Abstract
Pregnancy complications and poor birth outcomes can affect the survival and long-term health of children. The preconception period represents an opportunity to intervene and improve outcomes; however little is known about women’s mental health prior to pregnancy as a predictor of such outcomes. We sought to determine if and to what extent women’s preconception mental health status impacted subsequent pregnancy complications, non-live birth, and birth weight using a nationally representative, population-based sample. We used pooled 1996-2006 data from the nationally-representative Medical Expenditure Panel Survey (MEPS). Poor preconception mental health was defined as women’s global mental health rating of “fair” or “poor” before conception. Logistic regression was used to assess the association between preconception mental health and pregnancy complications, non-live birth, and having a low birth weight baby within the follow up period. Poor preconception mental health was associated with increased odds of experiencing any pregnancy complication (AOR 1.40, 95% CI: 1.02-1.92), having a non-live birth (AOR 1.48, 95% CI: 0.96-2.27), and having a low birth weight baby (AOR 1.99, 95% CI: 1.00-3.98), all controlling for maternal age, race/ethnicity, marital status, education, health insurance status, income, and number of children in the household. Significant racial and ethnic disparities exist for pregnancy complications and non-live births, but not for low birth weight. Women’s preconception mental health is a modifiable risk factor that stands to reduce the incidence of adverse pregnancy complications and birth outcomes.
Keywords: Women’s mental health, Pregnancy complications, Low birth weight, Non-live birth, Disparities
INTRODUCTION
Pregnancy complications and poor birth outcomes are serious global public health problems, causing substantial morbidity and mortality for mothers and their children. In the United States, obstetric outcomes, such as low birth weight (LBW;\2,500 g) and pregnancy complications, account for over 40% of all infant deaths [1] and have caused significant increases in childhood morbidity in recent years [2,3]. Because many risk factors for adverse obstetric outcomes can be identified and managed prior to pregnancy, recent recommendations have focused on improving women’s health during this critical preconception period [4].
Mounting evidence suggests a relationship between poor antepartum mental health and adverse obstetric outcomes [5,6]. However, there is a dearth of research examining the effects of mental health prior to pregnancy and subsequent obstetric outcomes [7-10]. Some evidence indicates that preconception mental health problems may be related to preterm birth [11] and pregnancy loss [12], whereas other studies have reported no association [13,14]. These conflicting results may be due to differences in study samples and the measurements of poor preconception mental health.
The purpose of this study was to determine if and to what extent women’s preconception mental health status impacted subsequent adverse maternal and pregnancy outcomes in a nationally representative, population-based sample of women. We hypothesized that preconception mental health would be significantly associated with pregnancy complications, having an outcome other than a live birth, and having a LBW baby. To our knowledge, our study is the first to examine the relationship between preconception mental health and multiple adverse maternal and pregnancy outcomes using nationally representative data.
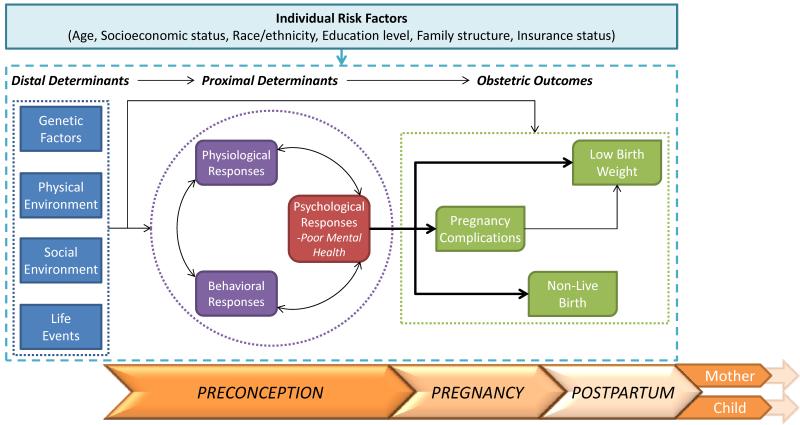
As seen in Fig. 1, our research draws upon Misra and colleagues’ [15] framework of perinatal health combining a life course developmental perspective [16,17] with a model of health determinants [18]. The integration of these theories emphasizes several key processes that inform our research questions and hypotheses. First, the perinatal framework posits that perinatal health and associated outcomes are influenced by cumulative effects of events across the lifespan and intergenerational effects. Second, multiple determinants and their interactions likely influence obstetric outcomes. Central to this framework is the idea that key health determinants prior to pregnancy have an important impact on obstetric outcomes. In our model we posit that distal determinants (including genetic, physical environment, social environment, and life events) can impact outcomes both directly and through more proximal preconception determinants including behavior, physiology, and perceived mental health. Based on these theories, our model illustrates that poor preconception mental health may increase the risk for pregnancy complications, having an outcome other than a live birth or a LBW baby, while accounting for individual-level risk factors.
Fig. 1. Conceptual framework of the preconception determinants of ddverse obstetric outcomes.
Figure 1 displays the conceptual framework that informs our work by combining Misra and colleagues’ framework of perinatal health, a life course developmental perspective, and a model of health determinants. As the perinatal framework posits that perinatal health and associated outcomes are influenced by cumulative effects of events across the lifespan and intergenerational effects, we display the trajectory of the maternal experience in this framework. The far left box represents distal determinants (including genetic, physical environment, social environment, and life events) that can impact outcomes through more proximal preconception determinants including behavior, physiology, and psychology, represented by the boxes within the circle. Specifically, our model illustrates that poor preconception mental health may increase the risk for several obstetric outcomes (far right box), while accounting for individual-level risk factors.
METHODS
Data
Data are from the household component (HC) of the 1996-2006 Medical Expenditure Panel Survey (MEPS), which collects information about medical conditions, health status, healthcare use, and expenditures. MEPS has an overlapping panel design, gathering information through five rounds of data collection over a two and a half year period, yielding a nationally representative sample of the civilian, non-institutionalized population of the U.S., with oversampling for blacks and Hispanics. Detailed methodology and a description of the MEPS data are available at http://www.meps.ahrq.gov/mepsweb/.
Additional data specific to pregnancy were obtained in each round if a woman in the household was pregnant (Pregnancy Detail Files). Because the pregnancy data are not publicly available, the Agency for Healthcare Research and Quality Data Center created a custom data set linking pregnancy data to the HC.
Sample
The outcomes of this analysis were staged across the course of a women’s pregnancy; thus, each outcome employed distinct exclusion criteria. Final samples included 3,373, 2,671, and 2,108 women to examine pregnancy complications, having an outcome other than a live birth (hereafter, non-live birth), and birth weight, respectively.
Women with singleton pregnancies included in the eleven panels of the Pregnancy Detail Files who had a non-zero person weight, preconception, antepartum, and complete covariate data were eligible for this analysis (n=3,933). Only one pregnancy per woman was included in the analyses. If a woman had more than one eligible pregnancy based on the previous inclusion criteria (n=452), a random number generator was used to randomly select a single pregnancy for inclusion in the analysis.
The final sample for the pregnancy complications analysis included 3,373 women who had a live birth or were still pregnant, excluding women with outcomes other than a live birth (n=534) and women with missing information on complications (n=26).
The final sample for the non-live birth analysis included only women with a birth outcome during the MEPS period, leaving 2,671 women for the analysis. Women were excluded if they were still pregnant during the MEPS period (n=1,160) or if they terminated their pregnancy by an abortion (n=102). Analysis including women who terminated pregnancies by an abortion are included in Appendix 1 (n=2,773).
The final sample for birth weight included 2,108 women. Women were excluded from these analyses if they had an outcomes other than a live birth (n=534), were still pregnant (n=1,160), or had missing information on their child’s birth weight (n=131).
Variables
Outcome Variables
Pregnancy Complications
To ascertain pregnancy complications, respondents were asked: “Looking at this card, which of these complications, if any, did (PERSON) experience during this pregnancy?” The responses included the following categories: (1) high blood pressure, toxemia, pre-eclampsia, or eclampsia; (2) anemia; (3) diabetes, gestational diabetes, or high blood sugar; (4) low lying placenta (placenta previa); (5) vaginal bleeding; (6) premature labor; or (7) none of these complications. Small sample sizes prohibited an examination of individual complications. Therefore a woman was considered to have a pregnancy complication if she indicated any one of the conditions (categories 1-6) during her pregnancy. A sensitivity analysis revealed no changes to our results when we considered the largest category of complications (high blood pressure, toxemia, pre-eclampsia, or eclampsia) versus: (1) any other complication (including: anemia, diabetes, gestational diabetes, high blood sugar, low lying placenta (placenta previa), vaginal bleeding, and premature labor); and (2) no complications.
Non-live Birth
To determine if the pregnancy resulted in an outcome other than a live birth respondents were asked: “Did (PERSON)’s {most recent pregnancy/next most recent pregnancy/pregnancy that we talked about last time} end in a live birth?” Respondents who reported that the pregnancy ended in a miscarriage or stillbirth were categorized as having a “non-live birth.” Women who reported that their pregnancy ended in an abortion were included in the non-live birth category in a sensitivity analysis (Appendix 1).
Birth weight
To determine birth weight women were asked: “How much did the baby weigh at birth?” Infant birth weight was categorized as LBW (<2,500 g), normal birth weight (NBW; 2,500-3,999 g), and high birth weight (HBW; >4,000 g in accordance with the CDC definition [19]).
Primary Predictor Variable
Self-reported mental health conditions and subjective measures of mental health status have been associated with mental health morbidity [20,21]. As this study examined the experience of poor preconception mental health, rather than diagnosis of a mental health condition, women were categorized as having poor preconception mental health only if they self-reported a global mental health rating of “fair” or “poor” when asked: “In general, would you say that your mental health is excellent, very good, good, fair, or poor?” [22] in any round prior to rounds in which they reported being pregnant. Women who self-reported a mental health condition of anxiety or depression but who did not report being symptomatic (poor global mental health rating) were not classified as having poor preconception mental health to prevent misclassification bias. The preconception period for which women could report poor mental health was between less than one and 18 months in length.
Race and ethnicity were examined in this study because of the well-documented association between race and birth outcomes [23]. Maternal race/ethnicity was determined by self-report based on standard options provided by the MEPS. We collapsed the MEPS race/ethnicity options into four mutually exclusive categories: white (non-Hispanic), black (non-Hispanic), other (non-Hispanic), and Hispanic. Other maternal and family sociodemographic variables included age (measured in the first year of the MEPS), education, marital/partner status, health insurance status (4 categories: private, 2 years of private insurance; public, 2 years of public insurance or 1 year of public insurance and 1 year of private insurance but continuously insured; partial, intermittent insurance coverage; and no insurance), family income as a percentage of the federal poverty level (FPL), and the number of children in the household (specifically, the number of children under 5 years of age and the number of children 5 to 17 years of age living in the household or family unit). All variables are comprised of mutually exclusive and exhaustive categories.
Analyses
Univariate comparisons for categorical sociodemographic and mental health variables were performed using Chi-squared analyses. Multivariable logistic regression models were developed to examine obstetric outcomes by preconception mental health status controlling for maternal race/ethnicity, age, marital status, education, health insurance status, income, and the number of children in the household. A variable for pregnancy complications was included in the model for birth weight because of the increased risk of LBW outcomes for women who experience pregnancy complications [13,24]. Adjusted odds ratios (AORs) and 95% confidence intervals (CIs) comparing obstetric outcomes for women in poor preconception mental health with women in good preconception mental health were estimated from the multivariable logistic models. Statistical significance was set at α ≤ 0.05; marginal statistical significance was set at 0.05 < α ≤ 0.10. Average adjusted predicted probabilities – marginal effects calculated for each observation in the data and then averaged – were generated from the regression models; standard errors of the marginal effects were calculated by the delta method. SAS 9.2 (SAS Institute Inc., Cary, NC) was used to construct the analytic files and STATA 11 (StataCorp LP, College Station, TX) was used to perform all analyses, accounting for the complex design of the MEPS. The standard errors were corrected due to clustering within strata and the primary sampling unit, and applied survey weights were used to produce estimates that account for the complex survey design, unequal probabilities of selection, and survey non-response.
The University of Wisconsin – Madison Health Sciences Institutional Review Board considered this study exempt from review because the data were already collected and deidentified.
RESULTS
Pregnancy Complications
Complications were reported in 34.3% (Table 1) of pregnancies. 8.7% of women with and 5.8% of women without pregnancy complications reported poor preconception mental health (6.8% overall, data not shown). There were significant differences between women with and without pregnancy complications in the distribution of race/ethnicity, marital status, education, insurance status, income, and number of school aged children in the household.
Table 1. Characteristics of sample by complications and multivariable analysis of the odds of any complication.
| Any Complication (vs. None) | ||||||||
|---|---|---|---|---|---|---|---|---|
| No Complications |
Any Complications |
Crude Odds Ratio |
95% Confidence Interval |
Adjusted Odds Ratio |
95% Confidence Interval |
|||
| TOTAL: weighted | 3,621,629 | 1,886,630 | ||||||
| weighted % | 65.7% | 34.3% | ||||||
| TOTAL: unweighted | 2,138 | 1,235 | ||||||
| unweight % | 63.4% | 36.6% | ||||||
| Maternal Characteristics | ||||||||
| Poor preconception mental healthb | ||||||||
| No | 94.2% | 91.3% | 1.00 | reference | 1.00 | reference | ||
| Yes | 5.8% | 8.7% | 1.56 | 1.14 | 2.13 | 1.40 | 1.02 | 1.92 |
| Age | ||||||||
| 14-19 | 10.6% | 11.3% | 1.03 | 0.78 | 1.35 | 0.79 | 0.57 | 1.10 |
| 20-24 | 25.8% | 27.0% | 1.01 | 0.78 | 1.30 | 0.89 | 0.67 | 1.17 |
| 25-29 | 26.8% | 27.8% | 1.00 | reference | 1.00 | reference | ||
| 30-34 | 24.9% | 21.1% | 0.82 | 0.63 | 1.06 | 0.92 | 0.71 | 1.20 |
| 35+ | 11.8% | 12.7% | 1.04 | 0.76 | 1.43 | 1.14 | 0.83 | 1.56 |
| Race/Ethnicityb | ||||||||
| White (Non-Hispanic)b | 62.8% | 57.2% | 1.00 | reference | 1.00 | reference | ||
| Black (Non-Hispanic)c | 12.0% | 17.0% | 1.55 | 1.23 | 1.96 | 1.35 | 1.05 | 1.74 |
| Other (Non-Hispanic) | 6.2% | 5.6% | 1.00 | 0.67 | 1.48 | 1.00 | 0.67 | 1.48 |
| Hispanic | 18.9% | 20.2% | 1.17 | 0.95 | 1.44 | 1.08 | 0.84 | 1.38 |
| Marital statusb | ||||||||
| Married, lives with partnerb | 71.9% | 66.2% | 1.00 | reference | 1.00 | reference | ||
| Never marriedb | 23.8% | 28.8% | 1.31 | 1.10 | 1.57 | 1.05 | 0.83 | 1.32 |
| Divorced, separated, widowed | 4.3% | 5.0% | 1.27 | 0.87 | 1.85 | 0.91 | 0.61 | 1.38 |
| Education statusa | ||||||||
| No or some high school | 21.9% | 24.9% | 1.05 | 0.85 | 1.30 | 0.97 | 0.76 | 1.24 |
| High school graduate | 26.9% | 29.0% | 1.00 | reference | 1.00 | reference | ||
| Some college | 20.9% | 22.3% | 0.99 | 0.77 | 1.28 | 1.06 | 0.81 | 1.39 |
| College or beyondb | 30.3% | 23.8% | 0.73 | 0.56 | 0.95 | 0.84 | 0.61 | 1.15 |
| Family Characteristics | ||||||||
| Health Insurance Statusc | ||||||||
| Private insurance onlyc | 66.6% | 59.1% | 1.00 | reference | 1.00 | reference | ||
| Any publicly funded insurancec | 18.9% | 25.4% | 1.52 | 1.21 | 1.90 | 1.19 | 0.87 | 1.62 |
| Partial insurance | 8.1% | 9.6% | 1.35 | 1.03 | 1.76 | 1.19 | 0.86 | 1.66 |
| No insurance | 6.5% | 5.9% | 1.02 | 0.75 | 1.38 | 0.88 | 0.63 | 1.24 |
| Ratio of family income to poverty thresholdc | ||||||||
| Below 100%c | 17.5% | 25.8% | 1.00 | reference | 1.00 | reference | ||
| 100-199% | 21.8% | 20.0% | 0.63 | 0.50 | 0.79 | 0.65 | 0.51 | 0.82 |
| 200-399% | 28.0% | 26.0% | 0.63 | 0.50 | 0.80 | 0.72 | 0.54 | 0.97 |
| 400%+a | 32.6% | 28.2% | 0.59 | 0.46 | 0.75 | 0.73 | 0.52 | 1.04 |
| Number of young children (age <5) | ||||||||
| None | 55.4% | 58.1% | 1.00 | reference | 1.00 | reference | ||
| One | 34.1% | 32.7% | 0.92 | 0.75 | 1.11 | 0.90 | 0.74 | 1.11 |
| Two+ | 10.5% | 9.2% | 0.84 | 0.63 | 1.11 | 0.80 | 0.60 | 1.07 |
| Number of school-aged children (age 5-17)a | ||||||||
| Nonea | 67.3% | 62.0% | 1.00 | reference | 1.00 | reference | ||
| Onea | 19.7% | 23.2% | 1.28 | 1.03 | 1.59 | 1.18 | 0.93 | 1.50 |
| Two | 8.8% | 10.5% | 1.30 | 1.00 | 1.69 | 1.13 | 0.82 | 1.54 |
| Three+ | 4.2% | 4.2% | 1.09 | 0.73 | 1.63 | 0.92 | 0.59 | 1.45 |
For univariate analysis,
P ≤ 0.05;
P ≤ 0.01;
P ≤ 0.001
Adjusted analysis controls for: preconception mental health, maternal age, race/ethnicity, marital status, education, health insurance, income, and number of children in the household
Source: 1996-2006 Medical Expenditure Panel Survey (MEPS). Data are weighted percentages unless otherwise indicated.
Note: Percentages may not sum to 100 due to rounding. The multivariable models included all variables listed in the table.
Women with poor preconception mental health had 40% higher odds of having pregnancy complications than women without such problems (AOR 1.40, 95% CI: 1.02-1.92; Table 1). Black (non-Hispanic) women had 35% higher odds of having pregnancy complications than white (non-Hispanic) women (AOR 1.35, 95% CI: 1.05-1.74). Women living at 100-199% and 200-399% of the FPL (AOR 0.65, 95% CI: 0.51-0.82; AOR 0.72, 95% CI: 0.54-0.97, respectively) were less likely to have pregnancy complications, compared to women living below 100% of the FPL.
Non-Live Birth
Over 18% of pregnancies resulted in a non-live birth (Table 2). The proportion of women reporting poor preconception mental health was significantly greater for women whose pregnancies resulted in a non-live birth than for women who had a live birth (non-live birth, 8.9% versus live birth, 5.7%, p<0.05; 6.3% overall). There were significant differences between women with and without non-live birth in the distributions of age, race/ethnicity, and insurance status.
Table 2. Characteristics of sample by live birth and multivariate analysis of the odds of non-live birth (excluding abortions.
| Non-live birth (vs. Live birth) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Live birth | Non-live birth | Crude Odds Ratio |
95% Confidence Interval |
Adjusted Odds Ratio |
95% Confidence Interval |
|||
| TOTAL: weighted | 3,611,467 | 796,668 | ||||||
| weighted % | 81.9% | 18.1% | ||||||
| TOTAL: unweighted | 2,239 | 432 | ||||||
| unweight % | 83.8% | 16.2% | ||||||
| Maternal Characteristics | ||||||||
| Poor preconception mental healtha | ||||||||
| No | 94.3% | 91.1% | 1.00 | reference | 1.00 | reference | ||
| Yes | 5.7% | 8.9% | 1.60 | 1.08 | 2.38 | 1.48 | 0.96 | 2.27 |
| Agec | ||||||||
| 14-19 | 10.2% | 7.7% | 0.91 | 0.59 | 1.38 | 0.67 | 0.35 | 1.26 |
| 20-24b | 26.0% | 18.7% | 0.86 | 0.59 | 1.26 | 0.74 | 0.49 | 1.10 |
| 25-29 | 27.4% | 22.9% | 1.00 | reference | 1.00 | reference | ||
| 30-34 | 23.9% | 24.7% | 1.23 | 0.89 | 1.71 | 1.38 | 0.96 | 1.97 |
| 35+c | 12.5% | 26.0% | 2.49 | 1.72 | 3.61 | 2.74 | 1.82 | 4.11 |
| Race/Ethnicitya | ||||||||
| White (Non-Hispanic) | 60.1% | 63.9% | 1.00 | reference | 1.00 | reference | ||
| Black (Non-Hispanic) | 13.5% | 11.5% | 0.80 | 0.57 | 1.11 | 0.73 | 0.49 | 1.08 |
| Other (Non-Hispanic) | 6.3% | 15.0% | 1.45 | 0.85 | 2.47 | 1.42 | 0.81 | 2.50 |
| Hispanica | 20.1% | 9.6% | 0.70 | 0.51 | 0.97 | 0.60 | 0.42 | 0.86 |
| Marital status | ||||||||
| Married, lives with partner | 71.7% | 69.6% | 1.00 | reference | 1.00 | reference | ||
| Never married | 24.1% | 23.1% | 0.99 | 0.72 | 1.36 | 1.64 | 1.04 | 2.58 |
| Divorced, separated, widowed | 4.2% | 7.3% | 1.80 | 1.10 | 2.96 | 1.84 | 1.07 | 3.17 |
| Education status | ||||||||
| No or some high school | 23.4% | 21.8% | 0.84 | 0.58 | 1.20 | 0.97 | 0.63 | 1.48 |
| High school graduate | 26.6% | 29.7% | 1.00 | reference | 1.00 | reference | ||
| Some college | 21.7% | 21.2% | 0.87 | 0.60 | 1.26 | 0.75 | 0.50 | 1.12 |
| College or beyond | 28.3% | 27.3% | 0.86 | 0.62 | 1.20 | 0.61 | 0.40 | 0.94 |
| Family Characteristics | ||||||||
| Health Insurance Statusc | ||||||||
| Private insurance only | 62.7% | 64.4% | 1.00 | reference | 1.00 | reference | ||
| Any publicly funded insurancec | 24.0% | 14.9% | 0.60 | 0.43 | 0.84 | 0.57 | 0.36 | 0.92 |
| Partial insurance | 7.6% | 9.2% | 1.18 | 0.77 | 1.79 | 1.10 | 0.68 | 1.77 |
| No insurancec | 5.7% | 11.6% | 2.00 | 1.31 | 3.04 | 2.20 | 1.46 | 3.32 |
| Ratio of family income to poverty threshold | ||||||||
| Below 100% | 21.5% | 18.4% | 1.00 | reference | 1.00 | reference | ||
| 100-199% | 21.1% | 23.5% | 1.30 | 0.89 | 1.89 | 1.10 | 0.71 | 1.70 |
| 200-399% | 27.2% | 25.9% | 1.11 | 0.78 | 1.58 | 0.87 | 0.55 | 1.39 |
| 400%+ | 30.2% | 32.2% | 1.25 | 0.88 | 1.77 | 0.98 | 0.60 | 1.61 |
| Number of young children (age <5) | ||||||||
| None | 59.5% | 61.5% | 1.00 | reference | 1.00 | reference | ||
| One | 31.6% | 29.1% | 0.89 | 0.68 | 1.17 | 0.98 | 0.73 | 1.33 |
| Two+ | 8.9% | 9.3% | 1.01 | 0.66 | 1.54 | 1.11 | 0.70 | 1.76 |
| Number of school-aged children (age 5-17) | ||||||||
| None | 64.0% | 62.6% | 1.00 | reference | 1.00 | reference | ||
| One | 21.9% | 18.6% | 0.87 | 0.62 | 1.21 | 0.76 | 0.53 | 1.09 |
| Two | 9.7% | 13.8% | 1.45 | 0.96 | 2.17 | 1.22 | 0.77 | 1.92 |
| Three+ | 4.4% | 5.1% | 1.18 | 0.71 | 1.96 | 0.83 | 0.47 | 1.47 |
For univariate analysis,
P ≤ 0.05;
P ≤ 0.01;
P ≤ 0.001
Non-live births include pregnancies which ended in a miscarriage or stillbirth
Adjusted analysis controls for: preconception mental health, maternal age, race/ethnicity, marital status, education, health insurance, income and number of children in the household
Source: 1996-2006 Medical Expenditure Panel Survey (MEPS). Data are weighted percentages unless otherwise indicated.
Note: Percentages may not sum to 100 due to rounding. The multivariable models included all variables listed in the table.
Women who reported poor preconception mental health showed a trend towards having a non-live birth as compared to women without such problems (AOR 1.48, 95% CI: 0.96-2.27, Table 2). Women over the age of 35 had almost three times the odds of having a non-live birth than women ages 25-29 (AOR 2.74, 95% CI: 1.82-4.11). Compared to women who were married or living with a partner, women who were never married had 64% higher odds (AOR 1.64, 95% CI: 1.04-2.58) and women who were divorced, separated, or widowed had 84% higher odds of having a non-live birth (AOR 1.84, 95% CI: 1.07-3.17). Hispanic women had 40% lower odds of having a non-live birth than white (non-Hispanic) women (AOR 0.60, 95% CI: 0.42-0.86). Women with any publicly funded insurance had 43% lower odds of having a non-live birth (AOR 0.57, 95% CI: 0.36-0.92); whereas uninsured women were more than twice as likely to have a non-live birth (AOR 2.20, 95% CI: 1.46-3.32) relative to women with private insurance. Women with a college degree or higher had 39% lower odds of having a non-live birth than women with a high school degree (AOR 0.61, 95% CI: 0.40-0.94).
Analyses including women who terminated their pregnancy by an abortion revealed 68% higher odds of having a non-live birth for women with poor preconception mental health (AOR 1.68, 95% CI: 1.11-2.55, Appendix 1).
Low Birth Weight
6.0% of infants were born LBW (Table 3). Poor preconception mental health was reported in a significantly higher proportion of mothers of LBW babies than mothers of NBW babies and HBW babies (LBW, 12.7% versus NBW, 5.7%; versus HBW, 1.2%; p<.01; 5.7% overall). Compared to mothers of NBW babies, mothers of LBW babies were more likely to be black (non-Hispanic), never married, partially insured, or have had a pregnancy complication.
Table 3. Characteristics of sample by birth weight and polychotomous multivariable analysis of the odds of low birth weight.
| Low Birth Weight (vs. Normal) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Low (< 2500 g) |
Normal (2500- 3999 g) |
High (4000+ g) |
Crude Odds Ratio |
95% Confidence Interval |
Adjusted Odds Ratio |
95% Confidence Interval |
|||
| TOTAL: weighted | 207,080 | 2,873,927 | 348,344 | ||||||
| weighted % | 6.0% | 83.8% | 10.2% | ||||||
| TOTAL: unweighted | 140 | 1,759 | 209 | ||||||
| unweight % | 6.6% | 83.4% | 9.9% | ||||||
| Maternal Characteristics | |||||||||
| Poor preconception mental healthc | |||||||||
| No | 87.3% | 94.3% | 98.8% | 1.00 | reference | 1.00 | reference | ||
| Yes | 12.7% | 5.7% | 1.2% | 2.39 | 1.27 | 4.49 | 1.99 | 1.00 | 3.98 |
| Ageb | |||||||||
| 14-19a | 9.9% | 10.4% | 3.8% | 1.05 | 0.47 | 2.33 | 0.73 | 0.27 | 1.97 |
| 20-24a | 33.2% | 26.6% | 16.7% | 1.38 | 0.74 | 2.57 | 1.28 | 0.64 | 2.55 |
| 25-29a | 23.6% | 26.1% | 36.6% | 1.00 | reference | 1.00 | reference | ||
| 30-34 | 19.3% | 24.7% | 27.3% | 0.86 | 0.47 | 1.59 | 1.06 | 0.55 | 2.03 |
| 35+ | 14.0% | 12.2% | 15.6% | 1.27 | 0.62 | 2.58 | 1.27 | 0.61 | 2.63 |
| Race/Ethnicityc | |||||||||
| White (Non-Hispanic)c | 51.3% | 59.8% | 73.2% | 1.00 | reference | 1.00 | reference | ||
| Black (Non-Hispanic)b | 22.1% | 13.1% | 7.9% | 1.96 | 1.11 | 3.47 | 1.59 | 0.85 | 2.95 |
| Other (Non-Hispanic) | 4.8% | 6.9% | 1.9% | 0.81 | 0.24 | 2.68 | 0.82 | 0.23 | 2.87 |
| Hispanic | 21.9% | 20.1% | 16.9% | 1.27 | 0.75 | 2.14 | 1.02 | 0.60 | 1.74 |
| Marital statusa | |||||||||
| Married, lives with partnera | 61.0% | 71.9% | 80.0% | 1.00 | reference | 1.00 | reference | ||
| Never marrieda | 32.7% | 23.8% | 17.4% | 1.62 | 0.98 | 2.68 | 1.16 | 0.58 | 2.34 |
| Divorced, separated, widowed | 6.2% | 4.3% | 2.6% | 1.71 | 0.80 | 3.68 | 1.05 | 0.47 | 2.32 |
| Education status | |||||||||
| No or some high school | 27.8% | 23.0% | 18.3% | 0.93 | 0.53 | 1.63 | 0.93 | 0.48 | 1.83 |
| High school graduate | 34.7% | 26.6% | 24.4% | 1.00 | reference | 1.00 | reference | ||
| Some college | 18.1% | 21.8% | 20.3% | 0.64 | 0.32 | 1.28 | 0.69 | 0.34 | 1.40 |
| College or beyonda | 19.4% | 28.6% | 37.1% | 0.52 | 0.29 | 0.94 | 0.76 | 0.40 | 1.43 |
| Any pregnancy complicationc | |||||||||
| No | 26.8% | 61.1% | 59.2% | 1.00 | reference | 1.00 | reference | ||
| Yes | 73.2% | 38.9% | 40.8% | 4.29 | 2.60 | 7.10 | 4.07 | 2.45 | 6.76 |
| Family Characteristics | |||||||||
| Health Insurance Statusa | |||||||||
| Private insurance only | 53.0% | 63.5% | 63.6% | 1.00 | reference | 1.00 | reference | ||
| Any publicly funded insurance | 26.0% | 23.7% | 23.6% | 1.31 | 0.78 | 2.22 | 0.87 | 0.46 | 1.65 |
| Partial insuranceb | 15.5% | 7.5% | 5.1% | 2.47 | 1.37 | 4.44 | 1.69 | 0.81 | 3.53 |
| No insurance | 5.4% | 5.2% | 7.8% | 1.24 | 0.56 | 2.72 | 0.96 | 0.36 | 2.54 |
| Ratio of family income to poverty threshold | |||||||||
| Below 100% | 25.7% | 21.1% | 21.0% | 1.00 | reference | 1.00 | reference | ||
| 100-199% | 27.5% | 21.0% | 16.0% | 1.07 | 0.61 | 1.88 | 1.29 | 0.73 | 2.31 |
| 200-399% | 24.8% | 27.0% | 30.7% | 0.75 | 0.42 | 1.37 | 1.01 | 0.52 | 1.93 |
| 400%+ | 21.9% | 30.9% | 32.3% | 0.58 | 0.31 | 1.10 | 1.01 | 0.41 | 2.50 |
| Number of young children (age <5) | |||||||||
| Nonea | 66.0% | 61.2% | 51.1% | 1.00 | reference | 1.00 | reference | ||
| Onea | 26.1% | 30.3% | 40.3% | 0.80 | 0.48 | 1.34 | 0.78 | 0.46 | 1.34 |
| Two+ | 7.9% | 8.5% | 8.6% | 0.86 | 0.39 | 1.94 | 0.79 | 0.34 | 1.81 |
| Number of school-aged children (age 5-17) | |||||||||
| None | 57.9% | 65.2% | 57.3% | 1.00 | reference | 1.00 | reference | ||
| One | 22.2% | 21.3% | 26.5% | 1.17 | 0.73 | 1.90 | 1.02 | 0.61 | 1.71 |
| Two | 14.5% | 9.1% | 11.8% | 1.79 | 0.96 | 3.35 | 1.53 | 0.80 | 2.93 |
| Three+ | 5.4% | 4.4% | 4.4% | 1.36 | 0.63 | 2.95 | 1.25 | 0.48 | 3.24 |
For univariate analysis,
P ≤ 0.05;
P ≤ 0.01;
P ≤ 0.001
Adjusted analysis controls for: preconception mental health, maternal age, race/ethnicity, marital status, education, health insurance, income, and number of children in the household
Source: 1996-2006 Medical Expenditure Panel Survey (MEPS). Data are weighted percentages unless otherwise indicated.
Note: Percentages may not sum to 100 due to rounding. The multivariable models included all variables listed in the table.
Having poor preconception mental health nearly doubled the odds of having a LBW baby (AOR 1.99, 95% CI: 1.00-3.98, Table 3), and having a pregnancy complication quadrupled the odds of having a LBW baby (AOR 4.07, 95% CI: 2.45-6.76). (See Appendix 2 for multivariable results for HBW compared to NBW).
Marital status attenuated the effect of race on LBW, such that the effect associated with being of black (non-Hispanic) race became statistically non-significant when marital status was added to the model (unadjusted OR 1.96, 95% CI: 1.11-3.47; adjusted for marital status OR 1.70, 95% CI: 0.91-3.18; fully adjusted OR 1.59, 95% CI: 0.85-2.95). The inclusion of marital status did not change the effect of poor preconception mental health (Data not shown; results available upon request).
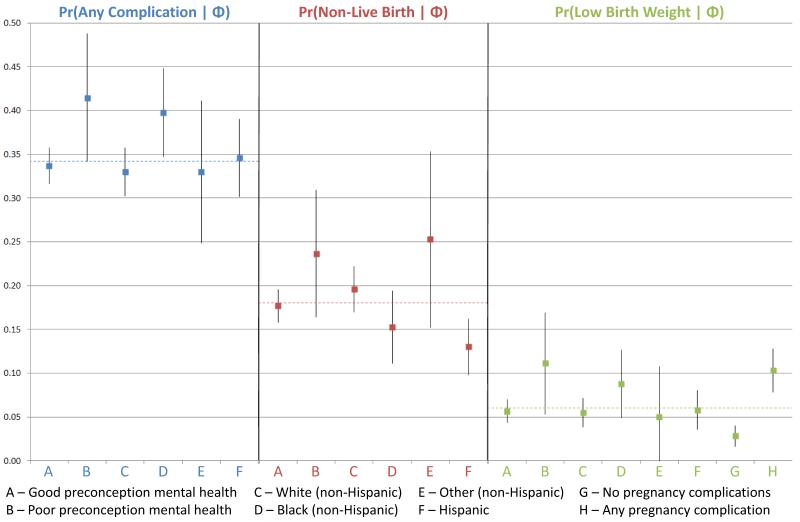
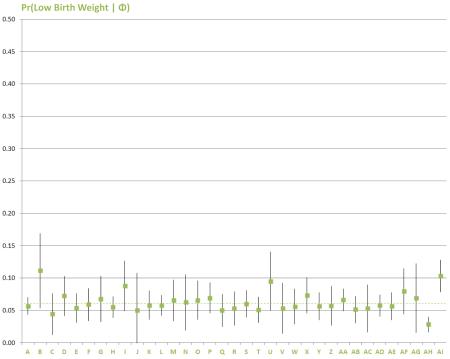
Figure 2 displays the average predicted probabilities of each of the three outcomes (any complication, non-live birth, and LBW, respectively) with respect to the main explanatory variables, controlling for maternal age, marital status, education, insurance, income, and the number of children in the household.
Fig. 2. Adjusted predicted probabilities of obstetric outcomes for main explanatory variables.
Figure 2 displays the adjusted average predicted probabilities of each of the three outcomes (any complication, non-live birth, and LBW, respectively) with respect to the main explanatory variables, controlling for maternal age, marital status, education, insurance, income, and the number of children in the household. The dashed lines on the chart represent the weighted prevalence estimates for each outcome. Delta method standard errors of the probabilities were used to construct 95% confidence intervals, which are represented by the vertical bars. Graphical depiction of the predicted probabilities of each outcome for all independent variables may be found in Appendix 3.
DISCUSSION
This nationally representative, population-based study showed that poor preconception mental health was the most significant risk factor for pregnancy complications, a possible risk factor for non-live birth, and a strong risk factor for LBW. Women who reported poor mental health before pregnancy were 40% more likely to have a pregnancy complication, almost 50% more likely to have a non-live birth, and nearly twice as likely to give birth to a LBW baby. Our findings draw attention to the importance of effective preconception care and have far reaching implications for the health and well-being of mothers and their babies.
Preconception mental health represents a vital modifiable risk factor for poor obstetric outcomes. Previous psychosocial interventions aimed at reducing poor pregnancy outcomes have had limited success [25,26], possibly because they were delivered during the antepartum period, rather than before pregnancy. There is ample evidence to suggest that poor maternal mental health leads to neuroendocrine alterations which have direct implications for fetal health [27-35]. Furthermore, chronic exposure to the pathophysiology of poor preconception mental health may alter a woman’s ability to respond to poor antepartum mental health, in addition to independently affecting obstetric outcomes. Moreover, recent studies have indicated that poor preconception mental health predicts both poor antepartum [36] and postpartum mental health [37]. Thus, interventions in the antepartum period may be too late to affect the psychobiological ramifications of poor preconception mental health.
Accordingly, a life course perspective on maternal and child health suggests that interventions aimed at preventing adverse obstetric outcomes may be most effective if they begin in the preconception period [17]. Women of all reproductive ages should be offered a continuum of health services that include preventive and primary care [17]. Promoting preconception health through screening, education, counseling, treatment, and/or referral [4] should occur in a variety of settings over the life course to ensure that women are effectively identified and treated for mental health problems, regardless of where or when they interface with the healthcare system. Research suggests that there are a substantial number of pregnant women screened in obstetrics settings who experience significant symptoms of depression, but are not adequately monitored or treated during this vulnerable time [38] and that disparities in treatment persist into motherhood [39]. In the absence of established guidelines [40] on addressing mental health issues in the preconception period, it is our hope that our findings will be a call to action.
Interestingly, receiving any publicly funded insurance, compared to private insurance, was the strongest protective factor against having a non-live birth in our study; while having no insurance significantly increased the likelihood of a non-live birth. This protective effect may operate through a number of pathways. Women who lack insurance may be severely limited in their ability to access healthcare services [41]; and non-live births are less likely when women receive early, comprehensive prenatal care [42-44]. Medicaid and other programs may play a critical role in providing access to affordable, comprehensive prenatal care for the women in greatest need. Furthermore, nutrition [45] and other health behaviors [46] have been suggested to play a role in the occurrence of non-live birth, and women on public insurance may also be enrolled in women, infants, and children (WIC) programs and thus may have better nutrition or other health behaviors as a result. Additionally, the “safety net” of public insurance may also help to reduce stress during the prenatal period, promoting better health and mental health for these women. Regardless of the mechanism, public insurance programs clearly play an important role in preventing negative obstetric outcomes. However, some women may only receive public coverage during and shortly after pregnancy. To promote preconception health and mental health over the life course, all women should have access to affordable, comprehensive care and expanding access to care via public insurance may help to ensure that all women enter pregnancy in optimal health.
Our finding that black women were more likely to have pregnancy complications than white women is consistent with other studies [47-53]. However, in contrast to previous research [54-56] we found no evidence of racial disparities in LBW. In our study, the relationship between black race and LBW was attenuated and non-significant after adjusting for marital status, age, and pregnancy complications, consistent with a prior study [57]. Several other studies have demonstrated that married women are at lower risk for LBW than unmarried women [58-61], perhaps due to social or emotional support they receive from their spouse. Ensuring that women have strong social support is an important modifiable risk factor for reducing the rate of LBW among black women. Many studies of black-white disparities in birth outcomes do not account for pregnancy complications [56,62,63]. Pregnancy complications may be part of the pathway between black race and LBW, and indeed our results show that black women are more likely than white women to experience pregnancy complications. Preventing complications should be a priority in reducing rates of LBW and future work should continue to investigate effective strategies.
Our study revealed significant socioeconomic risk factors for pregnancy complications, which may be reflective of unmeasured factors. Social class processes, including the effects of material deprivation, have been proposed as pathways between socioeconomic factors and birth outcomes [64]. Material deprivation may affect health by restricting access to safe neighborhoods or health-promoting services, or indirectly operate through individual behavior [64], such as smoking, which is typically more frequent among women who are unmarried, uneducated, or living in poverty [65,66,67]. Income may affect birth outcomes via the neighborhood context or social disparities [67]. The understanding that childhood socioeconomic factors are associated with obstetric outcomes in adulthood lends further support to adopting a life course perspective when examining the relationship between a women’s health and adverse birth outcomes [67].
Strengths and Limitations
To our knowledge, this is the first study to evaluate the association between preconception mental health status and adverse obstetric outcomes using national data. Our large sample size and rich data set allowed for simultaneous control of several sociodemographic and medical factors. Adjusting for these covariates did not change the robust relationship between preconception mental health and adverse obstetric outcomes.
Potential limitations should be considered when interpreting our results. First, it is possible that women who have experienced poor mental health to varying degrees throughout their lives may have been classified as not having poor preconception mental health, thereby underestimating our results. Moreover our independent variable was a single-item measure of self-rated mental health, as opposed to a diagnostic measure and therefore may not have fully captured women’s mental health status. However, many studies have correlated this measure with clinically significant symptoms and outcomes (e.g., [20,21,68]). Second, small sample sizes precluded us from being able to examine the role of preconception mental health on specific pregnancy complications. There may be different underlying etiologies for various complications that should be explored in future research (e.g., [69]). Third, pregnant women self-reported obstetric outcomes in the MEPS and therefore their response may be subject to recall bias or their report of such outcomes may have been influenced by their level of health literacy. Fourth, we were unable to assess maternal smoking behavior, as data on smoking was not collected until 2000, resulting in an inadequate sample size for evaluation. Fifth, the MEPS did not have information about women’s parity; however, we used the number of children in the household as a proxy for parity and it should be noted that this measure may not have adequately captured parity, as the biological relationships of household members are unknown and there may be other biological children living outside of the household. Finally, we were not able to account for other preconception factors (e.g., maternal intimate partner violence, social support, mastery, or self-esteem) that may play important mediating or moderating roles in the relationship between poor preconception mental health and adverse obstetric outcomes.
Conclusion
This nationally representative, population-based study showed that poor preconception mental health was the most significant risk factor for pregnancy complications, a possible risk factor for non-live birth, and a strong risk factor for LBW. As part of the efforts underway on many fronts to reduce risk for these deleterious obstetric outcomes, women and their providers should strive to identify and address poor mental health during the preconception period. Furthermore, policy changes aimed at increasing access to preconception care (including mental health screening and treatment) may be important for improving the health of women and their babies. These steps will ensure that women are in optimal mental health in the preconception period, which may be effective in reducing the incidence of poor obstetric outcomes.
Acknowledgments
We would like to acknowledge the generous funding that supported this research. WPW received funding from the University of Wisconsin Institute for Research on Poverty. LEW was supported by a grant from the Graduate School of the University of Wisconsin, Madison (PI: Witt) and a pre-doctoral NRSA Training Grant (T32 HS00083; PI: Smith). EWH was supported by NIH (T32 HD049302; PI: Sarto). We would also like to acknowledge the members of the Lifecourse Epidemiology and Family Health (LEAF) Lab (PI: Witt) for their review of the paper. We would also like to thank the anonymous reviewers for their helpful comments and suggestions.
Appendix 1 - Characteristics of sample by live birth and multivariate analysis of the odds of non-live birth (including abortions
| Non-live birth (vs. Live birth) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Live birth | Non-live birth | Crude Odds Ratio |
95% Confidence Interval |
Adjusted Odds Ratio |
95% Confidence Interval |
|||
| TOTAL: weighted | 3,611,467 | 965,703 | ||||||
| weighted % | 78.9% | 21.1% | ||||||
| TOTAL: unweighted | 2,239 | 534 | ||||||
| unweight % | 80.7% | 19.3% | ||||||
| Maternal Characteristics | ||||||||
| Poor preconception mental healthc | ||||||||
| No | 94.3% | 89.6% | 1.00 | reference | 1.00 | reference | ||
| Yes | 5.7% | 10.4% | 1.90 | 1.31 | 2.77 | 1.68 | 1.11 | 2.55 |
| Agec | ||||||||
| 14-19 | 10.2% | 8.9% | 1.02 | 0.69 | 1.52 | 0.67 | 0.37 | 1.22 |
| 20-24b | 26.0% | 18.4% | 0.82 | 0.59 | 1.15 | 0.68 | 0.47 | 0.99 |
| 25-29 | 27.4% | 23.6% | 1.00 | reference | 1.00 | reference | ||
| 30-34 | 23.9% | 25.0% | 1.21 | 0.90 | 1.64 | 1.44 | 1.05 | 1.96 |
| 35+c | 12.5% | 24.1% | 2.25 | 1.59 | 3.18 | 2.56 | 1.77 | 3.69 |
| Race/Ethnicityc | ||||||||
| White (Non-Hispanic) | 60.1% | 60.6% | 1.00 | reference | 1.00 | reference | ||
| Black (Non-Hispanic) | 13.5% | 13.1% | 0.96 | 0.71 | 1.32 | 0.78 | 0.55 | 1.12 |
| Other (Non-Hispanic)b | 6.3% | 11.9% | 1.88 | 1.19 | 2.97 | 1.90 | 1.19 | 3.03 |
| Hispanicb | 20.1% | 14.4% | 0.71 | 0.52 | 0.96 | 0.59 | 0.42 | 0.83 |
| Marital statusc | ||||||||
| Married, lives with partnerb | 71.7% | 64.1% | 1.00 | reference | 1.00 | reference | ||
| Never married | 24.1% | 26.7% | 1.24 | 0.94 | 1.62 | 2.14 | 1.43 | 3.20 |
| Divorced, separated, widowedc | 4.2% | 9.2% | 2.47 | 1.49 | 4.12 | 2.67 | 1.49 | 4.76 |
| Education status | ||||||||
| No or some high school | 23.4% | 22.7% | 0.87 | 0.64 | 1.18 | 0.98 | 0.68 | 1.42 |
| High school graduate | 26.6% | 29.7% | 1.00 | reference | 1.00 | reference | ||
| Some college | 21.7% | 21.5% | 0.88 | 0.64 | 1.23 | 0.77 | 0.53 | 1.13 |
| College or beyond | 28.3% | 26.1% | 0.82 | 0.58 | 1.16 | 0.63 | 0.40 | 0.97 |
| Family Characteristics | ||||||||
| Health Insurance Statusc | ||||||||
| Private insurance only | 62.7% | 63.0% | 1.00 | reference | 1.00 | reference | ||
| Any publicly funded insurancec | 24.0% | 16.3% | 0.67 | 0.50 | 0.91 | 0.56 | 0.37 | 0.86 |
| Partial insurance | 7.6% | 9.4% | 1.23 | 0.83 | 1.83 | 1.08 | 0.68 | 1.72 |
| No insurancec | 5.7% | 11.3% | 2.00 | 1.35 | 2.95 | 2.13 | 1.45 | 3.13 |
| Ratio of family income to poverty threshold | ||||||||
| Below 100% | 21.5% | 20.1% | 1.00 | reference | 1.00 | reference | ||
| 100-199% | 21.1% | 22.9% | 1.16 | 0.81 | 1.67 | 1.04 | 0.69 | 1.57 |
| 200-399% | 27.2% | 27.9% | 1.10 | 0.78 | 1.55 | 0.93 | 0.61 | 1.42 |
| 400%+ | 30.2% | 29.0% | 1.03 | 0.74 | 1.43 | 0.91 | 0.58 | 1.44 |
| Number of young children (age <5) | ||||||||
| None | 59.5% | 60.7% | 1.00 | reference | 1.00 | reference | ||
| One | 31.6% | 29.5% | 0.92 | 0.71 | 1.18 | 1.07 | 0.81 | 1.42 |
| Two+ | 8.9% | 9.8% | 1.08 | 0.75 | 1.55 | 1.24 | 0.83 | 1.86 |
| Number of school-aged children (age 5-17) | ||||||||
| None | 64.0% | 60.9% | 1.00 | reference | 1.00 | reference | ||
| One | 21.9% | 20.8% | 1.00 | 0.73 | 1.37 | 0.84 | 0.61 | 1.17 |
| Twoa | 9.7% | 13.5% | 1.46 | 1.01 | 2.11 | 1.17 | 0.77 | 1.78 |
| Three+ | 4.4% | 4.8% | 1.14 | 0.71 | 1.84 | 0.81 | 0.47 | 1.37 |
For univariate analysis,
P≤0.05;
P≤0.01;
P≤0.001
Non-live births include pregnancies which ended in a miscarriage, stillbirth, or abortion
Adjusted analysis controls for: preconception mental health, maternal age, race/ethnicity, marital status, education, health insurance, income and number of children in the household
Source: 1996-2006 Medical Expenditure Panel Survey (MEPS). Data are weighted percentages unless otherwise indicated
Note: Percentages may not sum to 100 due to rounding. The multivariable models included all variables listed in the table
Appendix 2 - Polychotomous multivariable analysis of the odds of high birth weight
| High Birth Weight (vs. Normal) | ||||||
|---|---|---|---|---|---|---|
| Crude Odds Ratio |
95% Confidence Interval |
Adjusted Odds Ratio |
95% Confidence Interval |
|||
| Maternal Characteristics | ||||||
| Poor preconception mental health | ||||||
| No | 1.00 | reference | 1.00 | reference | ||
| Yes | 0.20 | 0.05 | 0.78 | 0.22 | 0.06 | 0.86 |
| Age | ||||||
| 14-19 | 0.26 | 0.10 | 0.68 | 0.26 | 0.09 | 0.70 |
| 20-24 | 0.45 | 0.25 | 0.80 | 0.43 | 0.25 | 0.75 |
| 25-29 | 1.00 | reference | 1.00 | reference | ||
| 30-34 | 0.79 | 0.51 | 1.22 | 0.69 | 0.44 | 1.08 |
| 35+ | 0.91 | 0.51 | 1.62 | 0.85 | 0.44 | 1.64 |
| Race/Ethnicity | ||||||
| White (Non-Hispanic) | 1.00 | reference | 1.00 | reference | ||
| Black (Non-Hispanic) | 0.49 | 0.31 | 0.79 | 0.43 | 0.23 | 0.81 |
| Other (Non-Hispanic) | 0.23 | 0.08 | 0.64 | 0.21 | 0.08 | 0.58 |
| Hispanic | 0.69 | 0.47 | 1.00 | 0.67 | 0.43 | 1.05 |
| Marital status | ||||||
| Married, lives with partner | 1.00 | reference | 1.00 | reference | ||
| Never married | 0.65 | 0.40 | 1.06 | 1.18 | 0.64 | 2.17 |
| Divorced, separated, widowed | 0.55 | 0.21 | 1.47 | 0.60 | 0.22 | 1.65 |
| Education status | ||||||
| No or some high school | 0.87 | 0.50 | 1.50 | 0.88 | 0.50 | 1.55 |
| High school graduate | 1.00 | reference | 1.00 | reference | ||
| Some college | 1.02 | 0.59 | 1.76 | 0.96 | 0.55 | 1.67 |
| College or beyond | 1.41 | 0.86 | 2.33 | 1.28 | 0.74 | 2.23 |
| Any pregnancy complication | ||||||
| No | 1.00 | reference | 1.00 | reference | ||
| Yes | 1.08 | 0.75 | 1.56 | 1.07 | 0.73 | 1.56 |
| Family Characteristics | ||||||
| Health Insurance Status | ||||||
| Private insurance only | 1.00 | reference | 1.00 | reference | ||
| Any publicly funded insurance | 0.99 | 0.65 | 1.52 | 1.58 | 0.89 | 2.83 |
| Partial insurance | 0.68 | 0.35 | 1.31 | 0.96 | 0.45 | 2.06 |
| No insurance | 1.48 | 0.78 | 2.80 | 2.24 | 1.07 | 4.68 |
| Ratio of family income to poverty threshold | ||||||
| Below 100% | 1.00 | reference | 1.00 | reference | ||
| 100-199% | 0.76 | 0.45 | 1.31 | 0.62 | 0.35 | 1.10 |
| 200-399% | 1.14 | 0.67 | 1.97 | 0.97 | 0.50 | 1.87 |
| 400%+ | 1.05 | 0.60 | 1.83 | 0.85 | 0.40 | 1.83 |
| Number of young children (age <5) | ||||||
| None | 1.00 | reference | 1.00 | reference | ||
| One | 1.60 | 1.13 | 2.26 | 1.49 | 1.04 | 2.13 |
| Two+ | 1.22 | 0.67 | 2.21 | 1.14 | 0.60 | 2.19 |
| Number of school-aged children (age 5-17) | ||||||
| None | 1.00 | reference | 1.00 | reference | ||
| One | 1.42 | 0.99 | 2.02 | 1.52 | 1.03 | 2.25 |
| Two | 1.47 | 0.82 | 2.66 | 1.74 | 0.98 | 3.10 |
| Three+ | 1.13 | 0.52 | 2.46 | 1.23 | 0.46 | 3.33 |
Adjusted analysis controls for: preconception mental health, maternal age, race/ethnicity, marital status, education, health insurance, income, and number of children in the household
Source: 1996-2006 Medical Expenditure Panel Survey (MEPS)
Note: The multivariable models included all variables listed in the table
Appendix 3- Adjusted Predicted Probabilities of Obstetric Outcomes for All Explanatory Variables
Contributor Information
Whitney P. Witt, Department of Population Health Sciences, School of Medicine and Public Health, University of Wisconsin, 610 North Walnut Street, Office 503, Madison, WI 53726, USA, wwitt@wisc.edu
Lauren E. Wisk, Department of Population Health Sciences, School of Medicine and Public Health, University of Wisconsin, 610 North Walnut Street, Office 558, Madison, WI 53726, USA, wisk@wisc.edu
Erika R. Cheng, Department of Population Health Sciences, School of Medicine and Public Health, University of Wisconsin, 610 North Walnut Street, Office 530, Madison, WI 53726, USA, ercheng@wisc.edu
John M. Hampton, UW Carbone Cancer Center, Madison, WI, USA, jmhampton@uwcarbone.wisc.edu
Erika W. Hagen, Department of Population Health Sciences, School of Medicine and Public Health, University of Wisconsin, 610 North Walnut Street, Office 630, Madison, WI 53726, USA, ewarkentien@wisc.edu
References
- 1.Xu J, Kochanek K, Murphy S, Tejada-Vera B. National Vital Statistics Reports. Vol. 58. Hyattsville, MD: 2010. Deaths: Final Data for 2007; pp. 1–135. [PubMed] [Google Scholar]
- 2.Bhushan V, Paneth N, Kiely JL. Impact of improved survival of very low birth weight infants on recent secular trends in the prevalence of cerebral palsy. Pediatrics. 1993;91:1094–1100. [PubMed] [Google Scholar]
- 3.McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med. 1985;312:82–90. doi: 10.1056/NEJM198501103120204. [DOI] [PubMed] [Google Scholar]
- 4.Atrash HK, Johnson K, Adams M, Cordero JF, Howse J. Preconception care for improving perinatal outcomes: the time to act. Matern Child Health J. 2006;10:S3–11. doi: 10.1007/s10995-006-0100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67:1012–1024. doi: 10.1001/archgenpsychiatry.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alder J, Fink N, Bitzer J, Hosli I, Holzgreve W. Depression and anxiety during pregnancy: a risk factor for obstetric, fetal and neonatal outcome? A critical review of the literature. J Matern Fetal Neonatal Med. 2007;20:189–209. doi: 10.1080/14767050701209560. [DOI] [PubMed] [Google Scholar]
- 7.Precht DH, Andersen PK, Olsen J. Severe life events and impaired fetal growth: a nation-wide study with complete follow-up. Acta Obstet Gynecol Scand. 2007;86:266–275. doi: 10.1080/00016340601088406. [DOI] [PubMed] [Google Scholar]
- 8.Sjostrom K, Thelin T, Valentin L, Marsal K. Do pre-, early, and mid-pregnancy life events influence gestational length? J Psychosom Obstet Gynaecol. 1999;20:170–176. doi: 10.3109/01674829909075592. [DOI] [PubMed] [Google Scholar]
- 9.Khashan AS, McNamee R, Abel KM, et al. Rates of preterm birth following antenatal maternal exposure to severe life events: a population-based cohort study. Hum Reprod. 2009;24:429–437. doi: 10.1093/humrep/den418. [DOI] [PubMed] [Google Scholar]
- 10.Khashan AS, McNamee R, Abel KM, et al. Reduced infant birthweight consequent upon maternal exposure to severe life events. Psychosom Med. 2008;70:688–694. doi: 10.1097/PSY.0b013e318177940d. [DOI] [PubMed] [Google Scholar]
- 11.Gavin AR, Chae DH, Mustillo S, Kiefe CI. Prepregnancy depressive mood and preterm birth in black and white women: findings from the CARDIA Study. J Womens Health (Larchmt) 2009;18:803–811. doi: 10.1089/jwh.2008.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gold KJ, Dalton VK, Schwenk TL, Hayward RA. What causes pregnancy loss? Preexisting mental illness as an independent risk factor. Gen Hosp Psychiatry. 2007;29:207–213. doi: 10.1016/j.genhosppsych.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Haas JS, Fuentes-Afflick E, Stewart AL, et al. Prepregnancy health status and the risk of preterm delivery. Arch Pediatr Adolesc Med. 2005;159:58–63. doi: 10.1001/archpedi.159.1.58. [DOI] [PubMed] [Google Scholar]
- 14.Mustillo S, Krieger N, Gunderson EP, Sidney S, McCreath H, Kiefe CI. Self-reported experiences of racial discrimination and Black-White differences in preterm and low-birthweight deliveries: the CARDIA Study. Am J Public Health. 2004;94:2125–2131. doi: 10.2105/ajph.94.12.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Misra DP, Guyer B, Allston A. Integrated perinatal health framework. A multiple determinants model with a life span approach. American Journal of Preventive Medicine. 2003;25:65–75. doi: 10.1016/s0749-3797(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 16.Halfon N, Hochstein M. Life course health development: an integrated framework for developing health, policy, and research. The Milbank Quarterly. 2002;80:433–479. doi: 10.1111/1468-0009.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu MC, Halfon N. Racial and ethnic disparities in birth outcomes: a life-course perspective. Matern Child Health J. 2003;7:13–30. doi: 10.1023/a:1022537516969. [DOI] [PubMed] [Google Scholar]
- 18.Evans RG, Stoddart GL. Producing health, consuming health care. Social Science & Medicine. 1990;31:1347–1363. doi: 10.1016/0277-9536(90)90074-3. [DOI] [PubMed] [Google Scholar]
- 19.Committee on Scientific Evaluation of WIC Nutrition Risk Criteria, Institute of Medicine . WIC Nutrition Risk Criteria: A Scientific Assessment. National Academy Press; Washington, D.C.: 1996. [PubMed] [Google Scholar]
- 20.Mawani FN, Gilmour H. Validation of self-rated mental health. Health Rep. 2010;21:61–75. [PubMed] [Google Scholar]
- 21.Fleishman JA, Zuvekas SH. Global self-rated mental health: associations with other mental health measures and with role functioning. Med Care. 2007;45:602–609. doi: 10.1097/MLR.0b013e31803bb4b0. [DOI] [PubMed] [Google Scholar]
- 22.Cohen JW, Monheit AC, Beauregard KM, et al. The Medical Expenditure Panel Survey: a national health information resource. Inquiry. 1997;33:373. [PubMed] [Google Scholar]
- 23.Bryant AS, Worjoloh A, Caughey AB, Washington AE. Racial/ethnic disparities in obstetric outcomes and care: prevalence and determinants. Am J Obstet Gynecol. 2010;202:335–343. doi: 10.1016/j.ajog.2009.10.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Oppenraaij RH, Jauniaux E, Christiansen OB, Horcajadas JA, Farquharson RG, Exalto N. Predicting adverse obstetric outcome after early pregnancy events and complications: a review. Hum Reprod Update. 2009;15:409–421. doi: 10.1093/humupd/dmp009. [DOI] [PubMed] [Google Scholar]
- 25.Villar J, Farnot U, Barros F, Victora C, Langer A, Belizan JM. A randomized trial of psychosocial support during high-risk pregnancies. The Latin American Network for Perinatal and Reproductive Research. N Engl J Med. 1992;327:1266–1271. doi: 10.1056/NEJM199210293271803. [DOI] [PubMed] [Google Scholar]
- 26.Bryce RL, Stanley FJ, Garner JB. Randomized controlled trial of antenatal social support to prevent preterm birth. Br J Obstet Gynaecol. 1991;98:1001–1008. doi: 10.1111/j.1471-0528.1991.tb15338.x. [DOI] [PubMed] [Google Scholar]
- 27.Dunkel Schetter C. Psychological science on pregnancy: stress processes, biopsychosocial models, and emerging research issues. Annu Rev Psychol. 2011;62:531–558. doi: 10.1146/annurev.psych.031809.130727. [DOI] [PubMed] [Google Scholar]
- 28.Strohle A, Holsboer F. Stress responsive neurohormones in depression and anxiety. Pharmacopsychiatry. 2003;36:S207–214. doi: 10.1055/s-2003-45132. [DOI] [PubMed] [Google Scholar]
- 29.Wadhwa PD, Culhane JF, Rauh V, et al. Stress, infection and preterm birth: a biobehavioural perspective. Paediatr Perinat Epidemiol. 2001;15:17–29. doi: 10.1046/j.1365-3016.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- 30.Hobel CJ, Dunkel-Schetter C, Roesch SC, Castro LC, Arora CP. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks’ gestation in pregnancies ending in preterm delivery. Am J Obstet Gynecol. 1999;180:S257–263. doi: 10.1016/s0002-9378(99)70712-x. [DOI] [PubMed] [Google Scholar]
- 31.McGrath S, McLean M, Smith D, Bisits A, Giles W, Smith R. Maternal plasma corticotropin-releasing hormone trajectories vary depending on the cause of preterm delivery. Am J Obstet Gynecol. 2002;186:257–260. doi: 10.1067/mob.2002.119635. [DOI] [PubMed] [Google Scholar]
- 32.Pike IL. Maternal stress and fetal responses: evolutionary perspectives on preterm delivery. Am J Hum Biol. 2005;17:55–65. doi: 10.1002/ajhb.20093. [DOI] [PubMed] [Google Scholar]
- 33.Diego MA, Field T, Hernandez-Reif M, Cullen C, Schanberg S, Kuhn C. Prepartum, postpartum, and chronic depression effects on newborns. Psychiatry. 2004;67:63–80. doi: 10.1521/psyc.67.1.63.31251. [DOI] [PubMed] [Google Scholar]
- 34.Field T, Diego M, Dieter J, et al. Prenatal depression effects on the fetus and the newborn. Infant Behavior and Development. 2004;27:216–229. doi: 10.1016/j.infbeh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Lundy BL, Jones NA, Field T, et al. Prepartum depression effects on neonates. Infant Behavior and Development. 1999;22:121–137. [Google Scholar]
- 36.Witt WP, DeLeire T, Hagen EW, et al. The prevalence and determinants of antepartum mental health problems among women in the USA: a nationally representative population-based study. Arch Womens Ment Health. 2010;13:425–437. doi: 10.1007/s00737-010-0176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Witt WP, Wisk LE, Cheng ER, et al. Poor Prepregnancy and Antepartum Mental Health Predicts Postpartum Mental Health Problems Among US Women: A Nationally Representative Population-Based Study. Womens Health Issues. 2011;21:304–313. doi: 10.1016/j.whi.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marcus SM, Flynn HA, Blow FC, Barry KL. Depressive symptoms among pregnant women screened in obstetrics settings. J Womens Health (Larchmt) 2003;12:373–380. doi: 10.1089/154099903765448880. [DOI] [PubMed] [Google Scholar]
- 39.Witt WP, Keller A, Gottlieb C, et al. Access to Adequate Outpatient Depression Care for Mothers in the USA: A Nationally Representative Population-Based Study. J Behav Health Serv Res. 2009;38:191–204. doi: 10.1007/s11414-009-9194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atrash H, Jack BW, Johnson K. Preconception care: a 2008 update. Curr Opin Obstet Gynecol. 2008;20:581–589. doi: 10.1097/GCO.0b013e328317a27c. [DOI] [PubMed] [Google Scholar]
- 41.D’Angelo D, Williams L, Morrow B, et al. Preconception and interconception health status of women who recently gave birth to a live-born infant--Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 26 reporting areas, 2004. MMWR Surveill Summ. 2007;56:1–35. [PubMed] [Google Scholar]
- 42.Kirkham C, Harris S, Grzybowski S. Evidence-based prenatal care: Part I. General prenatal care and counseling issues. Am Fam Physician. 2005;71:1307–1316. [PubMed] [Google Scholar]
- 43.Javert CT. Prevention of habitual spontaneous abortion with early prenatal care. Bull N Y Acad Med. 1958;34:747–756. [PMC free article] [PubMed] [Google Scholar]
- 44.Katz V. Spontaneous and recurrent abortion: etiology, diagnosis, treatment. In: Katz V, Lentz G, Lobo R, Gershenson D, editors. Comprehensive Gynecology. Mosby Elsevier; Philadelphia, PA: 2007. pp. 359–388. [Google Scholar]
- 45.Baird DT, Cnattingius S, Collins J, et al. Nutrition and reproduction in women. Human Reproduction Update. 2006;12:193–207. doi: 10.1093/humupd/dmk003. [DOI] [PubMed] [Google Scholar]
- 46.Ness RB, Grisso JA, Hirschinger N, et al. Cocaine and tobacco use and the risk of spontaneous abortion. N Engl J Med. 1999;340:333–339. doi: 10.1056/NEJM199902043400501. [DOI] [PubMed] [Google Scholar]
- 47.Baraban E, McCoy L, Simon P. Increasing prevalence of gestational diabetes and pregnancy-related hypertension in Los Angeles County, California, 1991-2003. Prev Chronic Dis. 2008;5:A77. [PMC free article] [PubMed] [Google Scholar]
- 48.Caughey AB, Stotland NE, Washington AE, Escobar GJ. Maternal ethnicity, paternal ethnicity, and parental ethnic discordance: predictors of preeclampsia. Obstet Gynecol. 2005;106:156–161. doi: 10.1097/01.AOG.0000164478.91731.06. [DOI] [PubMed] [Google Scholar]
- 49.Thorpe LE, Berger D, Ellis JA, et al. Trends and racial/ethnic disparities in gestational diabetes among pregnant women in New York City, 1990-2001. Am J Public Health. 2005;95:1536–1539. doi: 10.2105/AJPH.2005.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Healy AJ, Malone FD, Sullivan LM, et al. Early access to prenatal care: implications for racial disparity in perinatal mortality. Obstet Gynecol. 2006;107:625–631. doi: 10.1097/01.AOG.0000201978.83607.96. [DOI] [PubMed] [Google Scholar]
- 51.Fiscella K. Race, perinatal outcome, and amniotic infection. Obstet Gynecol Surv. 1996;51:60–66. doi: 10.1097/00006254-199601000-00022. [DOI] [PubMed] [Google Scholar]
- 52.Anum EA, Brown HL, Strauss JF., 3rd Health disparities in risk for cervical insufficiency. Hum Reprod. 2010;25:2894–2900. doi: 10.1093/humrep/deq177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hulsey TC, Levkoff AH, Alexander GR, Tompkins M. Differences in black and white infant birth weights: the role of maternal demographic factors and medical complications of pregnancy. Southern Medical Journal. 1991;84:443–446. doi: 10.1097/00007611-199104000-00008. [DOI] [PubMed] [Google Scholar]
- 54.Alexander GR, Kogan MD, Himes JH, Mor JM, Goldenberg R. Racial differences in birthweight for gestational age and infant mortality in extremely-low-risk US populations. Paediatr Perinat Epidemiol. 1999;13:205–217. doi: 10.1046/j.1365-3016.1999.00174.x. [DOI] [PubMed] [Google Scholar]
- 55.Lu MC, Chen B. Racial and ethnic disparities in preterm birth: the role of stressful life events. Am J Obstet Gynecol. 2004;191:691–699. doi: 10.1016/j.ajog.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 56.Berg CJ, Wilcox LS, d’Almada PJ. The prevalence of socioeconomic and behavioral characteristics and their impact on very low birth weight in black and white infants in Georgia. Matern Child Health J. 2001;5:75–84. doi: 10.1023/a:1011344914802. [DOI] [PubMed] [Google Scholar]
- 57.Hessol NA, Fuentes-Afflick E, Bacchetti P. Risk of low birth weight infants among black and white parents. Obstet Gynecol. 1998;92:814–822. doi: 10.1016/s0029-7844(98)00310-x. [DOI] [PubMed] [Google Scholar]
- 58.Luo ZC, Wilkins R, Kramer MS. Disparities in pregnancy outcomes according to marital and cohabitation status. Obstet Gynecol. 2004;103:1300–1307. doi: 10.1097/01.AOG.0000128070.44805.1f. [DOI] [PubMed] [Google Scholar]
- 59.Hein HA, Burmeister LF, Papke KR. The relationship of unwed status to infant mortality. Obstet Gynecol. 1990;76:763–768. doi: 10.1097/00006250-199011000-00007. [DOI] [PubMed] [Google Scholar]
- 60.Abel MH. Low birth weight and interactions between traditional risk factors. J Genet Psychol. 1997;158:443–456. doi: 10.1080/00221329709596681. [DOI] [PubMed] [Google Scholar]
- 61.Rantakallio P, Oja H. Perinatal risk for infants of unmarried mothers over a period of 20 years. Early Hum Dev. 1990;22:157–169. doi: 10.1016/0378-3782(90)90182-i. [DOI] [PubMed] [Google Scholar]
- 62.Bruckner TA, Saxton KB, Anderson E, Goldman S, Gould JB. From paradox to disparity: trends in neonatal death in very low birth weight non-Hispanic black and white infants, 1989-2004. J Pediatr. 2009;155:482–487. doi: 10.1016/j.jpeds.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 63.Collins JW, Jr., David RJ, Simon DM, Prachand NG. Preterm birth among African American and white women with a lifelong residence in high-income Chicago neighborhoods: an exploratory study. Ethn Dis. 2007;17:113–117. [PubMed] [Google Scholar]
- 64.Rutter DR, Quine L. Inequalities in pregnancy outcome: a review of psychosocial and behavioural mediators. Soc Sci Med. 1990;30:553–568. doi: 10.1016/0277-9536(90)90154-k. [DOI] [PubMed] [Google Scholar]
- 65.Kalinka J, Laudanski T, Hanke W, Wasiela M. Do microbiological factors account for poor pregnancy outcome among unmarried pregnant women in Poland? Fetal Diagn Ther. 2003;18:345–352. doi: 10.1159/000071979. [DOI] [PubMed] [Google Scholar]
- 66.Kandel DB, Griesler PC, Schaffran C. Educational attainment and smoking among women: risk factors and consequences for offspring. Drug Alcohol Depend. 2009;104:S24–33. doi: 10.1016/j.drugalcdep.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blumenshine P, Egerter S, Barclay CJ, Cubbin C, Braveman PA. Socioeconomic disparities in adverse birth outcomes: a systematic review. Am J Prev Med. 2010;39:263–272. doi: 10.1016/j.amepre.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 68.Bramness JG, Walby FA, Hjellvik V, Selmer R, Tverdal A. Self-reported Mental Health and Its Gender Differences as a Predictor of Suicide in the Middle-Aged. American Journal of Epidemiology. 2010;172:160. doi: 10.1093/aje/kwq091. [DOI] [PubMed] [Google Scholar]
- 69.Ananth CV, Peltier MR, Chavez MR, Kirby RS, Getahun D, Vintzileos AM. Recurrence of ischemic placental disease. Obstetrics & Gynecology. 2007;110:128. doi: 10.1097/01.AOG.0000266983.77458.71. [DOI] [PubMed] [Google Scholar]