous anaphylactic activity of mouse antisera during the course of immunization. Life Sci. 1969;8:813–820. doi: 10.1016/0024-3205(69)90099-x. [DOI] [PubMed] [Google Scholar]
- Murphy DM, O'Byrne PM. Recent advances in the pathophysiology of asthma. Chest. 2010;137:1417–1426. doi: 10.1378/chest.09-1895. [DOI] [PubMed] [Google Scholar]
- Neaud V, Rosenbaum J. A red wine polyphenolic extract reduces the activation phenotype of cultured human liver myofibroblasts. World J Gastroenterol. 2008;14:2194–2199. doi: 10.3748/wjg.14.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira NP, Reis PA, Laranja GA, Pinto AC, Ajub CA, Felzenswalb I, et al. In vitro and in vivo toxicological evaluation of extract and fractions from Baccharis trimera with anti-inflamatory activity. J Ethnopharmacol. 2011;138:513–522. doi: 10.1016/j.jep.2011.09.051. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Oku H, Iwaoka E, Iinuma M, Ishiguro K. Allergy-preventive flavonoids from Xanthorrhoea hastilis. Chem Pharm Bull. 2007;55:675–678. doi: 10.1248/cpb.55.675. [DOI] [PubMed] [Google Scholar]
- Oliveira-Filho JC, Carmo PMS, Iversen A, Nielsen KF, Barros CSL. Experimental poisoning by Baccharis megapotamica var.weirii in búfalo. Pesq Vet Bras. 2012;32:383–390. [Google Scholar]
- Park CS, Kim TB, Lee KY, Moon KA, Bae YJ, Jang MK, et al. Increased oxidative stress in the airway and development of allergic inflammation in a mouse model of asthma. Ann Allergy Asthma Immunol. 2009;103:238–247. doi: 10.1016/S1081-1206(10)60188-3. [DOI] [PubMed] [Google Scholar]
- Patel M, Perrin K, Beasley R. Beta agonist use during asthma exacerbations: how much is too much? N Z Med J. 2011;124:77–80. [PubMed] [Google Scholar]
- Poynter ME, Irvin CG, Janssen-Heininger YM. Rapid activation of nuclear factor-kB in airway epithelium in a murine model of allergic airway inflammation. Am J Pathol. 2002;160:1325–1334. doi: 10.1016/s0002-9440(10)62559-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado CM, Leick-Maldonado EA, Yano L, Leme AS, Capelozzi VL, Martins MA, et al. Effects of nitric oxide synthases in chronic allergic airway inflammation and remodeling. Am J Respir Cell Mol Biol. 2006;35:457–465. doi: 10.1165/rcmb.2005-0391OC. [DOI] [PubMed] [Google Scholar]
- Rankin SM, Conroy DM, Williams TJ. Eotaxin and eosinophil recruitment: implications for human disease. Mol Med Today. 2000;6:20–27. doi: 10.1016/s1357-4310(99)01635-4. [DOI] [PubMed] [Google Scholar]
- Rogerio AP, Dora CL, Andrade EL, Chaves JS, Silva LFC, Lemos-Senna E, et al. Anti-inflammatory effect of quercetin-loaded microemulsion in the airways allergic inflammatory model in mice. Pharmacol Res. 2010;61:288–297. doi: 10.1016/j.phrs.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Roh GS, Shin Y, Seo SW, Yoon BR, Yeo S, et al. Proteome analysis of differential protein expression in allergen-induced asthmatic mice lung after dexamethasone treatment. J Proteomics. 2004;4:3318–3327. doi: 10.1002/pmic.200400930. [DOI] [PubMed] [Google Scholar]
- Rojas A, Cruz S, Ponce-Monter H, Mata R. Smooth muscle relaxing compounds from Dodonaea viscosa. Planta Med. 1996;62:154–159. doi: 10.1055/s-2006-957840. [DOI] [PubMed] [Google Scholar]
- Shi YH, Shi GC, Wan HY, Jiang LH, Ai XY, Zhu HX, et al. Coexistence of th1/th2 and th17/treg imbalances in patients with allergic asthma. Chin Med J (Engl) 2011;124:1951–1956. [PubMed] [Google Scholar]
- Shichijo M, Yamamoto N, Tsujishita H, Kimata M, Nagai H, Kokubo T. Inhibition of Syk activity and degranulation of human mast cells by flavonones. Biol Pharm Bull. 2003;26:1685–1690. doi: 10.1248/bpb.26.1685. [DOI] [PubMed] [Google Scholar]
- Shum BO, Rolph MS, Sewell WA. Mechanisms in allergic airway inflammation – lessons from studies in the mouse. Expert Rev Mol Med. 2008;10:e15. doi: 10.1017/S1462399408000707. [DOI] [PubMed] [Google Scholar]
- Slats AM, Janssen K, van Schadewijk A, van der Plas DT, Schot R, van den Aardweg JG, et al. Expression of smooth muscle and extracellular matrix proteins in relation to airway function in asthma. J Allergy Clin Immunol. 2008;121:1196–1202. doi: 10.1016/j.jaci.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Soares DG, Andreazza AC, Salvador M. Avaliação de compostos com atividade antioxidante em células da levedura Saccharomyces cerevisiae. Braz J Pharm Sci. 2005;41:95–100. [Google Scholar]
- Southam DS, Ellis R, Wattie J, Inman MD. Components of airway hyperresponsiveness and their associations with inflammation and remodeling in mice. J Allergy Clin Immunol. 2007;119:848–854. doi: 10.1016/j.jaci.2006.12.623. [DOI] [PubMed] [Google Scholar]
- Souza FCR, Gobbato NB, Prado CM, Martins MA, Maldonado EAL, Tibério IFLC. Effects of corticosteroids, antagonist cysteinyl leukotriene D4 and iNOS inhibition on the distal lung with chronic inflammation. Respir Physiol Neurobiol. 2012 doi: 10.1016/j.resp.2012.08.015. In press. [DOI] [PubMed] [Google Scholar]
- Taur DJ, Patil RY. Antiasthmatic activity of Ricinus communis L. roots. Asian Pac J Trop Biomed. 2011;1:S13–S16. doi: 10.1016/S2221-1691(11)60012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo AC, Arantes-Costa FM, Macchione M, Saldiva PH, Negri EM, Lorenzi-Filho G, et al. Salbutamol improves markers of epithelial function in mice with chronic allergic pulmonary inflammation. Respir Physiol Neurobiol. 2011;177:155–161. doi: 10.1016/j.resp.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Van Rensen EL, Evertse CE, van Schadewijk WA, van Wijngaarden S, Ayre G, Mauad T, et al. Eosinophils in bronchial mucosa of asthmatics after allergen challenge: effect of anti-IgE treatment. Allergy. 2009;64:72–80. doi: 10.1111/j.1398-9995.2008.01881.x. [DOI] [PubMed] [Google Scholar]
- Vieira RP, de Andrade VF, Duarte AC, Dos Santos AB, Mauad T, Martins MA, et al. Aerobic conditioning and allergic pulmonary inflammation in mice. II. Effects on lung vascular and parenchymal inflammation and remodeling. Am J Physiol Lung Cell Mol Physiol. 2008;295:L670–L679. doi: 10.1152/ajplung.00465.2007. [DOI] [PubMed] [Google Scholar]
- Voynow JA, Kummarapurugu A. Isoprostanes and asthma. Biochim Biophys Acta. 2011;1810:1091–1095. doi: 10.1016/j.bbagen.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel ER. Principles and methods for the morphometric study of the lung and other organ. Lab Invest. 1963;12:31–55. [PubMed] [Google Scholar]
- Wu MY, Hung SK, Fu SL. Immunosuppressive effects of fisetin in ovalbumin-induced asthma through inhibition of NF-κB activity. J Agric Food Chem. 2011;59:10496–10504. doi: 10.1021/jf202756f. [DOI] [PubMed] [Google Scholar]
- Zalewski CA, Passero LF, Melo AS, Corbett CE, Laurenti MD, Toyama MH, et al. Evaluation of anti-inflammatory activity of derivatives from aerial parts of Baccharis uncinella. Pharm Biol. 2011;49:602–607. doi: 10.3109/13880209.2010.537828. [DOI] [PubMed] [Google Scholar]
- Zhou HF, Xie C, Jian R, Kang J, Li Y, Zhuang CL, et al. Biflavonoids from Caper (Capparis spinosa L.) fruits and their effects in inhibiting NF-kappa B activation. J Agric Food Chem. 2011;59:3060–3065. doi: 10.1021/jf105017j. [DOI] [PubMed] [Google Scholar]
- Zimmermann N, Hershey GK, Foster PS, Rothenberg ME. Chemokines in asthma: cooperative interaction between chemokines and IL-13. J Allergy Clin Immunol. 2003;111:227–242. doi: 10.1067/mai.2003.139. [DOI] [PubMed] [Google Scholar]